Abstract
Exploration and activity are often described as trade-offs between the fitness benefits of gathering information and resources, and the potential costs of increasing exposure to predators and parasites. More exploratory individuals are predicted to have higher rates of parasitism, but this relationship has rarely been examined for virus infections in wild populations. Here, we used the multimammate mouse Mastomys natalensis to investigate the relationship between exploration, activity, and infection with Morogoro virus (MORV). We characterized individual exploratory behavior (open field and novel object tests) and activity (trap diversity), and quantified the relationship between these traits and infection status using linear regression. We found that M. natalensis expresses consistent individual differences, or personality types, in exploratory behavior (repeatability of 0.30, 95% CI: 0.21–0.36). In addition, we found a significant contrasting effect of age on exploration and activity where juveniles display higher exploration levels than adults, but lower field-activity. There was however no statistical evidence for a behavioral syndrome between these 2 traits. Contrary to our expectations, we found no correlation between MORV infection status and exploratory behavior or activity, which suggests that these behaviors may not increase exposure probability to MORV infection. This would further imply that variation in viral infection between individuals is not affected by between-individual variation in exploration and activity.
Keywords: animal personality, arenavirus, disease ecology, exploration, Mastomys natalensis, Morogoro virus
Animals explore their surroundings to reduce uncertainty in heterogeneous environments, gathering information about the availability of resources such as food (Tebbich et al. 2009) or mates (Schwagmeyer 1995). Such information-gathering behavior is referred to as intrinsic exploration (Hughes 1997), and there is a growing body of evidence that this behavior, together with others, shows consistent patterns between individuals over time and/or across situations (Sih et al. 2004; Réale et al. 2007; Bell et al. 2009) in a wide range of taxa (Gosling 2001; Bell 2007; Briffa and Weiss 2010). Such consistent differences in behavior are commonly referred to as personality traits (Réale et al. 2007), and can have a number of fitness consequences (Dingemanse and Réale 2005; Smith and Blumstein 2007). Shyer Trinidadian guppies Poecilia reticulata, for instance, exhibit stronger antipredator responses than bolder individuals (Brown et al. 2014). Personality can also influence reproductive success (Smith and Blumstein 2007), with high levels of exploration positively associated with reproductive success earlier in life both within (e.g., Tamias striatus; Montiglio et al. 2014) and across (e.g., muroid rodents; Careau et al. 2009) species.
The benefits of behavioral traits such as exploration, boldness, and activity may come with potential fitness costs if they increase the probability or rate at which individuals encounter predators (Boon et al. 2008; Jones and Godin 2010) and/or pathogens (Barber and Dingemanse 2010; Boyer et al. 2010). The magnitude of these fitness costs is predicted to co-vary with prey personality and predator foraging strategies or modes of pathogen transmission. For example, more exploratory chipmunks Tamias sibiricus have higher parasite loads than less exploratory individuals because they are more active and cover a larger area, which increases their encounter rate of parasites (Boyer et al. 2010). Alternatively, aggressive interactions may increase the transmission of some viruses via infectious saliva in bite wounds, as with Seoul virus (Glass et al. 1988; Klein et al. 2004). Aggressive behaviors may also correlate with other personality traits, such as boldness, thus forming a behavioral syndrome (Sih et al. 2004). Bold deer mice Peromyscus maniculatus, for instance, are 3 times more likely to be infected with Sin Nombre virus than shy deer mice, presumably because they engage more frequently in aggressive interactions that are predicted to increase the probability of virus transmission (Dizney and Dearing 2013). Similar relationships have been found in feral domestic cats Felis catus between boldness and the prevalence of Feline Immunodeficiency Virus, another virus transmitted via saliva (Natoli et al. 2005). Alternatively, if pathogens are shed into the environment via feces or urine, other personality traits such as exploration or activity may increase the likelihood of encountering contaminated environments and hence infection (Hughes et al. 2012).
Here, we use the multimammate mouse Mastomys natalensis as a model organism to investigate the relationship between 2 personality traits (exploration and activity), reproductive age, and infection with Morogoro virus (MORV). Viral RNA particles can be found in the blood of infected individuals up to 7 days after infection after which it declines rapidly, but they continue to shed virus particles in their excretions around up to 40 days after infection (Borremans et al. 2015b; Mariën et al. 2017). Still, it is unknown how long these excretions stay infectious in the environment. Transmission of MORV is mainly horizontal (Borremans et al. 2011) and is believed to occur via exposure to these virus particles excreted in feces, urine, and saliva (Borremans et al. 2015b), and thus potentially via direct contacts (e.g., grooming, licking, and mating) or through indirect exposure to virus particles in the environment. Infection appears to be acute, followed by a lifelong immunity, although a small proportion of animals seems to become chronically infected (Mariën et al. 2017).
We hypothesized that exploration and activity are drivers of MORV transmission in M. natalensis since the virus can potentially be transmitted via direct and indirect contacts. Mating in M. natalensis is believed to occur via a scramble competition mating system, in which males search competitively for females (Kennis et al. 2008), possibly in combination with a dominance hierarchy (Borremans et al. 2014). Male reproductive success in this species is correlated with weight, but is also highly heterogeneous, with a relatively small percentage (17–40%) of males recorded as fathering all offspring in a population (Kennis et al. 2008). Furthermore, territoriality is low during the breeding season and both males and females have overlapping home ranges (Borremans et al. 2014). This means that highly active or exploratory individuals of both sexes are more likely to enter home ranges of other individuals, which could lead to a higher probability of encountering MORV-infected individuals and excretions.
To test whether activity or exploration might play a role in the transmission of MORV in M. natalensis populations, we used field-based measures of activity, in combination with a series of behavioral trials to characterize exploration, and quantified the relationship between each individual’s personality traits and their MORV infection status. We hypothesized that exploration and activity would increase exposure to MORV. Specifically, we predicted that MORV-specific antibody prevalence should be higher in more exploratory and active individuals. In addition, we predicted that juveniles would be more exploratory than adults, as they are in a greater need to gather information about their environment (Hughes 1997; Biondi et al. 2013), but that adults would be more active than juveniles, because of their larger home ranges (Borremans et al. 2014) and a potential need to cover a larger area when searching for mates (Kennis et al. 2008).
Materials and Methods
Study site and species
Mastomys natalensis is the most common indigenous rodent in sub-Saharan Africa and a well-studied agricultural pest species (Leirs et al. 1994). The species’ reproductive cycle is strongly related to seasonal rainfall patterns, and populations can reach high densities in habitats where food is abundant (Leirs et al. 1994; Leirs et al. 1997). The analysis of movement patterns during a long-term field study has shown that male home ranges decrease and those of females increase during periods of high resource availability and population density (Borremans et al. 2014). During these periods, home ranges overlap greatly, indicating a low level of territoriality and reduced spatial activity. Home range sizes of both sexes are similar during the breeding season (Borremans et al. 2014).
We conducted fieldwork on the campus of the Sokoine University of Agriculture (SUA; Morogoro, Tanzania) between 29 July and 18 October 2013 (dry season—tail end of the breeding period). We trapped animals on 6 grids of 1 ha (100 traps in a 10 × 10 arrangement, 10 m among traps) in agricultural fields. Grids were spaced at least 700 m apart for spatial independence (Borremans et al. 2014). Within a trapping session, we implemented capture–mark–recapture trapping for 3 consecutive nights every 2 weeks for each grid, using Sherman LFA live traps (Sherman Live Trap Co., Tallahassee, FL) baited with a mix of peanut butter and maize flour. Traps were set in the evening and checked in the early morning and captured rodents were transported to the nearby SUA Pest Management Center for behavioral tests and blood sampling (details below). Rodents were released in the evening at their site of capture, after which we rebaited and re-set all traps. We conducted a total of 6 trapping sessions for all grids except 1, for which only 4 sessions were completed.
We used toe clipping to uniquely mark individuals at their first capture (Borremans et al. 2015a), and we recorded the weight, sex, and reproductive age (following Leirs et al. 1994) of individuals at each capture. We considered mice to be juvenile if signs of sexual activity could not be observed (scrotal testes in males; perforated vagina or pregnancy in females). In order to minimize any potential effects of stress, we recorded the behavior of each individual (see below for details) before blood sampling and toe clipping. Blood samples were taken from the retro-orbital sinus and preserved on pre-punched filter paper (∼15 μL/punch; Serobuvard, LDA 22, Zoopole, France). Saliva was collected by placing a small slip of filter paper into the mouth of the animal for approximately 20 s. If the animal urinated, a urine sample was collected on filter paper. Samples on filter paper were dried and stored in the dark, at ambient temperature (<28 °C) for 2 months, after which they were preserved at −20 °C as suggested by Borremans (2014). All experimental procedures were approved by the University of Antwerp Ethical Committee for Animal Experimentation (LA1100135) and adhered to the EEC Council Directive 2010/63/EU, and followed the Animal Ethics guidelines of the Research Policy of Sokoine University of Agriculture.
Behavioral trials
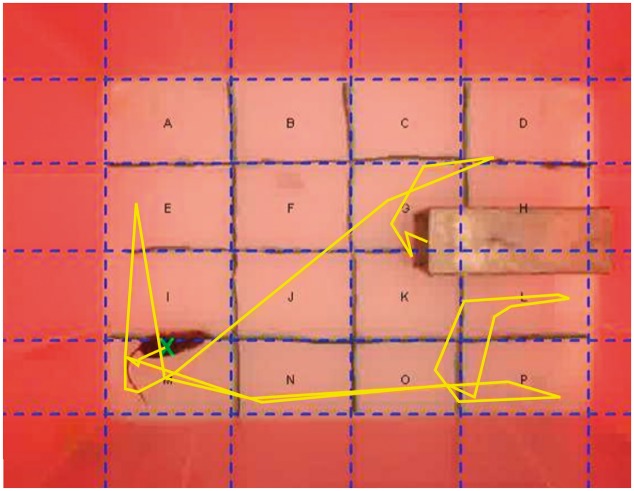
We conducted behavioral trials in the morning in 75 (L) × 55 (W) × 44 (H) cm semi-translucent arenas, the walls of which were covered with red plastic (Figure 1). We conducted trials under low-level natural daylight, which mice should have perceived as dark due to the red plastic sheets coating the walls, and recorded all trials using a digital video camera installed above each arena. Sixteen rectangles (19 × 13 cm) were marked on the floor of the arena to facilitate the automatic extraction of behavioral data (Figure 1). At the start of each trial, we placed a trap containing an individual at one end of the arena, with the trap opening facing the inside of the arena. The behavioral trial started when the trap was manually opened.
Figure 1.
The open field (OF) arena during data extraction, showing the arena walls (dark red) and floor (light red). The floor was divided into 16 rectangles (19 × 13 cm) and the yellow line tracks the animal’s movement.
Each behavioral trial consisted of 2 tests to quantify an individual’s exploratory behavior. First, an open field (OF) test which measured each individual’s reaction to a novel environment (Archer 1973). The OF test assumes that movement within the experimental arena is an index of exploration, as animals move around to investigate their surroundings (Dingemanse et al. 2002). After 5 min, the second test, a novel object (NO) test, began when we introduced a NO (a blue plastic box) into the arena, on the opposing side of the trap opening. In combination, these tests measure an individual’s exploration of a novel environment, and toward a NO (Réale et al. 2007). NO tests ran for 5 min, after which the animals were removed from the arena. The experimenter was only present at the start of the OF test, to open the trap, and at the beginning of the NO test for the introduction of the NO. To remove scent and dirt, we cleaned experimental arenas and NOs after every trial using 70% ethanol. Individuals were released at their point of capture following the completion of all behavioral tests and were held for a maximum of 5 h. Consecutive tests for individuals were separated by a minimum of 11 days (21 ± 9 days, mean ± SE).
Video analysis
We developed an imaging processing algorithm in R 3.0.2 (R Core Team 2013) to automatically extract behavioral data from the video files (code available on request): (i) locomotion, measured as the total number of times the animal changed squares, calculated separately for OF and NO tests (see Figure 1) and (ii) entrance latency, the time (in seconds) an animal took to leave the trap in the OF test, and after the introduction of the NO. If an animal did not leave the trap after 5 min in either test we recorded 300 s. Animals were not forced to leave the trap, as this would induce fear and/or anxiety behavior instead of exploration (Misslin and Cigrang 1986).
Detection and quantification of MORV RNA and antibodies against MORV
We analyzed blood, saliva, and urine samples at the University of Antwerp for MORV-specific IgG antibodies using immunofluorescence assay protocols described in Günther et al. (2009). Viral RNA extraction was performed on all samples using the QIAmp vRNA Mini Kit [Qiagen, Hilden, Germany; see Borremans et al. (2015b) for details]. RT-PCR protocols followed those described in Günther et al. (2009), with MoroL3359-forward and MoroL3753-reverse primers used to target a 340-nucleotide portion of the RNA-dependent RNA polymerase gene of MORV. We confirmed all amplicons by Sanger-sequencing at the Vlaams Instituut voor Biotechnologie (Antwerp, Belgium), and compared them to known MORV sequences using Geneious 7.0.6 (Kearse et al. 2012).
Statistical analysis
Individual exploratory behavior
We conducted 295 behavioral tests on 122 individuals (Nmale = 42, Nfemale = 80). All individuals were recorded at least twice (Nrecorded twice = 82, Nthree times = 30, Nfour times = 9, Nfive times = 1), which allowed us to estimate the repeatability of the behavioral responses measured in the behavioral tests (Réale et al. 2007). We used a principal component analysis (PCA) to reduce the number of behavioral variables from the OF and NO tests, and applied the Kaiser–Guttman criterion (eigenvalue >1; Kaiser 1991; Peres-Neto et al. 2005) when selecting the number of components to retain.
We used a linear mixed model (LMM) with maximum likelihood (Pinheiro and Bates 2000; Crawley 2012) to determine the effect of independent variables on the component (PCA) scores. We used sex (male/female), reproductive age (adult/juvenile), and a binomial variable describing whether it was the first time an individual had been caught and recorded (1 or 2, further referred to as first recording) as fixed effects, and a 3-way interaction between all the fixed effects. Grid and M. natalensis identity (ID) were included as random effects to correct for repeated measures effects, and to estimate the between- and within-individual variance required to calculate repeatability (Nakagawa and Schielzeth 2010; Wolak et al. 2012). To find the model that best fit our data, we removed statistically non-significant interactions and fixed effects from the model using a backward stepwise procedure (using P = 0.05 as the level to reject a fixed effect) implemented in the R package lmerTest (version 2.0; Kuznetsova et al. 2014). We used a likelihood ratio test (LRT) to determine the significance of the random effects, by comparing the final LMM with a linear model (LM) without ID or grid as a random effect; a P-value < 0.05 indicates that a significant amount of variance can be ascribed to between-individual variance (Martin and Réale 2008). Although we used the OF and NO tests to quantify exploration behavior, a single exploration value was needed for further analysis in the generalized linear model (GLM). We therefore used the best linear unbiased predictors (BLUPs) from the final LMM to generate a single exploration value (an individual index of personality) per individual. BLUPs provide estimates of the random effects (ID) independent of the other terms within the model, and are standardized to a mean of zero (Kruuk 2004; Martin and Réale 2008). They are less sensitive to extreme values within the data and are a more appropriate estimate for personality type than the mean of all measurements (Pinheiro and Bates 2000).
Trap diversity
We used the live-trapping data, and specifically trap diversity (the total number of unique trap locations in which an individual was trapped), to estimate individual activity in the field (Boyer et al. 2010). To test which factors affected activity, we ran a GLM with a Poisson error distribution, with activity as the dependent variable, and sex, reproductive age, trappability (total number of times an individual was trapped), and personality type (BLUP) as independent variables, together with a 2-way interaction between sex and reproductive age (Crawley 2012).
MORV infection status: We captured 776 different individuals (from 1,133 captures), on all grids during 108 trapping nights throughout the whole study period. We screened all individuals for MORV antibodies at least once during each recapture session in which it was encountered. All individuals that were recaptured during different trapping sessions (N = 220) were screened for MORV RNA at least twice. More details about the individuals’ initial infection state can be found in Mariën et al. (2017).
We tested how MORV antibody status and MORV RNA status (binary response for each test: positive or negative) in the full dataset varied as a function of sex, reproductive age, and their interaction using separate generalized linear mixed models (GLMMs) with binomial error distributions. We included capture grid as a random effect to control for variation in prevalence among the grids. Prevalence of MORV antibodies and MORV RNA, and their 95% confidence intervals, were calculated manually.
To test for the relationships between MORV infection status (antibodies, binary response) and personality type, we constructed a new GLM (with a binomial error distribution; Crawley 2012) with sex, reproductive age, trap diversity, and personality type (BLUP) as independent variables using the reduced, behavioral dataset. All statistical analyses were executed using R software 3.0.2 (R Development Core Team 2013).
Results
Individual exploratory behavior
The PCA reduced the number of exploratory variables to 2 components with an eigenvalue >1 (Table 1) which, combined, explained 86.80% of the total variance. The first component (PC1) explained 56.98% of the variance and was positively correlated with locomotion in both the OF and NO tests, and negatively with the latency measurements from both tests. The second component (PC2) explained 29.82% of the total variance. PC2 was positively correlated with locomotion during the NO test, but negatively with locomotion in the OF test (Table 1). We chose to retain only PC1 in our study as it explained the majority of the variance and because is strongly correlated with movement during the OF and NO which is an index of exploration (Dingemanse et al. 2002). Individual exploration types (BLUPS) were calculated from this component. From hereafter, we will refer to PC1 as exploration behavior (PC1) and the individual indices of exploration (BLUP) will be referred to as personality type.
Table 1.
Correlation of each behavior observed during the open field (OF) and novel object (NO) tests on Mastomys natalensis with the components of the principal component analysis (PCA)
| Behavioral variables | Component 1 (PC1) | Component 2 (PC2) |
|---|---|---|
| OF: Locomotion (no. squares crossed) | 0.553 | −0.411 |
| OF: Latency to leave trap | −0.508 | 0.521 |
| NO: Locomotion (no. squares crossed) | 0.455 | 0.554 |
| NO: Latency to leave trap | −0.478 | −0.503 |
| Total variance (%) | 56.98 | 29.82 |
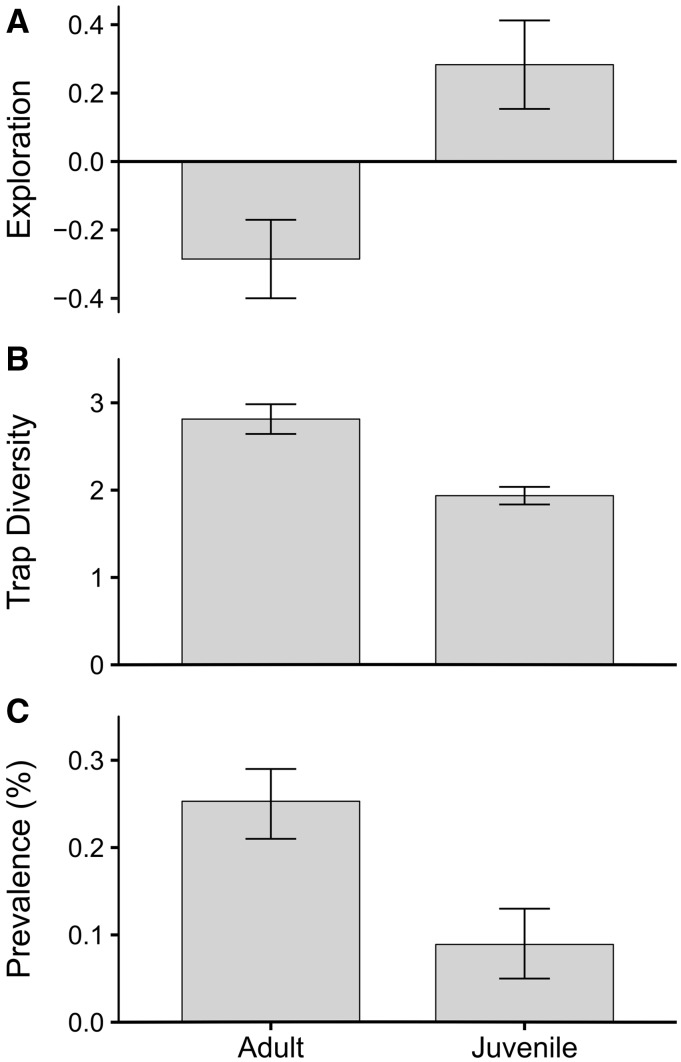
The LMM on exploration behavior (PC1) revealed a significant effect of reproductive age (Table 2), where juveniles were significantly more explorative than adults (coefficient ± SE = 0.564 ± 0.210; t114 = 2.687, P = 0.008; Figure 2A). There were no differences between the sexes or an effect of recording order (first vs. later recordings), and no interaction terms were significant (Table 2). There were no differences in exploration behavior (PC1) between the 6 grids (LRT χ2 = 0.00; P = 1) but M. natalensis ID explained a significant proportion of the variance in exploration behavior (LRT χ2 = 15.63; P < 0.001) and there were consistent differences in exploration behavior (PC1) through time between individuals with a repeatability of R = 0.30 (95% confidence interval 0.21–0.36).
Table 2.
Results from the stepwise reduction of the full linear mixed model (LMM) of the PC1 components (exploration behavior)
| PC1 (Exploration behavior) |
|||
|---|---|---|---|
| Dependent variables | df | F | P |
| First recording×reproductive age×sex | 1, 172 | 0.003 | 0.960 |
| First recording×reproductive age | 1, 175 | 0.759 | 0.385 |
| First recording×sex | 1, 175 | 2.238 | 0.137 |
| Reproductive age×sex | 1, 111 | 2.968 | 0.088 |
| First recording | 1, 178 | 0.143 | 0.706 |
| Sex | 1, 114 | 1.041 | 0.310 |
| Reproductive agea | 1, 114 | 7.219 | 0.008 |
Notes: Significant P-values (P < 0.05) are marked in bold.
Final model.
Figure 2.
Differences between adults and juveniles in mean (±SE) in (A) exploration behavior (PC1), (B) trap diversity (i.e., the total number of different trap locations in which an individual was trapped), and (C) MORV-specific antibody prevalence (MORV positive individuals divided by all individuals). Juveniles are significantly more exploratory than adults, but less active (lower trap diversity). MORV-specific antibody prevalence is significantly higher in adults than juveniles.
Trap diversity
Trap diversity (activity) was significantly positively correlated with the total number of times an individual was caught (trappability; coefficient ± SE = 0.106 ± 0.023, z119 = 4.596, P < 0.001). Independent of trappability, adult individuals were trapped in significantly more different traps than juveniles were (coefficient ± SE = −0.275 ± 0.122, z119 = −2.251, P = 0.024; Figure 2B), but there were no significant differences between sexes (P > 0.8) or a significant interaction between sex and age (P > 0.8). In addition, there was no effect of personality type (BLUP) on trap diversity (P > 0.5), and thus no statistical evidence for a behavioral syndrome between activity and exploration in M. natalensis.
MORV infection status
Throughout the whole study period, we found a prevalence of 0.18 for MORV antibody (95% confidence interval 0.11–0.24) and 0.23 for MORV RNA (95% confidence interval 0.17–0.29). We found no statistical differences between males and females in their MORV antibody [prevalence: males = 0.18 (95% confidence interval 0.09–0.25), females = 0.18 (95% confidence interval 0.12–0.24); coefficient ± SE = −0.06 ± 0.19, z772 = −0.32, P = 0.75] or MORV RNA infection status [prevalence: males = 0.23 (95% confidence interval 0.13–0.33), females = 0.23 (95% confidence interval 0.13–0.33); coefficient ± SE = 0.12 ± 0.31, z251 = −0.40, P = 0.69]. However, there were strong differences between reproductive ages, with prevalence significantly higher in adults than juveniles for both MORV antibody [prevalence: adult = 0.26 (95% confidence interval 0.17–0.35), juvenile = 0.13 (95% confidence interval 0.07–0.19); coefficient ± SE = −0.81 ± 0.19, z772 = −4.17, P < 0.001] and MORV RNA [prevalence: adult = 0.32 (95% confidence interval 0.20–0.43), juvenile = 0.17 (95% confidence interval 0.08–0.26); coefficient ± SE = −0.82 ± 0.30, z251 = −2.70, P = 0.007].
Of the individuals used in our behavioral study (i.e., those we recorded at least twice), 23 individuals were positive for MORV-specific antibodies at least once during the study (Nmales = 9, Nfemales = 14). MORV antibody prevalence in this group was 0.19 (95% confidence interval 0.14–0.24), similar to the MORV antibody prevalence of 0.18 in the entire dataset (95% confidence interval 0.11–0.24). Eleven individuals were seropositive throughout the whole study, indicating that they were infected with MORV prior to this study (Borremans et al. 2011). These individuals did not differ in weight from those that seroconverted during the study (F1 = 1.391, P = 0.252). Similar to the overall dataset, adults had a higher probability of testing positive for MORV-specific antibodies than juveniles in the reduced behavioral dataset (z1,118 = −2.956, P = 0.003, Figure 2C), and there were no differences in infection status between the sexes (z1,118 = 1.127, P = 0.202). There were no relationships between infection status and personality type (BLUP) or activity (P > 0.05; Table 3). Fifteen individuals within the behavioral dataset tested positive for MORV RNA (MORV RNA prevalence 0.12; 95% confidence interval 0.08–0.16); 11 of these were also MORV antibody positive. No statistical analyses were performed on the MORV RNA data due to the small sample size and its similarity to the antibody dataset.
Table 3.
Results from the generalized linear model (GLM) with infection (MORV antibody status) as a binomial variable
| Estimate ± SE | z-value | P–value | |
|---|---|---|---|
| Intercept | −0.986±0.679 | −1.453 | 0.146 |
| Trap diversity | −0.008±0.210 | −0.040 | 0.968 |
| Exploration (BLUP) | −0.202±0.450 | −0.448 | 0.654 |
| Reproductive age (juvenile) | −1.832±0.605 | −3.026 | 0.002 |
| Sex (male) | 0.712±0.532 | 1.339 | 0.180 |
Note: Statistically significant results (P < 0.05) are marked in bold.
Discussion
It has been hypothesized that consistent individual differences in exploratory behavior may influence parasite or pathogen infection status, but this relationship has been investigated for only a limited range of disease agents (Barber and Dingemanse 2010). In this study, we have provided evidence that M. natalensis expresses consistent individual differences, or personality types, in exploration behavior with an overall repeatability of 30%. Contrary to our expectations we found no relationship between individual’s MORV infection status and their exploration or activity level.
Exploration is an information-gathering behavior used for purposes such as assessing predation risk and investigating new food resources (Hughes 1997; Tebbich et al. 2009; Reader 2015). As predicted, juveniles were, on average, more exploratory than adults. Such a decline in exploration with age has been found in several other taxa, for example brown rats (Rattus norvegicus; Ray and Hansen 2005), corvids (Miller et al. 2015), and chimango caracaras (Milvago chimango; Biondi et al. 2013), and has been attributed to individuals’ need to gather information about their environment early in life (Reader 2015). Alternatively, because exploratory behavior can attract predators (Rödel et al. 2015), highly explorative individuals could be predated before reaching adulthood, hence adults may behave more carefully than juveniles due to experience (Rödel et al. 2015). It is also possible that juveniles are less efficient at gathering information and must therefore spend more time exploring their environment than adults to acquire the same amount of information (Biondi et al. 2013).
Although adult M. natalensis were less exploratory than juveniles, they were more active in their natural environments (i.e., visited a greater variety of traps), independent of the number of times they were trapped. These activity patterns in adults possibly stem from the timing of our study during the breeding season. On the one hand, female home ranges increase during this period, presumably to gather more food (Borremans et al. 2014). Males, on the other hand, are highly active in order to increase their reproductive success in the species’ scramble mating competition (Kennis et al. 2008). Nonetheless, we found no statistical evidence for a behavioral syndrome between activity and exploration. The absence of a behavioral syndrome between these 2 traits has been found in other species (Patterson and Schulte-Hostedde 2011; Carter et al. 2013, but see Boyer et al. 2010; Kekäläinen et al. 2014), and supports the results of a meta-analysis that showed that the average strength of the correlation between activity and exploration is weak (Garamszegi et al. 2012), and can depend on a range of environmental factors (e.g., predation pressure, Dingemanse et al. 2007).
Exploration and activity may have potential fitness costs if they increase individuals’ encounter rates with pathogens. Most individuals infected with MORV shed infectious particles acutely in their urine, feces, and saliva up to approximately 40 days after infection (Borremans et al. 2015b), although some individuals might become chronically infected (Mariën et al. 2017). More exploratory or active individuals may therefore have a higher probability of contacting infectious excretions and becoming infected. As antibodies indicate past infection and remain present in the host even after the virus is cleared (Mills et al. 2007; Günther et al. 2009; Borremans et al. 2015a), the higher antibody prevalence that we observed in adult M. natalensis is the result of cumulative opportunities to encounter the virus, as previously observed and discussed by Borremans et al. (2011), and also for other arenaviruses and host species (Demby et al. 2001; Mills et al. 2007). Nevertheless, we found no direct link between MORV infection status and exploration or activity. This may suggest that virus particles shed in the excretions of recent infected individuals are not as infectious as previously thought (e.g., compared with Lassa virus; Fichet-Calvet and Rogers 2009) and that MORV transmission may occur more commonly through direct contact (e.g., social interactions and mating) with infected conspecifics (Borremans et al. 2011). Our lack of significant results may also stem from our low sample size of MORV antibody positive individuals resulting in low statistical power.
If MORV transmission is strongly linked to direct contact with infected conspecifics rather than through infected environments, MORV RNA prevalence should then increase when social contacts between conspecifics increases (Drewe and Perkins 2014). We found that MORV RNA prevalence, a clear indication of recent infection, is significantly higher in adults than juveniles; similar patterns have been reported for Lassa virus, another arenavirus (Fichet-Calvet et al. 2008). Furthermore, we showed that adults are significantly more active than juveniles, which is likely to increase their probability of encountering infectious individuals (Kennis et al. 2008). Combined, these results suggest that direct contacts between individuals may be important for the transmission of MORV. If this is the case, there are multiple, non-mutually exclusive behaviors that would be expected to increase transmission of MORV. Aggressive behaviors, for example, increase transmission in other disease systems [e.g., hantavirus in: R. norvegicus (Klein et al. 2004) and P. maniculatus (Dizney and Dearing 2013)], but seem unlikely in M. natalensis due to their low levels of aggression (Veenstra 1958; Perrin et al. 2001). Alternatively, MORV could be transmitted during mating, as has been found in the Machupo arenavirus (Webb et al. 1975). While transmission during mating may indeed happen for MORV, on-going transmission in sexually inactive juvenile M. natalensis (Borremans et al. 2011), as indicated by high RNA prevalence, suggests that this is not the major mode of transmission during the study period. This however does not preclude the possibility that transmission during mating is the main mode of transmission during the low-density breeding season, when animals are almost exclusively sexually mature. Social contacts and position within the social network may be more important for virus transmission through direct contacts (Godfrey 2013; Drewe and Perkins 2014). Individuals with a large number of contacts, for instance, are expected to play a key role in acquiring and transmitting the virus (Lloyd-Smith et al. 2005; White et al. 2017). Strong heterogeneity in social contacts has indeed been found for M. natalensis (Borremans et al. 2016) but has not yet been linked to infection status. A more detailed study examining the relationships between sociability, social networks, personality, and MORV infection status could provide us with a greater understanding of MORV ecology and transmission dynamics.
It has been suggested that different personality types could vary in variations in disease susceptibility and/or transmission (Barber and Dingemanse 2010; Hawley et al. 2011; Barron et al. 2015; Ezenwa et al. 2016). While there has been a focus on the role of personality in disease transmission in tick (Boyer et al. 2010; Patterson and Schulte-Hostedde 2011; Bajer et al. 2015), trematode (Nakagawa et al. 2010; Koprivnikar et al. 2012; Seaman and Briffa 2015), and malarial (Dunn et al. 2011; Garamszegi et al. 2015; Garcia-Longoria et al. 2015) disease systems, more studies are acknowledging the importance of personality in viral models (Natoli et al. 2005; Dizney and Dearing 2013; Araujo et al. 2016). Our study provides the first evidence for the existence of personality types in M. natalensis, a significant pest species in sub-Saharan Africa (Leirs 1995), and reservoir host and vector for several important zoonotic infections (Frame et al.1970; Isaäcson 1975; Günther et al. 2009; Katakweba et al. 2012). We found that juveniles were typically more exploratory than adults under laboratory conditions, but also less active in the field. Nevertheless, we found no link between individuals’ exploratory behavior or activity and their MORV infection status, which may suggest that environmental transmission of MORV is not as prominent as we hypothesized. Together our results may indicate that exploration and activity might not increase the individual’s likelihood to come into contact with the virus suggesting that variation in viral infection between individuals is not affected by between-individual variation in exploration and activity.
Acknowledgments
We would like to thank the staff at the Pest Management Center (Sokoine University of Agriculture) for their excellent assistance during the fieldwork, specifically Shabani Lutea and Geofrey Sabuni. Thanks to Luc De Bruyn for statistical advice. We have no competing interests.
Funding
This work was supported by the University of Antwerp [grant number GOA BOF FFB3567 awarded to H.L.] and the Antwerp Study Centre for Infectious Diseases (ASCID). Flemish University Development Cooperation (VLIR-UOS), Tanzania travel grant [to B.V.B.] and B.V.B. is currently a research fellow of Research Foundation Flanders (FWO).
References
- Araujo A, Kirschman L, Warne RW, 2016. Behavioural phenotypes predict disease susceptibility and infectiousness. Biol Lett 12:20160480.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J, 1973. Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235. [DOI] [PubMed] [Google Scholar]
- Bajer K, Horváth G, Molnár O, Török J, Garamszegi LZ. et al. 2015. European green lizard Lacerta viridis personalities: linking behavioural types to ecologically relevant traits at different ontogenetic stages. Behav Process 111:67–74. [DOI] [PubMed] [Google Scholar]
- Barber I, Dingemanse NJ, 2010. Parasitism and the evolutionary ecology of animal personality. Philos Trans R Soc B Biol Sci 365:4077–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D, Gervasi S, Pruitt J, Martin L, 2015. Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Curr Opin Behav Sci 6:35–40. [Google Scholar]
- Bell AM, 2007. Evolutionary biology: animal personalities. Nature 447:539–540. [DOI] [PubMed] [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL, 2009. The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi LM, Guido J, Madrid E, Bó MS, Vassallo AI, 2013. The effect of age and sex on object exploration and manipulative behavior in a neotropical raptor, the Chimango caracara Milvago chimango. Ethology 119:221–232. [Google Scholar]
- Boon AK, Réale D, Boutin S, 2008. Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117:1321–1328. [Google Scholar]
- Borremans B, 2014. Ammonium improves elution of fixed dried blood spots without affecting immunofluorescence assay quality. Trop Med Int Health 19:413–416. [DOI] [PubMed] [Google Scholar]
- Borremans B, Hughes NK, Reijniers J, Sluydts V, Katakweba AAS. et al. 2014. Happily together forever: temporal variation in spatial patterns and complete lack of territoriality in a promiscuous rodent. Popul Ecol 56:109–118. [Google Scholar]
- Borremans B, Leirs H, Gryseels S, Günther S, Makundi R. et al. 2011. Presence of Mopeia virus, an African arenavirus, related to biotope and individual rodent host characteristics: implications for virus transmission. Vector Borne Zoonotic Dis 11:1125–1131. [DOI] [PubMed] [Google Scholar]
- Borremans B, Reijniers J, Hughes NK, Godfrey SS, Gryseels S. et al. 2016. Nonlinear scaling of foraging contacts with rodent population density. Oikos 126:792–800. [Google Scholar]
- Borremans B, Sluydts V, Makundi RH, Leirs H, 2015a. Evaluation of short-, mid- and long-term effects of toe clipping on a wild rodent. Wildl Res 42:143–148. [Google Scholar]
- Borremans B, Vossen R, Becker-Ziaja B, Gryseels S, Hughes N. et al. 2015b. Shedding dynamics of Morogoro virus, an African arenavirus closely related to Lassa virus, in its natural reservoir host Mastomys natalensis. Sci Rep 5:10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer N, Réale D, Marmet J, Pisanu B, Chapuis J-L, 2010. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J Anim Ecol 79:538–547. [DOI] [PubMed] [Google Scholar]
- Briffa M, Weiss A, 2010. Animal personality. Curr Biol 20:912–914. [DOI] [PubMed] [Google Scholar]
- Brown GE, Elvidge CK, Ramnarine I, Chivers DP, Ferrari MCO, 2014. Personality and the response to predation risk: effects of information quantity and quality. Anim Cogn 17:1063–1069. [DOI] [PubMed] [Google Scholar]
- Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM, 2009. Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct Ecol 23:150–156. [Google Scholar]
- Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R, 2013. Animal personality: what are behavioural ecologists measuring? Biol Rev 88:465–475. [DOI] [PubMed] [Google Scholar]
- Crawley MJ, 2012. The R Book. 1st edn. Chichester: John Wiley & Sons, Ltd. [Google Scholar]
- Demby AH, Inapogui A, Kargbo K, Koninga J, Kourouma K. et al. 2001. Lassa fever in Guinea: II. Distribution and prevalence of Lassa virus infection in small mammals. Vector Borne Zoonotic Dis 1:283–297. [DOI] [PubMed] [Google Scholar]
- Dingemanse N, Réale D, 2005. Natural selection and animal personality. Behaviour 142:1159–1184. [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ, 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav 64:929–938. [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R. et al. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138. [DOI] [PubMed] [Google Scholar]
- Dizney L, Dearing MD, 2013. The role of behavioural heterogeneity on infection patterns: implications for pathogen transmission. Anim Behav 86:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe JA, Perkins SE, 2014. Disease transmission in animal social networks In: Krause J, James R, Franks DW, editors. Animal Social Networks. Oxford: Oxford University Press; 95–110. [Google Scholar]
- Dunn JC, Cole EF, Quinn JL, 2011. Personality and parasites: sex-dependent associations between avian malaria infection and multiple behavioural traits. Behav Ecol Sociobiol 65:1459–1471. [Google Scholar]
- Ezenwa VO, Archie EA, Craft ME, Hawley DM, Martin LB. et al. 2016. Host behaviour–parasite feedback: an essential link between animal behaviour and disease ecology. Proc R Soc B Biol Sci 283:20153078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichet-Calvet E, LeCompte E, Koivogui L, Daffis S, Meulen JT, 2008. Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector-Borne Zoonotic Dis 8:41–48. [DOI] [PubMed] [Google Scholar]
- Fichet-Calvet E, Rogers DJ, 2009. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis 3:e388.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame JD, Baldwin JM, Gocke DJ, Troup JM, 1970. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg 19:670–676. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ, Markó G, Herczeg G, 2012. A meta-analysis of correlated behaviours with implications for behavioural syndromes: mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evol Ecol 26:1213–1235. [Google Scholar]
- Garamszegi LZ, Zagalska-Neubauer M, Canal D, Markó G, Szász E. et al. 2015. Malaria parasites, immune challenge, MHC variability, and predator avoidance in a passerine bird. Behav Ecol 26:1292–1302. [Google Scholar]
- Garcia-Longoria L, Møller AP, Balbontín J, de Lope F, Marzal A, 2015. Do malaria parasites manipulate the escape behaviour of their avian hosts? An experimental study. Parasitol Res 114:4493–4501. [DOI] [PubMed] [Google Scholar]
- Glass GE, Childs JE, Korch GW, LeDuc JW, 1988. Association of intraspecific wounding with hantaviral infection in wild rats Rattus norvegicus. Epidemiol Infect 101:459.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey SS, 2013. Networks and the ecology of parasite transmission: a framework for wildlife parasitology. Int J Parasitol Parasites Wildl 2:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling SD, 2001. From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86. [DOI] [PubMed] [Google Scholar]
- Günther S, Hoofd G, Charrel R, Röser C, Becker-Ziaja B. et al. 2009. Mopeia virus-related arenavirus in natal multimammate mice, Morogoro, Tanzania. Emerg Infect Dis 15:2008–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DM, Etienne RS, Ezenwa VO, Jolles AE, 2011. Does animal behavior underlie covariation between hosts’ exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integr Comp Biol 51:528–539. [DOI] [PubMed] [Google Scholar]
- Hughes NK, Kelley JL, Banks PB, 2012. Dangerous liaisons: the predation risks of receiving social signals. Ecol Lett 15:1326–1339. [DOI] [PubMed] [Google Scholar]
- Hughes RN, 1997. Intrinsic exploration in animals: motives and measurement. Behav Process 41:213–226. [DOI] [PubMed] [Google Scholar]
- Isaäcson M, 1975. The ecology of Praomys (Mastomys) natalensis in southern Africa. Bull World Health Organ 52:629–636. [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Godin J-GJ, 2010. Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc R Soc B Biol Sci 277:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H, 1991. Coefficient alpha for a principal component and the Kaiser–Guttman rule. Psychol Rep 68:855–858. [Google Scholar]
- Katakweba AAS, Mulungu LS, Eiseb SJ, Mahlaba TA, Makundi RH. et al. 2012. Prevalence of haemoparasites, leptospires and coccobacilli with potential for human infection in the blood of rodents and shrews from selected localities in Tanzania, Namibia and Swaziland. Afr Zool 47:119–127. [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M. et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekäläinen J, Lai Y-T, Vainikka A, Sirkka I, Kortet R, 2014. Do brain parasites alter host personality? Experimental study in minnows. Behav Ecol Sociobiol 68:197–204. [Google Scholar]
- Kennis J, Sluydts V, Leirs H, van Hooft WFP, 2008. Polyandry and polygyny in an African rodent pest species Mastomys natalensis. Mammalia 72:150–160. [Google Scholar]
- Klein SL, Zink MC, Glass GE, 2004. Seoul virus infection increases aggressive behaviour in male Norway rats. Anim Behav 67:421–429. [Google Scholar]
- Koprivnikar J, Gibson CH, Redfern JC, 2012. Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc R Soc B Biol Sci 279:1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk LEB, 2004. Estimating genetic parameters in natural populations using the “animal model”. Philos Trans R Soc Lond B Biol Sci 359:873–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen HB, 2014. lmerTest: Tests for Random and Fixed Effects for Linear Mixed Effect Models (lmer Objects of lme4 Package). R package version 2.0-6.
- Leirs H, 1995. Population Ecology of Mastomys natalensis (Smith, 1834): Implications for Rodent Control in Africa. A Report from the Tanzania-Belgium Joint Rodent Research Project (1986–1989). Publ. Agricultural. Brussels: Bellgian Administration for Development Cooperation.
- Leirs H, Stenseth NC, Nichols JD, Hines JE, Verhagen R. et al. 1997. Stochastic seasonality and nonlinear density-dependent factors regulate population size in an African rodent. Nature 389:176–180. [DOI] [PubMed] [Google Scholar]
- Leirs H, Verhagen R, Verheyen W, 1994. The basis of reproductive seasonality in Mastomys rats (Rodentia: Muridae) in Tanzania. J Trop Ecol 10:55–66. [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM, 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën J, Borremans B, Gryseels S, Broecke B, Vanden et al. , 2017. Arenavirus Dynamics in Experimentally and Naturally Infected Rodents. Ecohealth. doi:org/10.1007/S10393-017-1256-7. [DOI] [PubMed]
- Martin JGA, Réale D, 2008. Temperament, risk assessment and habituation to novelty in Eastern Chipmunks Tamias striatus. Anim Behav 75:309–318. [Google Scholar]
- Miller R, Bugnyar T, Pölzl K, Schwab C, 2015. Differences in exploration behaviour in common ravens and carrion crows during development and across social context. Behav Ecol Sociobiol 69:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JN, Alva H, Ellis BA, Wagoner KD, Childs JE. et al. 2007. Dynamics of oliveros virus infection in rodents in central Argentina. Vector Borne Zoonotic Dis 7:315–323. [DOI] [PubMed] [Google Scholar]
- Misslin R, Cigrang M, 1986. Does neophobia necessarily imply fear or anxiety? Behav Process 12:45–50. [DOI] [PubMed] [Google Scholar]
- Montiglio P-O, Garant D, Bergeron P, Messier GD, Réale D, 2014. Pulsed resources and the coupling between life-history strategies and exploration patterns in eastern chipmunks Tamias striatus. J Anim Ecol 83:720–728. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Coats J, Poulin R, 2010. The consequences of parasitic infections for host behavioural correlations and repeatability. Behaviour 147:367–382. [Google Scholar]
- Nakagawa S, Schielzeth H, 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc 85:935–956. [DOI] [PubMed] [Google Scholar]
- Natoli E, Say L, Cafazzo S, Bonanni R, Schmid M. et al. 2005. Bold attitude makes male urban feral domestic cats more vulnerable to Feline Immunodeficiency Virus. Neurosci Biobehav Rev 29:151–157. [DOI] [PubMed] [Google Scholar]
- Patterson LD, Schulte-Hostedde AI, 2011. Behavioural correlates of parasitism and reproductive success in male eastern chipmunks Tamias striatus. Anim Behav 81:1129–1137. [Google Scholar]
- Peres-Neto PR, Jackson DA, Somers KM, 2005. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput Stat Data Anal 49:974–997. [Google Scholar]
- Perrin MR, Ercoli C, Dempster ER, 2001. The role of agonistic behaviour in the population regulation of two syntopic African grassland rodents, the striped mouse Rhabdomys pumilio (Sparrman 1784) and the multimammate mouse Mastomys natalensis (A. Smith 1834) (Mammalia Rodentia). Trop Zool 14:7–29. [Google Scholar]
- Pinheiro JC, Bates DM, 2000. Mixed-Effects Models in S and S-PLUS. New York: Springer New York (Statistics and Computing; ). [Google Scholar]
- R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [cited 2017 August 15]. Available from: http://www.R-project.org/. [Google Scholar]
- Ray J, Hansen S, 2005. Temperamental development in the rat: the first year. Dev Psychobiol 47:136–144. [DOI] [PubMed] [Google Scholar]
- Reader SM, 2015. Causes of individual differences in animal exploration and search. Top Cogn Sci 7:451–468. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ, 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. [DOI] [PubMed] [Google Scholar]
- Rödel HG, Zapka M, Talke S, Kornatz T, Bruchner B. et al. 2015. Survival costs of fast exploration during juvenile life in a small mammal. Behav Ecol Sociobiol 69:205–217. [Google Scholar]
- Schwagmeyer PL, 1995. Searching today for tomorrow’s mates. Anim Behav 50:759–767. [Google Scholar]
- Seaman B, Briffa M, 2015. Parasites and personality in periwinkles Littorina littorea: infection status is associated with mean-level boldness but not repeatability. Behav Process 115:132–134. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE, 2004. Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT, 2007. Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455. [Google Scholar]
- Tebbich S, Fessl B, Blomqvist D, 2009. Exploration and ecology in Darwin’s finches. Evol Ecol 23:591–605. [Google Scholar]
- Veenstra AJF, 1958. The behaviour of the multimammate mouse Rattus (Mastomys) natalensis (A. Smith). Anim Behav 6:195–206. [Google Scholar]
- Webb PA, Justines G, Johnson KM, 1975. Infection of wild and laboratory animals with Machupo and Latino viruses. Bull World Health Organ 52:493–499. [PMC free article] [PubMed] [Google Scholar]
- White LA, Forester JD, Craft ME, 2017. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol Rev 92:389–409. [DOI] [PubMed] [Google Scholar]
- Wolak ME, Fairbairn DJ, Paulsen YR, 2012. Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137. [Google Scholar]