Abstract
Inbreeding negatively affects various life-history traits, with inbred individuals typically having lower fitness than outbred individuals (= inbreeding depression). Inbreeding depression is often emphasized under environmental stress, but the underlying mechanisms and potential long-lasting consequences of such inbreeding–environment interactions remain poorly understood. Here, we hypothesize that inbreeding–environment interactions that occur early in life have long-term physiological effects, in particular on the adult oxidative balance. We applied a unique experimental design to manipulate early life conditions of inbred and outbred songbirds (Serinus canaria) that allowed us to separate prenatal and postnatal components of early life conditions and their respective importance in inbreeding–environment interactions. We measured a wide variety of markers of oxidative status in adulthood, resulting in a comprehensive account for oxidative balance. Using a Bayesian approach with Markov chain Monte Carlo, we found clear sex-specific effects and we also found only in females small yet significant long-term effects of inbreeding–environment interactions on adult oxidative balance. Postnatal components of early life conditions were most persuasively reflected on adult oxidative balance, with inbred females that experienced disadvantageous postnatal conditions upregulating enzymatic antioxidants in adulthood. Our study provides some evidence that adult oxidative balance can reflect inbreeding–environment interactions early in life, but given the rather small effects that were limited to females, we conclude that oxidative stress might have a limited role as mechanism underlying inbreeding–environment interactions.
Keywords: canary, maternal effects, gene–environment interactions, hatching asynchrony
There is ample empirical evidence that inbreeding (mating between related individuals) negatively affects fitness-related traits like fecundity and survival (Keller and Waller 2002; Fox and Reed 2011). Inbreeding depression is often more pronounced in stressful compared to benign environments, indicating that inbred individuals show increased susceptibility to stressful environmental conditions in comparison to outbred individuals (Armbruster and Reed 2005; Fox and Reed 2011). Such inbreeding–environment interactions are important in many areas of biology, among others in conservation biology. However, the mechanisms that underlie inbreeding–environment interactions remain largely unknown, which prohibits a thorough understanding of both the causes and consequences of these phenomena (Kristensen et al. 2010).
Preventing oxidative stress [defined as an imbalance between the production of highly reactive substances and the capacity to neutralize such compounds with antioxidant defenses (Halliwell and Gutteridge 2007)] is an important component of stress resistance. Interestingly, genome-wide analyses show that inbreeding affects a wide variety of genes but has a disproportionate impact on genes involved in stress resistance and metabolism (Kristensen et al. 2005; Kristensen et al. 2006; Ayroles et al. 2009; Menzel et al. 2015), and that a number of these genes are also linked to the regulation of oxidative stress (Pletcher et al. 2002; Landis et al. 2004; Kristensen et al. 2005; Kristensen et al. 2010). Therefore, oxidative stress could be an important factor that underlies the detrimental effects of inbreeding (Okada et al. 2011; Freitak et al. 2014). Moreover, oxidative stress has been associated with early environmental stress (Haussmann et al. 2012; Marasco et al. 2013; Giordano et al. 2015; Zimmer and Spencer, 2015; Marasco et al. 2016). This suggests that inbreeding–environment interactions might affect the oxidative balance, which remains largely unexplored.
However, inbreeding–environment interactions and especially their consequences are by no means restricted to the moment at which environmental conditions are experienced. Individuals are particularly sensitive early in life when development occurs, and the environmental conditions that an individual experiences during this time frame may therefore have a major impact on the adult phenotype (Metcalfe and Monaghan 2001; Monaghan 2008). Inbreeding–environment interactions that occur early in life can therefore have potentially long-lasting consequences, and an inbreeding–environment interaction caused by early life stress may be reflected in stress sensitivity and levels of oxidative stress at adulthood.
Early life conditions that have the potential for life-long consequences can be separated in prenatal and postnatal conditions depending on during which period they act. In birds, prenatal conditions are formed by the conditions experienced within the egg. This often refers to differential maternal investment in egg size (Williams 1994; Royle et al. 1999), and egg components such as antioxidants (Royle et al. 1999; Royle et al. 2001; Blount et al. 2002; Royle et al. 2003) and hormones (Schwabl, 1993; Gil et al. 1999; Royle et al. 2001; Groothuis and Schwabl 2002). The early postnatal environment, on the other hand, consists of different factors such as sibling competition or the quality of parental care (at least in altricial birds that raise more than one chick at a time) (Forbes 2010; Mainwaring et al. 2010). Specifically, larger siblings are generally advantaged in food acquisition in comparison to smaller nestlings (Oddie, 2000; Royle et al. 2002), which is even noticeable under ad libitum conditions (de Boer et al. 2015). Therefore, sibling size hierarchies are a major determinant of the early environment of developing nestlings (Forbes 2010), which has been shown to interact with inbreeding (de Boer et al. 2015). Prenatal and postnatal conditions can not only have separate effects but also interactive effects, since prenatal (maternal) effects are thought to adjust offspring phenotype to the expected post-hatching conditions (Muller and Groothuis 2013).
In this study, we applied an experimental design that enabled us to examine the effects of inbreeding and prenatal and postnatal conditions simultaneously. We weight-matched inbred and outbred nestlings and placed them in either a size advantaged or size disadvantaged position in the within-brood hierarchy, mimicking hatching asynchrony (= postnatal conditions). The laying position of the egg from which they hatched (= prenatal conditions) either matched or mismatched with their position in the within-brood hierarchy. This experimental manipulation revealed substantial effects of postnatal conditions on early growth, with size advantaged nestlings growing faster than size disadvantaged nestlings. Birds that hatched from late laid eggs obtained a larger size at fledging than those hatched from early laid eggs, showing the importance of prenatal conditions too. Inbred birds grew slower than outbred birds, but this was independent of (dis)advantageous prenatal or postnatal conditions (see de Boer et al. 2016 for more details).
Here, we describe long-term effects of inbreeding in interaction with early life conditions and test if baseline values of oxidative damage and antioxidant protection in blood are affected at adulthood. To this end, we kept the birds that were reared in the experimental design under laboratory conditions after fledging. We then collected a sample of blood when the individuals were adult (i.e., fully sexually mature), but before they were mated. We measured a range of antioxidants and oxidative damage markers [damage to proteins (carbonyls), nitric oxide (NO)], enzymatic antioxidants (GPX, CAT, and SOD), and glutathione [ratio: GSH (reduced form)/GSSG (oxidized form)] that were designed to give us a comprehensive picture of the individual’s oxidative status.
We expect that disadvantageous conditions early in life could have long-term effects on oxidative balance in adulthood (Marasco et al. 2013; Zimmer and Spencer, 2015). Additionally, we expect that inbred birds have higher oxidative stress in comparison to outbred birds (Okada et al. 2011; Freitak et al. 2014), and that this should be especially noticeable when they were reared under disadvantaged conditions. That is, a size disadvantaged position in the within-brood hierarchy (i.e. low food availability due to high levels of sibling competition) (Forbes 2010), and/or mismatched prenatal and postnatal conditions (i.e., the laying position of the egg is mismatched with the position in the brood hierarchy) (Muller and Groothuis 2013).
Materials and Methods
Inbreeding and early life conditions
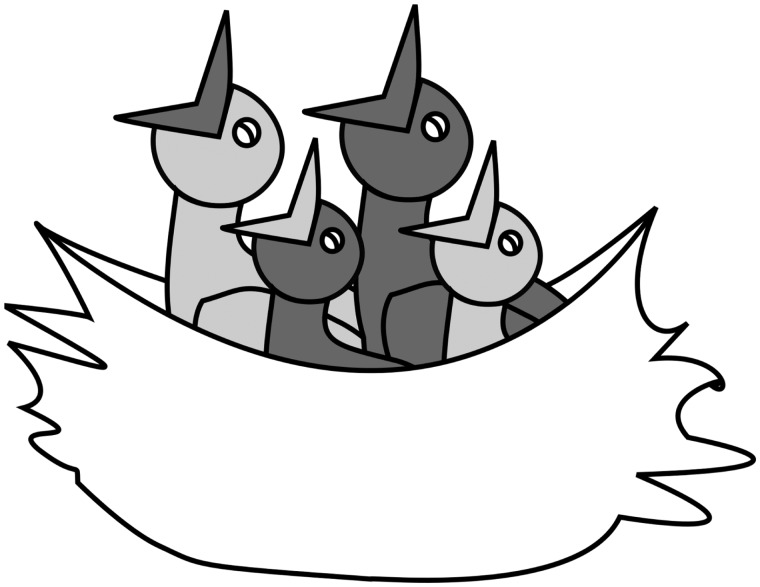
The parental generation of the birds used in this study originates from an outbred population kept at the University of Antwerp. In March 2014, two types of breeding pairs were created: full-sibling breeding pairs (= inbred offspring) and unrelated breeding pairs (= outbred offspring). For the here described study, a total of 140 (70 males, 70 females) nestlings were used. Inbred and outbred nestlings were combined in duos, based on body mass (< 0.2 g difference), and on the position in the laying sequence (= prenatal conditions, early laid eggs: first or second egg, or late laid eggs: third, fourth or fifth egg), and were then placed in a foster nest (=“experimental nest”) at maximum 2 days after hatching. Approximately half of these nests were reared by full sibling parents and half by unrelated parents. However, couple composition did not have an effect on nestling growth (de Boer et al. 2016). In each experimental nest, two duos of inbred and outbred nestlings were combined based on age, in such a way that each experimental nest contained an older, size advantaged duo, and a younger, size disadvantaged duo. The difference between the older/size advantaged and younger/size disadvantaged nestlings was 2 days in age, or if this was impossible at least 0.5 g difference in body mass at time of cross-fostering. The difference in age/size between the nestlings created an artificial sibling hierarchy within the nest, manipulating postnatal conditions (Figure 1).
Figure 1.
A schematic representation of our experimental set-up. Each experimental nest consisted of two pairs of weight-matched inbred (= dark grey) and outbred (= light grey) birds, that were placed either in a size advantaged position (= dark grey beaks), or in a size disadvantaged (= light grey beaks) position. Each pair of inbred and outbred birds hatched from either early laid eggs (=first or second laid egg) or late laid eggs (= all later laid eggs). Hatching from an early laid egg matched a size advantaged position in the nest, whereas hatching from a late laid egg mismatched a size advantage, and vice versa for birds reared in size disadvantaged positions.
Approximately half of the duos that were reared in a size advantaged position in the hierarchy hatched from early laid eggs (= matched prenatal and postnatal conditions; laying order of the egg corresponded to the position in the hierarchy), and half from late laid eggs (= mismatched prenatal and postnatal conditions), and vice versa for duos that were reared in size disadvantaged positions. Thus, four different experimental treatments in the early life (= prenatal and postnatal conditions) of the inbred and outbred focal birds can be distinguished: size advantaged/early laid egg (=matched conditions; inbred female N = 10, inbred male N = 8, outbred female N = 9, outbred male N = 12), size advantaged/late laid egg (=mismatched conditions; inbred female N = 8, inbred male N = 10, outbred female N = 9, outbred male N = 6), size disadvantaged/late laid egg (= matched conditions; inbred female N = 8, inbred male N = 14, outbred female N = 15, outbred male N = 9), and size disadvantaged/early laid egg (=mismatched conditions; inbred female N = 7, inbred male N = 4, outbred female N = 4, outbred male N = 7) (Figure 1). At fledging (± 25 days after hatching), a blood sample was taken from the brachial vein to determine sex with the use of PCR, and birds were from that time onwards housed in two groups separated by sex in large aviaries, with food and water available ad libitum.
Analyses of adult blood oxidative balance
The birds were exposed to a long light regime (14 h light: 10 h dark) from December 2014 onwards. This set off reproductive state, and when the birds were ∼1-year old (March 2015) they were weighed, and a blood sample was taken from the brachial vein. Blood samples were stored in a freezer (−80 °C) until laboratory analyses were conducted.
Blood markers of oxidative damage, antioxidant protection, and reactive nitrogen species production were measured according to established protocols. Specifically, we measured in red blood cells the concentration of protein carbonyls (marker of oxidative damage to proteins) according to Levine et al. (1990). Extracting buffer was used to dilute the red blood cells in order to obtain a concentration of 2 mg proteins/ml which was measured with the use of the Bradford protein assay (Bradford 1976). The concentration of protein carbonyls was noted as nanomoles per milligram proteins.
The activity of three antioxidant enzymes [glutathione peroxidase (GPX), superoxide dismutase (SOD) and catalase (CAT)] was assessed in 5 µL of red blood cells that were homogenized in 250 µL of extracting buffer (pH 7.4, 1.15% KCL and 0.02 M EDTA in 0.01 M PBS) with the use of a MagNALyser (Roche, Vilvoorde, Belgium). A micro-plate reader (Synergy Mx, Biotek Instruments Inc., Vermont, USA) was used for the activity measurements. Specifically, GPX activity was measured as the decrease in NADPH absorbance measured at 340 nm (Drotar et al. 1985), SOD activity was measured as the inhibition of nitroblue tetrazolium (NBT) reduction at 560 nm (Dhindsa et al. 1981) and CAT activity was measured as the rate of decomposition of H2O2 at 240 nm (Aebi 1984). Analyses were run in duplicate and inter-assay and intra-assay variation was <16%.
Further, the concentration of both reduced glutathione (GSH) and oxidized glutathione (GSSG) was measured in 2.5 µL of red blood cells using reversed-phase high-performance liquid chromatography (HPLC) with electrochemical detection (Shimadzu, Hai Zhonglu, Shanghai) according to AbdElgawad et al. (2015). The concentrations of GSH and GSSG were expressed as micromole per gram fresh weight of red blood cells. We used the GSH/GSSG ratio, which is a conventional index of redox state with higher values indicating lower oxidative stress (Jones, 2006). Finally, we measured in plasma the production of the free radical nitric oxide with a spectrophotometric assay (see for more details: Sild and Hõrak 2009; Vermeulen et al. 2016).
Statistical analyses
A total of six markers of oxidative balance [damage to proteins (carbonyls), nitric oxide (NO)], enzymatic antioxidants (GPX, CAT, and SOD), and glutathione [ratio: GSH (reduced form)/GSSG (oxidized form)] were analyzed with generalized linear mixed effects models using a Bayesian approach with Markov chain Monte Carlo (MCMC) algorithms implemented in the MCMCglmm R package (Hadfield 2010). We ran a preliminary model on data of all birds which included a fixed effect for sex in addition to the below mentioned fixed effects. This model revealed strong effects of sex on the oxidative balance (see results) and males and females were analysed separately to account for sex-specific resistance to oxidative stress. The fixed effects included in the models were: prenatal condition (hatched from early or late laid eggs), postnatal condition (size advantaged or size disadvantaged), and inbreeding status (inbred or outbred). The interactions between these effects were included, and the potential three-way interaction between all factors was also taken into account. Additionally, the weight of the birds at time of blood sampling was included as a fixed effect. Brothers and sisters were included in this study, as well as individuals that were reared together in an experimental nest. To control for potential confounding effects of these factors we included both the nest of origin and the rearing nest as random effects in the model.
All response variables had a Gaussian distribution and were scaled before the sex-specific models were run. MCMC chains were run for 505,000 iterations with a burn-in phase of 5000 iterations, and 1000 independent samples were taken from the posterior at intervals of 500 iterations. Convergence was determined by visual inspection of the traces and autocorrelation plots. The results are presented as the estimates of the sampled iterations with a 95% confidence interval. Statistical significance of the estimate can be assumed when confidence intervals do not overlap with zero. The correlation between parameters (X, Y) of the adult oxidative balance was calculated by dividing the covariance (cov(X, Y)) between parameters by the product of the standard deviations of the variance (SD(X)*SD(Y)).
The effect size of differences between inbred and outbred birds within each experimental group was calculated with the R package “compute.es” (Del Re 2013) using the mean, standard deviation, and sample size.
All analyses were performed in R (R Core Development Team 2014). In the analysis of CAT in females, and in the analysis of nitric oxide and protein carbonyls in males, we excluded one individual from the data that was considered an outlier (>0.5 Cook’s distance). All above described procedures have been approved by the ethical committee for animal experimentation at the University of Antwerp (file number 2011–86), and carried out in accordance with the relevant rules and guidelines.
Results
Baseline levels of all markers for oxidative balance except for nitric oxide significantly differed between males and females (estimate [95% CI]: carbonyls: −2.59 [−3.00; −2.11], P < 0.001; nitric oxide: 0.001 [−0.004; 0.006], P = 0.6; GPX: −0.006 [−0.011;-0.001], P = 0.016; CAT: 0.09 [0.04; 0.15], P < 0.001; SOD: 0.77 [0.68; 0.86], P < 0.001; GSH/GSSG: −2.22 [−3.41; −1.18], P < 0.001) (Table 1). In males, we find no effects of early life conditions and/or inbreeding in any of the parameters of oxidative balance (Table 2).
Table 1.
Differences in body mass and markers of the adult oxidative balance between non-breeding male and female Canaries
| Parameter | Mean ± SE females | Mean ± SE males |
|---|---|---|
| Body mass (g) | 19.37 ± 0.2 | 17.90 ± 0.2 |
| Protein carbonyls (nmol/mg proteins) | 3.00 ± 0.20 | 0.40 ± 004 |
| NO (mmol/L) | 0.004 ± 0.0002 | 0.006 ± 0.0006 |
| GPX (µmol NADPH/ mg prot/min) | 0.009 ± 0.0002 | 0.003 ± 0.0003 |
| CAT (µmol H2O2/ mg prot/min) | 0.15 ± 0.008 | 0.25 ± 0.02 |
| SOD (U/mg prot/min) | 0.27 ± 0.009 | 1.03 ± 0.04 |
| GSH/GSSG (each in µmol/ g fresh weight) | 4.43 ± 0.37 | 2.51 ± 0.32 |
Table 2.
Parameter estimates of Markov Chain Monte Carlo generalized linear mixed models for the effects of inbreeding, prenatal and postnatal early life conditions on different parameters of the adult oxidative balance
| Parameter | Fixed effects | Estimate | 95% CI | P | Estimate | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| Females | Females | Females | Males | Males | Males | |||
| Damage (proteins) | Carbonylsa | Inbreeding | −0.55 | [−1.81; 0.69] | 0.37 | −0.35 | [−1.63; 1.02] | 0.61 |
| Postnatal | −0.11 | [−1.06; 0.89] | 0.81 | −0.39 | [−1.73; 0.81] | 0.55 | ||
| Postnatal: Inbreeding | 1.38 | [−0.09; 2.90] | 0.06 | 1.04 | [−0.77; 2.62] | 0.23 | ||
| Prenatal | −0.24 | [−1.28; 0.73] | 0.68 | 0.09 | [−1.17; 1.21] | 0.88 | ||
| Prenatal: Inbreeding | 1.35 | [−0.15; 2.85] | 0.08 | 0.29 | [−1.21; 1.96] | 0.73 | ||
| Prenatal: Postnatal | 0.24 | [−1.11; 1.60] | 0.75 | 0.05 | [−1.55; 1.46] | 0.92 | ||
| Prenatal: Postnatal: Inbreeding | −2.65 | [−4.71; −0.77] | 0.014 | −0.15 | [−2.29; 1.97] | 0.90 | ||
| Weight | −0.06 | [−0.21; 0.08] | 0.45 | 0.11 | [−0.07; 0.26] | 0.19 | ||
| Nitric oxide | NOa | Inbreeding | 1.62 | [0.43; 2.99] | 0.016 | 0.26 | [−1.12; 1.57] | 0.70 |
| Postnatal | 1.10 | [−0.17; 2.07] | 0.07 | 0.72 | [−0.59; 1.95] | 0.26 | ||
| Postnatal: Inbreeding | −1.75 | [−3.29; −0.20] | 0.032 | −0.72 | [−2.45; 0.86] | 0.37 | ||
| Prenatal | 1.08 | [0.08; 2.21] | 0.05 | 0.07 | [−1.16; 1.17] | 0.92 | ||
| Prenatal: Inbreeding | −2.24 | [−3.61; −0.70] | 0.012 | −0.35 | [−2.06; 1.12] | 0.65 | ||
| Prenatal: Postnatal | −1.49 | [−3.17; −0.03] | 0.07 | 0.42 | [−1.00; 2.02] | 0.57 | ||
| Prenatal: Postnatal: Inbreeding | 2.25 | [0.38; 4.49] | 0.040 | 0.06 | [−1.84; 2.60] | 0.94 | ||
| Weight | 0.00 | [−0.18; 0.16] | 1.00 | 0.05 | [−0.13; 0.19] | 0.57 | ||
| Enzymatic antioxidants | GPX | Inbreeding | −1.82 | [−3.13; −0.51] | 0.006 | 0.07 | [−1.05; 1.03] | 0.90 |
| Postnatal | −0.43 | [−1.46; 0.55] | 0.39 | −0.22 | [−1.29; 0.95] | 0.72 | ||
| Postnatal: Inbreeding | 1.89 | [0.21; 3.25] | 0.026 | 0.14 | [−1.01; 1.45] | 0.86 | ||
| Prenatal | −0.03 | [−1.04; 1.13] | 0.93 | 0.24 | [−0.74; 1.25] | 0.65 | ||
| Prenatal: Inbreeding | 1.26 | [−0.23; 2.78] | 0.12 | −0.48 | [−1.67; 0.77] | 0.44 | ||
| Prenatal: Postnatal | −0.20 | [−1.61; 1.32] | 0.80 | 0.09 | [−1.41; 1.63] | 0.93 | ||
| Prenatal: Postnatal: Inbreeding | −1.08 | [−3.01; 0.84] | 0.26 | 0.33 | [−1.52; 1.77] | 0.67 | ||
| Weight | 0.00 | [−0.16; 0.14] | 0.94 | 0.06 | [−0.07; 0.22] | 0.42 | ||
| CATb | Inbreeding | −1.21 | [−2.43; 0.21] | 0.070 | 0.42 | [−0.86; 1.77] | 0.49 | |
| Postnatal | −1.06 | [−2.19; −0.003] | 0.050 | −0.03 | [−1.19; 1.40] | 0.96 | ||
| Postnatal: Inbreeding | 2.29 | [0.62; 3.71] | 0.006 | −0.95 | [−2.48; 0.76] | 0.23 | ||
| Prenatal | −0.49 | [−1.49; 0.72] | 0.38 | −0.38 | [−1.51; 0.73] | 0.50 | ||
| Prenatal: Inbreeding | 0.83 | [−0.74; 2.38] | 0.30 | −0.35 | [−1.89; 1.18] | 0.63 | ||
| Prenatal: Postnatal | 1.14 | [−0.53; 2.61] | 0.18 | −0.41 | [−2.16; 0.97] | 0.56 | ||
| Prenatal: Postnatal: Inbreeding | −1.83 | [−3.78; 0.31] | 0.086 | 1.57 | [−0.54; 3.55] | 0.14 | ||
| Weight | −0.10 | [−0.24; 0.06] | 0.21 | −0.06 | [−0.22; 0.11] | 0.47 | ||
| SOD | Inbreeding | −1.23 | [−2.41; 0.11] | 0.058 | 0.38 | [−0.99; 1.66] | 0.60 | |
| Postnatal | −0.34 | [−1.32; 0.67] | 0.50 | 0.18 | [−1.23; 1.57] | 0.80 | ||
| Postnatal: Inbreeding | 1.92 | [0.44; 3.60] | 0.022 | −0.30 | [−2.00; 1.35] | 0.73 | ||
| Prenatal | −0.08 | [−1.20; 0.87] | 0.89 | 0.01 | [−1.19; 1.21] | 0.99 | ||
| Prenatal: Inbreeding | 1.28 | [−0.39; 2.77] | 0.13 | 0.17 | [−1.51; 1.76] | 0.84 | ||
| Prenatal: Postnatal | −0.15 | [−1.50; 1.31] | 0.81 | −0.35 | [−2.03; 1.26] | 0.67 | ||
| Prenatal: Postnatal: Inbreeding | −1.60 | [−3.72; 0.40] | 0.13 | 0.23 | [−2.04; 2.56] | 0.87 | ||
| Weight | 0.01 | [−0.14; 0.16] | 0.86 | 0.03 | [−0.15; 0.20] | 0.76 | ||
| Glutathione | GSH/GSSG | Inbreeding | 0.10 | [−1.28; 1.49] | 0.88 | 0.60 | [−0.71; 1.78] | 0.38 |
| Postnatal | 0.44 | [−0.54; 1.69] | 0.43 | 0.24 | [−0.89; 1.53] | 0.72 | ||
| Postnatal: Inbreeding | −0.23 | [−2.10; 1.33] | 0.79 | −0.21 | [−1.77; 1.30] | 0.79 | ||
| Prenatal | 0.42 | [−0.81; 1.53] | 0.45 | 0.73 | [−0.42; 1.93] | 0.22 | ||
| Prenatal: Inbreeding | −0.23 | [−1.96; 1.26] | 0.78 | −0.65 | [−2.33; 0.82] | 0.45 | ||
| Prenatal: Postnatal | −0.82 | [−2.46; 0.81] | 0.30 | −0.93 | [−2.37; 0.52] | 0.20 | ||
| Prenatal: Postnatal: Inbreeding | 0.96 | [−1.33; 2.94] | 0.42 | 0.10 | [−2.09; 2.06] | 0.90 | ||
| Weight | −0.03 | [−0.20; 0.14] | 0.75 | −0.08 | [−0.25; 0.07] | 0.34 | ||
Significant effects in which confidence intervals do not overlap with zero are indicated in bold and P < 0.1 are indicated with italics.
aMales without 1 outlier.
bFemales without 1 outlier.
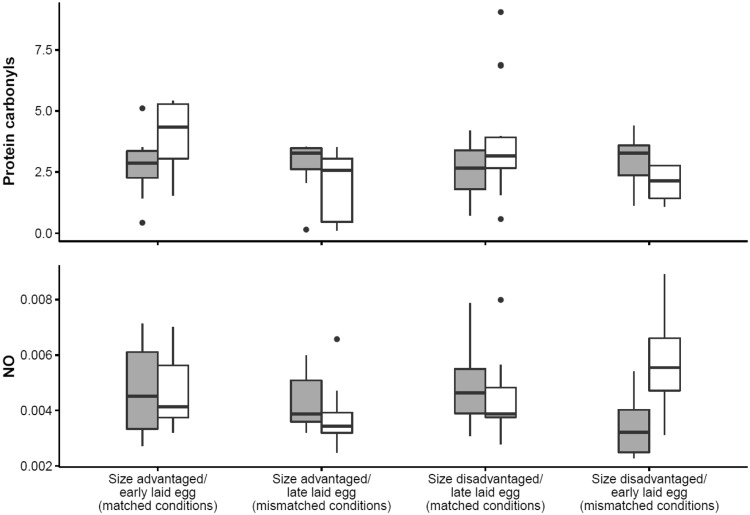
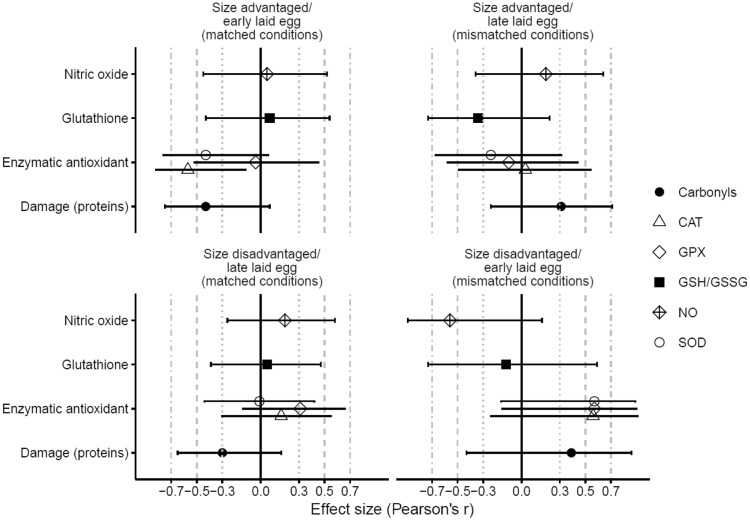
In females, the effects of inbreeding on protein carbonyls (marker of oxidative damage to proteins) differed according to prenatal and postnatal conditions (Table 2). In Figure 2, it can be observed that inbred females tended to have higher levels of oxidative damage in comparison to outbred females when hatched from an early laid egg and raised in a size disadvantaged position, and to a lesser extent when hatched from late laid eggs but raised in a size advantaged position, which are both conditions where the post-hatching environment mismatched with the laying position of the egg they had hatched from. Inbred females tended to have less oxidative damage to proteins than outbred females when hatched from an early laid egg and raised in size-advantaged position and to a lesser extent when hatched from late laid eggs and raised in size-disadvantaged position, which is under matched conditions. In Figure 3, it can be seen that the effect sizes are rather small and confidence intervals overlap with zero indicating non-significant differences between inbred and outbred females within the experimental groups.
Figure 2.
Differences in protein carbonyls (nmol/mg proteins) (top) and nitric oxide (NO, mmol/L) (bottom) between inbred (gray boxes) and outbred (white boxes) females canaries depended on prenatal and postnatal early life conditions. These early life conditions consisted of four experimental treatments: size advantaged/early laid eggs (= matched conditions; inbred females: N = 10, outbred females: N = 9), size advantaged/late laid eggs (= mismatched conditions; inbred females: N = 8, outbred females: N = 9), size disadvantaged/late laid eggs (= matched conditions; inbred females: N = 8, outbred females: N = 15), size disadvantaged/early laid eggs (= mismatched conditions; inbred females: N = 7, outbred females: N = 4).
Figure 3.
Effect sizes (± 95%CI) of the differences in markers of oxidative balance between inbred and outbred females in relation to early life conditions: size advantaged/early laid eggs (= matched conditions; inbred females: N = 10, outbred females: N = 9), size advantaged/late laid eggs (= mismatched conditions; inbred females: N = 8, outbred females: N = 9), size disadvantaged/late laid eggs (= matched conditions; inbred females: N = 8, outbred females: N = 15), size disadvantaged/early laid eggs (= mismatched conditions; inbred females: N = 7, outbred females: N = 4). Negative effect sizes represent higher values for outbred females in comparison to inbred females, and vice versa for positive effect sizes.
The effects of inbreeding on the nitric oxide concentration were also mediated by prenatal and postnatal conditions in females (Table 2). The negative effect of inbreeding was most noticeable in females that hatched from an early-laid egg and were placed in a size disadvantaged position. Further, inbred and outbred females did not show noteworthy differences in nitric oxide concentrations in other early life conditions (Figures 2 and 3).
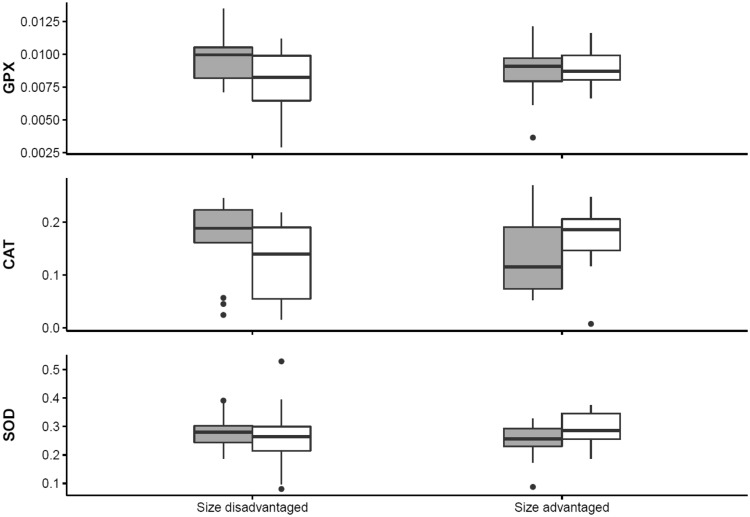
The activity of the enzymatic antioxidants GPX, CAT and SOD depended on inbreeding status in interaction with postnatal conditions in females. In Figures 3 and 4, it can be observed that inbred females that had been reared with a size disadvantage tended to have more enzymatic antioxidants than outbred females. On the other hand, inbred females tended to have less of the enzymatic antioxidant CAT when reared with a size advantage. These effects were rather small and for most of the experimental groups the confidence intervals of differences between inbred and outbred females overlapped with zero indicating non-significant effects (Figure 3). There were no significant effects of inbreeding and/or early life conditions on the GSH/GSSG ratio in females (Table 2). In both females and males, there were no significant effects of body mass on any of the parameters of oxidative balance (Table 2).
Figure 4.
Differences in the activity of enzymatic antioxidants GPX (µmol NADPH/mg prot/min), CAT (µmol H2O2/mg prot/min), and SOD (U/mg prot/min) between inbred (gray boxes) and outbred (white boxes) female canaries depended on the postnatal early life conditions. Postnatal conditions in the nest were manipulated by rearing birds with a size advantage (inbred females: N = 18, outbred females: N = 18) or with a size disadvantage (inbred females: N = 15, outbred females: N = 19).
The correlation between the various parameters of the adult oxidative balance is reported for males and females in Table 3. In females, the activity of GPX, CAT and SOD co-varied significantly. In males, the activity of CAT and SOD, and GPX and nitric oxide showed significant co-variation (Table 3).
Table 3.
The correlation between parameters of adult oxidative balance (X, Y) was calculated by dividing the covariance (cov(X.Y)) between parameters by the product of the standard deviations of the variance (SD(X)*SD(Y))
| Parameter | Carbonyls | NO | GPX | CAT | SOD | GSH/GSSG | |
|---|---|---|---|---|---|---|---|
| Females | Carbonyls | x | 0.04 | −0.02 | −0.04 | 0.03 | −0.15 |
| NO | x | x | 0.02 | −0.13 | −0.22 | −0.03 | |
| GPX | x | x | x | 0.43 | 0.65 | 0.06 | |
| CAT | x | x | x | x | 0.49 | 0.02 | |
| SOD | x | x | x | x | x | 0.24 | |
| GSH/GSSG | x | x | x | x | x | x | |
| Males | Carbonyls | x | 0.09 | 0.10 | −0.04 | −0.10 | −0.28 |
| NO | x | x | 0.21 | 0.17 | −0.03 | 0.01 | |
| GPX | x | x | x | 0.69 | 0.22 | 0.03 | |
| CAT | x | x | x | x | 0.22 | 0.16 | |
| SOD | x | x | x | x | x | 0.31 | |
| GSH/GSSG | x | x | x | x | x | x |
Confidence intervals of the covariance values that did not overlap with zero are indicated in bold.
Discussion
In this study, we investigated the long-term effects of inbreeding, prenatal and early postnatal conditions, and their potential interactive effects on adult oxidative balance. Our findings show that the effects of inbreeding were mediated by early postnatal conditions for the concentration of enzymatic antioxidants, and by prenatal and postnatal early life conditions for the damage to proteins and the nitric oxide concentration in females. Specifically, inbred females seemed to upregulate enzymatic antioxidants in adulthood when they had been reared under disadvantageous postnatal conditions. Inbred females that were reared under disadvantageous conditions also suffered more oxidative damage in adulthood than outbred females that were reared under disadvantageous conditions, but this effect was restricted to females that hatched from early laid eggs, i.e., were reared under mismatched pre- and postnatal conditions. Inbred females also had lower levels of nitric oxide than outbred females in this experimental group, which may be directly related to the increased levels of enzymatic antioxidants (Price et al. 2000). Nitric oxide is a very potent oxidant, and can have deleterious effects such as tissue damage when it is produced prolonged and excessively (Price et al. 2000; Vajdovich, 2008; Pamplona and Costantini, 2011). However, nitric oxide also has various important functions, such as protection against infection, and low levels of nitric oxide could therefore also relate to an impaired immune system (Vajdovich, 2008; Bichet et al. 2012).
There was no consistent effect of either prenatal or postnatal conditions or their combination on the adult oxidative balance, which makes it difficult to pinpoint what aspect of early life conditions caused the observed inbreeding–environment interactions in females. However, the inbreeding–environment interactions always included the postnatal conditions if significant, which suggests that the postnatal component of early life conditions may have been an important factor in inbreeding-environment interactions. Indeed, our experimental manipulation of the postnatal conditions had more pronounced effects on early growth and size at fledging than prenatal conditions (de Boer et al. 2016). Yet, neither postnatal nor prenatal conditions per se were clearly reflected in the adult oxidative balance. This is in contrast with previous studies showing effects of prenatal and/or postnatal conditions on the adult oxidative balance in birds (Marasco et al. 2013; Zimmer v Spencer, 2015) and the more general concept that conditions affecting early growth should be reflected in the adult phenotype, including physiology (Metcalfe and Monaghan, 2001; Monaghan, 2008). After fledging, all birds had ad libitum access to food with only limited competition, which may have alleviated any long-lasting effects of early life conditions, and such effects might be more apparent in the wild. However, both above-mentioned studies showing long-lasting effects of early life stress, which was manipulated via prenatal administration of corticosterone (Marasco et al. 2013; Zimmer and Spencer, 2015), were performed in captivity too. Although the developmental strategy of the precocial species used in those studies likely differs from that of the canary, this suggests that the lack of a clear effect of early life conditions in our study may not necessarily relate to the benign food conditions in captivity. It may also be that a different type of manipulation of early life conditions (e.g., exposure to stress hormones) than what we have done here has more pronounced effects on the adult oxidative balance (Marasco et al. 2013; Zimmer and Spencer, 2015).
Inbreeding affected the female oxidative balance always in interaction with early life conditions, suggesting that inbreeding per se does not necessarily relate to decreased resistance to oxidative stress. Unfortunately, studies that link inbreeding with oxidative stress are as yet extremely rare and restricted to insects (Okada et al. 2011; Freitak et al. 2014), which limits further conclusions. Inbreeding negatively affected growth in early life (de Boer et al. 2016), but those effects were transient and no more present in body mass at fledging and in adulthood, at which time we measured oxidative balance. Differences in the oxidative balance between inbred and outbred birds may therefore have been more pronounced early in life than in adulthood.
Our study shows a clear effect of sex on the adult oxidative balance. Oxidative damage to proteins, in particular, was on average 7.5 times higher in females than in males. Moreover, in females there was some evidence of inbreeding-environment interactions but in males there were no effects of early life conditions and/or inbreeding at all. Sex-specific effects on the oxidative balance have been previously reported in birds (Bize et al. 2008; Isaksson 2013; Marasco et al. 2013; Giordano et al. 2015). Females were, although the birds were not mated at the time of blood sampling, likely developing their ovaries, which is an energetically demanding process (Monaghan and Nager 1997; Nilsson and Råberg 2001). Thus, it could be that females were more constrained than males by costs associated with this process, causing the large differences in markers of oxidative balance between males and females. In addition, the oxidative balance is not necessarily the same across tissues (Speakman et al. 2015). We focused here on a variety of blood markers of oxidative balance, but perhaps the effects of inbreeding and/or early life conditions targeted other tissues in males than in females. Alternatively, the oxidative balance can vary with age which does not necessarily show the same pattern in males and females (Bize et al. 2008). Thus, there is also a possibility that the effects of inbreeding or early life conditions become apparent at a different life phase in males than in females, which could be an interesting topic for future studies.
Our study provides some evidence that inbreeding–environment interactions have long-term effects on adult oxidative balance. Our intricate experimental design allowed to test for the individual and combined effects of prenatal and postnatal components of such inbreeding–environment interactions simultaneously. However, this complex design did not allow us to obtain a large sample size and indeed the effects were rather small and also limited to females. Based on these data, we conclude that oxidative stress might have a limited role as a mechanism underlying inbreeding–environment interactions.
Author contributions statement
R.B., D.C., M.E., W.M. designed the experiment. R.B., D.C., G.C., H.E., H.A. contributed to the collection and analysis of the data. R.B. wrote the first draft. R.B., D.C., G.C., M.E., W.M. helped to improve the article, and all authors approved the final version for submission to the journal.
Acknowledgments
We thank Peter Scheys, Josie Meaney, and Geert Eens for their invaluable practical support, the members of the BECO group for useful discussions and Danny Huybrecht for helping with the HPLC analyses. We thank two anonymous reviewers for their helpful comments on an earlier version of this manuscript.
Funding
This study was funded by Research Foundation Flanders (11O5914N and 11O5916N granted to R.A.dB. and project G.0102.12 N granted to W.M. and M.E.).
References
- AbdElgawad H, Farfan-Vignolo ER, de Vos D, Asard H, 2015. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci 231:1–10. [DOI] [PubMed] [Google Scholar]
- Aebi H, 1984. Catalase in vitro. Method Enzymol 105:121–126. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Reed DH, 2005. Inbreeding depression in benign and stressful environments. Heredity 95:235–242. [DOI] [PubMed] [Google Scholar]
- Ayroles JF, Hughes KA, Rowe KC, Reedy MM, Rodriguez-Zas SL. et al. , 2009. A genomewide assessment of inbreeding depression: gene number, function, and mode of action. Conserv Biol 23:920–930. [DOI] [PubMed] [Google Scholar]
- Bradford MM, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Bichet C, Cornet S, Larcombe S, Sorci G, 2012. Experimental inhibition of nitric oxide increases Plasmodium relictum (lineage SGS1) parasitaemia. Exp Parasitol 132:417–423. [DOI] [PubMed] [Google Scholar]
- Bize P, Devevey G, Monaghan P, Doligez B, Christe P, 2008. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89:2584–2593. [DOI] [PubMed] [Google Scholar]
- Blount JD, Surai PF, Nager RG, Houston DC, Møller AP. et al. , 2002. Carotenoids and egg quality in the lesser blackbacked gull Larus fuscus: a supplemental feeding study of maternal effects. P Roy Soc B-Biol Sci 269:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer RA, Eens M, Fransen E, Müller W, 2015. Hatching asynchrony aggravates inbreeding depression in a songbird Serinus canaria: an inbreeding-environment interaction. Evolution 69:1063–1068. [DOI] [PubMed] [Google Scholar]
- de Boer RA, Eens M, Müller W, 2016. A loss of heterozygosity, a loss in competition? The effects of inbreeding, prenatal, and postnatal conditions on nestling development. Ecol Evol 6:7921–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re A, 2013. computees: Compute Effect Sizes. R package version 0.2–2.
- Dhindsa RS, Plump-Dhindsa P, Thorpe TA, 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. [Google Scholar]
- Drotar A, Phelps P, Fall R, 1985. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40. [Google Scholar]
- Forbes S, 2010. Social rank governs the effective environment of siblings. Biol Lett 7:346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CW, Reed DH, 2011. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65:246–258. [DOI] [PubMed] [Google Scholar]
- Freitak D, Bos N, Stucki D, Sundström L, 2014. Inbreeding-related trade-offs in stress resistance in the ant Formica exsecta. Biol Lett 10:20140805.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A, 1999. Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128. [DOI] [PubMed] [Google Scholar]
- Giordano M, Costantini D, Tschirren B, 2015. Sex-specific effects of prenatal and postnatal nutritional conditions on the oxidative status of great tit nestlings. Oecologia 177:123–131. [DOI] [PubMed] [Google Scholar]
- Groothuis TG, Schwabl H, 2002. Determinants of within- and among-clutch variation in levels of maternal hormones in black-headed gull eggs. Funct Ecol 16:281–289. [Google Scholar]
- Halliwell B, Gutteridge J, 2007. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death In: Free Radicals in Biology and Medicine.Vol. 4 Oxford: Oxford University Press; 187–267. [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM, 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. P Roy Soc B-Biol Sci 279: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson C, 2013. Opposing effects on glutathione and reactive oxygen metabolites of sex, habitat, and spring date, but no effect of increased breeding density in great tits Parus major. Ecol Evol 3:2730–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, 2006. Redefining oxidative stress. Antioxid Redox Sign 8:1865–1879. [DOI] [PubMed] [Google Scholar]
- Keller LF, Waller DM, 2002. Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241. [Google Scholar]
- Kristensen T, Sørensen P, Kruhøffer M, Pedersen K, Loeschcke V, 2005. Genome-wide analysis on inbreeding effects on gene expression in Drosophila melanogaster. Genetics 171:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T, Sørensen P, Pedersen K, Kruhøffer M, Loeschcke V, 2006. Inbreeding by environmental interactions affect gene expression in Drosophila melanogaster. Genetics 173:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen TN, Pedersen KS, Vermeulen CJ, Loeschcke V, 2010. Research on inbreeding in the ‘omic’ era. Trends Ecol Evol 25:44–52. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE. et al. , 2004. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci USA 101:7663–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I. et al. , 1990. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. [DOI] [PubMed] [Google Scholar]
- Mainwaring MC, Dickens M, Hartley IR, 2010. Environmental and not maternal effects determine variation in offspring phenotypes in a passerine bird. J Evol Biol 23:1302–1311. [DOI] [PubMed] [Google Scholar]
- Marasco V, Spencer KA, Robinson J, Herzyk P, Costantini D, 2013. Developmental post-natal stress can alter the effects of pre-natal stress on the adult redox balance. Gen Comp Endocr 191:239–246. [DOI] [PubMed] [Google Scholar]
- Marasco V, Herzyk P, Robinson J, Spencer KA, 2016. Pre-and post-natal stress programming: developmental exposure to glucocorticoids causes long-term brain-region specific changes to transcriptome in the precocial Japanese Quail. J Neuroendocrinol 28:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel M, Sletvold N, Ågren J, Hansson B, 2015. Inbreeding affects gene expression differently in two self-incompatible Arabidopsis lyrata populations with similar levels of inbreeding depression. Mol Biol Evol 32:2036–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P, 2001. Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Nager RG, 1997. Why don't birds lay more eggs? Trends Ecol Evol 12:270–274. [DOI] [PubMed] [Google Scholar]
- Monaghan P, 2008. Early growth conditions, phenotypic development and environmental change. Philos T Roy Soc B 363:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Groothuis TGG, 2013. Within-clutch variation in yolk testosterone as an adaptive maternal effect to modulate avian sibling competition: evidence from a comparative study. Am Nat 181:125–136. [DOI] [PubMed] [Google Scholar]
- Nilsson J-Å, Råberg L, 2001. The resting metabolic cost of egg laying and nestling feeding in great tits. Oecologia 128:187–192. [DOI] [PubMed] [Google Scholar]
- Oddie KR, 2000. Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912. [DOI] [PubMed] [Google Scholar]
- Okada K, Blount JD, Sharma MD, Snook RR, Hosken DJ, 2011. Male attractiveness, fertility and susceptibility to oxidative stress are influenced by inbreeding in Drosophila simulans. J Evol Biol 24:363–371. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Costantini D, 2011. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol-Reg I 301:R843–R863. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC. et al. , 2002. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol 12:712–723. [DOI] [PubMed] [Google Scholar]
- Price DT, Vita JA, Keaney JF Jr., 2000. Redox control of vascular nitric oxide bioavailability. Antioxid Redox Sign 2:919–935. [DOI] [PubMed] [Google Scholar]
- R Core Development Team, 2014. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Royle NJ, Surai PF, McCartney RJ, Speake BK, 1999. Parental investment and egg yolk lipid composition in gulls. Func Ecol 13:298–306. [Google Scholar]
- Royle NJ, Surai PF, Hartley IR, 2001. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav Ecol 12:381–385. [Google Scholar]
- Royle NJ, Hartley IR, Parker GA, 2002. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol Evol 17:434–440. [Google Scholar]
- Royle NJ, Surai PF, Hartley IR, 2003. The effect of variation in dietary intake on maternal deposition of antioxidants in zebra finch eggs. Funct Ecol 17:472–481. [Google Scholar]
- Schwabl H, 1993. Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sild E, Hõrak P, 2009. Nitric oxide production: an easily measurable condition index for vertebrates. Behav Ecol Sociobiol 63:959–966. [Google Scholar]
- Speakman JR, Blount JD, Bronikowski AM, Buffenstein R, Isaksson C. et al. , 2015. Oxidative stress and life histories: unresolved issues and current needs. Ecol Evol 5:5745–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdovich P, 2008. Free radicals and antioxidants in inflammatory processes and ischemia-reperfusion injury. Vet Clin N Am-Small 38:31–123. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Müller W, Eens M, 2016. Vitally important: does early innate immunity predict recruitment and adult innate immunity? Ecol Evol 6:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD, 1994. Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol Rev 69:35–59. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Spencer KA, 2015. Reduced resistance to oxidative stress during reproduction as a cost of early-life stress. Comp Biochem Phys A 183:9–13. [DOI] [PubMed] [Google Scholar]