Abstract
Background:
We investigated the relationship between sleep disturbance and cognitive decline or clinical conversion in individuals with normal cognition (CN), as well as those with mild cognitive impairment (MCI) and dementia due to Alzheimer’s disease (AD-dementia).
Methods:
Secondary analysis of 1,629 adults between 48 and 91 years old with up to 24 months of follow-up from the Alzheimer’s Disease Neuroimaging Initiative, a longitudinal cohort study.
Results:
Sleep disturbance was not associated with decline in memory, executive function, or global cognition. The presence of sleep disturbance did not significantly increase the risk of diagnostic conversion in CN, early MCI, or late MCI participants.
Conclusion:
This study investigated the effect of sleep disturbance on cognitive decline using several outcomes and does not support the hypothesis that sleep disturbance predicts subsequent cognitive decline.
Keywords: ADNI, Alzheimer’s Disease, sleep, sleep disruption, memory, executive function
I. Objective
Sleep disturbances are found at all stages of Alzheimer’s disease (AD) and are common in both patients with mild cognitive impairment (MCI) and dementia due to AD [1–4]. Among patients with AD, up to 44% experience either increased daytime sleepiness or difficulty sleeping at night [5]. Furthermore, sleep disturbances are a major contributor to the decision to institutionalize older individuals [6]. Investigating the association between sleep disturbance and cognitive decline may help define the role of sleep disturbance in neurodegenerative disease.
Several biomarker studies have supported the hypothesis that sleep disturbance is related to the pathogenesis of AD [7]. In healthy middle-aged men, a single night of unrestricted sleep has been reported to lead to a 6% decrease in CSF amyloid-β (Aβ) 42, whereas one night of sleep deprivation prevented this decrease [8]. Furthermore, shorter sleep duration and poor sleep quality have been associated with greater Aβ burden in older adults [9]. Together, these studies suggest that sleep may aid in the clearance of Aβ, while disrupted sleep may lead to Aβ accumulation that causes neurodegeneration due to AD.
Speculation that disrupted sleep may be involved in the pathogenesis of AD is also supported by preclinical studies using mouse models in which sleep deprivation has been associated with memory impairment and synaptic loss [10]. Furthermore, Aβ levels in the interstitial fluid of both wild-type and transgenic-AD mice has been observed to fluctuate with diurnal sleep patterns and to increase with sleep deprivation and infusion of orexin, a neuropeptide that regulates wakefulness. A similar pattern has been seen in the CSF of young, cognitively normal (CN) humans [11]. A study of two-photon imaging in mice has suggested that natural sleep or anesthesia induces fluxes of brain interstitial fluid that increases Aβ clearance [12].
The effect of disrupted sleep on cognition in CN adults has been evaluated in several cross-sectional and longitudinal cohort studies. These investigations have used both continuous and dichotomous measures of cognitive decline and have largely indicated that disrupted sleep in CN individuals increases the risk of cognitive decline [13–18]. Sleep disturbance has also been linked with increased risk of cognitive decline in individuals with already existing cognitive impairment. Nighttime behavioral disturbances, either disrupted nighttime sleep or daytime sleeping, increase the risk of progression from MCI to AD [19] and individuals with AD who had nighttime behavioral disturbances had increased mortality [20].
To further investigate the association between sleep disturbance and AD, we utilized data collected as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI). ADNI is an observational study of aging which enrolls individuals diagnosed as CN, early MCI (EMCI), late MCI (LMCI), or AD-dementia which allowed a robust assessment of risk across various diagnostic groups. We assessed the effect of sleep disturbance symptoms on both the change in cognitive performance over time, as well as the risk of progression to a more advanced stage of disease.
II. Methods
Data were obtained from the ADNI database (adni.loni.usc.edu) on 9/28/2014. ADNI was launched in 2003 as a public-private partnership, led by Michael W. Weiner, MD. All participants have given their informed consent and the study protocol has been approved by the committee on human research at each participating institution. At the time of download, the dataset contained 1629 adults (48 to 91 years old) with baseline data: 418 CN, 308 EMCI, 561 LMCI, and 342 AD-dementia. Detailed information describing diagnostic criteria can be found at www.adni-info.org. CN subjects had no memory complaints, normal memory performance, and absence of impairment in cognitive or function. EMCI subjects had a subjective memory concern, mildly abnormal memory performance, and preserved functional performance such that a diagnosis of AD-dementia could not be made. LMCI subjects had a subjective memory concern, memory performance that was abnormal and below that of EMCI subjects [21], and preserved functional performance such that a diagnosis of AD-dementia could not be made. AD-dementia subjects had a subjective memory concern, abnormal memory performance, and functional impairment meeting NINCDS/ADRDA criteria for probable AD [22].
Outcomes
Presence of sleep disturbance was determined by caregiver report on the Neuropsychiatric Inventory (NPI) or a brief questionnaire form of the NPI (NPI-Q). The NPI is a retrospective (up to 1 month) caregiver-informant interview that investigates 12 neuropsychiatric symptom domains: delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviors, nighttime behavioral disturbances, and appetite/eating disturbances [23]. A sleep disturbance symptom prior to conversion was considered to be present if a caregiver-informant reported the symptom during the study prior to conversion from CN to MCI, or MCI to AD-dementia. Since neuropsychiatric symptoms are more common among individuals with MCI and AD-dementia compared to those who are CN, symptoms reported at the conversion visit or the last study visit were excluded to minimize reverse causation. The primary outcomes used in this analysis included the AD Assessment Scale-cognitive sub-scale (ADAS11), a measure of global cognition, as well as a composite score of memory (ADNI-Mem) and a composite score of executive function (ADNI-EF) [24–26]. As an additional, primary outcome visit diagnosis was used to determine if a subject converted from CN to MCI (early or late) or MCI to AD-dementia. MMSE, Clinical Dementia Rating Scale sum of boxes (CDRsb), Geriatric Depression Scale (GDS), apolipoprotein E (APOE) genotype, medication use data, and medical comorbidities listed in Table 1 were utilized to characterize the study cohort. Sedative/hypnotic use was defined as use of a benzodiazepine or non-benzodiazepine GABA agonist for at least 1-month prior to conversion.
Table 1:
Participant Demographics
| CN | EMCI | LMCI | AD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSD | NSD | p-value | PSD | NSD | p-value | PSD | NSD | p-value | PSD | NSD | p-value | |
| n (%) | 66 (16%) |
352 | - | 118 (38%) |
190 | - | 190 (34%) |
371 | - | 153 (45%) | 189 | - |
| Age | 73.4±5.3 | 75.0±5.8 | 0.04* | 72.5±7.6 | 70.4±7.3 | 0.018* | 73.1±8.2 | 74.4±7.2 | 0.057 | 75.7±7.6 | 74.4±7.9 | 0.115 |
| Sex (% male) | 51.5 | 49.7 | 0.788 | 56.8 | 54.7 | 0.726 | 63.7 | 59.8 | 0.376 | 58.2 | 52.9 | 0.331 |
| Education | 16.6±2.3 | 16.2±2.8 | 0.314 | 15.5±2.7 | 16.2±2.6 | 0.018* | 16.0±2.8 | 15.8±3.0 | 0.656 | 15.5±3.0 | 15.0±3.0 | 0.110 |
| APOE Genotype (%) | ||||||||||||
| e3e3 | 71.2 | 72.8 | 0.956 | 47.9 | 62.4 | 0.013* | 48.7 | 44.1 | 0.472 | 36.6 | 31.4 | 0.418 |
| e3e4 | 25.8 | 24.6 | 41.0 | 33.3 | 40.2 | 41.9 | 43.1 | 50.3 | ||||
| e4e4 | 3.0 | 2.6 | - | 11.1 | 4.3 | - | 11.1 | 14.1 | - | 20.3 | 18.4 | - |
| Sedative/Hypn otic (%) | 12.1 | 6.8 | 0.137 | 17.8 | 10.0 | 0.048* | 10.0 | 4.0 | 0.005* | 7.2 | 5.3 | 0.467 |
| CDRsb | 0.0±0.1 | 0.0±0.1 | 0.470 | 1.4±0.8 | 1.2±0.7 | 0.024* | 1.7+1.0 | 1.6±0.9 | 0.485 | 4.6±1.7 | 4.3±1.7 | 0.079 |
| ADAS-11 | 6.2±3.4 | 6.0±2.9 | 0.591 | 8.1±3.5 | 7.7±3.5 | 0.252 | 11.3±4.6 | 11.6+4.6 | 0.420 | 19.4±7.2 | 19.4±6.6 | 0.991 |
| Comorbid Diagnoses (%) | ||||||||||||
| HTN | 51.5 | 44.3 | 0.281 | 54.2 | 44.7 | 0.105 | 48.4 | 48.8 | 0.935 | 54.2 | 47.1 | 0.188 |
| DM | 7.6 | 7.4 | 0.957 | 11.9 | 10.0 | 0.607 | 8.9 | 7.8 | 0.644 | 8.5 | 10.6 | 0.516 |
| CAD | 3.0 | 3.1 | 0.968 | 3.4 | 3.2 | 0.911 | 4.2 | 3.8 | 0.801 | 3.9 | 1.6 | 0.180 |
| GDS | 0.9±1.2 | 0.7+1.1 | 0.218 | 2.0±1.6 | 1.7+1.5 | 0.038* | 2.0±1.5 | 1.4+1.3 | <0.0005* | 1.7+1.4 | 1.6+1.5 | 0.436 |
PSD = positive for sleep disturbance symptoms, NSD = negative for sleep disturbance symptoms, CDRsb = Clinical Dementia Rating Scale - Sum of Boxes Score, ADAS-11 = Alzheimer’s Disease Assessment Scale-Cognitive Subscale, HTN = Hypertension, DM = Diabetes CAD = Coronary Artery Disease, GDS = Geriatric Depression Scale, ANOVA for continuous outcomes presented as mean±standard deviation, chi-square test for categorical outcomes,
p < .05
Statistical Analyses
Independent analyses were performed for each of the 4 baseline cognitive diagnoses: CN, EMCI, LMCI, or AD-dementia. A dichotomous variable was assigned as either positive for a sleep disturbance symptom prior to conversion (PSD), or negative for a sleep disturbance symptom prior to conversion (NSD). A comparison of baseline characteristics, including sleep disturbance, age, sex, level of education, sedative/hypnotic use, APOE genotype, CDR, MMSE, and ADAS11, as well as comorbidities of hypertension, diabetes, hypercholesterolemia, and coronary artery disease, for participants with 24 months of follow up data and those without 24 month follow up data was performed to assess for sources of attrition bias. In addition, a comparison of baseline characteristics between those with sleep disturbance symptoms and those without sleep disturbance symptoms prior to conversion was performed. Analysis of variance was used for continuous outcome variables and χ2 testing for categorical outcome variables.
To determine if differences in cognitive decline were dependent on presence of sleep disturbance prior to conversion, three repeated measures linear mixed models were used with ADAS11, ADNI-Mem, or ADNI-EF as the outcome variable and the interaction of sleep disturbance and time as the main explanatory variable. Age, sex, APOE genotype, education level, and sedative/hypnotic use were included as covariates since these are known risk factors for MCI or dementia that are routinely included in statistical models [27,28].
Survival curves of conversion from CN to MCI or AD-dementia, as well as conversion from MCI to AD-dementia were calculated using the Kaplan-Meier method. A Cox proportional hazards model was used to assess the risk of conversion based on sleep disturbance. The model used the same covariates as described above in addition to baseline ADAS11 score to adjust for disease stage. The confidence level for statistical inference was 95% (p<0.05). When interpreting statistical results, false discover rate (FDR) correction was applied since multiple outcome variables were assessed for each diagnostic group (ADNI-Mem, ADNI-EF, ADAS11, and conversion). Statistical analysis was performed using SPSS Statistics Version 21.0 (IBM Corp, Armonk, NY).
Power Analyses
For repeated measures linear mixed models, power analyses were performed using simulation with MATLAB R2015a and the Statistics Toolbox (The MathWorks, Inc., Natick, MA). Study data parameters were used for baseline mean cognitive scores (ADAS11, ADNI-EF, ADNI-Mem), standard deviation, and standard error of the parameter estimate for change in cognitive score over time. Presence of a sleep disturbance was the main explanatory variable and covariates were not included. For cox proportional hazards models, power analysis was performed using the proportion of subjects with PSD and the event rate for each baseline diagnosis[29]. Power Analyses were performed to assess the likelihood of a Type II error.
III. Results
Participant Characteristics
CN Participants.
Of the 418 CN participants who had a baseline visit, 66 (16%) were categorized as PSD. PSD participants were slightly younger compared to NSD participants (73.4 vs 75.0 years, p=0.040). There were no significant differences between PSD and NSD participants in sex, education, APOE genotype, sedative/hypnotic use, CDRsb, ADAS11, geriatric depression scale (GDS) score, or comorbid cardiovascular diagnoses at baseline (Table 1). There was no significant difference in the average follow-up time between PSD (22.6 months) and NSD (21.6 months) groups (p=0.158). Supplemental Table 1 contains a detailed summary of demographics stratified by diagnostic group and attrition.
EMCI Participants.
Of the 308 EMCI participants who had a baseline visit, 118 (38%) were categorized as PSD. PSD subjects were slightly older (72.5 vs 70.4 years, p=0.018) and less educated (15.5 vs 16.2 years, p=0.018) compared to NSD participants. PSD subjects were more likely to have APOE ε4 alleles (p=0.013). In addition, PSD subjects were more likely to be taking a sedative/hypnotic medication (17.8% vs 10.0%, p=0.048), had a slightly higher CDRsb (1.4 vs 1.2, p=0.024), and slightly higher GDS scores (2.0 vs 1.7, p=0.038). There were no significant differences between PSD and NSD participants in sex, ADAS11, or comorbid cardiovascular diagnoses at baseline (Table 1). There was no significant difference in the average follow-up time between PSD (21.3 months) and NSD (19.6 months) groups (p=0.070).
LMCI Participants.
Of the 561 LMCI participants who had a baseline visit, 190 (34%) were categorized as PSD. PSD subjects were more likely to be taking a sedative/hypnotic medication (10.0% vs 4.0%, p=0.005) and had slightly higher GDS scores (1.0 vs 1.4, p<0.005). There were no significant differences between PSD and NSD participants in age, sex, education, ADAS11, APOE genotype, CDRsb, ADAS11, or comorbid cardiovascular diagnoses at baseline (Table 1). There was no significant difference in the average follow-up time between PSD (20.9 months) and NSD (20.1 months) groups (p=0.215).
AD-dementia Participants.
Of the 342 AD participants who had a baseline visit, 153 (45%) were categorized as PSD. There were no significant differences between PSD and NSD participants in age, sex, education, ADAS11, APOE genotype, CDRsb, ADAS11, GDS score, sedative/hypnotic use, or comorbid cardiovascular diagnoses at baseline (Table 1). In AD participants, the PSD group had a significantly longer average follow-up time (17.8 months) compared to the NSD group (14.5 months (p=0.002).
Association between Sleep Disturbance and Longitudinal Cognitive Performance: Linear Mixed Models
The association between sleep disturbance and change in memory, executive function, or composite cognitive performance over time was investigated using a linear repeated measures mixed effects model with presence of sleep disturbance (PSD vs NSD) as the main explanatory variable and either change in ADNI-Mem score, change in ADNI-EF score, or change in ADAS11 score over time as the outcome variable (Table 2). Covariates included sex, APOE ε4 allele number, education level and sedative/hypnotic use, as well as all of their interactions with time (Supplemental Table 2).
Table 2:
Effect of Sleep Disturbance on Change in Cognition over Time
| CN | EMCI | LMCI | AD-dementia | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter Estimate |
p- value |
Parameter Estimate |
p- value |
Parameter Estimate |
p- value |
Parameter Estimate |
p- value |
|
| Memory | 0.003 | 0.169 | −0.001 | 0.542 | 0.000 | 0.799 | 0.002 | 0.335 |
| EF | 0.005 | 0.067 | 0.000 | 0.997 | −0.001 | 0.440 | −0.002 | 0.431 |
| ADAS-11 | −0.012 | 0.467 | 0.010 | 0.636 | −0.047 | 0.018* | 0.027 | 0.558 |
ADAS-11 = Alzheimer’s Disease Assessment Scale-Cognitive Subscale, EF = Executive Function, Parameter Estimates and p-values are for a repeated measures linear mixed effects model, PE = parameter estimate,
p < .05 prior to correction for multiple comparisons
CN Participants.
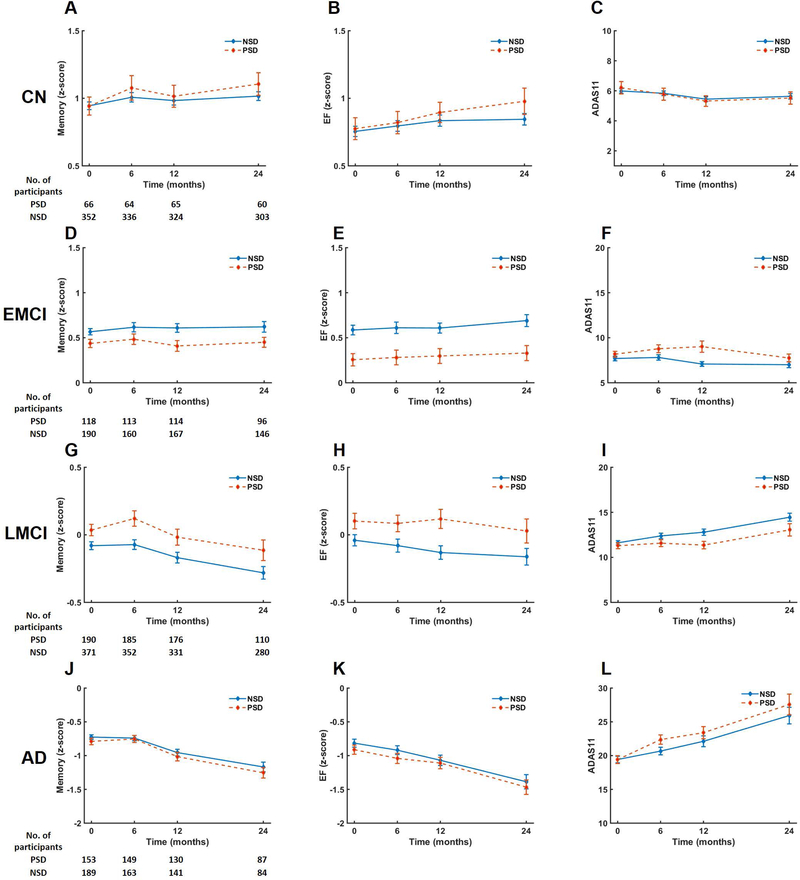
Within the CN sample, there was no significant decline in cognition over time as measured by ADNI-Mem (PE=−0.001, p=0.664), ADNI-EF (PE=0.015, p=0.361), and ADAS11 (PE=−0.038, p=0.634). The PSD and NSD groups did not differ in any measure of longitudinal cognitive performance, including ADNI-Mem, ADNI-EF, or ADAS11 (Figure 1).
Figure 1: Change in cognition over time for participants with sleep disturbance (PSD) and without sleep disturbance (NSD).
Memory (ADNI-Mem), executive function (ADNI-EF), and global cognitive (ADAS-11) performance over time for PSD and NSD groups stratified by baseline diagnosis of cognitively normal (CN), early MCI (EMCI), late MCI (LMCI), or AD-dementia (AD). Repeated measures linear mixed models were used to compare the effect of sleep group (NSD or PSD) on change in cognitive outcome over time. The model was adjusted for age, sex, APOE genotype, education level, and sedative/hypnotic use. The confidence level for statistical inference was 95% (p < 0.05). Plotted values are mean with error bars for standard error of the mean. FDR correction was performed for multiple comparisons.
EMCI Participants.
Within the EMCI sample, there was no significant decline in cognition over time as measured by ADNI-Mem (PE=0.014, p=0.053), ADNI-EF (PE=0.017, p = 0.146), and ADAS11 (PE=0.002, p=0.765). The PSD and NSD groups did not differ in any measure of longitudinal cognitive performance, including ADNI-Mem, ADNI-EF, or ADAS11 (Figure 1).
LMCI Participants.
Within the LMCI sample, there was no significant decline in cognition over time as measured by ADNI-Mem (PE=−0.006, p=0.722), ADNI-EF (PE=−0.011, p=0.281), and ADAS11 (PE=0.107, p=0.521). The PSD and NSD groups did not differ in longitudinal cognitive performance based on ADNI-Mem or ADNI-EF (Figure1). PSD was associated with a slower rate of ADAS11 increase (worsening) compared to the NSD group (Figure 1I, Table 2), but this was not significant after FDR correction for multiple comparisons (p=0.072).
AD-dementia Participants.
Within the AD-dementia sample, there was a significant decline in cognition over time as measured by ADNI-Mem (PE=−0.079, p<0.001), ADNI-EF (PE=−0.077, p<0.001), and ADAS11 (PE=1.649, p <0.001). The PSD and NSD groups did not differ in any measure of longitudinal cognitive performance, including ADNI-Mem, ADNI-EF, or ADAS11 (Figure 1).
In light of a previous report of non-demented adults [13,14], an additional analysis was performed as above with the exception that CN, EMCI, and LMCI were combined as a non-dementia cohort. PSD and NSD groups did not differ in longitudinal cognitive performance (Supplemental Table 3A).
Association between sleep disturbance and conversion: Cox Hazards Models
The association between sleep disturbance and risk of conversion to a more severe diagnostic group was investigated using Cox proportional hazards models with sleep disturbance as the main explanatory variable. Age, sex, APOE genotype, education level, sedative/hypnotic use, and baseline ADAS11 score were included as covariates (Supplemental Table 4).
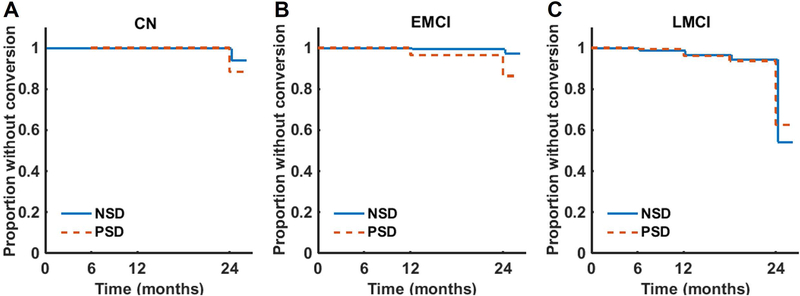
Twenty-six out of 393 participants (6.6%) with a baseline diagnosis of CN and at least one follow-up visit progressed to a diagnosis of MCI (EMCI or LMCI) over the two-year follow-up period (367 censored). Eighteen out of 281 (6.4%) participants with a baseline diagnosis of EMCI and at least one follow-up visit progressed to a diagnosis of AD-dementia (263 censored). One hundred and ninety-nine participants (36.9%) with a baseline diagnosis of LMCI and at least one follow-up visit progressed to AD-dementia (341 censored).
In the CN participants, PSD did not contribute to the risk of progression to MCI (HR=1.99, p=0.132, 95% CI for HR=0.813–4.918, Figure 2A). Notably, PSD did increase the risk of progression in the EMCI participants (HR 3.70, p=0.024, 95% CI for HR=1.19–11.52), but this finding was not significant after FDR correction for multiple comparisons (p=0.096). Finally, in the LMCI participants, PSD did not contribute to the risk of progression to AD-dementia (HR=0.87, p=0.371, 95% CI for HR=0.64–1.18, Figure 2C).
Figure 2: Conversion to a more impaired diagnostic group.
Survival curves were constructed using the Kaplan-Meier method. A Cox proportional hazards model was used to assess the time to conversion for individuals with a sleep disturbance (PSD) versus those without a sleep disturbance (NSD) prior to conversion. The model was adjusted for age, sex, APOE genotype, education level, and sedative/hypnotic use, as well as baseline ADAS-11 score to account for baseline disease stage. The confidence level for statistical inference was 95% (p < 0.05) and FDR correction was performed for multiple comparisons.
As with continuous measures, an additional analysis was performed as above with the exception that CN, EMCI, and LMCI were combined as a non-dementia cohort. PSD did not contribute to the risk of progression to either MCI or AD-dementia (Supplemental Table 3B).
Power Analyses
Power analyses were performed to aid in interpretation of our results. Depending on baseline diagnosis, the study had 80% power to detect group differences of 0.33–0.68 ADAS11 per year, 0.029–0.048 ADNI-EF per year, and 0.021–0.045 ADNI-Mem per year using repeated measures linear mixed models. Power to detect group differences was estimated by simulation over a range of rates of decline (Supplemental Figure 1, Supplemental Table 5). For the Cox proportional hazards models, the study had 80% power to detect a HR of 4.5 for participants with a baseline diagnosis of CN, 3.9 for participants with a baseline diagnosis of EMCI, and 1.5 for participants with a baseline diagnosis of LMCI.
IV. Discussion
We investigated the relationship between sleep disturbance and cognitive decline or conversion to a more advanced cognitive diagnosis in individuals who were CN or diagnosed with MCI or AD-dementia as part of the ADNI study. Overall, the presence of sleep disturbance symptoms did not affect the rate of decline in measures of global cognition, executive function, or memory performance. In addition, the presence of sleep disturbance symptoms did not increase the risk of conversion to a more advanced stage of disease. Since our analyses did not show a statistically significant effect of sleep disturbance on any measure, we performed power analyses to investigate the likelihood of a type II error. These analyses indicated that use of continuous measures with linear mixed models should be relatively sensitive compared to conversion (dichotomous) for detecting differences in cognitive decline between groups.
Our results differ from those of several previous prospective [13,15,16,18,19,30,31] or retrospective [32] cohort studies that reported an increased risk of cognitive decline and/or conversion to a more advanced stage of disease in individuals without dementia who have disrupted sleep. These studies have enrolled subjects who were CN [30,31] or not demented [13,15,16,18,19] and examined the risk for conversion to a more advanced diagnostic category [13,15,16,18,19,30–32] and—in one cohort, the Rush Memory and Aging Project—also measured cognitive decline [13,16]. In addition, sleep disturbance had an additive effect to increase risk in individuals who carried a copy of the APOE ε4 allele [33]. An advantage of our design—and that employed in the Rush Memory and Aging Project [13,16] —is the combined use of disease stage and measured cognitive outcomes. To mitigate the risk of a ceiling effect when measuring cognitive change over time, we utilized multiple cognitive outcomes that together are sensitive to cognitive changes in individuals [24–26].
Another advantage is the separate analyses by stage of disease, since different stages of disease may involve different degrees of diagnostic certainty and different rates of conversion/progression. Lim et al analyzed a single non-demented cohort [13,16], which likely encompassed our CN, EMCI, and LMCI groups. We therefore performed a post-hoc analysis, pooling these groups, but our results still differed. Another strength is our use of data from a multicenter study, which may enhance external validity. However, the ADNI cohort, like many samples recruited for observational studies of AD, is not nationally representative and may contain participants at disproportionate risk of cognitive decline. Because the attrition in ADNI is low over a 2 year follow up period, there is less risk of attrition bias (Figure 1). Finally, we controlled for confounders such as baseline cognitive performance, sedative/hypnotic use, and also used continuous measures of cognition that should be sensitive to changes over time, even prior to conversion to MCI or dementia.
Both questionnaire based sleep scales and actigraphy have been used to measure sleep disruption. Studies using actigraphy may have a strength of being objective measures that are not susceptible to recall bias. However, sleep scales such as the NPI [23] and Pittsburgh Sleep Quality Index [18] are subjective questionnaires that are validated and commonly used to measure sleep characteristics. One disadvantage of the NPI is that it cannot be used to determine the etiology of sleep disturbance (e.g. hypersomnia, insomnia, rapid eye movement sleep behavior disorder).
A major difficulty in the interpretation of studies in this field is the possibility of reverse causation--a common source of confounding in observational research [34]. In this setting, it may be difficult to dissociate whether sleep disruption modifies disease progression or is caused by AD and emerges as an early symptom. I.e., longitudinal studies that report a significant association may simply be identifying a feature of the disease, which is already well-established in cross-sectional studies [35].
Longitudinal studies that define sleep disturbance at baseline and then monitor participants for a sufficient follow-up period may be less susceptible to reverse causation. An important limitation of our study is thus the average follow-up period ranging from 16 months in participants with dementia at baseline to 22 months in participants with normal cognition at baseline. Many studies in this field have similar average follow-up periods ranging between 1 and 3.5 years [13,16,18,19,30]. Two studies with longer follow-up periods of 4.9 [15] and 7.3–7.7 years [31] examined conversion to MCI or AD dementia. Studies that utilize outcomes of incident MCI or dementia are likely to be particularly sensitive to reverse causation bias since the use of diagnostic conversion indicating dementia may lag behind the presence of cognitive or functional symptoms [34]. Longer follow up periods can help to minimize this bias and strengthen investigations of causality.
In summary, this study investigated the effect of sleep disturbance on cognitive and clinical outcomes and did not support the hypothesis that sleep disturbance is a risk factor for subsequent cognitive decline. These results raise important methodological questions about the evaluation of risk factors for clinical progression in AD.
Supplementary Material
Acknowledgements
APM was supported in part by the Yale Integrated Mentored Patient-Oriented Research Training (IMPORT) in Psychiatry (#5R25MH071584–07 – PI: Malison NIH/NIMH). This work was also supported by the National Institute on Aging [P50-AG047270].
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research &Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Disclosures
None of the authors have actual or potential conflicts of interest to disclose that are related to this research.
References
- 1.Wadsworth LP, Lorius N, Donovan NJ, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA: Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dementia and geriatric cognitive disorders 2012;34:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters ME, Rosenberg PB, Steinberg M, Tschanz JT, Norton MC, Welsh-Bohmer KA, Hayden KM, Breitner JC, Lyketsos CG, Cache County I: Prevalence of neuropsychiatric symptoms in CIND and its subtypes: the Cache County Study. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2012;20:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrester SN, Gallo JJ, Smith GS, Leoutsakos JM: Patterns of Neuropsychiatric Symptoms in Mild Cognitive Impairment and Risk of Dementia. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2016;24:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliwise DL, Mercaldo ND, Avidan AY, Boeve BF, Greer SA, Kukull WA: Sleep disturbance in dementia with Lewy bodies and Alzheimer’s disease: a multicenter analysis. Dement Geriatr Cogn Disord 2011;31:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitiello MV, Borson S: Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS drugs 2001;15:777–796. [DOI] [PubMed] [Google Scholar]
- 6.Pollak CP, Perlick D: Sleep problems and institutionalization of the elderly. Journal of geriatric psychiatry and neurology 1991;4:204–210. [DOI] [PubMed] [Google Scholar]
- 7.Lim MM, Gerstner JR, Holtzman DM: The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegenerative disease management 2014;4:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA: Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA neurology 2014;71:971–977. [DOI] [PubMed] [Google Scholar]
- 9.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM: Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA neurology 2013;70:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Meco A, Joshi YB, Pratico D: Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiology of aging 2014;35:1813–1820. [DOI] [PubMed] [Google Scholar]
- 11.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM: Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (New York, NY) 2009;326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M: Sleep drives metabolite clearance from the adult brain. Science (New York, NY) 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA: Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013;36:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim AS, Yu L, Costa MD, Leurgans SE, Buchman AS, Bennett DA, Saper CB: Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep 2012;35:633–640B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diem SJ, Blackwell TL, Stone KL, Yaffe K, Tranah G, Cauley JA, Ancoli-Israel S, Redline S, Spira AP, Hillier TA, Ensrud KE: Measures of Sleep-Wake Patterns and Risk of Mild Cognitive Impairment or Dementia in Older Women. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2016;24:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA: Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA neurology 2013;70:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe K, Nettiksimmons J, Yesavage J, Byers A: Sleep Quality and Risk of Dementia Among Older Male Veterans. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2015;23:651–654. [DOI] [PubMed] [Google Scholar]
- 18.Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, Hudon C: Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 2012;35:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters ME, Rosenberg PB, Steinberg M, Norton MC, Welsh-Bohmer KA, Hayden KM, Breitner J, Tschanz JT, Lyketsos CG, Cache County I: Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2013;21:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spalletta G, Long JD, Robinson RG, Trequattrini A, Pizzoli S, Caltagirone C, Orfei MD: Longitudinal Neuropsychiatric Predictors of Death in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 2015;48:627–636. [DOI] [PubMed] [Google Scholar]
- 21.Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR Jr., Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW, Alzheimer’s Disease Neuroimaging I: Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST: Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 24.Rosen WG, Mohs RC, Davis KL: A new rating scale for Alzheimer’s disease. The American Journal of Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging I: A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain imaging and behavior 2012;6:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging I: Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain imaging and behavior 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim YY, Ellis KA, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Villemagne VL, Maruff P: Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimers Dement 2013;9:538–545. [DOI] [PubMed] [Google Scholar]
- 28.Billioti de Gage S, Begaud B, Bazin F, Verdoux H, Dartigues JF, Peres K, Kurth T, Pariente A: Benzodiazepine use and risk of dementia: prospective population based study. BMJ 2012;345:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld DA: Sample-size formula for the proportional-hazards regression model. Biometrics 1983;39:499–503. [PubMed] [Google Scholar]
- 30.Burke SL, Maramaldi P, Cadet T, Kukull W: Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: dementia. Int Psychogeriatr 2016;28:1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JC, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, Phillips LS, Robinson JG, Kotchen JM, Johnson KC, Manson JE, Stefanick ML, Sarto GE, Mysiw WJ: Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement 2016;12:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Nettiksimmons J, Yesavage J, Byers A: Sleep Quality and Risk of Dementia Among Older Male Veterans. Am J Geriatr Psychiatry 2015;23:651–654. [DOI] [PubMed] [Google Scholar]
- 33.Burke SL, Maramaldi P, Cadet T, Kukull W: Neuropsychiatric symptoms and Apolipoprotein E: Associations with eventual Alzheimer’s disease development. Arch Gerontol Geriatr 2016;65:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF: Statins, cognition, and dementia-systematic review and methodological commentary. Nat Rev Neurol 2015;11:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Alberca JM, Lara JP, Cruz B, Garrido V, Gris E, Barbancho MA: Sleep disturbances in Alzheimer’s disease are associated with neuropsychiatric symptoms and antidementia treatment. The Journal of nervous and mental disease 2013;201:251–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.