Abstract
Objectives:
To describe the survival outcome of patients with borderline resectable or locally advanced pancreatic ductal adenocarcinoma (BR/LAPDAC) who have a pathologic complete response (pCR) following neoadjuvant chemoradiation.
Background:
Patients with BR/LA-PDAC are often treated with neoadjuvant chemoradiation in an attempt to downstage the tumor. Uncommonly, a pCR may result.
Methods:
A retrospective review of a prospectively maintained database was performed at a single institution. pCR was defined as no viable tumor identified in the pancreas or lymph nodes by pathology. A near complete response (nCR) was defined as a primary tumor less than 1 cm, without nodal metastasis. Overall survival (OS) and disease-free survival (DFS) were reported.
Results:
One hundred eighty-six patients with BR/LA-PDAC underwent neoadjuvant chemoradiation and subsequent pancreatectomy. Nineteen patients (10%) had a pCR, 29 (16%) had an nCR, and the remaining 138 (74%) had a limited response. Median DFS was 26 months in patients with pCR, which was superiorto nCR (12 months, P = 0.019) and limited response (12 months, P < 0.001). The median OS of nCR (27 months, P = 0.003) or limited response (26 months, P = 0.001) was less than that ofpCR (more than 60 months). in multivariable analyses pCR was an independent prognostic factorforDFS (HR = 0.45;0.22–0.93, P = 0.030) and OS (HR=0.41;0.17–0.97, P = 0.044). Neoadjuvant FOLFIRINOX (HR=0.47; 0.26–0.87, P =0.015) and negative lymph node status (HR=0.57; 0.36–0.90, P = 0.018) were also associated with improved survival.
Conclusions:
Patients with BR/LA-PDAC who had a pCR after neoadjuvant chemoradiation had a significantly prolonged survival compared with those who had nCR or a limited response.
Keywords: borderline resectable, locally advanced, neoadjuvant therapy, pancreatectomy, pancreatic ductal adenocarcinoma, pathologic complete response, survival
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with a poor prognosis. Despite advancements in surgical, systemic, and radiation therapies, mortality of PDAC is projected to surpass colorectal cancer to become the second leading cause of cancer-related death in the United States by 2030.1 Complete surgical resection remains the only potentially curative option. Even the most encouraging results in the era of “contemporary chemotherapy” reported a median overall survival of 28 months after resection and adjuvant chemotherapy.2,3 Patients with borderline resectable or locally advanced PDAC (BR/LA-PDAC) may have margin-negative surgery after a period of neoadjuvant chemotherapy or chemoradio-therapy.4 Patients with successfully resected BR/LA-PDAC following neoadjuvant therapy have similar overall survival when compared with those with initially resectable disease.5,6 Furthermore, neoadjuvant chemoradiation has increasingly been utilized even in patients with resectable PDAC due to its potential ability to reduce micrometastatic disease, increase likelihood of a complete resection, and offer a potential survival advantage.7
A pathological complete response (pCR) in PDAC is uncommon and observed in only 3% to 11% of resected specimen treated with neoadjuvant therapy.6,8–13 In esophageal and locally advanced rectal adenocarcinoma, a pCR is associated with greatly improved outcomes with lower rates of local recurrence and improved survival.14–16 Initial reports have suggested a similarly improved survival for PDAC patients with pCR.9–12 However, due to the rarity of a complete response in PDAC, pCR has been grouped with other significant responders in multivariable analysis. Thus, the independent prognostic value of pCR remains unclear. Small tumor size and negative nodal metastases are well-established prognostic indicators for improved long-term overall survival in patients with resectable PDAC following curative resection.17,18 Therefore, we defined tumors less than 1 cm without nodal metastases as “near complete response” (nCR) and used these as an established comparison cohort in this study.
In this study, we present the survival outcomes of a cohort of patients with BR/LA-PDAC who had a pCR or a nCR after neoadjuvant treatment. We evaluated the prognostic impact of pCR on survival.
METHODS
Patients with BR/LA-PDAC who presented to Johns Hopkins Hospital between 2008 and 2016 were identified from a prospectively maintained database and included in this study. Patients were identified with an initial diagnosis of BR/LA-PDAC as determined in our Pancreas Multidisciplinary Clinic or tumor board according to published definitions.19 Patients that failed to complete neoadjuvant chemoradiation therapy or did not proceed to surgery secondary to local disease progression, distant metastasis, or death were excluded from the study. Patients with death attributed to perioperative morbidity within 90 days of the operation were also excluded. The primary outcomes of interest were overall survival (OS) and disease-free survival (DFS).
Demographics, Clinicopathological, and Treatment Characteristics
The following features were extracted from our single-institutional prospective database: age, sex, location of tumor, type of neoadjuvant chemotherapy, type of radiation therapy, lymph node status, lymphovascular invasion, perineural invasion, margin status, type of surgery, requirement of vascular resection, postoperative complications, and treatment response. pCR was defined as an absence of any viable tumor in the pancreas or lymph nodes on final pathology. A nCR was defined as a primary tumor less than 1 cm of total measured area, without nodal metastases. The remaining neoadjuvant patients were deemed to be limited responders (LR). Identified cases of pCR had all histopathology, including the initial biopsies, re-reviewed by a pathologist with expertise in pancreatic malignancies.
All patients included in this study received neoadjuvant chemotherapy and radiation as either conventional external beam radiotherapy (CRT) or stereotactic body radiation therapy (SBRT). Chemotherapy regimens were selected at the discretion of the treating medical oncologist and were divided into single agent therapy, gemcitabine-based combination regimens (predominately gemcitabine/nab-paclitaxel or gemcitabine/capecitabine) and FOLFIRINOX (5-fluorouracil, leucovorin, oxaliplatin and irinotecan). Modified-FOLFIRINOX was commonly utilized at our institution and was not separated from full dose FOLFIRINOX therapy.
Patients who proceeded to the operating suite underwent pancreaticodudoenectomy, distal pancreatectomy, or total pancreatectomy as determined by the location and extent of the tumor. An R0 resection was defined as ≥1mm of margin free of malignant cells. Vascular resections were performed if involvement of the superior mesenteric vein, portal vein, celiac axis, or hepatic artery was appreciated. Vascular reconstruction was preferentially performed by primary repair. Patch venorrhaphy or interposition grafting was used only when primary repair was not feasible. Perioperative complications within 90 days from resection were scored by the Clavien grading system.20 Clavien grade III or greater were considered severe complications.
Follow-up and Survival
Follow-up data through August 2017 was retrieved from our database. The date of death was obtained from medical records, local obituary, or the Social Security Death Index. OS was calculated from the date of neoadjuvant therapy initiation to the date of death from any cause, or censored until the date of last follow-up. DFS was calculated from the time of surgery to the date of recurrence defined radiographically or confirmed patient death. Tissue diagnosis of recurrence was not routinely performed. Post-recurrence survival (PRS) was defined as the interval from documented recurrence to time of death or last follow-up.
Statistical Analysis
Statistical analysis was performed using Stata/MP 12.1 (Stata Corp, College station, TX). Categorical variables were expressed as percentages and were compared using a x2 or a Fisher exact test. Continuous variables were presented as median and interquartile range (IQR), and were compared using a Wilcoxon-Mann-Whitney test or a Kruskal-Wallis test. Survival analysis was performed using Kaplan-Meier survival estimates and a log-rank test. A P value less than 0.05 was considered statistically significant. Multivariable analysis included all variables significant on univariable analysis and was performed using a Cox proportional-hazards model.
RESULTS
Between 2008 and 2016, 186 patients with BR/LA- PDAC who completed neoadjuvant chemotherapy and radiation followed by pancreatectomy were identified. One hundred five patients were males (57%) and median age at the time of operation was 63 (IQR 69–57). Eighty-seven cancers (47%) were defined as borderline resectable, and 99 (53%) locally advanced.19 SBRT was utilized in 74 patients (40%), and CRT in the remaining 112 (60%). FOLFIRINOX was the most commonly utilized neoadjuvant regimen in 83 patients (45%) followed by combination gemcitabine therapy in 74 patients (40%) and singleagent chemotherapy in 29 (16%). Histopathologic specimens of the evaluable cohort were reviewed by pancreatic pathologists for the treatment effect. A pCR was observed in 19 patients (10%). Of the patients without a pCR, 29 (16%) had a nCR with the remaining 138 (74%) a limited response. Median follow-up for all the patients was 27 months (range 3–102). The median follow-up in the pCR group was 34 months (11–101), with 58% of pCR patients remaining alive, with a median follow-up of 41 months (24–101) in these living patients. Additional clinicopathologic features of the pCR and remaining groups were similar and summarized in Table 1.
TABLE 1.
Clinicopathologic Characteristics of Pathologic Complete Response (pCR) and Non-pCR Patients
| Variable | pCR (n = 19) | nCR + LR (n = 167) | P Value |
|---|---|---|---|
| Age, median years (IQR) | 62 (56–68) | 63 (57–69) | 0.84 |
| Male, n (%) | 11 (58%) | 94 (56%) | 0.89 |
| Radiation modality, n (%) | 0.09 | ||
| SBRT | 11 (58%) | 63 (38%) | |
| CRT | 8 (42%) | 104 (62%) | |
| Chemotherapy regimen, n (%) | 0.41 | ||
| FOLFIRINOX | 11 (58%) | 72 (43%) | |
| Multi-agent Gemcitabine based | 5 (26%) | 69 (41%) | |
| Single-agent | 3 (16%) | 26 (16%) | |
| Disease stage, n (%) | 0.16 | ||
| Borderline | 6 (32%) | 81 (49%) | |
| Locally advanced | 13 (68%) | 86 (51%) | |
| Operation procedure, n (%) | 0.97 | ||
| Pancreaticoduodenectomy | 14 (74%) | 120 (72%) | |
| Distal pancreatectomy | 4 (21%) | 39 (23%) | |
| Total pancreatectomy | 1 (5%) | 8 (5%) | |
| Vascular resection, n (%) | 5 (26%) | 74 (44%) | 0.13 |
| Resection margin, n (%) | 0.04 | ||
| R0 | 19 (100%) | 134 (80%) | |
| R1 | 0 (0%) | 33 (20%) | |
| Morbidity, n (%) | 0.25 | ||
| ≤Clavien–Dindo grade II | 16 (84%) | 120 (72%) | |
| ≥Clavien–Dindo grade III | 3 (16%) | 47 (28%) | |
| Lymphovascular invasion, n (%) | 0 (0%) | 44 (26%) | 0.002 |
| Perineural invasion, n (%) | 0 (0%) | 91 (55%) | <0.001 |
Values that met our defined P value of significant P < 0.05 were bolded. Additionally those that met our inclusion criteria for multivariate model (univariate P of <0.10) were also bolded.
Predictors of Survival
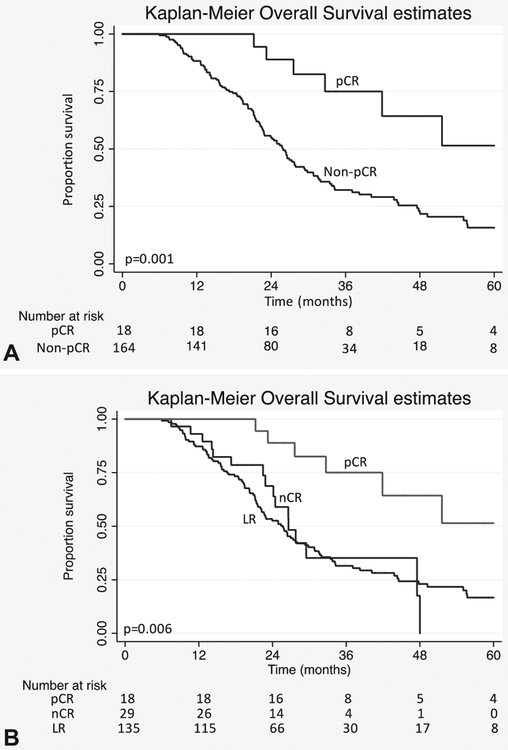
Four patients (2%), including 1 with pCR, died within 90 days after the operation due to perioperative morbidity and were excluded from the survival analysis. A median OS of 27 months was appreciated in the 182 evaluable patients. In the pCR group, median OS was not yet met at 60 months, which was longer than patients without a pCR (26 months, P = 0.001) (Fig. 1). With the additional subclassification of the nCR cohort in patients without a pCR, median OS remained superior in pCR when compared with both nCR (27 months, P = 0.003) and LR (26 months, P = 0.001). No difference was appreciated between the patients with nCR or LR (P = 0.773) (Fig. 1).
FIGURE 1.
A) Kaplan-Meier estimates of overall survival in patients with BR/LA-PDAC treated with neoadjuvant chemoradiation followed by pancreatic resection based on histological response. B) Kaplan-Meier estimates of overall survival in patients with BR/LA-PDAC treated with neoadjuvant chemo-radiation followed by pancreatic resection based on histological response. pCR v nCR (P = 0.003), pCR v LR (P = 0.001), LR v nCR (P = 0.773).
Results of univariable and multivariable analyses of predictors of OS are presented in Table 2. Unadjusted factors significantly associated with improved survival were pCR, neoadjuvant SBRT, neoadjuvant FOLFIRINOX, lack of perineural invasion, negative node metastases, and an R0 resection. On multivariable analysis, a pCR (HR 0.41, 95% CI 0.17–0.97, P = 0.044), and neoadjuvant treatment with FOLFIRINOX (HR 0.47, 95% CI 0.26–0.87, P = 0.015) remained independent predictors of OS. A trend was noted associating improved survival with a negative margin (HR 0.63, 95% CI 0.38–1.04, P = 0.069) and negative nodal status (HR 0.67, 95% CI 0.43–1.03, P = 0.071).
TABLE 2.
Univariable and Multivariable Cox Regression Analysis of Overall Survival in Patients With BR/LA-PDAC Who Underwent Neoadjuvant Chemoradiation Therapy
| Clinical Characteristics | Cohort (n = 182) | Univariable P Value | HR | 95% CI | Multivariable P Value |
|---|---|---|---|---|---|
| Response, n (%) | |||||
| Limited response | 135 (74%) | Ref. | |||
| Near complete response | 29 (16%) | 0.794 | 1.27 | 0.69–2.34 | 0.445 |
| Pathologic complete response | 18 (10%) | 0.002 | 0.41 | 0.17–0.97 | 0.044 |
| Age, n (%) | |||||
| < 60 years | 72 (40%) | Ref. | |||
| ≥60 years | 110 (60%) | 0.125 | |||
| Sex, n (%) | |||||
| Male | 103 (57%) | Ref. | |||
| Female | 79 (43%) | 0.197 | |||
| Radiation modality, n (%) | |||||
| CRT | 110 (60%) | Ref. | |||
| SBRT | 72 (40%) | 0.019 | 0.88 | 0.55–1.41 | 0.605 |
| Chemotherapy regimen, n (%) | |||||
| Single-agent | 27 (15%) | Ref. | |||
| Multi-agent Gemcitabine based | 74 (40%) | 0.284 | 0.75 | 0.45–1.24 | 0.255 |
| FOLFIRINOX | 81 (45%) | 0.001 | 0.47 | 0.26–0.87 | 0.015 |
| Disease stage, n (%) | |||||
| Borderline | 85 (47%) | Ref. | |||
| Locally advanced | 97 (53%) | 0.990 | |||
| Operation procedure, n (%) | |||||
| Pancreaticoduodenectomy | 131 (72%) | Ref. | |||
| Distal pancreatectomy | 42 (23%) | 0.267 | |||
| Total pancreatectomy | 9 (5%) | 0.838 | |||
| Vascular resection, n (%) | |||||
| No | 105 (58%) | Ref. | |||
| Yes | 77 (42%) | 0.498 | |||
| Resection margin, n (%) | |||||
| R1 | 23 (13%) | Ref. | |||
| R0 | 159 (87%) | 0.041 | 0.63 | 0.38–1.04 | 0.069 |
| Morbidity, n (%) | |||||
| ≥Clavien–Dindo grade III | 46 (25%) | Ref. | |||
| ≤Clavien–Dindo grade II | 136 (75%) | 0.140 | |||
| Lymphovascular invasion, n (%) | |||||
| Yes | 43 (24%) | Ref. | |||
| No | 112 (62%) | 0.269 | |||
| Perineural invasion, n (%) | |||||
| Yes | 90 (49%) | Ref. | |||
| No | 86 (47%) | 0.005 | 0.85 | 0.55–1.29 | 0.442 |
| Positive lymph node status, n (%) | |||||
| Yes | 52 (29%) | Ref. | |||
| No | 130 (71%) | 0.002 | 0.67 | 0.43–1.03 | 0.071 |
Values that met our defined P value of significant P < 0.05 were bolded. Additionally those that met our inclusion criteria for multivariate model (univariate P of <0.10) were also bolded.
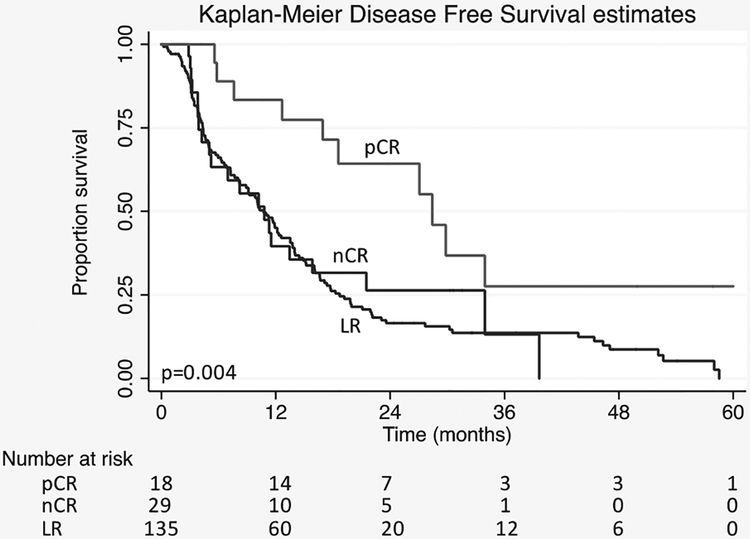
DFS was superior with Kaplan-M eier survival estimates and median DFS of 26 months in pCR cohort compared with 12 in nCR (P = 0.019) and 12 in LR groups (P < 0.001) (Fig. 2). No difference was appreciated between nCR and LR (P = 0.768). In 44% (n = 8) of pCR patients, no recurrence or death was observed. In the nCR (n = 8) and LR (n = 19) cohorts, only 27 patients (16%) did not have recurrence or death after surgery. Of the pCR patients with recurrence, 2 had loco-regional recurrence (20%) and 7 had distant recurrence including 3 patients with liver metastases, 1 with lung metastases, and 3 with carcinomatosis or multiple-site recurrence. The patterns of recurrence were similar in the non-pCR patients: with loco-regional recurrence documented in 30 (22%) patients and lung only metastases in 6 (4%). The remaining sites of distant recurrence included liver in 43 (31%) patients, carcinomatosis or mutliple-site recurrence in 33 (24%) patients, and other locations such as abdominal wall or ovary in 3 (2%) patients. Unfortunately, 1 (10%) patient with a pCR and 22 (16%) patients in the non-pCR cohort were identified as deceased without clear documentation of recurrence pattern. Despite similarities in recurrence patterns, a notable difference was appreciated in PRS, or the interval from recurrence to death. A PRS of only 6.4 months was observed in patients without a pCR compared with 11.2 months in those with a pCR (P = 0.029). Of patients with documented recurrence, 33% of those with a pCR compared with 16% of those without pCR remain alive with disease.
FIGURE 2.
Kaplan-Meier estimates of disease-free survival in patients with BR/LA-PDAC treated with neoadjuvant chemoradiation followed by pancreatic resection based on histological response. pCR v nCR (P = 0.019), pCR v LR (P < 0.001), LR v nCR (P = 0.768).
On univariable analysis, pCR, negative margins (R0), lack of lymphovascular invasion, lack of perineural invasion, and negative nodal metastases correlated with prolonged time to recurrence or death. On multivariable analyses, nodal metastases (HR 0.57, 95% CI 0.36–0.90, P = 0.018) and pCR (HR 0.45, 95% CI 0.22–0.93, P = 0.030) were independent predictors of improved DFS (Table 3).
TABLE 3.
Univariable and Multivariable Cox Regression Analysis of Disease-free Survival in Patients With BR/LA-PDAC Who Underwent Neoadjuvant Chemoradiation Therapy
| Clinical Characteristics | Cohort (n = 182) | Univariable P Value | HR | 95% CI | Multivariable P Value |
|---|---|---|---|---|---|
| Response, n (%) | |||||
| Limited response | 135 (74%) | Ref. | |||
| Near complete response | 29 (16%) | 0.713 | 1.21 | 0.71–2.06 | 0.489 |
| Pathologic complete response | 18 (10%) | 0.002 | 0.45 | 0.22–0.93 | 0.030 |
| Age, n (%) | |||||
| < 60 years | 72 (40%) | ref. | |||
| ≥60 years | 110 (60%) | 0.313 | |||
| Sex, n (%) | |||||
| Male | 103 (57%) | Ref. | |||
| Female | 79 (43%) | 0.588 | |||
| Radiation modality, n (%) | |||||
| CRT | 110 (60%) | Ref. | |||
| SBRT | 72 (40%) | 0.547 | |||
| Chemotherapy regimen, n (%) | |||||
| Single-agent | 27 (15%) | Ref. | |||
| Multi-agent Gemcitabine based | 74 (40%) | 0.308 | |||
| FOLFIRINOX | 81 (45%) | 0.185 | |||
| Disease stage, n (%) | |||||
| Borderline | 85 (47%) | Ref. | |||
| Locally advanced | 97 (53%) | 0.445 | |||
| Operation procedure, n (%) | |||||
| Pancreaticoduodenectomy | 131 (72%) | Ref. | |||
| Distal pancreatectomy | 42 (23%) | 0.622 | |||
| Total pancreatectomy | 9 (5%) | 0.536 | |||
| Vascular resection, n (%) | |||||
| No | 105 (58%) | Ref. | |||
| Yes | 77 (42%) | 0.908 | |||
| Resection margin, n (%) | |||||
| R1 | 23 (13%) | Ref. | |||
| R0 | 159 (87%) | 0.025 | 0.73 | 0.47–1.14 | 0.164 |
| Morbidity, n (%) | |||||
| ≥Clavien–Dindo grade III | 46 (25%) | Ref. | |||
| ≤Clavien–Dindo grade II | 136 (75%) | 0.133 | |||
| Lymphovascular invasion, n (%) | |||||
| Yes | 43 (24%) | Ref. | |||
| No | 112 (62%) | 0.072 | 1.11 | 0.71–1.73 | 0.647 |
| Perineural invasion, n (%) | |||||
| Yes | 90 (49%) | Ref. | |||
| No | 86 (47%) | 0.010 | 0.91 | 0.60–1.39 | 0.669 |
| Positive lymph node status, n (%) | |||||
| Yes | 52 (29%) | Ref. | |||
| No | 130 (71%) | <0.001 | 0.57 | 0.36–0.90 | 0.018 |
Values that met our defined P value of significant P < 0.05 were bolded. Additionally those that met our inclusion criteria for multivariate model (univariate P of <0.10) were also bolded.
DISCUSSION
This study presents our single institution cohort of pCR following neoadjuvant chemoradiation treatment in BR/LA-PDAC. Furthermore, we examine the association of pCR with survival. The magnitude of post-therapy response, and specifically a pCR, has been shown to be an important prognostic factor for survival in several malignancies.14–16 In pancreatic cancer, small tumor size, negative margins, and negative nodal metastases are the best established prognostic indicators for improved long-term OS following curative resection.17,18 Due to these established factors, the nCR group, as defined by <1 cm tumor with negative nodal metastases and negative margins, was used as an additional comparison group in our series. In this study, we found that BR/LA-PDAC patients with pCR had better survival, with the median not yet reached at 60 months, as compared with those with nCR or limited response. Median OS of the entire cohort was 27 months which is encouraging when compared with our institution’s historical cohort of over 2000 upfront pancreatic resections yielding a median OS of 19 months.17,21 Therefore, the prognostic impact of a pCR, which was significant for improved OS even within a preselected cohort, is notable. Moreover, in our study pCR was an independent prognostic factor for improved OS and DFS. These findings lend support to the use of neoadjuvant chemoradiation treatment in patients with BR/LA-PDAC and the use of pCR as an endpoint for future study.
Several prior studies have reported on patients with a pCR following neoadjuvant treatment for PDAC. After an extensive literature search, 21 studies and case series were identified with A) outcomes for patients receiving neoadjuvant chemoradiation for PDAC and B) at least 3 or more patients with a pCR (Table 4).9–13,22–37 The sample size of resected patients after neoadjuvant treatment ranged from 9 to 583 patients for a total of 2402 patients. Percentage of pCR ranged from 2% to 33% and pCR was observed in a total of 138 patients (6%), highlighting the rarity of a pCR in pancreatic cancer patients. Available recurrence and survival outcomes were encouraging and are shown in Table 4.
TABLE 4.
Literature Review of Prior Reports of Pathologic Complete Response in Pancreatic Cancer Following Neoadjuvant Therapy
| Reference | Institute (Country) | Inclusion Period | Resectability Status of Tumor | Neoadjuvant Regimen | % pCR | Recurrence and Survival Status of Patients With pCR |
|---|---|---|---|---|---|---|
| Snady et al (2000)25 | Mt. Sinai NY (USA) | 1989–1997 | 68 LAPC | CHT: 5-FU/Cisplatin RT: 54 Gy | 3/20 (15%) | NR |
| Calvo et al (2004)26 | Hospital G. Maranon (Spain) | 1998–2001 | 15 resectable | CHT: Tegafur RT: 45 −50.4 Gy | 3/9 (33%) | 3 pt alive with NED at 9. 25 and 36 mos. |
| Moutardier et al (2004)11 | Paoli-Calmettes (France) | 1996–2003 | 61 resectable | CHT: 5-FU/Cisplatin RT: 30–45 Gy | 3/40 (8%) | 1 pt died with liver mets at 27 mos. 2 pt alive with NED at 12 and 66 mos. |
| Turrini et al (2009)27 | Paoli-Calmettes (France) | 1996–2007 | 102 resectable | CHT: 5-FU/Cisplatin RT: 45 Gy | 8/62 (13%) | NR |
| White et al (2005)28 | Duke University (USA) | 1994–2005 | 51 BR + 16 LAPC | CHT: 5-FU RT: 30–45 Gy | 4/54* (7%) | 2 pt died of progression at 22 and 29 mos. 2 pt died with NED at 11 and 16 mos. |
| Katz et al (2008)29 | MD Anderson CC (USA) | 1999–2006 | 125 BR | CHT: multiple RT: 30–50.4 Gy | 4/66 (6%) | 1 pt developed mets. 1 pt died of lung cancer. 2 pt alive with NED |
| Chatterjee et al (2012)9 | MD Anderson CC (USA) | NR | NR | CHT: multiple RT: 30–50.4 Gy | 6/223 (3%) | 6 pt alive with NED. |
| Zhao et al (2012)10 | MD Anderson CC (USA) | 1995–2010 | NR | CHT: multiple RT: 30–50.4 Gy | 11/442 (3%) | 6 pt alive with NED at 10–194 mos (median 63). 3 pt died of other causes. 1 pt with 2nd primary PDAC at 84 mo† |
| Lee et al (2016)24 | MD Anderson CC (USA) | 2008–2012 | NR | CHT: multiple RT: 30–50.4 Gy | 3/167 (2%) | 3 pt alive with NED. |
| Cloyd et al (2017)30 | MD Anderson CC (USA) | 1990–2015 | NR | CHT: multiple RT: 30–50.4 Gy | 23/583 (4%) | 81 mos median survival for all pt. 4 pt developed distant recurrence |
| Tinkl et al (2009)31 | F. Alexander Univ. (Germany) | 1996–2006 | 120 LAPC | CHT: 5-FU or Gem/Cis RT: 50.4–55.8 Gy | 3/38 (8%) | NR |
| Chun et al (2011)12 | Fox Chase Center (USA) | 1987–2009 | NR | CHT: multiple RT: 50.4 Gy | 8/107 (7%) | NR |
| Rajagpalan et al (2013)32 | Univ. of Pittsburgh (USA) | 2008–2011 | 7 BR + 5 LAPC | CHT: multiple SBRT: 24–46 Gy | 3/12 (25%) | NR |
| Rose et al (2014)33 | VA Mason MC (USA) | 2008–2012 | 64 BR | CHT: Gem/Docetaxel RT: 50.4 Gy | 3/31 (10%) | NR |
| Pietrasz et al (2015)22 | AGEO-FRENCH cohort (France) | 2010–2013 | 47 BR + 33 LAPC | CHT: FFX RT: 54 Gy | 12/80 (15%) | NR |
| Hirata et al (2015)34 | Osaka Medical Center (Japan) | 2006–2011 | 104 resectable + 53 BR | CHT: Gem alone RT: 50 Gy | 6/157 (4%) | NR |
| Lee et al (2015)35 | Yonsei Univ. (South-Korea) | 2000–2012 | 35 resectable + 28 BR + 14 LAPC | CHT: Gem-based RT: 50–60 Gy | 9/86 (10%) | 4 pt with recurrence (2 liver. 2 peritoneum) and 5 pt alive with NED at 10–63 mos (median 21 mos) |
| Chuong et al (2016)13 | Univ. of Maryland (USA) | 2009–2012 | 36 BR | CHT: GTX RT: 32.5–40 Gy | 4/36 (11%) | 4 pt alive with NED |
| Rashid et al (2016)36 | Mofitt Cancer Center (USA) | 2006–2013 | 94 BR | CHT: GTX SBRT: 30–40 Gy | 10/55 (18%) | NR |
| Hashemi-Sadraei et al (2017)23 | Indiana University (USA) | 2008–2014 | 53 BR/LAPC | CHT: multiple RT: 45–54 Gy | 6/53 (11%) | 2 pt died (median 11 mos), 4 pt alive median survival 28.7 mos. 3 of which have recurred at median 20.4 mos |
| Mellon et al (2017)37 | Mofitt Cancer Center (USA) | 2010–2015 | 8 LAPC + 73 BR | CHT: multiple SBRT: 30–50 Gy | 6/81 (7%) | 6 pt alive with NED at median 33 mos. including 1 pt with liver met at 7 mos. treated with RFA and FFX. alive with NED at 27 mos. |
54/70 specimens available for examination.
Follow-up data available for 10/11 patients with pathologic complete response.
5-FU indicates 5-flourouracil; BR, borderline resectable; CHT, chemotherapy; FFX, FOLFIRINOX; Gem, gemcitabine; GTX, gemcitabine, docetaxel, and capecitabine; Gy, Gray; LAPC, locally advanced unresectable disease; mos; months; NR, not reported; pCR, pathologic complete response; pt, patient; RT, radiotherapy.
Statistically assessing the impact of pCR is difficult due to the small number of patients. Consequently, patients with pCR are often combined with other major response groups to capture the prognostic value of pCR on survival in multivariable models. A study from Fox Chase Center by Chun et al12 observed a major pathologic response (>95% fibrosis) in 21 patients of 107 patients (19%), including 8 patients (7%) with pCR. Median OS for major responders was 66 months and on subsequent multivariable analysis only major response was an independent predictor of OS (HR 2.26, 95% CI 1.11–4.61).12 Pietrasz et al22 defined no evidence of tumor and ypT0–1N0 as complete and major pathologic response, respectively. In their study, 21 of 80 patients (26%) had a major response, including 12 (15%) with pCR. No venous resection and a major response (HR 0.38, 95% CI 0.16–0.93) were independently associated with DFS on multivariable analysis.22 However, a retrospective study of 6 patients (11%) with pCR reported less robust survival, with early death in 2 patients, and recurrence at a median of 20.4 months in 3 of the 4 remaining living patients.23
Several studies from MD Anderson with overlapping inclusion periods have reported on patients with pCR. In a cohort described by Chatterjee et al, 6 patients (3%) with pCR and 36 patients (16%) with minimal residual tumor (<5% viable residual tumor) were grouped together as “response group 1” while all other 181 patients were assigned to “response group 2” (5% or more viable residual tumor).9 Subsequently, they showed in 2 separate multivariable analysis models that the pathologic response in group 1 was an independent prognostic factor for OS in both models, but not DFS. Also from MD Anderson, Lee et al24 reported 3 patients (2%) with a pCR and 18 patients (11%) with <5% residual carcinoma in the surgical specimen after neoadjuvant therapy. Conversely from the results presented by Chatterjee et al, multivariable analysis showed an independent association with improved DFS (P = 0.03), but not OS (P = 0.12).9 The authors concluded that the lack of statistical significance for OS may have been due to the relatively small number of patients. Of note, these trials were not exclusively limited to BR/LA-PDAC patients.
In our study, neoadjuvant FOLFIRINOX was also an independent prognostic factor for better OS in patients with BR/LA-PDAC who underwent neoadjuvant chemoradiation and pancreatic resection. We have seen increasing use of FOLFIRINOX regimen for the treatment of PDAC on the basis of the ACCORD trial demonstrating improved survival when compared with gemcitabine alone for patients with metastatic PDAC.38 Similar success has been reported on the utilization of FOLFIRINOX to convert advanced PDAC to resectability, resulting in encouraging OS rates.39,40 The combination of gemcitabine + nab/paclitaxel has similarly offered benefit over single agent gemcitabine alone in advanced PDAC.41 Two ongoing prospective clinical trials will better assess a potential difference between these 2 agents as neoadjuvant therapy for resectable (NCT02243007) and BR-PDAC (NCT02717091). As the number of patients receiving gemcitabine+nab/paclitaxel was limited in our cohort, any gemcitabine-based combination was grouped together and compared with FOLFIRINOX or single-agent therapy.
The use of neoadjuvant SBRT was associated with improved OS on univariable analysis. SBRT is a more recent treatment modality and thus was more frequently paired with aggressive multi-agent chemo-therapeutic regimens such as FOLFIRINOX. In our study, the use of SBRT did not maintain statistical significance when analyzed in a multivariable model, perhaps indicating confounding impact by the independent factors of chemotherapy and pCR. A prospective study comparing SBRT to CRT may help further identify if survival advantages do exist based on radiation modality. Concomitantly, the Alliance A021501 trial will evaluate the role of radiation in combination with chemotherapy comparing FOLFIRINOX versus FOLFIRINOX and SBRT followed by surgical resection in patients with BR-PDAC. An advantage of SBRT is that it can be delivered in only 5 days as opposed to 5 to 6 weeks with CRT, resulting in a shorter interval between neoadjuvant radiation therapy and surgical exploration and consequently allowing less time off from systemic chemotherapy. This may decrease the potential of systemic progression and improve quality of life.42 The expanded timing of CRT, however, allows better opportunity to develop and test new radiosensitizing therapeutics that may improve treatment effects.43 At this time, both SBRT and CRT are valid modalities with different benefits and do not appear in our cohort to independently impact survival.
DFS was similarly improved in the pCR cohort and response was identified as an independent prognostic factor on multivariable analysis. While a benefit was noted, a number of patients still had recurrence following a pCR contrary to some existing data that have reported a 0% recurrence rate in pCR cohorts.9 Patterns of recurrence in pCR patients were similar to both the non-pCR patients in this cohort and previously reported patterns in upfront resected PDAC.44 However, despite recurrence, a nearly 5 month longer median PRS was observed in pCR patients compared with the remaining cohort (11.2 vs 6.4; P = 0.029). This further suggests the important prognostic impact of a pCR on survival and as a surrogate marker for tumor biology. Moreover, these patients had a significant initial response to chemotherapy, and appear to continue to have beneficial treatment effect from adjuvant treatment following recurrence prolonging PRS. Our study is limited by our method of determining recurrence by radiographic change with a paucity of pathologic confirmation with biopsy. Furthermore, all the patients in our study underwent radiation and a large number of vascular resections were performed, both of which can increase the appearance of local inflammation often misinterpreted as recurrence on imaging. Despite this, 7 patients with pCR had distant recurrence including 2 with peritoneal carcinomatosis. Sampling error or the failure to identify viable cancer cells on pathologic analysis may be a potential contributing factor toward the recurrence noted in our pCR patients. Retrospective molecular analysis of the specimen of pCR patients who recurred may help further illuminate this question.
In summary, we found that pCR in patients with BR/LAPDAC following neoadjuvant chemoradiation therapy are associated with better survival than those with nCR or limited response. Additionally, pCR, the use of FOLFIRINOX, and a negative lymph node status were identified as independent prognostic factors for improved survival. These findings suggest that pCR is an important prognostic factor and viable endpoint for future study of neoadjuvant therapy in patients with PDAC.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. [DOI] [PubMed] [Google Scholar]
- 5.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang K, Lu W, Qin W, et al. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. Pancreatology. 2016;16:28–37. [DOI] [PubMed] [Google Scholar]
- 7.Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35:515–522. [DOI] [PubMed] [Google Scholar]
- 8.Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–522. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118: 3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moutardier V, Magnin V, Turrini O, et al. Assessment of pathologic response after preoperative chemoradiotherapy and surgery in pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2004;60:437–443. [DOI] [PubMed] [Google Scholar]
- 12.Chun YS, Cooper HS, Cohen SJ, et al. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann Surg Oncol. 2011;18: 3601–3607. [DOI] [PubMed] [Google Scholar]
- 13.Chuong MD, Frakes JM, Figura N, et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol. 2016;7:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590–1598. [DOI] [PubMed] [Google Scholar]
- 15.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–1167. [DOI] [PubMed] [Google Scholar]
- 16.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. JAm Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 18.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250: 187–196. [DOI] [PubMed] [Google Scholar]
- 21.He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford). 2014;16:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrasz D, Marthey L, Wagner M, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015;22(suppl 3):S1196–S1205. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi-Sadraei N, Gbolahan OB, Salfity H, et al. Clinical characteristics of patients experiencing pathologic complete response following neoadjuvant therapy for borderline resectable/locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Lee SM, Katz MH, Liu L, et al. Validation of a proposed tumor regression grading scheme for pancreatic ductal adenocarcinoma after neoadjuvant therapy as a prognostic indicator for survival. Am J Surg Pathol. 2016;40: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89: 314–327. [DOI] [PubMed] [Google Scholar]
- 26.Calvo FA, Matute R, Garcia-Sabrido JL, et al. Neoadjuvant chemoradiation with tegafur in cancer of the pancreas: initial analysis of clinical tolerance and outcome. Am J Clin Oncol. 2004;27:343–349. [DOI] [PubMed] [Google Scholar]
- 27.Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant 5 fluorouracilcisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology. 2009;76:413–419. [DOI] [PubMed] [Google Scholar]
- 28.White RR, Xie HB, Gottfried MR, et al. Significance ofhistological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol. 2005;12:214–221. [DOI] [PubMed] [Google Scholar]
- 29.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinkl D, Grabenbauer GG, Golcher H, et al. Downstaging of pancreatic carcinoma after neoadjuvant chemoradiation. Strahlenther Onkol. 2009;185: 557–566. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan MS, Heron DE, Wegner RE, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol. 2013;8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose JB, Rocha FG, Alseidi A, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. 2014;21:1530–1537. [DOI] [PubMed] [Google Scholar]
- 34.Hirata T, Teshima T, Nishiyama K, et al. Histopathological effects of preoperative chemoradiotherapy for pancreatic cancer: an analysis for the impact of radiation and gemcitabine doses. Radiother Oncol. 2015;114: 122–127. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Kang CM, Kim H, et al. Pathological complete remission of pancreatic cancer following neoadjuvant chemoradiation therapy; not the end of battles. Medicine (Baltimore). 2015;94:e2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rashid OM, Pimiento JM, Gamenthaler AW, et al. Outcomes of a clinical pathway for borderline resectable pancreatic cancer. Ann Surg Oncol. 2016;23:1371–1379. [DOI] [PubMed] [Google Scholar]
- 37.Mellon EA, Jin WH, Frakes JM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. 2017;56:391–397. [DOI] [PubMed] [Google Scholar]
- 38.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 39.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151:e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22:2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]