Abstract
Queens and workers of eusocial Hymenoptera are considered homologous to the reproductive and brood care phases of an ancestral subsocial lifecycle. However, the molecular mechanisms underlying the evolution of reproductive division of labor remain obscure. Using a brain transcriptomics screen, we identified a single gene, insulin-like peptide 2 (ilp2), which is always upregulated in ant reproductives, likely due to their higher nutritional state. In clonal raider ants, larval signals inhibit adult reproduction by suppressing ilp2, producing a colony reproductive cycle reminiscent of ancestral subsociality. Increasing ILP2 peptide levels overrides larval suppression, thereby breaking the colony cycle and inducing a stable division of labor. This suggests a simple model for the origin of ant eusociality via nutritionally determined reproductive asymmetries potentially amplified by larval signals.
Eusocial insects exhibit a reproductive division of labor in which queens lay eggs and workers perform other tasks (1). Eusociality in ants, and in many other Hymenoptera, likely evolved from a subsocial state in which a female wasp would lay an egg and then care for the resulting larva until pupation (1–3). Such brood care may have been induced by larval signals, and observations of extant subsocial wasps are consistent with this scenario (2–4). This temporal reproductive and behavioral plasticity was then modified into a fixed reproductive asymmetry between queens and workers in eusocial colonies (2, 5). This raises three important mechanistic questions: first, how are subsocial reproductive cycles regulated? Second, how is the eusocial reproductive division of labor regulated, i.e. what allows queens to lay eggs but prevents workers from doing so? And third, what is the evolutionary trajectory that gave rise to fixed eusocial division of labor from subsocial cycles? Here we suggest that, in ants, evolutionary innovations in insulin signaling may have played a crucial role in each case.
Eusociality evolved once in a common ancestor of ants and, with the exception of a few derived social parasites, all extant ants are eusocial (6) (Fig. 1). To identify conserved potential regulators of division of labor between reproduction and brood care in ants, we conducted an unbiased screen for differentially expressed genes between whole brains or heads of reproductives and non-reproductives across seven ant species, including four previously published datasets (Fig. 1; Tables S1, S2) (7–11). We sampled a range of reproductive strategies, from species with morphologically distinct queens and workers to queenless species. Among all 5,581 identified single-copy orthologs, we found only one such gene: insulin-like peptide 2 (ilp2). ilp2 was always significantly upregulated in reproductives (Fig. 1). Thus, the differential expression of ilp2 is likely conserved across ants. Consequently, the most recent common ancestor of ants likely had ilp2 expression that was high in reproductives and low in non-reproductives.
Figure 1: Brain gene expression in seven ant species identifies one conserved differentially expressed gene.
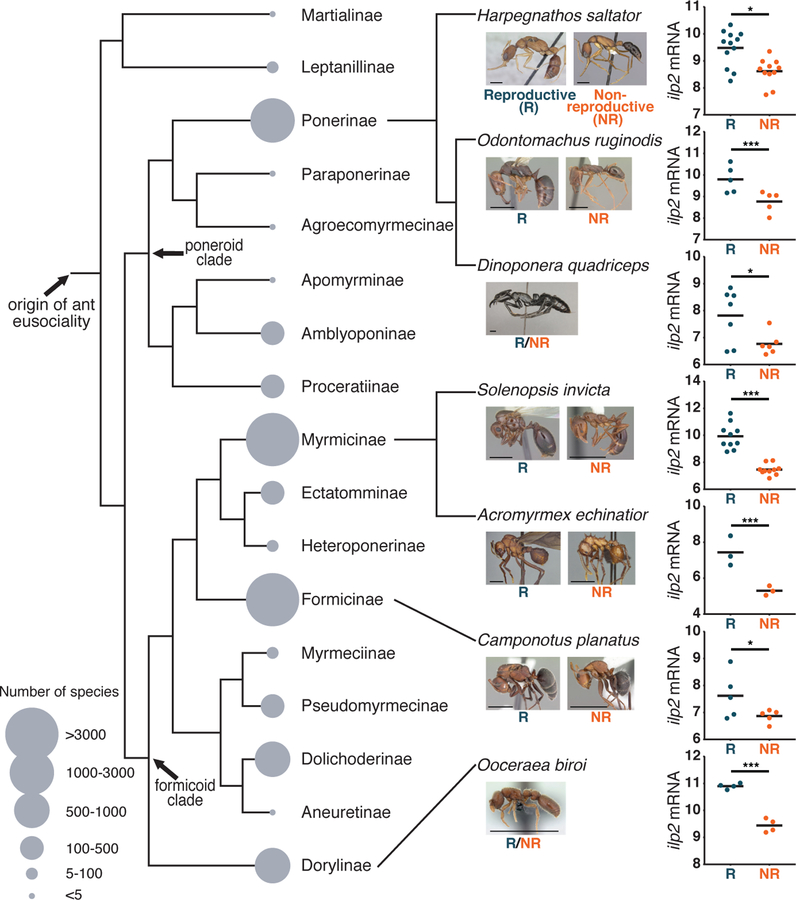
The figure shows the summary cladogram of the seven ant species used in this study in the context of the entire ant phylogeny with all subfamilies labeled. Five of the focal species have queens, while two (D. quadriceps and O. biroi) are queenless. Although H. saltator is not queenless, the data compared reproductive and non-reproductive workers (Table S1). The dot plots show variance-stabilized transformed read counts for ilp2. Blue and orange dots indicate reproductive and non-reproductive ants, respectively. Horizontal bars indicate means, and asterisks indicate statisically significant differences between groups (Wald test: * p<0.05; *** p<0.001). All images except for A. echinatior are from A. Nobile, S. Hartman, and E. Prado (www.antweb.org). Scale bars represent 2mm. The phylogeny is based on (30). Species numbers are from (6).
Although our approach is conservative and probably misses genes, it has the advantage of eliminating false positives. When we relaxed the statistical stringency for classifying genes as differentially expressed, our screen still returned ilp2 as the single candidate gene (Fig. S1). Relaxing other inclusion criteria divulged additional genes that might be expected to vary with reproductive state. For example, a total of 24 genes were consistently differentially expressed in subsets of five of the seven studied species (Fig. S2; Table S3). This list includes insulin-like peptide 1 (ilp1), as well as other genes implicated in insulin signaling (Fig. S3; Table S3). Non-single-copy orthologs were excluded from our screen. One example is vitellogenin (vg), a gene that has undergone repeated duplications in ants (12). The vitellogenin protein is a lipid carrier that provisions developing oocytes with yolk and constitutes a reliable indicator of female reproductive activity (12, 13). Studies of bees and other insects have shown that vitellogenin interacts with insulin signaling (14–16). vg indeed showed consistently higher expression in reproductives in our screen, even though this difference was not statistically significant in two of the ponerines (Fig. S3). These findings further bolster the conclusion that insulin signaling played a major role in the evolution of reproductive division of labor in ants.
Insulin regulates reproduction and food-seeking behavior across a wide range of organisms, making it a prime candidate for the regulation of subsocial cycles and eusocial division of labor (17). Most studied hymenopterans have two ILPs: ILP1 and ILP2 (Fig. S4). While ILP1 resembles insulin-like growth factor, ILP2 is similar to canonical insulin (Fig. S5) (11). In other holometabolous insects, these ILPs regulate larval growth, adult metabolism, and reproduction (17–19). Moreover, caste determination in most ant species relies on nutritional asymmetries during development: queen-destined larvae eat more than worker-destined larvae, which likely explains how queens acquire higher ILP2 levels (20). A study of Diacamma sp. found that the asymmetry in reproductive potential between ants was correlated with insulin receptor expression in the ovaries (21). This suggests a possible secondary mode of reproductive control downstream of ILPs that may augment the initial reproductive asymmetry reflected by differential ilp2 expression in the brain.
ILPs have not been studied functionally in eusocial insects in the context of reproductive division of labor between adults. However, insulin signaling has been implicated in other contexts, such as caste development and non-reproductive division of labor (18, 22–24). Current data from wasps and bees do not usually find ilp2 differentially expressed between adult queens and workers, suggesting that this expression pattern may be ant-specific (Table S5). This apparent inconsistency may be explained by the fact that eusociality has evolved independently in ants, bees, and wasps (1). While insulin signaling may have been co-opted repeatedly during social evolution, the details thus likely differ between independent lineages.
We used the clonal raider ant Ooceraea biroi to study ant ILP2. O. biroi has secondarily lost queens, resulting in a species in which workers reproduce synchronously and asexually (13, 25). Colonies alternate between reproductive and brood care phases. This colony cycle is regulated by the periodical presence of larvae, which suppress reproduction and induce brood care behavior in adults, and is reminiscent of the subsocial cycle presumed to precede eusociality in ants. Despite this unusual biology, O. biroi is eusocial. Workers display cooperative brood care, colonies contain overlapping generations of adults, and reproductive asymmetry exists within colonies (25).
We found that antibody-staining of ILP2 exclusively localized to the brain, primarily in a single medial cluster of ca. 15 cells in the pars intercerebralis (Fig. 2A–C, Fig. S6). These insulin-producing cells coincide in location with those of other insects (26, 27). Axons likely project to the corpora cardiaca, the only other brain region staining positive for ILP2 (Fig. S6–8). We quantified ILP2 in the insulin-producing cells, and found that its levels are higher in the brood care than in the reproductive phase (Fig. 2D, Fig. S6). Peptide levels are thus anti-correlated with transcription. This pattern is known from D. melanogaster, where the rate of ILP secretion correlates with the rate of ilp transcription (27). This suggests that the mechanisms of ilp expression and ILP secretion are conserved in holometabolous insects.
Figure 2: Larvae regulate ilp2 in adults.
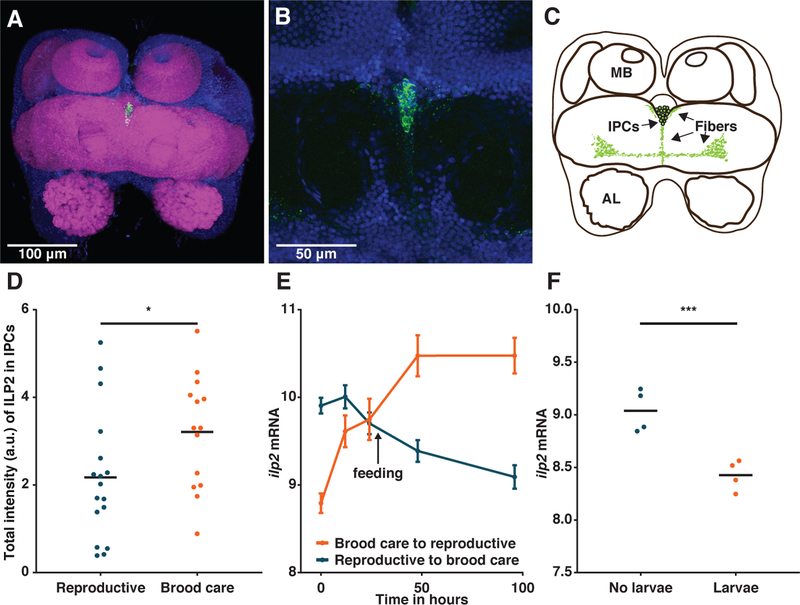
(A-C) Immunohistochemistry with anti-ILP2 antibody on an O. biroi brain localizes ILP2 peptide to a single cluster of insulin-producing cells (IPCs) in the pars intercerebralis (body-axis dorsal view). Green: anti-ILP2; blue: DAPI; magenta: phalloidin. MB: mushroom body; AL: antennal lobe. (D) Total intensity of ILP2 in the insulin-producing cells is higher in the brood care phase than in the reproductive phase (n≥14, t-test; p=0.046). (E) RNA-Seq time course shows that the addition of larvae downregulates ilp2, whereas the removal of larvae upregulates ilp2 (n≥4, time:transition interaction, Likelihood Ratio Test with 5% FDR correction; p<10−15). The black arrow indicates when ants with larvae were fed, i.e. changes in expression beyond that time point are confounded by differences in nutrition. Error bars depict SEM. Data from (28). (F) RNA-Seq on ant brains shows that under nutritionally controlled conditions, ilp2 is upregulated eight days after larvae are removed from O. biroi workers in the brood care phase (n=4, Wald test with 5% FDR correction; p<10−6). Data are variance-stabilized transformed read counts. Horizontal bars indicate means.
Because larvae regulate the O. biroi colony cycle, we asked whether larval communication altered ilp2 expression in adults. When larvae are removed from colonies in the brood care phase, ilp2 expression levels in adult brains increase dramatically within 12 hours (Fig. 2E) (28). This increase occurs under identical nutritional conditions. Conversely, when ants in the reproductive phase are given larvae, their ilp2 levels decrease (Fig. 2E). vgq, the vitellogenin gene upregulated in ant queens, responds similarly, albeit slower, to these changes (Fig. S9A), raising the possibility that ILP2 regulates reproduction at least partly by acting on vgq. Although this experiment is highly suggestive, the addition of larvae was always correlated with the removal of pupae, and changes in expression occurring after the 24h time point were confounded by nutritional differences. We therefore repeated this experiment without pupae and under nutritionally-controlled conditions. We removed larvae from colonies in the brood care phase, waited until the ants in these colonies activated their ovaries, and then compared brain gene expression between these and control colonies. Again, the removal of larvae increased ilp2 (Fig. 2F) and vgq (Fig. S9B) expression. This suggests that social signals can mediate insulin signaling independently of internal nutritional state, and that this is a key regulatory mechanism underlying the O. biroi colony cycle. Given the conserved association of caste and ilp2 expression in all ants, social regulation of ilp2 may also underlie the life cycle of the subsocial ancestor.
In D. melanogaster insulin signaling is necessary and sufficient to regulate the terminal differentiation of germline stem cells into oocytes. Moreover, it promotes yolk uptake in developing oocytes and is crucial for ovary activation (29). It is therefore plausible that the differential expression of ilp2 in ants has a causal role in regulating ovary activation and reproductive division of labor. We further hypothesized that if the regulation of ilp2 were freed, at least partially, from larval control, this would yield ants whose physiology is less susceptible to reproductive suppression. Such a mechanism would allow the evolution of distinct reproductive and non-reproductive castes from an ancestral subsocial cycle. To test this hypothesis, we injected synthetic O. biroi ILP2 mature peptide into workers in colonies with larvae. As a control, we injected the inactive B chain of this peptide (Fig. S11A) (19). Injecting ILP2 mature peptide caused strong ovary activation despite the presence of larvae (Fig. 3A–C, Fig. S10A). Higher doses of ILP2 caused ants to develop more eggs simultaneously (Fig. S10B,C), suggesting that quantitative differences in ILP2 levels vary the ants’ positions along a spectrum of reproductive potential. To ensure that ILP2 does not have inhibitory effects during the opposite phase of the colony cycle, we injected ants in the reproductive phase with ILP2, and found no detectable effect on ovary state (Fig. S11B,C).
Figure 3: ILP2 supplementation overrides larval suppression of adult reproduction.
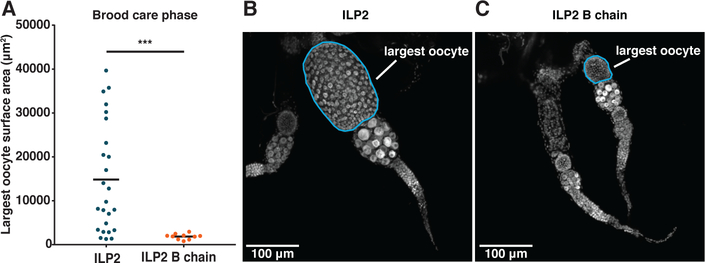
(A) Workers injected with 100 µM ILP2 in the brood care phase activate their ovaries relative to controls injected with 100 µM ILP2 B chain despite being in contact with larvae (n≥10, Welch’s t-test with Bonferroni correction (related data in Fig. S8); p=0.0005). (B and C) Confocal images of ovaries from ants injected with either 100 µM ILP2 (B) or 100 µM ILP2 B chain (C). Shown are the pairs of ovaries closest to the mean value from each treatment; the largest oocyte in each pair is circled in blue.
Finally, we hypothesized that, as developmental nutritional asymmetries determine caste in most ants, this might be a general and natural mechanism that produces asymmetries in baseline adult ILP2 levels and consequently in reproductive potential. While most O. biroi workers have two ovarioles, some (‘intercastes’) have four or more (25) (Fig. S12A,B). We found that these differences can be determined by the amount of food a larva receives (Fig. S13). Intercastes have longer and more active ovaries than regular workers in the brood care phase, suggesting that they are less sensitive to larval signals that suppress ovarian activity (Fig. 4A, Fig. S12C). This is consistent with previous work showing that some intercastes fail to regress their ovaries during the brood care phase (25). Finally, we found that the insulin-producing cells of intercastes contained more ILP2 than those of regular workers (Fig. 4B,C). As we have shown above, ILP2 peptide levels are negatively correlated with ilp2 expression, ovary state and, by extension, circulating ILP2 levels in workers between the different phases of the cycle, likely due to higher rates of peptide release during the reproductive phase (Fig. 2D). The phase-matched comparisons between different types of workers, on the other hand, show that intercastes consistently have higher ILP2 levels in their insulin-producing cells and, given their more active ovaries and decreased sensitivity to larval signals (25), it is likely that they also have consistently higher circulating ILP2 levels.
Figure 4: Intercastes respond less to larvae and have more ILP2 than regular workers.
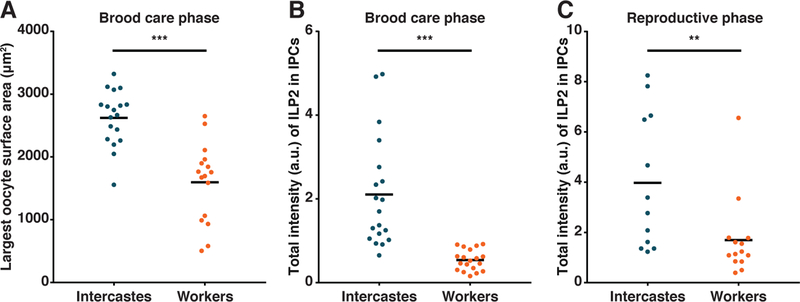
(A) Intercastes have more active ovaries than age-matched regular workers in the brood care phase, despite both being in contact with larvae (n≥16, Welch’s t-test; p<0.0001). (B) In the brood care phase (n=19, Mann-Whitney U test; p<0.0001) and (C) in the reproductive phase (n≥12, Mann-Whitney U test; p=0.0043), intercastes have more ILP2 in their insulin-producing cells than age-matched regular workers. Horizontal bars indicate means on all dot plots.
How the ancestral subsocial cycle was regulated remains unknown. However, assuming that similar mechanisms underlie the O. biroi colony cycle, our findings suggest a plausible scenario for the evolution of ant sociality. First, during the transition from solitary to subsocial, some signaling systems (probably including insulin signaling) in adults must have become responsive to larval signals. This allowed behavioral and physiological responses in adults to be appropriately modified for the nutritional requirements of the larvae. During the transition from subsocial to eusocial, increased developmental variation may have caused some adults to emerge from the pupa with low nutritional stores and low ILP2 levels. These sub-fertile individuals would have been more sensitive to larval signals that suppress reproduction and would consequently have foregone nest-founding and ovary activation and instead assumed brood care roles. Other adults, meanwhile, would have emerged with high nutritional stores and high ILP2 levels. These adults would have had reduced sensitivity to larval signals and would have been more likely to reproduce despite the presence of larvae. This reproductive asymmetry could then have been enhanced or modified by natural selection to ultimately produce the obligately reproductive queens and sterile workers of advanced eusocial species (Fig. S14). This scenario constitutes an explicit molecular version of Mary Jane West-Eberhard’s model for the evolution of hymenopteran eusociality (10).
Supplementary Material
ACKNOWLEDGEMENTS
We thank C. Zhao, H. Zebroski III, T. Tong, and the Rockefeller University Resource Centers for sequencing, peptide synthesis, and help with image analysis; M. Deyrup for assistance with ant collection; L. Olivos-Cisneros for dissecting brains; and L. Vosshall, C. Bargmann, B. Chait, H. Yan, C. Desplan, and the entire Kronauer Lab for helpful discussion. This is Clonal Raider Ant Project paper #9
Funding: This work was supported by grant 1DP2GM105454–01 from the NIH, a Searle Scholar Award, a Sinsheimer Scholar Award, a Hirschl /Weill-Caulier Trusts Award, a Klingenstein-Simons Award, a Pew Scholar Award, and an HHMI Faculty Scholar Award to D.J.C.K., a Leon Levy Neuroscience Fellowship (P.R.O.), a Rockefeller University Women & Science Fellowship (I.F-P.), and a Marie Curie international outgoing fellowship (PIOF-GA-2012–327992; R.L.)
Footnotes
Competing interests: The authors declare no competing interests
Data and materials availability: Raw sequence data are available through NCBI (BioProject PRJNA472392); scripts are available on GitHub (https://github.com/Social-Evolution-and-Behavior/insulin_signaling).
REFERENCES
- 1.Wilson EO, The Insect Societies (Belknap Press, 1971). [Google Scholar]
- 2.Wheeler WM, Ants; their structure, development and behavior (Columbia University Press, New York, 1910). [Google Scholar]
- 3.Hunt J, The evolution of social wasps (Oxford University Press, 2007). [Google Scholar]
- 4.Field J, The evolution of progressive provisioning. Behav. Ecol 16, 770–778 (2005). [Google Scholar]
- 5.West-Eberhard M, Flexible strategy and social evolution. Anim. Soc. Theor. facts, 35–51 (1987).
- 6.Ward PS, The Phylogeny and Evolution of Ants. Annu. Rev. Ecol. Evol. Syst 45, 23–43 (2014). [Google Scholar]
- 7.Libbrecht R, Oxley PR, Keller L, Kronauer DJC, Robust DNA methylation in the clonal raider ant brain. Curr. Biol 26, 391–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patalano S et al. , Molecular signatures of plastic phenotypes in two eusocial insect species with simple societies. Proc. Natl. Acad. Sci 112, 13970–13975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q et al. , Caste-specific RNA editomes in the leaf-cutting ant Acromyrmex echinatior. Nat. Commun 5, 4943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gospocic J et al. , The neuropeptide corazonin controls social behavior and caste identity in ants. Cell 170, 748–759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Materials and methods are available as supplementary materials at the Science website.
- 12.Corona M et al. , Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet 9, e1003730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxley PR et al. , The genome of the clonal raider ant Cerapachys biroi. Curr. Biol 24, 451–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badisco L, Van Wielendaele P, Vanden Broeck J, Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol 4, 202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corona M et al. , Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. U. S. A 104, 7128–33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsen K-A et al. , Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J. Exp. Biol 214, 1488–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth AL, Robinson GE, Evo-devo and the evolution of social behavior. Trends Genet 23, 334–41 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Azevedo SV, Hartfelder K, Amdam GV, Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.). J. Exp. Biol 216, 4347–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MR et al. , An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A 105, 5716–21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trible W, Kronauer DJC, Caste development and evolution in ants: it’s all about size. J. Exp. Biol 220, 53–62 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Okada Y et al. , Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp. J. Insect Physiol 56, 288–295 (2010). [DOI] [PubMed] [Google Scholar]
- 22.de Azevedo SV, Hartfelder K, The insulin signaling pathway in honey bee (Apis mellifera) caste development — differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol 54, 1064–1071 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Wheeler DE, Buck N, Evans JD, Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol 15, 597–602 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ament SA, Corona M, Pollock HS, Robinson GE, Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. U. S. A 105, 4226–31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teseo S, Kronauer DJC, Jaisson P, Châline N, Enforcement of reproductive synchrony via policing in a clonal ant. Curr. Biol 23, 328–32 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Riehle MA, Fan Y, Cao C, Brown MR, Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides 27, 2547–2560 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Géminard C, Rulifson EJ, Léopold P, Remote Control of Insulin Secretion by Fat Cells in Drosophila. Cell Metab 10, 199–207 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Libbrecht R, Oxley PR, Kronauer DJ, Time course analysis of the brain transcriptome during transitions between brood care and reproduction in the clonal raider ant. bioRxiv, 223–255 (2017).
- 29.LaFever L, Drummond-Barbosa D, Direct Control of Germline Stem Cell Division and Cyst Growth by Neural Insulin in Drosophila. Science 309, 1071–1073 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Borowiec ML et al. , Compositional heterogeneity and outgroup choice influence the internal phylogeny of the ants. bioRxiv, 173393 (2017). [DOI] [PubMed]
- 31.Bhatkar A, Whitcomb WH, Artificial Diet for Rearing Various Species of Ants. Florida Entomol 53, 229 (1970). [Google Scholar]
- 32.Bolger AM, Lohse M, Usadel B, Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabherr MG et al. , Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol 29, 644–652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM, BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Kircher M, Sawyer S, Meyer M, Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res 40, e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, Basic local alignment search tool. J. Mol. Biol 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 37.Robinson DF, Foulds LR, Comparison of phylogenetic trees. Math. Biosci 53, 131–147 (1981). [Google Scholar]
- 38.Dobin A et al. , STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anders S, Pyl PT, Huber W, HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Stoeckert CJ, Roos DS, OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Res 13, 2178–2189 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenzie SK, Fetter-Pruneda I, Ruta V, Kronauer DJC, Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. U. S. A 113, 14091–14096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto N et al. , An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J 276, 1221–1232 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Mizoguchi A, Okamoto N, Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front. Physiol 4, 217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veenstra JA, Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol 43, 49–63 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Southey BR, Sweedler JV, Rodriguez-Zas SL, Prediction of neuropeptide cleavage sites in insects. Bioinformatics 24, 815–825 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Katoh K, Kuma K, Toh H, Miyata T, MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33, 511–518 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stamatakis A, RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronquist F et al. , MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol 61, 539–42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mannerås-Holm L, Kirchner H, Björnholm M, V Chibalin A, Zierath JR, mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: effects of obesity and insulin resistance. Physiol. Rep 3, e12372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stout GJ et al. , Insulin/IGF-1-mediated longevity is marked by reduced protein metabolism. Mol. Syst. Biol 9, 679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shpigler H et al. , The transcription factor Krüppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol. Biol 10, 120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilaso M, Remnant EJ, Chapman NC, Oldroyd BP, Chanchao C, DNA methylation of Kr-h1 is involved in regulating ovary activation in worker honeybees (Apis mellifera). Insectes Soc 64, 87–94 (2017). [Google Scholar]
- 54.Kang P et al. , Drosophila Kruppel homolog 1 represses lipolysis through interaction with dFOXO. Sci. Rep 7, 16369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grozinger CM, Robinson GE, Endocrine modulation of a pheromone-responsive gene in the honey bee brain. J. Comp. Physiol. A 193, 461–470 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Hurov JB et al. , Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc. Natl. Acad. Sci. U. S. A 104, 5680–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleppe R, Martinez A, Døskeland SO, Haavik J, The 14–3-3 proteins in regulation of cellular metabolism. Semin. Cell Dev. Biol 22, 713–719 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Ramm G, Larance M, Guilhaus M, James DE, A Role for 14–3-3 in Insulin-stimulated GLUT4 Translocation through Its Interaction with the RabGAP AS160. J. Biol. Chem 281, 29174–29180 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Erion DM, Shulman GI, Diacylglycerol-mediated insulin resistance. Nat. Med 16, 400–2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monaco S et al. , Insulin stimulates fibroblast proliferation through calcium-calmodulin-dependent kinase II. Cell Cycle 8, 2024–2030 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Yao C et al. , Role of Fas-associated death domain-containing protein (FADD) phosphorylation in regulating glucose homeostasis: from proteomic discovery to physiological validation. Mol. Cell. Proteomics 12, 2689–700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He L et al. , Fas-associated protein with death domain (FADD) regulates autophagy through promoting the expression of Ras homolog enriched in brain (Rheb) in human breast adenocarcinoma cells. Oncotarget 7, 24572–84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rauschenbach IY et al. , Interplay of insulin and dopamine signaling pathways in the control of Drosophila melanogaster fitness. Dokl. Biochem. Biophys 461, 135–138 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Koupenova M, Ravid K, Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J. Cell. Physiol (2013), 10.1002/jcp.24352. [DOI] [PMC free article] [PubMed]
- 65.Johnston-Cox H, Eisenstein AS, Koupenova M, Carroll S, Ravid K, The Macrophage A2b Adenosine Receptor Regulates Tissue Insulin Sensitivity. PLoS One 9, e98775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez M, Flores C, Pearson J, Casanello P, Sobrevia L, Cell signalling-mediating insulin increase of mRNA expression for cationic amino acid transporters-1 and −2 and membrane hyperpolarization in human umbilical vein endothelial cells. Pflugers Arch. - Eur. J. Physiol 448, 383–94 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Suckow AT et al. , Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology 149, 6006–17 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ilany J et al. , Overexpression of Rad in muscle worsens diet-induced insulin resistance and glucose intolerance and lowers plasma triglyceride level. Proc. Natl. Acad. Sci. U. S. A 103, 4481–6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez-Diaz R et al. , Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med 17, 888–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira PG et al. , Transcriptome analyses of primitively eusocial wasps reveal novel insights into the evolution of sociality and the origin of alternative phenotypes. Genome Biol 14, R20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toth AL et al. , Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318, 441–4 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Standage DS et al. , Genome, transcriptome and methylome sequencing of a primitively eusocial wasp reveal a greatly reduced DNA methylation system in a social insect. Mol. Ecol 25, 1769–1784 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Jones BM, Kingwell CJ, Wcislo WT, Robinson GE, Caste-biased gene expression in a facultatively eusocial bee suggests a role for genetic accommodation in the evolution of eusociality. Proceedings. Biol. Sci 284, 20162228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jedlička P, Ernst UR, Votavová A, Hanus R, Valterová I, Gene expression dynamics in major endocrine regulatory pathways along the transition from solitary to social life in a bumblebee, Bombus terrestris. Front. Physiol 7, 574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


