Abstract
BACKGROUND:
The outcomes of sporadic pancreatic ductal adenocarcinoma (PDAC) patients with germline mutations of BRCA1/BRCA2 remains unclear. The prognostic significance of BRCA1/BRCA2 mutations on survival is not well established.
STUDY DESIGN:
We performed targeted next-generation sequencing (NGS) to identify BRCA1/BRCA2 germline mutations in resected sporadic PDAC cases from 2000 to 2015. Germline BRCA mutation carriers were matched by age and tumor location to those with BRCA1/BRCA2 wild-type genes from our institutional database. Demographics, clinicopathologic features, overall survival (OS), and disease-free survival (DFS) were abstracted from medical records and compared between the 2 cohorts.
RESULTS:
Twenty-two patients with sporadic cancer and BRCA1 (n = 4) or BRCA2 (n = 18) germline mutations and 105 wild-type patients were identified for this case-control study. The BRCA1/ BRCA2 mutations were associated with inferior median OS (20.2 vs 27.8 months, p = 0.034) and DFS (8.4 vs 16.7 months, p < 0.001) when compared with the matched wild-type controls. On multivariable analyses, a BRCA1/BRCA2 mutation (hazard ratio [HR] 2.10, p < 0.001), positive margin status (HR 1.72, p = 0.021), and lack of adjuvant therapy (HR 2.38, p < 0.001), were all independently associated with worse survival. Within the BRCA1/BRCA2 mutated group, having had platinum-based adjuvant chemotherapy (n = 10) was associated with better survival than alternative chemotherapy (n = 8) or no adjuvant therapy (n = 4) (31.0 vs 17.8 vs 9.3 months, respectively, p < 0.001).
CONCLUSIONS:
Carriers of BRCA1/BRCA2 mutation with sporadic PDAC had a worse survival after pancreatectomy than their BRCA wild-type counterparts. However, platinum-based chemotherapy regimens were associated with markedly improved survival in patients with BRCA1/BRCA2 mutations,with survival differences no longer appreciated with wild-type patients.
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease with a poor overall survival (OS), partly attributed to late diagnosis, early spread, and relatively ineffective systemic therapies showing benefit in only a subset of patients.1–4 The genetic and epigenetic heterogeneity of PDAC plays a role in its treatment resistance. Although activating mutations of genes such as KRAS and TP53 are prevalent in PDAC, a multitude of infrequently mutated genes have been identified, along with 4 specific patterns of chromosomal structure variation: stable, locally rearranged, scattered, and unstable.5,6 As a consequence, biomarkers that can accurately define subgroups of PDAC with different underlying biology are needed to match treatments to their underlying genetic pathway abberations.
Although most commonly observed as a sporadic disease, nearly 10% of PDAC cases are familial, defined as occurring in families with 2 or more affected firstdegree relatives.7 Within these familial patients, several clusters of germline mutations have been identified that lead to a propensity for malignancy including: BRCA1, BRCA2, PALB2, ATM, and DNA mismatch repair genes.7–9 These inherited familial mutations have also been identified in 3% to 5% of individuals with seemingly sporadic PDAC, perhaps as a result of incomplete penetrance.10,11
Germline BRCA1 and BRCA2 mutations are found in approximately 5% to 10% of familial PDAC and approximately 3% of apparently sporadic PDAC.12–15 The BRCA1 and BRCA2 proteins are involved in recognition and repair of double-stranded DNA via homologous recombination.16 DNA maintenance gene inactivation and subsequent repair deficiency may then impart sensitivity to DNA-strand-damaging cytotoxic agents such as platinum-based chemotherapy.17–19 The use of these platinum-based chemotherapeutics have conveyed a survival advantage in breast and ovarian cancer patients with BRCA mutations; however, only limited information has been reported regarding the therapeutic impact of BRCA status on platinums in patients with PDAC.20,21
The purpose of this study was to retrospectively investigate the association of BRCA1/BRCA2 mutations with survival in resected sporadic PDAC. Secondly, we aimed to investigate the relation of platinum-based adjuvant chemotherapy and the survival of BRCA mutated patients with PDAC.
METHODS
Patient selection
Patients with sporadic PDAC, undergoing resection at the Johns Hopkins Hospital between 2000 and 2015, were selected for targeted next generation sequencing (NGS). Exclusion criteria included known familial pancreatic cancer families (2 or more first degree relatives with PDAC), final pathology other than PDAC, and patients with surgery-related mortality within 90 days of resection. Patients with BRCA1/BRCA2 mutation were matched with additional patients with wild-type (WT) BRCA and other susceptibility genes analyzed in a 32-gene panel in 1:5 ratio. Case matching was performed by age and anatomic tumor location. Institutional Review Board approval was obtained for this study, with informed consent obtained from all patients.
Extraction and sequencing of DNA
Genomic DNA was extracted from banked frozen tissue from resected duodenum, or spleen using QIAamp DNA Micro Kit (QIAGEN) and quantified using Quantifiler (Thermo Fisher) according to the manufacturer’s instructions. Somatic tumor tissue was not sequenced for this study. Targeted sequencing was performed using Ion Torrent Proton platform next generation sequencing (Thermo Fisher). A 32-gene panel was used with known pancreatic cancer susceptibility genes (BRCA2, ATM, PALB2, BRCA1, CDKN2A, MLH1, MSH2, PRSS1, STK11, and TP53), known cancer susceptibility genes (MSH6, PMS2, CDH1, RAD51C, RAD51D, BUB1B, and FANCJ), and candidate pancreatic susceptibility genes (FANCA, FANCC, FANCG, FANCL, ARID1A, RECQL4, XRCC2, XRCC3, ERCC4, TERT, BAP1, BUB1, BUB3, and RNF43), as described in a previous study.11 Genetic data were analyzed using NextGENe Software (Soft genetics). Mutations with variants of unknown significance (VUS) were included in this study.
Demographics and clinicopathologic characteristics
Demographics and clinicopathologic features were obtained from a prospectively maintained pancreatic database including: medical history, family history of malignancy, type of operation, and neoadjuvant or adjuvant therapy. All resections were performed at the Johns Hopkins Hospital. Patients were routinely referred for chemotherapy and/or radiation therapy. Platinumbased chemotherapy included the use of cisplatin or oxaliplatin.
The following pathologic features were extracted from final pathology: tumor size, tumor infiltration extension, tumor differentiation grade, presence of lymph node metastases, microscopic perivascular and perineural invasion, and resection margin (R). The pancreatic neck, uncinate, and bile duct margins were assessed by experienced pancreatic pathologists, with R1 defined as a distance of tumor cells <1 mm from the closest resection margin and R0 when the distance was ≥1 mm.
Follow-up and survival
Patients were followed after pancreatectomy with CT scan of the chest, abdomen, and pelvis every 4 to 6 months for the first 2 years and yearly thereafter. Positron emission tomography or MRI were only used occasionally to study suspicious lesions identified on CT. Recurrence was defined as the imaging observation of distant metastases or progressing radiographic change within the surgical bed including the pancreas remnant or anastomosis sites. Biopsy confirmation of recurrence was not routinely performed. Disease-free survival (DFS) was calculated from the time of surgery to the documented date of recurrence or censored at the last date of follow-up. Overall survival was calculated from the date of diagnosis to the date of death or censored at the date of last follow-up. The date of death was obtained from medical records, local obituaries, or the Social Security Death Index.
Statistical analysis
Statistical analyses were performed using Stata/MP 12.1 (Stata Corp). Categorical variables were expressed as percentages of the group they were derived from and were compared using a chi-square or Fisher’s exact test. Continuous variables were presented as median with interquartile range (IQR), and were compared using a Kruskal-Wallis test. Kaplan-Meier survival curves and a log-rank test were used to estimate median survival and analyze survival outcomes between subgroups. Univariable analyses of demographic and clinicopathologic variables were performed using a Cox proportional hazards model. All factors with a value of p < 0.10 in univariable analysis were included as a covariable in multivariable regression analyses. A value of p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 658 patients with resected, presumably sporadic PDAC were included for analysis and sequenced via NGS. Twenty-two patients (3%) had germline BRCA1/ BRCA2 mutations (BRCA+): 4 with BRCA1 and 18 BRCA2 (eTable 1 contains further information about these mutations). An additional 213 (32%) patients had mutations (including VUS) identified in the remaining 30 cancer susceptibility genes in our NGS panel and were excluded. The remaining 423 patients (64%) were therefore of confirmed wild-type genotype and were matched by age and anatomic tumor location to the BRCA1/BRCA2 germline patients. We matched BRCA wild-type cases by age and anatomic tumor location (Table 1). Family history of cancer was similar in each group, both for the presence of any cancer (64% BRCA+ vs 61% WT, p = 0.689) or 1 relative with pancreatic cancer (9% BRCA+ vs 12% WT, p = 0.551). Neoadjuvant chemotherapy or radiation was received by 23% of the BRCA+ group compared with 16% of the WT group (p = 0.461). Most patients received adjuvant therapy (73% BRCA+ vs 79% WT, p = 0.648).
eTable 1.
Mutation Detail of BRCA-Mutated Patients
| Patient# | Age, y | Gene | Chromosome position | Amino acid change | Nucleotide change | Function |
|---|---|---|---|---|---|---|
| 1 | 66 | BRCA2 | 13:32911298–9 | p.K936Kfs | c.2808_2811delACAA | Frameshift* |
| 2 | 60 | BRCA2 | 13:32911298–9 | p.K936Kfs | c.2808_2811delACAA | Frameshift* |
| 3 | 83 | BRCA2 | 13:32911419 | p.S976Sfs | c.2928delC | Frameshift* |
| 4 | 63 | BRCA2 | 13:32914401 | p.S1970X | c.5909C>AC | Nonsense* |
| 5 | 62 | BRCA2 | 13:32914438 | p.S1982Rfs | c.5946delT | Frameshift* |
| 6 | 61 | BRCA2 | 13:32914438 | p.S1982Rfs | c.5946delT | Frameshift* |
| 7 | 57 | BRCA2 | 13:32914438 | p.S1982Rfs | c.5946delT | Frameshift* |
| 8 | 60 | BRCA2 | 13:32914438 | p.S1982Rfs | c.5946delT | Frameshift* |
| 9 | 59 | BRCA2 | 13:32932067 | Splice | c.7805+1G>A | Noncoding* |
| 10 | 65 | BRCA2 | 13:32972626 | p.K3326X | c.9976A>T | Nonsense* |
| 11 | 65 | BRCA2 | 13:32972626 | p.K3326X | c.9976A>T | Nonsense* |
| 12 | 76 | BRCA2 | 13:32893421 | p.Q92R | c.275A>G | Missense† |
| 13 | 68 | BRCA2 | 13:32912190 | p.A1233V | c.3698C>T | Missense† |
| 14 | 58 | BRCA2 | 13:32911703 | p.H1071Y | c.3211C>T | Missense† |
| 15 | 66 | BRCA2 | 13:32911794 | p.H1101R | c.3302A>G | Missense† |
| 16 | 54 | BRCA2 | 13:32912586 | p.C1365Y | c.4094G>A | Missense† |
| 17 | 56 | BRCA2 | 13:32915133 | p.T2214I | c.6641C>T | Missense† |
| 18 | 49 | BRCA2 | 13:32931943 | p.Q2561R | c.7682A>G | Missense† |
| 19 | 50 | BRCA1 | 17:41243887 | p.E1221X | c.3661C>A | Nonsense* |
| 20 | 58 | BRCA1 | 17:41276034 | fs | c.70_80delCAGATGGGACA | Frameshift* |
| 21 | 61 | BRCA1 | 17:41245975 | p.V525I | c.1573C>T | Missense† |
| 22 | 57 | BRCA1 | 17:41245975 | p.V525I | c.1573C>T | Missense† |
Known pathogenic variant.
Variant with unknown significance (VUS).
Table 1.
Demographics of BRCA-Mutated and Wild-Type Patients
| Variable | BRCA (n = 22) | Wild-type (n = 105) | p Value |
|---|---|---|---|
| Age, y, median (IQR) | 61 (57–65) | 61 (55–66) | 0.990 |
| Male sex, n (%) | 14 (64) | 48 (46) | 0.126 |
| Race, n (%) | 0.765 | ||
| Caucasian | 19 (86) | 88 (84) | |
| Non-Caucasian | 3 (14) | 17 (16) | |
| History of diabetes, n (%) | 6 (27) | 25 (24) | 0.566 |
| History of smoking, n (%) | 6 (27) | 29 (28) | 0.369 |
| Family history of cancer, n (%) | |||
| Any cancer | 14 (64) | 64 (61) | 0.689 |
| Pancreatic cancer | 2 (9) | 13 (12) | 0.551 |
| Neoadjuvant therapy, n (%) | 5 (23) | 17 (16) | 0.461 |
| Adjuvant therapy, n (%) | 16 (73) | 83 (79) | 0.648 |
Clinicopathologic characteristics
No significant difference was noted among clinicopathologic characteristics between the 2 groups (Table 2). Patients underwent either a pancreaticoduodenectomy (91% BRCA+ vs 90% WT) or a distal pancreatectomy (9% BRCA+ vs 10% WT) (p = 0.950). Total pancreatectomies were not performed in either group. Detailed pathologic features were well matched between the 2 groups including continuous tumor size, American Joint Committee on Cancer (AJCC) seventh edition T-stage (depth of tumor invasion), differentiation grade, presence of lymph node metastases, microscopic perivascular and perineural invasion, and resection margin status.
Table 2.
Clinicopathologic Features of BRCA-Mutated and Wild-Type Patients
| Variable | BRCA (n = 22) | Wild-type (n = 105) | p value |
|---|---|---|---|
| Operation procedure, n (%) | 0.950 | ||
| Pancreaticoduodenectomy | 20 (91) | 95 (90) | |
| Distal pancreatectomy | 2 (9) | 10 (10) | |
| Tumor size, cm, median (IQR) | 3 (2.5–3.7) | 3 (2.3–3.5) | 0.987 |
| Lymph nodes, median (IQR) | 20 (13–23) | 18 (14–26) | 0.674 |
| Positive nodal metastases, n (%) | 14 (64) | 76 (72) | 0.412 |
| Grade, n (%) | 0.202 | ||
| 1 | 0 (0) | 7 (7) | |
| 2 | 14 (64) | 47 (46) | |
| 3 | 8 (36) | 49 (48) | |
| T-stage, n (%) | 0.799 | ||
| T1 | 2 (9) | 8 (8) | |
| T2 | 5 (23) | 31 (30) | |
| T3 | 15 (68) | 64 (61) | |
| T4 | 0 (0) | 2 (2) | |
| Perivascular invasion, n (%) | 0.599 | ||
| Yes | 7 (32) | 55 (52) | |
| No | 8 (36) | 47 (45) | |
| Perineural invasion, n (%) | 0.937 | ||
| Yes | 19 (86) | 90 (86) | |
| No | 3 (14) | 15 (14) | |
| Resection margin, n (%) | 0.577 | ||
| R0 | 17 (77) | 75 (71) | |
| R1 | 5 (23) | 30 (29) |
IQR, interquartile range.
Survival outcomes
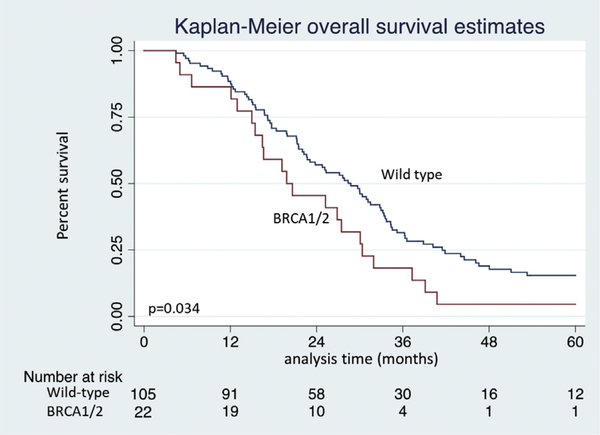
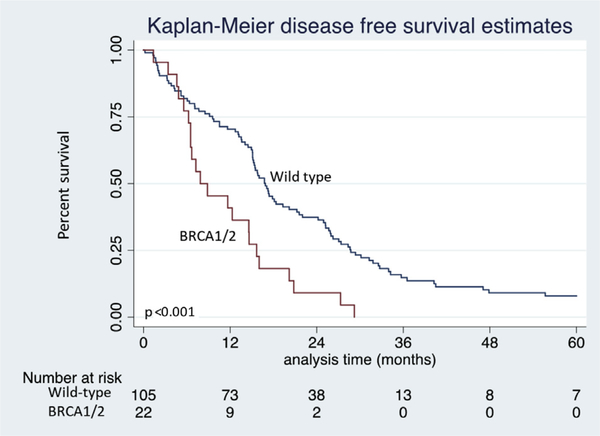
Median OS was inferior in patients with a germline BRCA1/BRCA2 mutation when compared with the matched wild-type control group (20.2 months vs 27.8 months, p = 0.034, Fig. 1). Likewise, median DFS was considerably shorter in those with BRCA1/BRCA2 mutations (8.4 months vs 16.7 months for WT, p < 0.001, Fig. 2).
Figure 1.
Kaplan-Meier overall survival estimates of BRCA1/BRCA2 germline mutation vs wild-type control patients after resection of pancreatic adenocarcinoma.
Figure 2.
Kaplan-Meier disease-free survival estimates of BRCA1/BRCA2 germline mutation vs wild-type control patients after resection of pancreatic adenocarcinoma.
A multivariable Cox regression model was generated to describe the strength of association of different mutational statuses with OS (Table 3). Within this model, a BRCA1/BRCA2 mutation was independently associated with inferior survival compared with the matched wild-type patients with resected sporadic PDAC (hazard ratio 2.10, p < 0.001). A positive microscopic margin status was a significant independent predictor of OS (HR 1.72, p = 0.021). Positive nodal status was included in the model due to an unadjusted univariable association (p = 0.081), but did not reach statistical significance on multivariable analysis (HR 1.44, p = 0.121). A different multivariable Cox regression model was used to assess the variables independently associated with inferior DFS (Table 4). Similarly, a BRCA1/BRCA2 mutation was associated with inferior DFS when compared with matched wild-type patients (HR 2.48, p < 0.001).
Table 3.
Univariable and Multivariable Cox Regression Analyses of Overall Survival in Patients Who Underwent Resection for Pancreatic Adenocarcinoma
| Clinical characteristic | Cohort(n = 127) | Univariable p Value |
|---|---|---|
| Mutation | ||
| Wild-type | 105 (83) | Reference |
| BRCA1/BRCA2 | 22 (17) | 0.036* |
| Hazard ratio | 2.10 | |
| 95% CI | 1.26–3.49 | |
| Multivariable p value | <0.001* | |
| Age, n (%) | ||
| <60 y | 64 (50) | Reference |
| ≥60 y | 63 (50) | 0.395 |
| Sex, n (%) | ||
| Male | 62 (49) | Reference |
| Female | 65 (51) | 0.315 |
| History of diabetes, n (%) | 31 (24) | 0.826 |
| History of smoking, n (%) | 35 (28) | 0.624 |
| Family history of cancer, n (%) | ||
| Any cancer | 78 (67) | 0.410 |
| Pancreatic cancer | 15 (12) | 0.432 |
| Neoadjuvant therapy, n (%) | 22 (17) | 0.313 |
| Adjuvant therapy, n (%) | 99 (80) | <0.001* |
| Hazard ratio | 0.348 | |
| 95% CI | 0.22–0.56 | |
| Multivariable p value | <0.001* | |
| Operative procedure, n (%) | ||
| Pancreaticoduodenectomy | 115 (91) | Reference |
| Distal pancreatectomy | 12 (9) | 0.776 |
| Positive nodal metastases, n (%) | 90 (71) | 0.081* |
| Hazard ratio | 1.44 | |
| 95% CI | 0.91–2.29 | |
| Multivariable p value | 0.121 | |
| Grade, n (%) | ||
| 1 | 7 (6) | Reference |
| 2 | 61 (48) | 0.821 |
| 3 | 57 (45) | 0.601 |
| T-stage, n (%) | ||
| T1 | 10 (7) | Reference |
| T2 | 36 (28) | 0.822 |
| T3 | 79 (63) | 0.370 |
| T4 | 2 (2) | 0.350 |
| Perivascular invasion, n (%) | ||
| No | 55 (43) | Reference |
| Yes | 62 (49) | 0.194 |
| Perineural invasion, n (%) | ||
| No | 18 (14) | Reference |
| Yes | 109 (86) | 0.891 |
| Resection margin, n (%) | ||
| R0 | 92 (72) | Reference |
| R1 | 35 (28) | 0.032* |
Statistically significant.
Table 4.
Univariable and Multivariable Cox Regression Analyses of Disease-Free Survival in Patients That Underwent Resection for Pancreatic Adenocarcinoma
| Clinical characteristic | Cohort(n = 127) | Univariable p Value |
|---|---|---|
| Mutation | ||
| Wild-type | 105 (83) | Reference |
| BRCA1/BRCA2 | 22 (17) | <0.001* |
| Hazard ratio | 2.48 | |
| 95% CI | 1.50–4.07 | |
| Multivariable p value | <0.001* | |
| Age, n (%) | ||
| <60 y | 64 (50) | Reference |
| ≥60 y | 63 (50) | 0.392 |
| Sex, n (%) | ||
| Male | 62 (49) | Reference |
| Female | 65 (51) | 0.492 |
| History of diabetes, n (%) | 31 (24) | 0.502 |
| History of smoking, n (%) | 35 (28) | 0.296 |
| Family history of cancer, n (%) | ||
| Any cancer | 78 (67) | 0.553 |
| Pancreatic cancer | 15 (12) | 0.949 |
| Neoadjuvant therapy, n (%) | 22 (17) | 0.847 |
| Adjuvant therapy, n (%) | 99 (80) | 0.080* |
| Hazard ratio | 0.633 | |
| 95% CI | 0.40–0.99 | |
| Multivariable p value | 0.047* | |
| Operative procedure, n (%) | ||
| Pancreaticoduodenectomy | 115 (91) | Reference |
| Distal pancreatectomy | 12 (9) | 0.684 |
| Positive nodal metastases, n (%) | 90 (71) | 0.236 |
| Grade, n (%) | ||
| 1 | 7 (6) | Reference |
| 2 | 61 (48) | 0.936 |
| 3 | 57 (45) | 0.688 |
| T-stage, n (%) | ||
| T1 | 10 (7) | Reference |
| T2 | 36 (28) | 0.984 |
| T3 | 79 (63) | 0.531 |
| T4 | 2 (2) | 0.549 |
| Perivascular invasion, n (%) | ||
| No | 55 (43) | Reference |
| Yes | 62 (49) | 0.429 |
| Perineural invasion, n (%) | ||
| No | 18 (14) | Reference |
| Yes | 109 (86) | 0.975 |
| Resection margin, n (%) | ||
| R0 | 92 (72) | Reference |
| R1 | 35 (28) | 0.132 |
Statistically significant.
Chemotherapy
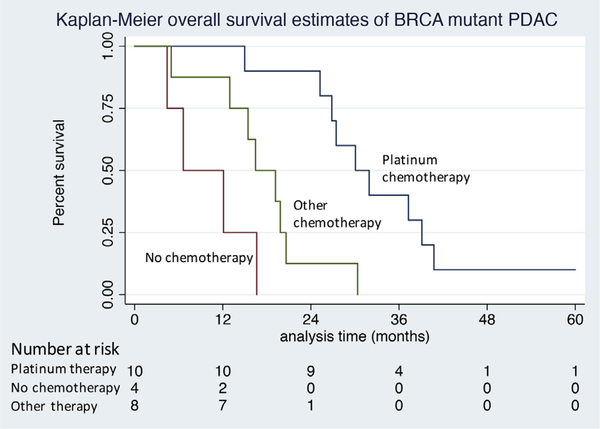
In the multivariable Cox regression model, adjuvant chemotherapy was independently associated with prolonged OS (29.9 months vs 16.6 months; HR 0.348, p < 0.001) and longer DFS (15.8 months vs 13.6 months; HR 0.633, p = 0.047). Receipt of neoadjuvant chemotherapy was not associated with DFS or OS. Within wild-type patients, there was no difference in median OS in patients who received platinum-based adjuvant compared with other chemotherapy regimens (33 vs 28 months, p = 0.897). However, within the group of patients with germline BRCA1/BRCA2 mutations, the use of platinum-based chemotherapy (n = 10) was associated with substantially longer OS than the use of alternative nonplatinum-based agents (n = 8) or failure to receive adjuvant therapy (n = 4) (31.0 vs 17.8 vs 9.3 months, p < 0.001, Fig. 3). Survival in wild-type patients who received chemotherapy was superior to that in BRCA1/2 mutant patients who underwent similar regimens (28.4 vs 17.8 months, p = 0.002); however, no survival difference was appreciated in patients receiving platinum therapy in both groups (WT, 32.7 months vs BRCA1/2, 31.0 months, p = 0.754).
Figure 3.
Kaplan-Meier overall survival estimates of BRCA1/BRCA2 germline mutated patients after resection of pancreatic adenocarcinoma stratified by type of chemotherapy received. Platinum-based chemotherapy vs other chemotherapy (p < 0.01) vs no chemotherapy (p < 0.01). Other chemotherapy vs no chemotherapy (p = 0.053).
DISCUSSION
The prognostic impact of germline BRCA1/BRCA2 mutations on sporadic PDAC survival is not well established.
This retrospective, single-institution, case-control study demonstrated, for the first time, that a germline BRCA1/BRCA2 mutation in patients with resected, sporadic PDAC was independently associated with inferior overall and disease-free survival compared with matched patients with a wild-type genotype. Additionally, BRCA mutants who received a platinum-based adjuvant chemotherapy had improved survival, similar to that of wildtype PDAC counterparts. This finding is of important clinical benefit and provides growing evidence that PDAC patients with a BRCA mutation may have inferior outcomes mitigated by “targeted” platinum-based chemotherapy.
Deleterious germline mutations are a well-established risk factor for asubset of PDAC,with many individuals carrying mutations despite not meeting familial criteria for genetic testing.10,11 Of these, mutations to the BRCA tumor suppressor genes are among the most frequently encountered. Mutations in BRCA are more commonly studied in the setting of breast or ovarian cancer.22,23 Although the association between BRCA mutations and PDAC in both the familial and seemingly sporadic case is known, the rarity of the diagnosis compounded by the infrequent nature of genetic testing has led to few studies of BRCA mutation and its impact on patient survival.24–28 In theory, BRCA mutations in PDAC may fall within the unstable genotype, representing a more mutagenic and aggressive tumor biology and subsequent worse survival.5
Multi-institutional studies by Golan and colleagues25,26 reported OS and clinical characteristics of PDAC in BRCA mutation carriers identified via polymerase chain reaction (PCR) analysis. Although a majority of patients in their cohort had unresectable disease, no significant difference was observed in median OS for patients with early stage disease when compared with OS in a matched cohort.25,26 Of note, in contrast to our study of seemingly sporadic PDAC, 32% of patients had familial PDAC. Their multi-institutional control cases did not have sequencing data to confirm the wild-type status. Furthermore, a large selection of their BRCA patients received neoadjuvant or adjuvant platinum-based treatment, perhaps contributing to the exceptional survival.25,26
Proteins in BRCA1 and BRCA2 are involved in recognition and repair of DNA damage via homologous recombination.16 Mutations and instability in these genes lead to the inability to repair double-strand DNA breaks and a subsequent sensitivity to platinum-based, DNAstrand-damaging cytotoxic agents.17–19 Use of these platinum-based chemotherapeutics has been effective in a high proportion of patients with breast and ovarian cancer with BRCA mutations, conveying a prolonged survival advantage.20,21 Given the dismal prognosis of PDAC and its notorious treatment resistance, there is great interest in identifying patient subsets that may have targetable therapeutic vulnerabilities. Promising results of platinumbased chemotherapy and the poly(ADP-ribose) polymerase inhibitors (PARPi) in BRCA mutation carriers with PDAC25,26,29–31 have led to further ongoing clinical trials (NCT02042378, NCT03140670).
Consistent with the current literature,32–35 we showed that adjuvant chemotherapy was associated with better surival. Certainly retrospective studies are limited due to selection bias. Patients may not make it to adjuvant therapy or have particular regimens selected for multiple reasons including patient performance status. In this study, both platinum-based chemotherapy and other predominately gemcitabine-based chemotherapies were associated with superior survival in wild-type patients than in those who did not recieve adjuvant therapy (p < 0.001). No difference was noted between the 2 groups of chemotherapy regimens (p = 0.897). However, the use of platinum-based chemotherapy in BRCA patients demonstrated a dramatic survival improvement, with median OS similar to that of WT patients (31.0 months vs 32.7 months, p = 0.754). This provides growing evidence that specific therapies can be targeted for a subset of patients with actionable mutations.
This study has several limitations worthy of mention. Due to its retrospective nature, it relied on self-reported family history when determining our seemingly sporadic cohort. Additionally, only the patients who proceeded to the operating suite for resection were included in analysis, excluding those with rapidly progressive or metastatic biology in both the wild-type and BRCA mutated groups. In-depth analysis of the impact of different neoadjuvant and adjuvant chemotherapy or chemoradiation regimen were beyond the scope of this study, as a large degree of chemotherapy heterogeneity existed within the total cohort. Furthermore, because our center is a tertiary surgical referral center, many patients opt for chemotherapy at local institutions, where the dosage and treatment details are difficult to obtain. The mitigation in worse OS and DFS observed in BRCA1/2 mutated patients who received platinum-based chemotherapy is certainly limited by selection bias in this retrospective study setting. Of note, 9 cases of BRCA mutations were variants with unknown significance (Table 1). The significance of VUS in BRCA remains unknown and clearly represents a clinical challenge; nonetheless, a correlation with worse outcomes was found in this study. In this study, only the germline was sequenced and biallelic inactivation of BRCA was not assessed within the tumor. Future efforts may show some of these patients with germline VUS to have tumor gene inactivation, potentially identifying additional pathogenic mutations. Finally, randomized controlled trials are necessary to prospectively assess the benefit of platinum agents in sporadic patients with BRCA1/2 mutations.
The growing ease and decreasing cost of gene sequencing in parallel with our growing knowledge of subsets of potentially targetable mutations further increases a push toward more ubiquitous sequencing of PDAC patients, even those without suspected familial disease. The outcomes presented from this study were all associations with solely germline mutations, so a sample of saliva or a simple cheek swab is all that is necessary to obtain information that could potentially assist with treatment direction and a survival impact. Hopefully, with further prospective study and technologic advancement, the future of PDAC treatment will follow this path, where germline sequencing may allow guidance to targeted therapy, such as platinum agents in BRCA carriers.
CONCLUSIONS
Our study demonstrated for the first time, that a germline BRCA1 or BRCA2 mutation in patients with resected sporadic PDAC infers an inferior overall and diseasefree survival when compared to survival in wild-type matched controls. However, the use of platinum-based chemotherapy was associated with improved survival, equivalent to that of wild-type counterparts. Prospective randomized trials will help further illuminate a potential treatment advantage in these select groups of patients.
Acknowledgment:
The authors are grateful to Lindsey Manos PA-C, DHPc and all of the multidisciplinary members of the Sidney Kimmel Pancreatic Cancer team, for their hard work and care for the patients presented in this study.
Abbreviations and Acronyms
- BRCA1
breast cancer 1
- BRCA2
breast cancer 2
- DFS
disease-free survival
- HR
hazard ratio
- NGS
next-generation sequencing
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- VUS
variants of unknown significance
- WT
BRCA1/BRCA2 wild-type
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 129th Annual Meeting, Hot Springs, VA, December 2017.
Contributor Information
Alex B Blair, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Vincent P Groot, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Georgios Gemenetzis, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Jishu Wei, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
John L. Cameron, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Oncology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Matthew J. Weiss, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Oncology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Michael Goggins, Departments of Oncology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Pathology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Medicine, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Christopher L. Wolfgang, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Oncology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Pathology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Jun Yu, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
Jin He, Departments of Surgery, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD; Departments of Oncology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, MD.
REFERENCES
- 1.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 2017. March 23 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biankin AV, Maitra A. Subtyping pancreatic cancer. Cancer Cell 2015;28:411–413. [DOI] [PubMed] [Google Scholar]
- 6.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg 2010;44:293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris AL, Roberts NJ, Jones S, et al. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer 2015;14:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts NJ, Norris AL, Petersen GM, et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov 2016;6:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holter S, Borgida A, Dodd A, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015;33: 3124–3129. [DOI] [PubMed] [Google Scholar]
- 11.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017;35:3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2007;16:342–346. [DOI] [PubMed] [Google Scholar]
- 13.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 2003;95:214–221. [DOI] [PubMed] [Google Scholar]
- 14.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res 2002;62:3789–3793. [PubMed] [Google Scholar]
- 15.Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002;108:171–182. [DOI] [PubMed] [Google Scholar]
- 17.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–921. [DOI] [PubMed] [Google Scholar]
- 18.Tutt AN, Lord CJ, McCabe N, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol 2005;70:139–148. [DOI] [PubMed] [Google Scholar]
- 19.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res 2005;11:7508–7515. [DOI] [PubMed] [Google Scholar]
- 20.Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 2009;115:359–363. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol 2011;22:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Wu J, Zhang C, et al. BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis. Oncotarget 2016;7:70113–70127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Broek AJ, Schmidt MK, van’t Veer LJ, et al. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PLoS One 2015;10:e0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Zhai K, Ke J, et al. BRCA1 missense polymorphisms are associated with poor prognosis of pancreatic cancer patients in a Chinese population. Oncotarget 2017;8: 36033–36039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan T, Sella T, O’Reilly EM, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer 2017;116: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol 2009;27:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo G, Lu Y, Jin K, et al. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther 2015; 15:1223–1231. [DOI] [PubMed] [Google Scholar]
- 30.Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 2011;16: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarchoan M, Myzak MC, Johnson BA 3rd, et al. Olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Oncotarget 2017; 8:44073–44081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621–633; discussion 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer 2009;100:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]