Abstract
Biliary tract cancers (BTC) are aggressive malignancies associated with resistance to chemotherapy and poor prognostic rates. Therefore, novel treatment approaches are in need. Immunotherapy represents a promising breakthrough that uses a patient’s immune system to target a tumor. This treatment approach has shown immense progress with positive results for selected cancers such as melanoma and nonsmall cell lung cancer. Initial preclinical data and preliminary clinical studies suggest encouraging mechanistic effects for immunotherapy in BTC offering the hope for an expanding therapeutic role for this disease. These approaches include targeted tumor antigen therapy via peptide and dendritic cell-based vaccines, allogenic cell adoptive immunotherapy, and the use of inhibitory agents targeting the immune checkpoint receptor pathway and multiple components of the tumor microenvironment. At this time demonstrating efficacy in larger clinical trials remains imperative. A multitude of ongoing trials aim to successfully translate mechanistic effects into antitumor efficacy and ultimately aim to incorporate immunotherapy into the routine management of BTC. With further research efforts, the optimization of dosing and therapeutic regimens, the identification of novel tumor antigens and a better understanding of alternative checkpoint pathway receptor expression may provide additional targets for rational combinatorial therapies which enhance the effects of immunotherapy and may offer hope for further advancing treatment options. Ultimately, the challenge remains to prospectively identify the subsets of patients with BTC who may respond to immunotherapy, and devising alternative strategies to sensitize those that do not with the hopes of improving outcomes for all with this deadly disease.
Keywords: Biliary Tract Cancers, Cancer Immunotherapy, Vaccines, Checkpoint Inhibitor Therapy, Cholangiocarcinoma, Adoptive T cell Therapy
Introduction
Biliary tract cancers (BTC) are groups of tumors that include intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma, and gallbladder carcinoma. Cholangiocarcinoma comprises an uncommon cancer type that is currently on the rise with a high mortality rate.1 Every year, nearly 5000 cases of ICC, coupled with over 11,000 cases of gallbladder carcinoma and extrahepatic cholangiocarcinoma cancer are estimated to occur in the United States.2,3 Globally the prevalence is varied, with reported incidences as high as 113 per 100,000 men and 50 per 100,000 women in Asia.1,4 Complete tumor extirpation or liver transplant are the only potentially curative treatment modalities, but even with these aggressive surgical approaches recurrence rates are as high as 50%.5–7 In addition, the vast majority of BTC patients are diagnosed with advanced and inoperable disease with a median survival of 3–6 months and 5-year survival of 5%−10% without treatment.8–10 With current standard treatment, these patients with advanced disease have only modest improvement in median overall survival (OS) of less than 1 year.11 Accordingly, novel modalities of treatment for BTC that are both effective and associated with durable responses are needed.
Cancer immunotherapy is among the biggest breakthroughs in the last decade of cancer research and has emerged as a promising treatment for many otherwise chemoresistant malignant diseases including melanoma, renal cell carcinoma, nonsmall cell lung cancer, and some forms of colon adenocarcinoma.12–14 It uses a mechanism to harness the patient’s immune system and kill tumor cells. In select malignancies, immunotherapy has proven to be well tolerated in the majority of patients with robust responses.12–14 Although many risk factors of BTC are associated with chronic inflammatory conditions, the use and study of targeted immunotherapy for these malignancies have been limited. Promising preclinical data and preliminary studies suggest that opportunities for treatment success may exist. These include enhancing the immune system via peptide or dendritic cell-based vaccines, adoptive T cell therapy, and the use of checkpoint inhibitor pathway blockade. Here we review the past, present, and future of immunotherapy use in BTC.
Host immune response in cancer
The normal human immune response functions to protect the host from foreign pathogens while remaining tolerant to self. The innate immune response is the first line of defense with natural killer cells, granulocytes, and phagocytes responding in minutes to hours. Pathogenic patterns are recognized and humoral mechanisms such as complement, help with rapid defense. In contrast the adaptive immune response is antigen specific, recruiting T cells for targeted destruction of foreign pathogens. The dendritic cells are professional antigen-presenting cells (APCs) that serve as a key link between innate and adaptive arms recognizing early inflammatory signals from the innate system and processing foreign antigens for future T cell maturation. Antigen specific effector T cells can then provide a cytotoxic and subsequent memory response to targeted pathogens (Fig 1).
Fig. 1.
Pathogens or tumor cells are initially targeted nonspecifically by the pattern recognizing innate immune response. Specific antigen is taken up and presented by dendritic cells to T cells, resulting in activation and differentiation into different effector subtypes including CD8+ cytotoxic T lymphocytes and CD4+ T helper cells. B cells are activated downstream and result in antibody production.
In the setting of malignancy, the immune system is often able to identify foreign antigens resulting from genetic abnormalities and may eliminate tumors early on in their development.15 Certain cancers, however, can eventually become clinically significant by avoiding immune destruction via mechanisms such as hijacking host autoimmunity defense receptor expression, recruiting immunosuppressive cells, secreting immunosuppressive cytokines, or other selective immune-editing that limits host immune system effectiveness.15–17 With this interchange in mind, there is noted variability in the infiltration of immune cells in different malignancies. Generally, across malignancies, increased adaptive cell tumor infiltration is correlated with improved outcomes. Correspondingly, in BTC, variability of immune cell infiltration has been appreciated with CD8+ and CD4+ cells noted in 30%−50% of resected specimens. Increased infiltration frequency of CD8+, CD4+, and dendritic cells in BTC has been associated with improved OS or reduced probability of metastasis.18–20 This serves as the rationale for incorporating immunotherapy in BTC in an attempt to enhance host immune responses so as to combat this deadly disease.
Vaccine therapy for BTC
Cancer vaccines have been designed to generate a host adaptive immune response to recognize and eliminate tumor cells. The host immune system is sensitized against tumor-specific antigens either by using whole cell vaccines, antigen specific peptide vaccines, or dendritic cells preloaded with antigen. The 2 antigens of particular interest for BTC are Wilm’s tumor protein 1 (WT1) and mucin protein 1 (MUC1).21,22 Mutations in WT1 and MUC1 are detected in multiple types of malignancies and are correlated with poor prognosis and therapeutic drug resistance.23,24 Although the clinical significance of these mutations in BTC is uncertain, WT1 mutations were found in up to 80% of biliary tumors.23 Similarly, 90% of BTC have been reported to overexpress MUC1 and is thought to be associated with worse OS.25
An open-labeled, dose-escalation phase I study of WT1 peptide vaccination in combination with gemcitabine in 8 patients with BTC was well tolerated without dose-limiting toxicity throughout the study.26 Stable disease at 2 months was observed in 50% of patients with a median survival of 288 days. WT1-specific T cells were detectable in 65% of patients after vaccination, although an association with positive outcomes was not noted.26 A phase I clinical trial of MUC1 peptide vaccine in 8 patients with advanced pancreatic and BTC was similarly well tolerated although disease progression was noted in 7 of 8 patients. A MUC1 specific response, as noted by increasing antiMUC1 IgG antibodies, was observed in the single patient with stable disease.27 Preclinical data has suggested treatment with 5-fluorouracil, gemcitabine, and Interferon gamma increases the expression of MUC1 and WT1 antigen in ICC cells, thus offering further rationale for combination therapies.28 Future phase II trials and use of therapeutic combination will offer additional insight to the possible benefit of peptide vaccination.
Vaccines with multiple peptide antigen targets outside of WT1 and MUC1 have also been investigated. Quadruple peptide vaccine therapy incorporating lymphocyte antigen 6 complex locus K (LY6K), TTK protein kinase, insulin like growth factor II mRNA binding protein 3 (IGF2BP3), and DEP domain-containing 1 (DEPDC1) were administered to 9 patients with unresectable BTC in a phase 1 study.29 Peptide-specific T cell responses were observed in 78% of patients and these responses in addition to developing a grade 2 local skin reaction at the vaccination site, were associated with a longer progression-free survival (PFS) and OS.29 The same group performed a phase I trial of vaccine with 3-peptide combination including: cell division cycle associated protein 1 (CDC1), cadherin 3 (CDH3), and kinesin family member 20A (KIF20A) in patients with advanced BTC.30 This triple peptide combination vaccine was well tolerated and resulted in peptide-specific T cell responses in all patients. Stable disease was observed in 5 of 9 patients although OS remained poor at 9.7 months. Of note, injection site reaction was prognostic of improved OS suggesting a benefit of immunologic reaction.30 Phase II trials are necessary to better determine the safety and efficacy of these combination peptide therapeutic options. Yoshitomi et al31 attempted a similar multipeptide vaccine in a phase II trial of advanced BTC patients, however, each vaccine was personalized, selecting up to 4 HLA matched peptides based on pre-existing host immunity. This personalized multipeptide vaccine was well tolerated and a T cell response to vaccine peptide was observed in 8 of 17 patients (47%) who completed the first cycle of vaccination and 4 of 7 (57%) following the second cycle.31 Although these immunologic data suggest feasibility and possibly future promise for peptide vaccine therapy, the effect on survival to date have been modest and must be investigated in larger-scale studies.
An alternative to peptide vaccine therapy is the use of antigen-pulsed dendritic cells to activate and upregulate a specific T cell response. Dendritic cells are the most effective subset of APCs, thus serving an important role in activating naïve T cells and regulating an optimal primary immune response. Dendritic cells can be pulsed with tumor associated antigens in an attempt to use their innate influence and generate an anticancer T-cell mediated immune response. WT1 and MUC1 pulsed dendritic cell vaccination was given to 65 patients with unresectable or recurrent BTC with and without combination chemotherapy.32 Of note, those patients who experienced fever after vaccination had improved OS on multivariate Cox proportional hazard analysis inferring that an immunologic response may be beneficial.32 Although well tolerated, objective clinical outcomes as a whole remained modest across the study with partial response in only 6% of patients and median survival of 7.2 months from the time of vaccination. A phase I/II study similarly used MUC1 loaded dendritic cell vaccine therapy, as adjuvant therapy in 12 resected BTC (n= 2) and pancreatic cancer patients.33 The vaccinations were well tolerated with a noted increase in activity of CD8+ and CD4+ T cells. Despite these T cell responses, vaccination did not induce anti-MUC1 antibody responses. Median OS was encouraging at 26 months with 33% of patients alive without evidence of recurrence over 4 years following resection.33 The specific effect of vaccination on these long-term survivors is uncertain as many patients had received neoadjuvant and adjuvant therapy and also owing to the small number of patients with BTC. Comparing vaccination in combination with chemotherapy vs chemotherapy alone would be beneficial in furthering our understanding of this approach.
Future searches for additional targetable, specific tumor associated antigens should continue with a hope to improve the efficacy of the vaccine approach. Annexin A2 is an antigen known to be expressed in multiple types of cancer including pancreatic cancer, breast cancer, colon cancer, and prostate cancer.34 Parasite associated BTCs have been reported to also upregulate Annexin A2.35 This protein has been identified as a metastasis-associated protein in pancreatic adenocarcinoma as its phosphorylation and surface translocation have demonstrated increased tumor invasion and metastasis formation. Subsequent inhibition of Annexin A2 pathways lead to decrease in tumor growth and metastasis formation in preclinical studies.36 This serves as one of many examples of possible future antigens that may serve as viable targets for improved BTC vaccine therapy and offers encouragement for ongoing preclinical studies.
Adoptive T cell therapy for BTC
An alternative approach to increasing host tumor specific immune response and intratumoral T cell infiltration is infusion of a patient’s own harvested tumor infiltrating lymphocytes (TIL).37 Adoptive T cell transfer increases the number of activated cytotoxic T lymphocytes in the tumor microenvironment than induced by a vaccine alone. In metastatic melanoma CD8+ enriched TIL infusion following a lymphodepleting preparative regimen led to an objective response in 58% of patients including 3 complete responders.38 Direct transfer of cellular immunity via adoptive immunotherapy has also been investigated for use in BTC in combination with dendritic cell vaccination.
Higuchi et al39 reported a case of >3 year long-term survival in a patient with lymph node positive ICC treated with adjuvant T cell-based adoptive therapy and tumor lysate pulsed dendritic cells. Tran et al40 reported an intriguing case of widely metastatic cholangiocarcinoma in which whole-exomic sequencing identified nonsynonymous mutations, which were individually transfected into APCs and cocultured with the patient’s harvested TIL to identify optimal reactivity. These specific reactive T cells were then expanded and transferred resulting in tumor regression up to 7 months following treatment.40 These encouraging individual cases have shown the feasibility of this treatment strategy and larger prospective trials as adjuvant therapy for postoperative ICC have thus been developed.41 A total of 36 patients received activated T cell transfer and were vaccinated with autologous tumor lysate pulsed dendritic cells. The median PFS was significantly improved at 18.3 months in patients receiving adjuvant immunotherapy compared to 7.7 months in those with adjuvant therapy alone (P= 0.005). OS was similarly encouraging, reaching 31.9 months in patients receiving adjuvant immunotherapy compared to 17.4 months in those without (P = 0.022).41 A randomized phase III trial is necessary to confirm the clinical effectiveness of adoptive T cell transfer and dendritic cell vaccine combination as adjuvant therapy for ICC.
Ongoing clinical trials continue to assess the benefit of adoptive immunotherapy in BTC with a specific aim of combining it with additional therapeutic approaches (Table 1). This includes the use of checkpoint inhibitor therapy at signs of progression (NCT01174121). Possible synergistic effects of adoptive T cell therapy and checkpoint inhibitors remains to be established.
Table 1.
Current immunotherapy clinical trials for advanced biliary tract tumors.
| Clinical trial number | Treatment regimen | Phase | Patients | Trial status |
|---|---|---|---|---|
| NCT01174121 | Adoptive immunotherapy with autologous tumor infiltrating lymphocytes following lymphocyte depletion plus pembrolizumab at progression | II | Metastatic GI tumors including cholangiocarcinoma | Currently enrolling |
| NCT02982720 | Pembrolizumab and PEG-intron | II | Advanced cholangiocarcinoma | Not yet open for enrollment |
| NCT02834013 | Nivolumab and ipilimumab | II | Advanced GI tumors including extrahepatic biliary carcinoma and intrahepatic cholangiocarcinoma | Currently enrolling |
| NCT02923934 | Ipilimumab and nivolumab | II | Advanced upper GI malignancies including cholangiocarcinoma | Not yet open for enrollment |
| NCT02703714 | Pembrolizumab and GM-CSF induction | II | Advanced biliary cancers | Currently enrolling |
| NCT02628067 | Pembrolizumab | II | Advanced solid tumors including BTC | Currently enrolling |
| NCT02054806 | Pembrolizumab | I | PDL1 positive advanced BTC | Currently enrolling |
| NCT03111732 | Pembrolizumab + capecitabine/oxaliplatin | II | Advanced BTC | Not yet open for enrollment |
| NCT02821754 | Durvalumab + tremelimumab + TACE/RFA or cryoablation | I/II | Advanced hepatocellular carcinoma and BTC | Currently enrolling |
| NCT03101566 | Nivolumab + gemcitabine/cisplatin or ipilimumab | II | Unresectable biliary tract cancer | Not yet open for enrollment |
GI, gastrointestinal.
Checkpoint inhibitors for BTC
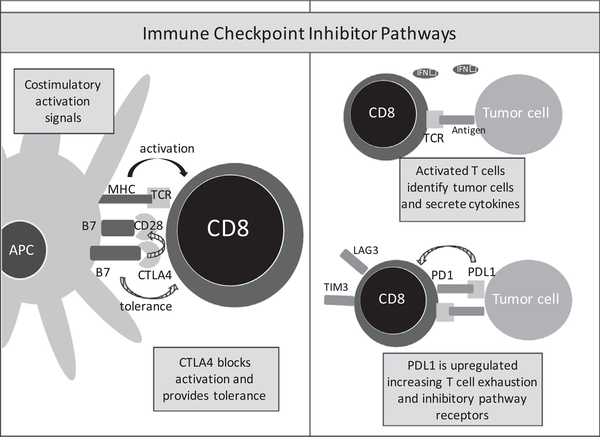
Coordinated intercellular communication via surface receptors mediates the immune response and provides homeostatic mechanisms to mitigate excessive damage by chronic inflammation as well as prevent autoimmunity.42 Immune checkpoints and other T-cell coinhibitory pathways are a major class of receptors that have gained growing attention as attractive immunotherapeutic targets. These receptors most prominently include programmed death-1 (PD1), its ligand (PDL1), and cytotoxic T-lymphocyte antigen-4 (CTLA4). These pathways operate to inhibit development, decrease function, or initiate cell death in effector cells as an evolutionary means to prevent excessive inflammation (Fig 2). However, tumor cells take advantage of these pathways serving as a prominent tumor immune evasion mechanism. Identification of these immunosuppressive pathways has led to the development of monoclonal antibodies to bind and block these inhibitory ligands or receptors potentiating underlying antitumor immune activity.
Fig. 2.
Immune checkpoint inhibitor pathways decrease T cell function, inhibit activation, and differentiation to prevent excessive inflammation. CTLA4 binds to the B7 complex of antigen-presenting cells (APC) blocking the costimulatory activation signal. PD1/PDL1 plays a role in the initiation of T cell exhaustion and upregulation of other inhibitory pathway receptors such as LAG3 or TIM3.
The PD1/PDL1 pathway plays a prominent role in the development of a tolerant tumor microenvironment (TME). PDL1 expressed on the surface of tumor cells, APCs and stroma interacts with PD1 on the surface of T cells and binding initiates T cell exhaustion.43,44 Tumors overexpress this mechanistic pathway, thus, contributing to T cell anergy and reducing their effector activity.45 The both Food and Drug Administration approved monoclonal antibodies, pembrolizumab (KEYTRUDA) and nivolumab (OPDIVO), have been developed to block the interaction between PD1 and its ligand. This blockade has shown encouraging results in multiple tumor types.12,13 Unfortunately, in advanced gastrointestinal malignancies, single agent therapy PD1 directed therapy has largely been ineffective with the noted exception of microsatellite unstable colorectal cancer.13
Preclinical data have suggested an encouraging future for targeting checkpoint pathways in biliary tract tumors. Multiple studies using immunohistochemistry have observed PD1/PDL1 expression in neoplastic cells and inflammatory cell aggregates in cases of ICC. Additionally, tumors with upregulated PD1/PDL1 have often correlated with worse clinical outcomes, suggesting a subset of these tumors may be candidates for checkpoint blocking agents.46–49 Gani et al reported PDL1 expression on cells in the tumor front in 72% of samples of resected ICC. This PDL1 expression was significantly associated with 60% reduction in OS compared to PDL1 negative counterparts.47 Sabbatino et al reported PD1 and PDL1 expression in 100% of resected ICC specimen and evidence of antitumor T-cell mediated immune responses as reflected by infiltration of lymphocytes with expression of activated HLA class II antigens. Although PDL1 or PD1 expression alone was not associated with clinical outcomes, negative or rare PDL1 expression coupled with high HLA class I expression was associated with significantly longer OS as compared with PDL1 expression, negative or low HLA class I antigen expression, or a combination of both.46 These studies provide rationale for prospective study in more detail of the prognostic impact of checkpoint expression in BTC as well as forging an initial pathway for immunotherapeutic clinical trials assessing the utility of checkpoint inhibition therapy in BTC in the future.
Currently a phase 1b trial is using PDL1 inhibitor monotherapy for patients with PDL1 positive advanced BTC. A total of 24 patients with 41% staining of the tumor stroma were enrolled in this study with interim analysis reports that checkpoint inhibitor therapy was well tolerated. There was evidence of modest antitumor activity with an overall response rate of 17.4% with 4 patients having a partial response, 4 with stable disease, and 12 with disease progression.50 Three patients did not have postbaseline tumor assessment at the time of interim analysis and 5 patients remain on treatment, including all responders.
An additional group of BTC that offers significant promise with checkpoint inhibitor therapy is the small subset with mismatch-repair deficiency. A phase 2 study evaluated the efficacy of pembrolizumab (PD1 inhibitor) monotherapy in patients with progressive metastatic carcinoma and noted impressive durable responses in patients with mismatch repair-deficient tumors. Although this study predominately studied colorectal cancer, a subset of mismatch repair-deficient noncolorectal cancer, including 4 cases of BTC, had similar encouraging outcomes with an objective response in 71% and PFS in 67% of these patients.51 Unfortunately, this mutation represents a rare subset in what is already an uncommon disease, and thus study in this isolated group of individuals is challenging.
Although PD1/PDL1 checkpoint inhibitor therapy has been the most popular pathway of study, targeted inhibition of multiple additional receptors such as T cell immunoglobulin and mucin domain-containing 3 (TIM3), indoleamine 2,3-dioxygenase (IDO), and lymphocyte activation gene 3 (LAG3) are being assessed in gastrointestinal malignancies including BTC.52–55 These evolutionary pathways are hijacked by tumor cells to inhibit development, decrease function, or initiate cell death in effector cells. Preclinical studies elaborating on the expression of these pathways in BTC and their clinical impact are ongoing but in the future, may offer direction to optimal pathways to target and provide additional rationale for combination therapies. Some success has been found in combining checkpoint inhibitors in immunogenic cancers such as melanoma,14,56,57 however, there is a paucity of data for BTC. Currently multiple phase I and II trials are assessing the impact of combination checkpoint inhibitor therapy in advanced BTC and may assist with maximizing future treatment strategies (Table 1). These include combinations such as ipilimumab (CTLA4 inhibition) and nivolumab (PD1 inhibition) (NCT02834013, NCT02923934, and NCT03101566) or durvalumab (PDL1 inhibition) and tremelimumab (CTLA4 inhibition) (NCT02821754). As the use of checkpoint inhibitors grows, trials are also assessing their combination with current first-line treatment approaches for advanced BTC including chemotherapy, transarterial catheter chemoembolization, and radiofrequency ablation (NCT03111732, NCT03101566, and NCT02821754). These preliminary studies will help further elucidate the effect of existing regimens on T cells, inhibitory cells, and checkpoint signals while also assessing potential synergistic effects with simultaneous checkpoint inhibition.
Immunotherapeutic approaches began in the metastatic setting in heavily treated patient populations. Their administration has evolved to being used in patients with potentially resectable tumors.58 In this setting, preclinical studies suggest that the scheduling of immunotherapy matters. Comparing the administration of neoadjuvant immune checkpoint therapy to adjuvant therapy alone, neoadjuvant immunotherapy appears to confer improved outcomes in preclinical models.59 Further clinical research is required to prove this concept in BTC.
Future directions
The outlook remains uncertain for the use of immunotherapy in BTC, but mounting evidence suggests encouraging mechanistic effects in this disease that may translate to its use as a valuable therapeutic tool in the future oncologist′s armamentarium. Further research focuses to demonstrate efficacy in larger studies and optimize existing regimens remains imperative while an effort to discover novel therapeutic targets or identify ideal therapeutic combinations continues.
Ultimately, it is unlikely that immunotherapy will be used generally in all patients with advanced BTC; however, a distinct subset may respond well to specific targeted therapy including adoptive cell therapy, dendritic pulse vaccine therapy, or checkpoint inhibition. Even rarer subsets such as those with mismatch repair-deficient disease have shown durable responses to checkpoint monotherapy with objective clinical benefit and improved outcomes.51 As our knowledge of genetic mutations expands, the ability to identify the groups that may best respond will improve. Nearly 40% of BTC have been found to harbor potentially targetable genetic alterations such as FGFR2, PRKACA, and ERBB2 suggesting a potential role for targeted molecular agents.60 Furthermore, the groups with worst outcomes were tumors with high mutational burden and a corresponding overexpression of immune checkpoint molecules.60 Therefore, a subset of BTC with specific genetic alterations may provide an opportunity for the combination of small molecule inhibition in combination with immunotherapy.61 Comprehensive genomic profiling may assist with precision-based therapy and offer significant promise for individualizing therapeutic options for patients with advanced BTC. The emerging challenge with immunotherapy involves acknowledging that a subset of patients substantially benefits from its use as monotherapy, and determining strategies to assist the majority remainder in overcoming resistance.
Acknowledgments
This work was supported in part by funds from the NCI T32 Grant no. 5T32CA126607–08 (A.B.) and the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (A.M.).
References
- 1.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: atrue increase? J Hepatol 2004;40(3):472–477. [DOI] [PubMed] [Google Scholar]
- 2.Facts Cancer & Figures 2016. Society AC, editor. Atlanta, GA: 2016. [Google Scholar]
- 3.Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. J Carcinog 2015;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54(1):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9(1):43–57. [DOI] [PubMed] [Google Scholar]
- 6.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153(6):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepaticcholangiocarcinoma. J Hepatobiliary Pancreat Surg 2001;8(2):154–157. [DOI] [PubMed] [Google Scholar]
- 8.Park J, Kim MH, Kim KP, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery,chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver 2009;3(4):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at asingle institution. Ann Surg. 2007;245(5):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med 1999;341(18): 1368–1378. [DOI] [PubMed] [Google Scholar]
- 11.Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinicaltrials. Chemotherapy 2014;60(1):13–23. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369(2): 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raval RR, Sharabi AB, Walker AJ, Drake CG, Sharma P. Tumor immunology and cancer immunotherapy: summary of the2013 SITC primer. J Immunother Cancer 2014;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffee EM. Immunotherapy of cancer. Ann N Y Acad Sci 1999;886:67–72. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreaticductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakakubo Y, Miyamoto M, Cho Y, et al. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer 2003;89(9):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshikiri T, Miyamoto M, Shichinohe T, et al. Prognostic value of intratumoral CD8þ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol 2003;84(4):224–228. [DOI] [PubMed] [Google Scholar]
- 20.Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109(10):2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: current status and emerging strategies.World J Gastrointest Oncol 2015;7(11):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi R, Yoshitomi M, Yutani S, et al. Current status of immunotherapy for the treatment of biliary tract cancer. Hum Vaccin Immunother 2013;9(5):1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatsuka S, Oji Y, Horiuchi T, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells.Mod Pathol 2006;19(6):804–814. [DOI] [PubMed] [Google Scholar]
- 24.Qi XW, Zhang F, Wu H, et al. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci Rep 2015;5:8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Roh SJ, Kim YN, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact.Oncol Rep 2009;22(3):649–657. [DOI] [PubMed] [Google Scholar]
- 26.Kaida M, Morita-Hoshi Y, Soeda A, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabinecombination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother 2011;34(1):92–99. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Ueno T, Kawaoka T, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tractcancer. Anticancer Res 2005;25(5):3575–3579. [PubMed] [Google Scholar]
- 28.Koido S, Kan S, Yoshida K, et al. Immunogenic modulation of cholangiocarcinoma cells by chemoimmunotherapy.Anticancer Res. 2014;34(11):6353–6361. [PubMed] [Google Scholar]
- 29.Aruga A, Takeshita N, Kotera Y, et al. Long-term vaccination with multiple peptides derived from cancer-testis antigenscan maintain a specific T-cell response and achieve disease stability in advanced biliary tract cancer. Clin Cancer Res 2013;19(8):2224–2231. [DOI] [PubMed] [Google Scholar]
- 30.Aruga A, Takeshita N, Kotera Y, et al. Phase I clinical trial of multiple-peptide vaccination for patients with advancedbiliary tract cancer. J Transl Med 2014;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshitomi M, Yutani S, Matsueda S, et al. Personalized peptide vaccination for advanced biliary tract cancer: IL-6,nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med 2012;3(3):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi M, Sakabe T, Abe H, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advancedbiliary tract cancer. J Gastrointest Surg 2013;17(9):1609–1617. [DOI] [PubMed] [Google Scholar]
- 33.Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine asadjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- 34.Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron 2011;4(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonglitthipagon P, Pairojkul C, Chamgramol Y, Mulvenna J, Sripa B. Up-regulation of annexin A2 in cholangiocarcinomacaused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol 2010;40(10):1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rucki AA, Foley K, Zhang P, et al. Heterogeneous stromal signaling within the tumor microenvironment controls themetastasis of pancreatic cancer. Cancer Res. 2017;77(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancerimmunotherapy. Nat Rev Cancer 2008;8(4):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudley ME, Gross CA, Langhan MM, et al. CD8þ enriched young tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 2010;16(24):6122–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higuchi R, Yamamoto M, Hatori T, Shimizu K, Imai K, Takasaki K. Intrahepatic cholangiocarcinoma with lymph nodemetastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: report of a case. Surg Today 2006;36(6):559–562. [DOI] [PubMed] [Google Scholar]
- 40.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4þ T cells in a patient with epithelial cancer. Science 2014;344(6184):641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cellvaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2012;19(2):171–178. [DOI] [PubMed] [Google Scholar]
- 42.Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targetedimmunotherapies to combat cancer. Nat Rev Cancer 2015;15(8):457–472. [DOI] [PubMed] [Google Scholar]
- 43.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4(5): 336–347. [DOI] [PubMed] [Google Scholar]
- 45.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironmentis driven by CD8(þ) T cells. Sci Transl Med 2013;5(200):200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbatino F, Villani V, Yearley JH, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease inintrahepatic cholangiocarcinoma. Clin Cancer Res 2016;22(2):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gani F, Nagarajan N, Kim Y, et al. Program death 1 immune checkpoint and tumor microenvironment: implications forpatients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2016;23(8):2610–2617. [DOI] [PubMed] [Google Scholar]
- 48.Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 2017;8(15):24644–24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 ontumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol 2009;100(6):500–504. [DOI] [PubMed] [Google Scholar]
- 50.Bang YJ, Doi T, De Braud F, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer 2015;51(3):s112. [Google Scholar]
- 51.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372(26): 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong D, Zhou Y, Chen W, et al. T cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphismsand susceptibility to pancreatic cancer. Mol Biol Rep 2012;39(11):9941–9946. [DOI] [PubMed] [Google Scholar]
- 53.Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreaticductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008;206(5): 849–854 [discussion 54–6]. [DOI] [PubMed] [Google Scholar]
- 54.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4–1BB, and CD40 as targets for cancerimmunotherapy. Curr Opin Immunol 2013;25(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheuk AT, Mufti GJ, Guinn BA. Role of 4–1BB:4–1BB ligand in cancer immunotherapy. Cancer Gene Ther 2004;11(3): 215–226. [DOI] [PubMed] [Google Scholar]
- 56.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreatedmelanoma. N Engl J Med 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forde PM, Smith K, Chaft JE, et al. Neoadjuvant anti-PD1, nivolumab, in early stage resectable non-small-cell lung cancer.ASCO Meeting Abstracts 2016;34(suppl 15):e20005. [Google Scholar]
- 59.Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6(12):1382–1399. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47(9):1003–1010. [DOI] [PubMed] [Google Scholar]
- 61.Lee H, Ross J. The potential role of comprehensive genomic profiling to guide targeted therapy for patients with biliary cancer. Ther Adv Gastroenterol 2017;10(6):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]