Abstract
Prenatal ethanol exposure (PE) leads to multiple cognitive and behavioral deficits including increased drug addiction risk. Previous studies have shown that the rearing environment plays a significant role in addiction propensity. In the present study, we investigated if environmental enrichment during development could be effective in lowering the PE-induced increase in addiction risk. To simulate heavy drinking during pregnancy in humans, pregnant Sprague-Dawley rats received ethanol (6 g/kg/day) or vehicle through intragastric gavage on gestation days 8–20. After weaning, the offspring were reared in either an enriched environment (EE) including neonatal handling and complex housing or an impoverished environment (IE) consisting of barren, single housing. Adult male offspring were then tested for locomotion, performance on the elevated plus maze, and amphetamine self-administration under a progressive ratio reinforcement schedule. Overall, EE rats, compared to IE rats, showed reduced locomotor activity in a novel environment and lower levels of anxiety, irrespective of prenatal treatments. Prenatal ethanol exposure increased amphetamine self-administration at both doses tested (0.02 and 0.05 mg/kg/infusion) and in each case EE relative to IE reversed this effect. These findings suggest that postnatal environmental complexity plays a determining role in addiction risk after PE.
Keywords: Addiction Risk, Neonatal Handling, Environmental Enrichment, Psychostimulant, Self-Administration, Impoverished Environment
1. Introduction
Prenatal ethanol exposure (PE) leads to a variety of physical, cognitive, and behavioral deficits, collectively referred to as fetal alcohol spectrum disorders (FASD). One of the deficits is increased risk of addiction to drugs of abuse (Alati et al., 2006; Barbier et al., 2009). Indeed, our previous work shows that PE leads to enhanced amphetamine conditioned place preference and self-administration in adult rats reared in the standard laboratory environment (Hausknecht et al., 2015; Hausknecht et al., 2017; Wang et al., under review). These observations are associated with PE-induced changes in the function and plasticity of excitatory synapses onto dopaminergic (DA) neurons located in the ventral tegmental area, the origin of the mesolimbic/cortical DA system (i.e., the brain reward pathway). These changes are considered critical cellular mechanisms of addiction (Choong, K. and Shen, R.Y., 2004; Hausknecht et al., 2015; Hausknecht et al., 2017; Xu and Shen, 2001).
So far, there are limited treatment options for PE-induced deficits (Murawski et al., 2015). Environmental intervention has been used to reduce impairments caused by PE (Gursky and Klintsova, 2017). Studies show that addictive behavior is influenced by rearing environments; an enriched environment (EE) can reduce addiction risk. Neonatal handling/short-term maternal separation and complex housing after weaning are two widely used approaches to decrease/protect against addictive behavior (Bardo et al., 2001; Moffett et al., 2007; Schwarz et al., 2011; Solinas et al., 2010). Furthermore, additional protective effects have been observed by combining these two approaches (Escorihuela et al., 1994; Fernández-Teruel et al., 2002; Pham et al., 1999). We have used the combined method previously in our laboratory (Wang et al., 2018). In the present study, this combined method was utilized to investigate if EE (neonatal handling followed by complex housing) could reverse PE-induced increased addiction risk.
2. Materials and Methods
2.1. Animals, Prenatal Ethanol Exposure, and Rearing Conditions
The methods used for breeding, prenatal ethanol exposure, and rearing have been reported before in detail (Choong, K. and Shen, R., 200,4; Wang et al., 2018). Briefly, during gestation days (GDs) 8–20, pregnant Sprague-Dawley rats (Envigo, Indianapolis, IN, USA) were gavaged intragastrically twice (6 h apart) every weekday, each with 3 g/kg ethanol (15% w/v) or vehicle (22.5% w/v sucrose water, isocaloric to ethanol). Single daily treatment with 4 g/kg solution was given on weekends. The PE treatment mimicked heavy prenatal ethanol exposure in humans (Eckardt et al., 1998; Shen et al., 1999). To equate caloric intake, controls were pair-fed with PE rats on GDs 8–20. Additionally, thiamine (8 mg/kg, i.m.; twice/week) was administered in both PE and control rats to avoid vitamin B1 deficiency caused by ethanol administration or pair-feeding. Our PE treatment causes no major stress (Hausknecht et al., 2015).
Cross-fostering was conducted along with culling on Postnatal day (PD) 1. The PE litters were fostered by extra dams that received no treatment and gave birth 2-days earlier; the control litters were cross-fostered among themselves (switching pups between 2-litters). Each litter was culled to 10 pups with ≤ 8 males. Pups from each litter were randomly assigned to either an enriched (EE) or an impoverished postnatal rearing environment (IE). We did not assign pups from the same litter into different rearing groups because the neonatal handling procedure involved the whole litter. Only males were used in the tests to limit the scope of the study.
The EE and IE rats were reared differently during early postnatal development and after weaning. Specifically, the EE rats received neonatal handling and were reared in complex housing post-weaning. Neonatal handling consisted of a short (15 min) maternal separation and handling procedure daily between PDs 2 and 20. This procedure has been reported to enhance maternal care (Pryce et al., 2001). Rats were weaned on PD 21 and were group housed (10 – 15/cage) in large four-story wire cages (64 × 92 × 160cm, Model: CG-71111, Drs. Forrest and Smith, Rhinelander, WI, USA) with 30 pet toys (pots, hideouts, ropes, wheels, etc.; Drs. Forrest and Smith). The toys were relocated or changed daily to create novelty. Control and PE rats were housed in different cages; littermates were always housed in the same cages.
The IE pups were left with their dams undisturbed by experimenters except for weekly cage change before weaning. From PD 21 onward, they were singly housed in small, hanging wire cages (17 × 24 × 20 cm) facing a wall and kept undisturbed. More details regarding the IE and EE conditions were described in Wang et al., 2018. Behavioral tests started when the rats were 8weeks old, with ≤ 3 rats/litter used in each test.
2.2. Locomotor Test and Elevated Plus Maze (EPM) Test
Locomotor activity was assessed in a novel environment as previously described (Gancarz et al., 2011). Eighty-nine rats (control, IE: n = 26 from 11 litters; PE, IE: n = 21/11 litters; control, EE: n = 21/9litters; PE, EE: n = 21/10litters) participated in this test (60 min). To assess rats’ anxiety levels, an EPM test was conducted in a subset of the rats (70 in total; control, IE: n = 15/10 litters; PE, IE: n = 14/10 litters; control, EE: n = 20/9litters; PE, EE: n = 21/10litters). They underwent the 5-min EPM test after being habituated to the testing room for 15 min.
2.3. Amphetamine Self-Administration
Amphetamine self-administration experiments were conducted after one week of operant pre-training and another week of recovery from the jugular vein catheterization surgery (Hausknecht et al., 2017). All rats were singly housed in standard plastic cages after surgery, to protect the catheters. As such, the post-weaning EE or IE rearing lasted for 6-weeks (until they were 9-weeks old).
Rats were trained to self-administer amphetamine (d-amphetamine hemisulfate, SigmaAldrich, St. Louis, MO, USA) at 0.02 mg/kg/infusion for 3-hours daily under a fixed ratio (FR) 1 and then FR2 schedules. One priming infusion was dispensed at the beginning of each FR session, followed by response-contingent amphetamine infusions. If rats self-administered 9 infusions/session for 2 FR1 and 1 FR2 sessions within 8 days, they then underwent two rounds of amphetamine self-administration experiments, one at 0.02 mg/kg/infusion for 6 daily sessions and, following 10 days of abstinence, another at 0.05 mg/kg/infusion for 6 more sessions. Both of these experiments were conducted under a progressive ratio (PR) schedule of reinforcement, as determined by 5e(0.25×infusion number)-5 (Richardson and Roberts, 1996; Vezina et al., 2002) with no priming infusions. Similar procedures have repeatedly been used, as described in earlier reports (Hausknecht et al., 2015; Hausknecht et al., 2017; Vezina et al., 2002; Wang et al., under review). Thirty-five (control, IE: n = 8/5 litters; PE, IE: n = 10/6 litters; control, EE: n = 9/5 litters; PE, EE: n = 8/6 litters) out of 44 rats satisfied the FR criterion to continue to the PR sessions. These rats were thus included in the statistical analyses.
2.4. Data Analysis
Locomotor activity was measured by a number of photobeam breaks, and the 1-h testing session was divided into twelve 5-min epochs. In the EPM test, the dependent variable (DV) was the percentage of time rats spent on the open arms. In the self-administration test, a number of amphetamine infusions obtained and responding time were the two DVs. Responding time referred to the duration between session initiation and the time point at which the last lever press was made, indicating motivation for drug seeking (Richardson and Roberts, 1996). Data outside of 2 standard deviations of the mean in each group were identified as outliers (2.7% in total) and brought down/up to the next highest/lowest level (Aguinis et al., 2013). Analysis of variance (ANOVA) was conducted, using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), with the significance level set at α = .05. Planned comparisons were used for pairwise comparisons after ANOVA to examine control vs. PE in the same rearing condition (IE or EE) or IE vs. EE with the same prenatal treatment (control or PE).
3. Results
3.1. Prenatal Ethanol Exposure Did Not Produce Major Teratogenic Effects.
The litter size was 14.21±.54 in controls and 14.43±.43 in PE rats. The pup body weight on PD 1 was 6.54±0.05 g in controls and 6.57±0.04 g in PE rats. There was no significant group difference in litter size or pup bodyweight, suggesting that PE did not produce major teratogenic effects.
3.2. Enriched Rearing Reduced Locomotor Responding to Novelty.
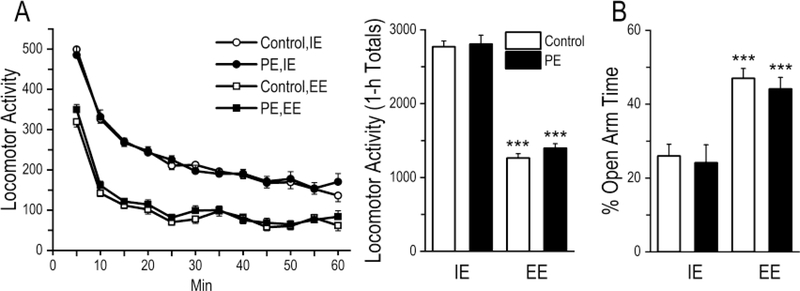
Prenatal ethanol exposure had no effects on locomotor activity. However, the EE rearing condition, relative to IE, decreased locomotion in both control and PE rats (rearing condition × 5min epoch interaction effect, F11,983 = 7.95, p < .001; litter effect: F37,983 = 5.52, p < .001, 3-way mixed ANOVA with litter as a nested variable, Fig. 1A).
Fig. 1.
Enriched environment (EE) led to behavioral phenotypes associated with lower drug addiction risk in rats with or without prenatal ethanol exposure (PE). (A) Rats reared in EE exhibited lower locomotor responding to novelty than rats reared in the impoverished environment (IE), regardless of prenatal treatments (control or PE). The left panel depicts photo beam breaks within 5-min epochs. The right panel is a summary plot for total photo beam breaks in 1 h. (B) Rats reared in EE showed lower anxiety than rats reared in IE, demonstrated by longer open arm stay/travel time (percentages within 5 min) in an elevated plus maze test, regardless of prenatal treatments (control or PE). Data are presented as Mean ± SEM. ***: p < 0.001, IE vs. EE rats in either the control or PE group, planned comparisons after ANOVA.
3.3. Enriched Rearing Produced Anxiolytic Effects.
Prenatal ethanol exposure did not affect the time staying/traveling on the open arms. The EE rearing condition, compared with IE, increased open arm time, indicating reduced anxiety levels in both control and PE rats (main effect of rearing condition: F1,31 = 41.91, p < .001; litter effect: F35,31 = 1.98, p < .05, 2-way ANOVA with litter as a nested variable, Fig. 1B).
3.4. Prenatal Ethanol Exposure Increased Amphetamine Self-Administration in Rats Reared in The IE Condition, Which Was Revered By EE.
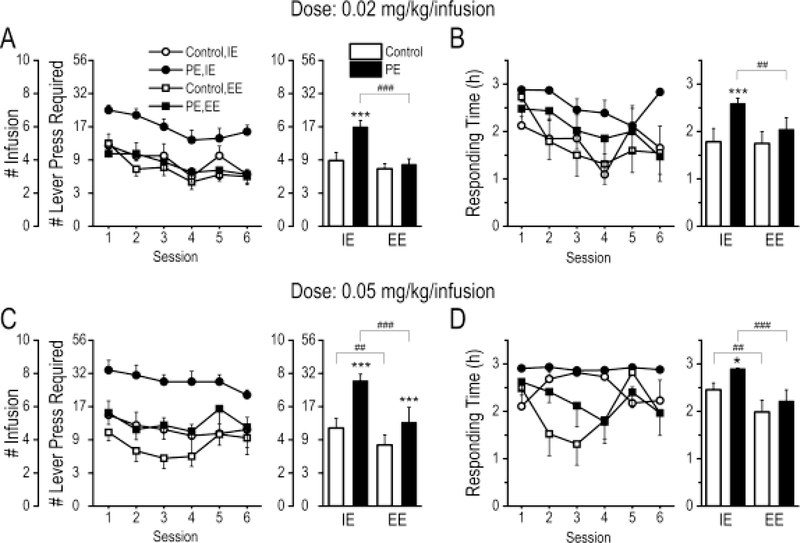
At both does of 0.02 and 0.05 mg/kg/infusion, PE rats reared in the IE condition self-administered more amphetamine (infusions) and had longer responding time than controls reared in the same condition. The effects were reversed by rearing PE rats in the EE condition. For number of infusions, a 4-way mixed ANOVA with litter as a nested variable (prenatal treatment: control vs. PE; rearing condition: IE vs. EE; dose: 0.02 vs. 0.05 mg/kg/infusion; session: 1–6) revealed an interaction effect between prenatal treatment and rearing condition (F1,13 = 18.63, p < .001), an interaction effect between dose and prenatal treatment (F1,31 = 9.23, p < .01), and a main effect of session (F5,155 = 6.88, p < .001), as well as a litter effect (F18,13 = 9.11, p < .001, Fig. 2A and C). For responding time, a 4-way mixed ANOVA with litter as a nested variable produced an interaction effect between rearing condition and session (F5,155 = 2.49, p < .05), main effects of dose (F1,31 = 16.66, p < .001) and prenatal treatment (F1,13 = 23.84, p < .001), and a litter effect (F18,13 = 4.80, p < .01, Fig. 2B and D).
Fig. 2.
Enriched environment (EE) reversed the increased drug addiction risk caused by prenatal ethanol exposure (PE), shown in amphetamine self-administration experiments under a progressive ratio (PR) schedule. At both doses of 0.02 and 0.05 mg/kg/infusion, PE rats reared in the impoverished environment (IE) obtained more amphetamine infusions (A & C) and had longer responding time (B & D) than controls reared in the same condition, but the effects were reversed by EE. Additionally, at the dose of 0.05 mg/kg/infusion, the protective effects of EE were also observed in control rats, in that EE, relative to IE, decreased amphetamine self-administration (C) and responding time (D) in control rats. Left panels depict daily sessions; right panels are summary graphs of averages across sessions. In A & C, Data are presented as Mean ± SEM number of amphetamine infusions obtained. The number of lever presses required under the PR schedule to obtain successive infusions of amphetamine is also shown (not cumulative requirement). In B & D, responding time refers to duration between session initiation and the time point at which the last lever press was made. *: p < .05, ***: p < 0.001, control vs. PE rats reared in either the IE or EE condition; ##: p < 0.01, ###: p < 0.001, IE vs. EE condition in either control or PE rats, planned comparisons after ANOVA.
The protective effects of EE were observed in PE rats at both amphetamine doses but were observed in controls only at the higher amphetamine dose of 0.05 g/kg/infusion (Fig. 2A and C). Similar group differences were observed in the responding time (Fig 2B and D).
4. Discussion
Data from the amphetamine self-administration experiments clearly show that PE rats reared in IE exert greater effort to obtain more amphetamine than controls reared in the same condition, indicating increased addiction risk in PE rats. This observation is reminiscent of results obtained in PE rats reared in the standard laboratory environment, who self-administer more amphetamine than controls reared in the same condition (Hausknecht et al., 2015; Hausknecht et al., 2017; Wang et al., under review). In fact, when we compared amphetamine self-administration at the dose of 0.02 mg/kg/infusion, there were no group differences between IE rats and rats reared in a standard laboratory environment, with or without PE (standard laboratory environment data from Wang et al., under review). Indeed, some investigators, including us, consider the standard rearing condition a simple, barren environment that typically leads to behavioral phenotypes (including addictive behaviors) similar to those observed in animals reared in an impoverished environment (Wang et al., 2018; Würbel, 2001).
Importantly, the PE-induced increase in amphetamine self-administration is substantially reversed by EE. The reversal effect by EE is also observed in control rats at the higher amphetamine dose of 0.05 mg/kg/infusion. This observation is consistent with previous studies showing that either neonatal handling or post-weaning complex housing reduces drug addiction risk. In the present study, we combine neonatal handling and post-weaning complex housing in order to maximize the EE effects. However, it remains unclear whether this method produced additive or synergistic effects. Therefore, future studies are needed to delineate the individual contributions of these two approaches in reducing the PE-induced increase in addiction risk. It is worth noting that the self-administration experiments were conducted over one month during which time all the rats were singly housed in standard plastic cages and no longer in the original rearing conditions. As such, the results suggest that rearing conditions during development persistently influence addictive behaviors. The finding also suggests that EE during development produces long-lasting protective effects and resiliency.
Currently, the specific mechanisms underlying the protective effects of EE are unclear. Neonatal handling and environmental enrichment induce a wide range of anatomical (e.g., enhanced neuron size, dendritic branching, and dendritic spine density; increased synaptogenesis and gliogenesis) and neurochemical (e.g., increased expression of growth factors) alterations. In addition, they have been shown, among other things, to ameliorate neuroinflammation, promote the development of neural circuitries, and modify synaptic function and plasticity (Fernández-Teruel et al., 2002; Ma et al., 2016; Schwarz et al., 2011; Solinas et al., 2010). It is possible that multiple changes take place collectively to mediate the effects of EE in reducing addiction risk after PE.
It has been shown that increased locomotor responding to novelty and anxiety are associated with augmented addiction risk (Gancarz et al., 2011; Spanagel et al., 1995). We do not observe a tight association between these behavioral phenotypes with a PE-induced increase in addiction risk. However, these behaviors are strongly modified by postnatal environments in that EE dramatically decreases locomotion and anxiety. These results emphasize that addiction risk after PE could be dissociated from emotionality measures and therefore needs to be directly studied. In addition, we did not observe an increase in locomotor activity or anxiety in PE rats, relative to controls, when they were reared in the IE condition. Indeed, in some studies, increased locomotor responding to novelty and anxiety are not detected after heavy PE, which may be influenced by multiple factors, such as PE timing and PE methods (Marquardt and Brigman, 2016). We have found that the PE paradigm used in the present study causes increased locomotion (Wang et al., under review) and anxiety (in the EPM task, unpublished data) in rats reared in a standard laboratory environment. Therefore, it is possible that the IE condition exerts major effects on locomotor responding to novelty and anxiety, thereby obscuring differences between control and PE rats reared in this condition.
Significant litter effects were found in every behavioral test in the present study, indicating correlations between littermates in the different behavioral measures. It is important to control for the disproportionate influence of any specific litter in studies involving prenatal treatments (Lazic and Essioux, 2013). As such, we used ≤ 3 rats/litter in each test and applied nested ANOVA (with litter as a nested factor) to enhance the rigor of the present study. In no case was an experimental group populated only by pups from one single litter. Naturally, it would be interesting to examine the specific litter differences, but it is beyond the scope of this study, considering a large number of litters involved in each test.
Taken together, we show that PE-induced increase in addiction risk is affected by postnatal rearing conditions. When reared in simple, impoverished environments, PE rats indeed exhibit increased addiction risk. In contrast, when they are reared in complex, enriched environments, this risk is substantially decreased. These conditions might represent two extreme rearing conditions in humans. At present, it is difficult to model all human rearing conditions. Future studies are required to understand how timing and/or the specific nature of postnatal environmental factors contribute to the levels of addiction risk following PE. The outcomes of such studies will provide further important insights into the determinants of behavioral deficits caused by PE and the development of intervention strategies for FASD.
Highlights.
Prenatal ethanol exposure leads to higher drug addiction risk.
Environmental enrichment reverses the increased addiction risk.
Environmental enrichment decreases locomotor responding to novelty.
Environmental enrichment produces anxiolytic effects.
Acknowledgements
This study was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (Grants AA12435 and AA019482 to R.S.). The authors thank Dr. Jerry B. Richards for his suggestions on the experimental design, and Millicent Nwankwo, Philip Slepian, and Alvin Wen for their help in data collection.
Role of Funding Source
The National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (Grants AA12435 and AA019482 to R.S.)
Footnotes
Author Disclosures
Conflicts of Interest No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguinis H, Gottfredson RK, Joo H, 2013. Best-practice recommendations for defining, identifying, and handling outliers. Organ. Res. Methods 16, 270–301. [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W, 2006. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch. Gen. Psychiatry 63, 1009–1016. [DOI] [PubMed] [Google Scholar]
- Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, Naassila M, 2009. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in Long Evans rats. Neuroscience 161, 427–440. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C, 2001. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 155, 278–284. [DOI] [PubMed] [Google Scholar]
- Choong K, Shen R, 2004. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience 126, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Choong K, Shen RY, 2004. Methylphenidate restores ventral tegmental area dopamine neuron activity in prenatal ethanol-exposed rats by augmenting dopamine neurotransmission. J. Pharmacol. Exp. Ther 309, 444–451. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B, 1998. Effects of moderate alcohol consumption on the central nervous system. Alcohol. Clin. Exp. Res 22, 998–1040. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Tobeña A, Fernández-Teruel A, 1994. Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behav. Brain Res 61, 169–173. [DOI] [PubMed] [Google Scholar]
- Fernández-Teruel A, Giménez-Llort L, Escorihuela RM, Gil L, Aguilar R, Steimer T, Tobeña A, 2002. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms? Pharmacol. Biochem. Behav 73, 233–245. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L, Richards JB, 2011. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav. Processes 86, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursky ZH, Klintsova AY, 2017. Wheel running and environmental complexity as a therapeutic intervention in an animal model of FASD. J. Vis. Exp 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht K, Haj-Dahmane S, Shen YL, Vezina P, Dlugos C, Shen RY, 2015. Excitatory synaptic function and plasticity is persistently altered in ventral tegmental area dopamine neurons after prenatal ethanol exposure. Neuropsychopharmacology 40, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht K, Shen Y-L, Wang R-X, Haj-Dahmane S, Shen R-Y, 2017. Prenatal ethanol exposure persistently alters endocannabinoid signaling and endocannabinoid-mediated excitatory synaptic plasticity in ventral tegmental area dopamine neurons. J. Neurosci 37, 5798–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Essioux L, 2013. Improving basic and translational science by accounting for litterto-litter variation in animal models. BMC Neurosci. 14, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-Y, Wang X, Huang Y, Marie H, Nestler EJ, Schlüter OM, Dong Y, 2016. Resilencing of silent synapses unmasks anti-relapse effects of environmental enrichment. Proc. Natl. Acad. Sci. U.S.A 113, 5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Brigman JL, 2016. The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol 51, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ, 2007. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol 73, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Moore EM, Thomas JD, Riley EP, 2015. Advances in diagnosis and treatment of fetal alcohol spectrum disorders: from animal models to human studies. Alcohol Res 37, 97–108. [PMC free article] [PubMed] [Google Scholar]
- Pham TM, Söderström S, Winblad B, Mohammed AH, 1999. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav. Brain Res 103, 63–70. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J, 2001. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev. Psychobiol. 38, 239–251. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD, 2011. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J. Neurosci 31, 17835–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Hannigan JH, Kapatos G, 1999. Prenatal ethanol reduces the activity of adult midbrain dopamine neurons. Alcohol. Clin. Exp. Res 23, 1801–1807. [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M, 2010. Prevention and treatment of drug addiction by environmental enrichment. Prog. Neurobiol 92, 572–592. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Shoaib M, Holsboer F, Landgraf R, 1995. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl.) 122, 369–373. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N, 2002. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J. Neurosci 22, 4654–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Hausknecht KA, Haj-Dahmane S, Shen R-Y, Richards JB, 2018. Decreased environmental complexity during development impairs habituation of reinforcer effectiveness of sensory stimuli. Behav. Brain Res 337, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Shen Y-L, Hausknecht K, Chang L, Haj-Dahmane S, Vezina P, Shen R-Y, Prenatal Ethanol Exposure Increases Risk of Psychostimulant Addiction (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würbel H, 2001. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci. 24, 207–211. [DOI] [PubMed] [Google Scholar]
- Xu C, Shen RY, 2001. Amphetamine normalizes the electrical activity of dopamine neurons in the ventral tegmental area following prenatal ethanol exposure. J. Pharmacol. Exp. Ther 297, 746–752. [PubMed] [Google Scholar]