Abstract
Literature on effects of equine therapy in individuals with autism spectrum disorder (ASD) has grown in recent times. Equine therapy is an alternative multimodal intervention that involves utilizing a horse to enhance core impairments in ASD. Recent systematic reviews in this area have several limitations including inclusion of populations other than ASD, assessment of a variety of animal-assisted interventions other than equine therapy, and a failure to conduct quantitative analyses to provide accurate effect size estimates. We conducted a focused systematic review to address these limitations. Our review suggested that equine therapy has beneficial effects on behavioral skills and to some extent on social communication in ASD. The evidence for positive effects of equine therapy on perceptuo-motor, cognitive, and functional skills is currently limited.
Keywords: Equine therapy, hippotherapy, therapeutic horseback riding, social communication, behavior, autism
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder that affects multiple subsystems including social communication, behavioral, cognitive, and perceptuo-motor domains. Core impairments in ASD include social communication difficulties as well as the presence of stereotyped and repetitive behaviors and interests (American Psychiatric Association, 2013). More specifically, social communication impairments include poor reciprocity during social interactions, reduced social gaze/eye contact, as well as delays in nonverbal and verbal communication (Mundy and Newell, 2007; Tager-Flusberg 1999; Dawson et al. 2004). In addition, children with ASD also demonstrate several comorbidities such as significant difficulties with behavioral and emotional regulation (i.e., anxiety, aggression, depression, hyperactivity, temper tantrums, and self-injurious behaviors) (Bodfish et al. 2000; Lecavalier 2006; Loh et al. 2007; Mazefsky, Pelphrey, and Dahl 2012) and cognitive difficulties in attentional focus, executive functioning, and working memory (Ozonoff, Pennington, & Rogers, 2006; Williams, Goldstein, & Minshew 2006). There is also substantial evidence for sensori-motor impairments in children with ASDs (Fournier et al. 2010; Bhat, Landa, & Galloway, 2011) including difficulty modulating sensory inputs (Tomchek and Dunn 2007; Baranek, Parham, and Bodfish et al., 2005) and pervasive motor impairments during coordinated arm/leg movements (Fournier et al. 2010; Green et al. 2009; Vilensky, Damasio, and Maurer 1981; Hallett et al. 1993), balance tasks (Minshew et al. 2004), as well as imitation and praxis tasks (Mostofsky et al. 2006; Dewey, Cantell, and Crawford 2007). Furthermore, comorbidities in sensori-motor performance correlate with and influence social communication performance of individuals with ASDs (Dziuk et al., 2007; MacDonald, Lord, & Ulrich, 2014). Limited movement exploration and motor clumsiness may lead to missed opportunities to develop social connections with peers and caregivers (Bhat, Landa, and Galloway 2011; Leary and Hill, 1996; Jansiewicz et al. 2006). Overall, individuals with ASD face difficulties in multiple developmental domains; this necessitates the use of multimodal interventions to effectively address both diagnostic impairments as well as comorbidities in this population.
Mainstream autism interventions based on principles of Applied Behavioral Analysis such as Discrete Trial Training (Lovaas, 1987), Pivotal Response Therapy (Koegel & Koegel, 2006), Early Start Denver Model (Rogers & Dawson, 2009), and Picture Exchange Communication System (Bondy & Frost, 2003) as well as more recent developmental approaches such as Floortime, Social Communication, Emotional Regulation and Transactional Support (SCERTS), Developmental Individual-Difference, Relationship-based model (DIR), etc. (Greenspan & Wieder 1997; Wieder & Greenspan, 2003; Prizant et al., 2006; Kasari et al. 2008; Landa et al., 2011) mainly focus on improving social communication, behavioral and academic skills of children with ASD. While ABA-based approaches use structured environments and incremental prompting to promote positive behaviors (Lovaas 1987; Bondy & Frost 2003; Mesibov, Shea, & Schopler 2004), developmental approaches focus on facilitating specific early social communication skills such as joint attention and imitation (Landa et al., 2011; Kasari et al. 2008). Although these approaches have substantial evidence for improving social communication and functional/academic skills, they may not address the sensori-motor needs of the children with ASD. Through this systematic review (SR), we summarize the evidence for the use of equine therapy as a multimodal intervention to address diagnostic impairments in social communication skills as well as behavioral and sensorimotor comorbidities in individuals with ASD.
Equine therapy involves activities completed in the presence of a horse, including mounted activities such as hippotherapy and therapeutic horseback riding as well as non-mounted, equine-focused activities such as grooming and caring for the horse (Lentini & Knox, 2015). Hippotherapy (HIP) involves purposeful manipulation of equine movement based on clinical reasoning of occupational therapists (OTs), physical therapists (PTs), and speech language pathologists (SLPs) to engage an individual’s sensory, neuromotor, and cognitive systems and achieve certain functional outcomes (American Hippotherapy Association, AHTA, 2017). The focus in HIP is on using the horse and its movement as a tool by allied health professionals to achieve therapeutic goals such as improvement of balance, sensory processing skills, arousal, etc. in clients. In contrast, in THR the focus is on teaching the student different types of riding skills (PATH International, 2017). A typical THR session involves mounted and non-mounted equine-assisted activities to promote the physical, cognitive, emotional, and social wellbeing of individuals with special needs (Ward et al., 2013). Compared to HIP accreditation, THR instructor certification is accessible to a broader group of trainers including special educators, counselors, as well as equestrians, following completion of online coursework and 25 hours of training experience.
To date, few SRs have been conducted on the effects of equine therapy in individuals with ASD (O’Haire, 2013, 2017; Lentini & Knox, 2015; Weise et al., 2016; McDaniel & Wood, 2017). O’Haire (2013, 2017) conducted a qualitative analysis of the effects of animal-assisted interventions as a whole (vs. equine therapy only) in ASD and reported positive effects in terms of social, communication/language, and stress/behavior, as well as a reduction in autism symptoms. Similarly, based on their broad review of 47 studies in individuals with diverse types of special needs, Lentini & Knox (2015) reported qualitative improvements in social emotional skills, self-regulation, self-esteem, and levels of anxiety and depression following equine therapy (Lentini & Knox, 2015). Wiese, Simpson, & Kumar (2016) reviewed 8 studies that assessed the effects of equine therapy on only social behavioral skills of individuals with ASD. Lastly, based on a comprehensive systematic review of equine-assisted interventions, McDaniel & Wood (2017) offered proof of concept evidence that equine therapies can benefit children and adolescents with ASD. Taken together, past SRs have focused on broader topics of animal-assisted interventions (vs. focusing on equine therapies), diverse populations (special needs with various diagnoses or mental health issues), and used more qualitative as opposed to quantitative analytical methods (none of the reviews assessed the quality of research evidence in conjunction with the size of treatment effects obtained in each study). The current study addresses these limitations by conducting a comprehensive (qualitative and quantitative) review of the effects of equine therapy on impairments in multiple subsystems/domains in individuals with ASD. Our first aim was to examine the effects of equine therapy on specific domains including social, communication, behavioral, and sensori-motor skills as well as broader functional outcomes including overall adaptive functioning and quality of life. Our second aim was to systematically assess the methodological quality (using Sackett’s level of evidence and PEDro scores) of the published research evidence to date and calculate the size of the treatment effects for all outcomes measured in individual studies included in the review.
Methods
Search Protocol
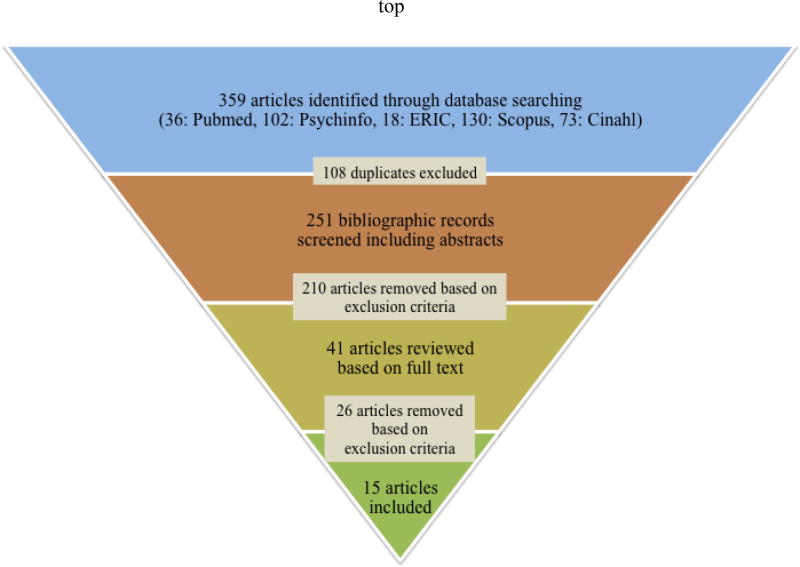
Studies were searched from five common electronic databases associated with the fields of health, psychology, allied health, and education i.e., PubMed (1950-Present), PsycINFO (1969-Present), ERIC (1966-Present), Scopus (1966-Present), and CINAHL (1937-Present). We searched for terms associated with “equine therapy” and “autism” (see details in Appendix 1). We also searched the reference sections of included articles and previously published reviews for additional relevant articles (see Figure 1 for details).
Figure 1.
A flowchart of the study selection process
Eligibility Criteria
Studies were screened based on the following inclusion criteria: a) peer-reviewed articles and b) studies reporting data on treatment effects of “equine” therapy using experimental or quasi-experimental study designs, and exclusion criteria: a) foreign language b) case reports, opinions, narrative reports, etc. c) animal-assisted or pet therapy not equine in nature, for example, using dolphins, dogs, etc., and d) unrelated topics, for example, PET imaging, etc.
Data Extraction and Evaluation
After applying the eligibility criteria, we were left with 15 articles in this review. We used the levels of evidence described by Sackett and colleagues (1997) to classify the methodological quality of studies. These guidelines provide systematic criteria that can be used to classify individual studies based on study design into 5 levels of evidence from Levels I–V. Level I studies provide the highest level of evidence and include randomized controlled trials whereas Level V studies include case reports and narrative statements. In addition, we used the Physiotherapy Evidence Database (PEDro) scale (Moseley et al., 2002) to evaluate the internal validity and interpretability of the randomized controlled trials (RCTs) and controlled clinical trials (CCTs) included in this review. The PEDro scale contains 11 items of which the last 10 items are scored to obtain the total PEDro score (Moseley et al., 2002). We coded each study for sample and study characteristics, methodological quality, assessment measures, dependent variables, and treatment effects (see Appendix 2 for the coding sheet). In addition to the qualitative coding of treatment effects based on the original study reports, we also report on quantitative effect size measures from each study. We used data reported in the original studies (wherever available) to calculate effect sizes using the standardized mean difference (d) index (Hedges, 1981, Lipsey & Wilson, 2001, Heudo-Medina & Johnson, 2011). We used the excel code developed by Heudo-Medina and Johnson to calculate effect sizes and their 95% confidence intervals for all studies (Heudo-Medina & Johnson, 2011). For studies that reported effect sizes within the original paper, we compared the reported (from original study) and calculated (based on our calculations) effect sizes. We also summarized the treatment effects reported per domain based on the reviewed studies, i.e. effects on social communication skills, motor skills, etc. All three authors who are physical therapists by training were involved in the search process, extraction of data, and coding of studies. For the purpose of reliability, all three authors coded five out of the 15 articles using the coding form listed in Appendix A. We used Intraclass correlation coefficients to calculate reliability on all the parameters coded for the individual studies. Each of the three authors achieved inter-rater reliability of over 87.5% after establishing consensus on scores they disagreed on. Each coder also established intra-rater reliability of over 99%. Following reliability, the remaining 10 articles were divided for review between the three authors.
Results
Description of Studies
Out of the 15 studies reviewed, 8 were conducted in the US, 2 in Spain (Tabares et al., 2012; Gracia-Gomez et al., 2014), 1 in Canada (Llambias et al., 2016), 1 in the UK (Anderson & Meints, 2016), 1 in Taiwan (Wuang et al., 2010), 1 in Italy (Borgi et al., 2016) and 1 in Slovakia (Steiner & Kertesz, 2015). All studies were published in peer-reviewed journals except one study, which was published as a peer-reviewed conference paper (Steiner et al., 2012). All included studies were published between 2009 and 2016, although, only one study mentioned the year of data collection in the published report (Wuang et al., 2010). Out of the 15 studies, 12 studies assessed the effects of THR whereas the remaining 3 studies assessed the effects of HIP in individuals with ASD.
Sample Characteristics
Our systematic review is based on a total of 428 subjects out of which 294 subjects received equine therapy and the remaining 134 subjects received some form of control intervention. The reviewed studies demonstrated wide variation in sample sizes ranging from 6 to 116 participants (Table 1A). Eleven out of the 15 studies included only children with diagnoses of ASD, 2 studies included a mixed sample of children with ASD and Asperger Syndrome (Bass et al. 2009; Kern et al. 2011), 1 study included children with ASD and Attention Deficit Hyperactivity Disorder (ADHD) (Llambias et al., 2016), and 1 study included children with ASD who also had comorbid disorders such as ADHD and Hypersensitivity and Sensory Integration Disorder (HSID) (Anderson & Meints, 2016).
Table 1.
| A: Study Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant Characteristics | Intervention Characteristics | |||||||||||
| First Author, Year |
Final Sample Size (E, C) |
Age M (SD) (Range) |
Diagnoses | Diagnostic Measure |
Other therapy |
Duration in weeks (Frequency sessions/week) |
Session time in mins |
Type (HIP, THR, SHR) |
Format (I or G) |
Therapy Provider Accreditation |
Past/recent Riding Experience |
C Group Interventions |
| Bass et al. (2009) | 34 (19, 15) | E: 6.95 (1.67) (5–10) C: 7.73 (1.65) (4–10) | ASD, AS | DSM IV | - | 12 (1) | 60 | THR | I | - | None | Waitlist |
| Wuang et al. (2010) | 60 (60, 0) | E only: F: 7.59 (0.31) M: 7.58 (0.34) Group Range: (6.42–8.75) | ASD | - | OT | 20 (2) | 60 | SHR, OT | I | n/a | None | OT |
| Kern, et al. (2011) | 24 (24, 0) | 7.8 (2.9) (3–12) | ASD, AS | PC, CARS | CNAT | 24 (1) | 60 | THR | I | - | None | n/a |
| Gabriels, et al. (2012) | 42 (26, 16) | 8.7 (−) (6–16) | ASD | PC, ABC-C, SCQ, ADOS | CNAT | 10 (1) | 60 | THR | G | PATH | <2 weeks in past 3 years | Waitlist |
| Steiner et al. (2012) | 26 (13, 13) | (−) (−) (10–13) | ASD | - | - | 5 (1) | 30 | THR | - | - | - | Special Pedagogy Exercises |
| Tabares, et al. (2012) | 8 (8, 0) | (−) (−) (8–16) | ASD | - | - | 4 (1) | 30 | HIP | I | AZE | Yes | n/a |
| Ajzenman et al. (2013) | 6 (6, 0) | 8.4 (2.5) (5–12) | ASD | DSM IV | - | 12 (1) | 45 | HIP | G | PATH | None | n/a |
| Jenkins et al. (2013) | 7 (4,3) | 9.5 (−) (6–14) | ASD | PC | - | 9 (1) | 60 | THR | G | PATH | None | Waitlist |
| Ward, et al. (2013) | 21 (21, 0) | 8.1 (−) (5–12) | ASD | DSM IV | SLP (all students), OT (20 students), PT (1 student) | 18 (1) | 60 | THR | G | PATH | None for 13 of the 21 students | n/a |
| García-Gómez, et al. (2014) | 16 (8, 8) | (−) (−) (7–14) | ASD | CARS | CNAT | 12 (2) | 45 | THR | - | PATH | None within 2 years | Medical and re-education treatment |
| Lanning, et al. (2014) | 18 (10, 8) | E: 7.5 (3.2) (4–15) C: 9.8 (3.2) (5–14) | ASD | - | - | 12 (1) | 60 | THR | I or G | PATH | None within 6 months | Social circles: Card games, Board games, Sensory activities |
| Gabriels, et al. (2015) | 116 (58, 58) | 10.2 (3.0) (6–16) | ASD | SCQ, ADOS, ABC-C | - | 10 (1) | 45 | THR | G | PATH | <2 hours EAAT within 6 months | Barn Activities |
| Anderson & Meints (2016) | 15 (15, 0) | 10 (3.8) (5–16) | ASD ADHD HSID | DSM (unspecifie d), PC | - | 5 (1) | 180 | THR | G | BHS, RDA | None | n/a |
| Borgi, et al (2016) | 28 (15, 13) | 8.6 (1.7) (6–12) | ASD | DSM IV, ICD-10 | CNAT | 25 (1) | 60–70 | THR | G | FISE | None | Waitlist |
| Llambias et al. (2016) | 7 (7, 0) | (−) (−) (4–12) | ASD (7) ADHD (2) | PC | SLP, PT, OT or BI | 9–12 (1) | 45–60 | HIP | I | PATH | None within 3 months | n/a |
| B: Methodological Quality | ||||||
|---|---|---|---|---|---|---|
| First Author, Year | Study Design | Control Group Allocation | Exclusion criteria: Past/recent riding experience? |
Checks on Treatment Fidelity/Integrity |
PEDro Score |
Level of Evidence |
| Bass et al. (2009) | RCT | Randomized | Yes | Not reported | 5 | I |
| Wuang et al. (2010) | Cross-Over Design | Non-randomized but post-hoc testing of baseline similarity | Yes | Yes | 4 | II |
| Kern, et al. (2011) | Pre-Post Design | n/a | Yes | Not reported | n/a | III |
| Gabriels, et al. (2012) | CCT | Non-randomized but post-hoc testing of baseline similarity | Yes | Not reported | 4 | II |
| Steiner et al. (2012) | CCT | Not randomized | Not reported | Not reported | 2 | II |
| Tabares, et al. (2012) | Pre-Post Design | n/a | Yes | Not reported | n/a | III |
| Ajzenman et al. (2013) | Pre-Post Design | n/a | Yes | Not reported | n/a | III |
| Jenkins et al. (2013) | Single-Case | Not randomized | Yes | Yes | n/a | IV |
| Ward, et al. (2013) | Single-Group Interrupted Time Series | n/a | No | Not reported | n/a | III |
| García-Gómez, et al. (2014) | CCT | Not randomized | Yes | Not reported | 4 | II |
| Lanning, et al. (2014) | CCT | Not randomized | Yes | Not reported | 2 | II |
| Gabriels, et al. (2015) | RCT | Randomized | Yes | Yes | 7 | I |
| Anderson & Meints (2016) | Pre-Post Design | n/a | Yes | Not reported | n/a | III |
| Borgi, et al (2016) | RCT | Randomized | Yes | Not reported | 5 | I |
| Llambias et al. (2016) | Single-Case Reversal | n/a | Yes | Yes | n/a | IV |
| C: PEDro scoring for RCT/CCT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year |
Eligibility criteria specified |
Random subject allocation |
Allocation concealment |
Baseline similarity of groups |
Blinding of subjects |
Blinding of therapists |
Blinding of assessors |
Measures of key outcomes |
Intention to treat |
Between group comparis ons |
Point measures & variability measures |
Total |
| Bass et al. (2009) | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Wuang et al. (2010) | Y | N | N | Y | N | N | Y | N | N | Y | Y | 4 |
| Gabriels, et al. (2012) | Y | N | N | Y | N | N | N | Y | N | Y | Y | 4 |
| Steiner et al. (2012) | Y | N | N | N | N | N | N | Y | N | N | Y | 2 |
| García-Gómez, et al. (2014) | Y | N | N | N | N | N | N | Y | Y | Y | Y | 4 |
| Lanning, et al. (2014) | Y | N | N | N | N | N | N | N | N | Y | Y | 2 |
| Gabriels, et al. (2015) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Borgi, et al (2016) | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
Legend: -: not reported; ABC-C: Aberrant Behavior Checklist-Community; ADHD: Attention Deficit Hyperactivity Disorder; ADOS: Autism Diagnostic Observation Schedule; AHA: American Hippotherapy Association; AS: Asperger Syndrome; ASD: Autism Spectrum Disorder; AZE: Association of Zootherapy of Extremadura; BHS: British Horse Society; BI: Behavioral Interventionist Services; C: Control group; CARS: Childhood Autism Rating Scale; CNAT: Continued Normal Activities and Therapies (Subjects did not alter normal routine—Specific therapies not specified); E: Experimental group; EAAT: Equine Assisted Activities and Therapies; FISE: Federazione Italiana Sport Equestri; G: Group; HIP: Hippotherapy (Equine-assisted occupational or physical therapy); HSID: Hypersensitivity and Sensory Integration Disorder; ICD-10: International Statistical Classification of Disease and Related Health Problems; I: Individual; M: Mean; n/a not applicable; OT: Occupational Therapy; PATH: Professional Association of Therapeutic Horsemanship International; PC: Physician Confirmation/Diagnosis; PIW: Pre-Intervention Waiting Period; PT: Physical Therapy; RDA: Riding for the Disabled; SCQ: Social Communication Questionnaire; SHR: Simulated Horseback Riding; SLP: Speech Language Pathology Services; THR: Therapeutic Horseback Riding
Legend: CCT: Controlled Clinical Trial; n/a: not applicable; N: No; RCT: Randomized Control Trial; Y: Yes Sackett’s levels of evidence: Level 1: Systematic reviews, meta-analyses, RCTs, Level II: Two-group, non-randomized studies, Level III: One-group non-randomized studies, Level IV: Descriptive studies (single-subject designs), Level V: Case reports/expert opinions/consensus statements
Legend: See common legend below 2C
Only four studies reported the use of standardized autism-specific measures such as the Autism Diagnostic Observation Schedule (ADOS), Social Communication Questionnaire (SCQ), or Childhood Autism Rating Scale (CARS) to establish the diagnosis of participants (Gabriels et al., 2012; Gabriels et al. 2015; García-Gómez et al. 2014; Kern et al. 2011). Eleven other studies reported having confirmed diagnosis of subjects based on expert clinical opinion of a licensed health professional and four of the remaining studies did not report on the method of confirmation of subjects’ diagnoses (Table 1A). Seven out of the 15 studies also reported on some additional baseline variables such as Intelligence Quotient (IQ) and language use of subjects to characterize their adaptive skills and functional status.
All studies provided equine therapy to children or adolescents between 3 and 16 years (Table 1A); interestingly, our literature search did not reveal any studies that provided interventions to adults with autism. In terms of gender distribution of subjects, two studies did not provide information on the male-to-female ratio of subjects in their final post-attrition sample (Lanning et al., 2014; Ajzenman et al., 2013). Out of the total 404 subjects included in the remaining 13 studies, 328 subjects were males and 76 were females. In terms of sample selection criteria, 13 out of the 15 studies excluded children with previous riding experience, 1 study included both children with and without prior riding experience (Tabares et al., 2012), and 1 study did not report on children’s previous riding experiences (Steiner et al., 2012, Table 1A). Studies accounting for previous horseback riding experience either excluded subjects with previous riding experience or defined a time period prior to the intervention (ranging from 3 months to 3 years) in which subjects must have limited to no riding experience.
Study Characteristics
A variety of study designs were employed in the included studies (Table 1B for details). Out of the three randomized controlled trials (RCT) that qualified as level I evidence, 2 studies (Bass et al., 2009; Borgi et al., 2016) employed a waitlist control group, whereas in the third study (Gabriels et al., 2015), the control group engaged in horsemanship activities involving a stuffed horse, with no contact with live horses. The PEDro scores for these 3 studies ranged from 5–7/10 (see Table 1B & IC).
Out of the five studies that were classified as Level II evidence, one study (Wuang et al., 2010) used a crossover design, whereas the remaining 4 studies were controlled clinical trials (Gabriels et al., 2012; Steiner et al., 2012; Garcia-Gomez et al., 2014; Lanning et al., 2014). All 5 studies employed non-randomized controls; however, only 2 of these studies conducted post-hoc testing to ascertain baseline similarity of the experimental and control groups on select variables including age, gender, perceptuo-motor performance, nonverbal IQ, and presence of seizures (Wuang et al., 2010; Gabriels et al., 2012). Four of these Level II studies provided conventional therapies for control group subjects including occupational therapy (OT) (Wuang et al., 2010), special pedagogy exercises (Steiner et al., 2012), medical and re-education treatment (Garcia-Gomez et al., 2014), and social circles (Lanning et al., 2014) while the last study (Gabriels et al., 2012) used a waitlist control group. In terms of PEDro scoring, all level II studies, scored in the range of 2–4/10 (see Table 1B & 1C for details).
Our review included 5 Level III studies that employed a single group and compared outcomes within this group from pretest to posttest, or at multiple time points in an interrupted fashion (Kern et al., 2011; Tabares et al., 2012; Ajzenman et al., 2013; Ward et al., 2013; Anderson & Meints, 2016). Lastly, 2 studies in this review employed a single subject design and were qualified as Level IV evidence (Jenkins & DiGennaro-Reed, 2013 (here on Jenkins et al.); Llambias et al., 2016). Although the Jenkins et al. (2013) study non-randomly assigned 4 of their 7 subjects to a treatment group and the remaining 3 to a waitlist control group, they did not conduct any between-group comparisons and only reported on individual data, therefore justifying their classification as Level IV evidence. Since PEDro scores can only be calculated for RCTs or CCTs (Moseley et al., 2002), we could not report on these scores for the 7 Level III and IV studies in this review.
Outcome Measures and Assessments
Given that ASD is a disorder that affects several sub-systems, many studies in this review assessed the impact of equine therapies on more than one skill domain using a combination of subjective and objective measures including standardized tests, quantitative measures, observational measures, parent/teacher-rated questionnaires, and video coding to assess treatment efficacy (Table 2A & 2C). Specifically, 12 out of the 15 studies used parent/teacher-rated questionnaires such as the Sensory Profile (SP), Vineland Adaptive Behavior Scale (VABS), Ages & Stages Questionnaire (ASQ) etc.; 6 studies used standardized tests administered by expert clinicians, for instance, the Childhood Autism Rating Scale (CARS), Sensory Integration and Praxis Tests (SIPT), Bruininks Oseretesky Test of Motor Proficiency (BOT), etc.; 3 studies used video coding or observational measures to assess changes in subjects’ behaviors during training sessions; 2 studies used objective quantitative measures including kinematic analysis and force plate data; and 1 study used physiological measures including salivary hormonal levels to assess the effects of equine therapy (see Tables 2A and 3A for details). For the outcomes evaluated, only 7 studies reported reliability of the assessed variables. In terms of blinding assessors for clinical assessments or video coding, three studies blinded assessors to grouping/treatments (Borgi et al., 2016; Gabriels et al., 2015; Wuang et al., 2010), two other studies blinded assessors/coders to study goals but not treatment groups (Anderson & Meints, 2016; Llambias et al. 2016), two studies admitted to not following blinding procedures (Ajzenman et al., 2013; Gabriels et al., 2012), and the remaining 8 studies did not specify details about blinding (see Table 3A). In terms of the maintenance of treatment gains, only 4 of the 15 studies assessed the long-term effects of equine therapies through follow-up testing which was conducted at 1 month (Llambias et al., 2016), 1.5 months (Ward et al., 2013), 3 months (Steiner et al., 2012), and 6 months post intervention (Wuang et al., 2010).
Table 2.
| A: Study-wise list of dependent variables & results | ||||
|---|---|---|---|---|
| First Author, Year |
Domains/Variables Tested | Type of effect (B/W) |
Measures | Measures/variables showing improvement |
| Bass et al. (2009) | Social and Sensory processing skills | B | SRS, SP | SRS overall and Social motivation subscale scores; Overall score and all sub-scales of SP except fine motor/perceptual subscale |
| Wuang et al. (2010) | Motor and Sensory processing skills | B | BOT, TSIF | All subtests of BOT and TSIF |
| W | All subtests of BOT and TSIF | |||
| Kern, et al. (2011) | Social Communication, Behavioral, and Sensory processing skills and QOL | W | CARS, TPCIS, SP, QOL ESS, TSS | CARS, QOL ESS |
| Gabriels, et al. (2012) | Adaptive Behavior, Motor, Sensory Processing, and Social Communication skills | B | ABC- C, VABS, BOT, SIPT | All subscales of ABC-C except Inappropriate speech |
| W | All subscales of ABC-C except Inappropriate speech; Adaptive behavior composite and Social, Communication, and Daily living, subscales of VABS; BOT-2 short form scores; Verbal and postural praxis subtests of SIPT | |||
| Steiner et al. (2012) | Social Communication and Motor skills | B | PAC, Length of gait cycle | All subscales of PAC; length of gait cycle |
| W | All subscales of PAC; length of gait cycle | |||
| Tabares, et al. (2012) | Physiological variables as proxy for social skills | W | Hormone levels: Cortisol, Progesterone and Cortisol/Progesterone | Cortisol, Progesterone and Cortisol/Progesterone hormone levels |
| Ajzenman et al. (2013) | Adaptive Behavior, Social Communication skills, Postural Stability, Functional participation | W | VABS, CACS, postural variables | Adaptive behavior composite and Social and Communication subscales of VABS; Self-care, low demand leisure, and social interaction subscales of CACS; All postural stability variables |
| Jenkins et al. (2013) | Behavioral, Affective, Communication, and Motor skills | B | CBCL-TRF, video coding of affect, problem behavior, and communication skills | No effect on any variables |
| W | Behavioral video coding of Posture | Improvements in posture | ||
| Ward, et al. (2013) | Social Communication, Behavioral, and Sensory Processing skills | W | GARS, SPSC | Autism index and social interaction scales of GARS; Registration, sensitivity, school factor 1, school factor 4, auditory, visual, and touch subscales on SPSC |
| García- Gómez, et al. (2014) | QOL and Behavioral skills | B | QOLQ | Interpersonal relationship and social inclusion subscales of QOLQ |
| W | BASC | Aggressiveness sub-scale of BASC | ||
| Lanning, et al. (2014) | QOL | B | Pediatric QOL, CHQ | No improvement on any variables |
| W | Physical health summary score, psychosocial health summary score, social functioning, emotional functioning, and physical functioning subscales of Pediatric QOL | |||
| Gabriels, et al. (2015) | Social Communication, Adaptive Behavior, Motor, and Sensory Processing skills | B | PPVT, VABS, ABC-C, SALT, SIPT, BOT, SRS | Irritability and hyperactivity subscales of ABC-C; Social cognition and social communication scales of SRS; # of words and # of different words on SALT |
| Anderson & Meints (2016) | Adaptive behavior, Social Communication, Problem solving, and Affective skills | W | ASQ, VABS, EQ, SQ, EQ/SQ | ASQ, EQ, Maladaptive behavior composite of VABS |
| Borgi, et al (2016) | Adaptive behavior, Social Communication, Motor, and Executive Functioning skills | B | VABS, TOLT | Social, communication, and daily living subscales of VABS; Planning time, execution time, total time, # of correct solutions, and # of rule violations on the TOLT |
| W | Social and motor subscales of VABS; Planning time on TOLT | |||
| Llambias et al. (2016) | Social skills | W | Engagement measured through behavioral video coding | Engagement |
| B: Study-wise list of reported and calculated effect sizes | |||||||
|---|---|---|---|---|---|---|---|
| First Author, Year |
Reporte d ES (Y/N) | Type of effect (B/W) |
Magnitude of reported ES | Magnitude of calculated ES | CI range for ES | # of ES per measure where CI does not include 0 | Comments |
| Bass et al. (2009) | N | B | None | SRS (2): 0.38–0.45; SP (5): 0.39–0.85 | SRS (2): −1.13 to 0.3; SP (5): −0.29 to 1.56 | SRS: 0; SP: 1 (inattention/distractibility subscale) | Although the study has a between-group design, they did not report between-group ESs. Instead they calculated within-group ESs for each group and tested the significance of these effects using dependent t tests within each group. ESs are also not reported for all measures and across both groups. Poor agreement between reported and calculated ESs |
| Y | W | SRS (1): 0.66; SP (1): 0.059 | SRS (1): 0.31; SP (1): 0.54 | SRS (1): −0.8 to 0.18; SP (1): 0.03 to 1.06 | SRS: 0; SP: 1 | ||
| Wuang et al. (2010) | Y | B | BOT & TSIF: All effects were reported to be large using partial eta-squared | BOT (16): 0.56–7.98; TSIF (14): 1.78–5.29 | BOT (16): 0.04 to 9.5; TSIF (14): 1.18 to 6.35 | BOT: 16; TSIF: 14 | This study did not report actual values of between-group effect sizes using Cohen’s d. They assessed magnitude of between-group effects using partial eta-squared values obtained from their MANOVA run. Fairly good agreement between reported and calculated within-group effect sizes. |
| Y | W | BOT (22): 4.50–13.31, retention of gains at follow-up; TSIF (14): 5.08–15.40, retention of gains at follow-up | BOT (16): 4.39–9.60, retention of gains at follow-up; TSIF: (14): 3.73–15.00, retention of gains at follow-up | BOT (16): 3.12 to 10.17; TSIF: (14): 2.64 to 19.15 | BOT: 16; TSIF: 14 | ||
| Kern, et al. (2011) | Y | W | CARS(2): 0.36–0.50; No calculations provided for QOL ESS | CARS (2): 0.36–0.50; Insufficient information to calculate ES for QOL ESS | CARS (2): −0.81 to 0.098 | CARS: 0 | Excellent agreement between reported and calculated ESs |
| Gabriels, et al. (2012) | N | B | None | ABC-C (4): 0.69–0.87; | ABC-C (4): 0.11 to 1.47; | ABC-C: 4 | Good triangulation of ES estimates using Means & SDs, t values, and p values. |
| N | W | ABC-C (4): 0.63–0.81; VABS (4): 0.19–0.35; BOT (1): 0.50; SIPT (2): 0.38–0.45 | ABC-C (4): −0.29 to −1.17; VABS (4): −0.05 to 0.68; BOT (1): 0.15 to 0.85; SIPT (2): 0.04 to 0.79 | ABC-C: 4; VABS: 1 (adaptive total score); BOT: 1; SIPT: 2 | |||
| Steiner et al. (2012) | N | B | None | Data not available to make calculations | Not applicable | Not applicable | Study does not report any data. Only figures provided. Also the type of stats used were mentioned, no further details of statistical results provided |
| N | W | ||||||
| Tabares, et al. (2012) | N | W | None | Hormonal changes (6): 0.33–2.09 2 ES could not be calculated due to insufficient data | Hormonal changes (6): −2.09 to 3.81 | Hormonal changes: 2 (cortisol and progesterone levels from pre 1st session to post 1st session) | It is not exactly clear from the report how certain variables were calculated for statistical analysis. Also there are some discrepancies between values reported in the text versus in one of the Figures and the abstract. |
| Ajzenman et al. (2013) | Y | W | VABS: Social (2): 0.35–0.36, Communication (2): 0.47–1.19, ABC (1): 0.39; CACS (3): 0.62–0.91; Postural variables (9): 0.2–1.91 | VABS: Social (2): 0.32–0.34, Communication (2): 0.41–1.01, ABC (1): 0.37; CACS (3): 0.44–0.78; Postural variables: Reliable calculation not possible due to lack of Means and SDs | VABS: Social (2): -0.76 to 1.42, Communication (2): −0.7 to 2.46, ABC (1): −0.73 to 1.46; CACS (3): −0.5 to 2.08; | VABS: 0, CACS: 0; | Good agreement between reported and calculated ESs for VABS; Some discrepancies for CACS; ESs could not be reliably computed and corroborated for motor variables due to insufficient data |
| Jenkins et al. (2013) | N | B | None | Data not available to make calculations | Not applicable | Not applicable | - |
| N | W | None | |||||
| Ward, et al. (2013) | N | W | None | GARS (6): 0.35–0.51; SPSC (15): 0.24–0.67 | GARS (6): −0.99 to 0.12; SPSC (15): −0.22 to 1.18 | GARS: 1 (social interaction); SPSC: 7 | - |
| García-Gómez, et al. (2014) | Y | B | QOLQ (2): 2.05–2.43 | QOLQ (2): 1.95–2.31 | QOLQ (2): 0.75 to 3.57 | QOLQ: 2 | Fairly good agreement between reported and calculated ESs |
| Y | W | BASC (1): 0.22 | BASC (1): 0.21 | BASC (1): −1.04 to 0.63 | BASC: 0 | ||
| Lanning, et al(2014) | N | B | None | No significant effects | Not applicable | Not applicable | |
| N | W | Pediatric QOL (5): 0.62–0.68 | Pediatric QOL (5): −0.17 to 1.48 | Pediatric QOL: 0 | |||
| Gabriels, et al. (2015) | Y | B | ABC-C (2): 0.5–0.53; SRS (2): 0.41–0.63; SALT (2): 0.54 | ABC-C (2): 0.40–0.43 SRS (2): 0.44–0.62; SALT (2): 0.21–0.30 | ABC-C (2): −0.8 to −0.03 SRS (2): −0.99 to - 0.07; SALT (2): −0.15 to 0.66 | ABC-C: 2; SRS: 2; SALT: 0 | The study reported between-group ESs only. Good agreement between reported and calculated ES estimates for SRS, but some discrepancies for ABC-C and SALT, probably due to a different method of ES calculation used in the study |
| Anderson & Meints (2016) | N | W | None | ASQ (1): 0.06; VABS (1): 0.11; EQ (1): 0.12 | ASQ (1): −0.6 to 0.49; VABS (1): −0.66 to 0.44 EQ (1): −0.43 to 0.67 |
ASQ: 0; VABS: 0; EQ: 0 | - |
| Borgi, et al. (2016) | N | B | None | VABS (2): 0.14–0.4; TOLT (1): 0.76 | VABS (2): −0.7 to 1.5; TOLT (1): −1.54 to 0.02 |
VABS: 0; TOLT: 0 | Problems in triangulating calculated values of ES using Means & SEs and F values. The paper reported that sample sizes were different for each of the outcomes measured. These differential sample sizes were used for our ES calculations. But probably the paper used some method of data imputation prior to statistical testing, which was not clearly specified in the paper. Hence, possibly the difficulties in triangulation |
| N | W | Data not provided to calculate within-group ES for 3 variables on VABS and 5 variables on TOLT. | Not applicable | Not applicable | |||
| Llambias et al. (2016) | Y | W | Engagement: 100% IRD for all 7 subjects | No data provided to confirm results | Not applicable | Not applicable | - |
| C: Domain-specific effects of hippotherapy | ||||||
|---|---|---|---|---|---|---|
| Domains/ Variables Assessed |
Measures used | Studies using the measure | # of studies with significant effects |
# of studies with non- significant effects |
ES magnitude (Total # of ES calculated: B & W) |
# of ES where CI does not include 0 |
| Social Communication | SRS, VABS, ABC-C, CARS, GARS, SALT, PPVT, ASQ, PAC, TPCIS, behavioral coding | Bass et al., 2009, Kern et al., 2011; Jenkins et al., 2013; Ward et al., 2013; Gabriels et al., 2012, 2015; Anderson & Meints, 2016; Borgi et al., 2016; Llambias et al., 2016; Steiner et al., 2012; Ajzenman et al., 2013 | 9 | 2 | Small to large (21: 6B & 15W) | 3: 2B & 1W |
| Behavioral | BASC, ABC-C, VABS, EQ, SQ, CBCL-TRF, CARS, GARS, behavioral coding | García-Gómez et al. 2014; Gabriels et al., 2012, 2015; Jenkins et al., 2013; Anderson & Meints, 2016, Ward et al., 2013; Kern et al., 2011 | 5 | 2 | Small to large (19: 6B & 13W) | 11: 6B &5W |
| Sensory | SP, TSIF, SPSC | Bass et al., 2009; Wuang et al., 2010; Kern et al., 2011; Ward et al., 2013 | 3 | 1 | Small to large (56: 19B & 37W) | 42: 14B & 28W |
| Motor | BOT, PAC, VABS, postural and gait variables, behavioral coding | Wuang et al., 2010; Gabriels et al., 2012, 2015; Ajzenman et al., 2013; Jenkins et al., 2013; Steiner et al., 2012; Borgi et al., 2016 | 4 | 3 | Small to large (44: 16B & 28W) | 43: 16B & 27W |
| Cognition/Executive functioning | TOLT | Borgi et al., 2016 | 1 | - | Medium (1: 1B) | 0 |
| Functional participation | CACS | Ajzenman et al., 2011; | 1 | - | Medium to large (3: 3W) | 0 |
| QOL | Pediatric QOL, CHQ, QOL ESS, custom-developed | Kern et al., 2011; García-Gómez et al. 2014; Lanning et al., 2014; | 2 | 1 | Medium to large (7: 2B & 5W) | 2: 2B |
| Physiological | Cortisol and Progesterone levels | Tabares et al., 2012 | 1 | - | Small to large (6: 6W) | 2: 2W |
Note: Effect size (ESs) have been calculated and reported only for variables and measures where significant effects were reported in the original study. Effect sizes have been reported as absolute values in terms of magnitude only (for instance, for some variables a negative ES implies improvement). The numbers in parentheses reported next to the measure in columns 3 & 4 indicate the number of effect sizes calculated per measure. In case of multiple effect sizes calculated per measure, an ES range has been reported. Whenever possible, an attempt was made to triangulate ES values (for instance, if the study provide means and SDs as well as change values from pre- to post-intervention, ESs were calculated using both methods and checked for agreement). In addition, we have also reported Confidence intervals (CI) for effect sizes. In cases where multiple effect sizes have been calculated per outcome measure, CI ranges have been reported. Lastly, we have also mentioned the number of statistically significant effect sizes (CI do not include 0) per outcome measure for each study.
Legend: See common legend below 2C
Note: In between-group studies, if for a specific variable, significant within-group effects were reported in the study in the absence of significant between-group effects, the study has been categorized as not demonstrating a significant effect for that variable/outcome (the effect was not robust enough to be detected at a between-groups level). The qualitative effect size magnitude classification reported in the last column is based on the ESs reported in the original papers (wherever applicable); if unavailable in the original report, our calculated ESs have been used for the coding. ES magnitude coding: Small: 0.2–0.49, Medium: 0.50–0.79, Large ≥ 0.8. We have also provided the # of effect sizes (between-group and within-group) where the CI does not include 0.
Common Legend for Tables 2A & 2B & 2C: B: Between-group effect; W: Within-group effect; CI: Confidence interval; SRS: Social Responsiveness Scale; SP: Sensory Profile; BOT: Bruininks Oseretsky Test of Motor Proficiency; TSIF: Test of Sensory Integration Function; CARS: Childhood Autism Rating Scale; TPCIS: Timberlawn Parent-Child Interaction Scale; QOL ESS: Quality of life enjoyment and satisfaction survey; TSS: Treatment Satisfaction Survey; SP: Sensory Profile; ABC-C: Aberrant Behavior Checklist-Community; VABS: Vineland Adaptive Behavior Scales; SIPT: Sensory Integration and Praxis Tests; PAC: Pedagogical Analysis and Curriculum; CACS: Child Activity Card Sort; CBCL-TRF: Childhood Behavior Checklist – Teacher Rating Form; GARS: Gilliam Autism Rating Scale; SPSC: Sensory Profile School Companion; QOLQ: Quality of Life Questionnaire; BASC: Behavior Assessment System for Children; CHQ: Child Health Questionnaire; PPVT: Peabody Picture Vocabulary Test; SALT: Systematic Analysis of Language Transcripts; ASQ: Ages & Stages Questionnaire; EQ: Empathizing Quotient; SQ: Systemizing Quotient; TOLT: Tower of London Test of Executive Functioning; MANOVA: Multivariate Analysis of Variance
Table 3.
| A: Types of assessors/raters and methods for blinding | ||||
|---|---|---|---|---|
| First author (year) |
Type of assessment | Blinding of assessor (s) |
Raters/Informants | |
| Authors/ Research staff |
Parent/ Teacher |
|||
| Bass et al. (2009) | Parent questionnaires | None specified | x | |
| Wuang et al. (2010) | Clinical assessments | Blinded to groups | x | |
| Kern, et al. (2011) | Observational assessment, Questionnaires | None specified | x | x |
| Gabriels, et al. (2012) | Questionnaires, Parent interview, Clinical assessments | Not blinded | x | x |
| Steiner et al. (2012) | Observational assessment, Kinematic and kinetic gait analysis | None specified | x | x |
| Tabares, et al. (2012) | Biochemical | None specified | x | |
| Ajzenman et al. (2013) | Parent questionnaires/interviews, Motion/force analysis |
Not blinded | x | x |
| Jenkins et al. (2013) | Teacher questionnaire, Video recordings for behavioral coding | None specified | x | x |
| Ward, et al. (2013) | Parent/expert questionnaire | None specified | x | x |
| García-Gómez, et al. (2014) | Parent questionnaires | None specified | x | |
| Lanning, et al. (2014) | Parent questionnaires | None specified | x | |
| Gabriels, et al. (2015) | Parent questionnaires, Clinical assessments | Blinded to treatment | x | x |
| Anderson & Meints (2016) | Parent questionnaires, interviews | Blinded to study goals | x | |
| Borgi, et al (2016) | Parent interview, Clinical assessment | Testers blinded to group | x | x |
| Llambias et al. (2016) | Video coding of behaviors | Blinded to study goals | x | x |
| B: Recommendations for equine therapy treatment parameters | |
|---|---|
| Characteristics | Recommendations for Clinicians |
| Duration | 30–60 minutes per session |
| Frequency | 1–2 sessions per week |
| Time | 3 to 6 months (1 month minimum) |
| Type | Therapeutic horseback riding or hippotherapy |
| Setting | Horse barn and nearby trails and/or therapy room for carryover activities |
| Environment | Outdoor and indoor environments |
| Providers | Certified riding instructors or OT/PT/SLP clinicians with hippotherapy certification |
| Assistants | 2 side walkers, therapist/instructor could be horse leader. |
| Components |
|
Treatment effects
Out of the 15 studies, 6 reported only within-group effects, 2 reported only between-group effects, and 7 studies reported both between- and within-group effects (see Table 2C). Although the critical first step in this literature is to assess if equine therapy is a feasible and useful treatment modality for individuals with ASD, equally if not more important is the accurate estimation of the size of this treatment effect, if present and its classification as small, medium, or large according to standard conventions (Cohen, 1988). Effect sizes (ES) and their confidence intervals (CI) are required to understand the clinical meaningfulness of research findings (Page, 2014; Sullivan & Fein, 2012). Interestingly, only 6 studies in our review reported ES for some of the outcomes (see Table 2B). Even out of these 6 studies, Wuang et al. (2010) did not provide exact values of between-group ES. Similarly, although Bass, Duchowny, & Llabre (2009) conducted a between-group study, they only reported within-group ES (see Table 2B). Therefore, wherever possible, we calculated ES and their 95% CI using data reported in the original studies. Accordingly, we could calculate ES for 6 more studies that did not provide these estimates in the original report (see Table 2B). However, we could not calculate ES for the 3 remaining studies due to insufficient data provided in the published report (Llambias et al., 2016; Jenkins et al., 2013; Steiner et al., 2012). Comparisons between the calculated and reported ES are provided in Table 2B. We have also provided 95% CI ranges for calculated ES and details of the number of calculated ES per study where the 95% CI does not include 0 (implying a truly significant non-zero treatment effect at 5% significance level) (see Table 2B). Next, we summarize the salient findings of this review in a domain-wise manner.
Social communication skills
This domain was most frequently assessed in equine therapy studies, with 9 out of the 11 studies that assessed social communication outcomes reporting improvements in skills following equine interventions (Table 2C). Several studies employed multiple measures to evaluate therapy-related changes in skills – 8 studies used generic standardized developmental tests or questionnaires (such as Childhood Autism Rating Scale (CARS), Vineland Adaptive Behavior Scales (VABS) etc.), 3 studies used measures specific to social communication skills (such as Social Responsiveness Scale (SRS), Peabody Picture Vocabulary Test (PPVT), etc.), and 2 studies used video coding to code for relevant behaviors during training sessions (see Tables 2A & 2C). In terms of ES, there was considerable variability in the reported/calculated estimates across studies (see Table 2C). However, the 5 methodologically strongest Level I and II studies in this group as identified using Sackett’s classification system and PEDro scores suggested that equine therapies have small to medium size effects on social communication skills (Gabriels et al., 2012, 2015; Bass et al., 2009, Borgi et al., 2016, Steiner et al., 2012). For instance, Gabriels et al. (2015) reported that a 10 week intervention of THR in individuals with ASD led to improvements in the social cognition (ES = 0.41) and social communication (ES = 0.63) sub-scales of the SRS as well as in the total number of different and new words spoken (ES = 0.54) as assessed using the SALT test. Along the same lines, Bass et al. (2009) who also used the SRS scale, reported improvements in the overall scores (ES = 0.45) and the social motivation subscale (ES = 0.38) following a 12-week THR intervention. In contrast, Borgi et al. (2016) reported modest between-group improvements on the socialization sub-domain (ES = 0.14) of the VABS following a 6-month THR intervention. Overall, out of the 21 ES estimates we calculated only 3 ES had CI that did not include 0 (Table 2C). Taken together, there is limited evidence supporting the use of equine therapies for facilitating social communication skills in individuals with ASD.
Behavioral skills
Out of the 7 studies that assessed the effects of equine therapies on behavioral skills including stereotyped/problem behaviors, affective responses, irritability and hyperactivity, and overall ability to regulate behaviors and moods, 5 studies found positive effects of equine-assisted activities (Table 2C). In terms of outcome measures, 4 studies used assessments that specifically evaluated behavioral skills such as the Childhood Behavior Checklist (CBCL) and the Aberrant Behavior Checklist (ABC), whereas only 2 studies relied solely on generic assessments such as the CARS to evaluate effects of equine therapies on behavioral skills in individuals with ASD (see Tables 2A & 2C). Although there was variability in ES estimates provided/calculated for these 7 studies, the 3 Level I & II studies in this review reported small to moderate effects of equine therapy on behaviors of children with ASD (Gabriels et al., 2015; Gabriels et al., 2012; Garcia-Gomez et al., 2014). Gabriels et al. (2012) found medium to large between-group effect sizes for improvements in the irritability (ES = 0.87), lethargy/social withdrawal (ES = 0.81), stereotypy (ES = 0.69), and hyperactivity (ES = 0.80) subscales of the ABC in a pilot study involving a 10-week THR intervention in 42 children and adolescents with ASD compared to a waitlist control group. In a more recent, well-controlled RCT of identical duration by the same group, they found more moderate but significant between-group improvements in the irritability (ES = 0.5) and hyperactivity (ES = 0.53) sub-domains of the ABC in 58 individuals with ASD (Gabriels et al., 2015). The third Level II study by Garcia-Gomez et al. (2014) used the teacher-rated behavioral questionnaire, Behavior Assessment System for Children (BASC), as the outcome measure and found small within-group improvements (ES = 0.22) in only the aggressiveness domain of the questionnaire following 3 months of THR training in 8 children with ASD. Our own calculations suggested that out of the 19 ES computed for behavioral skills, the CI of 11 ES did not include 0 (Table 2C). Overall, current literature provides modest evidence for the use of equine therapies to alleviate behavioral impairments in ASD.
Sensory skills
Sensorimotor skills are currently not considered a part of the cardinal diagnostic features of ASD; however, recently there is growing evidence for the presence of perceptuo-motor impairments across the lifespan in ASD (Green et al., 2009; Bhat et al., 2011; Bedford, Pickles, & Lord., 2015; Ben-Sasson et al., 2009; Baranek et al., 2005; Srinivasan et al., 2015). We found that 3 of the 4 studies that assessed sensory skills reported positive effects following therapy (see Table 2C). Three studies using parent/teacher-rated questionnaires (such as the Sensory Profile (SP) and the Sensory Profile School Companion (SPSC)), while the last study used a clinician-administered test called the Test of Sensory Integration Function (TSIF) (see Tables 2A & 2C). Out of the 4 studies, 2 were classified as Level I or II evidence (Bass et al., 2009; Wuang et al., 2010) whereas the remaining 2 studies provided Level III evidence (Kern et al., 2011; Ward et al., 2013). Although the same outcome measure was used by Bass et al. (2009) and Kern et al. (2011), the former study reported positive effects of 12-weeks of equine therapy whereas the latter found no changes in the SP following 24-weeks of equine therapy (see Table 2A & 2B). Furthermore, although the Bass et al. (2009) study was an RCT, the authors did not calculate between-group ES (see Table 2B). Our own calculations suggested that ES varied from small to large across the different subscales of the SRS: overall score (ES = 0.46), sensory seeking (ES = 0.39), attention and distractibility (ES = 0.85), sensory sensitivity (ES = 0.48), and sedentary (ES = 0.59). In the Wuang et al. (2010) study, the authors employed a cross-over design and found that a 20-week intervention involving simulated horseback riding led to large-sized improvements on all the subscales of the TSIF; our calculated between-group effect sizes (ES range = 1.78–5.29) corroborate the authors’ claims. Out of the total 56 ES calculated from data presented in the 4 studies, 42 ES were significant (CI did not include 0, see Table 2C). Overall, the review suggests promising positive (small to large sized) effects of equine therapies on sensory skills in ASD.
Motor skills
Four out of the seven studies that assessed gross and fine motor skills suggested positive effects following equine therapy (see Table 2C). To evaluate motor skills, 4 studies used standardized tests (such as the Bruininks Oseretsky Test of Motor Proficiency (BOT), the Sensory Integration and Praxis Tests (SIPT), and the motor subscale of the VABS), 1 study used force plates to assess postural sway during quiet stance (Ajzenman et al., 2013), 1 study used kinematic measures to assess gait of subjects (Steiner et al., 2012), and 1 study used video-coding (Jenkins et al., 2013) to assess children’s ability to maintain upright posture while seated on a horse (see Tables 2A & 2C for details). Five of these 7 studies qualified as Level I or II evidence (see Tables 1B and 2C). Amongst the methodologically strong studies, 2 studies reported improved motor skills following equine intervention (Wuang et al., 2010; Steiner et al., 2012), whereas the remaining 3 did not find robust between-group differences in motor skills following equine therapy sessions (Gabriels et al., 2012, 2015; Borgi et al., 2016). Out of the studies that supported the use of equine-assisted activities in ASD, we could corroborate the findings of large ES as reported by Wuang et al. (2010) on the BOT test. In contrast, although Steiner et al. (2012) reported positive effects of THR on gait of subjects, they did not report actual ES or include data that would allow independent calculation of ES. Although both the remaining Level III and IV studies in this group suggested positive effects of equine therapy on motor skills, they either did not allow independent calculation of ES (Jenkins et al., 2013) or reported a wide range of ES across dependent variables (Ajzenman et al., 2013). Although 43 of the 44 calculated ES in this set of studies reported ES that were significantly different from 0 at a 95% CI, note that the Wuang study alone contributed to around 40 ES estimates (Table 2C). Taken together, presently there is only weak evidence for positive treatment effects (varying in magnitude from small to large) of equine therapy on motor skills.
Functional participation and quality of life measures
Four studies (2 Level II and 2 Level III evidence) evaluated changes in children’s functional participation and quality of life (QOL) following short-term equine therapy interventions (see Table 2C for details). In terms of functional participation, following a 3-month HIP intervention, children increased their participation (ES range: 0.62–0.81) in age-appropriate leisure and self-care activities on the Child Activity Card Sort test (Ajzenman et al., 2011) (Note: our ES estimates based on data from the report are more conservative and CI of ES include 0, see Tables 2B & 2C). In terms of QOL, Lanning et al. (2014) and Kern et al. (2011) used standardized measures such as the Pediatric Quality of Life Generic Core Scales (PedsQL), the Child Health Questionnaire (CHQ), and the General Activities Subscale of the Quality of Life Enjoyment and Satisfaction Questionnaire (QOL ESS), whereas Garcia-Gomez et al. (2014) developed a custom questionnaire to assess QOL. While large improvements (ES range = 2.05 – 2.43) were found on the custom-developed QOL questionnaire following a 3-month THR intervention, the other two studies reported lack of definitive improvements in QOL of subjects due to the equine interventions provided (Lanning et al., 2014; Kern et al., 2011). Moreover, out of the total 7 ES calculated from these studies, the CI of only 2 ES did not include 0 (Table 2C). It may well be the case that the relatively limited duration of interventions (12–24 weeks) provided by some of the studies in this group (Lanning et al., 2014; Kern et al., 2011) may have been insufficient to produce substantial changes in the QOL of the participants. To summarize, the current state of literature in this field does not allow us to comment on the effect of equine therapy on QOL and functional participation of individuals with ASD.
Other skills
Only 1 Level I study (Borgi et al., 2016) assessed the effects of a 6-month equine therapy intervention on cognitive skills, specifically executive functioning and found that compared to a waitlist control group children with ASD reduced the latency of their first move (ES = 0.76, but CI includes 0) during a problem solving task following THR. Another study that assessed the effect of HIP on salivary cortisol and progesterone levels as a proxy for social skills, found large reductions in cortisol levels (ES range = 0.96 – 2.09) and small to large increases in progesterone levels (ES range = 0.33–1.59) in 8 children with ASD (Tabares et al., 2012). However, we caution readers while interpreting the results of this study since it was methodologically poor (Level III) and had inadequate reporting standards.
Long-term effects of equine therapies
The utility of any therapy depends not just on its immediate effects, but also more importantly on its long-term carryover effects and the ability to generalize learned skills to novel contexts. Interestingly, only 4 studies in this review assessed long-term effects of equine therapy and the generalization of treatment effects to settings not involving horses (Wuang et al., 2010; Llambias et al., 2016; Steiner et al., 2012; Ward et al., 2013). Their results are mixed. Ward et al. (2013) and Steiner et al. (2012) found that although there were short-term benefits following equine therapy, children’s behaviors returned to almost baseline levels following withdrawal of the intervention. On the other hand, Wuang et al. (2010) and Llambias et al. (2016) found sustained improvements in behaviors even after the completion of the equine therapy intervention. Overall, the conflicting evidence on long-term effects of equine-assisted therapies limits our ability to draw definitive conclusions regarding the sustenance of treatment effects following equine interventions in ASD.
Intervention Characteristics
The studies included in this review assessed the effects of relatively short-term equine-assisted interventions in children with ASD (Table 3B). The mean duration in weeks of the delivered intervention was 12.67 (SD = 6.47 weeks, range = 4–25 weeks). Almost all studies provided sessions at a frequency of one session per week, with the exception of 2 studies that provided therapy twice a week (García-Gómez et al. 2014; Wuang et al. 2010). The median session duration was 60 minutes (N = 9), with an average time of 61 minutes (SD = 34.7 min, range: 30–180 min). The average number of sessions in the intervention was 14.8 (SD = 9.8 sessions, range: 4–40 sessions).
Fourteen out of the 15 studies employed live horses for the purpose of intervention, whereas 1 study used innovative exercise equipment, Joba® that provided a simulated horse riding experience (Wuang et al., 2010). Out of the studies that used live horses, 11 provided therapeutic horseback riding (THR) treatment whereas the remaining 3 studies used hippotherapy (HIP) (see Table 1B). Six out of the 11 THR studies and 2 out of the 3 HIP studies explicitly stated that the delivered intervention adhered to standard guidelines for equine-assisted activities as laid down by national-level governing organizations such as the Professional Association of Therapeutic Horsemanship (PATH) International, the Spanish Equestrian Federation, Italian Equestrian Federation, or the American Hippotherapy Association (see Table 1A for details). In terms of certification of instructors, 8 out of the 11 THR interventions and all 3 HIP studies stated that riding instructors were certified by national-level horsemanship organizations (see Table 1A for details). Out of the 14 studies that used live horses for therapy, 10 studies reported using assistants (trained personnel or parents) to guide/help children during therapy, 2 studies did not use assistants (Borgi et al., 2016; Llambias et al., 2016), and the remaining 2 studies provided no details on support staff (Tabares et al., 2012; Steiner et al., 2012). However, only 2 studies that used assistants explicitly reported training them using an internally developed program specific to the riding facility (Anderson & Meints, 2016; Gabriels et al., 2012).
Studies that provided THR typically focused on (1) horsemanship skills that aimed at developing the child’s bond with their horse, and (2) riding skills targeted towards enhancing children’s motor, social communication, emotional, and cognitive well-being (Table 3B). Horsemanship skills involved grooming, feeding, taking care of, and leading horses. Riding skills include training children to mount and dismount from their horses, followed by more complex skills including walking, trotting, halting, steering, turning, and walking their horse around cones. In addition, a few studies (Bass et al., 2009; Ward et al., 2013; Borgi et al., 2016; Gabriels et al., 2012) also included horse-mounted games such as “Simon says”, catch and throw, dropping a ring on a pole, ball and cup games, etc. that focused on training desired skills within an enjoyable and interactive context. Typically, THR sessions started with some warm up exercises and activities to prepare the horse, followed by riding exercises/games on the horse, and finally leading the horse back to the stables and bidding farewell to the horse and the instructors. Amongst the HIP studies, Tabares et al. (2012) structured their therapy similar to a THR session. In contrast, Llambias et al. (2016) and Ajzenman et al. (2013) studies involved certified OTs using horses as an integral part of their therapy session to improve children’s balance, postural control, motor planning and sequencing, fine motor, sensory regulation, and attentional skills. For instance, subjects were encouraged to maintain balance in different positions as the horse was led through different paths or participants practiced functional and social skills through games involving obstacle courses, ball catching, carrying objects, and grabbing and dropping rings (Llambias et al., 2016 and Ajzenman et al., 2013). The only study that provided simulated riding experience using exercise equipment focused on training the child to ride on the horse simulator in seated and lying down positions and also engaging the child in games meant to facilitate their cognitive, affective, and social skills (Wuang et al., 2010).
Out of the 14 studies, in 6 studies the intervention was tailored to the individual skills/needs of the child (Wuang et al., 2010; Gabriels et al., 2012; Jenkins et al., 2013; Lanning et al., 2014; Anderson & Meints, 2016; Llambias et al., 2016). For instance, the specific activities chosen or the format of the intervention (individual or group therapy) were tailored to the interests and abilities of the child. Finally, only 4 studies reported on procedures for ensuring treatment implementation fidelity across training sessions for study subjects (Gabriels et al. 2015; Jenkins et al. 2013; Llambias et al. 2016; Wuang et al. 2010).
Discussion
Summary of Results
In the present review, we aimed to assess the quality and quantity of evidence in support of equine therapies as an adjunct therapy tool for individuals with ASD. While past reviews have provided a qualitative summary of the literature in this field, ours is the first review to report on the actual size of the treatment effects by calculating effect size estimates and their 95% CI wherever possible from data presented in individual study reports. We believe that a fair judgment of the merits of equine therapy as a treatment tool for ASD require consideration of the methodological quality of studies (PEDro rating and Sackett’s level of evidence) in conjunction with the magnitude and direction of treatment effects on different outcomes.
Our review was based on 15 experimental and quasi-experimental studies that assessed the impact of THR and HIP interventions in 294 children and adolescents with ASD. In terms of methodological quality (Sackett et al., 1997), a little over half (8 out of 15) of these studies offered Level I or II evidence, whereas the remaining qualified as Level III or IV evidence. Eight of the studies designed their interventions by adhering to standard guidelines offered by national or international equine therapy certification agencies. Although most of the studies provided an adequate description of the study procedures including the setting of the study, personnel involved, activities conducted, etc. it was surprising that only about a quarter of the studies actually assessed treatment fidelity/integrity across training weeks. Similarly, only 4 of the studies assessed long term benefits or generalization of training following short-term equine experiences in individuals with ASD.
Equine therapy by its very nature has the potential to impact multiple subsystems. Accordingly, we found that most studies assessed the effects of equine therapies on multiple skills in ASD. As is clear from Table 2C, maximum work so far has been dedicated towards evaluating equine therapy effects on social communication, behavioral, and motor outcomes. Going by dual criteria of amount of positive evidence (# of studies demonstrating gains and # of significant nonzero ES) and strength of positive evidence (level I or II studies), our review suggests consistent and reliable positive effects of short-term equine therapies behavioral skills in ASD. Although similar promising evidence is emerging with respect to the utility of equine therapies in enhancing social communication, sensory and perceptuo-motor skills in ASD, given the small number of studies (of high quality) in this area and somewhat conflicting results across studies using the same outcome measures (for example, Bass et al., 2009 and Kern et al., 2011 for the Sensory Profile), we see a clear need for rigorous research in these domains. Moreover, in case of sensory-motor skills, currently, the literature seems biased as a result of the few studies that calculated multiple ES (Wuang et al., 2010; Ward et al., 2013). Lastly, the current state of evidence on equine therapies does not allow us to make any claims with respect to the effects of these therapies on cognitive skills, physiological variables, functional skills or QOL of individuals with ASD.
Clinical Implications
In this section, based on our review, we provide recommendations pertaining to assessment and treatment, for clinicians working with children and adolescents with ASD. In terms of assessment measures, a majority of studies used caregiver-rated questionnaires followed by standardized tests to assess dependent variables (Table 3A). In a related vein, some studies used broad assessment tools that evaluated treatment effects on multiple developmental domains, whereas others used tools specific to the domain assessed. The choice of instruments typically depends on multiple factors such as participants’ functional skills including their ability to comprehend and comply with instructions, their ability to imitate others, their motor and intellectual skill levels, etc. Nevertheless, we recommend that wherever possible, clinicians use a combination of qualitative and quantitative tools (parent questionnaires, standardized tests, video coding) to assess the impact of equine-assisted activities in ASD. Similarly, by using both domain-general and domain-specific measures, clinicians will be able to gauge impact of the intervention on the overall development of the child as well as detect subtle and more-nuanced treatment effects within specific domains (see Table 2C for details).
In terms of treatment, our review suggests that in order to obtain appreciable behavioral changes in individuals with ASD, equine-based interventions should be provided for at least 1 month; although more robust improvements are seen only if the equine activities are continued for at least 3 to 6 months. A longer duration of intervention provides the individual sufficient time to get accustomed to the horse, overcome the anxiety associated with novel activities, and build a rapport with the horse. Interventions were mostly provided at the frequency of 1 session/week (with few studies providing 2 sessions/week) with each session ranging from 30–60 minutes in duration (see Table 3B). Therapeutic activities practiced should focus on horsemanship skills, riding skills, as well as games and group activities that focus on addressing the core social communication and perceptuo-motor impairments in ASD. Given the novelty and dynamic nature of the activities involved, clinicians may need 1 to 3 trained volunteers to assist participants with mounting-dismounting, guiding, and steering the horse, and also provide cues and prompts as needed during the session. Since ASD is a spectrum disorder and the challenges faced by individuals with this diagnosis varies significantly, we recommend that clinicians tailor the intervention to the specific needs of the individual, including the choice of activities, level of support provided, individual or group nature of the training, amount of practice provided, and the progression of activities during training.
Limitations and future directions for research
Although we demonstrated preliminary support for the utility of equine therapy in ASD, our review highlights several lacunae in this literature that need to be addressed in future studies. There is a clear need for large sample size studies that employ narrow and clear inclusion criteria to study the impact of equine therapies on homogenous samples of individuals with ASD in terms of age range, diagnosis, adaptive behavior, and functional skills. Moreover, future studies need to ensure methodological rigor by using standardized tests for confirming participant diagnosis, employing well-matched control groups, random assignment of subjects, blinding of assessors, and strict controls on treatment fidelity. In order to strongly establish the utility of equine therapy in the treatment of ASD, more studies need to assess the short term and long term effects of equine therapy (THR and HIP) compared to conventional modes of therapy on multiple subsystems (social communication, sensory-motor, behavioral, and cognitive) and functional skills in individuals with ASD across the lifespan.
In the current review, we attempted to go beyond providing a qualitative summary of the current equine therapy literature by calculating quantitative ES estimates for each skill domain. We found that several studies did not report ES appropriate to their study design and also did not provide enough data to enable us to independently calculate ES. For some other studies that allowed independent ES estimation, there were conflicts between reported and calculated ES. Future studies should ensure adequate reporting of ES along with their 95% or 99% CI or at least provide necessary data to allow estimation of ES and their CI. Such reporting standards would enable a quantitative synthesis of the equine therapy literature in the form of a meta-analysis. A meta-analysis would be able to provide an aggregate estimate of the treatment effect in ASD and systematically account for the large heterogeneity in effect sizes (see Table 2C) using moderator analyses.
Conclusions
Our literature review aimed at summarizing the evidence to date on the effects of equine-assisted activities and therapies including both THR and HIP in individuals with ASD. We searched several relevant health-related databases and finally selected and reviewed 15 articles assessing the impact of equine therapies on several different outcomes in children and adolescents with ASD. Although there was considerable variability between studies in terms of sample characteristics, intervention characteristics, outcomes and assessment tools used, as well as the magnitude and direction of treatment effects reported for different types of outcomes, our combined qualitative and quantitative analysis suggested promising immediate effects of short-term equine therapy interventions on behavioral skills in ASD. There is currently some limited evidence supporting the utility of equine therapies in enhancing social communication and perceptuo-motor in individuals with ASD. The effects of equine-assisted activities on cognitive and functional skills/QOL of individuals with ASD are at present poorly researched. Future studies should employ methodologically rigorous study designs with large sample sizes to evaluate the role of equine therapies in the standard-of-care treatment of individuals with ASD.
Acknowledgments
Funding:
This work was supported by the Leadership Education in Neurodevelopmental and Related Disabilities (LEND) grant from the Maternal & Child Health Bureau (MCHB) of the Health Resources and Service Administration (HRSA) under grant number T73MC30116 (PI: Beth Mineo). This work was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod).
Appendix 1: Search terms for databases
(“hippotherapy” OR “horse-riding” OR “therapeutic horseback riding” OR “horseback riding” OR “therapeutic riding” OR “equine therapy” OR “pet therapy” OR “animal-assisted” OR “equine-assisted” OR “horse therapy” OR “equine movement” OR “equine facilitated therapy” OR “equine-assisted therapy”[MeSH Terms] OR “animal-assisted therapy”[MeSH Terms])
AND (“Autistic Disorder”[MeSH Terms] OR “autism” OR “autistic” OR “autism spectrum disorder” OR “ASD” OR “ASDs”)
Appendix 2: Final coding sheet per study
Coding Form: Effects of Equine Therapy on Individuals with Autism Spectrum Disorder: A
Systematic Review
Coder: ___________________
Date of coding: ______________________
Study characteristics
Study identifier (one string): 1st three letters of the 1st author, publication year, 01, 02.. __________
Author Names: ________________________________________________________
Year of publication: __________________
-
Full citation of publication (APA style): ___________________________________
_________________________________________________
_________________________________________________
_________________________________________________
Source of study : ________ (1= journal, 2 = conference paper)
Location of study: ________________
Year of data collection _______________
Sample characteristics
Total sample size: ___________
Age of subjects: _____________ (Mean (SD) and range)
Diagnosis of subjects: ____________________________ (ASD, AD, PDD-NOS, PDD)
-
Measures used to establish diagnosis:
(0 = standardized tests used, 1 = unstandardized observational measure, 2 = parent report/questionnaire)
_________________________________________________
_________________________________________________
_________________________________________________
Gender of subjects (# of males & females): _____________________________________
Type of subjects included (High/Moderate/Low functioning) OR (Verbal - phrases or more), Low-verbal (few words), Non-verbal (no words)): ______________________________
IQ of subjects: ____________________ (Mean (SD) and range)
-
Final # of subjects who participated in the study (Post attrition):
_________________________________________________
-
Socioeconomic status:
_________________________________________________
-
Ethnicity:
_________________________________________________
-
Inclusion criteria:
_________________________________________________
-
Exclusion criteria:
_________________________________________________
Methodological quality of studies
-
Study design:
_________________________________________________
_________________________________________________
________ (RCT/CCT/Pre-post designs, cross over designs, single-subject design, single-subject reversal designs)
-
Study design (1 = Between-subjects design, 2 = Within-subjects design):
_________________________________________________
-
PEDro score for methodological quality (for RCTs and CCTs only):
______________________
Rating of 0–10. See pdf.
-
Type of control group used:
_________________________________________________
1 = random assignment of individuals
2 = matching individuals on some variable (specify variable: _____________________) and then random assignment
3 = tried to ensure some comparability of the non-equivalent control group by for example: matching on some variable.
_________________________________________________
4 = non-equivalence of comparison group was not addressed
5 = no control group (pre-post design)
Follow-up (1= present, 2 = absent & # ): ______________________________
Intervals between follow ups: _______________________
Checks on treatment fidelity/integrity:
Experimental group characteristics
# of participants: ________________________
Total # who completed the study (after attrition): ____________________
Gender (# of males & females) : ___________________
Frequency of intervention: ___________________________
Time of intervention (per session): _____________________
Duration of intervention (days/weeks/months): ______________________
-
Type of intervention: ___________________________________
(1= Hippotherapy, 2 = Therapeutic Horseback Riding, 3 = Other type)
-
Components of intervention (all that apply):
____Horseback Riding ___Horsemanship (grooming, feeding, returning to stable)
Intervention design (Individual sessions/group therapy) : ___________________
-
Intervention provider (all that apply):
OT/PT___ AHTA Licensed ___ Equine Expertise___Other (Specify training of provider): _______
Previous riding experience of subjects in years and months: ________________
Pre-requisites of intervention: _________________ (what is this, barn, safety equipment, etc??)
-
Other therapies subjects were receiving at the same time (specify): Along with details of Frequency, Intensity, Time, and Type - Provide details of when the child is receiving this therapy
_________________________________________________
_________________________________________________
Control group characteristics
# of control groups: _________________
-
# of participants in each control group:
_________________________________________________
-
Total # who completed the study (after attrition) in each control group:
_________________________________________________
Gender (# of males & females) in each control group: __________________________
Frequency of intervention: ___________________________________________
Time of intervention (per session): _______________________________________
Duration of intervention (days/weeks/months): _____________________________
-
Type of intervention in control groups:
_________________________________________________
_________________________________________________
Other interventions that the control group is receiving if any (if mentioned in the paper)
Dependent variables
-
Type of dependent
variable: _________________________________________________
(1 = Social measures example: joint attention behaviors
2 = Communication skills such as verbal and non-verbal skills (gestures))
3 = Behavioral problems – aggression, self-injurious behaviors
4 = Sensory problems like hyper-responsiveness to sensory stimuli
5 = Motor behaviors like repetitive behaviors, stereotypy, balance, coordination
6 = Quality of life outcomes
7 = Self-care skills and activities of daily living
8 = Cognitive skills (some studies have looked at executive function)
9 = Hormonal changes
10 = Others (specify ______________________________ )
-
Variables analyzed (Duration or Rate or Frequency of something)
_________________________________________________
_________________________________________________
-
Instrument/s used for assessment of dependent variable (if multiple DVs mention all different scales used):
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
-
Type of scale/measure used for assessing dependent variables:
(1 = Standardized scale, 2 = observational measure, 3 = video coding, 4 = questionnaires, 5 = other
(specify ________________________________________________)
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
-
Reliability of assessments used:
_________________________________________________
-
Time point of measurement of dependent variables:
_________________________________________________
-
# of dependent variables for which effect sizes will be obtained from the study:
_________________________________________________
-
Direction of effect (1 = positive, 2 = negative, 3 = no effect) per DV:
_________________________________________________
_________________________________________________
_________________________________________________
-
Type of statistic used per DV:
_________________________________________________
_________________________________________________
_________________________________________________
5=Effect Sizes using eta-squared, Cohen’s d, other effect size statistic
4=ANOVA
3=t-test
2=chi-square tests to compare ordinal/nominal scores of change with training
1=correlations for reliability of measures.
Note: additive scores can be given if more than one method was used.
Footnotes
Conflict of Interest Statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Achenbach TM, Rescorla LA. Child behavior checklist for ages 6 to 18. Burlington: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Ajzenman HF, Standeven JW, Shurtleff TL. Effect of hippotherapy on motor control, adaptive behaviors, and participation in children with autism spectrum disorder: a pilot study. American Journal of Occupational Therapy. 2013;67(6):653–63. doi: 10.5014/ajot.2013.008383. [DOI] [PubMed] [Google Scholar]
- American Hippotherapy Association. [Accessed June 16, 2017];Hippotherapy as a treatment. Available at: http://www.americanhippotherapyassociation.org/hippotherapy/hippotherapy-as-a-treatment-strategy/
- Anderson S, Meints K. Brief Report: The Effects of Equine-Assisted Activities on the Social Functioning in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2016;46(10):3344–52. doi: 10.1007/s10803-016-2869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres AJ. Sensory integration and praxis tests. Los Angeles, CA: Western Psychological Services Clinic; 2007. [Google Scholar]
- Bass MM, Duchowny CA, Llabre MM. The effect of therapeutic horseback riding on social functioning in children with autism. Journal of Autism and Developmental Disorders. 2009;39(9):1261–7. doi: 10.1007/s10803-009-0734-3. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development. 3. San Antonio, TX: Harcourt assessment, Inc; 2006. [Google Scholar]
- Baranek G, Parham L, Bodfish J. Sensory and Motor Features in Autism: Assessment and Intervention. In: Volkmar FPR, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders. Hoboken, NJ: Wiley; 2005. pp. 831–857. [Google Scholar]
- Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research. 2016;9(9):993–1001. doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Bhat A, Landa R, Galloway JC. Perspectives on motor problems in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91(7):1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of Repetitive Behavior in Autism: Comparisons to Mental Retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/A:1005596502855. [DOI] [PubMed] [Google Scholar]
- Bondy A, Frost A. Communication Strategies for Visual Learners. In: Lovaas OI, editor. Teaching Individuals with Developmental Delays: Basic Intervention Techniques. Austin, TX: Pro-Ed; 2003. pp. 291–304. [Google Scholar]
- Borgi M, Loliva D, Cerino S, Chiarotti F, Venerosi A, Bramini M, Cirulli F. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2016;46(1):1–9. doi: 10.1007/s10803-015-2530-6. [DOI] [PubMed] [Google Scholar]
- Bruininks R, Bruininks B. Bruininks-Oseretsky Test of Motor Proficiency - Second Edition (BOT2) Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- Bruininks RH, Woodcock RW, Weatherman RF, Hill BK. Scales of independent behavior-Revised. Itasca, Illinois: Riverside publishing company; 1996. [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early Social Attention Impairments in Autism: Social Orienting, Joint Attention, and Attention to Distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, Crawford SG. Motor and Gestural Performance in Children with Autism Spectrum Disorders, Developmental Coordination Disorder, and Or Attention Deficit Hyperactivity Disorder. Journal of the International Neuropsychological Society. 2007;13:246–256. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Dunn W. The sensory profile school companion. San Antonio, TX: Pearson Publishing; 2006. [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4) Austin, TX: Pearson Assessments; 2007. [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla M, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. Journal of Autism and Developmental Disorders. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Pan Z, Dechant B, Agnew JA, Brim N, Mesibov G. Randomized Controlled Trial of Therapeutic Horseback Riding in Children and Adolescents With Autism Spectrum Disorder. Journal of American Academy Child Adolescent Psychiatry. 2015;54(7):541–9. doi: 10.1016/j.jaac.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gomez A, Risco ML, Rubio JC, Garcia-Pena IM. Effects of a program of adaptive therapeutic horse-riding in a group of ASD children. Electronic Journal of Research in Educational Psychology. 2014;12(1):107–128. [Google Scholar]
- Gilliam JE. The Gilliam autism rating scale. 2. Austin, TX: Pro-Ed, Inc; 2006. [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, Baird G. Impairment in Movement Skills of Children with Autistic Spectrum Disorders. Developmental Medicine and Child Neurology. 2009;51(4):311–316. doi: 10.1111/j.1469-8749.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of Autistic Adults. Archives of Neurology. 1993;50(12):1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6(2):107–128. [Google Scholar]
- Huedo-Medina TB, Johnson BT. Estimating the standardized mean difference effect size and its variance from different data sources: A spreadsheet. Storrs, CT, USA: Authors; 2011. [Google Scholar]
- Henderson SE, Sugden DA. Movement Assessment Battery for Children. London: Psychological Corporation; 1992. [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor Signs Distinguish Children with High Functioning Autism and Asperger’s Syndrome from Controls. Journal of Autism and Developmental Disorders. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jenkins SR, DiGennaro-Reed FD. An Experimental Analysis of the Effects of Therapeutic Horseback Riding on the Behavior of Children with Autism. Research in Autism Spectrum Disorders. 2013;7(6):721–740. doi: 10.1016/j.rasd.2013.02.008. [DOI] [Google Scholar]
- Kasari C, Paparella T, Freeman S, Jahromi LB. Language Outcome in Autism: Randomized Comparison of Joint Attention and Play Interventions. Journal of Consulting and Clinical Psychology. 2008;76(1):125–137. doi: 10.1037/0022-006X.76.1.125. [DOI] [PubMed] [Google Scholar]
- Kaur M, Gifford T, Marsh K, Bhat A. The effects of robot-child interactions on bilateral coordination skills of typically developing children and one child with autism between 4 to 7 years of age. Journal of Motor Learning and Development. 2013;1(2):31–37. doi: 10.1123/jmld.1.2.31. [DOI] [Google Scholar]
- Kern JK, Fletcher CR, Garver CR, Mehta JA, Grannemann BD, Knox KR, Trivedi MH. Prospective trial of equine-assisted activities in autism spectrum disorder. Alternative Therapies. 2011;17(3):14–20. http://www.ncbinlm.nih.gov/pubmed/22164808. [PubMed] [Google Scholar]
- Lanning BA, Baier ME, Ivey-Hatz J, Krenek N, Tubbs JD. Effects of equine assisted activities on autism spectrum disorder. Journal of Autism and Developmental Disorder. 2014;44(8):1897–907. doi: 10.1007/s10803-014-2062-5. [DOI] [PubMed] [Google Scholar]
- Llambias C, Magill-Evans J, Smith V, Warren S. Equine-Assisted Occupational Therapy: Increasing Engagement for Children with Autism Spectrum Disorder. American Journal of Occupational Therapy. 2016;70(6):7006220040p1–p9. doi: 10.5014/ajot.2016.020701. [DOI] [PubMed] [Google Scholar]
- Leary MR, Hill DA. Moving on: Autism and Movement Disturbance. Mental Retardation. 1996;34:39–53. [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and Emotional Problems in Young People with Pervasive Developmental Disorders: Relative Prevalence, Effects of Subject Characteristics, and Empirical Classification. Journal of Autism and Developmental Disorders. 2006;(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lentini JA, Knox MS. Equine-Facilitated Psychotherapy with Children and Adolescents: An Update and Literature Review. Journal of Creativity in Mental Health. 2015;10:278–305. doi: 10.1080/15401383.2015.1023916. [DOI] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, Calif: Sage publications; 2001. [Google Scholar]
- Lin JK. Test of Sensory Integration Function Manual. Taipei, Taiwan: Psychological Corporation; 2004. [Google Scholar]
- Loh A, Soman T, Brian J, Bryson SE, Roberts W, Szatmari P, Smith IM, Zwaigenbaum L. Stereotyped Motor Behaviors Associated with Autism in High-Risk Infants: A Pilot Videotape Analysis of a Sibling Sample. Journal of Autism and Developmental Disorders. 2007;37(1):25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule. second. Torrance, CA: Western Psychological Services; 2012. (ADOS-2) manual (part 1): Modules 1–4. [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lovaas OI. Behavioral Treatment and Normal Educational and Intellectual Functioning in Young Autistic Children. Journal of Consulting and Clinical Psychology. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Lord C, Ulrich DA. Motor skills and calibrated autism severity in young children with autism spectrum disorder. Adapted Physical Activity Quarterly. 2014;31(2):95–105. doi: 10.1123/apaq.2013-0068. [DOI] [PubMed] [Google Scholar]
- McDaniel P, Wood W. Autism and Equine-Assisted Interventions: A Systematic Mapping Review. J ournal of Autism and Developmental Disorders. 2017;47(10):3220–3242. doi: 10.1007/s10803-017-3219-9. [DOI] [PubMed] [Google Scholar]
- Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the Physiotehrapy Evidence Database (PEDro) Australian Journal of Physiotherapy. 2003;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Pelphrey KA, Dahl RE. The Need for a Broader Approach to Emotion Regulation Research in Autism. Child Development Perspectives. 2012;6:92–97. doi: 10.1111/j.1750-8606.2011.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov GB, Shea V, Schopler E. The TEACCH Approach to Autism Spectrum Disorders Springer 2004 [Google Scholar]
- Miller JF, Chapman RS. SALT: A computer program for the Systematic Analysis of Language Transcripts. Madison, WI: University of Wisconsin; 2000. [Google Scholar]
- Minshew NJ, Sung KB, Jones BL, Furman JM. Underdevelopment of the Postural Control System in Autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental Dyspraxia is Not Limited to Imitation in Children with Autism Spectrum Disorders. Journal of the International Neuropsychological Society. 2006;12(3):314–326. doi: 10.1017/S1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Mundy P, Newell L. Attention, joint attention, and social cognition. Current Directions in Psychological Science. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME. Animal-assisted intervention for autism spectrum disorder: a systematic literature review. Journal of Autism and Developmental Disorders. 2013;43(7):1606–22. doi: 10.1007/s10803-012-1707-5. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive Function Deficits in high-functioning Autistic Individuals: Relationship to Theory of Mind. Journal of Child Psychology and Psychiatry. 2006;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- PATH International. [Accessed June 16, 2017];PATH International home page. 2011. 2017 Available at: http://www.pathintl.org/
- Pierce K, Schreibman L. Increasing Complex Social Behaviors in Children with Autism: Effects of Peer-Implemented Pivotal Response Training. Journal of Applied Behavior Analysis. 1995;28(3):285. doi: 10.1901/jaba.1995.28-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett D, Rosenberg J, Muir G, Haynes B, Richardson W. Evidence based medicine: what it is and what it isn’t. British Medical Journal. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVillis RF, Daly K. Toward Objective Classification of Childhood Autism: Childhood Autism Rating Scale (CARS) Journal of Autism and Developmental Disorders. 1980;10(1):91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Srinivasan S, Kaur M, Park I, Gifford T, Marsh K, Bhat A. The Effects of Rhythm and Robotic Interventions on the Imitation/Praxis, Interpersonal Synchrony, and Motor Performance of Children with Autism Spectrum Disorder (ASD): A Pilot Randomized Controlled Trial. Autism Research and Treatment. 2015;2015:736516, 18. doi: 10.1155/2015/736516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Kertesz Z. Effects of therapeutic horse riding on gait cycle parameters and some aspects of behavior of children with autism. Acta Physiologica Hungarica. 2015;102(3):324–35. doi: 10.1556/036.102.2015.3.10. [DOI] [PubMed] [Google Scholar]
- Squires J, et al. Early detection of developmental problems: Strategies for monitoring young children in the practice setting. Journal of Developmental and Behavioral Pediatrics. 1996;17(6):420–427. doi: 10.1097/00004703-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Tabares C, Vicente F, Sanchez S, Aparicioc S, Alejoc S, Cuberob J. Quantification of hormonal changes by effects of hippotherapy in the autistic population. Clinical Neurochemistry. Neurochemical Journal. 2012;6(4):311–316. [Google Scholar]
- Tager-Flusberg H. A Psychological Approach to Understanding the Social and Language Impairments in Autism. International Review of Psychiatry. 1999;11(4):325–334. doi: 10.1080/09540269974203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory Processing in Children with and without Autism: A Comparative Study using the Short Sensory Profile. The American Journal of Occupational Therapy. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Damasio AR, Maurer RG. Gait Disturbances in Patients with Autistic Behavior: A Preliminary Study. Archives of Neurology. 1981;38(10):646–649. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Sparrow SS, Goudreau D, Cicchetti DV. Social deficits in autism: An operational approach using the vineland adaptive behavior scales. Journal of the American Academy of Child & Adolescent Psychiatry. 1987;26(2):156–161. doi: 10.1097/00004583-198703000-00005. [DOI] [PubMed] [Google Scholar]
- Ward SC, Whalon K, Rusnak K, Wendell K, Paschall N. The association between therapeutic horseback riding and the social communication and sensory reactions of children with autism. Journal of Autism and Developmental Disorders. 2013;43(9):2190–8. doi: 10.1007/s10803-013-1773-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. New York: Psychological Corporation; 1949. [Google Scholar]
- Weise C, Simpson R, Kumar S. The Effectiveness of Equine-Based Therapy in the Treatment of Social and Behavioural Aspects of Children with Autism Spectrum Disorder: A Systematic Review. Internet Journal of Allied Health Sciences and Practice. 2016;14(3):12. [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. The Profile of Memory Function in Children with Autism. Neuropsychology. 2006;20(1):21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig E, Semel E, Secord W. Clinical Evaluation of Language Fundamentals –Fifth Edition (CELF-5) Pearson Publications; 2013. [Google Scholar]
- Wuang YP, Wang CC, Huang MH, Su CY. The effectiveness of simulated developmental horse-riding program in children with autism. Adapted Physical Activity Quarterly. 2010;27(2):113–26. doi: 10.1123/apaq.27.2.113. [DOI] [PubMed] [Google Scholar]