Abstract
Nitric oxide (NO)-releasing chitosan oligosaccharides were modified with ester functional groups to examine how the mucoadhesive nature of the scaffold impacts the ability of NO to degrade mucins from human bronchial epithelial cell cultures and clinical sputum samples collected from patients with cystic fibrosis (CF). Agarose gel electrophoresis experiments indicated that the mucoadhesive NO-releasing chitosan oligosaccharides degraded both the purified mucins and sputum, while control scaffolds (without NO release or mucoadhesive ligands) had no effect on mucin structure. Microscopic observations of sputum treated with the mucoadhesive NO-releasing chitosan oligosaccharide confirmed degradation of the mucin and DNA networks. Similarly, the viscosity and elasticity of sputum were reduced upon treatment with the mucoadhesive NO-releasing chitosan, demonstrating the potential utility of these NO-releasing scaffolds as mucolytic agents.
Keywords: mucolytic, nitric oxide, mucins, cystic fibrosis
Graphical abstract
INTRODUCTION
Cystic fibrosis (CF) is a life-limiting, genetically inherited disorder affecting roughly 30,000 people in the United States of America (US) and 70,000 people worldwide.1,2 A defect in the CF transmembrane conductance regulator protein results in the accumulation of mucus in and obstructs normal function of the lungs, liver, pancreas, gastrointestinal tract, and reproductive system.3 As mucus accumulates and thickens in the CF lungs, airway clearance is impaired, fostering bacteria colonization.4 This thickened mucus layer generates an environment that protects bacteria from immune cells and promotes biofilm formation.5 Chronic infections are accompanied by persistent inflammation and ultimately a decline in lung function.5 As a result, respiratory complications related to chronic bacterial infections are the leading cause of death for CF patients.6
Many CF therapeutics have focused on treating the symptoms of the disease as it progresses, specifically by combating bacterial infection and reducing mucus viscoelasticity and accumulation. We have previously reported on the bactericidal action of NO-releasing chitosan oligosaccharides against CF-relevant Pseudomonas aeruginosa in planktonic and biofilm-based cultures.7,8 The broad spectrum antibacterial action of NO makes this compound an attractive alternative to traditional antibiotics for which many bacteria have developed resistance. The next logical step in applying NO-releasing chitosan as a CF therapeutic involves understanding the effects of both NO and the chitosan backbone on the viscoelastic properties of mucus. In addition, the role of chitosan mucoadhesion on NO delivery must be investigated to understand the true potential of NO-releasing chitosan oligosaccharides as a CF treatment candidate.
As a result of favorable properties including mucoadhesivity,9,10 biodegradability,9,11 and low cytotoxicity,9 researchers have begun to consider the use of chitosan for pulmonary drug delivery applications. The study of chitosan’s mucoadhesive properties, in particular, has become an active area of research. Electrostatic interaction between chitosan’s positively charged amines and the negatively charged sialic acid residues of mucins9,12,13 is believed to enhance chitosan retention in the airways and therefore increase time of action.9,14 In fact, chitosan has been reported to facilitate the delivery and improve the efficacy of antibiotic-releasing particles to the lungs.15–19 On the other hand, mucoadhesion could potentially affect drug penetration into the mucus. Klinger-Strobel et al. described the benefits of modifying chitosan with polyethylene glycol (PEG)14 to facilitate better drug action on human cervicovaginal mucus,20,21 bacterial biofilms,22 and CF sputum.20–23 Herein, we modified chitosan oligosaccharides with functional groups that would control mucoadhesive properties while maintaining comparable NO-release characteristics to understand the effects of scaffold mucoadhesion on the mucolytic action of NO.
EXPERIMENTAL SECTION
Materials
Medium molecular weight chitosan (viscosity 200–800 cP), 2-methylaziridine, ethyl acrylate, tert-butyl acrylate, sulfopropyl acrylate potassium salt, sodium dodecyl sulfate (SDS), dithiothreitol (DTT), bovine serum albumin (BSA), and type II gastric pig mucin (GPM) were purchased from Sigma-Aldrich (St. Louis, MO). Nitric oxide gas was purchased from Praxair (Sanford, NC). Argon (Ar), NO calibration (26.85 ppm, balance N2), and nitrogen (N2) gases were purchased from Airgas National Welders (Durham, NC). Sodium methoxide was purchased from Acros Organics (Geel, Belgium).
Tris-acetate-EDTA (TAE) buffer (10×) was purchased from Mediatech, Inc. (Manassas, VA) and diluted 1:10 in distilled water prior to use. Saline-sodium citrate (SSC) buffer (20×) was purchased from Promega Corporation (Madison, WI) and diluted 1:5 to obtain 4× SSC buffer. Dulbecco’s phosphate buffered saline (DPBS, 1×) was purchase from Life Technologies (Carlsbad, CA). Powdered milk (Drink ‘n Mix) was purchased from Walmart (Durham, NC). Neutral buffered formalin (NBF, 10 vol %) was purchased from Sigma-Aldrich (St. Louis, MO). Phosphate buffer (10 mM, pH 6.5) and phosphate buffered saline (10 mM, pH 6.5) were prepared in-house using common laboratory salts and reagents.
Anti-MUC5B antibody (H-300) was purchased from Santa Cruz Biotechnology (Dallas, TX). Anti-MUC5AC antibody (45M1) was purchased from Abcam (Cambridge, MA). Secondary antibodies (IRDye 800CW Donkey anti-Mouse IgG and IRDye 680RD Donkey anti-Rabbit IgG) were purchased from LI-COR Biosciences (Lincoln, NE). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was purchased from Life Technologies (Carlsbad, CA).
Distilled water was purified using a Millipore Milli-Q UV Gradient A-10 system (Bedford, MA). All common laboratory salts and reagents were purchased from Fisher Scientific (Pittsburgh, PA). All materials were used without further purification unless specified otherwise.
Culture washings containing mucus were collected from primary human bronchial epithelial (HBE) cell cultures from a patient with CF as previously described.24 Briefly, primary cell cultures obtained from excess surgical tissue (UNC-Chapel Hill Tissue Core Facility) were grown on 0.5 mm pore-sized Millicell cell culture inserts (Millipore, Bedford, MA) in air–liquid interface media (UNC Chapel Hill Tissue Core Facility) for a minimum of 6 weeks until the cultures developed cilia and well-defined periciliary liquid (PCL) and mucus layers. Washings were collected by adding 150 μL of PBS per 1 cm2 of culture area after 2 d of mucus accumulation. Subsequently, these washings were treated immediately with chitosan oligosaccharides for analysis.
Sputum samples were collected from CF patients by spontaneous expectoration. The samples were stored in sterile containers at −20 °C until use.
Synthesis of 2-Methylaziridine Modified Chitosan Oligosaccharides
Polymeric chitosan was oxidatively degraded into chitosan oligosaccharides as described previously.25 Briefly, medium molecular weight chitosan (2.5 g) was dissolved in 15 wt % hydrogen peroxide (50 mL) and stirred at 85 °C for 1 h. Insoluble, nondegraded chitosan was removed by filtration. Water-soluble oligosaccharides were collected via precipitation in acetone, washed copiously with ethanol, and dried in vacuo. A Ubbelohde viscometer was used to measure the viscosity of the chitosan oligosaccharides in a solution of sodium chloride (0.20 M) and acetic acid (0.10 M) at 25 °C. The molecular weight was calculated to be 4.41 ± 0.04 kDa using the classic Mark–Houwink equation (η = 1.81 × 10−3 M0.93).26
The water-soluble chitosan oligosaccharides were then modified with 2-methylaziridine (Scheme 1). Chitosan oligosaccharides (0.50 g) were dissolved in stirred water (10.00 mL). Hydrochloric acid (12.1 M, 27.5 μL), water (250 μL), and 2-methylaziridine (178 μL, 1:1 molar ratio to primary amines on the unmodified chitosan oligosaccharide) were then added to this solution, with continuous stirring for 5 d at 25 °C and 24 h at 85 °C. The resulting 2-methylaziridine-modified chitosan oligosaccharides (COS) were precipitated in acetone, washed with methanol to remove excess 2-methylaziridine, and dried in vacuo.
Scheme 1.
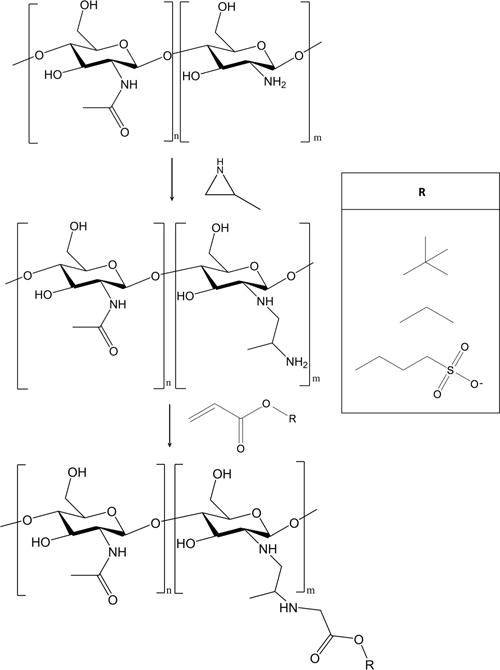
Chitosan Oligasaccharide Modification with 2-Methylaziridine and Subsequent Functionalization with Acrylates
Ester Modification of Chitosan Oligosaccharides
The mucoadhesive properties of COS were altered by the Michael addition of acrylates to the amino groups to form structurally distinct saturated ester functional groups on the scaffold backbone (Scheme 1). Ethyl acrylate (EA, 2.08 mL) and tert-butyl acrylate (TBuA, 2.78 mL) were added to COS (500 mg) in a solution of water (6.00 mL), methanol (14.00 mL), and ammonium hydroxide (1.00 mL). Methanol was excluded from the reaction solvent for the addition of sulfopropyl acrylate (SPA) because the SPA is water-soluble. More specifically, SPA (4.43 g) was added to COS (500 mg) in a solution of water (20.00 mL) and ammonium hydroxide (1.00 mL). A 10-fold molar excess of acrylate (vs primary amine) was used for all reactions to maximize the acrylate addition. After 72 h of stirring at room temperature, the resulting ester-modified COS was precipitated with acetone, collected via centrifugation, and washed with methanol to remove excess reagent. The SPA product was washed copiously with ethanol. The resulting EA-, TBuA-, and SPA-modified COS (COS-EA, COS-TBuA, and COS-SPA, respectively) were dried in vacuo overnight and stored at room temperature. Removal of unreacted acrylate from the products was verified by the disappearance of the vinyl protons in the 1H NMR spectra. Ester-modified COS was characterized by 1H NMR (Bruker 400 MHz DRX spectrometer) to determine the degree of substitution and product purity. Representative 1H NMR peaks were as follows:
COS: 1H NMR (D2O, 400 MHz) δ-0.8–1.1 (br, 3H), 1.9 (s, 3H), 2.3–2.9 (br, 4H), 3.3–4.0 (br, 5H), 4.4 (s, 1H).
COS-EA: 1H NMR (D2O, 400 MHz) δ-0.8–1.1 (br, 6H), 1.9 (s, 3H), 2.3–2.9 (br, 4H), 3.3–4.0 (br, 5H), 4.1 (s, 2H), 4.4 (s, 1H).
COS-TBuA: 1H NMR (D2O, 400 MHz) δ-0.8–1.1 (br, 12 H), 1.9 (s, 3H), 2.3–2.9 (br, 4H), 3.3–4.0 (br, 5H), 4.4 (s, 1H).
COS-SPA: 1H NMR (D2O, 400 MHz) δ-0.8–1.1 (br, 6H), 1.9 (s, 3H), 2.3–2.9 (br, 4H), 3.3–4.0 (br, 5H), 4.1 (s, 2H), 4.4 (s, 1H).
Calculations used to determine the degrees of deacetylation and substitution, and FT-IR characterization data are provided in Supporting Information.
Synthesis of NO-Releasing Chitosan Oligosaccharides
To impart NO storage and release, N-diazeniumdiolate NO donors were formed on the secondary amines of the COS and ester-modified COS.25 Modified chitosan oligosaccharides (15 mg) were dissolved in a solution of water (300 μL), methanol (700 μL), and 5.4 M sodium methoxide (25 μL) in a 1 dram vial equipped with a stir bar. The open vial was placed in a 160 mL Parr general purpose stainless steel pressure vessel and stirred vigorously. Oxygen was removed from the reaction vessel by purging with argon (10 s, 8 bar) thrice, followed by three longer argon purges (10 min, 8 bar). The vessel was then filled with potassium hydroxide-purified NO gas (10 bar) for 72 h at room temperature. Afterward, the argon purging procedure was repeated to remove unreacted NO. N-Diazeniumdolate-modified chitosan oligosaccharides (COS-NO, COS-EA-NO, COS-TBuA-NO, and COS-SPA-NO) were precipitated in acetone, collected via centrifugation to yield a yellow powder, dried in vacuo, and stored at −20 °C until further study.
Chemiluminescence Detection of NO Release
A Sievers 280i Chemiluminescence Nitric Oxide Analyzer (Boulder, Colorado) was used to quantify NO release. Prior to analysis, the instrument was calibrated with air passed through a NO zero filter (0 ppm of NO) and 26.8 ppm of NO standard gas (balance N2). The N-diazeniumdiolate modified chitosan oligosaccharides (1.0 mg) were immersed in 30 mL of deoxygenated PBS (pH 6.5) at 37 °C whereupon released NO was carried by N2 gas to the detector at a flow rate of 80 mL/min. Additional N2 flow was supplied to the sample flask at 200 mL/min to match the collection rate of the instrument. Analysis was terminated once NO concentrations fell below 10 ppb NO/mg COS-NO. Additionally, a PerkinElmer Lambda 40 UV/vis spectrometer was used to obtain UV–vis spectra of 0.1 mg/mL solutions of all compounds in 50 mM sodium hydroxide. This basic solution was used in order to avoid undesirable N-diazeniumdiolate NO donor degradation that begins immediately at neutral pH.
Turbidimetric Titrations of Mucins
Gastric pig mucin (960 mg) was dissolved in 250 mL of sterile phosphate buffer (PB) at 4 °C for 18 h. The mucin suspension was centrifuged (1,500g, 4 °C, 0.5 h) to remove insoluble components. The resulting mucin solution was stored in sterile containers at 4 °C for up to 1 week prior to use. Solutions of mucin and ester-modified chitosan oligosaccharides were prepared by combining 236 μL of the purified mucin solution with 34 μL of the chitosan oligosaccharide solutions (3.6–54.5 mg/mL in sterile PB) in a 96-well plate. The mucin and chitosan solutions were incubated for 1 h at 37 °C with gentle shaking (100 rpm) after which the absorbance was read at 540 nm using a Thermoscientific Multiskan EX plate reader. A corrected absorbance was obtained after subtracting the absorbance of the chitosan oligosaccharides (i.e., without mucin) from the COS-mucin absorbance.
Zeta Potentials of Mucin–Chitosan Oligosaccharide Aggregates
Gastric pig mucin (10.0 mg) was dissolved in 10.00 mL of sterile PB at 4 °C for 18 h. The mucin suspension was centrifuged (1,500g, 4 °C, 0.5 h) to remove insoluble components. The resulting mucin solution was stored in sterile containers at 4 °C for up to 1 week prior to use. Modified chitosan oligosaccharide solids were added to the mucin solution, vortexed until dissolved, and incubated for 1 h at 37 °C. Lower concentrations of mucin and chitosan oligosaccharides were used in this assay to prevent corrosion of the electrochemical cell for the zeta potential measurements. The zeta potential (i.e., surface charge) of the mucin–chitosan aggregate was determined using a Malvern Zetasizer Nano-ZS equipped with a 10 mW HeNe laser (633 nm) and a NIBS detector at an angle of 173°.
Gel Electrophoresis
Concentrated stocks of chitosan oligosaccharides (COS and COS-SPA) and NO-releasing chitosan oligosaccharides (COS-NO and COS-SPA-NO) or DTT (20 μL) were added to HBE mucus (40 μL), stirred gently, and incubated at room temperature for 2 h with gentle rocking. As CF sputum contains proteolytic enzymes, the incubation time was decreased to 1 h to reduce enzymatic degradation of the sample.
Agarose Mucin Gel Electrophoresis
Following treatment, samples were separated by electrophoresis as previously described.27 In brief, samples (40 μL) were loaded onto a 0.8 wt % agarose gel in 1× TAE buffer with 1 wt % SDS for electrophoretic mucin separation at 80 V for 90 min. The gel was subsequently reduced with 10 mM DTT for 20 min. Mucins were transferred by vacuum (45 mbar, 1.5 h) in 4× SSC buffer onto a nitrocellulose membrane with a pore size of 0.45 μm (Optitran BA-S 85 membrane, GE Healthcare Life Sciences, Piscataway, NJ). Following blocking of nonspecific interactions with powdered milk (3 wt % in DPBS) for 1 h, mucins were detected by exposure to diluted primary antibodies raised against MUC5AC and MUC5B (0.1 μg/mL in 3% milk) overnight (3 °C). The membranes were washed thrice with DPBS (10 min) and fluorescently labeled with secondary antibodies (0.2 μg/mL in 3 wt % powdered milk, 1 h, 25 °C). The gels were subsequently washed in DPBS again and then analyzed using a LI-COR Odyssey CLx Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Migration distances were quantified using ImageJ (National Institute of Health, Bethesda, MD). For CF sputum samples, migration distances were normalized to the PBS-treated sample to account for the large degree of heterogeneity.
Confocal Microscopy
Prior to loading onto the agarose gel, CF sputum samples (5 μL) exposed to COS, COS-NO, COS-SPA, or COS-SPA-NO were carefully smeared on glass microscopy slides to prevent mechanical disruption of mucin and the DNA network. The samples were fixed with NBF (10 vol %), washed with DPBS, and blocked with BSA (3 wt % in DPBS) for 1 h at room temperature. Mucin networks were visualized by immunohistochemical detection. First, MUC5AC and MUC5B were identified via exposure to primary antibody solutions (0.4 and 0.2 μg/mL for mouse anti-MUC5AC and rabbit anti-MUC5B, respectively). The slides were washed with DPBS three times (10 min) prior to exposure to secondary antibodies (1 μg/mL Alexa 488 and 594 antirabbit and mouse, respectively) and DAPI (5 μg/mL) for 1 h at 25 °C to facilitate quantitative measurement. The slides were then washed with DPBS (10 min) and mounted with Fluorsave (Calbiochem). Confocal images were obtained with an Olympus FV 1000 (Olympus, Hamilton, Bermuda) using a 20× objective.
Parallel Plate Rheology
Spontaneously expectorated sputum from one CF patient collected at a single time point was used for rheological measurements. Concentrated solutions of COS-NO (27.8 μL) were added to 250 μL aliquots of sputum to achieve final COS-NO concentrations of 0, 5, 10, and 20 mg/mL. Sputum samples were slowly rotated at room temperature for 1 h. The rheological properties of the treated samples were measured via amplitude sweep experiments over a stress range of 0.025–50 Pa at a single frequency (1 Hz) on a Bohlin Gemini Rheometer (Malvern Instruments, Worcestershire, UK) with a 20 mm diameter parallel plate set to a gap thickness of 50 mm. Rheological measurements were performed at 23 °C to minimize sample dehydration. The elastic modulus (G′) and viscous modulus (G″) were determined from the linear regimes as previously reported.28 All values are reported as the mean ± standard error of the mean (SEM) for a minimum of three separately evaluated aliquots of the treated sputum sample.
Statistical Analysis
All values are reported as the mean ± standard deviation for three or more pooled experiments unless otherwise noted. Statistical significance was determined using the two-tailed Student’s t test.
RESULTS AND DISCUSSION
Synthesis of NO-Releasing Mucoadhesive Chitosan Oligosaccharides
The mucoadhesive nature of chitosan is believed to be derived from electrostatic and hydrophobic interactions as well as hydrogen bonding between chitosan and mucins. Of these interactions, electrostatic attractions between the positively charged primary amine on chitosan and the negatively charged sialic acid and ester sulfate groups on mucins predominate.13 Sogias et al.13 and Mencchicchi et al.12 have demonstrated that blocking the primary amines on chitosan with acetyl groups (i.e., decreasing the deacetylation of the chitosan) reduces its ability to bind and aggregate mucin in solution. To determine how chitosan mucoadhesion affects NO delivery and efficacy, three structurally distinct ester modifications were used to sterically block the primary amines on 2-methylaziridine-modified chitosan oligosaccharides (COS) (Scheme 1). Acrylates were chosen as the route to modification because propenoates covalently bind to chitosan under mild synthetic conditions. Specific modifications were also selected based on their commercial availability. Ethyl acrylate and tert-butyl acrylate were compared to determine if increasing the steric bulk from an ethyl group to a tert-butyl group altered the mucoadhesive nature of COS. To reduce mucoadhesion, a negatively charged acrylate (sulfopropyl acrylate) was employed to electrostatically repel mucus in addition to sterically hindering the primary amine. Reaction conditions for the Michael addition of acrylates to the COS scaffold were optimized to maximize modification efficiency. All reactions were carried out at room temperature as heating to 50 °C produced undesirable side-reactions between ethyl acrylate and the chitosan backbone (data not shown). The primary amine on COS (RNH3+, pKa ∼ 10) is deprotonated in basic solution (pH 12), thereby increasing reactivity toward the β-carbon of the acrylate vinyl group. In this manner, the modification of COS with TBuA is enhanced from 11 ± 1% at pH 7 (i.e., neutral conditions) to 83 ± 15% at pH 12. The resulting, optimized reaction yielded similar modification efficiencies for all three ester modifications: 86 ± 4% for COS-EA, 83 ± 15% for COS-TBuA, and 98 ± 15% for COS-SPA. Differences between modification efficiencies for the three distinct saturated ester groups were not significant as determined by one-way ANOVA (F (2,10) = 1.42, p = 0.31).
To impart NO storage and release, N-diazeniumdiolate NO donors were formed at the secondary amine sites on the modified chitosan oligosaccharides via exposure to high pressures of NO gas.25 Nitric oxide storage was tuned by maintaining constant solvent ratios (3:7 water/methanol) and base concentrations for all scaffolds, resulting in similar NO payloads ([NO]total ∼ 0.4 μmol/mg) and release kinetics (t1/2 ∼ 30 min) for both the unmodified COS and ester-modified scaffolds (Table 1). A sample NO-release curve can be found in the Supporting Information. While NO release from N-diazeniumdiolates may be altered by charge stabilization or hydrophobicity imparted from local functional groups,29 the comparable NO-release kinetics of these materials were somewhat expected as the ester modifications did not significantly alter the hydrophobicity of the materials. Indeed, the similar NO-release half-lives exhibited herein indicate that the NO-release kinetics of ester-modified chitosan oligosaccharides are generally more influenced by the hydrophobicity of the chitosan backbone rather than the exterior functional groups.
Table 1.
NO-Release Properties of Ester-Modified Chitosan Oligosaccharides PBS (pH 6.5, 37 °C)a
| [NO]totalb (μmol/mg) | [NO]maxc (ppb/mg) | t(l/2)d (min) | tde (h) | |
|---|---|---|---|---|
| COS-NO | 0.44 ± 0.11 | 2800 ± 600 | 36.1 ± 2.8 | 7.85 ± 0.75 |
| COS-EA-NO | 0.42 ± 0.16 | 3200 ± 1100 | 28.8 ± 5.2 | 7.62 ± 0.67 |
| COS-TBuA-NO | 0.42 ± 0.09 | 2500 ± 400 | 25.3 ± 8.6 | 7.74 ± 0.74 |
| COS-SPA-NO | 0.39 ± 0.12 | 3300 ± 1100 | 28.9 ± 4.3 | 6.72 ± 1.92 |
All values are reported as the mean ± standard deviation for 3 or more pooled experiments.
Total NO.
Max NO Flux.
Half-life.
Duration of NO-release.
The formation of the N-diazeniumdiolate NO donor was characterized using UV–vis spectroscopy. For all compounds, UV–vis spectra showed an absorbance maximum at 253 nm following exposure to high pressures of NO gas, confirming N-diazeniumdiolate NO donor formation.30 Absorbance peaks at or near 350 nm were not observed, indicating little or no formation of potentially carcinogenic nitrosamines.31 The absorbance spectra for each of the modified chitosan oligosaccharides are provided in the Supporting Information.
Mucoadhesion of Saturated Ester- and 2-Methylaziridine-Modified Chitosan Oligosaccharides
Turbidimetric titrations of gastric pig mucin (GPM) with ester-modified COS were performed to determine the mucoadhesive properties of the chitosan oligosaccharide scaffolds. In the presence of low concentrations of mucoadhesive polymers, mucins form light scattering self-assembled complexes.13,32,33 As such, the turbidity of mucin solutions (as measured by corrected absorbance) is expected to increase with the addition of mucoadhesive scaffolds, resulting in increased absorbance measurements. At larger concentrations, mucoadhesive polymers have been shown to disaggregate mucin–polymer complexes.13 Alternately, the turbidity (i.e., absorbance) of mucin solutions should remain constant in the presence of muco-inert scaffolds. Turbidity was monitored at 540 nm to maximize sensitivity toward GPM-chitosan complexes while minimizing the absorbance of free chitosan oligosaccharides in solution (λmax = 375 nm).
The turbidity of GPM solutions increased rapidly (nearly 4-fold) at low concentrations (≤3 mg/mL) of the COS, COS-EA, and COS-TBuA scaffolds, indicating significant mucoadhesion with mucins in solution (Figure 1). At concentrations ≥4.5 mg/mL, disaggregation of chitosan mucin complexes was observed for COS and COS-TBuA. The addition of COS-SPA to GPM solutions resulted in a slight increase in the turbidity of the solutions at high concentrations. As electrostatic interactions between the positively charged chitosan and negatively charged mucins facilitate mucoadhesion,12,13,34 the corresponding electrostatic repulsion between the mucins and negatively charged sulfonate group of COS-SPA likely circumvented the formation of chitosan–mucin aggregates at low concentrations. At larger concentrations (>3 mg COS-SPA/mL), small increases in turbidity were observed and attributed to the attractive forces (e.g., hydrophobic or hydrogen bonding) between the chitosan oligosaccharide backbone and mucin particles.13
Figure 1.
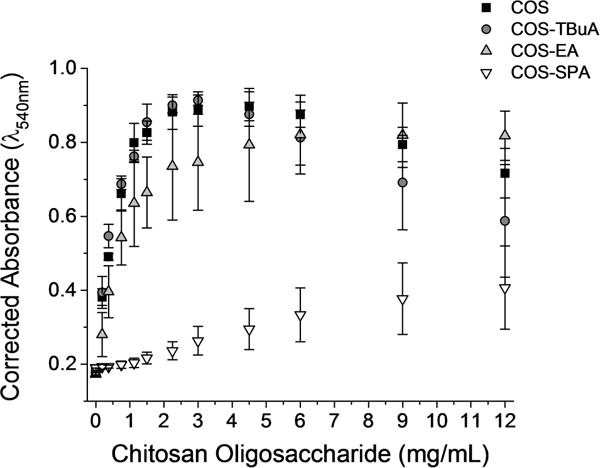
Turbidimetric titration of gastric pig mucin (GPM) with modified chitosan oligosaccharide scaffolds without NO in phosphate buffer (10 mM, pH 6.5).
While the TBuA and EA modifications were expected to sterically block the mucoadhesive primary amines on the COS scaffold, any steric hindrance did not significantly alter the mucoadhesion compared to the unmodified COS scaffold. The retained mucoadhesive properties are likely the result of insufficient steric blocking of the positively charged amine or hydrophobic interactions between the modified group and the mucins. To determine the extent of mucoadhesion due to electrostatics, the turbidity assay was repeated in PBS (Supporting Information). The addition of sodium chloride to the solution minimizes electrostatic interactions between chitosan and mucins.13 For all modified chitosan oligosaccharides, the addition of sodium chloride reduced the turbidity of the solutions, indicating that electrostatic attraction between the chitosan oligosaccharides and mucins was retained even after the chitosan was modified to prevent interactions between the positively charged amine and negatively charged groups on the mucins.
Zeta potential measurements of chitosan–mucin aggregates corroborated the turbidimetric titration results (Figure 2). At pH 6.5, blank GPM solutions exhibited a zeta potential of −9.8 ± 0.5 mV. Adding chitosan oligosaccharides modified with neutral or positively charged terminal groups (COS, COS-TBuA, and COS-EA) to dilute mucin solutions resulted in increased zeta potentials. Treatment of GPM solutions with negatively charged COS-SPA did not alter the measured zeta potential at the concentrations tested, further demonstrating the reduced mucoadhesive properties of COS-SPA. Only low concentrations of chitosan oligosaccharide could be tested in this assay because larger concentrations corroded the electrode on the zeta potential cell.
Figure 2.
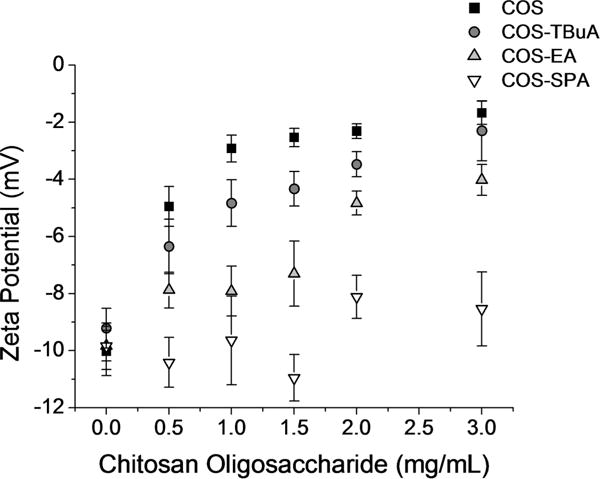
Zeta potential measurements of gastric pig mucin (GPM)-chitosan aggregates formed upon the addition of chitosan oligosaccharides (without NO) to mucin solutions (1 mg/mL in 10 mM PB at pH 6.5).
Electrophoretic Separation of Purified Mucus Following Treatment with Chitosan Oligosaccharides
To determine the effects of NO on mucin size (i.e., molecular weight), mucus collected from CF HBE cultures was separated by agarose gel electrophoresis following treatment with increasing concentrations of control chitosan scaffolds (i.e., non-NO-releasing) and NO-releasing chitosan oligosaccharides. The mucolytic action of the NO-releasing chitosan oligosaccharides was evaluated using both MUCB and MUC5AC, the key mucins responsible for gel formation in the airway. The concentrations of these mucins are known to increase during pulmonary exacerbations in CF.35 Strongly and weakly mucoadhesive chitosan oligosaccharides (COS and COS-SPA, respectively) were used to evaluate the effects of scaffold mucoadhesion on mucin migration. These modifications were chosen because the difference in the charge of their terminal functional groups (NH3+ for COS and SO3− for SPA) were hypothesized to cause differences in their interactions with negatively charged mucins. Any increase in the mucin migration distance after exposure to chitosan oligosaccharides would reflect a change in size and/or charge of the mucin multimers, suggesting a beneficial therapeutic effect in the treatment of CF mucus.
Compared to treatment with blank PBS, mucin migration was not affected by exposure to control COS and COS-SPA scaffolds regardless of the scaffold’s mucoadhesive properties (Figure 3). Treatment with the weakly mucoadhesive NO-releasing COS-SPA (COS-SPA-NO) scaffold similarly had no impact on mucin migration. However, treatment with the strongly mucoadhesive COS-NO increased the migration distances of both MUC5AC and MUC5B mucins relative to controls (p ≤ 0.05) in a dose-dependent manner at 10 mg/mL (Figure 4). The increase in mucin migration may be attributed exclusively to the pharmacological effects of NO since treatment with the control scaffold alone did not affect migration. In this manner, the reduced size of the mucin multimers leads to faster migration. Of note, scaffold mucoadhesion is necessary for effective NO delivery as treatment with COS-SPA-NO did not alter the migration of MUC5AC or MUC5B. We hypothesize that poor scaffold-mucin association with negatively charged COS-SPA does not facilitate localized NO release, thereby requiring greater doses for equivalent therapeutic activity. We have reported similar observations for bacteria and NO-releasing silica and dendrimer macromolecular scaffolds.36 Indeed, the association of dendrimers37–39 and silica40–43 with bacteria membranes improve the antimicrobial action of NO release as a result of more targeted and localized NO delivery. Clearly, the design of mucoadhesive NO-releasing scaffolds would be equally advantageous for ensuring target proximity and increased therapeutic action.
Figure 3.
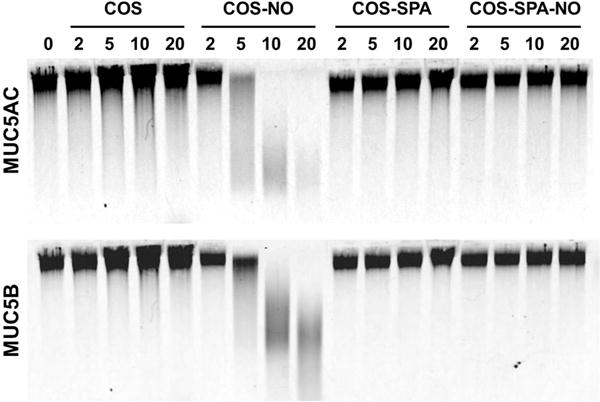
Representative Western blot of MUC5AC and MUC5B mucins from HBE culture washings from a donor with cystic fibrosis treated with COS, COS-NO, COS-SPA, and COS-SPA-NO for 2 h at 25 °C at concentrations ranging from 0 to 20 mg/mL.
Figure 4.
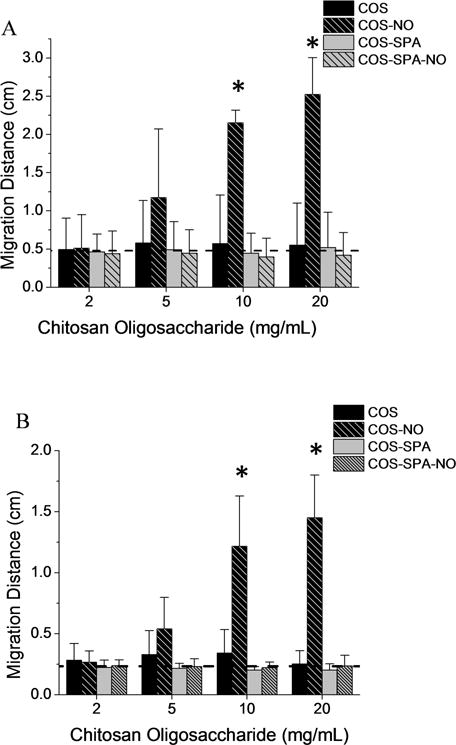
Migration distances of (A) MUC5AC and (B) MUC5B mucins from CF-HBE culture washings following treatment with modified chitosan oligosaccharides for 1 h at room temperature. The migration distances of mucin treated with equal volumes of PBS are denoted with dashed horizontal lines. All values are presented as the mean ± standard deviation for 3 or more pooled experiments. Asterisks (*) indicate significant differences (p < 0.05) relative to treatment with PBS (0 mg/mL).
Electrophoretic Separation of CF Sputum Following Treatment with Chitosan Oligosaccharides
While NO released from COS-NO increased the migration of polymeric mucins, the complexity of CF sputum (e.g., high concentrations of DNA, bacteria, inflammatory proteins, and cells) may influence NO delivery and potency. We thus evaluated the effects of COS, COS-NO, COS-SPA, and COS-SPA-NO on mucins in CF sputum via agarose gel electrophoresis. As with HBE mucus, treatment of CF sputum with control (non-NO-releasing) chitosan scaffolds and COS-SPA-NO did not affect mucin migration (Figure 5). In contrast, migration of MUC5AC and MUC5B increased following treatment with COS-NO at concentrations ≥ 10 mg/mL (Figure 6), further supporting the mucolytic activity of NO for this NO-release scaffold. Of note, the concentration of chitosan oligosaccharides used in this assay is higher than the concentration used in the turbidity assay. The concentration of mucins is also higher in the sputum (approximately 6.5 mg/mL)44 than the mucin concentration used in the turbidity assay (3.0 mg/mL); however the ratio of chitosan to mucin (mg/mg) is similar in both assays. Others have argued that muco-inert scaffolds are more effective for drug delivery in CF airways due to improved mucus penetration.14 However, the pharmacological target of many such drugs lies beyond the mucus layer, whereas COS-NO targets the accumulated, adherent mucus itself. Indeed, the mucolytic activity of NO-releasing chitosan oligosaccharides appeared to be dependent upon the mucoadhesive properties of the COS scaffold for both HBE mucus and CF sputum, with the weakly mucoadhesive COS-SPA-NO scaffold demonstrating no beneficial therapeutic effect.
Figure 5.
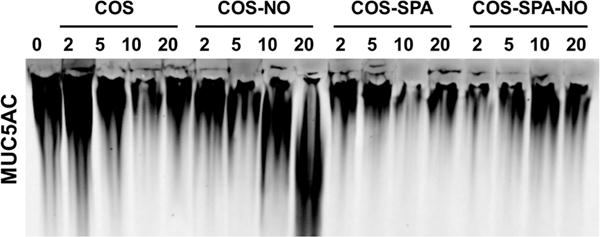
Representative Western blot of MUC5AC mucins from CF sputum treated with COS, COS-NO, COS-SPA, and COS-SPA-NO for 1 h at 25 °C at concentrations ranging from 0 to 20 mg/nL. Similar trends were observed for MUC5B.
Figure 6.
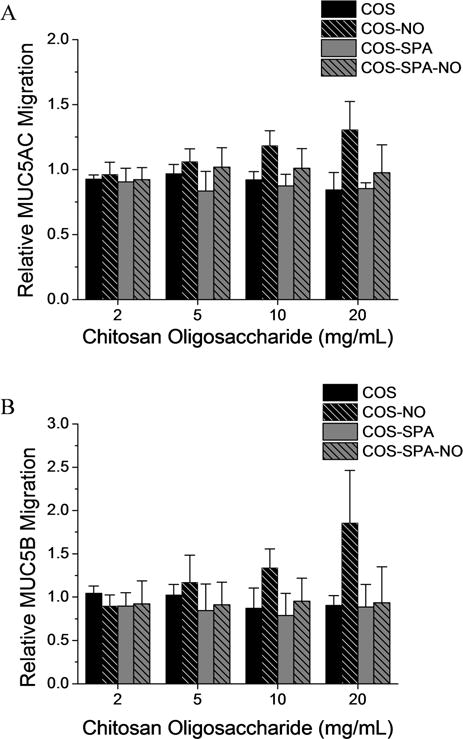
Relative migration distances of (A) MUC5AC and (B) MUC5B mucins from CF sputum following treatment with modified chitosan oligosaccharides for 1 h at room temperature. Migration distances were normalized to CF sputum samples treated with an equal volume of PBS. All values are presented as the mean ± standard deviation for n = 3 or more pooled experiments.
Fluorescent Microscopy of the CF Sputum
To visualize NO-mediated changes in the network formed by mucins and DNA in the CF sputum, treated samples were imaged with a confocal laser scanning microscope. In the PBS-treated samples (Figure 7A–D), networks of MUC5AC (red) and MUC5B (green) were intertwined, forming three-dimensional architectures characterized by thick mucin filaments (Figure 7A, white arrows) and web-like mucin sheets. As detected by intense DAPI (blue) staining, intact neutrophils were also embedded within the three-dimensional mucin network. Upon treatment with chitosan oligosaccharides, the intracellular DNA signal was diminished while the extracellular signal was enhanced throughout the sputum samples, likely the result of neutrophil and other inflammatory cell death (Figure 7F, J, N, and R).45 Treatment with COS-SPA and COS-SPA-NO had no other discernible effects on the CF sputum architecture.
Figure 7.
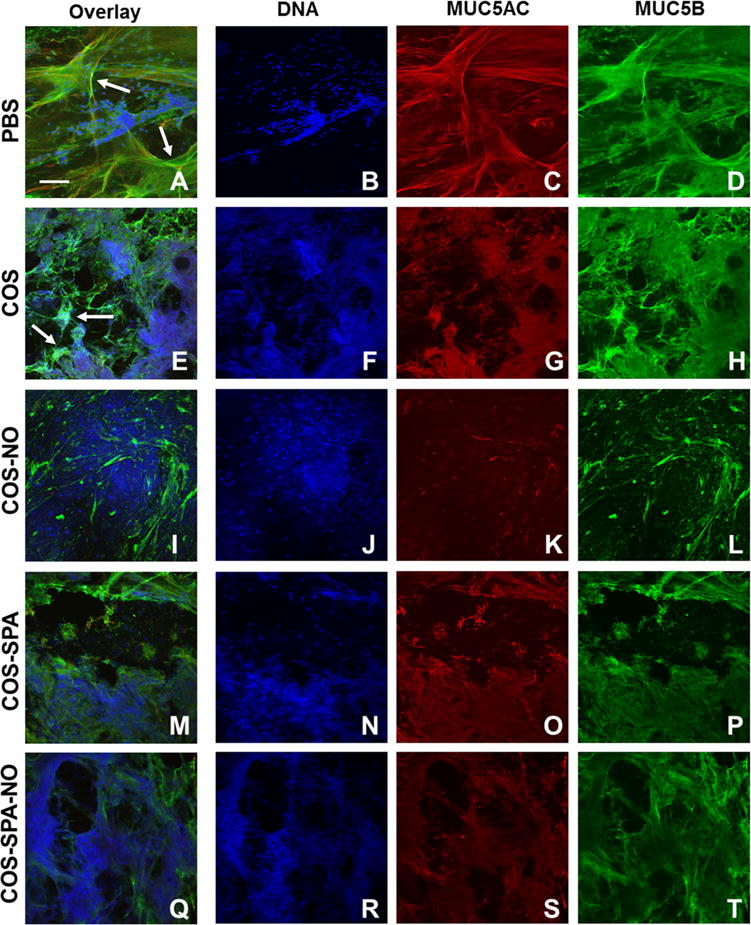
Confocal microscopy images of CF sputum treated with PBS (A–D) or 20 mg/mL COS (E–H), COS-NO (I–L), COS-SPA (M–P), or COS-SPA-NO (Q–T) for 1 h at 25 °C. Arrows indicate long strands of mucins in untreated samples (A) which aggregate and form clumps upon treatment with COS (E).
The positively charged chitosan variants, COS and COS-NO, greatly altered the appearance of the mucin networks in CF sputum. The COS control alone induced mucin clustering as evidenced by both signal intensification and size reduction of the mucin sheets (Figure 7E, white arrows), indicating that the COS-mucin electrostatic interactions persisted in the complex viscoelastic environment of CF sputum. Nitric oxide release via COS-NO further degraded the mucin network, as depicted by a lower overall mucin signal and relaxed mucin network (Figure 7K,L). Samples treated with COS-NO (Figure 7 K–L) lacked the long filaments of mucin that were abundant in the PBS-treated samples (Figure 7A, white arrows). These microscopic observations suggest intense disruption of the mucin network by COS-NO. The NO-mediated mucin disentanglement is expected to reduce sputum elasticity since high mucin entanglement correlates with elevated sputum elasticity.5
Treatment with COS-SPA-NO did not alter the sputum network (Figure 7Q–T). Electrostatic repulsion between the chitosan oligosaccharide backbone and negatively charged mucins prevented effective chitosan oligosaccharide penetration into the most dense mucin matrix. In this manner, the NO release from COS-SPA-NO was ineffective at altering the mucin network.
CF Sputum Rheology
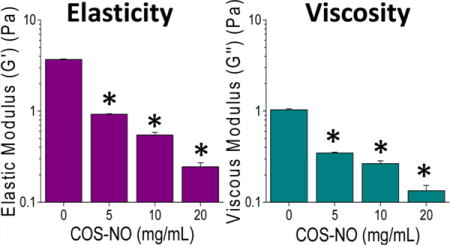
Mucolytics are designed to decrease the biophysical properties of mucus, thereby increasing mucociliary clearance and pulmonary function.44,46 Parallel plate rheology was used to measure both the elastic (G′) and viscous (G″) moduli, and evaluate how treatment with COS-NO affected the biophysical properties of CF sputum. After treatment with COS-NO for 1 h at room temperature, dose-dependent reductions in CF sputum viscosity and elasticity were observed relative to controls (Figure 8). At the lowest concentration tested (5 mg/mL), COS-NO reduced sputum elasticity and viscosity by 74.9 ± 0.4 and 66.3 ± 1.2%, respectively, compared to PBS-treated controls. Treatment of the CF sputum with a 4× concentration of COS-NO (20 mg/mL) reduced the sputum elasticity and viscosity by 93.4 ± 0.7 and 87.0 ± 2.0%, respectively. These results indicate that COS-NO is highly effective at decreasing the viscoelastic properties of CF sputum at short (1 h) exposure periods.
Figure 8.
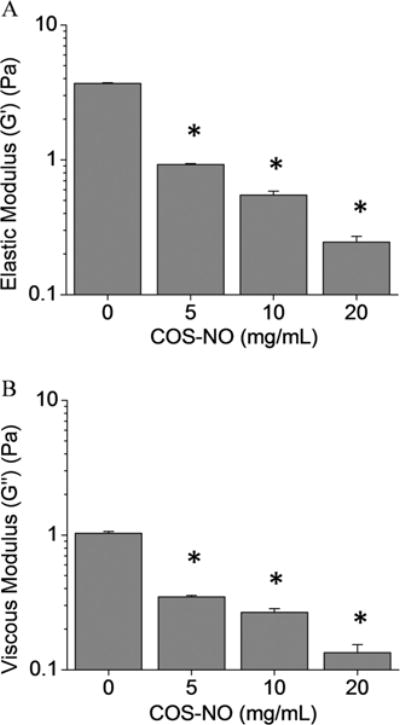
(A) Elastic and (B) viscous moduli of CF sputum following treatment with COS-NO for 1 h at 25 °C. Values presented as the mean ± standard error of the mean for n = 3 triplicate measurements. Asterisks (*) indicate significant differences (p < 0.05) relative to treatment with PBS (0 mg/mL).
While it is difficult to compare rheological data in the literature due to widely varying exposure and measurement parameters,47 the COS-NO treatment resulted in similar changes in sputum viscoelasticity as conventional mucolytic therapies used to treat CF (e.g., N-acetylcysteine, dornase alfa). For example, Seagrave et al. reported that N-acetylcysteine treatment (30 μM) for 24 h decreased the viscosity and elasticity of HBE mucus by an order of magnitude versus controls.28 Shah et al. observed reduced expectorated sputum viscosity and elasticity (59 and 68%, respectively) in patients with CF following treatment with dornase alfa for 10 d (2.5 mg, twice daily).48 While slightly greater concentrations of COS-NO were required to similarly alter sputum rheological properties relative to the N-acetylcysteine and dornase alfa used previously, longer exposure times and/or greater NO payloads would decrease the required therapeutic dose for equivalent action. Indeed, we employed a shorter therapeutic exposure compared to that in previous reports (1 h compared to 24 h28 or 10 daily treatments48) and achieved similar reductions in CF sputum viscosity and elasticity, demonstrating the utility of COS-NO as a potential mucolytic agent.
The diminished elastic and viscous moduli of CF sputum may also be attributed to alterations in the DNA network via NO-mediated DNA cleavage.49–51 Importantly, the electrophoretic separation and confocal microscopy results indicate that NO actively alters the mucin network. Studies are underway to determine the mechanism by which the NO alters the rheological properties of the sputum.
While these studies clearly indicate the mucolytic action of nitric oxide-releasing scaffolds, several questions remain for further development of any potential therapeutic. As NO may be genotoxic,52 the cyto- and genotoxicity of these materials should be characterized prior to clinical studies. Likewise, NO-release kinetics may similarly alter the mucolytic potential similar to how it influences antibacterial action.25,36,53
CONCLUSIONS
Herein, we report the mucolytic action of NO-releasing chitosan scaffolds as a means to decrease the viscoelasticity of CF sputum by reducing mucin size and damaging the three-dimensional mucin network. While inhalation of NO gas has been proposed as a mucolytic therapy previously, our work establishes the potential benefits of NO release from a mucoadhesive scaffold for direct delivery into the mucus plug. In all experiments, the mucoadhesive properties of the chitosan oligosaccharide scaffolds significantly enhanced NO’s potency. The ability to target NO release to CF mucus should lower the required NO dose for therapeutic action while simultaneously lessening any potential effects on other NO-mediated processes. As NO is also a potent antibacterial agent,7,8,25,36,54–56 this work suggests the dual-action potential of NO-releasing chitosan oligosaccharides as antibacterial and mucolytic agents for the treatment of CF.
Supplementary Material
Acknowledgments
We thank Dr. Scott Donaldson of the UNC School of Medicine, Department of Pulmonary Diseases and Critical Care Medicine for providing CF sputum samples. We would also thank Mr. Lei Yang for collecting the FT-IR spectra of our materials.
Funding
Financial support for this work was provided by the National Institutes of Health (AI112029, HL108808), National Science Foundation (110281 and 1462992), and Cystic Fibrosis Foundation (EHRE07XX0, EHRE16XX0). We acknowledge support from UNC EFRC Center for Solar Fuels (“Energy Frontier Research Center” funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001011), and UNC SERC (“Solar Energy Research Center Instrumentation Facility” funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy under Award Number DE-EE0003188) for the FT-IR instrumentation used to characterize our materials.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.7b00039.
Deacateylation and degree of substitution calculations; UV–vis and FT-IR spectra; representative NO-release curve; and turbidimetric titrations in phosphate buffered saline (PDF)
ORCID
Mark H. Schoenfisch: 0000-0002-2212-0658
Notes
The authors declare the following competing financial interest(s): M.S. is a co-founder and maintains a financial interest in Novan, Inc. and Novoclem Therapeutics, Inc. Both companies commercialize macromolecular nitric oxide storage and release vehicles for clinical indications.
References
- 1.Høiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 2011;9(1):32–39. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treggiari MM, Rosenfeld M, Retsch-Bogart G, Gibson R, Ramsey B. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr Pulmonol. 2007;42(9):751–756. doi: 10.1002/ppul.20665. [DOI] [PubMed] [Google Scholar]
- 3.Ehre C, Ridley C, Thornton DJ. Cystic fibrosis: an inherited disease affecting mucin-producing organs. Int J Biochem Cell Biol. 2014;52:136–145. doi: 10.1016/j.biocel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM, Superfine R, Rubinstein M, Iglewski BH. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2006;103(48):18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Sun Yoon S, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: Rethinking antibiotic treatment strategies and drug targets. Adv Drug Delivery Rev. 2002;54(11):1425–1443. doi: 10.1016/s0169-409x(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 6.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15(2):194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reighard KP, Schoenfisch MH. Antibacterial action of nitric oxide-releasing chitosan oligosaccharides against Pseudomonas aeruginosa under aerobic and anaerobic conditions. Antimicrob Agents Chemother. 2015;59(10):6506–6513. doi: 10.1128/AAC.01208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reighard KP, Hill DB, Dixon GA, Worley BV, Schoenfisch MH. Disruption and eradication of P.aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling. 2015;31(9–10):775–787. doi: 10.1080/08927014.2015.1107548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grenha A, Al-Qadi S, Seijo B, Remuñán-López C. The potential of chitosan for pulmonary drug delivery. J Drug Delivery Sci Technol. 2010;20(1):33–43. [Google Scholar]
- 10.Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36(8):981–1014. [Google Scholar]
- 11.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Delivery Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Menchicchi B, Fuenzalida JP, Bobbili KB, Hensel A, Swamy MJ, Goycoolea FM. Structure of chitosan determines its interactions with mucin. Biomacromolecules. 2014;15(10):3550–3558. doi: 10.1021/bm5007954. [DOI] [PubMed] [Google Scholar]
- 13.Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9(7):1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- 14.Klinger-Strobel M, Lautenschläger C, Fischer D, Mainz JG, Bruns T, Tuchscherr L, Pletz MW, Makarewicz O. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis-where do we stand? Expert Opin Drug Delivery. 2015;12(8):1351–1374. doi: 10.1517/17425247.2015.1007949. [DOI] [PubMed] [Google Scholar]
- 15.Manca M-L, Mourtas S, Dracopoulos V, Fadda AM, Antimisiaris SG. PLGA, chitosan or chitosan-coated PLGA microparticles for alveolar delivery?: A comparative study of particle stability during nebulization. Colloids Surf B. 2008;62(2):220–231. doi: 10.1016/j.colsurfb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Park J-H, Jin H-E, Kim D-D, Chung S-J, Shim W-S, Shim C-K. Chitosan microspheres as an alveolar macrophage delivery system of ofloxacin via pulmonary inhalation. Int J Pharm. 2013;441(1):562–569. doi: 10.1016/j.ijpharm.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Jain D, Banerjee R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J Biomed Mater Res, Part B. 2008;86(1):105–112. doi: 10.1002/jbm.b.30994. [DOI] [PubMed] [Google Scholar]
- 18.Osman R, Kan PL, Awad G, Mortada N, Abd-Elhameed E-S, Alpar O. Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int J Pharm. 2013;449(1):44–58. doi: 10.1016/j.ijpharm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Ungaro F, d’Angelo I, Coletta C, di Villa Bianca RDE, Sorrentino R, Perfetto B, Tufano MA, Miro A, La Rotonda MI, Quaglia F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Controlled Release. 2012;157(1):149–159. doi: 10.1016/j.jconrel.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine. 2011;6(2):365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang BC, Dawson M, Lai SK, Wang Y-Y, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci U S A. 2009;106(46):19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forier K, Messiaen A-S, Raemdonck K, Deschout H, Rejman J, De Baets F, Nelis H, De Smedt SC, Demeester J, Coenye T. Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine. 2013;8(6):935–949. doi: 10.2217/nnm.12.129. [DOI] [PubMed] [Google Scholar]
- 23.Suk JS, Lai SK, Wang Y-Y, Ensign LM, Zeitlin PL, Boyle MP, Hanes J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30(13):2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DB, Button B. Establishment of respiratory air–liquid interface cultures and their use in studying mucin production, secretion, and function. Methods Mol Biol. 2012;842:245–258. doi: 10.1007/978-1-61779-513-8_15. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Slomberg DL, Schoenfisch MH. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials. 2014;35(5):1716–1724. doi: 10.1016/j.biomaterials.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maghami GG, Roberts GA. Evaluation of the viscometric constants for chitosan. Makromol Chem. 1988;189(1):195–200. [Google Scholar]
- 27.Ramsey KA, Rushton ZL, Ehre C. Mucin Agarose Gel-Electrophoresis: Western Blotting for High-Molecular Weight Glycoproteins. J Visualized Exp. 2016;2016(112):e84153. doi: 10.3791/54153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res. 2012;13:98. doi: 10.1186/1465-9921-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riccio DA, Schoenfisch MH. Nitric oxide release: part I. Macromolecular scaffolds. Chem Soc Rev. 2012;41(10):3731–3741. doi: 10.1039/c2cs15272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102(4):1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 31.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Nitric oxide donors: chemical activities and biological applications. Chem Rev. 2002;102(4):1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 32.Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur J Pharm Sci. 2000;10(4):251–257. doi: 10.1016/s0928-0987(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 33.Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization of chitosan hydrochloride–mucin rheological interaction: influence of polymer concentration and polymer: mucin weight ratio. Eur J Pharm Sci. 2001;12(4):479–485. doi: 10.1016/s0928-0987(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 34.Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–764. doi: 10.1002/mabi.201000388. [DOI] [PubMed] [Google Scholar]
- 35.Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175(8):816–821. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter AW, Schoenfisch MH. Nitric oxide release: Part II. Therapeutic applications. Chem Soc Rev. 2012;41(10):3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Slomberg DL, Shah A, Schoenfisch MH. Nitric oxide-releasing amphiphilic poly (amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules. 2013;14(10):3589–3598. doi: 10.1021/bm400961r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules. 2012;13(10):3343–3354. doi: 10.1021/bm301109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worley BV, Schilly KM, Schoenfisch MH. Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly (amidoamine) Dendrimers. Mol Pharmaceutics. 2015;12(5):1573–1583. doi: 10.1021/acs.molpharmaceut.5b00006. [DOI] [PubMed] [Google Scholar]
- 40.Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano. 2008;2(2):235–246. doi: 10.1021/nn700191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter AW, Slomberg DL, Rao KS, Schoenfisch MH. Influence of scaffold size on bactericidal activity of nitric oxide-releasing silica nanoparticles. ACS Nano. 2011;5(9):7235–7244. doi: 10.1021/nn202054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl Mater Interfaces. 2013;5(19):9322–9329. doi: 10.1021/am402618w. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter AW, Worley BV, Slomberg DL, Schoenfisch MH. Dual action antimicrobials: nitric oxide release from quaternary ammonium-functionalized silica nanoparticles. Biomacromolecules. 2012;13(10):3334–3342. doi: 10.1021/bm301108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dou J, Xu Q, Tan C, Wang W, Du Y, Bai X, Ma X. Effects of chitosan oligosaccharides on neutrophils from glycogen-induced peritonitis mice model. Carbohydr Polym. 2009;75(1):119–124. [Google Scholar]
- 46.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192(2):182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro-and macrorheology of mucus. Adv Drug Delivery Rev. 2009;61(2):86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah PL, Scott SF, Knight RA, Marriott C, Ranasinha C, Hodson ME. In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax. 1996;51(2):119–125. doi: 10.1136/thx.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol. 1996;9(5):821–827. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- 50.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res Fundam Mol Mech Mutagen. 1999;424(1):37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 51.Duan J, Kasper DL. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology. 2011;21(4):401–409. doi: 10.1093/glycob/cwq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felley-Bosco E. Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev. 1998;17(1):25–37. doi: 10.1023/a:1005948420548. [DOI] [PubMed] [Google Scholar]
- 53.Backlund C, Worley B, Sergesketter A, Schoenfisch MH. Kinetic-dependent killing of oral pathogens with nitric oxide. J Dent Res. 2015;94:1092–8. doi: 10.1177/0022034515589314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seabra AB, Justo GZ, Haddad PS. State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol Adv. 2015;33:1370. doi: 10.1016/j.biotechadv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl Microbiol Biotechnol. 2010;88(2):401–407. doi: 10.1007/s00253-010-2733-x. [DOI] [PubMed] [Google Scholar]
- 56.Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, Marschal M, Miller CC, Riethmüller J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection. 2016;44(4):513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


