Abstract
Incorporation of fish age into the assessment of status and trends for persistent, bioaccumulative and toxic chemicals in the Great Lakes has become an important step for the U.S. EPA’s Great Lakes Fish Monitoring and Surveillance Program (GLFMSP). A slowing in the rate of decline for total PCBs in Lake Huron beginning in 2000, led the Program to complete a retrospective analysis to assess how chemical contamination may be influenced by fish age. Analytical results suggest that fish age is an important variable when assessing contaminant trends and that the Program needed to revise its compositing scheme to group fish according to age, rather than by length, prior to homogenization and chemical analysis. An Interlaboratory comparison study of multiple age structures was performed to identify the most appropriate age estimation structure for the Program. The lake trout (Salvelinus namaycush) maxillae was selected, over the otolith, as the most precise, accurate, and rapidly assessed structure for the Program when compared between laboratories and against the known age from the coded wire tag (CWT). Age-normalization practices can now be implemented when assessing contaminant concentrations and trends for the GLFMSP.
Keywords: Fish, Bioaccumulation, Age, Maxillae, Otolith
Introduction
Changes in Great Lakes food webs have had several serious repercussions on the health of Great Lakes fish over the past 20 years, including changes in bioaccumulation potential of contaminants in top predator species such as lake trout (Salvelinus namaycush) and walleye (Sander vitreus) (Zhou et al., 2017a, 2017b; Pagano et al., 2018). Food web structures have been stressed by nutrient availability, presence of invasive species, declines in prey availability, increases in predator density, density dependent growth and increases in predator-prey ratios and overall predation pressures (Tsehaye et al., 2014; He et al. 2015 and 2016; USGS, 2016; Lake Michigan Lake Trout Working Group Report, 2016). Each of those stress factors could result in slower growing fish which might contribute to higher chemical concentrations of persistent and bioaccumulative compounds. For example, in Lakes Huron and Michigan, there are signs of increasing oligotrophication in open waters (Barbiero et al., 2012; Barbiero et al. (this issue); and Bunnell et al., 2014). Additionally, changes in invertebrate communities have the potential to impact the size and composition of prey fish communities due to resource availability and exploitative competition and ultimately top predator species, like lake trout (Salvelinus namaycush), Chinook salmon (Oncorhynchus tshawytscha), and walleye (Sander vitreus) (He et al., 2016 and Barbiero et al., 2012).
The U.S. EPA Great Lakes National Program Office (GLNPO) Great Lakes Fish Monitoring and Surveillance Program (GLFMSP) has been monitoring chemical concentrations of whole body top predator fish species in the Great Lakes since the 1970s (GLFMSP Quality Management Plan (QMP), 2012) (Carlson et al., 2010; Carlson and Swackhamer, 2006; Chang et al., 2012; De Vault et al., 1996; McGoldrick and Murphy, 2016). The target species is lake trout in Lakes Superior, Huron, Michigan, and Ontario and walleye in Lake Erie. In 2013, after several years of collection and comparison of lake trout and walleye (2008–2011), lake trout replaced walleye as the target species in the eastern basin of Lake Erie. Historically, lake trout in the size range of 600–700 mm were targeted with an assumption that they represented fish between the ages of six and eight years old (Elrod et al., 1996; Madenjian et al., 1998). Similarly, walleye in the size range of 400–500 mm were targeted with an assumed age of four and six years old based on broad assumptions regarding age/size relationships (GLFMSP QMP, 2012). The GLFMSP composite scheme identified that fish were to be grouped, based on length, into 10 composites of five fish each, homogenized, and then analyzed for contaminant concentrations. Beginning in 2003, fish age was assessed post homogenization from saved structures using various methods (otolith, scale, fin clip, and/or coded wire tags (CWTs)) and recorded for future use. Fish age was not assessed for the GLFMSP prior to 2003.
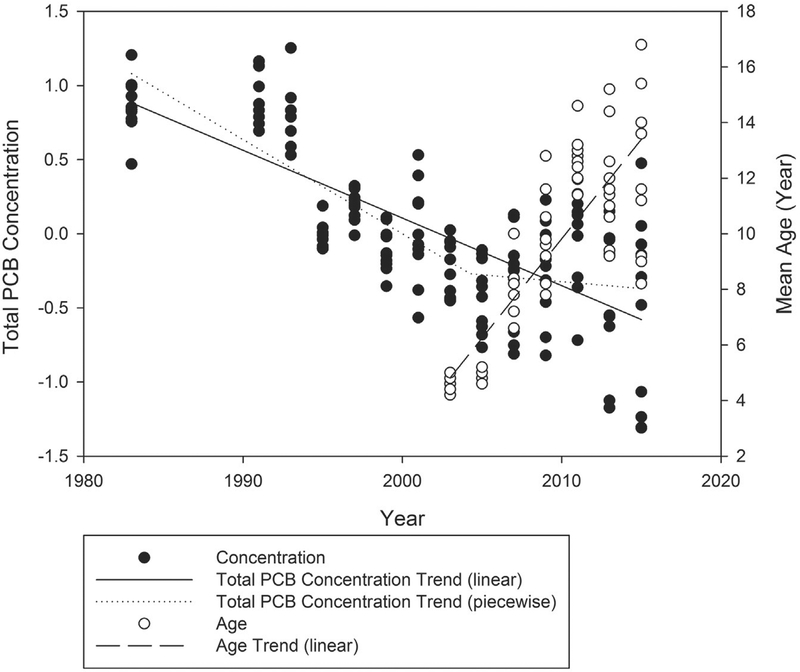
As part of routine assessments of status and trends of persistent, bioaccumulative, and toxic chemicals, the GLFMSP identified an increase in both the annual mean total PCB concentrations and the range in concentration of the ten fish composites from the Port Austin (Odd Year) site in Lake Huron (Fig. 1) in the early 2000’s, resulting in a slowing in the rate of decline of the total PCB concentration trend (Fig. 2, Total PCB Concentration piecewise trend dotted line). An apparent increase was observed in both the average age and the range of ages of composites at the Port Austin site beginning in approximately 2003 (Fig. 2, Age Trend linear dashed line). While the long term trend for total PCBs at the Port Austin site in Lake Huron continued to decline (Fig. 2, Total PCB Concentration linear trend solid line), the combination of increasing mean age of composites and the total PCB concentration trend indicated the need to investigate the source of the change and led to a retrospective review of age for GLFMSP samples in all lakes. Results of the review indicated that fish in the target size range were exceeding the target age range at some stations (Fig. 3–e).
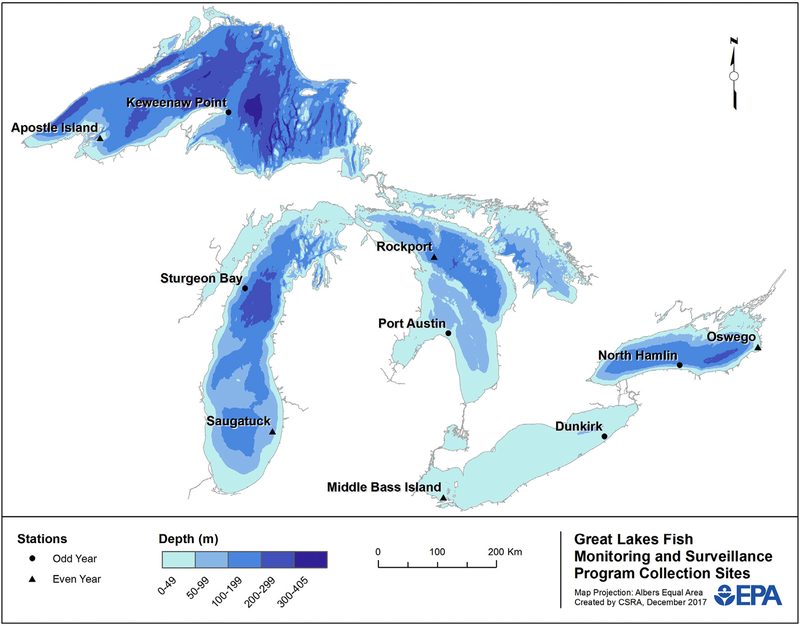
Fig. 1.
Great Lakes Fish Monitoring and Surveillance Program collection locations. Sites indicated with a circle are sampled in odd years and sites indicated with a triangle are sampled in even years.
Fig. 2.
Total PCB concentration and mean age in whole body lake trout composites from the Lake Huron at Port Austin Great Lakes Fish Monitoring and Surveillance Program site from 1983. Long term negative trends in total PCB concentration with time were found to be significant at the 95% confidence level for both the linear ((t-statistic (t) = −6.0432, p-value (p) = <0.0001, and number of observations (N) = 14) solid black line) and the first segment of the two-part piecewise linear regression ((t = −3.1474, p = 0.0255, and N = 7) dotted line). The second segment of the two-part piecewise linear regression was not fond to be significant at the 95% confidence level ((t = −0.6829, p = 0.5250, and N = 7) dotted line). The age of the collected lake trout increased significantly over time at the 95% confidence level (t = 5.4575, p = 0.0028, and N = 7) between 2003 and 2015.
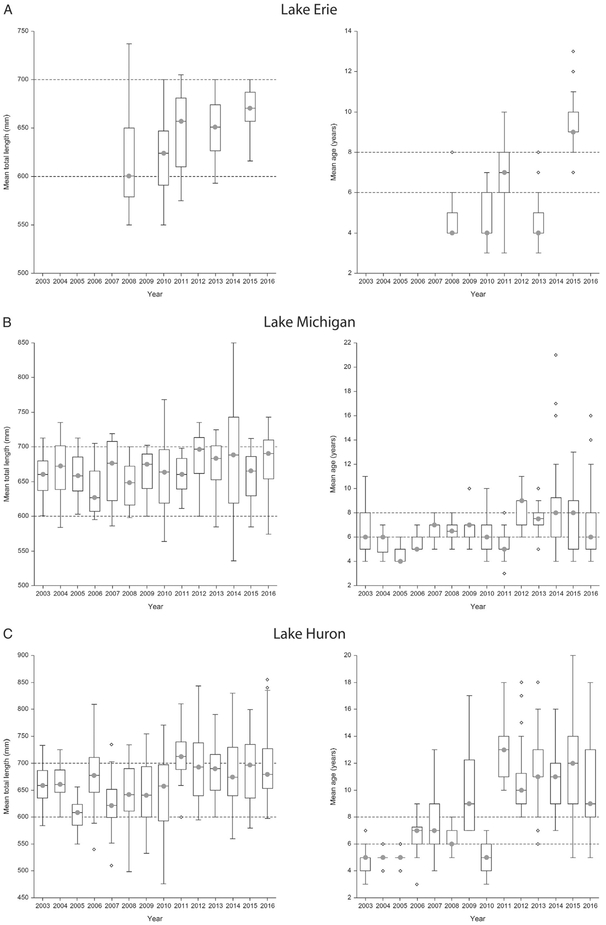
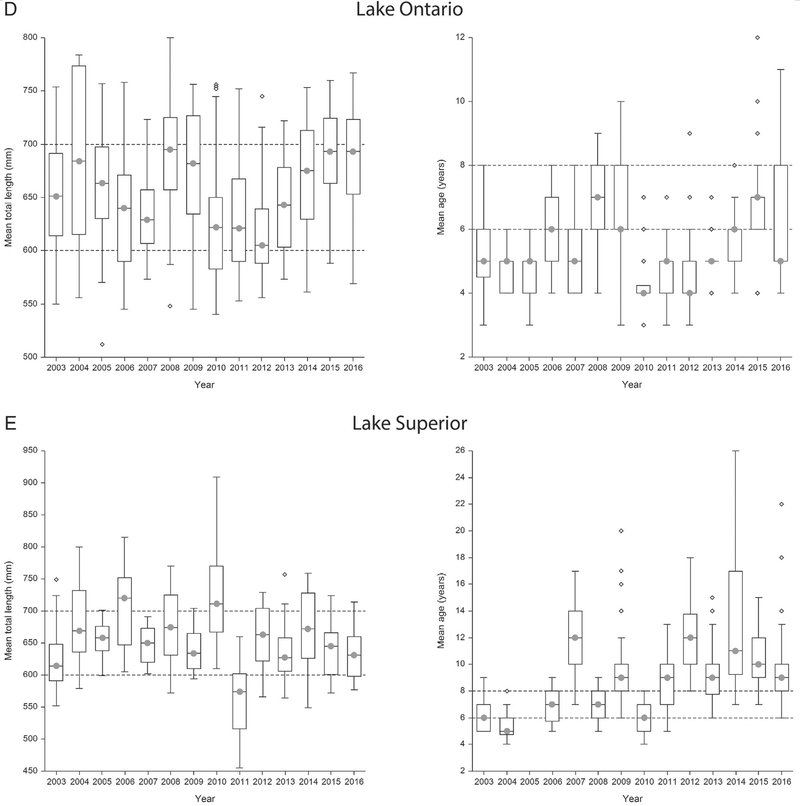
Fig. 3.
a–e Age and size ranges of lake trout collected for Great Lakes Fish Monitoring and Surveillance Program from Lake Erie (a), Lake Michigan (b), Lake Huron (c), Lake Ontario (d), and Lake Superior (e). The plots on the left identify that the majority of samples are collected in the target length range (600–700 mm) as identified by the dashed lines. The plots on the right identify that the same samples are not within the target age range (6–8 years old) with Lakes Huron and Superior exceeding the target age range most often since 2007.
The GLFMSP considered the number of documented environmental stresses in the Great Lakes that may impact fish growth and age and determined that older, and potentially more contaminated, fish can confound the long term trend assessments for the GLFMSP. Additionally, age was assessed post compositing which likely resulted in a high variability of contaminant concentrations within individual composites, making it difficult to estimate the total variability around the site mean and indicating the need to consider age in interpreting chemical results. Age contaminant relations were developed and documented by the GLFMSP and other sources which has resulted in age-normalizing when calculating contaminant concentrations (Zhou et al., 2017a, 2017b; Fernando et al., 2017; Zhou et al., 2017a, 2017b; Omara et al., 2015; Pagano et al., 2018; Sackett et al., 2013; Doetzel, 2007). To our knowledge, the application of maxillary age enumeration to age-normalize whole fish samples for contaminant concentration analysis and trends is a novel practice.
To normalize ages, a revision of the compositing scheme was needed in order to age fish quickly prior to homogenization, to better interpret data, and control for the effect of age on chemical analysis. The coordinated efforts of two laboratories contributed to a multi-year inter-laboratory comparison between the use of otoliths and maxillae for assigning lake trout ages prior to fish sample homogenization. Aquatec Biological Sciences, Inc. (Aquatec), the current homogenization laboratory supporting the GLFMSP (2011–present), and the Michigan Department of Natural Resources (MDNR), a long term sample collection partner of GLFMSP and experienced in the use of maxillae to estimate lake trout ages (Wellenkamp et al., 2015), participated in the inter-laboratory study.
Lake trout used in the study included those from all sampling locations in Lakes Superior, Michigan, Huron and Ontario during the 2013–2016 field seasons and the 2013 and 2015 collection from the eastern basin of Lake Erie. An additional 10 lake trout were also collected from the eastern basin of Lake Erie in 2014. This study was designed to 1) assess the influence of age versus size in compositing practices and 2) determine the most appropriate fish structure for the GLFMSP to use for fish age estimation prior to homogenization by analyzing accuracy and precision of maxillae and otoliths. Although the otolith structure has been widely used for age determination of lake trout, Wellenkamp et al. (2015) have documented and identified several advantages of the maxilla structure for age determination, such as ease of extraction. Incorporation of this method into the GLFMSP has identified additional benefit to the program including rapid age assessment prior to compositing and reduced fluid/tissue loss from structure removal for contaminant analysis.
Methods
Sampling and obtaining age structures
In the current GLFMSP sampling design, top predator fish are collected at two sites in each of the Great Lakes, with sites alternating within each lake annually (Fig. 2). Lake trout are collected in all lakes, and walleye are collected at one site located in the western basin of Lake Erie. Lake trout have been selected as the species of GLFMSP focus because they are the primary top predator species in the Great Lakes. Lake trout do not inhabit the shallow waters of Lake Erie; and therefore walleye were chosen as the top predator species for the GLFMSP in the western basin of this lake (GLFMSP Quality Management Plan (QMP), 2012). Field crews collect 50 fish from each lake annually according to sample collection standard operating procedures (SOPs) (GLFMSP Quality Assurance Project Plan (QAPP) for Sample Collection Activities, 2012). The Program design specifies that lake trout between 600 and 700 mm and walleye between 400 and 500 mm, with target ages of six to eight years and four to six years respectively, should be collected; and that fish of a similar size should be composited together to reduce the impact of size variation on contaminant trend data.
The inter-laboratory comparison was limited to lake trout collected from Lakes Superior, Michigan, Huron and Ontario during the 2013, 2014, 2015 and 2016 field seasons, as well as lake trout collected from Lake Erie during the 2013, 2014 and 2015 field seasons, were used for this study (Total N = 899 samples & CWT N = 263 (Electronic Supplementary Material (ESM; Table S1).
Field methods were developed with MDNR to establish uniform criteria for removal and preservation of the maxillae from lake trout in 2013. Otoliths and CWTs were removed from fish in the homogenization laboratory according to the laboratory’s methods that were developed for the GLFMSP.
Quality control
As a quality control measure, multiple annuli determinations for each lake trout were conducted by Aquatec. When determinations differed by more than one year, a consensus was reached on a final age and the annuli enumeration was reported.
MDNR implemented an annual training phase for new laboratory technicians to maintain quality control. Each year and for each set of lake trout samples, age assignments were conducted first for CWT tagged known-age fish and fin-clipped fish. The fin clip, combined with an initial read of the maxilla section, allowed for the finding of the true age. This procedure minimized potential drift and inconsistency in assigning fish ages based on the maxilla sections. To account for changes in staffing through the study, new staff members were trained by qualified staff until the new staff individual was able to demonstrate accurate age assessments from both age structures. The training used known-age samples from the previous year. All otolith and maxilla images were stored and shared using a File Transfer Protocol (FTP) website. Image files were named using the fish sample IDs.
Inter-laboratory study
Each year, Aquatec completed the initial age assessment using otoliths; and MDNR completed the initial age assessment using maxillae. In the first year (2013), the two labs collaborated to assign lake trout ages from the age structure processed in one of the two labs, and accomplished initial training for using both structures. In the subsequent years (2014–2016), blind reads (i.e., reading the image without knowledge of any information except the date of capture) and secondary final reads (i.e., confirmation of the blind age assignment with supplemental information such as fin clips from marked fish to aid in year/class assignment) were completed on all lake trout maxillae. Otoliths did not undergo secondary final reads as maxillae were used to composite fish prior to homogenization. The study team identified three consecutive direct comparisons of age using multiple structures as the target to adequately assess accuracy and precision to determine the best age structure for the GLFMSP.
Upon receipt of maxillae, MDNR prepared cross sections and photographed images based on the procedures described in Wellenkamp et al. (2015). MDNR then conducted a blind and secondary final read of maxilla sections for every individual lake trout sample and posted the digital images of each cross section on the FTP website, and a blind-read age was assigned by Aquatec.
MDNR secondary final read results were used to group fish by age, prior to homogenization, into the standard ten-fish composites. Chemical analysis, age normalization, and data interpretation were then completed by the GLFMSP analytical chemistry grantee, Clarkson University, for a suite of chemical contaminants (Zhou et al., 2017a, 2017b; Fernando et al., 2017; Zhou et al., 2017b; Omara et al., 2015; Pagano et al., 2018).
Aquatec removed otoliths from the lake trout and initially stored them dry. An otolith from each fish (when available) was mounted in resin (West System 105 epoxy resin by West Marine) and hardened with West Marine hardener. Cross-sections of the otoliths were prepared using a Buehler Isomet low speed saw equipped with a diamond blade. The resultant section was examined microscopically (20–40× magnification) and digitally photographed using a Motic MP3 microscope-mounted camera. Aquatec enumerated annuli from these photographs and posted digital images of all cross sections on the FTP website where they could be viewed and then assigned a blind age by MDNR.
Post age assignment review
We met annually to compare the age assignments from the two labs and discuss problematic structures, including those with poor image quality and irregular characteristics that prevented age assignment. All age results, including CWT assigned ages, were compiled for a measure of precision (reproducibility between laboratories) and accuracy (comparison of assessed age against known age from CWTs). The maxillae structure was used to group samples into composites prior to homogenization, but the primary age enumeration structure did vary for post age assignment over the study period (2013–2016) (ESM Table S2) due to the refinement of the age enumeration methods. Primary age enumeration structures for individual composites are defined as the structure most frequently used among the five fish within that composite. For example, when an otolith was identified as the most accurate age for 3 individuals within the composite and a maxilla was identified as the most accurate for the remaining 2 individuals in a composite, the otolith is identified as the primary age structure for the composite.
Statistical analysis
Statistical models presented in the figures were fit using SigmaPlot Version 12.0 (Systat Software, Inc., San Jose, California USA, www.systatsoftware.com) and SAS Version 9.4 (SAS Institute, Inc., Cary, NC, www.sas.com). Trends in age and concentration over time were fit using linear and two piecewise regression models, with total PCB concentration log-transformed prior to performing the analyses. For these models, the annual means of the log-transformed total PCB concentration were used to avoid pseudoreplication resulting from using multiple measurements in a single year. An example of the time trend analysis of fish growth and PCB concentrations for a long term monitoring station is given in Fig. 2 (with actual data for this figure given in the ESM Table S3 and statistical results in Table S4. For models evaluating the effect of compositing techniques on concentration and age, linear regression models were fit.
Results and discussion
Fish aging was added to the GLFMSP as a routine procedure in 2003. However, at that time, the Program’s aging procedures primarily relied on scale ages in addition to fin-clip information, and age assignment was not completed prior to lake trout sample homogenization. Age enumeration methods for the GLFMSP have been refined since age was incorporated into the Program in 2003 (ESM Table S2). A retrospective analysis of GLFMSP age results indicated that since 2007, lake trout in the target size range had consistently exceeded the target age range in Lakes Superior, Michigan and Huron (Fig. 3a–e) as assessed by otolith, fish scales, and fin clips.
In 2011, the GLFMSP primarily relied upon otoliths and CWTs (when available) for aging, as they were considered the most reliable and accurate aging structures. At that time, 100% of lake trout collected from Lake Huron exceeded the target age range (6–8 years old), and 54% of samples exceeded the target age range in Lake Superior. Fish were within the target age range in Lakes Ontario and Michigan. Those age assignments, however, were not used as part of the compositing procedure prior to sample homogenization. Rather, composites were identified based on size and age was identified post homogenization.
For the inter-laboratory comparison study years, 2013–2016, the GLFMSP relied on multiple age structures. In 2016, results indicate that the GLFMSP target age range was exceeded 65% of the time in Lake Superior, and 70%, 22%, and 1% of the time in Lakes Huron, Michigan and Ontario respectively (Fig. 3a–e). Results of the inter-laboratory study clearly indicate that fish age is a critical variable when assessing trends of chemical contaminates for the GLFMSP and that identifying accurate and rapid age assignments prior to sample homogenization assists the Program to account for the effects of age on contaminant accumulation.
Additionally, this assessment identified that for a given size range, the age range may be large and that age composition can vary substantially from lake to lake (Fig. 3a–e). In Lake Michigan, as well as Lake Ontario, where alewives (Alosa pseudoharengus) remain the most important component of lake trout diet, some small increases in total length of lake trout samples, even within the target size range, have led the mean age of lake trout samples to substantially exceed the target age range. This is due to general slowed growth of adult lake trout in lake ecosystems that lack large bodied prey fish (Elrod et al., 1996; Madenjian et al., 1998). In Lake Huron, major declines in the abundance of alewives during the middle 1990s, and the complete collapse of ale-wives in 2003, have substantially further reduced lake trout growth and body condition (He et al., 2015, 2016). In Lake Superior, noticeable declines in lake trout growth are due to increases in lake trout abundance (Sitar et al., 2014; Pratt et al., 2016).
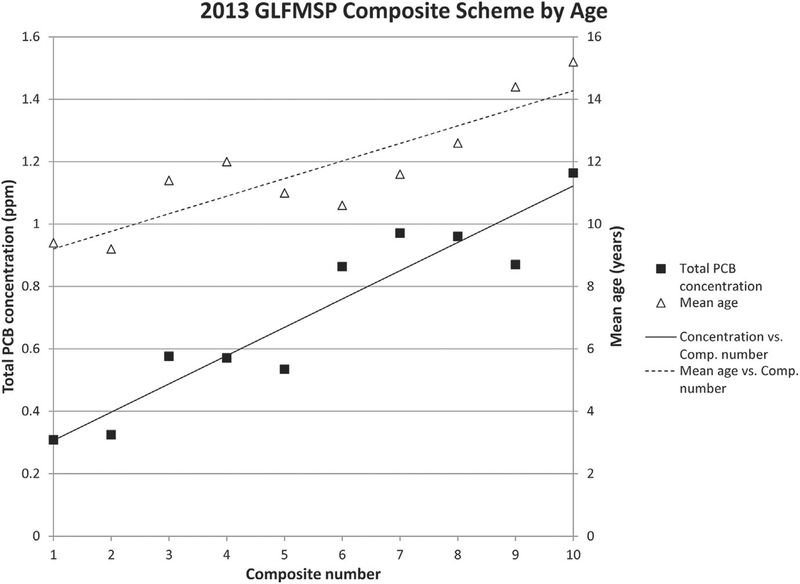
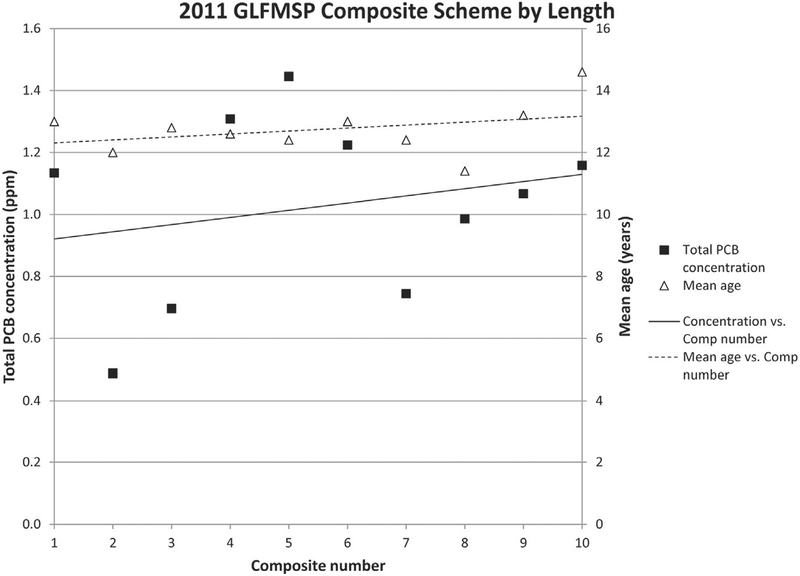
As part of the study, the GLFMSP composite scheme was revised from grouping fish based on length to grouping fish based on age assessed from maxilla enumeration. A comparison of total PCB concentration of individual composites from the Port Austin site was completed in two consecutive sampling years, 2011 and 2013, using the two different compositing schemes. The results indicated that total PCB concentration are more closely correlated with age when composites are grouped by age assessed prior to homogenization (Fig. 4) rather than by length (Fig. 5). The correlation between age and contaminant concentrations (r = 0.8) is much greater in 2013 (Fig. 4) than in 2011 (r = 0.3) (Fig. 5). The regression slopes are significant at the 95% confidence level for both concentration and age in 2013, but neither are significant in 2011. The strong relationship between contaminant concentration across composites provides a good indication that by grouping composites by age, we have decreased variability in contaminant concentration within each composite which will allow for a more accurate estimate of variability around the site mean and lead to more confident interpretation of trends. Note that the fish age distribution was slightly wider in 2013 than in 2011 (See actual data for Figs. 4 and 5 in ESM). Because this increased age variability would likely mean that the concentration variability between fish also would be greater for 2013 compared to 2011, the observed impact of age-based compositing on the composite/concentration association seen in 2013 would not likely have been as strong if it were applied to the 2011 data. Results of the inter-laboratory study clearly indicate that prior to sample homogenization, accurate and rapid age assignments are required for GLFMSP samples and will assist the Program to account for the effects of age on contaminant accumulation.
Fig. 4.
2013 Great Lakes Fish Monitoring and Surveillance Program lake trout compositing scheme by length from the Port Austin, Lake Huron site. Mean total PCB concentrations are represented by black squares and mean ages for individual composites are represented by black triangles. A linear trend is plotted for both mean total PCB concentration, solid line, and for mean age, dashed line. Lake trout composites are arranged according to similar fish age (youngest fish in first composite and age increases left to right) which was assessed prior to compositing. Correlation between PCB concentration and mean age is r = 0.80. The regression slopes are significant at the 95% confidence level for both concentration and age (2013 Total PCB concentration: t = 8.00, p < 0.0001 and 2013 Mean Age: t = 5.27, p = 0.0008).
Fig. 5.
2011 Great Lakes Fish Monitoring and Surveillance Program lake trout compositing scheme by length from Port Austin, Lake Huron. Mean total PCB concentrations are represented by the black squares and mean ages for individual composites are represented by black triangles. A linear trend is plotted for both mean total PCB concentration, solid line, and for mean age, dashed line. Lake trout composites were arranged according to similar sized fish and age was assessed post compositing. Correlation between PCB concentration and mean age is r = 0.30. Neither regression slopes are significant at the 95% confidence level for concentration or age (2011 Total PCB concentration: t = 0.68, p = 0.515 and 2011 Mean Age: t = 1.04, p =0.331).
Precision
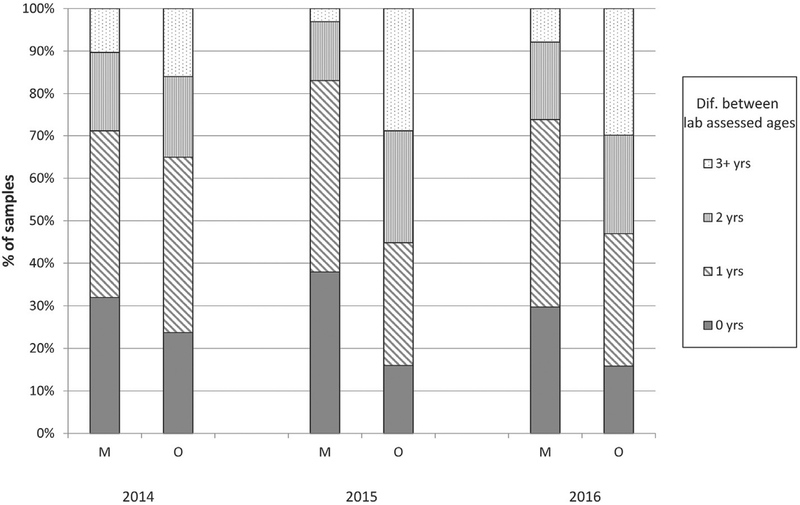
Based on age assignments from the inter-laboratory study, precision of a given method was assessed through the measures of absolute differences in the number of years between the two laboratory-determined ages (categorized as 0, ±1, 2, or 3+ years), (Fig. 6). Differences varied between years and structures; however, maxilla-based age assignments from the two laboratories agreed about 76% of the time within ±1 year and 93% of the time within ±2 years, while otolith-based age assignments from the two laboratories had much lower agreement at 52% and 75%, within ±1 and ±2 years, respectively. These across–laboratory results indicated that using the maxillae provided more reproducible age assignments than using otoliths for GLFMSP samples.
Fig. 6.
Frequency of absolute difference between age assignments by MDNR and Aquatec (Precision). M: using maxilla section. O: using otolith section.
Accuracy
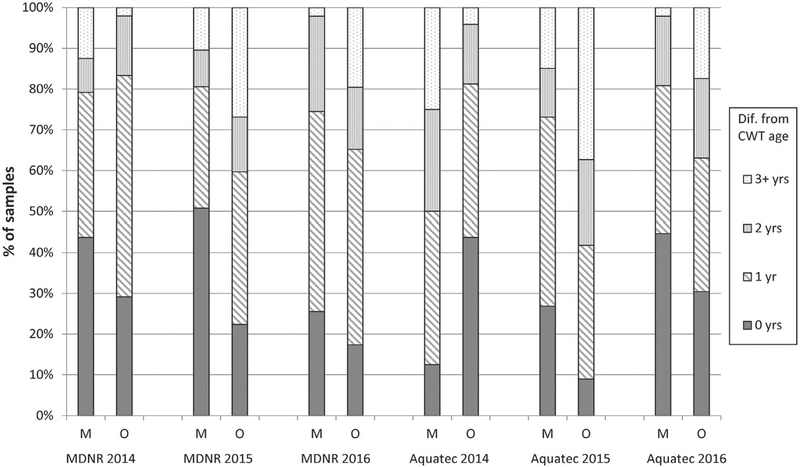
The study also included an assessment of the accuracy of each aging method by comparing blind age assignment for each structure against known ages from CWTs (Fig. 7). Overall, 73% of maxilla estimated ages were within ±1 year of the known CWT age and about 89% were within ±2 years of the known CWT age, while 66% of otolith estimated ages were within ±1 year of the known CWT age and 82% were within ± 2 years of the known CWT age. Similar to the assessment of precision, using maxillae provided more accurate age estimations than using otoliths for GLFMSP samples.
Fig. 7.
Frequency of absolute difference between age assigned and the known age based on coded wire tags (CWT) (Accuracy). M: evaluating the primary maxilla age assigned by MDNR. O: evaluating the primary otolith age assigned by Aquatec.
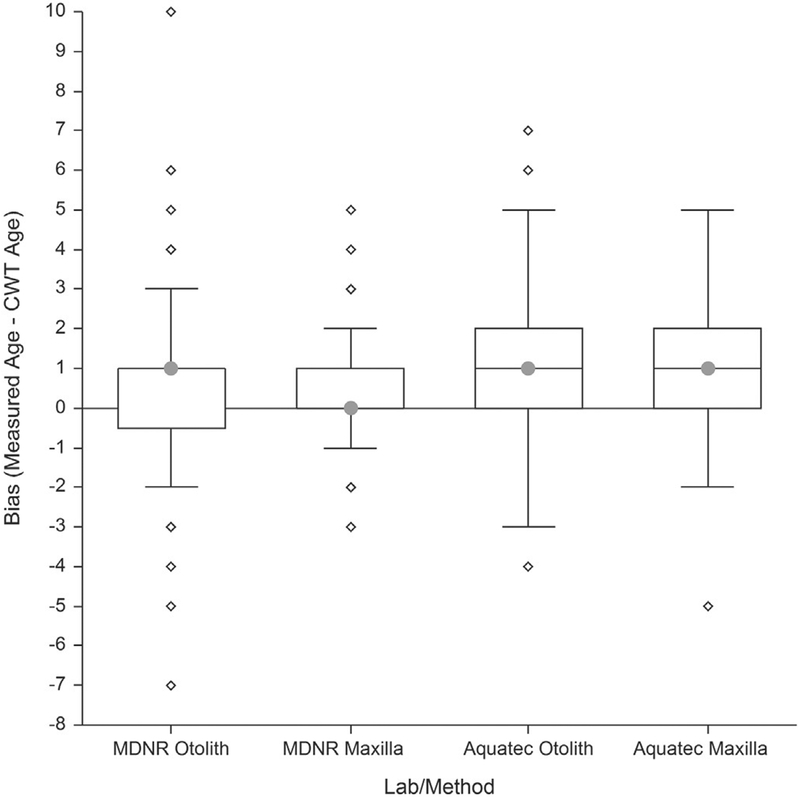
Bias was also assessed by subtracting the known CWT age from the blind age assignment for each structure (Fig. 8). This assessment indicated that 1) both labs tend to overestimate age using both structures and 2) there is no apparent difference between structures in the magnitude of bias. An additional review of those age assignments based on blind reads of maxilla sections was completed and indicated that those biases using the maxilla method are easily corrected by further clarifying and communicating age assignment decision rules applied to the lake trout samples from a specific lake. Thus, accuracy of the maxilla method can be further improved in future applications.
Fig. 8.
Box plots showing bias distribution based on data from 2014 to 2016. Bias was calculated by subtracting the known coded wire tag age from the blind measured age for each structure of the two structures by each of the two labs.
We also identified that a greater number of problematic structures were identified in Lake Ontario than in other Lakes and that age determination for those samples were also the least accurate. The underlying reason is unknown, but large numbers of hatchery-reared lake trout were collected in this lake and hatchery practices may be a contributing factor. Meanwhile, the highest precision achieved across the two labs was for lake trout collected from Lake Superior, where most samples were wild lake trout. Increases in precision and accuracy of age assignments for lake trout collected from fisheries and ecosystems undergoing continued changes over time will improve the comparability of contaminant protocols utilizing composite samples.
Conclusion
We found that the precision of the maxillary age enumeration method was greater between the two laboratories than for the otolith age enumeration. Based on higher accuracy, precision, and smaller bias, the maxillary enumeration method will be used by the GLFMSP into the future for lake trout samples from all lakes. Maxilla age, in addition to fish size, will now be used by the GLFMSP to direct the compositing scheme prior to homogenization to minimize the effects of age on contaminant results and allow the GLFMSP to better interpret contaminant trends. GLFMSP fish will continue to be collected in the target size range (600–700 mm for lake trout and 400–500 mm for walleye) in the immediate future. The decision to utilize the maxillae as the primary aging structure for the GLFMSP is significant as it will allow for rapid and accurate age identification prior to homogenization. Consequently the Program can incorporate the added benefit of field extractions, reduced lab processing and reduced fluid loss from the thawing and extraction of the otolith in the lab prior to homogenization (He, 2014; Wellenkamp et al., 2015). It will be important, however, to continue to validate the maxilla enumeration method against CWTs (when available) in the post age assessment review annually to confirm consistency of methods for final age reporting.
The revised age assignment procedure has been incorporated in the GLFMSP. Annual age assignments to GLFMSP samples will be added to the publicly available Great Lakes Environmental Database (GLENDA) for use by natural resources and fishery management agencies in the Great Lakes. Age data will continue to be an important variable for consideration in interpretation of persistent and bioaccumulative chemical results.
In the future, the GLFMSP will need to further consider how this shift in age structure is affecting the long term Program’s assessment of status and trends of persistent, bioaccumulative, and toxic chemicals. Areas for additional work and refinement include 1) decision rule refinement to aid in age determination that reflect ecological and hatchery supported fishery differences among lakes, 2) evaluation of the appropriateness of the GLFMSP target size collection and 3) continued evaluation of the accuracy of maxilla structures against known ages determined from CWTs and marked fish, when available. There is an expectation that the U. S. Fish and Wildlife Service’s Mass Marking Program will significantly increase the number of marked lake trout in the Great Lakes. Evaluation of the maxilla method against the true ages from marked lake trout samples collected to support the GLFMSP will allow for improved accuracy and precision of the maxilla age determination method.
Supplementary Material
Acknowledgements
Funding for this work was provided by the Great Lakes Restoration Initiative under the United States Environmental Protection Agency, grant number 00E01172 and Contract number EP-C-15–012, Work Assignment 2–14, with CSRA LLC. The views expressed in this publication are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. We wish to thank the Clarkson University Research Consortium, GLFMSP analytical chemistry partners, and to give a special thanks to James Johnson, retired (MDNR) for his encouragement and dedication to this project.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jglr.2018.05.006.
References
- Barbiero RP, Lesht BM, Warren GJ, 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. J. Great Lakes Res. 38, 368–380. [Google Scholar]
- Barbiero RP, Lesht BM, Warren GJ, Reavie ED, Rudstam LG, Watkins JM, Kovalenko KE, Karatayev AY, 2018. . this issue. Insights on Drivers of Recent Changes in Nutrients and Lower Food Web Structure in Lake Michigan and Lake Huron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell DB, Barbiero RP, Ludsin SA, Madenjian CP, Warren GJ, Dolan DM, Brenden TO, Briland R, Gorman OT, He JX, Johengen TH, Lantry BF, Lesht BM, Nalepa TF, Riley SC, Riseng CM, Treska TJ, Tsehaye I, Walsh MG, Warner DM, Weidel BC, 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. BioSci. 64, 26–39. [Google Scholar]
- Carlson DL, Swackhamer DL, 2006. Results from the U.S. great lakes fish monitoring program and effects of lake processes on contaminant concentrations. J. Great Lakes Res. 32 (2), 370–385. [Google Scholar]
- Carlson DL, DeVault DS, Swackhamer DL, 2010. On the rate of decline of persistent organic contaminants in lake trout (Salvelinus namaycush) from the Great Lakes, 1970– 2003. Environ. Sci. Technol 44 (6), 2004–2010. [DOI] [PubMed] [Google Scholar]
- Chang F, Pagano JJ, Crimmins BS, Milligan MS, Xia X, Hopke PK, Holsen TM, 2012. Temporal trends of polychlorinated biphenyls and organochlorine pesticides in Great Lakes fish, 1999–2009. Sci. Total Environ. 439, 284–290. [DOI] [PubMed] [Google Scholar]
- De Vault DS, Hesselberg R, Rodgers PW, Feist TJ, 1996. Contaminant Trends in lake trout and walleye from the Laurentian Great Lakes. J. Great Lakes Res. 22 (4), 884–895. [Google Scholar]
- Doetzel LM, 2007. A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Toxicology Graduate Program. University of Saskatchewan, Saskatoon. [Google Scholar]
- Elrod JH, O’Gorman R, Schneider CP, 1996. Bathythermal distribution, maturity and growth of lake trout strains stocked in U. S. Waters of Lake Ontario, 1978–1993. J. Great Lakes Res. 22 (3), 722–743. [Google Scholar]
- Fernando S, Renaguli A, Milligan M, Pagano J, Hopke P, Holsen T, Crimmins B, 2017Comprehensive analysis of the Great Lakes top predator fish for novel halogenated organic contaminants by GCxGC-HR-ToF. Environ. Sci. Technol 52 (5), 2909–2917. [DOI] [PubMed] [Google Scholar]
- GLFMSP Quality Assurance Project Plan (QAPP) for Sample Collection Activities https://www.epa.gov/sites/production/files/2016-02/documents/glfmsp_qapp_version_2_111312_merged_508_0.pdf [accessed 16-1-/2017].
- GLFMSP Quality Management Plan (QMP) https://www.epa.gov/sites/production/files/2016-02/documents/glfmsp_qmp_version_2_final_111312_508.pdf [accessed 16-1-/2017].
- Great Lakes Environmental Database (GLENDA) https://www.epa.gov/great-lakes-legacy-act/great-lakes-environmental-database-glenda [accessed 16-1-/2017].
- He JX, 2014. Workshops on lake trout age assignments in annual stock assessment: accuracy and precision of methods specific to age ranges and length bins 2014 Project Progress Report. Great Lakes Fishery Commission. [Google Scholar]
- He JX, Bence JR, Madenjian CP, Pothoven SA, Dobiesz NE, Fielder DG, Johnson JE, Ebener MP, Cottrill AR, Mohr LC, Koproski SR, 2015. Coupling age-structured stock assessment and fish bioenergetics models: a system of time-varying models for quantifying piscivory patterns during the rapid trophic shift in the main basin of Lake Huron. Can. J. Fish. Aquat. Sci 72 (1), 7–23. [Google Scholar]
- He JX, Bence JR, Roseman EF, Fielder DG, Ebener MP, 2016. Using time-varying asymptotic length and body condition of top piscivores to indicate ecosystem regime shift in the main basin of Lake Huron: a Bayesian hierarchical modeling approach. Can. J. Fish. Aquat. Sci 73 (7), 1092–1103. [Google Scholar]
- Lake Michigan Lake Trout Working Group Report http://www.glfc.org/pubs/lake_committees/michigan/LTWG_docs/Lake%20Trout%20Working%20Group%20Report%202016.pdf [accessed 18-14-2018].
- Madenjian CP, DeSorcie TJ, Stedman RM, 1998. Maturity Schedules of lake trout in Lake Michigan. J. Great Lakes Res. 24 (2), 404–410. [Google Scholar]
- McGoldrick DJ, Murphy EW, 2016. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008–2012). Environ. Pollut 217, 85–96. [DOI] [PubMed] [Google Scholar]
- Omara M, Crimmins BS, Back BC, Hopke PK, Chang FC, Holsen TM, 2015. Mercury biomagnification and contemporaryfood web dynamics in Lakes Superior and Huron. J. Great Lakes Res. 41, 473–483. [Google Scholar]
- Pagano JJ, Garner AJ, McGoldrick DJ, Crimmins BS, Hopke PK, Milligan MS, Holsen TM, 2018. Corrected trends and toxic equivalence of PCDD/F and CP-PCBs in lake trout and walleye from the Great Lakes: 2004–2014. Environ. Sci. Technol 52 (2), 712–721 (January 16). [DOI] [PubMed] [Google Scholar]
- Pratt TC, Gorman OT, Mattes WP, Myers JT, Quinlan HR, Schreiner DR, Seider MJ, Sitar SP, Yule DL, Yurista PM, 2016. The State of Lake Superior in 2011. Great Lakes Fish. Comm. Spec. Pub.16_01 Available from:. http://www.glfc.org/pubs/SpecialPubs/Sp16_01.pdf.
- Sackett DK, Cope WG, Rice JA, Aday DD, 2013. The influence of fish length on tissue mercury dynamics: implications for natural resource management and human health risk. Int. J. Environ. Res. Public Health 10, 638–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitar SP, Jasonowicz AJ, Murphy CA, Goetz FW, 2014. Estimates of skipped spawning in lean and siscowet lake trout in southern Lake Superior: implications for stock assessment. Trans. Am. Fish. Soc 143 (3), 660–672. [Google Scholar]
- Tsehaye I, Jones ML, Bence JR, Brenden TO, Madenjian CP, Warner DM, 2014. Assessing the balance between predatory consumption and prey dynamics in the Lake Michigan pelagic fish community. Can. J. Fish. Aquat. Sci 71 (4):627–644. 10.1139/cjfas-2013-0313. [DOI] [Google Scholar]
- USGS (U.S. Geological Survey), 2016. Compiled Reports to the Great Lakes Fishery Commission of the Annual Bottom Trawl and Acoustics Surveys for 2015 [online].Available at:. http://www.glfc.org/pubs/lake_committees/common_docs/CompiledReportsfromUSGS2016.pdf [accessed 16-1-/2017].
- Wellenkamp W, He JX, Vercnocke D, 2015. Using maxillae to estimate ages of lake trout. N. Am. J. Fish Manag. 35, 296–301. [Google Scholar]
- Zhou C, Cohen MD, Crimmins BA, Zhou H, Johnson TA, Hopke PK, Holsen TM, 2017a. Mercury temporal trends in top predator fish of the Laurentian Great Lakes from 2004 to 2015: are the concentrations still decreasing? Environ. Sci. Technol 51, 7386–7394. [DOI] [PubMed] [Google Scholar]
- Zhou C, Pagano J, Crimmins BA, Hopke PK, Milligan MS, Murphy EW, Holsen TM, 2017b. Polychlorinated Biphenyls and Organochlorine Pesticides Concentration Patterns and Trends in Top Predator Fish of Laurentian Great Lakes from 1999 to 2014 this Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.