Abstract
We are in the midst of a technological revolution that is providing new insights into human biology and cancer. In this era of big data, we are amassing large amounts of information that is transforming how we approach cancer treatment and prevention. Enactment of the Cancer Moonshot within the 21st Century Cures Act in the USA arrived at a propitious moment in the advancement of knowledge, providing nearly US$2 billion of funding for cancer research and precision medicine. In 2016, the Blue Ribbon Panel (BRP) set out a roadmap of recommendations designed to exploit new advances in cancer diagnosis, prevention, and treatment. Those recommendations provided a high-level view of how to accelerate the conversion of new scientific discoveries into effective treatments and prevention for cancer. The US National Cancer Institute is already implementing some of those recommendations. As experts in the priority areas identified by the BRP, we bolster those recommendations to implement this important scientific roadmap. In this Commission, we examine the BRP recommendations in greater detail and expand the discussion to include additional priority areas, including surgical oncology, radiation oncology, imaging, health systems and health disparities, regulation and financing, population science, and oncopolicy. We prioritise areas of research in the USA that we believe would accelerate efforts to benefit patients with cancer. Finally, we hope the recommendations in this report will facilitate new international collaborations to further enhance global efforts in cancer control.
Part 1: Introduction
In a US State of the Union address in 2016, President Barack Obama called on US Vice President Joe Biden to lead a new, national Cancer Moonshot to accelerate efforts to prevent, diagnose, and treat cancer—to achieve a decade of progress in just 5 years. The resulting Cancer Moonshot Task Force brought together all relevant federal agencies and fostered more than 70 private sector collaborations to focus on transforming cancer research and care. The Blue Ribbon Panel (BRP) was established as part of this programme and produced a report to guide federal funding decisions, reflecting the combined effort of government, private industry, researchers, oncologists, patients, advocates, and philanthropic organisations. The report made ten recommendations and suggested three demonstration projects (panel 1).1 Following the change in the US Government’s administration in 2017, the spirit and ambitions of the Cancer Moonshot lives on through the many institutions and hospitals across the country and Joe Biden, along with his wife, Jill Biden, went on to form the Biden Cancer Initiative, a non-governmental organ-isation to champion enhanced research efforts to tackle the growing cancer epidemic.
Panel 1: The recommendations and demonstration projects from the Blue Ribbon Panel report 2016.
Ten recommendations
Network for direct patient engagement
Cancer immunotherapy clinical trials network
Therapeutic target identification to overcome drug resistance
A national cancer data ecosystem for sharing and analysis
Fusion oncoproteins in childhood cancers
Symptom management research
Prevention and early detection: implementation of evidence-based approaches
Retrospective analysis of biospecimens from patients treated with standard of care
Generation of human tumour atlases
Development of new enabling cancer technologies
Demonstration projects
Paediatric immunotherapy translational science network
Advancing cancer prevention: Lynch Syndrome demonstration project
Emergent technologies for intra- and extra-tumour pharmacotyping
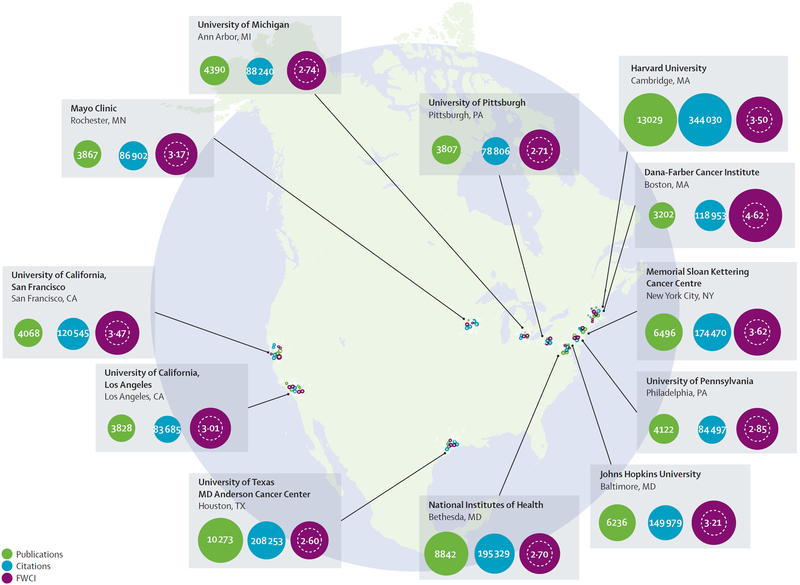
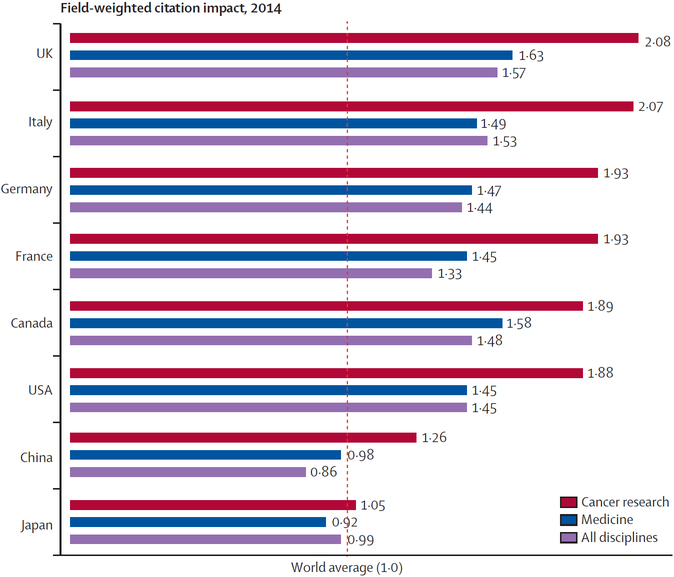
The BRP recommendations were built on recent discoveries and rapidly accumulating big data that provide new insights into how cancers develop and progress.2 Although specific research institutes in the USA produce a substantial body of highly-cited research outputs (figure 1), the USA lags behind other high-income countries, on the basis of field-weighted citation impact, in cancer research (figure 2). To ensure that cancer research in the USA continues to be world-leading, it is imperative that investment is concentrated into specific research areas. The BRP report, by necessity, could only devote a small amount of attention to each recommendation. This Commission was borne out of one of the public–private partnerships in the original Cancer Moonshot Task Force under the Obama administration. Our goals were to take a deeper look at the BRP’s ten original recommendations, provide more insights to bolster these recommendations, and suggest a more detailed plan for their implementation. To accomplish this, the Commission convened a group of cancer experts, including many who contributed to the BRP report, along with national experts in areas that required more in-depth analysis, along with international experts to identify ways to move these priorities into globally relevant points of discussion and possible collaboration.
Figure 1: A selection of leading US cancer research institutes along with publication output, total citations, and field-weighted citation impact (FWCI), 2011–15.
World average for FWCI 1.00. Adapted with permission from the Elsevier Cancer Research Report.3
Figure 2: Field-weighted citation impact in cancer research, medicine, and all disciplines for selected countries in 2014.
Source: Scopus. Adapted with permission from the Elsevier Cancer Research Report.3
This report is divided into areas of research priorities. Each priority area is considered equally important to change cancer as we know it, and the list is therefore not arranged in any order of priority. Within each subject area, we highlight specific recommendations that will both directly improve cancer treatment and care, and are ready for immediate action. We also explore topics that were beyond the scope of the BRP report, but are of equal importance and must be considered in the overall landscape. These additional areas include surgical oncology, radiation oncology, nuclear medicine and imaging, and oncopolicy. Some priorities have been broken down to focus on more specific areas including early detection and prevention, genetics and epigenetics, drug development, and big data. Similar to the BRP report, new technologies are discussed within each section, rather than as a separate section, to provide specific examples of why these technologies are needed to further advance the transformation of science into new interventions. Many of the important overlapping themes that emerged from the BRP recommendations are highlighted in this report. First and foremost is the need to consider health disparities and population sciences, including dissemination of cancer care, in all of the recommendations. Patient outcomes are greatly affected by racial, cultural, and socioeconomic background and access to equivalent care. At a time when health-care policy is a political target, we must make sure these issues are considered as integral components. A second and equally important theme is that of data-sharing. In this era of big data, all sectors of the cancer community must come together to identify ways to share information in real time while satisfying the needs of every participant. We must move away from silos and become patient-focused. Thought-leaders in academia, government, industry, patient advocacy, and foundations should all help ensure success.
The importance of patient-centred priorities in science cannot be understated. As a component of this ambition, the BRP recommended developing an ecosystem that is accessible to patients. This report strongly supports developing data systems that allow patients to input their own personal data for use by the cancer community and, in return, provide outputs to patients that allow them to identify the most scientifically sound clinical trials for which they might be eligible. We need to empower patients to take part in contributing their data and allow them to have access to the information in real time so they can make scientifically sound decisions for their continued care. The BRP refers to this process as preregistration for clinical trials.
Three other priority areas are prevention, paediatric cancers, and survivorship. Cancers in children, adolescents, and young adults have different biologies to adult cancers. Yet these differences have not been taken into account in drug development. We provide more specific recommendations on how to tackle these rare, but deadly cancers in young patient groups. Until recently, both prevention and survivorship had not received sufficient scientific focus. However, in this era of targeted and precision medicine, patients with cancers that were once lethal are now living longer with cancer as a chronic condition. We have identified specific areas of research that are needed to combat the long-term morbidities associated with these new treatments. Additionally, advances in technologies make it possible to identify small changes in healthy tissue at the earliest stages of cancer development, which opens up new opportunities to intercept premalignancies and prevent cancer development, but these need to be balanced pragmatically with the risks of overtreatment.
For cancer research in the USA to succeed, all sectors of the cancer research community must come together, work beyond national borders whenever necessary, and make sure that each has a stake in the outcomes. Improving patient access and quality of cancer care is a fundamental pillar for success. Expanding access, decreasing disparities, and improving patient-centred outcomes is crucial. Although our recommendations are meant to be a foundation for implementation, we are cognisant of the complexity of diverse stakeholders and the fluidity of politics in the USA. However, we hope that the threat of cancer, which affects people of all ideologies, will galvanise the entire community to catalyse the ambitions of the original Cancer Moonshot initiative. We also hope these recommendations will facilitate international collaborations to accelerate global efforts to tackle cancer control more effectively than at present.
Part 2: Prevention
Only half of patients who develop cancer can be cured with existing therapies; the other half will die of their disease. Therapeutic interventions have improved, converting some previously fatal cancers to ones that can be treated like chronic diseases. However, even under these circumstances, patients will suffer chronic morbidities that limit their quality of life. For this reason, the BRP identified the development of cancer prevention approaches as a scientific priority. Available chemoprevention is associated with severe adverse effects that hinder wide-scale dissemination to healthy populations at risk for cancer. In this section, we focus on the challenges and opportunities in developing both precision-based and immune-based cancer prevention that is safe and without substantial (short-term or long-term) severe adverse effects. We also discuss lifestyle changes that affect cancer development. Our considerations for potential interventions will focus primarily on current understanding and knowledge gaps of both the genetic and inflammatory signals that drive the initiation of premalignancies and facilitate cancer development. Although equally important, behavioural approaches to control smoking, obesity, and sedentary behaviour that modify environmental exposures and cancer risk have been recently reviewed elsewhere.4,5
Lifestyle and cancer
More than half of cancers are preventable.4 In particular, diet, nutrition, physical inactivity, chronic emotional stress, inadequate social support, and obesity are thought to be important contributors to increasing global cancer incidence.5 Changes in these lifestyle behaviours might reduce the cancer burden: our genes are a predisposition, not necessarily our fate. Our genetic background, however, affects how and where tumours develop, which could improve screening and pre-emptive preventive designs.6
In a large pooled analysis of 1·44 million participants (186 932 cancer cases) and their self-reported physical activity,7 high levels of leisure-time physical activity were associated with reduced risks of 13 cancer types. In a large meta-analysis8 of cancer mortality from 71 studies, the highest levels of physical activity were associated with significantly reduced all-cancer mortality, especially in cancer survivors. Occupational sedentariness was an independent risk factor for premenopausal breast, colon, and endometrial cancer.9 Mortality meta-analysis data10 (including cardiac and cancer status) from more than 1 million adults showed complex joint effects of physical activity and sedentary behaviour. Wholefoods, plant-based diets, and the Mediterranean diet are protective against various types of cancer, cardiovascular disease, ageing, and obesity.11
A responsible approach to lifestyle medicine for cancer is to integrate the best of traditional and non-traditional methods in prevention and treatment interventions. For example, comprehensive lifestyle changes might slow progression of localised prostate cancer. In one study,12 men who had chosen active surveillance were randomly assigned to a non-intervention control group or to an intensive lifestyle intervention. The lifestyle modifications included a wholefoods, plant-based diet (vegetarian, reduced fat and refined carbohydrates), stress-management techniques (including yoga and meditation), moderate exercise (such as walking), and social support and community (love and intimacy). After 1 year, prostate-specific antigen concentrations had decreased significantly in the experimental group but increased in the control group. Intriguingly, results of further studies showed that gene expression changed within 3 months of lifestyle adaptation. 48 protective genes were upregulated and 453 genes were downregulated—including genes and oncogenes known to affect oxidative stress, chronic inflammation, and the regulation of prostate, breast, and colon cancer.12 Telomerase activity also increased by 30%,13 and after 5 years, there was a significant increase in telomere length and a significant correlation between degree of lifestyle change and changes in telomere length.14 These same lifestyle changes have also been shown to reverse the progression of coronary heart disease, with fewer cardiac events at 5 years,15 and to prevent type 2 diabetes, hypertension, and hypercholesterolaemia.16 Chronic perceived stress is inversely associated with telomere length and associated with cancer and heart disease, but can be ameliorated by exercise and other lifestyle changes.17
In the Physicians’ Health Study,18 men with localised prostate cancer who ate a mostly western diet had 2·5 times higher risk of prostate cancer-related death than men in the lowest quartile after 9·9 years. By contrast, men who ate a more plant-based diet had a 36% lower risk of death from all causes. Men with prostate cancer who ate a lot of vegetables, fruit, fish, and whole grains and had a Mediterranean diet had better survival than men on western diets.18 Dietary effects might be important for specific subgroups. For example, although fruit and vegetable intake had no effect on overall breast cancer risk in the EPIC cohort,19 results of a pooled analysis of 993 466 women from 20 prospective cohort studies (followed for 11–20 years) showed a significant inverse association between vegetable intake and risk of oestrogen receptor-negative breast cancer20—a provocative finding that has been confirmed in several cohorts.21 For example, the results of the PREDIMED randomised controlled trial22 suggested that a Mediterranean diet supplemented with extra-virgin olive oil prevented breast cancer.
Lifestyle changes have such diverse and far-reaching benefits in many chronic diseases driven by chronic inflammation, oxidative stress, and impaired immune function, which can be beneficially affected by healthy lifestyle changes. For example, obesity produces an inflammatory state characterised by nuclear factor κB activation, increased production of interleukin 6 and other cytokines, and macrophage polarity switching.23 Animal protein, especially from red meat, significantly increases risk and progression of colorectal cancer, type 2 diabetes, and all-cause mortality.24,25 Furthermore, the heterocyclic amines in cooked meat are known carcinogens. High-protein diets can reduce beneficial microbiota (and metabolites) and immune protection.26 The effects of whole grain and fibre-rich diet in colon cancer vary by gut microbiota.27 High-fat diets can induce intestinal progenitor cells to a more stem cell-like fate, increasing tumour incidence. Regular consumption of beverages with high sugar content also increases cancer risk. Despite being calorically equivalent, fructose and glucose are biochemically quite different. Fructose is almost exclusively metabolised in the liver by ketohexokinase. Wild-type mice fed a western diet (high in fat and fructose) developed severe fatty liver and non-alcoholic steatohepatitis (NASH). Ketohexokinasedeficient mice fed the same diet were largely protected from NASH. Such outcomes strongly suggest that overeating fructose promotes obesity and metabolic syndrome, which are linked to many cancers.28 By contrast, carbohydrate-containing wholefoods like legumes, non-starchy vegetables, fruits, and whole grains have been shown to reduce cancer risk.
Diet and lifestyle choices are key drivers of obesity and changes in the microbiome. High-fat diets cause changes in the microbiome that increase the risk of obesity and other chronic diseases.29 The association between obesity and cancer is such that the International Agency for Research on Cancer has classified the evidence of a causal link as sufficient for certain cancers. Global estimates suggest that 1 billion people are overweight and that 2 billion people will be overweight by 2030. These data suggest that overeating could be the largest avoidable cause of cancer in non-smokers. Obesity is a major cause of cancer risk and mortality, including fatty liver disease-related hepatocellular carcinoma—the cancer with the most rapidly increasing incidence in the USA (especially in Hispanic men). Gut microbiota-induced metabolites promote obesity-linked immune escape in hepatic tumorigenesis.30 Circadian disruption is also associated with an increased incidence of obesity, diabetes, and cancer. Circadian dysregulation of farnesoid X receptor (FXR) promoted fatty acid-induced hepatocellular carcinoma in mice,31 providing biological plausibility for the chemopreventive benefit of an FXR ligand in patients with non-cirrhotic, non-alcoholic steatohepatitis.32
In summary, lifestyle choices have a substantial effect on the risk of developing many common cancers. Increasing evidence indicates that comprehensive lifestyle changes could prevent cancer progression, and it is therefore crucial that public health measures are strengthened to better promote these interventions as part of a more holistic approach to cancer control. The interplay between immunity and diet, lifestyle, environmental, inflammatory, and metabolomic factors, and the multibiome (including viruses and protozoa) have important implications for prevention strategies.
Chemoprevention
Results of the first randomised controlled trial of precision-based chemoprevention (EPOC)33 were reported in 2016, beginning a new era of molecular selection in this field.34 Primary findings from the EPOC study showed that erlotinib did not prevent oral cancer in patients with high-risk oral premalignant lesions (where high risk was defined by preset loss-of-heterozygosity profiles). However, when efficacy was analysed according to development of rash, erlotinib-treated patients with grade 2–3 rash at month 1 had statistically significant improvement in oral cancer-free survival compared with patients with grade 1 or no rash who were treated with erlotinib. Recent data suggest that the rash associated with EGFR inhibitors is mediated at least in part by induced immunity.35 Correlative EPOC immune profiling studies, including PD-L1 expression patterns36 in oral precancers are ongoing.
Perhaps the most promising precision-based approach to cancer prevention in the near future involves molecular selection for repurposed low-dose aspirin (on the basis of the prostaglandin pathway status and other approaches).37,38 In 2016, the US Preventive Services Task Force (USPSTF) recommended low-dose aspirin for colorectal cancer prevention, a major milestone in the field of chemoprevention.39 In view of aspirin’s potential adverse effects (eg, bleeding), tailoring aspirin use is a high priority. Data from a series of recent studies38 of colorectal neoplasia, prostaglandins, and aspirin support a precision-based approach in this setting: aspirin reduced colorectal cancer risk in patients with high expression of 15-hydroxyprostaglandin dehydrogenase mRNA in the normal mucosa adjacent to cancerous tissue.40 Somatic mutations and germline variants of PIK3CA and BRAF and HLA class I antigen expression affect aspirin efficacy in colorectal cancer prevention and are therefore promising leads under investigation prospectively.38 Furthermore, data from prospective cohort studies show that regular aspirin use is associated with a lower risk of colorectal cancer and a reduced number of tumour-infiltrating lymphocytes, suggesting that aspirin could be particularly effective at preventing tumours that rely on an immunosuppressive microenvironment for growth and progression.
Extensive investigation of the genetics of various hereditary forms of cancer have aided our understanding of sporadic neoplasia. Research of tumours in patients carrying BRCA1 or BRCA2 mutations has led to paradigm-changing, precision-based therapy with poly ADP-ribose polymerase inhibitors,41 which have preventive activity in Brca1-deficient mice.42 Similarly, Lynch syndrome is a model of immune oncology for sporadic high-level microsatellite instability tumours and immune prevention.43,44 Germline defects in NER genes can cause xeroderma pigmentosum, a rare autosomal recessive genetic disorder associated with UV-induced DNA damage, mutational signatures, and very high risk of non-melanoma skin cancer that can be reduced using bacterial DNA repair enzymes or nicotinamide,45 which prevent UV-induced immune suppression and enhance DNA repair. Advances in understanding the convergence of Wnt and EGFR signalling in familial adenomatous polyposis, a disease characterised by germline mutations in APC, led to a breakthrough trial of combinatorial chemoprevention with erlotinib and sulindac after standard prophylactic colectomy, which reduced duodenal neoplasia, the leading cause of death.46 The risk of myeloma is increased more than 30-fold in patients with the inherited lipid-storage disorder Gaucher disease, characterised by germline GBA mutations, due in part to lysolipid-induced chronic inflammation and genomic instability. Lysolipid substrate reduction in Gba1-deficient mice decreases the risk of gammopathies.47 which led to the discovery that in nearly 25% of all cases of monoclonal gammopathy of undetermined significance (MGUS) or multiple myeloma, the underlying clones might be driven by lipid antigens such as inflammation-associated bioactive lipids, which has important prevention implications in various high-risk groups.47 Patients with Gaucher disease and high-risk African cohorts have an increased incidence of polyclonal gammopathies,48 suggesting that polyclonal B-cell activation might be a less genetically complex pre-MGUS phase.
Universal colorectal cancer tumour testing for mismatch repair deficiency to screen for Lynch syndrome is a paradigm-changing approach for identifying inherited cancer risk that has become standard practice and the main trigger for confirmatory germline testing.49 An analysis of sequencing data in The Cancer Genome Atlas showed rare germline mutations (eg, in BRCA1, BRCA2, FANCM, MSH6) in 4–19% of cancer types, unselected for family history.50 Germline variants and somatic events are also intricately linked, with specific haplotypes of JAK2 Val617Phe in myeloproliferative neoplasms51 and EGFR exon 19 microdeletions and Thr790 mutations in non-small-cell lung cancer (NSCLC).52 In a pan-cancer study6 (22 tumour types, about 6000 tumours) integrating common germline loci with somatic changes, inherited variation was found to affect somatic evolution of neoplasia by directing where (organ site) and how (which genes are affected transcriptionally) cancer develops, highlighting the remarkable prospect of anticipating and intercepting key early events during tumour development. Multigene testing is broadening the spectrum of cancer risk linked to various hereditary syndromes, frequently identifying individuals with high-penetrance germline mutations that are unexpected from clinical history (eg, colorectal cancer in patients with BRCA1 or BRCA2 mutations).41,53
Immunological prevention: leveraging the recent revolution in immunotherapy
The past several years have seen a revolution in our knowledge of how the immune system interacts with cancers (panel 2). A number of crucial immune-inhibitory pathways within the tumour micro environment prevent immune recognition of cancers. As a direct result of this new knowledge, new immune oncology drugs have been developed and several have been approved for the treatment of subsets of genetically defined melanomas, NSCLCs, head and neck cancers, and bladder cancers. One of these drugs, pembrolizumab, will soon be approved for microsatellite instability high tumours.
Panel 2: The immune system and its recognition of cancerous cells.
The immune system has the potential to recognise cancer and precancer cells as foreign and kill them
The T lymphocyte or effector T cell becomes activated to kill the tumour cell by recognising those components in the cells that distinguish them from their normal counterparts; T cells can be stimulated or engineered to recognise unique molecular features of tumour cells
Further research is needed to direct T cells to recognie precancer and cancer cells as foreign and to overcome a disabling, immunosuppressive microenvironment; identification of novel premalignancy antigens and strategies to disrupt the immunosuppressive properties in the premalignant microenvironment are pivotal
The interaction between the immune system and precancerous tissue is a fundamental principle that is applicable to all or nearly all organ and cell types
Development of anti-cancer vaccines to eliminate cancers “before they develop and become malignant is an aspirational goal; as in cervical cancer, where vaccination against human papilloma virus might eventually eliminate the disease, the development of cancer vaccines to stimulate T cells to recognise precancer cells as foreign will hopefully prevent other cancers
Immunological interventions, specifically cancer vaccines, could also be key to realising precision-based prevention in certain types of neoplasia characterised by various germline mutations. Vaccines have been used successfully in preventing virus-associated cervical cancer (human papillomavirus [HPV] vaccine) and hepatomas (hepatitis B virus vaccine). For example, cancers that arise in the setting of Lynch syndrome have high-level microsatellite instability and widespread accumulation of somatic frameshift mutations, which result in immunogenic neoantigens that are thought to underlie the success of PD1 immune checkpoint inhibitors in patients with cancers associated with Lynch syndrome.36 These breakthroughs have advanced immunotherapy for mismatch repair-deficient or high-level microsatellite instability cancers, and evidence of frameshift peptide immune surveillance (and intact MHC class 1) even in healthy patients with Lynch syndrome have generated interest in the possibility of using immune-based prevention strategies (eg, cancer vaccines against predictable frameshift mutation-derived peptides). Aspirin is also a standard of care for Lynch syndrome. Whether aspirin mediates an immuno-permissive, preneoplastic microenvironment deserves further investigation. As another example, a specific mutational signature of single-base substitutions has been found in tumour samples with pathogenic germline or somatic BRCA1 or BRCA2 variants, attributed to failure of DNA double strand repair by homologous recombination. This mutational signature has been observed in breast, ovarian, pancreatic, gastric, and oesophageal cancers. In breast, ovarian, and pancreatic cancers, this BRCA mutational signature has been associated with markers of increased antitumour immunity, strongly suggesting a role for immune-based mechanisms to prevent or treat such cancers.
Building on this knowledge, major effort is needed to identify the immune-relevant antigens expressed by early premalignant lesions. Safe and effective vaccine-based approaches will be needed to successfully deliver these antigens and induce protective immunity in people at risk. These vaccines might be in the form of peptides, proteins, or viral or bacterial vectors. The identification of potent adjuvants that can specifically target at least one innate pathway and alter the developing inflammation within the premalignant lesion to favour an anticancer response is important to vaccine development. Preclinical mouse models that recapitulate the early genetic progression from normal to premalignant tissue at a time equivalent to individuals in their late teens and early adulthood will be necessary to study the inflammatory changes associated with genetic alterations that drive cancer development and to study approaches to intercepting cancer development. Existing mouse models fall short by providing the expressed genes from the time of birth.
Goals for developing approaches to cancer prevention
The overall goal is to create and implement a national strategy to discover and assess novel targeted approaches for cancer interception. These approaches will probably include precision-based interventions ranging from small molecules to vaccine interventions. Although many interventions are already approved for cancer prevention, immune-based approaches are particularly promising because they are a form of precision medicine with relatively few side-effects compared to conventional chemotherapy.
To accelerate advances in cancer prevention, we propose a comprehensive programme that includes constructing a human cancer atlas that builds on The Cancer Genome Atlas. The purpose of this human cancer atlas would be to catalogue and link the genetic, epigenetic, and inflammatory pathways for each of several tumour types and their precursor lesions. A translational programme would provide rigorous testing of novel interventions that overcome fundamental obstacles to successful prevention. Crucial to the success of this initiative is the development of early detection approaches to identifying people at risk of different cancers and biomarkers to optimise potential interventions. Early detection approaches can be accelerated by connecting the biological data in the human cancer atlas with risk factors linked to specimens analysed to generate the atlas.
Examples of successful precision-based prevention are listed in panel 3.
Panel 3: Examples of successful precision prevention.
Elegant molecular studies are redefining the range of cancers linked to various hereditary cancer predisposition syndromes and are helping to identify somatic alterations that occur before a malignancy develops; these advances are leading to novel preventive strategies.54
BRCA1
PARP inhibitors are one of the most compelling forms of precision cancer therapy in various forms of cancer associated with mutation in BRCA1 or BRCA2 and have been shown to delay breast cancer tumour development in Brca1-deficient mice42
Although most breast cancers arising in patients who carry BRCA1 mutations are oestrogen-negative, tamoxifen use appears to be associated with a reduced risk, particularly for contralateral breast cancer,37 likely because of female hormones in the early ontogeny of BRCA1-associated breast cancer
Elegant studies of luminal progenitors in BRCA1 models and RANK-ligand blockade in Brca1-deficient mice55 support a planned international trial of denosumab, a RANK-L inhibitor in BRCA1-mutation carriers; this drug is already approved by the US Food and Drug Administration for bone loss and has a well-established record of safety
Synchronous inhibition of both COX-2 and EGFR have synergistic effects in reducing the burden of intestinal adenomas in APCmin mice, which serve as a model for familial adenomatous polyposis (FAP); combined sulindac and erlotinib in patients with FAP had striking efficacy in reducing the burden of duodenal neoplasia, a leading cause of mortality in FAP.46
Universal colorectal cancer testing for mismatch repair deficiency to screen for Lynch syndrome is a paradigm-changing approach to identifying inherited cancer risk and has become standard practice and the main trigger for confirmatory germline testing.49
Patients at high risk of pancreatic neoplasia caused by germline mutations benefit from imaging-based early detection research.56
Low-dose aspirin, recommended in 2016 for colorectal cancer prevention by the US Preventive Services Task Force, is a possible precision medicine approach to change standard of care in cancer prevention by including molecular selection (based on prostaglandin pathway; eg, 15-hydroxyprostaglandin dehydrogenase mRNA expression in the normal mucosa adjacent to colorectal cancer) and somatic mutations and germline variants of PIK3CA and BRAF, and HLA class I antigen expression and key immune effects.
Xeroderma pigmentosum is a rare autosomal recessive genetic disorder associated with UV-induced DNA damage and very high risk of non-melanoma skin cancer that can be reduced using bacterial DNA repair enzymes or nicotinamide.45
Gaucher disease confers a very high risk of monoclonal gammopathy of undetermined significance and myeloma in part through lysolipid-induced chronic inflammation and genomic instability; lysolipid substrate reduction in Gba1-deficient mice decreases the risk of gammopathies and is now being explored in a clinical trial.47
In-depth mechanistic research of the farnesoid X receptor (FRX) ligand31 adds further biological plausibility to the first real signal of clinical benefit (including reduced inflammation and fibrosis) in a randomised controlled trial of the FXR-ligand obeticholic acid in the hepatocellular carcinoma precursor non-alcoholic steatohepatitis32
Overdiagnosis and overtreatment of patients with Barrett’s oesophagus is prevented in a precision-based approach to risk stratification using combined molecular assays from a single Cytosponge sample.57
The Premalignant Cancer Atlas (PMCA) project
The rate-limiting step in developing and implementing precision-based prevention approaches has been our limited understanding of precancer biology, which stands in contrast with the extensive knowledge of advanced disease. Although the seminal genetic model of tumori-genesis was defined in the colon nearly 30 years ago,58 limited numbers of adenomas have been analysed by next-generation sequencing.59 The interaction between immunity and neoplasia is now established as a fundamental principle of cancer development and progression. Premalignant lesions are regions of histologically abnormal tissue that often precede invasive carcinoma. These lesions can be found in diagnostic biopsies in patients under suspicion of cancer and in samples obtained from patients at increased risk for cancer during screening procedures (eg, pancreatic cysts). Some of these lesions will progress to invasive carcinoma, although many will remain stable or regress. The histological features of these lesions have been characterised for many cancers, yet comprehensive profiles of the tumour microenvironment and genomic, transcriptomic, and epigenomic alterations are not well defined, making it difficult to develop risk stratification and intervention strategies. Recent advances in next-generation sequencing and computational biology are shedding light on premalignant genomic mutational signatures.
To move this field of medical research from anecdotal examples of progress to full-blown successes that prevent cancer, large-scale, systematic effort is needed to longitudinally map the biology of premalignancies. Specifically, a programmatic approach must bring together cancer biologists, biochemists, immunologists, nuclear and mitochondrial geneticists, computational biologists, engineers, epidemiologists, and experts from other key disciplines to develop premalignancy roadmaps and optimise strategies for cancer prevention. The US National Cancer Institute’s PMCA project, which came from the BRP recommendations, is designed to evaluate the current state of the science and provide feedback to the National Cancer Institute leadership for a concerted effort to comprehensively profile premalignant lesions and provide a blueprint for feasibility and pilot studies in key organ sites. The optimal goals of this programme should be to: (1) form a national group of experts who will design a tissue collection of banked and prospectively collected premalignant specimens at different stages of premalignancy and from different organ sites; (2) apply existing genomic, proteomic, and immune assays to delineate the signals within these lesions to create a three-dimensional analysis of each lesion; and (3) develop a national database similar to The Cancer Genome Atlas database with links to clinical annotations that can be accessed by any investigator. The resulting tissue collection and clinical annotation from this effort would provide a national resource with which to enhance understanding of the biology of premalignancy and identify new targets for interception.
Pathways identified from the proposed PCMA project should provide additional molecules for chemoprevention targeting, antigens for vaccine targeting, and candidates for biomarker development that are crucial for screening and early detection.
A long-term goal for the PCMA should be to include all human cancers and their premalignant lesions (solid and haematologic), with a special focus on paediatric cancers. Initially, the project should concentrate on at least two major adult and two major paediatric cancers and their associated premalignancies. Using archived specimens, the initial workflow should include the complete annotation of 1000 premalignancies and their tumours for the pilot study. These data will serve as a general resource and training set for subsequent efforts, similar to that of The Cancer Genome Atlas. Use of archived samples will accelerate population of the PMCA database. The highest priority, yielding the data set with highest value, will be to collect and annotate biomarker data using samples that are collected over time from each patient.
Actual choice of indications for the initial training set will depend on the availability of sample collections that permit the planned analyses. This decision can be made after consideration by the steering committee and its advisors. A next step would be to analyse the evolution of cancer from premalignant to malignant disease, to identify genes (eg, mutational signatures), neoantigens, and signalling pathways that can be studied for their value as biomarkers in screening and early detection, and to develop interceptional approaches.
Longitudinal analysis of premalignancies should be another goal. Large-scale longitudinal and systematic mapping of the molecular and cellular determinants of premalignant lesion progression to invasive carcinoma will provide genomic and immune targets for disease interception. Focusing on haematological premalignancies has several advantages, including the ease of repeatedly acquiring neoplastic cells to study their clonal evolution over time.
Comprehensive single-cell and circulating DNA omics studies will have a key role in improving our understanding in disease pathogenesis. Hundreds of individual cells can be monitored, thus overcoming fundamental limitations of analysing bulk-cell populations and allowing precise study of intraclonal and microenvironment architecture and crosstalk in the process and timing of transformation.
Data for each premalignant lesion and accompanying biospecimens should include all relevant information. The PMCA database should be constructed using an open-source, flexible structure that will permit the entry and relational searching of all forms of data, from sequence to imaging information. Priority should be given to specific key areas (panel 4).
Panel 4: Priorities for the Premalignant Cancer Atlas database.
Patient demographics
Age, sex, and ethnicity
Geographic residence and so-called exposome
Lifestyle factors, including quantitative measures of physical activity and sedentary behavior
Medical history
Treatment history and outcome
Non-oncology pharmaceutical history
Radiological imaging
Genomics
RNAseq, from bulk tissue and single cell
Patient exome sequence to identify single nucleotide polymorphisms
Mutant neo-epitope discovery (in coordination with the Antigen Discovery and Tumor Microenvironment programme)
Mutational status for common cancer susceptibility genes (eg, deleterious mutations in BRCA, p53, PALB2, mismatch repair genes)
Mitochondrial DNA and function
Neo-epitopes
Cancer testis antigens, differentiation antigens, overexpressed shared antigens, viral antigen discovery
T-cell receptor technology
Multiplexed immunohistochemistry or immunofluorescence analysis of tumour sections
T-cell infiltrates
Myeloid or monocytic cell infiltrates
Stromal architecture
Metabolism
Microbiota (gut, lung, skin)
Preclinical models for interception strategies
A diverse array of genetically engineered preclinical models (eg, mice, zebrafish, organoids), technologies (eg, single-cell and cell-free DNA analyses), and disciplines (eg, immunology, biochemistry, genetics, imaging, and cell biology) are now being leveraged to study premalignant biology. Emerging techniques and models of progenitors and mutational processes to link cell lineage to clonal evolution include induced pluripotent stem cells and CRISPR/Cas9 editing to map the evolution of myeloid neoplasia, and cell-fate dynamics, reprogramming, and lineage-specific regulation of progenitor cells, potentially at the single-cell level, to identify and target cancers for early destruction.60–62 Data from these new models suggest that some premalignant lesions progress to cancer via fundamental epigenetic or transcriptional reprogramming to a progenitor-like state required for driver mutations to induce tumorigenesis.63 For example, recent studies in BRAF Val600Glu and p53-null zebrafish suggest that initiation of malignant transformation within a so-called cancerised field requires fundamental epigenetic reprogramming of these premalignant cells into an embryonic state via transcription factor-mediated reactivation of genes typically expressed only in neural crest progenitor cells.63 This reprogramming involves the binding of multiple transcription factors and generation of superenhancer regions. The zebrafish model data provides evidence that the earliest stages of tumorigenesis involve reprogramming to an embryonic cell state. Such data suggest that tumour-initiating cells can be identified and potentially targeted for early destruction through their ability to reactivate an embryonic epigenetic state. Widespread epigenetic field defects have been observed in apparently normal breast tissue adjacent to breast cancer64 and are also associated with inflammation-related cancers, such as Helicobacter pylori-induced neoplasia,65 where next-generation sequencing has revealed more cancer pathway-related genes affected by DNA methylation than by genetic alterations.66 Although zebrafish are an important model for studying cancer stem cells, genetically engineered mouse models provide important opportunities to study the links between early genetic changes and the resultant shaping of the precancerous microenvironment. This environment forms as a dynamic process that is unique to the specific genetic changes and the reactive stromal responses that occur as different tumours develop.
Antigen identification for targeted interception
We propose the creation of a robust national PMCA network of investigators who follow people at high risk for cancer development. This initiative would rely on a national strategy to discover neoantigens associated with different germline susceptibility genes and to assess novel immunotherapies that target these expressed genes and intercept premalignant progression. The PMCA network must incorporate detailed biochemical and enzymological studies on purified protein complexes to decipher the precise, context-dependent function of chromatin and other epigenetic modifiers and somatic mutations in precancer development and progression.67 This network should take advantage of a standardised baseline protocol (tissue acquisition, antigen identification, and biomarker interrogation) to acquire a deep understanding of the types of antigens that develop early and affect cancer development and to identify the early signals that form barriers to immune recognition.
The elaborate interaction between the immune system and neoplasia involves an increasingly complex cellular microenvironment and dynamic interactions between host genetics, environmental factors, and microbes in shaping the immune response. The PMCA network should focus on identifying premalignant antigens, identifying immune-suppressive signals in the premalignant microenvironment that prevent T-cell activation and entry into premalignant lesions (and mechanisms of checkpoint signalling, such as T-cell metabolism), testing new combinations of checkpoint and immune enhancers (adjuvants) informed by these biomarker studies, developing animal models appropriate for these immune studies, and developing and testing cancer vaccines informed by target identification, with safe adjuvants such as aspirin and metformin that could help overcome such immune resistance.
Technology development
The PMCA should promote the development of new technologies and computational methods that would support its mission and contribute to the emerging database (panel 5). Single-cell technologies are being applied to precancers, including ductal carcinoma in situ (DCIS). For example, findings from single-cell sequencing of DCIS samples have shown intralesion genetic heterogeneity in gene copy number, suggesting complex and distinct evolutionary processes involved in early DCIS and subclonal selection in invasive disease. Multicolour fluorescent in-situ hybridisation to evaluate clonal evolution at single-cell resolution in Barrett’s oesophagus has showed extensive genetic diversity in progressors.68 A whole-exome, single-cell sequencing method has been developed to assess genetic heterogeneity and tested on a patient with premalignant JAK2-negative myeloproliferative neoplasms (essential thrombo cythaemia).69 A major challenge is to develop single-cell technology to study spatial proximity and temporal dynamics between cells, integrating individual cellular states into models of functioning tissues, including interactions of precancer, immune cells, and other components of the microenvironment. Such technologies would allow discoveries that could revolutionise our fundamental understanding of neoplasia biology.70 Leveraging The Human Cell Atlas and other major initiatives, including novel single-cell technology, will be crucial.
Panel 5: New technologies and computational methods.
Radiological imaging methods
Nuclear medicine imaging methods: metabolic probes, immune PET
Imaging of premalignant lesions
Liquid biopsy technology—sensitive and specific enough to monitor premalignancies (eg, image advanced PanINs)40
Quantitative imaging of cell distribution and function in biopsy samples
Cross-referencing to datasets in The Cancer Genome Atlas
Facile approaches to T-cell epitope identification and T-cell receptor diversity
Single-cell transcriptome analyses in unprocessed tissue
Multiplexed in-situ hybridisation transcriptome analyses
Multiplexed morphological, immunohistochemical, and molecular analyses in fixed tissue
Conclusions
Precision-based cancer prevention is now possible. The technologies are available to identify the earliest genetic changes and their associated expressed mutant proteins. Once known, these targets can be used to develop safe and effective targeted chemoprevention and novel vaccine approaches (panel 6). Coupled with lifestyle interventions, successful precision prevention strategies will have the most long-term effect on eradicating cancers for future generations.
Panel 6: Priorities that will facilitate the development of safe and effective chemopreventive and immunoprevention strategies.
Develop a Premalignant Cancer Atlas (PMCA) that links genetic, epigenetic, proteomic, and inflammatory characteristics with clinical information.
A comprehensive, dynamic, and easily searchable PMCA database will be crucial to advancing understanding of how cancer development can be intercepted
Data should be gathered from the proposed high-risk national network and from the external cancer research community, and it should be freely accessible to all researchers and to the general public; the resulting biology will engage technology and pharmaceutical companies
Develop companion preclinical models to study inflammatory responses to specific genetic alterations that drive cancers
New genetically engineered mouse models are needed to uncover the inflammatory pathways associated with each specific genetic alteration that drives different cancers and to develop vaccine approaches for interception that are based on the different genetic drivers and associated neoantigens that cause different cancers to develop
Develop biomarker assays that can be used to identify people at risk of genetically defined cancers
Novel, non-invasive technologies are now available to detect low levels of genetic alterations in small volumes of serum and plasma
Liquid biopsies should be developed for new targets identified from the PMCA
Develop non-invasive imaging technologies for early detection of pre-malignancies
Imaging technologies are on the precipice of moving from assessing anatomical changes and metabolic changes within tumours to studying specific molecular targets
As new candidate proteins expressed by early premalignancies are identified, efforts are needed to translate this information into new imaging diagnostics
Develop vaccines for interception
Crucial to this programme is identifying antigens that can be recognised by the immune system and developing vaccines or engineered immune cells to target these antigens
The overriding goals are: (1) activate and redirect our own immune systems to attack and kill all precancers; and (2) develop anticancer vaccines as potent as current polio, diphtheria, and rubella vaccines that will protect future generations from developing cancer
Part 3: Early cancer detection, population sciences, and public health
Epithelial cancers develop over a long period of time, with a natural history that can extend for several decades, offering recurring opportunities to detect early preinvasive changes to fully developed disease (eg, cervical, colorectal, breast, prostate, and aerodigestive cancers). This wide window of opportunity is key for the targeted deployment of multiple strategies (existing and emergent) for cancer prevention and early detection, in the clinic and at the population level.71 Existing approaches to cancer prevention, screening, and early detection have been generic and generally driven by sex or age categories, and assessments of risk have been imprecise. The technologies are at hand to increase the precision and sensitivity of existing tools for prevention, screening, and early detection, with targeting and tailoring to specific population subgroups and attention to risk-based variable screening intervals.72 New knowledge about risk factors arising from research advances in molecular epidemiology, genetics, environmental exposures, infectious diseases, and behavioural and lifestyle factors have helped refine risk assessment and diagnostic testing for early cancer detection. Just as precision-based oncology strategies are being used to target biological pathways within the tumour, precision-based approaches should be created and applied to cancer prevention and early detection; however, these must be implemented cautiously to avoid both underdiagnosis and overdiagnosis.
The value of population sciences and public health
How can the population sciences and public health be brought to bear on this situation? Epidemiology is a powerful tool, and when combined with our increasing knowledge of the genetic risk for specific cancers and environmental exposures (exogenous and endogenous), it can help to focus on relevant high-risk or predisposed population groups. Other population and social science strategies, including health policy research, are important for population-level implementation and adoption of effective, precision-based, and early detection approaches because they can provide guidance on the most effective, efficient, and judicious use of resources on the basis of who is most likely to benefit, with attention to approaches and specific settings (clinic or community) that are most likely to be successful for specific segments of the population. Many inequities in health-care delivery magnify disparities in cancer outcomes for many people. Ensuring the routine access to preventive cancer-care services such as early detection is an essential first step and does not require new discoveries, but rather extends services that are effective to all who should receive them. Implementation in the USA of the Affordable Care Act in recent years has increased attention to these issues, but the full potential of its goals in cancer screening and prevention are yet to be realised.
The BRP, by necessity, could only devote a small amount of attention to early detection of cancer, but it made high-level recommendations for implementing evidence-based approaches to prevention and early detection, noting the potential for large reductions in mortality as a result of reduced incidence and early diagnosis of cancers. The BRP also highlighted the need for researching ways to increase the uptake of prevention and early detection programmes in populations with greatest need, such as those with cancer health disparities. As an example, they cited case finding and early detection of families with Lynch syndrome as a demonstration project to include all individuals and families at risk for additional cancers. This recommendation capitalises on known hereditary cancer predisposition genes, affecting perhaps 10–15% of incident colon and endometrial cancer cases and detectable in tumour specimens. But, additional approaches should be considered. For example, all young patients with colon cancer could have universal genetic testing73 since tissue testing can be imperfect and other cancer predisposition genes could be the source of early-onset disease and since some individuals might not meet traditional criteria for a Lynch syndrome diagnosis.
Although such specialised demonstration projects should be done, other important priorities related to the early detection of cancer at the population level also need attention. Fundamental to these efforts is the need to identify and address the determinants of cancer health disparities through basic science research, population-based strategies, and health-system interventions. Some disparities in care relate to poor-quality delivery of standard screening procedures such as breast cancer screening, as was seen in a community assessment in Chicago, IL, USA.74,75 As new cancer screening services are implemented in various communities (eg, lung cancer screening with low-dose CT), quality standards must be in place to ensure that the benefits of early detection accrue and that the harms of screening are limited.76 Quality standards for all cancer screening tests, whether based on laboratory tests or imaging, must be prioritised if they are to deliver on the promise of early detection.
Implementing the recommendations
Here we expand on several priorities that have immediate opportunities if there is attention to science, evidence, and implementation of guidelines.
First, an example for consideration is prevention of HPV-associated malignancies. Universal immunisation of the entire population is a tremendous opportunity to reduce the burden of cervical, anogenital, and oral cancers. Young girls and boys should be immunised, and opportunistic strategies should be taken to immunise people who have missed out on this baseline immunisation, such as college-age students and young adult or middle-aged women who are HPV negative. With universal implementation of HPV vaccination, gains in cancer prevention would probably be similar to what has been achieved with immunisation against hepatitis-B virus and reduction in the incidence of hepatocellular carcinoma.77 Furthermore, the most recent guidelines for cervical cancer screening must be consistently implemented.78 In particular, screening should be avoided in women for whom screening has no value (and potential harm) in cytological evaluation (ie, women younger than 21 years, women older than 65 years with no prior abnormality, and women with hysterectomy and surgical removal of the cervix), which could amount to tremendous cost savings in clinical assessments and laboratory fees. The appropriate use of the combination of cytology and HPV cervical screening in women older than 30 years can identify high-risk individuals and reduce the frequency of screening of low-risk individuals. Recommended cervical cancer screening strategies are a good example of the way in which cancer screening in general can be refined by applying state-of-the-art scientific knowledge. However, changing the behaviour of clinicians, health systems, and women to adhere to these recommendations is a major challenge in implementation science. We call for similar approaches to be developed for other cancer screening programmes (eg, colorectal, breast, prostate, lung cancer) that are currently based on fairly crude risk algorithms, mainly defined by age. In the absence of randomised or prospective observational trials to guide precision-based screening for many of the major cancers, we recommend harnessing the power of big data in innovative simulation and modelling studies to guide practice. For example, what might be lost in terms of population-level mortality benefit by going to a 3-year, 5-year, or 7-year faecal immunochemical test screening schedule for some subgroups deemed to be at the lowest risk levels for colorectal cancer?
Second, we focus on the importance of cancer screening and risk reduction among cancer survivors.79 About 15% of new incident cancers occur in cancer survivors.80,81 This patient population, now close to 16 million in the USA, are already engaged in the cancer care system, understand the challenges of a cancer diagnosis, and might be at high risk for second cancers related to heredity, behavioural risks (eg, tobacco use, UV radiation, infections), and exposures to cancer treatment. Targeting this high-risk population with screening appropriate to age and exposure, risk-reduction surgery (in case of hereditary predisposition genes), and intensive surveillance could ensure earlier detection. Some of these individuals might be appropriate for chemoprevention strategies and behavioural and lifestyle changes focused on reducing risk. Having a cancer diagnosis is a teachable moment for many individuals, and clinicians and health systems need to seize this opportunity in an organised way.82 To some extent, that has been the call since the US Institute of Medicine’s report in 2006 recommended greater coordination of post-treatment care for cancer survivors.83 To date, implementation of the recommendations in that report has been minimal with respect to changes in clinical practice for adult cancer survivors. In terms of cancer prevention and early detection, the cancer survivor population must be the focus of our attention.
Third, we call attention to the needs of the health-care workforce if their services are to be effective and save lives. Inequities in access and delivery of cancer-related services, whether screening, behavioural and lifestyle interventions, genetic testing and counselling, or follow-up services after abnormal tests, are dependent on an organised health system that focuses on the population and the individual. As cancer screening and diagnosis becomes more refined and effective, a larger clinical workforce will be needed to explain the rationale for screening, the targeting of high-risk individuals, the need for genetic testing, and potential for risk reduction. For example, too few genetic counsellors exist to test people with known cancer, let alone unaffected family members. The maldistribution of these services, especially in rural and subsegments of urban areas, has led to enormous disparities in care. This affects large segments of the population that have much to gain through earlier detection and prevention strategies (eg, smoking cessation and lung cancer screening in the rural parts of southern USA). Attention to the needs of the workforce and health-care delivery system will be a crucial component of any strategy to reduce the burden of cancer in the USA. The National Cancer Institute can have an important part in expanding the community and regional workforce through its Comprehensive Cancer Centers, which are already strategically engaged with their catchment areas. If adequately supported, these cancer centres can train an allied health workforce that extends into the community to ensure translation and implementation of early detection services.
Conclusions
Six priority areas have the potential for high reward by increasing the yield from cancer screening and early detection, while reducing the potential for overuse and overdiagnosis (panel 7). Big data has been heralded as important for learning what works clinically in the real world,84 yet there is an emerging opportunity to use these strategies in multilevel modelling of cancer aetiology and risk in the population. Especially important are the inclusion of environmental, behavioural, and health-care resource factors in modelling approaches and outcomes. Screening programmes save lives, as was demonstrated in Delaware, where population-based colorectal cancer screening was implemented and treatment provided to all citizens of the state,85 long before the implementation of the Affordable Care Act. It is time to move the revolution in science and medicine into screening and early detection, with the new technologies being applied to cervical cancer screening as an example. A one-size-fits-all strategy can no longer be used for cancer screening because it is wasteful and difficult to implement. Implementing screening tailored by age and risk factors will be essential for increased accuracy, as is now being considered for breast cancer screening and mammography intervals. These strategies will have a great effect on minimising morbidities and mortality from cancer in future generations.
Panel 7: Priorities for early cancer detection, population sciences, and public health.
Harness the power of big data to develop and model more precise approaches to cancer screening, taking into consideration factors at multiple levels from genetics, biology, environmental exposures, including behavioural factors, resource availability, and policy environment
Expand the diversity of databases and exchange of knowledge of high-risk alleles (particularly across racial and ethnic groups and underserved populations to address disparities) and of polygenic classification of risks for common cancers (eg, breast, lung, colon, prostate)
Refine cancer screening protocols to reflect the state of knowledge of disease epidemiology, risk profiles, and early detection testing methods (eg, implement and adopt new cervical cancer screening recommendations, with tailored protocols and testing strategies)
Use state-of-the-art implementation science strategies to ensure delivery of screening and early detection for cancer to the right people at the right time, reinforcing precision principles and evidence-based guidelines
Focus cancer screening and early detection on cancer survivors
Expand the workforce with capability to provide genetic counselling and testing for patients with cancer and their families and with more general expertise in targeting and fostering adherence to cancer screening services in high-risk people
An additional priority is a focus on understanding how screening and early detection strategies should be delivered in the real world. Contemporary implementation science strategies must be applied to ensure that high-level, evidence-based interventions are actually used in the community and delivered to the target population.86 One of the populations at highest risk of developing cancer are cancer survivors. These individuals often are not aware of the risk for second cancers (based on genetic susceptibility, common exposures, treatment-related second cancers), and are not receiving appropriate screening. For example, patients with head and neck cancers are at risk for other tobacco-related malignancies and should receive lung cancer screening. Finally, there is an important need for an expanded professional workforce with training in cancer genetics to provide counselling and genetic testing for patients with cancer and their families. Many new patients and cancer survivors need this testing to help in initial treatment decisions and subsequent management. A well-trained professional workforce could help with follow-up cancer screening and adherence to preventive interventions in individuals at high risk for cancer based on hereditary predisposition. This is essential for the achievement of precision prevention.
Part 4: Drug discovery, development, and delivery
The challenge of developing breakthrough therapies in cancer lies in its genetic, biological, and clinical heterogeneity. It stands to reason that successful drug development would emerge from an intense focus on patient needs and coordinated integration of academic researchers, pharmaceutical drug developers, and clinical disease experts. However, drug approvals are expensive and have a high risk of failure. Several factors within the traditional drug discovery process contribute to this situation. One important factor is the inefficient and incomplete transfer of knowledge and information between academia, industry, and clinical domains throughout the lifecycle of the drug development process. This inefficiency produces enormous expenses and exposes many patients in clinical trials to futile therapies. We believe that improved integration of academic science and industrial execution for projects that are prioritised by clinical need could vastly improve efficiencies within the system, yielding both improved validation of drug targets earlier at substantially reduced costs and more precise definition of patient responder subpopulations that would inform the design of clinical trials.87
An unprecedented increase in the number of therapies have been approved for marketing by the US Food and Drug Administration (FDA) in the past 2–3 years,88 but this continues at immense costs, with hundreds of drugs failing in clinical trials. The Tufts Center for Drug Development (Boston, MA, USA) estimates that bringing a single new therapy to the market costs US$2·6 billion.89 One way to reduce late-stage clinical development expenses is through stringent milestone-setting metrics, ultimately through discontinuing most projects early in their lifecycles when costs are relatively low, rather than carrying questionable projects forward and allowing costs to accumulate.
Unbiased and rigorous biological studies to prioritise drug targets
A fundamental aspect of novel therapeutic development should be rigorous validation of their merits, both from a biological perspective and informed by the ability to identify patient subpopulations that might best benefit from the new treatment. Probable resistance mechanisms should be identified before clinical trials. Stringency in early decision making is essential to prioritise the abundance of potential targets brought forward by colleagues from academia. First, reproducibility and consistency between model systems in basic science affects the probability of success of drug discovery and development programmes; the research community must commit to independently validate all potential targets before investing substantial resources in drug discovery.90 Personnel and resources need to be available for an unbiased, rigorous prioritisation approach to selecting targets, using tools that have been (and continue to be) successfully designed in academia (eg, genetically-modified mouse models, patient-derived xenografts, CRISPR-engineered isogenic models) to ensure that unfit projects are terminated early in the process. With this early due diligence, a large proportion of the doomed projects that would otherwise proceed into costly stages of drug development could be ended early.91
Linking molecular targets with biology
Questions related to mechanisms of action, the molecular link to the targeted disease, the role of a potential target in disease maintenance, and potential resistance or redundancy issues are of fundamental importance and should be fully addressed as a project moves into drug discovery. Given the exponential costs associated with drug discovery and development, early stopping rules are essential. The high risk of failure in late-stage clinical trials, particularly in oncology, is often because drugs are tested in the wrong patient populations. The profound degree of intratumoral heterogeneity in many cancer types may be of paramount importance when selecting appropriate targeted therapies.92 Although a great deal of useful data is available to position therapeutics in likely responder subpopulations, many clinical trials are still not designed to optimise patient enrolment on the basis of a preclinically validated biomarkers.93
In-depth biological analyses of targets are needed to determine the context in which the activity of such targets would be rate-limiting for the tumour. These analyses would inform the design of biomarker-driven trials in specific patients (smart trials) and ensure that drugs are tested in defined disease subsets rather than attempting to capture broader market opportunities. Administering investigational drugs to the right patients will avoid unnecessary toxic effects and increase the likelihood of trial success. Heavy investment in translational biology also yields financial benefits in clinical testing by reducing time to read-out of activity in proof-of-clinical concept studies, resulting in early activation of pivotal clinical trials. Most importantly, early investment in defining optimal targets for smart trials is the most ethical path forward because it reduces the risk to patients of receiving an ineffective therapy.
Maintaining influence through clinical proof of concept
Partnering to carry out projects after the successful early clinical trials at reduced overall costs could inspire changes in the pharmaceutical industry that improve clinical trial outcomes and reduce drug pricing in the longer term. Although it is not feasible for every cancer centre to establish a drug development operation or for every pharmaceutical company to build basic science and translational research laboratories, we do believe that closer collaboration between academia and industry is imperative. This proposed model could be scaled and used to change the drug development environment. Rigorous adherence to target validation and preclinical testing will reduce costs by allowing only properly vetted, suitable drug candidates to advance. Fewer resources will be wasted on expensive, late-stage clinical trials for drugs that are unlikely to benefit patients. The handful of academic centres that have recently moved in this direction should be able to export the knowledge gained and share it with colleagues in academia and the pharmaceutical industry.94
With a focus on the patient and the ability to do innovative, biomarker-intensive clinical trials, it would be important, whenever possible, to maintain strong influence on product development through clinical proof of concept. The intention would be to avoid situations where a therapy gets used in an inappropriate clinical trial or wrong endpoints are used because of business-driven market considerations that are not based on the science. Controlling clinical development through early clinical trials takes substantial resources but ensures that the mission of tackling unmet clinical need, including orphan indications that are often passed over by commercial entities, is accomplished. We believe that by asking tough questions about a novel therapy’s effect on the tumour at the molecular and pathophysiological level, as well as on the patients’ overall performance and quality of life, drugs can be identified that will most effectively combine with standard-of-care treatment. The goal should be to go from incremental increases in progression-free survival to cures that preserve the quality of life for survivors.
Large comprehensive cancer centres or consortia of smaller cancer centres could adopt such programmes and create a learning system by integrating drug discovery and development expertise with cancer biology, genetics, and clinical expertise and infrastructure. The expanding, IT-based integration of clinical and research data allow the field to establish new and deeper understanding of the evolution of a cancer’s molecular and functional architecture upon treatment. Together, this organisational framework will increase the probability of success in drug development.
Sustainability
The ultimate success of the model described above will be the demonstration that transformative drugs can be designed and delivered to patients more quickly and at a lower cost than with typical industry-driven efforts. It is a long-term goal to have the model of clinically informed, integrated drug discovery recognised as a new standard for oncology therapeutics, and, if successful, might equally be applied to drug development for other diseases that are difficult to cure (eg, Alzheimer’s disease).
An important aspect of this strategy is linked to leveraging governmental, philanthropic, and corporate funding sources. Early project out-licensing to large pharmaceutical companies might not be the optimal way to create value for the patient. Priorities in drug development in the pharmaceutical industry often change because of strategic redirection and perceived (or real) lack of commercial prospects. By relinquishing drug development rights early, projects might be terminated, or drug tests might be deprioritised for commercially undesirable patient populations (rare cancers) or done in a broad range of unselected tumour types, just to expand the market of a drug but decreasing the likelihood of positive outcomes in the clinic.
The cancer research community should be driven by the intention to find cures, even for the smallest population of patients and for the rarest cancers. For example, only one in three patients with breast cancer respond to trastuzumab.95 By better defining the patient subpopulation through a validated model of responder identification, instead of taking an all-comer HER2-positive approach, the one patient who is likely to respond to the treatment could be selected and the other two patients could be spared from the toxic effects of a drug with no effect. Academic centres therefore need to retain strong influence on the development programme at early stages of clinical testing (phase 1b). To do so, funding must be leveraged from philanthropy, the US National Institutes of Health, not-for-profit sources, and, in the longer term, the revenues secured through milestone and royalty payments from licensed assets.
Conclusions
The proposed model will do more than simply decrease the cost of drug development. This science-driven drug discovery approach will also improve patient access to cures. Academic, governmental, and drug development entities will recognise the value of integrated collaboration with drug developers, basic academic researchers, and clinical disease stakeholders because they can share data and costs. In turn, academic centres will see the value of investing in these efforts, as revenues from successful projects will provide additional support for their organisation, an idea that will probably become increasingly attractive as health-care expenditures become regulated and clinical reimbursements progressively decrease. The entry of multiple entities into the marketplace should reduce costs across the industry, while simultaneously enhancing innovation.
These recommendations align with the Cancer Moonshot Task Force recommendation to enhance public–private partnerships and are based on common-sense solutions that would, if implemented, simultaneously benefit the primary players in the industry: pharmaceutical companies, payers, and patients (panel 8). Drug developers would realise returns on their investments more quickly through a fast, rational, and science-driven FDA approval process. Payers would benefit from cheaper drug costs resulting from increased competition within the industry. Finally, and most importantly, patients would realise gains through more rapid access to life-saving medicines. Partnering with and educating policymakers on the wisdom of reassessing laws that slow drug development, stifle competition between pharmaceutical companies, and adversely affect the health of patients with cancer will be essential.
Panel 8: Priorities for drug discovery, development, and delivery.
We recommend the development of an organisational mechanism that fosters the creation of dedicated pharmaceutical-like research and development units within academic centres. These units would enable efforts in the discovery of new, impactful therapies. To stringently validate concepts before entering into costly clinical trials, these units would move discoveries into early proof-of-concept clinical trials by leveraging the deep scientific and clinical knowledge of academic medical centres.
Target discovery and validation
Invest in disruptive technologies to support comprehensive and unbiased efforts at novel target discovery and validation, using state-of-the-art systems and technologies in relevant model systems (patient-derived xenograft and syngeneic models), motivated by rigorous science and stringent ‘go–no go’ criteria
Target advancement requiring a comprehensive understanding of target mechanism of action, contribution to disease pathogenesis, and defining the context in which the target is rate-limiting to develop a clinical path hypothesis
Drug discovery
Sustain investment in developing high-quality small-molecule drugs and biological modalities suitable for advancement into first-in-human clinical trials, with appropriate overall profile to engage target safely and effectively in humans and suitable for use in combination with standard of care agents
Drug discovery must be driven by experienced cross-functional teams with track records of successful drug development
Delivery systems
Focus investment in novel drug delivery technologies and new therapeutical modalities to achieve proof-of-concept in human beings (eg, novel delivery technologies to enhance delivery of drug directly to the tumour and thereby sparing vital organs, novel treatment approaches such as PROTACs/degronimids for protein degradation, approaches to therapeutically intervene with microRNAs, and cell-based therapies)
Seek to enhance the arsenal of therapeutics available to both clinicians and patients
Preclinical development and translational studies
Develop a strong translational package in collaboration with clinical disease experts to position the programmes for early-stage clinical trials in defined patient populations
Focus on translational, innovative patient-derived models cemented on strong foundations of the pharmacological audit trail, including optimal drug exposure, target engagement, modulation of target biology, intermediate biomarkers of response, efficacy, and assessment of mechanism of acquired resistance
Clinical development
Design informative, biomarker-rich, and focused phase 1 clinical trials in biomarker-stratified patient populations to rigorously test hypotheses in 30–50 patients
Trials that do not reach clearly defined levels of clinical efficacy should not proceed to later stage investigation to reduce inefficient use of funds and resources
Part 5: Genomic and immune analysis of tumour cells and the tumour microenvironment
As the USA takes on the three aims of improving cancer care, improving cancer outcomes, and making better investments in cancer care, at least one target is hidden in plain sight—ensuring that technology developed in the past decades is used to characterise c ancer cells and their associated normal cells at the molecular level, and that use of these technologies leads to new knowledge and to the precise identification of matching targeted or immunotherapies. The use of modern technologies to characterise the cancer cell and supporting cells in the tumour microenvironment is integral to understanding three key areas: identifying patients who are most likely to respond to a drug or treatment; identifying patients at risk for adverse reactions; and monitoring response to treatment, either to adjust dosing or to detect emerging resistance.
Molecular characterisation of the tumour genome
The use of next-generation sequencing technology, and its attendant decreasing costs, to generate comprehensive data about DNA-based and RNA-based differences between tumour and normal cells in individual cancers and sophisticated computational analysis of these data has transformed knowledge of the cancer genome landscape in the past 8 years. DNA sequencing of a tumour genome was first described in 2008,96 and huge efforts have ensued since to characterise the genomes of thousands of cancers from multiple tissues. However, almost all studies have focused on treatment-naive disease, since metastatic or recurrent cancers are difficult to obtain. The few genomic comparisons of primary diagnostic specimens with their metastases have shown important information about the genetic determinants of treatment resistance and the changes in heterogeneity that occur. In view of the frequency of treatment refractory disease and the poorly described relations between matched metastatic and primary cancers, we encourage studies that actively bank such metastatic lesion samples through rapid autopsy programmes for comparison with the primary cancer. For tumour types where metastatic lesions are frequently removed surgically for curative intent, such as colorectal, brain, and ovarian cancers, there are rich opportunities to perform these comparisons without autopsy. The simultaneous analysis of DNA and RNA from these samples will enable a full characterisation of changes in patients’ immune responses, which will further enrich strategies to treat refractory metastatic disease.
Molecular characterisation of the tumour microenvironment
Both the tumour and immune microenvironment can be characterised with next-generation sequencing-based methods and analyses. This is important because cancers should elicit immune responses, but these responses are suppressed in the tumour microenvironment. In particular, tumour-killing immune cells are recruited into the tumour and either activated or repressed within the tumour microenvironment by epigenetic effects that range from cell–cell interactions97,98 to soluble signalling factors and metabolites.99,100 Most of these factors are potentially druggable. Effector T cells have tumour-killing potential that varies widely as epigenetic factors affect T-cell differentiation from naive to exhausted.101 This naturally leads to a focus on the immune and tumour microenvironment at the genomic, transcriptomic, proteomic, and metabolic levels, with particular emphasis not only on classification but also on understanding how the various molecular interactions within that environment lead to emergent behaviours,102 ranging from tumour-killing activity to adverse side-effects. Of central importance are experimental and computational methods to resolve these molecular interactions at the level of a single cell or a few cells, with time, to yield predictive models.
Advances in transcriptional and genomic profiling, enabled by increasingly cost-effective next-generation sequencing tools, are now readily integrated with microchip platforms for single-cell analysis.103 Single-cell analyses that quantitatively and simultaneously capture multiple levels of molecular information within the intact tumour microenvironment are the next big step in tissue analysis for resolving interactions between tumour and immune cells. As the various generations of single-cell analytical methods mature, the value they provide for biomarker identification will probably evolve to guide clinical decision-making. A related bioengineering challenge is the development of in-vitro models (ie, so-called organ on a chip models104) that recapitulate tumour–immune cell interactions. The characterisation of the tumour microenvironment as a potential therapeutic target is also important and must be supported.
RNAseq: multiplex information from a single data type
One key approach to improving understanding and characterisation of tumours and their microenvironment is analysis of multiplex RNAseq data generated by next-generation sequencing. Methods for generating RNAseq libraries have improved tremendously in the past 2 years. Complex libraries are now constructed from tiny amounts of input RNA, even from single cells. By producing paired-end sequencing data from these libraries, we can add highly valuable information to genomic data from cancers, especially for the comparison of pretreatment and post-treatment tissue biopsies or resections. Similarly, the ability to analyse the resulting datasets by a multitude of different algorithmic approaches has also advanced rapidly in the past 2 years, showing important characterisations of the cancer and its microenvironment (panel 9).
Panel 9: RNAseq.
RNAseq provides a quantitative characterisation of genes being expressed and their level of expression
RNAseq data can verify the existence of hard-to-detect DNA variants such as fusion genes resulting from deletion, translocation, or inversion of chromosomes
Analysis of the gene expression profiles can be correlated with genomic data to evaluate pathway activation or repression and hence inform therapeutic decision making
RNA expression also can reveal tissue subtypes within a given tissue histological sample; a temporal series of tumours across therapeutic interventions can be used to determine whether this subtype assignment has changed
Advanced analyses can identify alternative splicing that might be tumour-specificRNAseq data can contain information about long non-coding RNAs and their relative expression, which can change with response or resistance to therapy and might be prognostic
The immune microenvironment of cancer cells can be assessed by mining RNAseq data and interpreting the classes of immune molecules that are active in the tumour mass; new algorithms such as CIBERSORT can characterise the infiltrating immune cell types in the tumour mass from RNAseq data without an initial isolation of immune cells
RNA can be specifically processed to yield information about the T-cell receptor repertoire and whether therapy (including immunotherapy) has expanded or contracted the diversity of the repertoire
Assay regulation and reimbursement
To advance and progress molecular analysis of tumour cells and their microenvironment will require an infrastructure of appropriate regulation and reimbursement. A regulatory framework with appropriate reference standards would improve patient and provider confidence in next-generation sequencing-based assay results, and more patients will receive precision therapies appropriate for their cancers. The hope is that these resources will prevent ineffective therapies, decrease variation in patient outcomes while improving outcomes overall, and limit adverse events.
Obtaining coverage and reimbursement is the primary obstacle to widespread adoption of next-generation sequencing-based cancer profiling tests. Commercial insurers decide whether a patient will need such testing before they cover the cost of targeted therapy, yet no standard guidelines exist to determine what is reimbursed. A clear strategy towards standardised reimbursement is needed.
Data repository and data standards
Remarkable amounts of data are generated in laboratories and clinics all over the world with regard to genomic and immune analysis of tumour and surrounding cells, but each undertaking generates data with its own unique terms and structure. These big data will fuel advances in cancer care only if included in health-care electronic data systems, applying standardised reporting methods to codify the information and render it shareable across electronic record systems. Along with data-sharing, a universal patient consent language must be adopted that reflects the reality of cloud-based computing and the safeguards against personal data identification. Use of such information would expand exponentially with the adoption of common data elements, data standards, and data collection standards and with the establishment of core technical standards and functions.
Conclusions
The expanded characterisation of tumour and surrounding cells has yielded tremendous new information to advance cancer treatment. The ongoing priorities in this area are listed in panel 10.
Panel 10: Priorities for genomic and immune analysis of tumour and peritumor cells.
Collect matching metastatic tissue with primary tissue
Characterise the tumour environment and advance technologies to enable such study
Renew focus on RNAseq studies of tumour and the microenvironment
Develop regulatory and reimbursement steps for molecular analysis of tumour and microenvironment for routine patient management
Establish national data collection and annotation standards
Part 6: Expediting access to cancer drugs for research and patient access to clinical trials
An essential step toward reducing the burden of cancer is to increase the availability of anticancer drugs for research and expand patient access to clinical trials. Everything that is known about chemoprevention and medical treatment of cancer today is because of a strong drug discovery ecosystem and the commitment of previous generations of patients, families, researchers, and doctors to maintain a high quality in clinical trials. Opportunities also certainly exist to greatly accelerate progress in drug discovery and innovations for patient and tumour characterisation, clinical trial design, regulatory processes, and endorsement and democratisation of participation in clinical trials. The ultimate goal is to align research and care in a seamless continuum such that all patients have access to clinical trials as part of standard care and their clinical course and experience informs future research.
In this section we review opportunities to improve drug development and cancer treatment. First, the process of drug discovery and credentialing has expanded greatly to include preclinical testing in in-vitro, cell, organoid, and animal models and with computational approaches. A key bottleneck for researchers is access to promising molecules to test novel hypotheses. Efforts like the US National Cancer Institute’s Drug Formulary should be better supported by both government and private entities so that the decision to accept or reject a drug can be made quickly and early in its development. As combination therapy is often useful, efforts to test rational combinations from different sources in the preclinical setting should be incentivised. Drug development has slowed in certain cancers. For example, the FDA has approved only three drugs for paediatric cancers in the past three decades. Efforts such as the Priority Review Voucher, an industry incentive to enhance drug development for paediatric rare diseases, should be maintained or perhaps expanded.
Promote novel clinical trial designs
The research field of oncology has been an especially fertile ground for high-quality clinical trials. The traditional progression through phase 1, 2, and 3 trials has substantially improved clinical care by enabling evidence-based care. Although extraordinarily useful, this continuum is no longer sufficient. Advances in molecular characterisation of both tumour and host and new opportunities to collect and analyse large amounts of data mean that new approaches to clinical investigation must be developed. The clinical trial design should be tailored to the questions asked.
Preoperative and so-called window of opportunity trials with appropriate biospecimen acquisition should continue across disease types where feasible. The use of liquid biopsies, if validated, could expand this design to even more tumour types and might give early signals about the efficacy of a drug or therapeutic approach. Molecularly driven basket and umbrella trials should also be more frequently considered.105,106
Longitudinal biospecimen sampling and analysis should be pursued to improve understanding of tumour heterogeneity and evolution and will ultimately guide clinical management of the individual patient. The fact that both anatomical site of origin and aberrant molecular pathways are important in predicting tumour response or resistance to an agent supports the use of trials agnostic to organ-specific cancer and trials with expanded access that have the ability to rapidly include a new cohort without the burden of launching a new trial. Such trials could also be extended to include children older than 12 years.
Finally, the oncology community has been slow to embrace large simple trials or trials that focus on the real world, and a concerted effort should be made to ascertain whether and when such designs might be useful in cancer research. The desire to explore these types of trial designs will need to be balanced with a focus on tailoring therapy to the individual tumour and patient.107 Opportunities to share data from all types of sources should be encouraged.108
Reducing regulatory and financial burden
The regulatory and financial burdens associated with clinical trials have escalated in the past several decades and these have become a deterrent for patients, doctors, and payers. Propelled by well-intentioned efforts to enhance patient safety, the regulatory structure has paradoxically become so burdensome that it blocks access to trials, thereby depriving many patients of state-of-the-art care. Although ensuring patient safety is an absolute requirement, the regulatory environment surrounding clinical trials should be re-engineered to eliminate duplication and redundancy (eg, the increasing use of a central institutional review board is reducing unnecessary and unhelpful reviews for multisite trials). This redesign would also decrease the cost of clinical trials, a goal of utmost importance for all stakeholders. In the long term, failure to complete well-designed biologically driven clinical trials will be even more expensive if the human and economic burden of cancer is not reduced now.
The USA has been at the forefront of clinical cancer research for many years, thanks in part to a system that underwrote unfunded aspects of clinical trials. This informal system of subsidy has unravelled in recent years, with damaging consequences. In the future, the shared responsibility for clinical trials must be recognised. In particular, patients should expect to participate in a clinical trial as part of standard care, and the cost should be covered by payers, research funding agencies, and the pharmaceutical industry similar to the approaches already adopted in other high-income countries. Cancer patients in the USA who are insured through Medicare, Medicaid, and the Veterans Administration should be afforded full coverage for participation in clinical trials. Solutions to reduce regulatory burden and underwrite cost of care in clinical trials will be absolutely essential to the goal of disseminating access to clinical trials to a large population and thereby increasing the value and generalisability of findings.
Optimisation of the drug approval process through the USA and worldwide
The FDA has worked intensively to speed up the approval process for new drugs while ensuring efficacy and safety. Multiple new pathways to approve a drug and promote ongoing monitoring have been introduced. One barrier within the FDA was its organisational structure, which inadvertently separated key parts of the approval process for cancer drugs into different groups. The newly designated FDA Center of Excellence for Oncology brings key groups under a single umbrella to facilitate expert review of new products and bring them to patients as quickly as possible. A rapid review process will ensure that useful drugs reach to market expeditiously and make it possible to limit the use of expanded access programmes.109
Cancer is a problem worldwide, and international collaborations in trial design could promote trials that yield impactful results in multiple countries. Collaboration between drug regulatory agencies like the European Medicines Agency and the FDA would also benefit patients by decreasing regulatory burden and cost.
Democratisation of clinical trials
Ideally, participation in clinical trials should be an option to everyone. Indeed, participation in a clinical trial at some point in a patients’ journey with cancer should be the expectation rather than the exception. Achievement of this goal will require several changes in approach. Careful examination of eligibility criteria for clinical trials, reducing them to the minimum needed for an informative and feasible trial, is crucial for the generalisability of results.
Clinical trials must include large populations that have not been traditionally served.110 For example, age is an important risk factor for most cancers, yet elderly patients are often excluded from clinical trials because of eligibility criteria, which might be too restrictive, or insurance coverage that makes their trial participation too expensive. Similarly, regulatory change should mandate the testing of drugs in children when childhood and adult cancers have a common molecular footprint. Industry is not required to test drugs in children unless there is a common indication. Given that children do not have the predominant adult cancer indications (ie, breast, lung, colon, and prostate cancer), development of new therapies to treat children with cancer is greatly lagging behind drug development for adult cancers. Treatment of paediatric cancers has improved, thanks in large part to the robust clinical trial ethos that permeates care of paediatric patients with cancer, it is important to maintain focus on these patients. Although comparably few in number, children with cancer have especially great opportunity for gains from cure in terms of years of productive life.
People from minority ethnic backgrounds are also often underrepresented in clinical trials, a failure of particular importance because some minority populations are rapidly becoming majority populations. Underserved populations, such as those with low socioeconomic status or living in rural areas, have also been traditionally underrepresented in trials, but their inclusion is needed to make findings more generalisable. In all cases, a solution to minimise financial burden of clinical trial participation must be identified so that participants are not penalised for their willingness to volunteer.
Conclusions
A burgeoning scientific understanding of cancers underlies the development of new therapies. Clinical trial participation, rather than be perceived as a burden or risk that should be avoided or left to others, must become the norm for clinical care. This culture change will require expanded access to drugs for preclinical discovery, support for innovation in the science, implementation of new clinical trial designs, a reduction of the regulatory burden, roll-out of planned changes in the drug approval process, and advocacy for change in financing and payment for clinical trials by shared responsibility across all stakeholders (panel 11).
Panel 11: Priorities to expedite access to cancer compounds for research and patient access to clinical trials.
Prioritise efforts to provide access to promising compounds for researchers to test novel hypotheses and combinations
Promote novel clinical trial designs (eg, basket and umbrella trials), and pursue longitudinal biospecimen sampling and analysis to understand tumour evolution and guide drug development and patient management
Reduce regulatory and financial burden for trials with strategies such as central institutional review boards and appropriate coverage by all third party payers
Optimise the drug approval process through the FDA and around the world through collaboration between the newly designated FDA Center of Excellence for Oncology and sister regulatory agencies
Democratise clinical trials by making them available to people from special or underserved populations such as the elderly, children, and people from minority ethnic backgrounds or with low socioeconomic status
Part 7: Applying advanced technologies to catalyse cancer breakthroughs
Cancer is a complex disease with phenotypic and functional heterogeneity that demands an interdisciplinary approach to accelerate progress in prevention, detection, and therapeutics.111 With a growing appreciation of the cancer’s molecular landscape and the associated complexities, the research community has broadened collaborations across scientific disciplines to understand the varied biological signatures of these diseases and engineer options that can help improve the lives of patients.112
An important aspect of the Cancer Moonshot initiative was the underlying premise to prioritise technological capabilities that can catalyse new scientific breakthroughs.113 In this section, we highlight several approaches that have benefited from convergence in the engineering and life sciences and have potential to affect the oncology landscape.
Why the timing is right
Recent progress has furthered our understanding of cancer and produced innovative tools to combat the disease. This progress also made the timing of the Cancer Moonshot a particularly ripe opportunity for a coordinated response across the scientific community. In the 15 years since the completion of the Human Genome Project, the cost of sequencing technologies has decreased dramatically,114,115 fundamental understanding of the molecular pathogenesis of cancer has leapt forward,116 and the ability to manipulate human and microbial cells has fuelled a new wave of therapeutic applications.117 Furthermore, advances in converging technologies (eg, nanotechnology and computing) have brought engineers and biologists together to leverage novel miniaturisation tools from the world of semiconductors, sensing devices, and other technical marvels to shape cancer care. In short, the knowledge and ability is now available to act on a molecular level. In this section, we highlight several approaches that have benefited from convergence in the engineering and life sciences and that have the potential to shape the oncology landscape.
Advanced technologies for the detection, monitoring, and characterisation of tumours
Early detection of cancer is a pillar of effective treatment. Modern technologies enable detection and monitoring of rare tumour cells or their cell-free products, such as nucleic acids, at early stages of the disease.118 Nanoscale probes can be engineered to travel through the bloodstream, to detect and measure biological processes inside the tumour microenvironment, and to emit signals that can be detected in body fluids like blood or urine.119,120 Key bottlenecks associated with clinical trial design could be relieved by using microdose drug-delivery technologies that provide in-situ exposure of cancer cells to drugs, thus offering the potential to increase throughput and personalise drug-response data.121 Alternative test strategies using patient-derived organoids and engineered tissue models can also recapitulate essential features of cancer progression by preserving the architecture and diversity of cell types in tumours. These models could be used together with clinical diagnosis tools to map a preferred treatment plan on an individualised basis.122
The phenotypic and functional plasticity of tumour cells has led to a particular priority on engineering precise means of closely monitoring patient outcomes. For example, microdevices that capture and analyse circulating tumour cells or cell clusters could enable real-time monitoring and management of the disease.118 Clinically, these platforms also allow investigators to study patterns of tumour drug-resistance, postsurgical relapse, and metastatic dissemination. Monitoring strategies should be developed together with dosing approaches to optimise effective treatment options.
A key ongoing challenge in cancer imaging is how to extract sufficiently high-resolution information from solid tumours to guide therapy and accurately predict treatment outcomes. Multifunctional particle systems can offer novel models of visualisation. For example, nanoscale imaging probes have been shown to aid the intraoperative delineation of tumour margins.123 These high-resolution imaging technologies could lead to more accurate characterisation of tumours, especially when combined with other assessment methods such as radiological and nuclear imaging, PET imaging, and high-throughput interrogation methods using genomics or proteomics.
Therapeutic approaches to improve delivery of antitumour cargoes
The trove of genome sequencing data, such as that from The Cancer Genome Atlas, is enabling researchers to identify a new generation of promising targets for cancer treatments. Nonetheless, most targets are not accessible by antibody-based therapeutics or traditional small-molecule approaches. RNA silencing or RNA-guided genome editing could target otherwise undruggable cancer pathways. Proof of concept therapeutics using interfering RNA or cancer vaccines using messenger RNA (to transiently express a target protein) have made important strides toward clinical translation.124,125
The unique properties of nanomaterials, including size, shape, surface chemistry, and biodegradability, have been exploited to enhance drug delivery and modulate pharmacokinetic profiles (eg, drug circulation time, solubility, tissue penetration, tunable release), reduce toxicity, and improve therapeutic efficacy. For example, limitations associated with extracellular stability of RNA-based therapeutics are being addressed using nanoparticles, and these approaches are being developed for clinical use.126 Another approach is synchronised or staggered combination delivery using nanoparticles to reduce toxic effects or to overcome challenges associated with tumour drug resistance.127 Reformulation of existing drugs using nanoparticles is aimed to either repurpose treatments previously approved by the FDA or to repurpose promising drug candidates to broaden intervention options for patients.
Programmable immune cells for customised antitumour response
Despite exciting progress in immunotherapy, challenges remain in identifying susceptible patient populations and widening the therapeutic index of adoptive T-cell transfer. Most approaches have focused on modulating the native immune response against disease. Additionally, meeting the demands of a large population in need of personalised cell-based therapies with existing manufacturing methods remains a huge challenge.65
Advanced technologies to increase tissue specificity, limit endogenous cell responses, and decrease off-target toxicity have been explored with engineered tumour-specific T-cell receptors and chimeric antigen receptors (CARs). For example, new synthetic biology methods using engineered Notch receptors are being explored to redirect T-cell responses.128 Researchers have also explored the possibility of using nanocarriers to selectively deliver tumour-specific CAR genes into host T cells in situ.129 Overall, these approaches might eventually allow clinicians to programme immune cells with a wide set of features and functions that can only be induced by specific cues associated with the tumour micro environment.
Conclusions
The above examples are just a few possible approaches that are poised to be brought to bear on existing clinical challenges. Convergence is predicated on harnessing the strength of interdisciplinary fields and recognising that often the experts in any area might have solutions to the problems at hand, but the right minds had not been engaged in the context of the unique challenges posed by cancer care. Ultimately, the explosion of molecular-level detective work, cell-by-cell and patient-by-patient, using nanoscale tools and techniques to disrupt intracellular and intercellular pathways, means that we are hopefully entering a period of perfect conditions to accelerate progress both at the bench and the bedside. These efforts will advance the state of the science and have a crucial part in making the goals of Cancer Moonshot a reality.
Part 8: Cancer immunotherapy, combination therapy, and precision oncology
Advances in biology and technological developments have revolutionised immunotherapy. In the past few years, a number of immunotherapies have been developed to block inhibitory signals in cancers and activate T-cell-mediated anticancer responses, with FDA approvals for the treatment of a number of deadly cancers.130 Importantly, these responses are often durable with minimal toxicities, providing improved quality of life for patients with previously terminal diagnoses. In this section, we highlight the state of the field of cancer immunotherapy, describe the challenges and opportunities, and provide a vision for cancer immunity as a fundamental treatment for curing the most devastating cancers. Ultimately, immunotherapy will become a first-line therapy for many cancers, which when combined with other treatments such as localised radiation, oncogene-targeted therapies, and even low-dose chemotherapy, will augment efficacy while reducing the side-effects of these previously established treatments.
Checkpoint inhibition
Use of immunotherapy as a routine cancer treatment modality has progressed enormously in the past 5 years. The approval of the CTLA-4 blocking antibody ipilimumab for metastatic melanoma in 2011 was followed by a succession of regulatory approvals of PD-1 pathway-blocking antibodies for what is still a growing list of cancer diagnoses (melanoma, NSCLC, renal-cell carcinoma, bladder cancer, head and neck squamous-cell carcinoma, Hodgkin’s lymphoma) and of combined checkpoint blockade for patients with metastatic melanoma. Immune checkpoint-blocking antibodies are now widely available as standard options for patients with these diseases, with other drugs being assessed in clinical trials. Although the long-term durability of these responses is still to be resolved, a substantial proportion of patients treated with checkpoint inhibitors are in remission years after therapy.
Despite these unprecedented advances, the molecular determinants of response and resistance to immunotherapy drugs are still being discovered. For non-responding cancers and patients, other clinical applications under investigation include targeting other immune regulatory pathways (eg, CD137, OX40, GITR, TIGIT, and LAG-3) and the use of oncolytic viruses and targeted therapies to promote immunogenic death.131,132 The use of PD-1 or PD-L1 immune checkpoint inhibitors has been helped by measurements of PD-L1 expression in tissue from several tumour types, although the correlation between expression and clinical response remains dubious. Emerging data also suggest that tumour mutational burden133 or microsatellite instability44 could provide additional refinements in identifying tumours that will respond to these drugs. Even if these readouts are eventually combined in some way, the field still lacks a categorical molecular means to enrich for likely responders across the spectrum of immunotherapy drugs and tumour types. Overcoming this barrier will rely on new insights into the components of antitumour immunity that go awry and the development of advanced immunoprofiling capabilities to show the deficits in any given tumour. With new imaging approaches, immunologically cold tumours can be identified, and the efficacy of new therapeutics that augment T-cell trafficking into tumours will be assessed dynamically. Research efforts to bring forth such capabilities should ideally be guided by a precision oncology-inspired vision of simultaneously identifying the crucial immunological lesions operant in any given tumour and pinpointing those therapeutic modalities or combinations that are most likely to overcome these lesions in individual patients. Equally pivotal insights that propel progress in immuno therapeutic design might emerge from systematic studies of intrinsic and acquired resistance to existing drugs. Indeed, deep investigation of tumour resistance mechanisms was one of the key recommendations of the BRP. Emerging insights might eventually suggest new therapeutic avenues. Examples include genetic alterations of antigen processing and presentation, the response to interferon γ, and tumour cell states that reduce a cancer’s immunogenicity. Overall, concerted investment into comprehensive efforts that define the basis of response and resistance to immuno therapies has exceptional potential to speed up additional gains that will benefit many more patients with cancer.
Genomics, epigenetics, and the microenvironment in cancer immunotherapy
The genomic revolution, along with dramatic improvement in the efficiency and cost of sequencing, has transformed the community’s understanding of tumour immunity. Increasing evidence suggests that in many cases, the success of checkpoint inhibition correlates with the mutational load in the cancer and the ability to induce T-cell responses to the tumour neoantigens.131 In clinical trials, investigators are exploring the feasibility and activity of patient-specific T-cell therapies and vaccines designed to promote immunity to epitopes encoded by mutations in individual patients’ tumours. These efforts will interrogate tumour cells at multiple sites and exploit the tumour mutations. The identification of early mutations could ensure generalised responses. Equally important is the notion of shared antigens, including embryonic antigens, modified proteins, and splice variants, that can be targeted by T cells or biospecifics. In some settings and in a subset of patients, shared driver mutations in oncogenes such as KRAS might function as antigenic epitopes that can be recognised and exploited in T-cell therapies and potentially vaccines.134 This is particularly important in prostate cancer and pancreatic adeno-carcinoma, where only a few genomic mutations are observed. The potency, selectivity, and adaptability of immune therapies offer the potential to overcome resistance to traditional oncogenic pathway-targeted therapies. Future research to define the genomic, immune, and clinical profile of patients most likely to respond to immune therapies is needed to determine mechanisms of activity and optimal clinical use. Routine genomic sequencing and proteomic analysis of tumours to create personalised medicines will transform immuno-therapy for an increasing numbers of cancers. In the future, checkpoint inhibitors will be used in combination with genetic and epigenetic modifiers that enhance tumour immunogenicity and promote effector T-cell responses to enhance sensitivity or overcome resistance. Finally, as more is learned about the immune system and tumour environment, there is increased awareness of the complex role of the epigenetic and environmental control of immunity. Reprogramming of effector T cells and regulatory cells in the tumour microenviroment, combined with the specialised role of the microbiome in determining the outcome of checkpoint inhibitors,135 directly align with the BRP’s initiatives that focus on the role of nutrition, stress, health status, and economics in the success of cutting-edge immunotherapies.
T-cell therapies
Advances in cell therapy have focused on both CARs and T-cell receptor (CAR-T) transgenic constructs. Patients with acute lymphoblastic leukaemia (ALL) and B cell lymphomas have very high response to CD19-targeted CAR-T cell therapy, and other targets are being explored (eg, B-cell maturation antigen for multiple myeloma, interleukin 13 receptor α1 for glioblastomas, and mesothelin for various solid tumours). T cells have also been engineered to recognise relatively tumour-specific targets, and bispecific fusion proteins redirecting T cells to specific peptide MHC complexes or surface molecules have entered clinical trials. One such fusion construct, blinatumomab, has received regulatory approval for relapsed ALL. The great potential of cellular therapy in cancer is best exemplified by the anti-CD19 CAR-T cell therapy of childhood ALL, achieving durable complete responses and potential cures in the setting of multiple relapsed disease.136 Avoidance of the acute toxic effects of cytokine release syndrome (CRS), although allowing for the persistence of cells to provide prolonged memory response, are current goals of this approach.137 Combination immune and cellular therapies will probably be needed to ensure that checkpoint-inhibitor therapy abrogates T-cell exhaustion, that T-cells infiltrate solid tumours, and that responses are durable.
The complexity and costs of individual cellular therapies are major hurdles to this approach, as are early relapses due to tumour-antigen shedding or mutation. Exciting new directions include the use of cutting-edge gene editing technologies to alter cell intrinsic checkpoints.138 Additional goals include avoiding unwanted auto-immunity and CRS, creating universal T-cell therapy, systems-based approaches to regulating receptor expression and function, and additional payloads to manipulate the tumour microenvironment and enhance activity against solid tumours. As a consequence, cellular therapies will become more cost effective as new nonviral approaches to gene transduction, universal off-the-shelf anticancer cells, and armed T cells that codeliver additional therapeutics such as checkpoint inhibitors locally become a single combination therapy.
Building a cohesive partnership between government, industry, and philanthropy
One of the BRP’s recommendations was to create a national Clinical Trials Biorepository Network for Immunotherapy. This imperative should not be perceived as a one-size-fits-all approach or become limited to the actual conduct of clinical trials. As highlighted above, the dynamic advances in cancer immunotherapy is fostering the birth and maturation of a new discipline. One clear observation in the past decade is that the advances in cancer immunology-based drug development would not have occurred without individual discovery, entrepreneurism, and deep basic research efforts. These advances reflect the clear value of strong partnerships between the government, industry, philanthropy, and the public in advancing the effort. Organisations such as the Cancer Research Institute, Stand Up To Cancer, and the Society for Immunotherapy of Cancer have been instrumental in supporting immunotherapy efforts at a time of limited resources from the US National Cancer Institute. Within the US National Cancer Institute, both intramural programmes and networks such as the Cancer Immunotherapy Trials Network have continued to push innovation and drug development, even when most of the cancer research community did not appreciate the value of immunotherapy as a cancer treatment modality. A few pioneering companies, were also willing to work with innovative immunotherapy scientists in academia to advance novel immunotherapies in the early days. Cancer Moonshot is the latest in a collaborative effort to bring more targeted funding to cancer research and, as highlighted by the BRP, an emphasis on cancer immunotherapy and a large concerted clinical trials network is recommended. Actual achievements from this legislation and associated recommendations will not be realised without the continued partnerships of those identified above together with continued support from public-focused, non-profit organisations such as the Friends Of Cancer Research, from philanthropists such as Sean Parker and Michael Bloomberg, from the small biotechnology industry, and from the White House and Congress. Finally, equally important has been the support from patient advocacy groups and patients who are willing to participate in clinical trials and provide the biospecimens that are essential to advance understanding of why some patients respond to immunotherapy and others do not.
The next step is a robust implementation plan that builds on these cooperative efforts. Collaborative models should be piloted within the US National Institutes of Health, FDA, and beyond to accelerate discovery through collaboration. These efforts are already evident from the number of PD-1-based combination therapies that are in the clinic and under investigation for multiple tumour types, which so far have involved more than 700 clinical trials and more than 125 000 patients. Many efforts have been made to establish networks, consortia, cooperative groups, and non-profit organisations focusing on advancing immunotherapy. To ensure efficiency and avoid redundancy, a first step is to identify and create a registry of all interested parties. This should be followed by understanding the capabilities and strengths of each party to create synergies and leverage existing expertise. The goal is a common vision and alignment around selecting and prioritising the most promising therapy combinations, developing innovative clinical trial designs, and deeply interrogating clinical and mechanistic data. The classical approach to generating safety data first and then identifying the ideal patient subpopulation is no longer a viable option; it could be aided by preclinical studies as a means for patient selection on the basis of the biology of the drug in question. Patients could then be selected for early progressive studies. Patient selection, or at least an idea about the subpopulation, should be suggested from preclinical studies and then validated early in progressive phase studies. The guidelines should also address the level of clinical activity that needs to be demonstrated for a therapy combination to advance. Finally, radical new approaches to sharing data are needed. It is time to break down the silos and barriers to advancing knowledge for the best interests of patients who are waiting for new cures. Emerging data clearly show that the future of immunotherapy will be in personalised combinations. The US National Institutes of Health should encourage multifaceted, highly focused, and new immunotherapy efforts, working across forprofit and non-profit entities and different disciplines to build bridges between research and drug development.
Conclusions
We have highlighted the extraordinary advances in cancer immunology and immunotherapy for some of the most deadly forms of cancer. The recommendations of the BRP reflected both these achievements and the potential of this endeavour. Immunotherapy, once a forgotten field of research relegated to the smallest meeting rooms and lowprofile journals, has become the rising star of cancer treatment. But the field is still in its infancy. Now is the time for a coordinated and concerted effort that rewards innovative science (panel 12). Advances in the treatment and cure of patients with this devastating disease will rely on a deterministic framework that builds on discoveries through innovative partnerships, a sharp focus on advancing the science, and the clinical application of novel cancer immunotherapies. However, the ultimate goal for all cancer researchers is to develop enough insights to prevent cancer. As part of the Cancer Moonshot effort, there must be a commitment to primary prevention using social science, epidemiology, and behaviour change to reduce the risk of cancers. Primary prevention must also be an imperative for the cancer immunology community. The immune system is perhaps the most sensitive and diverse of biological systems, able to detect and destroy even the most minimal differences between self and non-self. Just as the polio and smallpox vaccines led the vaccine revolution in the past century, which included the development of HPV vaccines to prevent cervical cancer, we hope that cancer vaccines will lead the way to eradicating non-viral cancers in the future.
Panel 12: Priorities for cancer immunotherapy, combination therapy, and precision oncology.
Promote a precision oncology-inspired vision of simultaneously identifying the important immunological lesions in any given tumour and pinpointing those therapeutic modalities or combinations that are most likely to overcome these lesions in individual patients
To determine mechanisms of activity and optimal clinical use, develop an integrated, standardised approach to defining the genomic, immune-based, and clinical profile of patients who are most likely to respond or who are at risk of developing side-effects to immune therapies
Develop and recommend a standardised clinical and biomarker-based discovery approach to guide the exploration of novel immunotherapy combinations, both pre-clinically and clinically
Prospectively assemble a collection of immunotherapy refractory or resistant tumour specimens from which to discover new targetable pathways
To advance our understanding of underlying mechanisms of immune activation and the breakdown of tolerance, create a large data repository of adverse events related to checkpoint inhibition and immune enhancers, focusing on autoimmune, cytokine-release syndrome, and other immune-related syndromes
Build a cohesive partnership between government, industry, and philanthropy to establish networks, consortia, and cooperative groups focusing on advancing immunotherapy
Part 9: Paediatric oncology
The steady improvement in survival of children and adolescents with cancer over the past 50 years is one of the major victories of modern medicine. Although certain childhood malignancies have seen little or no progress, and redoubled effort is certainly necessary for these diseases, more than 80% of children diagnosed with cancer in high-income countries can now expect long-term cure.139 This extraordinary success has resulted in an ever-expanding population of paediatric cancer survivors, projected to reach 500 000 people in 2020 in the USA alone.140 However, most survivors face a lifetime of medical issues; more than 81% of survivors live with at least one severe, disabling, or life-threatening health condition.141–144 The life expectancy of survivors is shortened on average by 10 years compared with healthy individuals,145 roughly equivalent to the decrement in the life expectancy of smokers.146 We must therefore strive to cure more children, but also to inflict less life-long harm.
The chance for cure is not equally distributed across the population. Vulnerable subgroups, including children from low-income families or non-white households, have outcomes far inferior to those achievable.147 Adolescents and young adults, as a group, have inferior outcomes compared to those of younger children.148 Broadening the lens, the proportions of cure for children living in low-income and middle-income countries (LMICs) lag far behind those in high-income countries.
Cancer Moonshot is an unprecedented opportunity to galvanise the paediatric oncology community, catalyse further dramatic improvements in curative treatment, and reduce the short-term and long-term toxic effects of cancer and its therapy. In this section, we recapitulate the BRP’s recommendations for paediatric cancers and suggest additional research priorities for investment.
The BRP recommendations
In most of the BRP recommendations, paediatric cancer is either the sole focus or a major focus: of the ten recommendations, seven explicitly reference paediatric cancers (table). These recommendations represent an unparalleled chance to develop a new generation of efficacious therapies. The remaining recommendations, although without explicit reference to children, also have paediatric relevance. For instance, a recommendation focusing on early detection clearly has paediatric applications. Some paediatric cancers are increasingly understood to be the first manifestation of germline mutations;152 screening patients for cancer predisposition is therefore increasingly important. Discovery of a genetic predisposition has obvious implications for screening and early detection, both in the child and in affected family members. In children with hereditary cancer predisposition syndromes, clinical surveillance protocols to detect asymptomatic tumours have already improved long-term survival.151 Thus, our first recommendation is to strongly support that every BRP recommendation is fully applied to children with cancer.
Table:
Paediatric applications of the Blue Ribbon Panel (BRP) recommendations
| Paediatric applications explicitly discussed in the BRP report | Paediatric applications | |
|---|---|---|
| Network for direct patient engagement | Yes | Highly relevant for children with cancer; existing childhood cancer cooperative trial groups offer a platform upon which such networks could be built |
| Cancer immunotherapy translational science network | Yes | The BRP called for a network that focused on the particular issues with this approach specific to children, such as the paucity of identified cancer neoantigens and absence of response to immune checkpoint blockade; the recommendation went further to suggest a nationwide paediatric translational science network that would support the clinical trials group |
| Therapeutic target identification to overcome drug resistance | Yes | The BRP recognised that mechanisms of drug resistance might differ between children and adults and specifically recommended that childhood malignancies be studied in parallel with adult malignancies |
| A National cancer data ecosystem for sharing and analysis | No | Although no mention of paediatrics specifically, this recommendation is highly relevant for children with cancer and their caregivers; existing childhood cancer cooperative trial groups offer a platform upon which such networks could be built |
| Fusion oncoproteins in childhood cancer | Yes | Specific to childhood cancers, many of which are driven by fusion oncoproteins |
| Symptom management research | Yes | Very brief mention of need for symptom management to improve quality of life for survivorsof childhood cancers; 80% of children are cured of their disease, but 81% of survivors experience at least one severe disabling disorder; a focus on prevention, development of age-appropriate, patient-reported outcome tools, and evidence-based management algorithms are urgently needed149,150 |
| Prevention and early detection: implementation of evidence-based approaches | No | Although most childhood cancers are not currently amenable to prevention or early detection, many germline tumour predisposition syndrome first manifest as a paediatric tumour; these children and their affected family members are in need of the development and implementation of evidence-based and effective screening programmes;151 moreover, many childhood cancer survivors are at risk of developing a second treatment-related malignancy, and more effective prevention and screening strategies must be implemented |
| Retrospective analysis of biospecimens from patients treated with standard of care | No | Although the BRP noted that a large barrier to this goal is the low percentage of adults who enrol in trials within the National Cancer Institute’s National Clinical Trials Network, the opposite is true for paediatric cancers; most paediatric patients enrol on a clinical trial and agree to tumour banking; leveraging the clinical trials data and biobanked specimens collected under the auspices of, for example, the Children’s Oncology Group ought to be prioritised |
| Generation of human tumour atlases | Yes | The need to include paediatric cancers in this initiative is highly visible in the BRP report |
| Development of new enabling cancer technologies | No | Paediatric cancers are not explicitly mentioned in this recommendation, yet the need for innovative technologies, such as intra-tumour and extra-tumour pharmacotyping, is equally relevant to childhood cancers |
The BRP recommendations outline tremendous opportunities for paediatric oncology. However, to fully transform the childhood cancer landscape, we suggest four additional research investment priorities: survivorship, cancers in adolescents and young adults, reductions in health disparities, and global oncology.
Survivorship
Findings from pioneering cohort studies such as the Childhood Cancer Survivor Study153 and the St Jude Lifetime Cohort154 have demonstrated the serious physical and psychosocial consequences of childhood cancer treatment.141–144,155 The cumulative prevalence of at least one serious, life-threatening, or disabling condition in cancer survivors aged 45 years is 81%.141 The cumulative incidence of serious adverse health conditions in a cancer survivor aged 24 years is comparable to that of an untreated individuals aged 50 years.156 Paediatric oncologists who are aware of these toxic effects have modified protocols wherever possible, and consequently risks of serious late effects are decreasing.157 But more can, and should, be done. Targeted drugs might be less toxic, but cannot be assumed to be the panacea, since little is known about potential long-term effects of novel small-molecule drugs like tyrosine kinase inhibitors, checkpoint inhibitors, immunotherapy drugs, or cell-based immunotherapies.
To substantively improve these long-term outcomes, we must develop a multidisciplinary programme that spans the research continuum, from basic science to clinical research to implementation science. Risk prediction modelling would inform treatment decisions. Ideally, in view of emerging evidence that host genomics affects the risk of developing specific late effects, such risk prediction models would not only include demographic, clinical, and treatment-level variables, but also pharmacogenomic data.158,159 Prioritising the development of drugs that specifically ameliorate toxic effects could allow effective chemotherapeutics to remain in use. The development of cellular and animal models to simultaneously assess chemoprotection against important off-target toxic effects (cardiotoxicity, ototoxicity, neurotoxicity) and antitumour effects will allow for more confident introduction of chemoprotective agents. Carefully cataloguing the after-effects of treatment with developmentally appropriate, patient-reported outcomes is essential to the research. Paediatric oncologists must expand upon the excellent work done by the vanguards of survivorship research in developing transition plans, evidence-based screening guides, and prevention strategies with health-care delivery research to augment implementation. These efforts must engage the paediatric and adult cancer communities and collaborate with adult primary care providers. A goal should be to create a network of centres of excellence to coordinate survivorship-related research. The goals of this network should be to develop biological models, risk prediction tools, screening strategies, efficacious chemoprotective agents, and implementation and health-care delivery research and to provide expert clinical care (panel 13). Although many survivorship issues are also relevant to adult patients with cancer, unique considerations in patients exposed to cancer therapy as children will require research specific to this population.
Panel 13: Specific research priorities related to childhood cancer survivorship.
Create rigorous and clinically relevant risk prediction models that incorporate genomic, demographic, treatment exposures data from the patient
Develop biological and animal models of specific late effects, including both acute side-effects with long-term consequence (eg, osteonecrosis, ototoxicity) and long-term effects (eg, cardiotoxicity)
Identify novel chemoprotective agents to prevent specific late effects
Develop and disseminate evidence-based screening strategies for physical and psychosocial late effects that minimise long-term morbidity and mortality
Identify health-care delivery models that reach the maximum number of childhood cancer survivors
Create a network of centres of excellence to coordinate survivorship-related research
Adolescent and young adult oncology
Adolescents and young adults with cancer are a vulnerable subpopulation whose outcomes have not improved to the same extent as those of young children.160 The cancers in adolescents and young adults are different to those of children and older adults because they represent a transition period between paediatric-like, embryonal cancers derived from mesodermal tissues and adult-like carcinomas derived from epithelial tissues. The most common cancers in this age range (eg, Hodgkin’s disease, germ-cell tumours, thyroid cancer, sarcomas) are rare compared with the more common cancers in adults.161 More than 90% of adolescents and young adults in North America receive their care in community practices where expertise in treating cancers in adolescents and young adults might be limited.162,163 Because most community practices do not participate in the US National Cancer Institute’s National Clinical Trial Network, this subpopulation has not benefited from the standardisation of care, the incremental improvement of sequential clinical trials, or the insights derived from linking biospecimens to clinical outcomes.164 Many other possible mechanisms could explain the survival gap in this group of patients, such as unfavourable tumour biology, unique pharmacokinetics, diagnostic delay, and poor adherence to prescribed therapies.165–168
If fully applied to adolescents and young adults, the BRP recommendations will revolutionise oncology for these patients. For example, the BRP recommendation to develop a federated network of tumour profiling services accessible to patients and linked with opportunities for clinical trial participation could have a particularly important and positive effect on these patients.
In addition to existing BRP recommendations, two major recommendations specific to cancer treatment for adolescents and young adults are warranted. First, a clinical trial network specific to this subpopulation should be developed to encourage the development and reach of clinical trials specifically designed for this age group. This network could be independent, but would ideally represent collaborative efforts between adult and paediatric groups. The success of such trials has already been demonstrated. Investigators in both the Dana-Farber Cancer Institute ALL Consortium169 and the US Intergroup study C10403170 examined the use of paediatric-style ALL treatment protocols in adolescents and young adults and found markedly increased survival compared with traditional adult protocol-based treatment. Second, research on the most effective health-care delivery model for adolescents and young adults with cancer is essential. Despite the evidence outlined above, investigators in California found that the percentage of adolescents and young adults with ALL receiving appropriate treatment actually decreased from 31% in 2008–12 to 21% in 2013–14.163 According to findings from the population-based AYA-HOPE study,171 nearly 30% of adolescents and young adults across settings did not receive appropriate medical therapy. Several jurisdictions have built alternative models of cancer care delivery for this age group; for example, the UK has built a nationwide network of cancer units for adolescents and young adults.172 Although the BRP denotes issues such as coverage and fragmentation of care delivery as beyond the scope of their report, an assessment of these novel, mature mechanisms of cancer care delivery would inform future initiatives for adolescents and young adults. Goals should include improving cancer outcomes by fully applying each BRP recommendation to this population, fostering a clinical trial network for clinical and biological research that is specifically for adolescents and young adults, and identifying effective cancer care delivery models for this age group.
Reduction of health disparities
The BRP report focuses on reducing health disparities, particularly in trial enrolment, tumour sample collection, and the uptake of screening and prevention strategies. Disparities in short-term and long-term childhood cancer outcomes by race, ethnic background, and socioeconomic status also exist.147,166,173 Research elucidating the mechanisms underlying these disparities is essential.
A seminal example in the USA involves adherence to oral chemotherapy for treatment of ALL. When non-adherence was defined as taking less than 95% of prescribed doses of 6-mercaptopurine, proportions were highest in patients of non-white ethnic background, low annual household income, or low parental educational level.166 Non-adherence strongly correlated with risk of relapse: the cumulative incidence of relapse in children adhering to treatment was 5% compared with 17% of children who did not adhere.166 A remarkable 47% of the relapses in children could be attributed to non-adherence. The impact of effective interventions targeting factors such as non-adherence in disadvantaged subpopulations could therefore match or even exceed that of novel therapeutic agents. To design and assess effective targeted interventions, a goal should be to determine the mechanisms underlying dispairities in childhood cancer outcomes associated with socioeconomic status and ethnic background.
Global oncology
Most children with cancer live in LMICs, and their outcomes lag far behind those in high-income countries because of delayed diagnosis, poor access to cancer care, increase treatment-related mortality, abandonment of therapy, and inadequate health-care systems.64,174 Addressing this global disparity is a moral imperative, as noted by the BRP Paediatric Working Group. Novel insights into aetiology, cancer biology, and treatment innovations that can only be learned through research in collaboration with LMICs will benefit all children with cancer. For instance, investigators in an international collaboration identified genetic polymorphisms associated with thiopurine toxicity has a major implication for care of subgroups of children in all settings.175 The explosion of data arising from tumour genome sequencing comes almost exclusively from high-income countries; sequencing of samples from LMICs might yield important implications for therapy for children in both LMICs and high-income countries. Innovative programmes such as medical telecommunication could improve access to quality cancer care for children in LMICs and have application to underserved populations in high-income countries.176,177 A goal should be to establish international research networks for biological, epidemiological, clinical, and health-services research in partnership with paediatric cancer centres in LMICs to identify insights into cancer aetiology and treatment innovations with maximum benefit to children with cancer who live in LMICs.
Conclusions
The BRP recommendations represent an unparalleled opportunity to galvanise childhood cancer research. Fully applying these recommendations to paediatric patients, coupled with additional priorities pertaining to survivor-ship (panel 13), cancers in adolescents and young adults, health disparities, and global oncology (panel 14) will allow for truly revolutionary advances at every stage of the cancer journey for all populations.
Panel 14: Priorities for paediatric oncology.
Ensure that each BRP recommendation is fully applied to children with cancer
Create a network of centres of excellence to coordinate survivorship-related research that includes the development of biological models, risk prediction tools, screening strategies, efficacious chemoprotective agents, and implementation and health-care delivery research
Improve cancer outcomes for adolescents and young adults by fully applying each BRP recommendation to this population; foster clinical trial networks specifically for clinical and biological research of cancers in adolescents and young adults, and identify effective cancer care delivery models
Determine the mechanisms underlying disparities in childhood cancer outcomes between populations of differing socioeconomic status and between racial and ethnic groups to design and evaluate targeted interventions
Establish international research networks that conduct biological, epidemiological, clinical, and health services research in partnership with childhood cancer centres in LMICs to identify insights into cancer aetiology and treatment innovations that can only be discovered through work outside high-income countries
Part 10: Supportive care
The goals of Cancer Moonshot are to dramatically accelerate efforts to end cancer as we know it. About 15·5 million (5%) of the American population are cancer survivors, and this population is expected to grow to 26·1 million survivors by 2040.178 As part of the Cancer Moonshot initiative, it is therefore important to focus on the care that is needed to return people to their fullest possible functioning in society after a cancer diagnosis. Supportive care, including survivorship, palliative, and end-of-life care, holistically addresses the physical, emotional, and social needs of people with cancer, irrespective of age, and is an essential component of care from the time of diagnosis throughout the persons’ life.179 However, investment and progress in supportive care is incomparable with the advances in understanding, diagnosis, and treatment of cancer. Inadequate supportive care can be expensive by causing increased health-care use (including emergency department visits, unplanned hospital admissions,180 and decreased adherence181,182), affecting treatment outcomes, and possibly reducing survival.183–186 Increased symptom burden reduces quality of life for patients and increases distress to caregivers. Successful supportive care focuses on managing disease and treatment-related symptoms, improving quality of life, assuring preference-concordant treatment, and improving adherence to recommended treatments, and it is associated with increased survival.187,188 In this section we address general and specific priority areas ready for accelerated progress in this area.
Basic, translational, and clinical science to improve symptom management
Despite growing attention to the health issues affecting patients after cancer, too little attention is given to the causes and mechanisms of these problems. Of particular importance is the need to fund research to better understand the biochemical basis for each of the specific symptoms and toxic effects, alone and combined, in patients as a result of cancer and cancer therapy. As with management of the cancer, the oncology community needs to move from understanding the aetiology of disease to developing and testing interventions and addressing the underlying pathology, rather than merely treating the symptoms. Basic science studies of genetic predisposition to toxic effects and pharmacogenetics are needed. Achievements to date include an understanding of the genomic basis for aromatase-associated arthralgias189,190 and associated chemotherapy-induced peripheral neuropathy.191 Additional funding is needed for translational studies that bridge laboratory data to treatment in the clinic and home and for clinical trials to investigate drug mechanisms and evaluate potential therapies in patients.
Effective pharmacological and non-pharmacological treatment strategies for common symptoms
Although support for research of novel approaches to treating and preventing cancer has been, and will always be, of paramount importance in cancer research, there is a crucial need to fund research in supportive oncology that could benefit patients with cancer of all ages. Supportive oncology includes various therapies or interventions that might prevent, minimise, or reverse symptoms associated either with the cancer or with the manifestations associated with the various drugs or therapeutic approaches used to treat the cancer. Many therapies have meaningful benefit for common cancer-related symptoms, but availability and access (in the case of non-pharmacological therapies) to many of these treatment strategies are limited. An example of a nonpharmacological approach to controlling cancer pain is the use of the scrambler, which uses surface electro-stimulation to modulate and decrease neuropathic pain. Unfortunately, this intervention has not been extensively tested and not widely available.192 Drug–disease or drug–drug interactions (in the case of traditional pharmacological approaches) warrant strengthened efforts to identify new drugs (or non-pharmacological approaches) to effectively mitigate common constellations of symptoms emanating from cancer treatment or the cancer,193 and to develop mechanistic and predictive models for treatment of adverse effects and symptom risk, taking into account genomics, age, function, and comorbidities.
With increasing numbers of patients surviving cancer, in many cases with persistence of symptoms associated with, or as sequelae of, cancer treatment, it has become increasingly important to devote time and resources to improving symptom management.194 Among the topics deserving further evaluation are symptoms that appear to be associated with diverse cancers (eg, anorexia or cachexia),195 lymphoedema,196 other symptoms often associated with cancer treatment (eg, hot flushes),197 sexual health,198 chemotherapy-induced peripheral neuropathy,199 cognitive changes,200,201 and fatigue.202
Optimising quality cancer care for symptom management
Some evidence-based symptom management guidelines exist, but they have not been fully implemented or disseminated nationally. These symptom management guidelines should be identified, harmonised, and coordinated. Barriers to coordinated development and dissemination should be identified and mitigated.
Patient-reported outcomes are an important component of assessing symptoms and the effectiveness of management strategies.188 Systems for identifying patient-reported symptoms need to be integrated and linked with evidence-based symptom guidelines through electronic health records to support point-of-care assessment and management and to encourage self-management.
The quality of cancer care delivery could also be optimised by investigating the role of comorbidities and multimorbidity-related drug regimens on symptom burden, optimising treatment adherence strategies to prevent or manage symptom burden, and developing, evaluating, and implementing symptom-related self-management programmes.
Survivorship care
The growing number of cancer survivors is a success story, but also brings new public health challenges. Since the 2005 report by the US Institute of Medicine,83 the growth of survivorship care has been marked by new research and clinical programmes to assess and address the multidimensional needs of cancer survivors, including screening for recurrence and secondary cancers, addressing the psychological effect of cancer, managing side-effects of therapy, screening for late effects of treatment, and assisting with development of or return to a healthy lifestyle. Each of these domains presents research questions related to how to screen cancer survivors, how, where, and by whom the research questions can be optimally addressed, and how to integrate research into patient care so outcomes can be improved after the intensive phases of initial cancer care delivery. Some answers can be applied across broad populations of patients, whereas others will be specific to one cancer type, recipients of a particular treatment, or patients in a particular age group. The past decade can be characterised as one of growing awareness of the issues facing cancer survivors, and the next decade must be one of defining evidence-based strategies to address these needs. We have defined several important areas for investment that would improve the care of cancer survivors.
First, definitive descriptive work is needed to define the short-term (<5 years) and long-term (≥5 years) needs of patients of all ages after completion of initial cancer therapy. Clinicians and patients need a clear picture of what to expect in the months and years after treatment for each specific cancer type and treatment modality. Research is needed to better define the short-term side-effects of specific therapies and their natural history, identify those side-effects and therapies that contribute to the greatest morbidity and any that pose a threat to mortality, and develop evidence-based approaches to screening, management, and, where possible, prevention of these problems. Health issues that can persist for years after treatment must be identified. For example, chemotherapy-induced peripheral neuropathy is a common side-effect of commonly-used chemotherapy drug classes such as taxanes and platinums. For many patients, this side-effect is a transient problem that resolves within months, but for other patients, it can lead to lifelong pain and disability, including increasing the risk of falls with ageing.203,204 Clinicians need reliable tools to predict who is at greatest risk, improved methods of diagnosis, and evidence-based approaches for prevention and management delivered at the point of care. Chemotherapy-induced peripheral neuropathy can be caused by a single drug, but most people treated for cancer have multiple problems that need to be addressed. The ability to identify all similar problems for patients and to address each of them must be greatly improved.
Second, evidence-based approaches must be developed to predict and screen for problems based on expected prevalence in a given patient population, taking into account treatment and patient-specific predictive factors like age. For example, overt cardiac toxic effects from anthracycline therapy is rare. However, as awareness of this issue has grown and with the emergence of the cardio-oncology field, it has become clear that many patients experience subclinical cardiac changes that can progress to clinical heart failure with time. Strategies to identify patients at greatest risk are in development, and prevention and early detection seems to greatly reduce associated morbidity.205–208 Similarly, not all patients will experience long-term emotional distress after a cancer diagnosis, but for those who do, it can have a profound effect on quality of life, social and physical functioning, and health-care use.209,210 Evidence-based tools to screen for distress in patients with cancer should be validated, tailored, and widely implemented for cancer survivors. Once distress is reported, access to relevant services should be made available, and reassessment of distress should occur to evaluate effectiveness of intervention.
Third, there is an unmet need for evidence-based approaches to a wide range of short-term, long-term, and late physical and emotional complications that can arise as a result of the cancer and therapy. Much of oncology is necessarily disease-based, and programmes to diagnose, appropriately stage, and manage specific cancers are designed with the goal to cure or control the disease. The disease-based approach, and increasingly the molecular approach to cancer management, has markedly improved survival for many cancer types. However, relatively little research has been done to define the optimal management of the many physical, emotional, and social consequences of cancer therapy that can cause morbidity and even early mortality. Research is needed to improve understanding of the causes and contributing factors underlying the complications arising after cancer diagnosis and treatment, such as fatigue, cognitive impairment, early cardiovascular disease, neuropathy, infertility, pulmonary toxicity, immunocompromise, metabolic syndrome, anxiety, depression, and fear of recurrence. With a fundamental understanding of these complications, the community can develop and test interventions to prevent or manage each of these issues in individuals with these problems or who are at high risk.
Finally, health services research is needed to identify the optimal methods of health-care delivery after completion of initial cancer treatment. Much of the research to date has focused on survivorship care plans as a method to improve communication with patients and enhance care coordination between oncology specialists and primary care. There are many other important aspects of delivering quality care to cancer survivors, and greater attention to questions related to the location of care, models of shared care among primary care and specialists, telehealth, reducing unnecessary health care use, and addressing behaviour change to improve lifestyle and wellness must be funded to improve survivorship care.
Palliative care
Palliative care centres on the patient and family to optimise quality of life by anticipating, preventing, and treating suffering. Palliative care throughout the duration of illness involves addressing physical, intellectual, emotional, social, and spiritual needs and facilitating patient autonomy, access to information, and choice.179,211 Patients at any age and at any stage of cancer should be provided palliative care along with curative treatment. In 2017, the American Society of Clinical Oncology recommended that “patients with advanced cancer be referred to interdisciplinary palliative care teams (consultation) that provide inpatient and outpatient care early in the course of disease, alongside active treatment of their cancer”.187 These recommendations emanated from numerous clinical trials in which patients receiving palliative care had consistent improvement in quality of life and symptoms and inconsistent increases in survival (but no decrease in survival).
Although palliative care improves quality of life and reduces overall symptom burden, many questions remain with respect to the key components of palliative care and the best strategies to reduce symptom burden, especially in patients with metastatic cancer, comorbidities, or advanced age. We have identified several research priorities that should be invested in to improve the care of cancer survivors.
First, clarity is needed to understand which aspects of palliative care have the greatest effect on a patient’s quality of life and outcomes. Palliative care has a proven effect on an array of quality-of-life domains, yet it is unclear which components of palliative care (eg, comprehensive assessment, symptom management, promotion of prognostic awareness, reduction in late chemotherapy) improves outcomes the most.
Second, research is needed to ascertain how palliative care is best delivered to all patients in need, irrespective of age, given resource constraints. In the USA, only 250 doctors and nurses are trained in palliative medicine annually, despite estimates that 6000–10 000 palliative care specialists are needed to cover inpatient needs; this estimate does not account for the need for palliative care specialists in the community-based setting.212 These workforce constraints affect the amount of time it takes for a patient with cancer to access palliative care.211 In view of resource constraints, telehealth and mHealth could become important in expanding the provision of palliative care to those in need. Nurse-led telephone coaching sessions, an approach to delivering palliative care in the ENABLE study183 and other studies, was associated with improvements in quality of life, 1-year survival, and resource use. More studies are needed to improve understanding of how symptom monitoring through mHealth applications and telehealth can most effectively be integrated into cancer care as a way of extending palliative care access and delivery.
Third, effort is needed to determine how best to identify, treat, and advise patients about treatment-related adverse effects and symptom risk that are affected by age, function, and comorbidities. New promising therapies exacerbate prognostic uncertainty, unknown treatment side-effects, and uncertainty about ideal treatment duration, especially for elderly patients and patients with functional impair ments or multiple comorbidities. Clinical trials that establish the safety and efficacy of new therapies often exclude or have limited representation of these populations and do not collect patient-reported outcomes. Additionally, patients with cancer prioritise outcomes like function and treatment burden in different ways. Early and dedicated palliative care services can aid the identification of treatment goals and provide decision-making support. However, oncologists and palliative care providers alike have little guidance on how best to approach decisions that involve emerging precision medicine therapies, especially in the context of multimorbidity. How best to support patients, their caregivers, and health-care providers in clinical decision making in an era of precision medicine, and how best to optimise patients’ attainment of their overall health-care goals are crucial questions. Patient-reported outcome data are needed to support clinical discussions with patients about the likelihood of survival, toxic effects, and suffering, with efforts to provide precision medicine or immunotherapy late in the course of illness. Research in communication, decision making, and bioethics must address these complex, but vitally important issues for individual patients, clinicians, and the broader society.
As survivorship is focused on a person with cancer from the time of diagnosis and for the rest of life, we cannot end this section without discussing end-of-life care research needs. The US Institute of Medicine213 identified areas for further research to improve patient-centred and family-oriented care. These areas included communication of preferences and shared decision-making, advanced care planning over time (especially in paediatric and adolescents), better prognostic models, effect on caregivers, and effective models for care. The US National Institutes of Nursing Research does a lot of research on end-of-life issues and have identified the following research gaps: living with uncertainty; the costs of caregiving; communication; interventions for ethnically and culturally diverse populations; and transitions in care.214,215 We have much to learn from our patients216,217 and our European colleagues218 in this area. Although some foundations fund end-of-life research,219 overall federal funding for end-of-life research is small and should be expanded.
Conclusions
Scientific and clinical advances in symptom management must be accelerated throughout the cancer continuum for all ages. Our recommendations are listed in panel 15.
Panel 15: Priorities for supportive care.
Increase understanding of the basic mechanisms of common symptoms, including genetic predisposition, pharmacogenomics, pathophysiology, and response to interventions
Support the development of an evidence-based screening and triage system to identify and address needs of cancer survivors with high burden of symptoms or emotional distress
Identify the most effective elements of palliative care interventions and, through health services research, identify means to provide these important aspects of care to all patients with cancer
Part 11: Radiation oncology
Tremendous technological progress has been made in radiation oncology in the past three decades, with the advent of software and hardware inventions that integrate three-dimensional tumour imaging with highly accurate treatment delivery methods. Consequently, patients treated for common malignancies have better tumour control and fewer side-effects than ever before. In many instances, such as selected head and neck, lung, bladder, uterine cervix, and prostate cancers, radiotherapy—sometimes combined with a radiosensitising agent—offers a non-invasive, organ-sparing, potentially curative treatment with equivalent long-term outcomes and often fewer toxic effects than more invasive approaches.
Modern radiotherapy technology can also provide high-value care. The precision achieved with new treatment systems not only reduces toxic effects, but also allows radiation oncologists to shorten the overall treatment schedule for many patients. Stereotactic techniques enable non-invasive cranial and extracranial tumour ablation, unlocking novel management strategies in oligometastatic disease.
Key challenges ahead include optimising the benefit of radiation therapy in the metastatic setting, identifying the best ways to combine radiation therapy with new molecular targeted or immunomodulatory agents, deepening our understanding of tumour and normal tissue radiobiology to inform the rational design of new radiosensitisers and radioprotectors, devising ways to individualise radiation treatments, and probing clinical opportunities for alternative forms of radiotherapy, such as charged particles.
New applications of radiotherapy
A vision put forth 20 years ago is now being tested in phase 3 clinical trials. In the mid-1990s, Hellman and Weichselbaum220 proposed that an intermediate state of cancer dissemination, oligometastasis, existed between purely localised disease and widespread metastatic disease, and that judicious use of local therapy (radiotherapy, surgery, or other ablative modality) on oligometastasis gives patients long-term and disease-free survival. A preponderance of indirect evidence lends credence to this theory.221 Phase 1 studies establishing the safety of ablative radiotherapy for metastases in the lung, liver, and spine222–224 were followed by phase 2 studies evaluating the efficacy of stereotactic radiotherapy in more narrowly defined clinical settings.225–227
Phase 3 studies have been launched, and early results are promising. Results of a multi-institutional study228 showed that local therapy (in this study, nearly always radiotherapy) for patients with a limited number of metastatic sites of NSCLC can extend progression-free survival; median overall survival has not been reached. Other studies in the USA and internationally (eg, NCT02364557, NCT02759783, NCT02089100, NCT02893332, NC01446744, and NCT02417662) will inform our collective understanding of which patients with oligometastatic disease benefit from local interventions to known disease sites. However, optimising patient and modality selection (radiotherapy versus other means) will take years of additional research. Moreover, with improved systemic therapies, patients with widely metastatic disease can revert to an induced oligometastatic state where they might likewise benefit from local therapy as with de-novo oligometastatic disease, adding yet another clinical nuance to evaluate.
Combination of radiotherapy and systemic treatments
To date, the only setting in which adding a targeted drug to radiotherapy has improved survival is the combination of cetuximab and radiotherapy in patients with locally advanced head and neck cancer.229 Interestingly, although cetuximab is assumed to primarily inhibit epidermal growth factor, immunomodulatory effects might also contribute to its mechanism of action,230 highlighting the need to understand the crosstalk between complex signalling events. The current system of pharmaceutical development tends to favour the development of agents as monotherapy rather than as enhancers of radiotherapy or other cytotoxic interventions. Important opportunities might therefore be missed, especially for drugs without stand-alone cytotoxicity.231 Examples of potential radiosensitising drugs with little independent antitumour efficacy are inhibitors of poly ADP-ribose polymerase, a DNA repair enzyme, and Wee-1, a cell cycle modulator.232
One of the most tantalising observations in recent years is the abscopal effect whereby patients receiving immunotherapy, either a checkpoint inhibitor or other immunostimulatory drug, show a response in both irradiated and non-irradiated sites.233–236 Preclinical data indicate that radiation synergises with immunotherapy in several ways. Radiation promotes release of tumour antigens237 and induces chemokines that recruit T cells into the tumour.238,239 Radiation can also increase expression of death receptors,240,241 MHC class 1 proteins,242,243 costimulatory molecules,244 and stress-induced ligands245,246 that enhance tumour-cell recognition and killing by T lymphocytes. Radiation overcomes tumour resistance to anti-PD1 therapy by inducing interferon β, which upregulates MHC class 1 expression. However, radiation also has immunosuppressive effects through a variety of mechanisms.247–251 Understanding the interactions between radiation and the different types of novel immune therapies is therefore crucial to be able to exploit beneficial synergies and avoid counterproductive combinations.
New directions in radioprotection and functional restoration
Apart from technical improvements that reduce toxic effects of radiotherapy in normal tissue, pharmacological approaches to radioprotection have included the use of the free radical scavenger amifostine, but this drug is not widely adopted because of side-effects and marginal efficacy.252 A newer radioprotection strategy is to target radiation-induced signalling pathways that mediate the underlying physiological changes. For example, inhibition of prolyl hydroxylases, enzymes that promote degradation of hypoxia inducible factor-2, can protect against lethal irradiation in mice.253 Several prolyl hydroxylase inhibitors are being tested in clinical trials for the treatment of anaemia and might warrant investigation as radioprotectors.
Stem-cell therapy via cell transplantation or in-situ activation can also restore function after radiation damage.254 Preclinical studies have identified stem cells in adult murine salivary glands that restore function when transplanted into irradiated recipient glands.256 Alternatively, awareness of the anatomical locations of normal stem cells can be exploited to inform design of the internal radiation dose distribution so that these cells are spared. Trials to assess stem-cell dose avoidance are underway in multiple clinical settings.
New technologies in radiotherapy: genomics, imaging, charged particles, and radionuclides
The approach to prescribing radiation dose to solid cancers is based primarily on knowledge of the tumour location, histology, and radiosensitivity of surrounding normal tissues without accounting for the intrinsic sensitivity of tumour or normal tissues in individual patients. Biomarkers that allow the dose to be titrated for individual patients would be valuable.
One way to approach this challenge is to use liquid biopsies. For example, blood samples can be analysed for circulating tumour DNA or circulating tumour cells.256,257 Such non-invasive tests can easily be repeated to collect information about the genotype of tumours being irradiated when tumour biopsies are not available, quantitatively estimate tumour heterogeneity as a biomarker, assess early response during radiotherapy to determine dose escalation or de-escalation, and recognise radiographically occult residual disease after radiotherapy to identify patients at high risk for recurrence. For example, the presence of residual, circulating, tumour-derived Epstein-Barr virus DNA after radiotherapy in patients with nasopharyngeal cancer predicts outcome.258 Modification of adjuvant therapy based on post-treatment Epstein-Barr virus DNA concentration is being explored in a prospective trial (NCT02135042). In view of recent advances in technologies to detect circulating tumour DNA,259 similar approaches could probably be extended to most other tumour types.
Biomarkers that can predict outcomes before initiating radiation is another important advance. For example, mutations in the KEAP1/NRF2 pathway predicted an increased risk of local relapse in patients with locally advanced NSCLC treated with high-dose radiotherapy with or without chemotherapy.260 Another candidate indicator is the genome-based model for adjusting radiotherapy dose, a composite metric involving a quantitative assessment of the expression of ten individual genes to determine a radiosensitivity index and an equation accounting for the radiation dose schedule.261
Other non-invasive methods of response assessment include imaging-based approaches with a novel radioactive tracer, radiomics, and functional imaging. First, 18F-labelled fluorodeoxyglucose (18F-FDG) and 18F-labelled fluoromisonidazole (18F-MISO) PET-CT provide metabolic-specific and hypoxia-specific imaging, respectively, and trials to test response-adapted modification of radiotherapy dose or target volume on the basis of early responses detected with these tracers are ongoing for stage 3 lung cancer and head and neck cancer (NCT01507428, NCT00606294). Radiomics involves computer-aided analyses of imaging features not readily apparent to human visual inspection. This technology is in its infancy, but might yield clinically relevant predictions of radiotherapy effects.262 Finally, four-dimensional CT scanning methods to characterise breathing-related motion for radiotherapy planning could also be applied to derive a quantitative estimate of regional lung function.263,264 A clinical trial is underway (NCT02528942) to assess the use of this information to avoid unnecessary exposure of highly functional regions of lung during radiotherapy in patients with locally advanced primary lung cancer.
Nearly all external beam radiotherapy is administered with photons, high-energy x-rays that are gradually absorbed as they traverse the patient. With the use of multiple beam angles and modulation of the intensity of the beam cross section, exposure of adjacent normal organs to the radiation dose can be minimised. The physical properties of charged particles (eg, protons and carbon ions) differ from photons. In essence, charged particles steadily deposit some energy up to a certain depth of tissue, the so-called Bragg peak, where almost all remaining energy is deposited in a sudden burst and beyond which there is very little exit dose. By using particles of varying energy, the Bragg peak can be smeared out to cover the full depth of the tumour. The absence of exit dose beyond the Bragg peak is appealing in numerous clinical settings if it can be exploited to safely reduce toxic effects and the risk of secondary tumours induced by radiation in normal tissues, a consideration of special importance in the paediatric cancer population, for example.
A reduced risk of side-effects could make proton therapy a cost-effective alternative for paediatric medulloblastoma,265 but careful comparative analyses will be necessary to determine the degree of clinical advantage in that setting. In one prospective series,266 proton therapy was associated with fewer long-term toxic effects relative to photon-based treatment, and disease control and survival were equivalent.267 However, some uncertainty persists about the exact depth of the Bragg peak for proton and carbon ions within patients,268 and the incomplete understanding of the biological effect of protons relative to photons, especially for CNS tumours, is a source of concern.269 Another major challenge for particle therapy in its current form is the expense. These advantages and disadvantages have been reviewed in more detail elsewhere.270
In a Cancer Moonshot-sponsored project, researchers at Stanford University, CA, USA, and the Palo Alto Veterans Affairs Medical Center, CA, USA, are exploring new indications for protons and carbon ions and building a less expensive particle therapy facility. Among new lines of investigation to pursue is the question of how to exploit certain biological effects of particle therapy that differ from photons, focusing on tumour stem cells or modulation of systemic immunity.271
The development and clinical administration of soluble radionuclides for infusional therapy generally involves collaboration between radiation oncologists, nuclear medicine specialists, and interventional radiologists. One surprising advancement in this area is the demonstration that calcium-mimetic 223Ra, which has an osteotropic tendency, and can increase survival in selected patients with metastatic prostate cancer.272 Other recent work in this clinical area is typical of the more common approach to modern radionuclide therapy, namely an effort to link a radioactive moiety with a tumour-specific antibody.273 Challenges remains to develop effective radio immuno-therapy for solid tumours while sparing normal surrounding tissues, and further refinement of this approach is much needed for clinical use.274
Conclusions
The substantial progress in radiation oncology in recent times has brought substantial advances in cancer outcomes, and we anticipate further breakthroughs in the coming years as important studies mature. With continued support for cutting-edge investigations (panel 16), there is reason for optimism that radiation oncology will keep pace with other fast-moving sectors of cancer care and offer better treatments to patients in the years to come.
Panel 16: Priorities for radiation oncology.
Test the role of radiation or other localised therapy in clinical trials to extend survival and disease-free survival in the oligometastatic setting
Although novel targeted therapies are providing patients with a much better outlook than ever before, some resistant clonogens will invariably emerge, and there is a need to interdigitate local therapy strategically
Investigate combinations of radiotherapy with immunotherapy or other novel molecular drugs
Data from preclinical and early-phase clinical trials indicate that radiation can help harness the full power of immunotherapy, and additional work is needed to optimise the combination
Novel drugs that block DNA repair or cell-cycle progression might have minimal independent antitumour efficacy, but great value when combined with radiotherapy, and these possibilities must be explored
Develop protective and restorative interventions for normal tissue effects of radiation
Molecular biology methods offer new insights into the cellular effects of radiation and how to mitigate them in normal tissues; progress in this area would help not only patients with cancer, but other patients who are unwittingly exposed to high doses of radiation
Build biological and radiographic tools to personalise radiation therapy for individual patients
Measurement of circulating tumour cells, circulating tumour DNA, and novel molecular imaging, among other techniques, might empower radiation oncologists to select the ideal amount of treatment and region of treatment for patients with cancer to maximise chances of tumour control and minimise chances of side-effects
Explore the value of heavy ion therapy
Physical and biological features of protons, carbon ions, and other heavy particles offer potential clinical advantages, but much more knowledge is needed on how to exploit their full potential, and creative engineering is needed to reduce the cost of the technology
Part 12: Nuclear medicine and imaging
Precision oncology relies on highly specific targeting of cancer or other key cells in the tumour microenvironment and identifying malignant lesions to help select a treatment that will be effective. In-vitro diagnostics, imaging, and radionuclide therapy have important roles in tailoring treatment to an individual’s unique biology, making therapy more effective and reducing costs.
The combination of imaging and therapeutics can deliver cancer-targeting and pharmacokinetic information that can suggest whether a therapeutic approach could be effective. Once a therapy is administered, these approaches can be used to assure the therapy is working or needs to be changed. This combination can therefore be a highly cost-effective measure for expensive therapies. Imaging is also useful for prediction and assessment of drug-induced, end-organ toxic effects and can be used to detect subclinical drug-related toxic effects that could have major implications on management decisions, especially if early imaging studies also suggest the treatment is ineffective.
In this section, we highlight specific examples to show the importance of molecular imaging—in the clinic or close to clinical translation—in cancer detection, treatment selection, and treatment monitoring.
Precision oncology
Imaging can guide individualised and highly specific cancer treatment and is therefore a key element of precision oncology. Imaging offers many opportunities for targeted cancer screening, staging, and therapeutic response evaluation.275 Sophisticated image analysis, including big data methods such as machine learning, can extract information from images that is not readily observed by visual interpretation. Molecular imaging is well suited for guiding targeted therapy.276,277 In addition to detecting and staging cancer, often more accurately than anatomical imaging, molecular imaging can be used to measure regional molecular features and assess therapeutic target expression across the full body burden of disease, serving as a predictive marker and identifying target heterogeneity. Serial quantitative molecular imaging also provides a robust early indication of therapeutic efficacy and, importantly, of therapeutic futility. This combination makes molecular imaging a powerful tool for guiding effective and cost-effective cancer therapy.278
Findings from early studies showed the unique capability of molecular imaging as a cancer biomarker. In patients with breast cancer, highly specific PET ligands can be used to measure regional oestrogen receptor and HER2 expression, which correlate quantitatively with invitro data,279,280 identify site-to-site heterogeneity in advanced disease,281 and predict response to therapy.282,283 Similarly, 18F-FDG PET data can show response to therapy as early as a few days or weeks into treatment for several cancers, including lymphoma, breast cancer, lung cancer, and gastrointestinal stromal tumours.284–287 PET tumour proliferation imaging compounds such as 18F-labelled fluorothymidine (18F-FLT) also allow earlier response assessment (within 1–2 weeks) for both chemotherapy288 and targeted therapy,289 and can also indicate toxic effects for proliferative organs such as bone marrow. Combined imaging to measure regional target expression and assess early therapeutic response is especially powerful for guiding therapy. In the ZEPHIR study,290 pretherapy HER2 PET imaging and early serial 18F-FDG yielded 100% negative and positive predictive value for complete response to HER2-targeted breast cancer therapy.
These early examples and findings from other multicentre trials using novel PET probes284,290–292 set the stage for the integration of molecular imaging into precision oncology. Research and future trials should focus on the methods that can have the largest effect on cancer therapy by helping to direct tissue sampling to the most informative sites, selecting patients most likely to respond to targeted therapy, and identifying therapeutic futility early, saving patients the toxic effects and cost of therapies.
Imaging in immuno-oncology
Extrinsic host-derived factors are pivotal to promoting malignancy.293 For example, inflammation is one of two new enabling characteristics of cancer.111 Immune cells often make up most of the tumour mass, confounding standard efforts to monitor immunotherapy. Immuno-therapy involves using immunomodulatory antibodies, cancer vaccines, or cells to treat cancer.294 One cannot simply use conventional Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 anatomical criteria to monitor immunotherapy because such therapy can have vastly different effects on the anatomic image of a tumour despite similar, often positive, patient out comes.295,296 To address this difficulty, a consensus guideline known as iRECIST, a modified RECIST version 1·1 for immune-based therapeutics, has been developed for clinical trial protocols, but has not yet been validated to guide clinical practice.297 18F-FDG PET, a sensitive metabolic technique for detecting tumour progression, can also be somewhat misleading in guiding immunotherapy, even using the PERCIST criteria298 because immune-cell influx causes a metabolic flare, suggesting a need for more specific molecular imaging agents.
The tumour immune microenvironment must be better understood. The goal for development of immunologically targeted molecular imaging compounds is to understand the tumour microenvironment noninvasively. For example, although checkpoint blockade has had arguably the most profound effect on cancer of any therapy in recent memory, only about 20% of patients have a durable response.299 This might be due to heterogeneous and dynamic expression of key receptors on tumours or immune cells, which could be addressed with imaging in near-real time.300 Another interesting option is to image adverse effects, which are not uncommon with current immunotherapies301 (eg, lethal neuroinflammation caused by CAR-T cell therapy302). An individual’s potential side-effects can be assessed by molecular imaging of specific pathways and cells.
Despite the proliferation of immunotherapy trials, molecular imaging is seldom used. According to one review,303 484 clinical trials involved imaging, but only 15 trials involved molecular rather than anatomical imaging, nearly half of which used 18F-FDG, with its attendant problems. The strategies used to target the relevant immune cells are as varied and complex as immunotherapy itself, including ex-vivo labelling of,303–305 or introduction of,306–309 imaging reporters to adoptively transferred cells, immunoimaging with radiolabelled antibodies310,311 or antibody fragments,312,313 and radiopharmaceuticals of low molecular weight.314 All strategies are being pursued in parallel, and therapeutic clinical trials that integrate them are beginning to appear. Crucial to this effort will be collaboration between immunologists, oncologists, and imaging specialists to obtain the most informative compounds for each indication in immunooncology.
Theranostics
The term theranostics is a portmanteau of therapeutics and diagnostics, coined to refer to systematic integration of targeted diagnostics and therapeutics, in alignment with the concept of precision medicine.315–319 The imaging counterpart of a theranostic compound identifies whether or not, and to what extent, a particular biological target is present in a particular disease process such as cancer, and thereby identifies those subset of patients who would benefit from the companion therapeutic drug. This concept is especially important in view of the remarkable molecular heterogeneity between individual cells within an individual tumour, between cancers of the same type, and between the primary tumour and its metastases. Theranostics has long been important in the history of nuclear medicine, and the list of theranostic companions, and interest in their use, is increasing as more basic knowledge about relevant biological markers is acquired and as new radiopharmaceuticals that target these biomarkers are developed.320,321 Imaging has also been used to select the radiation dose and to minimise or avoid toxic effects on normal tissues in radio-pharmaceutical therapies (eg, theranostics such as anti-CD20 antibodies, and emerging peptide radionuclide therapies).322–324
Since the early development of theranostics with radioiodine in thyroid diseases, research and clinical use of other theranostic agents have increased dramatically.325,326 Major strides have been made in understanding the underlying biology of cancer and improving methods for designing and synthesising targeted theranostic drugs. For example, metaiodobenzylguanidine has been used for many years for diagnostic imaging and treatment of neuroblastoma, paraganglioma, and phaeochromocytoma.327 More recent examples include drugs that target somatostatin receptors in neuroendocrine tumours and prostate-specific membrane antigen (PSMA) in prostate cancer. Clinical trials are underway to assess the advantages of theranostics in oncology. Early results in the setting of neuroendocrine tumours, using 68Ga-labelled DOTATATE for imaging and 90Y-labelled or 177Lu-labelled DOTATATE for peptide receptor radionuclide therapy have been encouraging. Likewise, early findings with 68Ga-labelled PSMA for imaging and 177Lu-labelled PSMA for prostate radioligand therapy indicate clinical efficacy and manageable toxic effects. Other theranostic drugs labelled with 68Ga or 177Lu have been used to target chemokine receptor 4 ligands in myeloproliferative disorders and in cancers that overexpress gastrin-releasing peptide receptor.316,317,328–330 With this rich historical context and recent major advances, theranostics should be considered a priority for further research and development for precision medicine.
Integrated diagnostics
Integrated diagnostics combining both in-vitro diagnostics331 and imaging (in-vivo diagnostics) holds great promise for the early detection and management of cancer. Several scenarios exist in which integrated diagnostics could eventually become a cost-effective approach. For example, in ovarian cancer detection, a blood test in high-risk patients can be done at specific frequencies to assess blood biomarkers. If the test is positive, then the individual proceeds to a molecular imaging test (eg, a targeted microbubble ultrasound study). First, a low-cost, high-sensitivity, moderate-specificity test will produce false positives. However, a second test with molecular imaging could hopefully intercept many of these false positives.332 Integrated diagnostics could also be done nearly simultaneously. This would improve overall sensitivity and specificity in distinguishing benign from malignant disease compared with either approach alone. This approach has been initially validated in lung cancer by combining 18F-FDG PET-CT with blood biomarkers.333,334 Another application for integrated diagnostics could be use of in-vitro diagnostics at key time points when molecular imaging would have been done, thereby reducing costs.
Integrated diagnostics could potentially be used for prognostication after therapy. Breast cancer studies were the first to integrate 18F-FDG PET-CT imaging and circulating tumour cell counts for surveillance of patients with metastatic breast cancer, with a potential advantage of measuring circulating tumour cells over 18F-FDG PET-CT335–337 for post-therapy prognosis. However, 18F-FDG PET-CT tumour uptake was reported as being potentially useful for patients with less than five circulating tumour cells. These data illustrate the complexity of integrated diagnostics when both in-vitro and in-vivo diagnostics are useful for predicting patient outcomes.
Conclusions
Research and future trials should focus on methods that can have the largest effect on cancer detection and therapy. A series of key steps are needed to move this field forward: combinations of in-vitro and in-vivo diagnostics in specific cancer applications need testing; the limitations of integrated diagnostics need defining; decision management algorithms to quantify the cost-effectiveness of integrated diagnostics need developing; wearable diagnostics that could help with the issue of invitro diagnostic timing need developing and validating; and finally, discovery and thorough validation for in-vitro and in-vivo biomarkers must continue. Carefully planned prospective studies of theranostics versus more standard therapies should be conducted.
The move towards precision medicine will be facilitated by supporting research, education, and training on targeted radionuclide therapy and increased production of imaging and therapeutic radioisotopes.338 Collaboration is needed, and stakeholders include federal agencies, academia, and the pharmaceutical industry.339–341 Standardisation across devices, patients, and time is necessary and will be addressed by the Quantitative Imaging Biomarkers Alliance (QIBA), and the FDG-PET Uniform Protocols for Imaging in Clinical Trials will inform the QIBA FDG PET Profile281 for clinical trials of oncological therapies. For nuclear oncology to be successful, coordination between the FDA and the US Center for Medicare and Medicaid Services must be streamlined to ensure reimbursement of the cost of developing new compounds for radionuclide imaging and therapy.340 Our top five priorities for this area are listed in panel 17.
Panel 17: Priorities for nuclear medicine and imaging.
Develop and validate cost-effective molecular imaging strategies to allow stratification of patients and to predict and monitor therapeutic response for the major classes of therapeutics
Develop and use in-vitro diagnostics and in-vivo molecular imaging to optimise earlier detection of cancer
Use big data and analytics to integrate data from multiple sources for informed cancer detection and management
Develop and validate key multitargeted theranostic approaches that include radionuclide therapy, with the goal to improve patient outcome and quality of life and to reduce toxic effects
Develop and validate decision models to quantify the cost-effectiveness of integrating molecular imaging into clinical cancer management strategies
Develop streamlined strategic approaches to regulatory approval, reimbursement, and clinical integration of new molecular imaging and theranostic agents into cancer care
Part 13: Surgical oncology
Despite tremendous advances in cancer genomics, transcriptomics, proteomics, metabolomics, and other omics; the advent of rational drug design based on structural biology and specific molecular targets; the renaissance of immunotherapy for cancer; the emergence of advanced imaging, nanotechnology, bioinformatics, large data networks, cancer epidemiology, and biostatistics; and the improvements in conventional systemic therapy and radiation therapy; complete surgical resection of solid tumours still cures more patients than all other therapies combined.
John Hunter established many of the first modern theories of cancer care in the 1700s. Specifically, Hunter advocated for total removal of tumours and potential areas of lymphatic spread, emphasising the so-called constitutional effects of cancer. For hundreds of years, surgery has been central to cancer treatment, while effective treatments other than resection have only become available in the recent history of oncology. Even in the modern era of multidisciplinary care, surgeons remain on the front line of cancer treatment and are integral members of any multimodality team-based approach. Surgeons are often the first providers to diagnose and treat cancer, to advise patients about treatment options, to refer patients for adjuvant therapy and clinical trials, and to coordinate multidisciplinary care.
Surgical investigators lead or actively participate in basic and translational cancer research efforts. Many more surgeons actively participate in the design, implementation, and accrual of patients to clinical trials. Other cancer surgeons are leaders in health services and health outcomes research, generating information about patient outcomes, quality of care, and the economics of cancer care delivery. As such, surgeons are involved in every aspect of research and treatment.
The Cancer Moonshot Task Force identified five key strategic goals (panel 18),342 and the BRP outlined ten key research opportunities to complement the Task Force’s activities.1 In light of the historical and current clinical and research contributions of surgeons in cancer management, it was somewhat perplexing to note that surgery is not mentioned in the Cancer Moonshot Task Force’s 29-page report or in the BRP’s 57-page report. Although not surprising that the Task Force and the BRP focused on scientific advances to prevent and eradicate cancer, one wonders if perhaps the importance of cancer surgery was overlooked. In view of how surgical treatment of solid cancers is likely to remain central to any potential curative framework, articulating how surgical oncology can contribute to achievement of the objectives outlined in the Cancer Moonshot and BPR reports is crucial.
Panel 18: The five key strategic goals identified by the Cancer Moonshot Task Force.
Catalyse new scientific breakthroughs
Unleash the power of data
Accelerate bringing new therapies to patients
Strengthen prevention and diagnosis
Improve patient access and care
Future goals of surgical oncology in research, technology development, and patient care
Successful accomplishment of the Cancer Moonshot goals will rely on an integrated, multidisciplinary approach that includes cancer surgeons. In particular, several of the priorities require analysis of high-quality tumour tissue. Formalin-fixed, paraffin-embedded tissue cannot be used in many important studies, so surgeons will have an essential role in real-time procurement of fresh or frozen tumour specimens for research. Surgeons can be, and should also act as, important collaborators in developing and planning expanded, clinically annotated biorepositories. Surgeons will remain integral in the design and implementation of the next generation of innovative clinical trials and in the collection and analysis of clinical, translational, and basic science data.
Improving patient access and quality of cancer care is a fundamental pillar of Cancer Moonshot. Progress in expanding access, decreasing disparities, and improving patient-centred outcomes must necessarily involve surgeons since they are often the first to treat patients with newly diagnosed cancer. Given the national burden of cancer, resection of a solid tumour is usually the most efficacious and cost-effective means to treat cancer in many resource-limited areas. Unfortunately, access to equitable high-quality and high-value surgical care is far from universal. Although many complex solid malignancies are treated at cancer centres or specialised centres, most patients with cancer who undergo surgery in the USA (about 80%) are treated by general surgeons in community practice. Expertise associated with greater experience and standardisation of care pathways improves outcomes. In light of the growing burden of cancer in the USA, Cancer Moonshot should include development of a national strategy to strengthen the cancer provider workforce.
The important first step in ensuring access to high-quality surgical cancer care starts with improved education of surgeons and other providers. The Society of Surgical Oncology has been a national and international leader in defining rigorous standards for the training of surgical oncologists. This leadership has led to the recognition of Complex General Surgical Oncology as a board-certified specialty by the American Board of Surgery. Equally important is the need to better train and provide continuing education to general surgeons who often provide cancer care in certain areas with low socioeconomic status. A commitment to improve access to surgical care in underserved areas is likely to have far-reaching effects on national cancer mortality. Access to appropriate cancer surgery varies considerably. For example, in the UK, access to liver resection in patients with colorectal liver metastases varies ten-times among English National Health Service hospitals.343 Furthermore, inequalities in the clinical and research environment can undermine a patient’s ability to receive multidisciplinary care and that patient’s access to surgery. The commitment to broaden access to surgery must extend to underserved areas around the world. The recent effort to establish a global curriculum for surgical oncology training highlights this need.344
The BRP identified symptom management as a key initiative. Surgeons have an essential role in the surgical palliation of many patients with cancer. The role of palliative surgical procedures is evolving and requires research to better understand the balance between preventive and pre-emptive treatment, the probability of achieving specific patient-defined goals, morbidity, and the value of palliative intervention to each individual patient.
The BRP’s initiative for the development of new, enabling cancer technologies should be broadened to include innovation in minimally invasive surgery, image-guided surgery, tumour ablation, intraoperative margin assessment, and other modalities that will improve surgical outcomes. Advances in technology to allow greater precision in preoperative assessment, intraoperative planning, and assessment of completeness of tumour resection using mass spectrometry, fluorescent compounds, and other cellular and molecular diagnostics will likely improve the ability of surgeons to completely resect tumours and spare normal tissues.345 Technological improvements in robotics and natural orifice translumenal endoscopic surgery will allow more routine extirpation of tumours with smaller, or no, scars at all. Technology development must be coupled with judicious decision-making around its most effective use, avoiding unnecessary expense and futile treatments. Research into enhanced recovery after surgery that focuses on regional versus general anaesthesia, avoidance of opioids, and a more expedited functional recovery of patients also has important implications for patients.
The American College of Surgeons has a long history of leading key cancer programmes including the Commission on Cancer, the National Accreditation Program for Breast Centers, the American Joint Committee on Cancer, and cosponsorship of the National Cancer Database. Accomplishing the BRP’s goal of establishing a national cancer data ecosystem for sharing and analysis will rely on the cooperation, coordination, and integration of existing nationwide surgical cancer programmes. Any such large-scale database initiatives will require collection, sharing, and analysis of surgery-specific variables and annotation of clinical surgical data to correlate with basic science discoveries. However, informative data analysis cannot be accomplished without attention to components of surgical decision-making, technical considerations, and completeness of tumour resection. Priority should be given to incorporating existing, large-scale surgical data collection efforts into any national cancer data ecosystem.
As cancer therapy moves away from traditional cytotoxic chemotherapy and toward more targeted (often orally-available) therapies and immunotherapies, the role of surgical oncologists will continue to evolve. Resection is needed in at least half of all cancer cases, and this demand for cancer surgery is expected to increase over the next 15 years because of increasing cancer incidence.346 Paradoxically, as systemic, personalised, and targeted medicine evolves, the role for surgery is likely to expand. Specifically, as systemic therapy becomes more effective, surgery for patients with cancers that have been down-staged or who have resistant sites of disease could expand the proportion of patients being indicated for surgery. To this end, surgeons will need to be increasingly involved in the administration of perioperative systemic therapy, and in research to design better methods to assess treatment response (eg, enhanced imaging techniques, circulating tumour cells, novel biomarkers), to optimise the timing of surgical resection, or to determine whether resection is needed at all. Patients receiving systemic treatments in randomised controlled trials need relevant surgical input to assess whether subsequent surgery aimed at curative treatment would be appropriate.347,348
Engagement of surgeons in the design and conduct of perioperative (ie, neoadjuvant and adjuvant) systemic therapy clinical trials will be crucial to the success of any cooperative group study design efforts. Greater involvement of cancer surgeons in team science will improve the quality of trial design and opportunities for correlative translational studies involving biological specimens. The surgical leadership of clinical trial efforts such as the National Surgical Adjuvant Breast and Bowel Project established the importance of surgeons in generating clinical trial data that meaningfully improves patient care. As such, increasing the fraction of patients in the USA who are enrolled in perioperative, correlative clinical trials that integrate surgery and systemic therapy will rely on strategies directed at increasing surgeon participation. Greater engagement of surgeons will improve clinical trial accrual.
Conclusions
Our priorities in surgical oncology (panel 19) are similar to other disciplines involved in cancer research and therapy, and are in line with Cancer Moonshot and BRP initiatives. Surgery remains the cornerstone of potentially curative therapy for solid malignancies. Cancer surgeons are often involved in patient care throughout the continuum of diagnosis, treatment, adjuvant therapy, and long-term follow-up. Therefore, we should not overlook the opportunity to engage the surgical community in the search for better care for patients with cancer.
Panel 19: Priorities for surgical oncology.
Improve patient access to surgical care as resection of solid tumours is the cornerstone of curative therapy for most patients
Eliminate disparities in cancer care through education, training, and innovative programmes that disseminate best practices and multidisciplinary care to all patients
Stimulate innovation in surgical technology and perioperative treatment that will improve outcomes, reduce complications, and enhance recovery
Integrate existing surgical cancer data collection efforts into larger data network initiatives
Expedite progress in developing new cancer therapies by improving adult clinical trial accrual
Part 14: Big data and enhanced data-sharing
Big data is redefining the science and management of cancer, with an unprecedented variety, volume, and speed of acquisition.349,350 This new, data-rich reality, deriving in large measure from the convergence of advanced technologies such as next-generation sequencing, nano-technology, and imaging with the molecular and clinical sciences, portends a future of precision medicine in which patients receive molecularly targeted therapies and individualised disease management.351,352 However, the integration, interrogation, management, and leverage of the ever-increasing pace and volume of these multidimensional data resources present challenges that range from improving data quality and security to achieving required interoperability across the cancer enterprise. These problems are not unique to oncology. Lessons learned and models from areas such as finance, astrophysics, and commerce have overcome many of the same impediments faced by the oncology community. Through a combination of practical solutions to support the use of imperfect data and the development of market-driven, patient-centric analytics, these and other approaches offer instructive models that can be adopted to create high-value oncology data resources. In this section, we summarise aspects of the oncology big data conundrum and highlight solutions-oriented approaches to bridge the big data world of oncology with a more functional future enterprise that seamlessly integrates research and clinical care.
Big data challenges in oncology
Although still young, the big data revolution is well underway in biomedicine, especially in oncology. The volume of data from advanced, technology-enabled discovery is already exceeding Moore’s law, the benchmark exponential pace of computer processor growth in the past 50 years.353 More than 90% of the digital data created to date across all fields was produced in the past 2 years, and only 1% of these data have been analysed.353 This trend is projected to continue for the foreseeable future, as billions of smart devices will have an ever-increasing need for computer processing power and data cloud storage. Like so many other fields, the digitisation of oncology is becoming a reality, and oncology is already facing similar problems related to data quality, sharing, integration, and analytics.
Embracing fuzzy data
Cancer is complex at every level, and the data challenges are correspondingly so. Each of the more than 200 types of cancer can be classified into numerous molecularly defined subtypes. Cancer data might include pathological and radiological images, treatment records, laboratory data, clinical trial records, and even cellular and molecular data. Specialists of specific cancers might have their own culture of discovery and data requirements across the research and care continuum, resulting in countless datasets of variable or unknown quality. This problem has ignited a difficult public conversation about the reproducibility of biomedical research data from a substantial amount of published work. There is no single solution to this data reproducibility problem since it might stem from any aspect of the discovery process, from the choice of samples and technologies to the experimental design, or the overall absence of standards to provide a reliable foundation for end-to-end data creation and reproducible analysis.354 The difficulties associated with reproducing biomedical research data are emblematic of the state of oncology big data—often consisting of disconnected data resources of unknown (or low) quality and with limited capability for interoperability and data sharing.352,355
Because cancer is a diverse set of diseases that are almost all molecularly and clinically heterogeneous, and because use-cases that could help unify standards are uncommon, data quality in oncology has historically been (and continues to be) highly variable. Data collection and storage platforms for clinical trials and medical records are equally variable, resulting in insufficient high quality, open source, multidimensional datasets and a cancer research and care enterprise that is rarely interoperable. This unfortunate history means that far too much data in oncology are relegated to so-called data tombs, where they remain unused for the benefit of either research or patients. This is especially unfortunate for certain communities with low patient volumes because these unused resources could facilitate and speed progress in underserved areas of oncology research such as the paediatric and rare cancers.
Other research fields have faced these challenges and learned to value imperfect (fuzzy) data from various sources.356,357 The oncology community must, in concert with a more sophisticated, standards-informed oncology data ecosystem,358 find ways to use the vast amounts of existing fuzzy oncology data in innovative new models. One approach could be wider use of so-called oncology data lakes. These repositories receive and store raw data indefinitely. These data lakes are ideal for collecting data that define neither the potential users nor value up front, but rather let the users ask their own specific questions and design approaches for data analysis. This economically feasible approach accepts so-called scruffy data from disparate sources and, if the resource is properly structured, can encourage and support submission of both research and clinical data for analysis across the cancer research-to-care continuum.
Long-term development of standards and other strategies for data collection and management should be designed to produce high-quality data with appropriate metadata and provenance.354 Standards to support end-toend approaches for data generation, curation, sharing, and the interoperability needed to drive data integration and analysis requires leadership from the US National Institutes of Health and US National Cancer Institute. The private sector can also participate in creating platform solutions for data-sharing through public–private partnerships and as primary providers of solutions. However, although both the government and private sectors can provide support through various initiatives and mechanisms, the mandate and work needed to accomplish these very audacious goals must rest with the affected oncology research, clinical, and patient communities.
Possible solutions to the big-data conundrum
Multiple solutions-driven efforts from across the enterprises are needed to allow cancer research communities to make the best use of existing oncology data and, in parallel, ensure the development of the interoperable big-data system (or systems) envisioned by the BRP. Indeed, a parallel strategy must also embrace incremental solutions that make use of existing, and often fuzzy, data to gain much needed insights into how molecular changes manifest in cancer patients. This will require federal agency support for new initiatives that effectively use existing oncology data and set the stage for a more comprehensive solution. For example, the government could fund community efforts to develop standards-based, high-quality, linked datasets to address both of these needs.
Interoperability and data-sharing
There is broad recognition in the cancer research community that data-sharing in oncology is a crucial scientific strategy and that, in clinical trials, it is becoming seen as an ethical imperative. In that respect, data-sharing has been a requirement of all US National Institutes of Health grantees since 2003. A broader mandate, issued to all government agencies by the President’s Science Advisor in 2013,359 elaborated the expectation that this policy would apply to all of biomedicine and beyond. There are clearly caveats in terms of patient confidentiality and other protections afforded under the Health Insurance Portability and Accountability Act 1996, but overall, data-sharing should be an integral part of the work of the entire oncology research-to-care continuum. Although the emphasis on data-sharing is often focused on basic and translational oncology research, data-sharing between the investigators that do the myriad clinical studies and trials that comprise clinical research is equally important.
Addressing the needs of individual cancer patients requires predictable and reproducible treatment strategies based on data-sharing models with the statistical power to develop knowledge bases across cancer types, patient characteristics, and clinical experiences. Powerful tools such as next-generation sequencing are empowering oncologists to determine the molecular profile of individual patients.351 However, the benefits of precision medicine remain to be proven for individual cancers and across the landscape of cancer types and subtypes. Similarly, the benefits of cancer immunotherapy can be profound for individual patients, but the challenge of identifying those patients is a major barrier to broad implementation.
Obstacles to data-sharing range from the technical challenges inherent in establishing interoperable systems to counter-intuitive reward structures that drive data protectionism. Existing reward structures that are too often responsible for a reluctance to share data must be recognised as a major problem by all segments of the cancer research community so that realistic solutions can be developed. As Former US Vice President Joe Biden noted, assuming patient data is appropriately protected, any data generated as part of a federally supported grant must be made public.
Overall, the technical challenges associated with data-sharing and interoperability in oncology, while difficult, are fixable through models that establish data standards and provide infrastructure for accessibility. For example, the general deployment of cloud computing and associated storage solutions are providing solutions to problems with file sizes and transfer in areas such as genomics, which has previously made data-sharing extremely difficult. There are also increasingly comprehensive opportunities to share clinical data derived from cancer types or subtypes, clinical experiences, personal wellness devices, and personal medical records. Furthermore, the ability to generate more robust data through the regular practice of medicine by leveraging electronic medical records and decision-support systems must be explored.
The good news is that just as the research community learns to use fuzzy data, and as more comprehensive oncology data ecosystems are built, data-sharing based on questions such as “tumours like this one” and “patients like this one” or shared clinical experiences can be achieved in federated models. These approaches are already being undertaken and supported. Together with other patient-centric strategies, these approaches can be used to build models across a data-sharing continuum350 that, with attention to key technical and data quality requirements, will provide a new generation of data resources to support powerful, high patient-value analytics.
Realistic data-sharing frameworks
Creating systems that seamlessly connect research to clinical care is perhaps the greatest challenge in oncology today. Fortunately, these are solvable problems.
Existing oncology data collected in the past decades is often stored in disconnected silos. The silo designation might be a consequence of why or how the data was originally collected and reflects long-held views that data (be it from basic science or clinical sources) is proprietary to an investigator or programme. As the need for data sharing and publicly available datasets become an expectation of the oncology research and clinical communities, these old views are falling away. Although an integrated (standards-informed) oncology data ecosystem is a worthy goal in the long term, some coalitions have begun collecting and curating molecular and clinical data with a goal of making these resources publicly available. These pilot programmes are in keeping with the BRP recommendations that these types of initiatives be pursued in parallel with efforts to develop the infrastructure needed to support an oncology data ecosystem.
For example, in relating to applying data quality standards, the American Association for Cancer Research’s GENIE (genomics) Project publicly released de-identified genomic data from eight institutions, which included data from 19 000 patients and 59 cancer types.360 Instructive models from areas outside oncology, such as the National Science Foundation’s ORION project, are examples of approaches to integrating and using low-quality data collected by coalitions. These examples, and several others, show that an incremental approach to using existing data is possible when there is a commitment to ensuring patient privacy, transferring data across institutions and from international sites, and overcoming the logistics of large-scale data-sharing for public release, among other challenges.
These problems are solvable through collaborative scientific initiatives354 that, taken together, can establish networks to support a new generation of urgently needed analytics. Although not a perfect solution, these innovative networks and consortia based on specific questions (or strategies) that ultimately create high-value public oncology data resources offer a way forward. For example, social media approaches could be established to connect researchers or provide a patient commons for data input. Overall, an integrated, strategic, federated approach could be employed to create a first-generation framework for interoperability and oncology data-sharing. The approach would leverage existing resources, be scalable, and, if structured and overseen properly, provide invaluable insights and direction for the development of a prospective national oncology data system. Finally, and perhaps of greatest importance, this approach would begin the process of building the analytics that are so essential to improving the lives of patients with cancer.350
Conclusions
In this era of big data generation, the development of a national data ecosystem that facilitates data-sharing must be an important priority, in addition to several other key areas (panel 20). Many groups are already developing systems that are accessible to the greater community. Continued progress will rely on bringing all sectors of the cancer community together to take part in this opportunity and to break down existing silos that inhibit progress. Platforms must be developed to integrate all forms of data acquired through different technologies, including fuzzy and patient-reported data. Such a system would accelerate progress in understanding different cancers and in identifying new approaches for cancer detection, prevention, and treatment.
Panel 20: Priorities for big data and enhanced data-sharing.
Encourage and fund the creation of innovative oncology data lakes
A consortia approach would facilitate the process of accepting existing datasets for analysis and building new repositories to address specific needs, while also addressing the incentives needed to share data
Such an approach could provide both a substantive foundation for the development of data resources from existing data (scruffy data and otherwise) and networks or frameworks for new initiatives
Develop a new and strategic initiative focused on attracting and rewarding real innovation in analytical and machine-learning models built using oncology data
Interpreting vast amounts of oncology data for knowledge building that ties molecular science to clinical outcomes requires new approaches that integrate new ideas and technologies (eg, machine learning, artificial intelligence) and learning from other disciplines and sectors to create new generations of analytic tools
Rewarding this work is needed to incentivise oncology researchers (and researchers from other fields) to advance the state of mathematical models and analytics designed to analyse and manage oncology data
Create new training programmes for data scientists to attract the best and brightest, both nationally and internationally, to commit to a career in oncology big data
These programmes are needed immediately to address what is quickly becoming a critical shortage of talented scientists in all aspects of oncology data management and analysis
Bioinformatics and data science, which are now organic and integral parts of the research process, will increasingly be required in oncology to realise the vision of precision medicine and to ensure patient benefit from the big data revolution
Individuals with hybrid training in both data analytics and oncology will be the future leaders who know both the important questions to be asked and the methods needed to answer such questions
Part 15: Health disparities, health-system reform, oncopolicy, and regulation
Advances in cancer care must be matched by provision of high-quality care to patients in need. In the USA, and worldwide, costs of cancer care are high and increasing, and this threatens access to care and the potential for all patients to achieve the best possible outcome. Research is needed to better understand the current status of cancer care delivery, the impact of costs of care, and the disparities in access and outcome. Innovations both in the clinic and in policy could improve access to high-quality care while ensuring that such care is financially sustainable for the individual patient and for society. In this section, we outline details of the health services and policy research agenda that must complement ongoing basic, translational, and clinical research through the 21st Century Cures Act to ensure that the benefits of this research reach the patients they are intended to help.
Background
Health-care costs in the USA are the highest in the world and continue to increase faster than average inflation. In 2015, the US Centers for Medicare & Medicaid Services estimated that total costs of care in the USA were US$3·2 trillion dollars, increasing more than 5% per year, and accounting for close to 18% of the gross domestic product.361 These costs are unsustainable, and they threaten access to care and other national spending priorities (including research to improve care). Cancer care contributes roughly 5% of total health-care costs, but spending for cancer therapy (novel drugs, specifically) has been one of the primary drivers of increased health-care spending in recent years.362 In addition to high societal costs for care, direct out-of-pocket costs to patients in the USA threaten access to cancer care and contribute to additional burdens on patients and families associated with a cancer diagnosis.363 High direct costs of care can limit adherence to therapy and result in personal bankruptcy.364–366
As a result of concerns over costs of cancer care and access, attention has been focused on defining the value of cancer care so that high-value care can be promoted in the clinic and at the policy level, and low-value care can be discouraged or eliminated. The American Society of Clinical Oncology (ASCO) through its Task Force on Value in Cancer Care has promoted conversations between oncologists and patients about costs of care and more realistic conversations about options and care preferences at the end of life.367,368 Recently, ASCO and other groups, including the European Society for Medical Oncology and the National Comprehensive Cancer Network, have proposed value frameworks to guide clinicians and policy makers in defining value for specific interventions.369 One of the challenges in American cancer policy is that agencies supporting research of comparative effectiveness in cancer therapy have been explicitly prohibited from supporting cost-effectiveness research. Approval of cancer drugs and coverage decisions by the US Centers for Medicare & Medicaid Services are, by statute, not subject to cost-effectiveness considerations. In this context, oncology payment reform is intended to incentivise high-value care with mechanisms such as episode-based payments. Multiple demonstration projects sponsored by the US Centers for Medicare & Medicaid Services and private payers are underway, and proposals to replace the current payment system for oncology care have been offered.370 Similarly, patients and doctors are increasingly willing to discuss costs of care in the clinic, but the optimal content and timing of these discussions and the effect on costs of care, access, and disparities have not been established.371
Concerns with costs of care are closely tied to concerns about access and disparities in cancer care and outcomes. Despite one of the highest health-care expenditures in the world, the USA—an increasingly diverse nation—is characterised by the fact that health outcomes in minority and other underserved and marginalised populations do not fare as well as the majority.372–375 Cancer management, prevention, diagnosis, treatment, and research is inferior for minority and populations with low socioeconomic status, culminating in substantially reduced survival.373–375
Several factors seem to contribute to these differences, including inadequate access to prevention, diagnosis, treatment, and research; minority community suspicion of the health-care system and of doctors from other cultures; poverty, lack of health insurance; stigmas associated with cancer and death; linguistic and literacy barriers; impaired nutrition and other resources; comorbidities; and poor expectations of outcome from cancer treatment.373–375 This inequity in the health-care system, collectively termed disparity of care, is not unique to the USA.376,377 Although many relevant definitions exist, the US Department of Health and Human Services defines health disparities as differences in the incidence, prevalence, mortality, and burden of diseases and other adverse health conditions that exist among specific population groups.
Irrespective of the specific community, cure for most advanced cancers remains elusive. However, data indicate that if all of the population in the USA had the outcomes of college-educated, white men, the mortality would be reduced by at least 25%.378 Clinical practice and research in oncology are changing rapidly, and optimal treatment often correlates with involvement in clinical research or the early implementation of its products. In association with research, patients might have to tolerate exposure to incompletely defined levels of hazard and the necessity of dealing with detailed informed consent documentation, non-routine patterns of care, and the consequently high levels of uncertainty. These issues could present particular problems for minority and other underserved populations. Overextended local medical practitioners in underserved communities might not be current with the latest developments in cancer care and technology and are thus unable or unwilling to encourage their patients to gain the benefits of all the available opportunities. These factors combine to make cancer care a particularly difficult issue for minority and other underserved populations.
One of the most fundamental issues in any discussion of disparities in medical care that are based on race or ethnic background is the definition of these terms. Self-identified race or ethnicity (SIRE) is widely used as an index, but is subject to the vagaries of family legend. It has been suggested that this parameter is more useful as a sociocultural index than for serious biomedical research379 and that specific genetic studies are more informative in this context. The alternative view is that, for whatever reason, genetic variations are associated with SIRE and that this index should not be dismissed.380 Rebbeck and colleagues381 propose that, with the paucity of extant information, SIRE and genetic information should be integrated and applied to disparities research to the extent possible, a view that seems pragmatic and sensible.
On an international basis, disparities in health care are predominantly associated with poverty, increased age, and geographical isolation,374–376 whereas in the USA, race and ethnicity are additional factors,382 even if this is based on self-identification. Notwithstanding that national cancer statistics are predominantly based on SIRE, there is clear evidence that increased incidence of cancer in minority groups and underserved populations is in many cases accompanied by reduced survival.372,373,383,384 The total incidence of cancer is higher in African Americans than in white Americans,383,384 with particular differentials in cancers of the prostate, lung, breast, and colon. Even when the stage of the disease is considered, survival figures are inferior in propensity-matched African Americans and Latinos. The perceivable gap in incidence and mortality has not narrowed appreciably in the past 50 years.374,383
Poverty is a confounding variable that could explain an important element of the outcome differences. Koroukian and colleagues385 have shown a substantial reduction in cure of curable cancers among Medicaid recipients compared with insured populations. Low educational levels also correlate with inferior outcomes.386 In this context, we outline research priorities in the area of oncology health policy, health-care delivery, and disparities research that should be considered as part of the 21st Century Cures Act research agenda.
Defining high-value and low-value care
With limited resources, high costs, and ongoing clinical need for safer and more effective interventions in virtually all cancer settings, clinicians, payers, and policy makers must be able to define and prioritise high-value care over low-value care. Most of the frameworks for value rely on clinical research to establish the efficacy and toxic effects of therapy in a population. Beyond clinical trials, population-based data are needed to confirm, in real-world settings, the outcomes that are reported in controlled clinical trials. These population-based studies should address cost-effectiveness and compare effectiveness to evaluate the relative merits of available therapeutic options. The focus of this research should be on achieving clinically meaningful benefit rather than simply demonstrating any statistically significant, but clinically marginal advance in efficacy or safety. Investment in the infrastructure to support collection of population-based data and development of platforms to aggregate data are needed. As the understanding of value in oncology is refined, it will be necessary to examine the threshold for initial approval of new cancer drugs, the effect that accelerated approval has on access to effective therapy, and the effect of after-market research and phase 4 trials. Approval, use, and effect of high-cost, non-drug interventions such as robotic surgery and proton-beam radiation therapy must also be analysed.
One of the most promising opportunities to improve patient outcomes, while also making care more efficient, is the identification of validated biomarkers to guide patient selection for targeted therapy. There is a need for investment in prospective studies of biomarkers and support for population-based, real-world studies to define the clinical utility and effect of available and emerging genomic platforms. Similarly, novel technologies, including circulating tumour cell assays, and innovation in radiographic assessment that can identify response or resistance to therapy early in the course of care could potentially translate into improved outcomes and more rational and efficient care.
Delivery of high-quality, affordable cancer care
Once high-value and low-value care have been defined, interventions at the policy level and clinical level that promote high-value care and discourage low-value care must be developed and tested. Evaluation of pilot programmes related to payment reform (such as episode-based, bundled payments) and care pathways will determine the effect of these initiatives on patients, providers, health-care use, and health-care costs.387 The effect of enhanced cost-transparency and explicit consideration of cost in the oncology clinic should also be measured. The effect of such discussions on access to care, financial burden to patients, and disparities (which could be exacerbated by discussions of cost) are largely unknown.
As comparative effectiveness data emerge to guide cancer care, the diffusion of new information and barriers to change in practice should be examined, both when adoption of a new intervention is indicated, when an intervention can be safely omitted, or when a less expensive but equally effective option is available. Prominent examples of low-value care include those that were flagged by ASCO and the American Society for Radiation Oncology in the Choosing Wisely Campaign,388 which identified oncology practices of little value to patients, such as baseline scans in asymptomatic early stage breast cancer or prostate cancer. Despite these findings, clinicians can be slow to abandon some of these practices.389 Similar examples include hypofractionation in early stage breast cancer and omission of radiotherapy for elderly women with small oestrogen receptor-positive breast cancers.390,391
A crucial issue in oncology care in the years ahead will be to assess the effect of any new federal health-care reform legislation that changes coverage and insurance-benefit design. Reform could affect cancer prevention, out-of pocket costs, and access to high-cost cancer therapy. Equally important will be to understand the effect on cancer outcomes in patients who do not have health insurance, have high deductibles and coinsurance on access and adherence, and for whom cancer care imposes a financial burden.
The cost and availability of generic and orphan drugs in oncology should be assessed. Shortages of important generic drugs have become common in oncology.392 The causes and consequences of drug shortages must be better understood, and interventions must be designed to manage and prevent such shortages in the future. Costs of cancer drugs and the connections between cost, access, adherence, and outcomes is and will remain a priority for cancer policy research. industry-based and charitable patient-assistance programmes that promote patient access to high-cost drugs, but potentially help sustain high costs merit further research.393 Both empirical research and policy initiatives should aim to support innovation while promoting access to affordable drugs that reflect value to patients, rather than simply what the market will bear.
Understanding and eliminating cancer disparities
Priorities for cancer disparities research can be divided into observational studies to identify disparities related to prevention, diagnosis, or treatment, and interventions intended to reduce or eliminate disparities.
Many factors contribute to the reduced participation by minory groups in programmes of cancer prevention.394–396 Similar factors pertain to each of the specific areas of disparity, including poverty and little or no family support, social habits (diet, low exercise levels, industrial toxin exposure), attitudes and access to health care, and intercurrent medical problems such as obesity.394,396 In addition to smoking, consumption of unhealthy diets, and greater exposure to dangerous workplaces, minority populations often use genetic testing programmes that identify individuals at increased risk of cancer who could benefit from preventive strategies less than the majority population.397
Delay in diagnosis of cancer is also found in minority populations and in groups with low socioeconomic status. This disparity is associated with inadequate health insurance and access to health care, absence of a regular home for health care, attitudes and beliefs, and health provider factors (eg, overload, errors of clinical practice, language barriers, social stereotyping).373,394–396,
Some medical services are inferior in underserved and impoverished areas,394,398 possibly reflecting differences in resources, staffing, patient population, and clinician overload and burnout. Competing comorbid causes of death399 and differences in cancer stage at presentation and treatment received400 could add to these outcomes. More information should be acquired prospectively to allow optimal solutions to be developed.
Each of the major issues has potential solutions, but practical and fiscal commitment from government and communities will be necessary to ensure major progress in a timely fashion. Safety nets, community-linked patient navigator and access systems, improved social support and medical homes, and increased community health education are likely to improve outcomes such as stage at presentation and survival.
Several innovative approaches have been initiated and are being assessed. The ASCO Health Disparities Task Force creates specific scientific content for each annual scientific meeting and has expanded minorities-directed content on the ASCO patient-facing website, allocated funds for research in disparities of cancer care, and taken initiatives to increase mentorship and training for young doctors and scientists from minority populations with assistance of a generous grant from the Susan G Komen Foundation. A college loan repayment programme has helped retain young oncologists in underserved areas with large minority populations. Another Task Force is studying the costs of cancer care and implications for minority populations. ASCO’s stance has been codified in a position statement401 listing the following imperatives: (1) improvement of access to high-quality health care; (2) improvement of awareness of health-care disparities; (3) increased diversification and cultural awareness training of the oncology workforce; (4) expanded research of cancer disparities; (5) increased diversification of clinical trials; and (6) enhancement of patient involvement in their own care.
Regulation of cancer research
The final policy-related research priority we wish to highlight is the regulation of cancer research itself. Regulation and costs of research are tied to the costs of cancer therapy and the time it takes to bring novel therapies and associated diagnostics to the clinic. The regulation of cancer research must be assessed to identify opportunities to make it more efficient and thus reduce the burden and cost associated with clinical trials. The importance of surrogate endpoints, such as pathological complete response to neoadjuvant therapy, should be examined and validated systematically across different cancers. The process of scientific review and institutional review board merits ongoing evaluation to improve effectiveness and efficiency and to reduce redundancy. Similarly, initiatives to leverage health information technology, reduce manual data collection, and streamline research processes to reduce cost and patient burden should be supported. As changes to the Federal Policy for the Protection of Human Subjects (the Common Rule) are established, the effect of these changes on both clinical trials and health-services research in oncology should be investigated. The effect of accelerated approval and breakthrough therapy designation should be studied. Finally, there is an ongoing need to address disparities in research itself and to identify areas of oncology where the burden of disease merits strengthened research investment.402
Although funding from the 21st Century Cures Act will focus on cancer policy and disparities affecting cancer care in the USA, most of these issues will also have global relevance. Defining value, promoting evidence-based practice, identifying opportunities to enhance sustainable access to high-quality care for all patients, and improving the regulation of cancer research are international challenges. The ongoing investment in basic, translational, and clinical research should be supported by policy-oriented health-services research that is designed to ensure that the advances in oncology continue and improve outcomes for all patients (panel 21).
Panel 21: Priorities for health disparities, health system reform, oncopolicy, and regulation.
Define high-value and low-value care
Deliver high-quality affordable cancer care
Eliminate cancer disparities
Re-examine regulation of cancer research
Part 16: Summary and call for action
The cancer research community has embraced the extraordinary opportunity afforded by Former US Vice President Joe Biden when he initiated the Cancer Moonshot. Few foresaw the remarkable energy, creativity, and scientific dialogue and redirection that emerged between February and October, 2016, with the publication of the BRP report. This Commission delves further into the topics and outlines, with decisiveness, key priorities to achieve the Cancer Moonshot goal of achieving a decade of progress in cancer treatment in just 5 years. 1 year in, and we have witnessed new research collaborations, initiatives, and funding opportunities in line with Cancer Moonshot. Most importantly, the entire cancer research community has weighed-in to create momentum seen few times in the history of science.
Despite headwinds of federal budget priorities, clinical practice interruption of clinical research, and ongoing regulatory and administrative hurdles, some things are clear: the blueprint laid out by the BRP and this Commission should occupy our priorities in the next 4–5 years. Few of us have the capacity to see decades into the future for the next new cancer discovery, so we need to collectively focus on these initiatives and suggestions and look forward to validation and success in some areas, redirection in others, and expect that these efforts will lead to superseding breakthroughs that yield the next major impact on patients’ lives.
The obligation of cancer investigators, federal agencies, universities and research institutes, and private philanthropic supporters worldwide is to heed the provisions outlined in this Commission for direction and investment, and to meet the timeline for impact. Delays affect the lives of patients and their families and limit a return to a normal life.
We have identified measures of success and deliverables with metrics and timelines for each of the sections in this report (panel 22). It will serve as a guide for investigators and resource allocation. A note on how to approach this set of goals comes from the wisdom of the Former US Vice President Joe Biden himself: seek the best-in-class collaborators, reduce barriers, and operate efficiently while sharing data and reducing unnecessary competition. All fields of cancer research, treatment, and prevention are included in this global appraisal of opportunities to improve cancer health in the USA and the rest of the world. The time for action is now.
Panel 22: Research priorities—a call for action.
Precision cancer prevention
- Measures
- Premalignant Cancer Atlas
- Immunotherapeutics for prevention
- Mutational targets for intervention
- Improved public health prevention campaigns
- Increased research in lifestyle medicine (including nutrition, exercise, stress management, smoking cessation, and social support) and the association with cancer risk and prevention
- Metrics
- More than 10 000 patient biospecimens (2–4 years)
- Cancer epitope vaccines (1–4 years) and epitope therapeutics (2–6 years)
- Enhanced national, state, and institutional messaging and interventions on healthier and more active lifestyles (1–2 years)
- Large randomised trials to assess effectiveness of lifestyle medicine in reducing the prevalence of common cancers (2–6 years)
Early detection and public health
- Measures
- Big data from electronic medical records to identify high risk populations
- Increased screening and genetic testing
- Focus on cancer survivors’ second cancers
- Metrics
- Algorithms for high-risk populations (1–2 years)
- Increase rates of screening for breast, prostate, and colon cancer (1–2 years)
- Improve selective screening of high risk populations (2–4 years)
Drug discovery and development
- Measures
- Expand library of molecular targets
- Improve lead compound throughput and preclinical testing
- Facilitate ‘go–no go’-based approach in early-phase clinical trials
- Partner development costs
- Metrics
- Expanded cancer centre drug pipeline (2–6 years)
- Facilitated preclinical testing (1–2 years)
- Rapid throughput of phase 1–2 trials (2 years)
- Reduced development cost with reduced commercial pricing (4–6 years)
Precision tumour assessments
- Measures
- Molecular assessment of primary and metastatic tumours and their microenvironment
- Immune infiltration of tumours
- Shared data for genomics, immunomics, and microenvironment
- Metrics
- Reduced price and gained reimbursement for biomarkers (2–4 years)
- Use of big data for treatment decisions (1–4 years)
- Improved therapeutics towards immunomics and microenvironment (2–6 years)
Expediting patient access to new drugs with expanded clinical trials
- Measures
- Assess special populations (paediatrics, underserved, minority ethnic groups)
- Reduce regulatory burden for early phase trials
- Increase access to include just-in-time protocol activation and multisite management
- Metrics
- Increaesed participation of paediatric and underserved patients on clinical trials (4 years)
- Greater accrual to early-phase trials (2–6 years)
Immunotherapy
- Measures
- Identify cancer epitopes based on mutational spectra
- Immunotheraputic cancer profiling; record and manage adverse immune events
- Expand repertoire of immunotheraputics
- Metrics
- Expanded therapeutics for cancer epitopes (2–4 years)
- Expand T-cell and checkpoint therapeutics (2–6 years)
Paediatric oncology
- Measures
- Apply Blue Ribbon Panel recommendations to children
- Create centres to coordinate survivorship research
- Increase research into cancers in adolescents and young adults, and increase access to clinical trials
- Define effect of socioeconomic status and racial ethnic background on disparities in childhood cancer outcomes
- Establish international research networks for childhood cancer centres in low-income and middle-income countries
- Metrics
- Increase proportion of adolescent and young adult patients with cancer who are treated at large centres (4 years)
- Increase access to novel drugs and clinical trials (1–2 years)
- Develop survivorship interventions to reduce morbidity of treatments (2 years)
Supportive oncology
- Measures
- Expand research and therapeutic efforts into treatment-related symptom management and prevention, survivorship and after-effects, and palliative care
- Establish care guidelines with easy access
- Metrics
- Establish national norms for symptom management related to cancer, treatment, and survivorship (2–4 years)
- Identify effective interventions (2–6 years)
Radiation oncology
- Measures
- Expand oligometastatic radiation therapy
- Evaluate immunomodulatory approaches of radiation in combination with by checkpoint inhibitors or novel antibodies
- Redouble efforts in radioprotection
- Individualise radiation on the basis of genomics and imaging
- Assess carbon and proton for therapeutic efficacy
- Metrics
- Increased use of radiation for oligometastasis (2 years)
- Combination therapies approved for immune and radiation treatments (2–4 years)
- Genomic modulated radiation dosing (2–6 years)
Nuclear medicine and imaging
- Measures
- Develop pathway-based PET imaging for detection and early evidence of treatment efficacy, including immunotherapeutics
- Broaden theranostic agents for the assessment and treatment of more cancers
- Work more closely with FDA for review and approval of imaging compounds
- Metrics
- FDA-approved PET imaging agents (2–4 years)
- FDA-approved theranostic agents (1–4 years)
Surgical oncology
- Measures
- Develop surgical oncology technology in minimally invasive surgery, image-guided surgery, tumour ablation, and intraoperative margin assessment
- Involve surgeons in therapeutic management, especially with genomic-based therapies and immunotherapies
- Enhanced training and education
- Metrics
- New minimally invasive surgery, image-guided surgery, tumour ablation, and intraoperative margin assessment (2–5 years)
- Enhanced data and biospecimen collection (2 years)
- Implement new educational programmes to train the surgeons of the future (4–6 years)
Data-sharing and big data analysis
- Measures
- Collect experiential, clinical patient data lakes to assess collective responses, treatment efficacy, toxicity, and long-term tolerance; use such data to reduce the cost of investigation and hasten drug approvals
- Facilitate interoperability of data-sharing and machine learning
- Improve guidance for data-sharing across health systems
- Enable patients to self-direct access to their electronic medical records
- Metrics
- Expanded data lakes with information about treatment, toxic effects, and FDA approvals (2–3 years)
- Improved data-sharing metrics (2 years)
- Expanded patient-directed data donations (3–4 years)
Health disparities and access to care
- Measures
- Improve cost-effectiveness recommendations and acceptance into practice
- Reduce unnecessary imaging, testing, and treatment
- Shorten time to treat
- Bring treatment sites and navigators to underserviced communities
- Continue efforts to promote innovative care in the community setting, linking to academic cancer centres
- Improve peer review at the US National Institutes of Health and all federal agencies
- Metrics
- Improved novel therapy access in community, rural, and underserved sites (2–4 years)
- Decreased cancer mortality in community, rural, and underserved communities (6 years)
- Improved peer-review processes (2–6 years)
For HPV vaccination and cancer prevention see https://www.cdc.gov/vaccines/vpd/hpv/
For the NIH Data Sharing Policy see https://grants.nih.gov/grants/policy/data_sharing/
For the Orion database system see http://orion.cs.purdue.edu/
For the ASCO patient-facing website see to www.cancer.net
Footnotes
Declarations of interests
DBA serves on the Board of Directors for Tempus, Applied Proteomics, and the Biden Foundation Cancer Initiative. BMA reports personal fees from Bristol-Myers Squibb, Abbvie, Schlesinger Health Associates, and Precision Health Economics. KCA reports personal fees from Millennium Takeda, Bristol-Myers Squibb, and Gilead. JAB reports personal fees from Arcus and FlxBio, and grants and personal fees from Juno and Pfizer. AJB reports personal fees from NuMedii, Personalis, Roche, Genentech, and Pfizer. DM reports personal fees from G ealthcare and Blue Earth Diagnostics, and grants from Siemens Heathcare. ALF reports consulting and stock from Decibel Therapeutics. JH is the Founder and Director of PACT Therapeutics and of Isoplexis, and has equity in Isoplexis. EMJ reports grants and personal fees from Aduro Biotech and grants from Bristol-Myers Squibb. DKM reports stock in Carevive. KMcM is on the Board of Directors for Provectus Biopharmaceuticals, on the Scientific Advisory Board for Elucida Oncology, and holds a patent on characterising melanoma using RNA, miRNA, and polypeptides. NJM is a full-time employee of Flatiron Health, and has a patent pending for systems and methods for matching patients with clinical trials. DO reports personal fees from Sharecare for consultancy. MGP has patents pending for various theranostic imaging agents. Q-TL reports grants from Varian Medical Systems and Redhill Biopharma, and travel assistance from Bristol-Myers Squibb. RW is a consultant for Nihon MediPhysics. JDW reports grants from Bristol-Myers Squibb, Merck, MedImmune, Genentech, and Immunocore and is a consultant for Bristol-Myers Squibb, Merck, MedImmune, and Genentech. AA is a consultant for Genentech, AtlasMDX, Third Rock Ventures, Pfizer, Sun Pharma, and Bluestar, receives research support from Sun Pharma, is a co-founder of Tango Therapeutics, and holds patents on the use of PARP inhibitors jointly with AstraZeneca. All other authors declare no competing interests. LG’s contributions to this report is solely related to his research at the Massachusetts Institute of Technology.
References
- 1.National Cancer Institute. Cancer Moonshot. Blue Ribbon Panel Report 2016. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf (accessed Sept 30, 2017).
- 2.Lowy DR, Collins FS. Aiming high—changing the trajectory for cancer. N Engl J Med 2016; 374: 1901–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsevier. Cancer research Current trends & future directions. Amsterdam: Elsevier, 2016. [Google Scholar]
- 4.Emmons KM, Colditz GA. Realizing the potential of cancer prevention—the role of implementation science. N Engl J Med 2017; 376: 986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr J, Anderson CA, Lippman SM. Physical activity, sedentary behavior, diet, and cancer: an update and emerging new evidence. Lancet Oncol 2017; 18: e457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter H, Marty R, Hofree M, et al. Interaction landscape of inherited polymorphisms with somatic events in cancer. Cancer Discov 2017; 7: 410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure–time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016; 176: 816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capozzi LC, Nishimura KC, McNeely ML, Lau H, Culos–Reed SN. The impact of physical activity on health–related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med 2016; 50: 325–38. [DOI] [PubMed] [Google Scholar]
- 9.Johnsson A, Broberg P, Johnsson A, Tornberg AB, Olsson H. Occupational sedentariness and breast cancer risk. Acta Oncol 2017; 56: 75–80. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016; 388: 1302–10. [DOI] [PubMed] [Google Scholar]
- 11.Di Daniele N, Noce A, Vidiri MF, et al. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017; 8: 8947–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol 2005; 174: 1065–70. [DOI] [PubMed] [Google Scholar]
- 13.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008; 9: 1048–57. [DOI] [PubMed] [Google Scholar]
- 14.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 2013; 14: 1112–20. [DOI] [PubMed] [Google Scholar]
- 15.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998; 280: 2001–7. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One 2010; 5: e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Kenfield SA, Van Blarigan EL, et al. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev Res 2015; 8: 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emaus MJ, Peeters PH, Bakker MF, et al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am J Clin Nutr 2016; 103: 168–77. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst 2013; 105: 219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farvid MS, Chen WY, Michels KB, Cho E, Willett WC, Eliassen AH. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ 2016; 353: i2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo E, Salas-Salvado J, Donat-Vargas C, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 2015; 175: 1752–60. [DOI] [PubMed] [Google Scholar]
- 23.Castoldi A, Naffah de Souza C, Camara NO, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol 2015; 6: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF–1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014; 19: 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samraj AN, Pearce OM, Laubli H, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA 2015; 112: 542–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature 2016; 535: 65–74. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol 2017; 3: 921–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyssiotis CA, Cantley LC. Metabolic syndrome: F stands for fructose and fat. Nature 2013; 502: 181–82. [DOI] [PubMed] [Google Scholar]
- 29.Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care 2015; 18: 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo TM, Kamachi F, Watanabe Y, et al. Gut microbiota promotes obesity–associated liver cancer through pge2–mediated suppression of antitumor immunity. Cancer Discov 2017; 7: 522–38. [DOI] [PubMed] [Google Scholar]
- 31.Kettner NM, Voicu H, Finegold MJ, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 2016; 30: 909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015; 385: 956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.William WN Jr, Papadimitrakopoulou V, Lee JJ, et al. Erlotinib and the risk of oral cancer: the Erlotinib Prevention of Oral Cancer (EPOC) randomized clinical trial. JAMA Oncol 2016; 2: 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauman JE, Grandis J. Oral cancer chemoprevention–the end of EPOC, the beginning of an epoch of molecular selection. JAMA Oncol 2016; 2: 178–9. [DOI] [PubMed] [Google Scholar]
- 35.Im JS, Herrmann AC, Bernatchez C, et al. Immune-modulation by epidermal growth factor receptor inhibitors: implication on anti–tumor immunity in lung cancer. PLoS One 2016; 11: e0160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.William WN Jr, Uraoka N, Peng SA, et al. Immune profiling of oral pre-malignant lesions (OPLs): an Erlotinib Prevention of Oral Cancer (EPOC) study biobank analysis. J Clin Oncol 2017; 35 (suppl): 1545. [Google Scholar]
- 37.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol 2016; 2: 762–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016; 16: 173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan AT, Ladabaum U. Where do we stand with aspirin for the prevention of colorectal cancer? The USPSTF recommendations. Gastroenterology 2016; 150: 14–18. [DOI] [PubMed] [Google Scholar]
- 40.Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15–hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med 2014; 6: 233re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman N. Realizing the promise of cancer predisposition genes. Nature 2014; 505: 302–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To C, Kim EH, Royce DB, et al. The PARP inhibitors, veliparib and olaparib, are effective chemopreventive agents for delaying mammary tumor development in Brca1–deficient mice. Cancer Prev Res (Phila) 2014; 7: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD–1 blockade. Clin Cancer Res 2016; 22: 813–20. [DOI] [PubMed] [Google Scholar]
- 44.Le DT, Uram JN, Wang H, et al. PD–1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin–cancer chemoprevention. N Engl J Med 2015; 373: 1618–26. [DOI] [PubMed] [Google Scholar]
- 46.Samadder NJ, Neklason DW, Boucher KM, et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA 2016; 315: 1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal immunoglobulin against lysolipids in the origin of myeloma. N Engl J Med 2016; 374: 555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhodapkar MV. MGUS to myeloma: a mysterious gammopathy of underexplored significance. Blood 2016; 128: 2599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017; 3: 464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu C, Xie M, Wendl MC, et al. Patterns and functional implications of rare germline variants across 12 cancer types. Nat Commun 2015; 6: 10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell PJ. Somatic and germline genetics at the JAK2 locus. Nat Genet 2009; 41: 385–86. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, He L, Ramirez J, et al. Functional EGFR germline polymorphisms may confer risk for EGFR somatic mutations in non-small cell lung cancer, with a predominant effect on exon 19 microdeletions. Cancer Res 2011; 71: 2423–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol 2017; 35: 1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yurgelun MB, Chenevix-Trench G, Lippman SM. Translating germline cancer risk into precision prevention. Cell 2017; 168: 566–70. [DOI] [PubMed] [Google Scholar]
- 55.Nolan E, Vaillant F, Branstetter D, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1–mutation carriers. Nat Med 2016; 22: 933–9. [DOI] [PubMed] [Google Scholar]
- 56.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long–term prospective follow-up studies from three European expert centers. J Clin Oncol 2016; 34: 2010–19. [DOI] [PubMed] [Google Scholar]
- 57.Ross-Innes CS, Chettouh H, Achilleos A, et al. Risk stratification of Barrett’s oesophagus using a non–endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2: 23–31. [DOI] [PubMed] [Google Scholar]
- 58.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science 2013; 339: 1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borras E, San Lucas FA, Chang K, et al. Genomic landscape of colorectal mucosa and adenomas. Cancer Prev Res (Phila) 2016; 9: 417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imielinski M, Guo G, Meyerson M. Insertions and deletions target lineage-defining genes in human cancers. Cell 2017; 168: 460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotini AG, Chang CJ, Chow A, et al. Stage-specific human induced pluripotent stem cells map the progression of myeloid transformation to transplantable leukemia. Cell Stem Cell 2017; 20: 315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nestorowa S, Hamey FK, Pijuan Sala B, et al. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood 2016; 128: e20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufman CK, Mosimann C, Fan ZP, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 2016; 351: aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta S, Howard SC, Hunger SP, et al. Childhood cancers In: Gelband H, Jha P, Sankaranaryanan R, Horton S, eds. Disease control priorities, 3rd edn Washington, DC: World Bank, 2015. [Google Scholar]
- 65.Plumridge H. New costly cancer treatments face hurdles getting to patients. Wall Street Journal, October 6, 2014. https://www.wsj.com/articles/new–costly–cancer–treatments–face–hurdles–getting–to–patients–1412627150 (accessed May 9, 2017). [Google Scholar]
- 66.Yoda Y, Takeshima H, Niwa T, et al. Integrated analysis of cancer-related pathways affected by genetic and epigenetic alterations in gastric cancer. Gastric Cancer 2015; 18: 65–76. [DOI] [PubMed] [Google Scholar]
- 67.Hu D, Gao X, Cao K, et al. Not all H3K4 methylations are created equal: Mll2/COMPASS dependency in primordial germ cell specification. Mol Cell 2017; 65: 460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez P, Timmer MR, Lau CT, et al. Dynamic clonal equilibrium and predetermined cancer risk in Barrett’s oesophagus. Nat Commun 2016; 7: 12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou Y, Song L, Zhu P, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 2012; 148: 873–85. [DOI] [PubMed] [Google Scholar]
- 70.Tanay A, Regev A. Scaling single-cell genomics from phenomenology to mechanism. Nature 2017; 541: 331–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer 2003; 3: 243–52. [DOI] [PubMed] [Google Scholar]
- 72.Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2016; 66: 95–114. [DOI] [PubMed] [Google Scholar]
- 73.Vilar E, Stoffel EM. Universal genetic testing for younger patients with colorectal cancer. JAMA Oncol 2016; 3: 448–49 [DOI] [PubMed] [Google Scholar]
- 74.Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Caus Control 2009; 20: 1681–88. [DOI] [PubMed] [Google Scholar]
- 75.Grabler P, Dupuy D, Rai J, Bernstein S, Ansell D. Regular screening mammography before the diagnosis of breast cancer reduces black:white breast cancer differences and modifies negative biological prognostic factors. Breast Cancer Res Treat 2012; 135: 549–53. [DOI] [PubMed] [Google Scholar]
- 76.The National Academies of Science, Engineering, Medicine. Health and Medicine Division. Implementation of lung cancer screening: proceedings of a workshop Washington, DC: The National Academies Press, 2016. [PubMed] [Google Scholar]
- 77.Chang M-H, You S-L, Chen C-J, et al. , and the Taiwan Hepatoma Study Group. decreased incidence of hepatocellular carcinoma in hepatitis b vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009; 101: 1348–55. [DOI] [PubMed] [Google Scholar]
- 78.Moyer VA, Force USPST. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156: 880–91. [DOI] [PubMed] [Google Scholar]
- 79.Campo RA, Rowland JH, Irwin ML, Nathan PC, Gritz ER, Kinney AY. Cancer prevention after cancer: changing the paradigm—a report from the American Society of Preventive Oncology. Cancer Epidemiol Biomarkers Prev 2011; 20: 2317–24. [DOI] [PubMed] [Google Scholar]
- 80.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271–89. [DOI] [PubMed] [Google Scholar]
- 81.Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016; 122: 3075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganz PA. A teachable moment for oncologists: cancer survivors, 10 million strong and growing! J Clin Oncol 2005; 23: 5458–60. [DOI] [PubMed] [Google Scholar]
- 83.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academies Press, 2006. [Google Scholar]
- 84.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol 2010; 28: 4268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grubbs SS, Polite BN, Carney J, et al. eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol 2013; 31: 1928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell SA, Chambers DA. Leveraging implementation science to improve cancer care delivery and patient outcomes. J Oncol Pract 2017; 13: 523–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Workman P, Draetta GF, Schellens JH, Bernards R. How much longer will we put up with $100,000 cancer drugs? Cell 2017; 168: 579–83. [DOI] [PubMed] [Google Scholar]
- 88.Vivot A, Jacot J, Zeitoun JD, Ravaud P, Crequit P, Porcher R. Clinical benefit, price and approval characteristics of FDA–approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol 2017; 28: 1111–16. [DOI] [PubMed] [Google Scholar]
- 89.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ 2016; 47: 20–33. [DOI] [PubMed] [Google Scholar]
- 90.Macleod MR, Michie S, Roberts, et al. Biomedical research: increasing value, reducing waste. Lancet 2014; 383: 101–04. [DOI] [PubMed] [Google Scholar]
- 91.Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature 2012; 483: 531–33. [DOI] [PubMed] [Google Scholar]
- 92.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017; 168: 613–28. [DOI] [PubMed] [Google Scholar]
- 93.Jardim DL, Groves ES, Breitfeld PP, Kurzrock R. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat Rev 2017; 52: 12–21. [DOI] [PubMed] [Google Scholar]
- 94.Jarvis LM. Academic drug discovery centers adapt to shifts in funding sources. Universities are pushing their novel molecules into clinical trials. Chemical Engineering News 2017; 95: 16–18. [Google Scholar]
- 95.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–26. [DOI] [PubMed] [Google Scholar]
- 96.Ley TJ, Mardis ER, Ding L, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 2008; 456: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 98.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Wang Y, Hwang ES, He Y-W. Interleukin–10: an immune–activating cytokine in cancer immunotherapy. J CLin Oncol 2016; 34: 3576–78. [DOI] [PubMed] [Google Scholar]
- 100.Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 2015; 5: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD–1 blockade. Science 2016; 354: 1160–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tirosh I, Izar B, Prakadan SM, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016; 352: 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Macosko EZ, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 2015; 161: 1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benam KH, Novak R, Nawroth J, et al. Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Systems 2016; 3: 456–66. [DOI] [PubMed] [Google Scholar]
- 105.Simon R. Genomic alteration-driven clinical trial designs in oncology. Ann Intern Med 2016; 165: 270–78. [DOI] [PubMed] [Google Scholar]
- 106.Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res 2015; 21: 4786–800. [DOI] [PubMed] [Google Scholar]
- 107.Najafzadeh M, Schneeweiss S. From trial to target populations—calibrating real-world data. N Engl J Med 2017; 376: 1203–05. [DOI] [PubMed] [Google Scholar]
- 108.Bertagnolli MM, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med 2017; 376: 1178–81. [DOI] [PubMed] [Google Scholar]
- 109.Blumenthal GM, Goldberg KB, Pazdur R. Drug development, trial design, and endpoints in oncology: adapting to rapidly changing science. Clin Pharmacol Ther 2017: 101: 572–74. [DOI] [PubMed] [Google Scholar]
- 110.Beaver JA, Ison G, Pazdur R. Reevaluating eligibility criteria-balancing patient protection and participation in oncology trials. N Engl J Med 2017: 376: 1504–05. [DOI] [PubMed] [Google Scholar]
- 111.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 112.Sharp PA, Cooney CL, Kastern MA, et al. The third revolution: the convergence of the life sciences, physical sciences and engineerinG. 2011. http://research.uc.edu/Libraries/Advanced_Seminar_Documents/MIT_White_Paper_on_Convergence_pd.sflb.ashx (accessed Feb 17, 2017).
- 113.Singer DS, Jacks T, Jaffee E. A U.S. “Cancer Moonshot” to accelerate cancer research. Science 2016; 353: 1105–06. [DOI] [PubMed] [Google Scholar]
- 114.Hayden EC. The $1,000 genome. Nature 2014; 507: 295. [DOI] [PubMed] [Google Scholar]
- 115.Mardis ER. DNA sequencing technologies: 2006–2016. Nat Protoc 2017; 12: 213–18. [DOI] [PubMed] [Google Scholar]
- 116.Harris TJR, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol 2010; 7: 251–65. [DOI] [PubMed] [Google Scholar]
- 117.Cox DBT, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med 2015; 21: 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kelley SO, Mirkin CA, Walt DR, Ismagilov RF, Toner M, Sargent EH. Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat Nanotechnol 2014; 9: 969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwon EJ, Dudani JS, Bhatia SN. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat Biomed Eng 2017; published online April 10. DOI:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwong GA, von Maltzahn G, Murugappan G, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol 2013; 31: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jonas O, Landry HM, Fuller JE, et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med 2015; 7: 284ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 2015; 21: 1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phillips E, Penate-Medina O, Zanzonico PB, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med 2014; 6: 260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moderna Therapeutics. Merck and Moderna announce strategic collaboration to advance novel mRNA-based personalized cancer vaccines with KEYTRUDA (pembrolizumab) for the treatment of multiple types of cancer. June 29, 2016. https://www.modernatx.com/newsroom/press-releases/merck-and-moderna-announce-strategic-collaboration-advance-novel-mrna-based (accessed May 8, 2017).
- 125.Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 2013; 3: 406–17. [DOI] [PubMed] [Google Scholar]
- 126.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer 2010; 11: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morton SW, Lee MJ, Deng ZJ, et al. A Nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal 2014; 7: ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roybal KT, Williams JZ, Morsut L, et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 2016; 167: 419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith TT, Stephan SB, Moffett HF, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol 2017; 12: 813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14: 561–84. [DOI] [PubMed] [Google Scholar]
- 132.Swart M, Verbrugge I, Beltman JB. Combination Approaches with Immune–Checkpoint Blockade in Cancer Therapy. Front Oncol 2016; 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD–1 blockade in non–small cell lung cancer. Science 2015; 348: 124–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tran E, Robbins PF, Lu Y-C, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016; 375: 2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.University of Texas M D Anderson Cancer Center. Gut bacteria associated with cancer immunotherapy response in melanoma. ScienceDaily, February 21, 2017. [Google Scholar]
- 136.Johnson LA, June CH. Driving gene-engineered T cell immunotherapy of cancer. Cell Res 2017; 27: 38–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang E, Xu H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol 2017; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res 2016; 23: 2255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute, 2016. [Google Scholar]
- 140.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013; 12: 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhakta N, Liu N, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016; 17: 1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gurney JG, Krull KR, Kadan-Lottick NS, et al. Social outcomes in the Childhood Cancer Survivor Study Cohort. J Clin Oncol 2009; 27: 2390–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Recklitis CJ, Diller LR, Li X, Najita J, Robison LL, Zeltzer LK. Suicidal ideation in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol 2010; 28: 655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller LR. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med 2010; 152: 409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013; 368: 341–50. [DOI] [PubMed] [Google Scholar]
- 147.Bona K, Blonquist TM, Neuberg DS, Silverman LB, Wolfe J. Impact of socioeconomic status on timing of relapse and overall survival for children treated on Dana-Farber Cancer Institute ALL Consortium protocols (2000–2010). Pediatr Blood Cancer 2016; 63: 1012–18. [DOI] [PubMed] [Google Scholar]
- 148.Lewis DR, Seibel NL, Smith AW, Stedman MR. Adolescent and young adult cancer survival. J Natl Cancer Inst Monogr 2014; 49: 228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Flank J, Robinson PD, Holdsworth M, et al. Guideline for the treatment of breakthrough and the prevention of chemotherapy-induced nausea and vomiting in children with cancer. Pediatr Blood Cancer 2016; 63: 1144–51. [DOI] [PubMed] [Google Scholar]
- 150.Miller MM, Donald DV, Hagemann TM. Prevention and treatment of oral mucositis in children with cancer. J Pediatr Pharmacol Ther 2012; 17: 340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Villani A, Shore A, Wasserman JD, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: 11 year follow–up of a prospective observational study. Lancet Oncol 2016; 17: 1295–305. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. New Engl J Med 2015; 373: 2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27: 2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity–grading of long–term health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2016; 26: 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reulen RC, Winter DL, Lancashire ER, et al. Health-status of adult survivors of childhood cancer: A large-scale population-based study from the British childhood cancer survivor study. Int J Cancer 2007; 121: 633–40. [DOI] [PubMed] [Google Scholar]
- 156.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life–threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol 2014; 32: 1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016; 374: 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ross CJ, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 2009; 41: 1345–49. [DOI] [PubMed] [Google Scholar]
- 159.Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol 2012; 30: 1422–28. [DOI] [PubMed] [Google Scholar]
- 160.Stevens MC. The ‘Lost Tribe’ and the need for a promised land: the challenge of cancer in teenagers and young adults. Eur J Cancer 2006; 42: 280–81. [DOI] [PubMed] [Google Scholar]
- 161.Bleyer A, Barr R. Cancer in young adults: 20 to 39 years of age: overview. Semin Oncol 2009; 36: 194–206. [DOI] [PubMed] [Google Scholar]
- 162.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic speciatly care for older adolscents in Utah. J Clin Oncol 2007; 25: 4616–21. [DOI] [PubMed] [Google Scholar]
- 163.Muffly L, Kichtensztajn D, Shiraz P, et al. Adoption of pediatric-inspired acute lymphoblastic-leukemia regimens by adult oncologists treating adolescents and young adults: a population-based study. Cancer 2017; 123: 122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hay AE, Rae C, Fraser GAM, et al. Accrual of adolescents and young adults with cancer to clinical trials. Curr Oncol 2016; 23: e81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bisogno G, Compostella A, Ferrari A, et al. Rhabdomyosarcoma in adolescents: a report from teh AIEOP Soft Tissue Sarcoma Committee. Cancer 2012; 118: 821–27. [DOI] [PubMed] [Google Scholar]
- 166.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non–Hispanic white children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol 2012; 30: 2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brasme JF, Morfouace M, Grill J, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol 2012; 13: e445–59. [DOI] [PubMed] [Google Scholar]
- 168.Canner J, Alonzo TA, Franklin J, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescents/young adult and younger patients. Cancer 2013; 119: 4162–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.DeAngelo DJ, Stevenson KE, Dalhberg SE, et al. Long-term outcome of a pediatric inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 2015; 29: 526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stock W, Luger SM, Advani AS, et al. Favorable outcomes for older adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL): early results of US Intergroup Trial C10403. Blood 2014; 124: 796. [Google Scholar]
- 171.Potosky AL, Harlan LC, Albritton K, et al. Use of appropriate initial treatment among adolescents and young adults with cancer. J Natl Cancer Inst 2014; 106: dju300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carr R, Whiteson M, Edwards M, Morgan S. Young adult cancer services in the UK: the journey to a national network. Clin Med 2013; 13: 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One 2014; 9: e89482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gupta S, Rivera-Luna R, Ribeiro R, Howard SC. Pediatric oncology as the next global child health priority: the need for national childhood cancer strategies in low- and middle-income countries. PLoS Med 2014; 11: e1001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moriyami T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016; 48: 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hazin R, Qaddoumi I. Teleoncology: current and future applications for improving cancer care globally. Lancet Oncol 2010; 11: 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Santiago TC, Jenkins JJ, Pedrosa F, et al. Improving the histopathologic diagnosis of pediatric malignancies in a low-resource setting by combining focused training and telepathology strategies. Pediatr Blood Cancer 2012; 59: 221–25. [DOI] [PubMed] [Google Scholar]
- 178.Office of Cancer Survivorship, Division of Cancer Control & Population Sciences, National Cancer Institute. Statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html (accessed Sept 21, 2017).
- 179.Dahlin C. Clinical practice guidelines for quality palliative care, 3rd edn Pittsburgh, PA: National Consensus Project for Quality Palliative Care, 2013. [Google Scholar]
- 180.Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 2011; 29: 2683–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat 2011; 130: 681–89. [DOI] [PubMed] [Google Scholar]
- 182.Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat 2013; 138: 325–28. [DOI] [PubMed] [Google Scholar]
- 183.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015; 33: 1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gilbertson-White S, Aouizerat BE, Jahan T, Miaskowski C. A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliat Support Care 2011; 9: 81–102. [DOI] [PubMed] [Google Scholar]
- 185.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small–cell lung cancer. N Engl J Med. 2010; 363: 733–42. [DOI] [PubMed] [Google Scholar]
- 186.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009; 302: 741–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017; 35: 96–112. [DOI] [PubMed] [Google Scholar]
- 188.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ingle JN, Schaid DJ, Goss PE, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 2010; 28: 4674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boora GK, Kulkarni AA, Kanwar R, et al. Association of the Charcot-Marie-Tooth disease gene ARHGEF10 with paclitaxel induced peripheral neuropathy in NCCTG N08CA (Alliance). J Neurol Sci 2015; 357: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boora GK, Kanwar R, Kulkarni AA, et al. Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med 2016; 5: 631–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Majithia N, Smith TJ, Coyne PJ, et al. Scrambler Therapy for the management of chronic pain. Support Care Cancer 2016; 24: 2807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Astrup GL, Hofsø K, Bjordal K, et al. Patient factors and quality of life outcomes differ among four subgroups of oncology patients based on symptom occurrence. Acta Oncol 2017; 56: 462–70. [DOI] [PubMed] [Google Scholar]
- 194.Loprinzi CL, Barton DL, Jatoi A, et al. Symptom control trials: a 20–year experience. J Support Oncol 2007; 5: 119–28. [PubMed] [Google Scholar]
- 195.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990; 82: 1127–32. [DOI] [PubMed] [Google Scholar]
- 196.Loprinzi CL, Kugler JW, Sloan JA, et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med 1999; 340: 346–50. [DOI] [PubMed] [Google Scholar]
- 197.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med 1994; 331: 347–52. [DOI] [PubMed] [Google Scholar]
- 198.Barton DL, Wender DB, Sloan JA, et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central Cancer Treatment Group protocol N02C3. J Natl Cancer Inst 2007; 99: 672–79. [DOI] [PubMed] [Google Scholar]
- 199.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 2002; 98: 195–203. [DOI] [PubMed] [Google Scholar]
- 200.Myers JS, Koleck TA, Sereika SM, Conley YP, Bender CM. Perceived cognitive function for breast cancer survivors: association of genetic and behaviourally related variables for inflammation. Support Care Cancer 2017; 25: 2475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bender CM, Merriman JD, Gentry AL, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer 2015; 121: 2627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv 2016; 10: 51–61. [DOI] [PubMed] [Google Scholar]
- 203.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016; 159: 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kolb NA, Smith AG, Singleton JR, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 2016; 73: 860–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tashakori Beheshti A, Mostafavi Toroghi H, Hosseini G, Zarifian A, Homaei Shandiz F, Fazlinezhad A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: a double-blind randomized trial. Cardiology 2016; 134: 47–53. [DOI] [PubMed] [Google Scholar]
- 206.Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016; 37: 1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 2015; 313: 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leandro-Garcia LJ, Inglada-Perez L, Pita G, et al. Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet 2013; 50: 599–605. [DOI] [PubMed] [Google Scholar]
- 209.Huang IC, Brinkman TM, Armstrong GT, Leisenring W, Robison LL, Krull KR. Emotional distress impacts quality of life evaluation: a report from the Childhood Cancer Survivor Study. J Cancer Surviv 2017; 11: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lebel S, Tomei C, Feldstain A, Beattie S, McCallum M. Does fear of cancer recurrence predict cancer survivors’ health care use? Support Care Cancer 2013; 21: 901–06. [DOI] [PubMed] [Google Scholar]
- 211.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016; 19: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010; 40: 899–911. [DOI] [PubMed] [Google Scholar]
- 213.Institute of Medicine. 2015. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press, 2015. [PubMed] [Google Scholar]
- 214.Csikai EL. Developing the science of end-of-life and palliative care research: National Institute of Nursing Research summit. J Soc Work End Life Palliat Care 2011; 7: 291–99. [DOI] [PubMed] [Google Scholar]
- 215.Adams LS, Miller JL, Grady PA. The spectrum of caregiving in palliative care for serious, advanced, rare diseases: key issues and research directions. J Palliat Med 2016; 19: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khan SA, Gomes B, Higginson IJ. End-of-life care—what do cancer patients want? Nat Rev Clin Oncol 2014; 11: 100–08. [DOI] [PubMed] [Google Scholar]
- 217.Davies JM, Gao W, Sleeman KE. Using routine data to improve palliative and end of life care. BMJ Support Palliat Care 2016; 6: 257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sigurdardottir KR, Haugen DF, Bausewein C, et al. , and Project PRISMA. A pan-European survey of research in end-of-life cancer care. Support Care Cancer 2012; 20: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Prina LL. Foundation funding for palliative and end-of-life care. Health Aff (Millwood) 2017; 36: 1340–42. [DOI] [PubMed] [Google Scholar]
- 220.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13: 8–10. [DOI] [PubMed] [Google Scholar]
- 221.Lo SS, Moffatt-Bruce SD, Dawson LA, et al. The role of local therapy in the management of lung and liver oligometastases. Nature Rev Clin Oncol 2011; 8: 405–16. [DOI] [PubMed] [Google Scholar]
- 222.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi–institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009; 27: 1572–78. [DOI] [PubMed] [Google Scholar]
- 223.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi–institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009; 27: 1579–84. [DOI] [PubMed] [Google Scholar]
- 224.Chang EL, Shiu AS, Lii MF, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image–guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys 2004; 59: 1288–94. [DOI] [PubMed] [Google Scholar]
- 225.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1–2 trial. Lancet Oncol 2012; 13: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol 2014; 4: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014; 32: 3824–30. [DOI] [PubMed] [Google Scholar]
- 228.Gomez DR, Blumenschein GR, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first–line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncology 2016; 17: 1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous–cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–78. [DOI] [PubMed] [Google Scholar]
- 230.Srivastava RM, Trivedi S, Concha-Benavente F, et al. STAT1 induced HLA class I upregulation enhances immunogenicity and clinical response to anti-EGFR mAb cetuximab therapy in HNC patients. Cancer Immunol Res 2015; 3: 936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Morris ZS, Harari PM. Interaction of radiation therapy with molecular targeted agents. J Clin Oncol 2014; 32: 2886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chalmers AJ. Science in focus: combining radiotherapy with inhibitors of the DNA damage response. Clin Oncol (R Coll Radiol) 2016; 5: 279–82. [DOI] [PubMed] [Google Scholar]
- 233.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 2035. [DOI] [PubMed] [Google Scholar]
- 235.Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic antimelanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85: 293–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin–2: tumor and immunological responses. Sci Transl Med 2012; 4: 137ra74. [DOI] [PubMed] [Google Scholar]
- 237.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105: 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-γ production within the tumor microenvironment influences antitumor immunity. J Immunol 2008; 180: 3132–39. [DOI] [PubMed] [Google Scholar]
- 239.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181: 3099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003; 170: 6338–47. [DOI] [PubMed] [Google Scholar]
- 241.Ifeadi V, Garnett–Benson C. Sub-lethal irradiation of human colorectal tumor cells imparts enhanced and sustained susceptibility to multiple death receptor signaling pathways. PLoS One 2012; 7: e31762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Garnett CT, Palena C, Chakarborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004; 64: 7985–94. [DOI] [PubMed] [Google Scholar]
- 243.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vereecque R, Buffenoir G, Gonzalez R, et al. γ-ray irradiation induces B7. 1 expression in myeloid leukaemic cells. Br J Haematol 2000; 108: 825–31. [DOI] [PubMed] [Google Scholar]
- 245.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436: 1186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim J, Son Y, Park S, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med 2006; 38: 474. [DOI] [PubMed] [Google Scholar]
- 247.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res 2015; 75: 2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys 2007; 68: 499–507. [DOI] [PubMed] [Google Scholar]
- 249.Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol 2012; 2: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kuo P, Bratman SV, Shultz DB, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res 2014; 20: 5558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Deng L, Liang H, Burnette B, et al. Irradiation and anti–PD–L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 2000; 18: 3339–45. [DOI] [PubMed] [Google Scholar]
- 253.Taniguchi CM, Miao YR, Diep AN, et al. PHD inhibition mitigates and protects against radiation–induced gastrointestinal toxicity via HIF2. Sci Transl Med 2014; 6: 236ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Benderitter M, Caviggioli F, Chapel A, et al. Stem cell therapies for the treatment of radiation–induced normal tissue side effects. Antioxid Redox Signal 2014; 21: 338–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 2008; 3: e2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 2014; 4: 650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chaudhuri AA, Binkley MS, Osmundson EC, Alizadeh AA, Diehn M. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol 2015; 25: 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Le QT, Zhang Q, Cao H, et al. An international collaboration to harmonize the quantitative plasma Epstein–Barr virus DNA assay for future biomarker-guided trials in nasopharyngeal carcinoma. Clin Cancer Res 2013; 19: 2208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Med 2014; 20: 548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jeong Y, Hoang NT, Lovejoy A, et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov 2017; 7: 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort–based study. Lancet Oncol 2016; 18: 202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cunliffe A, Armato SG, Castillo R, et al. Lung texture in serial thoracic computed tomography scans: correlation of radiomics–based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys 2015; 91: 1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vinogradskiy Y, Koo PJ, Castillo R, et al. Comparison of 4–dimensional computed tomography ventilation with nuclear medicine ventilation–perfusion imaging: a clinical validation study. Int J Radiat Oncol Biol Phys 2014; 89: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vinogradskiy Y, Schubert L, Diot Q, et al. Regional lung function profiles of stage I and III lung cancer patients: an evaluation for functional avoidance radiation therapy. Int J Radiat Oncol Biol Phys 2016; 95: 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mailhot Vega RB, Kim J, Bussière M, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer 2013; 119: 4299–307. [DOI] [PubMed] [Google Scholar]
- 266.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016; 17: 287–98. [DOI] [PubMed] [Google Scholar]
- 267.Eaton BR, Esiashvili N, Kim S, et al. Clinical outcomes among children with standard–risk medulloblastoma treated with proton and photon radiation therapy: a comparison of disease control and overall survival. Int J Radiat Oncol Biol Phys 2016; 94: 133–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Andreo P. On the clinical spatial resolution achievable with protons and heavier charged particle radiotherapy beams. Phys Med Biol 2009; 54: N205. [DOI] [PubMed] [Google Scholar]
- 269.MacDonald SM, Laack NN, Terezakis S. Humbling advances in technology: protons, brainstem necrosis, and the self-driving car. Int J Radiat Oncol Biol Phys 2017; 97: 216. [DOI] [PubMed] [Google Scholar]
- 270.Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol 2014; 32: 2855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Moncharmont C, Guy JB, Wozny AS, et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in head and neck squamous cell carcinoma (HNSCC). Oncotarget 2016; 7: 47738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. [DOI] [PubMed] [Google Scholar]
- 273.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration–resistant prostate cancer with 117Lu labeled PSMA-617. J Nuc Med 2016; 57: 1170–76. [DOI] [PubMed] [Google Scholar]
- 274.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer 2015; 15: 347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yankeelov TE, Mankoff DA, Schwartz LH, et al. Quantitative imaging in cancer clinical trials. Clin Cancer Res 2016; 22: 284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kelloff GJ, Krohn KA, Larson SM, et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res 2005; 11: 7967–85. [DOI] [PubMed] [Google Scholar]
- 277.Weissleder R, Schwaiger MC, Gambhir SS, Hricak H. Imaging approaches to optimize molecular therapies. Sci Transl Med 2016; 8: 355ps16. [DOI] [PubMed] [Google Scholar]
- 278.Mankoff DA, Farwell MD, Clark AS, Pryma DA. Making molecular imaging a clinical tool for precision oncology: a review. JAMA Oncol 2016; 3: 695–701. [DOI] [PubMed] [Google Scholar]
- 279.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010; 87: 586–92. [DOI] [PubMed] [Google Scholar]
- 280.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med 2008; 49: 367–74. [DOI] [PubMed] [Google Scholar]
- 281.Kurland BF, Peterson LM, Lee JH, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med 2011; 52: 1541–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol 2006; 24: 2793–99. [DOI] [PubMed] [Google Scholar]
- 283.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 2001; 19: 2797–803. [DOI] [PubMed] [Google Scholar]
- 284.Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med 2013; 54: 1862–68. [DOI] [PubMed] [Google Scholar]
- 285.Kostakoglu L, Gallamini A. Interim 18F-FDG PET in Hodgkin lymphoma: would PET–adapted clinical trials lead to a paradigm shift? J Nucl Med 2013; 54: 1082–93. [DOI] [PubMed] [Google Scholar]
- 286.Van den Abbeele AD, Gatsonis C, de Vries DJ, et al. ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med 2012; 53: 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol 2006; 24: 3282–92. [DOI] [PubMed] [Google Scholar]
- 288.Contractor KB, Kenny LM, Stebbing J, et al. [18F]-3’Deoxy-3’-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin Cancer Res 2011; 17: 7664–72. [DOI] [PubMed] [Google Scholar]
- 289.Sohn HJ, Yang YJ, Ryu JS, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res 2008; 14: 7423–29. [DOI] [PubMed] [Google Scholar]
- 290.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol 2016; 27: 619–24. [DOI] [PubMed] [Google Scholar]
- 291.Gerstner ER, Zhang Z, Fink JR, et al. ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma Using 18F–FMISO PET and MRI. Clin Cancer Res 2016; 22: 5079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kostakoglu L, Duan F, Idowu MO, et al. A phase ii study of 3’–Deoxy–3’–18F–fluorothymidine PET in the assessment of early response of breast cancer to neoadjuvant chemotherapy: results from ACRIN 6688. J Nucl Med 2015; 56: 1681–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Feng Y, Martin P. Imaging innate immune responses at tumour initiation: new insights from fish and flies. Nat Rev Cancer 2015; 15: 556–62. [DOI] [PubMed] [Google Scholar]
- 294.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol 2012; 13: 1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gilles R, de Geus–Oei LF, Mulders PF, Oyen WJ. Immunotherapy response evaluation with (18)F-FDG-PET in patients with advanced stage renal cell carcinoma. World J Urol 2013; 31: 841–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tumeh PC, Radu CG, Ribas A. PET imaging of cancer immunotherapy. J Nucl Med 2008; 49: 865–68. [DOI] [PubMed] [Google Scholar]
- 297.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (suppl 1): 122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long–Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33: 1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chatterjee S, Lesniak WG, Miller MS, et al. Rapid PD–L1 detection in tumors with PET using a highly specific peptide.Biochem Biophys Res Commun 2017; 483: 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hey SP, Kesselheim AS. The FDA, Juno Therapeutics, and the ethical imperative of transparency. BMJ 2016; 354: i4435. [DOI] [PubMed] [Google Scholar]
- 303.Juergens RA, Zukotynski KA, Singnurkar A, Snider DP, Valliant JF, Gulenchyn KY. imaging biomarkers in immunotherapy. Biomark Cancer 2016; 8 (suppl 2): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ehlerding EB, England CG, McNeel DG, Cai W. Molecular imaging of immunotherapy targets in cancer. J Nucl Med 2016; 57: 1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Adonai N, Nguyen KN, Walsh J, et al. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci USA 2002; 99: 3030–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Su H, Chang DS, Gambhir SS, Braun J. Monitoring the antitumor response of naive and memory CD8 T cells in RAG1−/− mice by positron-emission tomography. J Immunol 2006; 176: 4459–67. [DOI] [PubMed] [Google Scholar]
- 307.Najjar AM, Manuri PR, Olivares S, et al. Imaging of sleeping beauty–modified CD19–specific T cells Expressing HSV1–thymidine kinase by positron emission tomography. Mol Imaging Biol 2016; 18: 838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lee HW, Yoon SY, Singh TD, et al. Tracking of dendritic cell migration into lymph nodes using molecular imaging with sodium iodide symporter and enhanced firefly luciferase genes. Sci Rep 2015; 5: 9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Keu KV, Witney TH, Yaghoubi S, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med 2017; 9: eaag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chatterjee S, Lesniak WG, Gabrielson M, et al. A humanized antibody for imaging immune checkpoint ligand PD–L1 expression in tumors. Oncotarget 2016; 7: 10215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel radiotracer for immunoPET imaging of PD–1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjug Chem 2015; 26: 2062–69. [DOI] [PubMed] [Google Scholar]
- 312.Rashidian M, Keliher EJ, Bilate AM, et al. Noninvasive imaging of immune responses. Proc Natl Acad Sci USA 2015; 112: 6146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mall S, Yusufi N, Wagner R, et al. Immuno–PET imaging of engineered human T cells in tumors. Cancer Res 2016; 76: 4113–23. [DOI] [PubMed] [Google Scholar]
- 314.Kim W, Le TM, Wei L, et al. [18F]CFA as a clinically translatable probe for PET imaging of deoxycytidine kinase activity. Proc Natl Acad Sci USA 2016; 113: 4027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Baum RP, Kulkarni HR. Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy—the Bad Berka Experience. Theranostics 2012; 2: 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Warner S. Diagnostics + therapy=theranostics. The Scientist, August 30, 2004. [Google Scholar]
- 317.Lütje S, Heskamp S, Cornelissen AS, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status theranostics. Theranostics 2015; 5: 1388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconj Chem 2011; 22: 1879–903. [DOI] [PubMed] [Google Scholar]
- 319.Alberti C. From molecular imaging in preclinical/clinical oncology to theranostic applications in targeted tumor therapy. Eur Rev Med Pharmacol Sci 2012; 16: 1925–33. [PubMed] [Google Scholar]
- 320.Taieb D, Hicks RJ, P K. Nuclear medicine in cancer theranostics: beyond the target. J Nucl Med 2016; 57: 1659–60. [DOI] [PubMed] [Google Scholar]
- 321.Del Vecchio S, Zannetti A, Fonti R, Pace I, Salvatore M. Nuclear imaging in cancer theranostics. J Nucl Med Mol Imaging 2007; 51: 152–63. [PubMed] [Google Scholar]
- 322.Matthay KK, Weiss B, Villablanca JG, et al. Dose escalation study of no–carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: new approaches to neuroblastoma therapy consortium trial. J Nucl Med 2012; 53: 1155–63. [DOI] [PubMed] [Google Scholar]
- 323.Bodei L, Cremonesi M, Ferrari M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with (90) Y-DOTATOC and (177)Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 2008; 35: 1847–56. [DOI] [PubMed] [Google Scholar]
- 324.Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine 131I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 2000; 96: 1259–66. [PubMed] [Google Scholar]
- 325.Silberstein EB. Radioiodine: the classic theranostic agent. Semin Nucl Med 2012; 42: 164–70. [DOI] [PubMed] [Google Scholar]
- 326.Ahn B-C. Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. Biomed Res Int 2016; 1680464: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sisson JC, Yanik GA. Theranostics: evolution of the radiopharmaceutical meta–iodobenzylguanidine in endocrine tumors. Semin Nucl Med 2012; 42: 171–84. [DOI] [PubMed] [Google Scholar]
- 328.Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka Experience since 2013. J Nucl Med 2016; 57: 97S–104S. [DOI] [PubMed] [Google Scholar]
- 329.Hermann K, Schottelius M, Lapa C, et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med 2016; 57: 248–51. [DOI] [PubMed] [Google Scholar]
- 330.Dalm SU, Bakker IL, de Blois E, et al. 68Ga/177Lu–NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J Nucl Med 2017; 58: 293–99. [DOI] [PubMed] [Google Scholar]
- 331.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 332.Lutz AM, Willmann JK, Drescher CW, et al. Early diagnosis of ovarian carcinoma: is a solution in sight? Radiology 2011; 259: 329–45. [DOI] [PubMed] [Google Scholar]
- 333.Carlsson A, Nair VS, Luttgen MS, et al. Circulating tumor microemboli diagnostics for patients with non-small-cell lung cancer. J Thorac Oncol 2014; 9: 1111–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nair VS, Keu KV, Luttgen MS, et al. An observational study of circulating tumor cells and (18)F-FDG PET uptake in patients with treatment-naive non-small cell lung cancer. PLoS One 2013; 8: e67733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.De Giorgi U, Mego M, Rohren EM, et al. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J Nucl Med 2010; 51: 1213–18. [DOI] [PubMed] [Google Scholar]
- 336.De Giorgi U, Valero V, Rohren E, et al. Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J Clin Oncol 2009; 27: 3303–11. [DOI] [PubMed] [Google Scholar]
- 337.De Giorgi U, Valero V, Rohren E, et al. Circulating tumor cells and bone metastases as detected by FDG–PET/CT in patients with metastatic breast cancer. Ann Oncol 2010; 21: 33–39. [DOI] [PubMed] [Google Scholar]
- 338.NSAC Isotopes Subcommittee. Meeting isotope needs and capturing opportunities for the future: the 2015 long range plan for the DOE-NP isotope program. Washington, DC: US Department of Energy, 2015. [Google Scholar]
- 339.Fahey F, Zukotynski K, Capala J, Knight N. Targeted radionuclide therapy: proceedings of a joint workshop hosted by the National Cancer Institute and the Society of Nuclear Medicine and Molecular Imaging. J Nucl Med 2014; 55: 337–48. [DOI] [PubMed] [Google Scholar]
- 340.Fahey F, Zukotynski K, Jadvar H, Capala J. Proceedings of the Second NCI–SNMMI workshop on targeted radionuclide therapy. J Nucl Med 2015; 56: 1119–29. [DOI] [PubMed] [Google Scholar]
- 341.Graham MM, Wahl RL, Hoffman JM, et al. Summary of the UPICT protocol for 18F–FDG PET/CT imaging in oncology clinical trials. J Nucl Med 2015; 56: 955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Report of the Cancer Moonshot Task Force: executive summary. https://medium.com/cancer-moonshot/report-of-the-cancer-moonshot-task-force-executive-summary-e711f1845ec (accessed Oct 1, 2016).
- 343.Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010; 97: 1110–18. [DOI] [PubMed] [Google Scholar]
- 344.Are C, Berman RS, Wyld L, Cummings C, Lecoq C, Audisio RA. Global curriculum in surgical oncology. Ann Surg Oncol 2016; 23: 1782–95. [DOI] [PubMed] [Google Scholar]
- 345.Azagury DE, Dua MM, Barrese JC, et al. Image–guided surgery. Curr Probl Surg 2015; 52: 476–520. [DOI] [PubMed] [Google Scholar]
- 346.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015; 16: 1193–224. [DOI] [PubMed] [Google Scholar]
- 347.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010; 11: 38–47. [DOI] [PubMed] [Google Scholar]
- 348.Köhne CH, Poston G, Folprecht G, et al. FOLFIRI plus cetuximab in patients with liver–limited or non–liver–limited RAS wild–type metastatic colorectal cancer: a retrospective subgroup analysis of the CRYSTAL study. Eur J Surg Oncol 2016; 42: 1540–47. [DOI] [PubMed] [Google Scholar]
- 349.Andreu-Perez J, Poon CC, Merrifield RD, Wong ST, Yang GZ. Big data for health. IEEE J Biomed Health Inform 2015; 19: 1193–208. [DOI] [PubMed] [Google Scholar]
- 350.Bellazzi R. Big data and biomedical informatics: a challenging opportunity. Yearb Med Inform 2014; 9: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.He KY, Ge D, He MM. Big data analytics for genomic medicine. Int J Mol Sci 2017; 18: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shaikh AR, Butte AJ, Schully SD, Dalton WS, Khoury MJ, Hesse BW. Collaborative biomedicine in the age of big data: the case of cancer. J Med Internet Res 2014; 16: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Toga AW, Dinov D. Sharing big biomedical data. J Big Data 2015; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Nature. Big data: community cleverness required. Nature 2008; 455: 1. [DOI] [PubMed] [Google Scholar]
- 355.Chow SC, Kong Y. On big-data analytics in biomedical research. J Biomed Biostat 2015; 6: 236. [Google Scholar]
- 356.Badawi O, Brennan T, Celi LA, et al. Making big data useful for health care: a summary of the inaugural MIT Critical Data Conference. JMIR Med Inform 2014; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Pearson RK. Mining imperfect data: dealing with contamination and incomplete records. Philadelphia: SIAM, 2005. [Google Scholar]
- 358.Miller K. NIH launches a united ecosystem for big data. 12 big data to knowledge centers of excellence funded. January 8, 2015. http://biomedicalcomputationreview.org/content/nih-launches-united-ecosystem-big-data (accessed May 9, 2017).
- 359.Executive Office of the President, Office of Science and Technology Policy. Increasing access to the results of federally funded scientific research (memo). February 22, 2013. https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/ostp_public_access_memo_2013.pdf (accessed May 9, 2017).
- 360.AACR. Project GENIE publicly releases large cancer genomic data set. January 5, 2017. http://www.aacr.org/Newsroom/Pages/News–Release–Detail.aspx?ItemID=994#.WG5wXlPyu71 (accessed May 9, 2017). [DOI] [PubMed]
- 361.US Centers for Medicare & Medicaid Services. National health expenditure fact sheet. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html (accessed May 23, 2017).
- 362.Schrag D. Reimbursing wisely? CMS’s trial of Medicare Part B Drug Payment Reform. N Engl J Med 2016; 374: 2101–05. [DOI] [PubMed] [Google Scholar]
- 363.Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 2016; 122: 283–89. [DOI] [PubMed] [Google Scholar]
- 364.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol 2014; 32: 306–11. [DOI] [PubMed] [Google Scholar]
- 365.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst 2014; 106: dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013; 32: 1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol 2009; 27: 3868–74. [DOI] [PubMed] [Google Scholar]
- 368.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol 2011; 29: 755–60. [DOI] [PubMed] [Google Scholar]
- 369.Bentley TG, Cohen JT, Elkin EB, et al. Validity and reliability of value assessment frameworks for new cancer drugs. Value Health 2017; 20: 200–05. [DOI] [PubMed] [Google Scholar]
- 370.Polite BN, Miller HD. Medicare innovation center oncology care model: a toe in the water when a plunge is needed. J Oncol Pract 2015; 11: 117–19 [DOI] [PubMed] [Google Scholar]
- 371.Peppercorn J. Financial toxicity and societal costs of cancer care: distinct problems require distinct solutions. Oncologist 2017; 22: 123–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.American College of Physicians. Racial and ethnic disparities in health care. A position paper of the American College of Physicians. Ann Intern Med 2004; 141: 226–32. [DOI] [PubMed] [Google Scholar]
- 373.Hanes MA, Smedley BD. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academy of Sciences, 1999. [PubMed] [Google Scholar]
- 374.Institute of Medicine of the National Academies. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academy of Sciences, 2003. [PubMed] [Google Scholar]
- 375.Raghavan D. Disparities in cancer care: challenges and solutions. Oncology 2007; 21: 493–506. [PubMed] [Google Scholar]
- 376.Symonds RP, Lord K, Mitchell AJ, Raghavan D. Recruitment of ethnic minorities into cancer clinical trials: experience from the front lines. Brit J Cancer 2012; 107: 1017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Jones LA, Chilton JA, Hajek RA, Iammarino NK, Laufman L. Between and within: international perspectives on cancer and health disparities. J Clin Oncol 2006; 24: 2204–08. [DOI] [PubMed] [Google Scholar]
- 378.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–36. [DOI] [PubMed] [Google Scholar]
- 379.Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med 1996; 125: 675–79. [DOI] [PubMed] [Google Scholar]
- 380.Rosenberg NA, Pritchard JK, Weber JL, et al. : Genetic structure of human populations. Science 2002; 298: 2381–85. [DOI] [PubMed] [Google Scholar]
- 381.Rebbeck TR, Halbert CH, Sankar P. Genetics, epidemiology, and cancer disparities: is it black and white? J Clin Oncol 2006; 24: 2164–69. [DOI] [PubMed] [Google Scholar]
- 382.Satcher D. From the Surgeon General: eliminating global cancer disparities. JAMA 2000; 284: 2864. [DOI] [PubMed] [Google Scholar]
- 383.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population based trends in cancer treatment. J Natl Cancer Inst 2005, 97: 1407–27. [DOI] [PubMed] [Google Scholar]
- 384.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 385.Koroukian SM, Bakaki P, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer 2012; 118: 4271–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst 2007; 99: 1384–94. [DOI] [PubMed] [Google Scholar]
- 387.Reinke T. CMS wants to remodel cancer payment, care. Manag Care 2016; 25: 12–15. [PubMed] [Google Scholar]
- 388.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol 2012; 30: 1715–24. [DOI] [PubMed] [Google Scholar]
- 389.Ramsey SD, Henry NL, Gralow JR, et al. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol 2015; 33: 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA 2014; 312: 2542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Palta M, Palta P, Bhavsar NA, Horton JK, Blitzblau RC. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: changes in practice patterns after publication of Cancer and Leukemia Group B 9343. Cancer 2015; 121: 188–93. [DOI] [PubMed] [Google Scholar]
- 392.Jagsi R, Spence R, Rathmell WK, et al. Ethical considerations for the clinical oncologist in an era of oncology drug shortages. Oncologist 2014; 19: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zafar SY, Peppercorn JM. Patient financial assistance programs: a path to affordability or a barrier to accessible cancer care? J Clin Oncol 2017: 35: 2113–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Wolff M, Bates T, Beck B, et al. Cancer prevention in underserved African American communities: barriers and effective strategies. A review of the literature. WMJ 2003; 102: 36–40. [PubMed] [Google Scholar]
- 395.Brown DR, Fouad MN, Basen-Engquist K, et al. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol 2000; 10: S13–21. [DOI] [PubMed] [Google Scholar]
- 396.Ramirez AG, McAlister A, Villarreal R, et al. Prevention and control in diverse Hispanic populations: a national initiative for research and action. Cancer 1998; 83 (suppl): S1825–29. [Google Scholar]
- 397.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 2005, 293: 1729–36. [DOI] [PubMed] [Google Scholar]
- 398.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg 2006, 243: 281–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 2005, 294: 1765–72. [DOI] [PubMed] [Google Scholar]
- 400.Wisnivesky JP, McGinn T, Henschke C, Hebert P, Iannuzzi MC, Halm EA. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med 2005, 171: 1158–63. [DOI] [PubMed] [Google Scholar]
- 401.Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D. American Society of Clinical Oncology policy statement—disparities in cancer care. J Clin Oncol 2009; 27: 2881–85. [DOI] [PubMed] [Google Scholar]
- 402.Aggarwal A, Lewison G, Idir S, et al. The state of lung cancer research: a global analysis. J Thorac Oncol 2016; 11: 1040–50. [DOI] [PubMed] [Google Scholar]