Abstract
Concentration patterns and temporal trends of legacy persistent, bioaccumulative and toxic (PBT) contaminants were determined using the Great Lake Fish Monitoring and Surveillance Program (GLFMSP) top predator fish data from 1999 to 2014 and applying Kendall-Theil robust regression after cluster-based age normalization. For most Great Lakes sites, significant decreasing concentration trends ranging from −4.1% to −21.6% per year (with the only exception being mirex in Lake Erie walleye) were found for PBTs including polychlorinated biphenyls (PCBs), dichloro-diphenyl-trichlorethane (DDTs), dieldrin, endrin, chlordane, oxychlordane, nonachlor, mirex, and hexachlorobenzene (HCB) reflecting the successful historical and ongoing reduction of fugitive releases and remediation efforts in the U.S. and Canada including physical removal (dredging) coupled with sediment sequestration. Generally, lower concentrations and faster decreasing trends are observed in western/northern sampling sites compared to eastern/southern sites as the former sites are generally more remote from population centers and industrial activities. PCBs, which can be released from ongoing sources, have the highest concentration, the second slowest decreasing trend, and increasing mass fractions of the contaminants studied suggesting that they will continue to be the legacy contaminant of greatest concern into the future.
Keywords: Legacy contaminants, Lake trout, PBTs, Trends, Age normalization
Introduction
The United States banned or restricted the use of many persistent, bioaccumulative, and toxic (PBT) contaminants over the past several decades because of their persistence in the environment, and potential risk to humans and the environment (Chang et al., 2012; Clement et al., 2012; Cornwell et al., 2015; USEPA, October, 2014). For example, hexachlorobenzene (HCB) was phased out in the 1960s (ATSDR, September, 2002), dichloro-diphenyl-trichlorethane (DDT) and mirex were restricted in the 1970s (Chang et al., 2012; USEPA, 2004), dieldrin, endrin, and chlordane were banned in the 1980s (Chang et al., 2012; USEPA, 2003) and polychlorinated biphenyls (PCBs) were banned in the 1970s (Marvin et al., 2004a).
However, these PBTs can still be widely detected in the environment, and some legacy contaminants have been released into the environment even after their manufacture has been terminated. For example, PCBs currently may be released from leaking transformers, building sealants, brownfield sites, contaminated soils and sediments, and waste handling and recycling facilities (Diamond et al., 2010; Melymuk et al., 2013; Robson et al., 2010), and HCB (manufactured as an agriculture chemical) has also been emitted as by-product or impurity from some manufacturing processes including chlorinated solvent production and base metal smelting, and the incineration of wastes and sewage sludge (Leger, 1991; Luscombe and Costner, 2001; USATSDR, Aug., 2015).
The United States and Canada have been collecting and measuring concentrations of legacy contaminants in the Great Lakes (GL) fish, for example lake trout (Salvelinus namaycush) and walleye (Sander vitreus) since the late 1970s as part of what is now called the Great Lakes Fish Monitoring and Surveillance Program (GLFMSP) in the U.S. (Chang et al., 2012; Zananski et al., 2011; Zhou et al., 2017) and in Canada the Sports Fish Contaminant Monitoring Program (SFCMP) (Bhavsar et al., 2007) and the Fish Contaminants Monitoring and Surveillance Program (FCMSP) (McGoldrick and Murphy, 2016). Additionally, PCBs were designated as a Chemical of Mutual Concern (CMC) by the Governments of Canada and the United States in February of 2014, under the Great Lakes Water Quality Agreement (GLWQA). The GLWQA directs Canada and the U.S. to target CMCs for action through development of binational strategies that may address research, monitoring, surveillance and pollution prevention and control provisions (GLWQA, 2015).
The concentrations of these legacy contaminants in the environment and biota vary significantly due to many complex factors including their release history, persistence, transport potential, bioaccumulation potential, ecosystem characteristics, and changes in climate (Macksasitorn et al., 2015; Melymuk et al., 2014; Ng and Gray, 2011; Paterson et al., 2016). For most legacy contaminants, Lakes Superior (LS) and Huron (LH), which are remote to urban (population centers and industry) and agricultural areas, had lower concentrations (Chang et al., 2012; Gewurtz et al., 2008) while Lakes Michigan (LM), Erie (LE), and Ontario (LO), which are closer to areas with large populations, industrial influences and agricultural activity, have higher concentrations (Chang et al., 2012; El-Shaarawi et al., 2011). However, there are exceptions to this pattern for contaminants that were not used extensively within the GL basin and whose primary pathway to the GLs is through long-range atmospheric transport and deposition. For these chemicals (like toxaphene), LS and LH have higher concentrations because of their larger surface areas and long water residence times (Xia et al., 2012). PCBs and DDTs are the dominant organic contaminants in fish tissue and can be up to 10–100 times higher in average concentration than other organic contaminants (Chang et al., 2012; McGoldrick and Murphy, 2016).
Concentrations have decreased in most lakes over the past several decades due to management actions and voluntary industrial actions that phased out the use of these chemicals (Dellinger et al., 2014; El-Shaarawi et al., 2011). In general, the trend pattern can be described as a rapid decrease after the initial phase-out period followed by a slow to no decrease or even apparent concentration increases, especially in the lower lakes (Carlson et al., 2010; French et al., 2011; Sadraddini et al., 2011). The recent concentration decreases in the legacy contaminant concentration in fish were mostly slower than expected (or predicted) due to the complexity of the ecosystem, such as the long response time after reducing inputs, changes in lake trophic status and food webs due to invasive species, resuspension from sediments, changing fish growth rates, and changes in climate (Carlson et al., 2010; Cornwell et al., 2015; Ng and Gray, 2011; Stow et al., 2004; USEPA, 2002).
The magnitude of PBT bioaccumulation in fish tissue varies with the species, size, and age of the fish and is largely controlled by the trophic status of a lake (El-Shaarawi et al., 2011; Mahmood et al., 2013; Paterson et al., 2016). The GL have undergone significant changes in trophic structure over the past decade with the introduction of invasive species, i.e. round goby and dreissenid mussels (Cornwell et al., 2015; Crane and Einhouse, 2016; Lepak et al., 2015; Warner and Lesht, 2015). These changes may have affected the extent of PBT bioaccumulation and resulting trends in top predator fish. For GLFMSP, fish have been historically collected and grouped using length as an age metric to minimize the influence of different bioaccumulation times (ages) on contaminant concentration trends. However, due to the changes in lake food webs due to invasive species and eutrophication, the age of the same size lake trout (a long-lived fish) varied significantly, especially in LM, LH and LS, which will impact bioaccumulation patterns (Drouillard et al., 2009; Russell et al., 1999). Our previous trend results (1999 to 2009) assessed contaminants based upon an assumed age based on the length of the fish (i.e. age to length ratio was assumed to be constant) because fish age was not determined prior to 2004 (Chang et al., 2012).
The current study updates legacy PBT concentration temporal trends in GL top predator fish using PBT concentration and fish age data from 2010 to 2014 (Chang et al., 2012). This time period includes significant trophic perturbations that have altered fish growth rates, so fish age normalization was needed. These data were combined with earlier data to provide long-term overall (GL regional) concentration trends from 1999 to 2014 without fish age normalization (fish age data were only available since 2004) and age-normalized trends from 2004 to 2014. Trends for legacy PBTs, including PCBs, DDTs, dieldrin, endrin, chlordane, oxychlordane, nonachlor, HCB, and mirex, in GL top predator fish (lake trout and walleye) were determined using non-parametric trend test methods after clustering-based age normalization. As PCBs had the highest concentrations of these chemicals, PCB ratio trends are also discussed. Overall, this work provides information on historical, current, and future legacy contaminant behavior in GL top predator fish. Insights from this analysis of legacy PBT bioaccumulation in GL fish provides critical insight into the impact of food web perturbations on concentration trends that is necessary to better understand and manage legacy PBTs in the Great Lakes.
Methods
Sampling, contaminants analysis, and QA/QC
The sampling information and analysis methods were described in detail in our previous papers (sample handling and preparation details given in the Electronic Supplementary Information (ESM) Table S1) (Chang et al., 2012; Zananski et al., 2011; Zhou et al., 2017). In brief, 50 fish samples (600–700 mm length lake trout from LH, LM, LO and LS, and 400–500 mm length walleye from LE) were collected and composited into ten composite samples of five fish in each lake annually for contaminant analysis. The concentrations in the composited samples are well correlated with concentrations in additional individual fish caught from the same location and analyzed individually (data shown in ESM Fig. S9). Fish were collected from alternating sites for odd years and even years in each lake, as shown in ESM Fig. S1. Generally, even sites are shallower than odd year sites (except for LH). The two sites were chosen to represent offshore fishing grounds and based on their proximity to urban and manufacturing centers are loosely classi-fied into either industry influenced or non-industry influenced areas in each of the lakes (ESM Table S1) (GLFMSP, 2004; USEPA, 2012). Note that the designation of industrial or non-industrial influenced site was based on the relative conditions in each lake (site proximity and magnitude of industrial areas in each lake). For example, the non-industrial site in LE likely has more industrial influence than the industrially influenced site in LS.
The samples were extracted by an accelerated solvent extractor (ASE 350, Dionex, Sunnyvale, CA) with dichloromethane (DCM), lipids were removed via gel permeation chromatography (GPC, Waters, Milford, CA), and then the extracts were fractionated over 4% deactivated silica into two fractions with hexane (F1) followed by 50:50 hexane:DCM (F2), which was used for PCBs (F1) and OC pesticides analysis (F2) (DDT, dieldrin, endrin, cis-chlordane, trans-chlordane, oxychlordane, cis-nonachlor, trans-nonachlor, mirex, and HCB) using GC-ECD. Compound identification was confirmed with a mass spectrometry detector in electron capture negative ion mode (GC/MS-ECNI, Agilent 7890/5975 MSD). Note that only total DDT (t-DDTs, sum of p-p′ DDD, p-p′ DDE, o-p′ DDT, p-p′ DDT) and total PCBs (t-PCBs, 119 PCB congeners were analyzed as shown in ESM Table S7) were used for the trend analysis reported here. The concentration distributions of DDTs and PCBs congeners are presented in the ESM Fig. S7. National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1946 samples were analyzed in each 10-sample batch of field samples. For QA/QC control, PCB-14, PCB-65, PCB-166, PCB-209, PCT-3, and PCT-5 (pentachloroterphenyl) were added as surrogates to evaluate extraction efficiency; the average recoveries were 87 ± 14%, 82 ± 14%, 100 ± 12%, 98 ± 15%, 89 ± 13%, and 91 ± 15%, respectively. All PCB and OC pesticide masses in method blanks were below the limits of detection (LOD) defined as the mass associated with the average method blank plus standard deviation times Student’s t statistic. No samples included in this analysis were below the LOD.
Fish age analysis
Fish ages were determined using multiple approaches, including otoliths, fin clips, coded wire tags (CWT), scales, and maxillae by the homogenization lab. The maxillae estimation method, developed at the Michigan Department of Natural Resources (MDNR) (Wellenkamp et al., 2015), became the only aging technique used other than CWT in 2012. This change increased the speed of sample aging, allowed samples to be composited according to known ages instead of assumed ages based on length, and resulted in minimal fluid and tissue loss from the sample. The measured ages before and after this change were combined when available as there were no significant difference between maxillary age (new method) and otolith age (old method) (ANOVA analysis, p 0.12).
Contaminants clustering and age normalization
The age normalization method based on hierarchical clustering for one contaminant (fish mercury) was developed in a previous paper (Zhou et al., 2017). The relationship between age and contaminants concentrations presented here were determined to be nonlinear and year dependent, similar to what was found previously for mercury (Zhou et al., 2017). In this study, the age normalization method was adjusted to account for the multiple contaminants. A second hierarchical clustering analysis was added to separate the PBTs into different groups based on the linear correlations between PBTs that had similar bioaccumulation rates (Paterson et al., 2016). The age normalization method for multiple contaminants was performed as follows: 1) linear correlation coefficients were calculated between legacy contaminants; 2) the two most correlated contaminants were used to create a new contaminant group by adding their concentrations together; 3) Step 1 was performed again using a new contaminant until all the contaminants were in a group; 4) contaminant clusters were determined using a correlation coefficient threshold (0.5); 5) age normalization was performed for each contaminant cluster using the same method as described previously (Zhou et al., 2017).
The age normalization for each contaminant cluster was performed as: 1) regression lines between age and total contaminant concentration in the contaminant cluster were calculated for each year; 2) the distance between each year was calculated based on the equations obtained in step 1. The distance was determined using the average distance of each data point in one group to the other regression line (this approach considers both the distance of data points in the groups and the similarity of the regression lines); 3) hierarchical clustering was performed to define the clusters based on the distances obtained in step 2. Since hierarchical clustering will generate new group combinations (by combining the data points), the distance within new groups was recalculated in each step of hierarchical clustering; 4) linear regression equations were determined for each cluster obtained in step 3; and 5) concentrations were normalized to the average age (6.77 years) based on the equations obtained in step 4. A flowchart detailing the steps used for contaminant clustering and age normalization is shown in ESM Fig. S3.
Statistical analysis
The overall trends in the GL region were analyzed for six major contaminants for which long-term (1999–2014) monitoring data (t-PCBs, t-DDTs, dieldrin, chlordane, oxychlordane, and nonachlor) are available. Note that an overall trend analysis for the GL region from 2004 to 2014 following age normalization was also performed using these six major contaminants. In this analysis, all the lake trout data from all sites and lakes (except walleye from in Lake Erie) were combined to present a regional temporal trend. Trends after age normalization from 2004 to 2014, lake-by-lake, with even and odd year sites analyzed independently if the age normalized concentrations had significant differences between the sites based on multi-way analyses of variance (n-way ANOVA), were determined for eight legacy contaminants (the above six plus HCB and mirex). The trends in the t-PCBs ratio (t-PCB concentration/total legacy contaminant concentrations) in each lake are also presented.
N-way ANOVA test was performed to compare the concentration difference between year, fish age, fish species, contaminant species, and sampling sites. Nonmetric multidimensional scaling (NMDS) was then used to investigate the legacy contaminant patterns for each site. Non-parametric Kendall’s tau test and Kendall-Theil robust line (with Sen’s slope) were used for trends and breakpoint analysis (Granato, 2006; Huang et al., 2015; Kendall, 1948; Litaor et al., 2016; Stonevičius et al., 2014) using KTRLine software developed by the U.S. Geological Survey (USGS). The statistical analyses were performed using SPSS 22 (2014 IBM Corporation) and MATLAB 2016b.
Results and discussion
Contaminants patterns
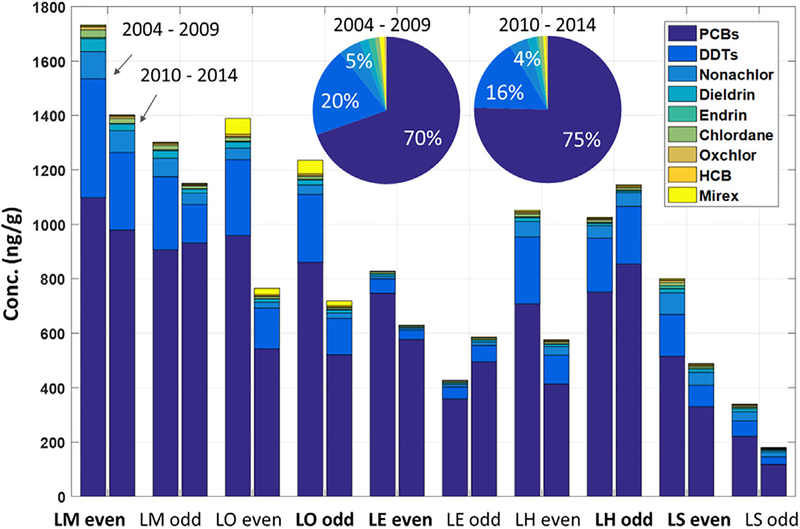
t-PCBs, t-DDTs, and nonachlor concentrations dominate the concentration profile and represent >95% of the total mass measured (Fig. 1). Average concentrations for all samples averaged into early (2004–2009) and later (2010–2014) periods are 730 (2004–2009) and 580 (2010–2014), 206 (2004–2009) and 125 (2010–2014), 50 (2004–2009) and 34 (2010–2014) ng/g for t-PCBs, t-DDTs, and nonachlor, respectively. The industry influenced sites for most lakes (LE, LM, LH and LS) had statistically significant (two sample t-tests p < 0.01) higher average total legacy contaminant concentrations than non-industrial influenced sites (32%, 21%, 24% and 61% higher for LE, LM, LH and LS, respectively) (ESM Fig. S8). LO sites had only small differences (<5%) likely because of the contaminant input from the Niagara river (Marvin et al., 2004a) was evenly distributed in the surficial sediment across the three major depositional basins in LO (Marvin et al., 2002) and the proximity of all of the lake to major urban areas. The concentration difference among the lakes is inversely related to the relative distance to major population centers and industrial areas, which supports previous work that atmospheric sources are important determinants of fish legacy contaminant concentrations (Melymuk et al., 2014; Shunthirasingham et al., 2016; Sun et al., 2007) and suggests that lake trout ranges are at least somewhat limited relative to the size of the lakes.
Fig. 1.
Mean concentrations of nine legacy contaminants for the earlier period (2004–2009) and recent period (2010–2014) for each site (bar chart) and for all samples (pie chart). Note that a site name in bold font indicates the site with more industrial influence for that lake.
However, LE, one of the most urban influenced lakes due to the proximity of Toledo, Cleveland and Detroit (Mahmood et al., 2013; Sun et al., 2007) has relative lower concentrations for most contaminants (Fig. 1). This result is probably because the top predator fish species in LE is wall-eye, which generally have a lower bioaccumulation rate than lake trout (Carlson et al., 2010; Chang et al., 2012). Our ANOVA analysis results support previous studies as it identified a significant concentration difference (p < 0.01) between lake trout and walleye in Lake Erie, with walleye having lower concentrations (440 ng/g total legacy contaminants on average) compared with lake trout (610 ng/g total legacy contaminants on average) (detailed results are presented in ESM Table S2).
For the other four lakes, LM, which is one of the most urban influenced lake due to the proximity to the highly populated and industrial cities, such as Chicago, and Milwaukee, and the industrial areas centered around Gary, IN, has the highest concentrations, LH and LO have intermediate concentrations, and LS, which is the least urban (in terms of population and industrial proximity) influenced lake, has the lowest concentrations for most contaminants. The low concentrations seen in LS are also likely due to lower inputs from non-atmospheric sources (runoff and riverine inputs) as it is the headwater lake for the GL (Chang et al., 2012; Peverly et al., 2015) and generally not downwind of urban/industrial areas. Note that relatively high mirex concentrations were detected in LO fish (36.4 ng/g on average, the highest concentration in the GLs and the third highest contaminant concentration in this lake) probably due to historical manufacture and releases from manufacturing sites into the Niagara and Oswego River and other locations within the lake’s watershed (Comba et al., 1993; Shunthirasingham et al., 2016; USEPA, 2010). Most sites had negative concentration differences (indicating decreasing concentrations) between the earlier period (2004–2009) and current period (2010–2014). Note that higher current concentrations were only found at the LE odd site because lake trout, which have higher PBTs concentrations, were sampled there after 2010 and at the LH odd site which had the fastest increasing fish age as shown in ESM Fig. S2 as older fish can have larger amounts of accumulated PBTs.
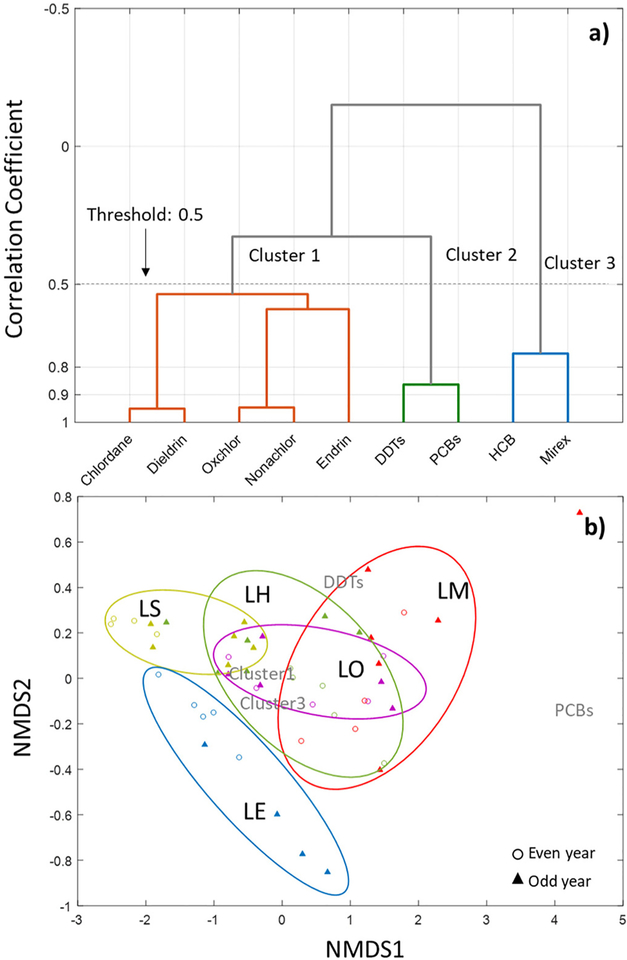
The bioaccumulation pattern of legacy contaminants in fish can be similar depending on specific chemical characteristics (Kelly et al., 2007; Paterson et al., 2016). Concentration correlations grouped the contaminants into three clusters (Fig. 2a): Cluster 1 contains chlordane, dieldrin, oxychlordane, nonachlor and endrin which are non-aromatic contaminants with average Kows ranging from 5.2 to 6.2; Cluster 2 contains t-PCBs and t-DDTs which both have benzene rings and have higher bioaccumulation potentials; note that the major DDT congeners observed in fish was p-p′ DDE (contributed 83% mass with log Kow =6.5), and the major PCB congeners observed in fish were hexa and hepta PCBs (contributed 60% of the mass with log Kows ranging from 7.3 to 8.3); Cluster 3 contains HCB and mirex that contain benzene rings and have lower bioaccumulation potential with log Kow ranging from 5.3 to 5.7 compared with Cluster 2 (log Kows are listed in ESM Table S3). Site specific contaminant concentration patterns are presented in NMDS plots (Fig. 2b), which indicates unique patterns for LE sites (no overlapping areas with other lakes) as two different fish species were included in the model for LE; for LS, most points are in the negative x-axis and for LM most points are in the positive x-axis indicating LS and LM have significantly different patterns.
Fig. 2.
a) Legacy contaminant clustering results based on concentration correlations (groups were separated when the correlation coefficient larger than 0.5). b) A non-metric multidimensional scaling (NMDS) plot comparing legacy contaminant patterns in each site. Note that panel a is clustered by contaminant and panel b is clustered by site.
Fish age trends
Significant increasing annualized age trends (%/yr) were found for LH (7.2% even year site; 9.9% odd year site), LM (4.8% even year site; 3.7% odd year site) and LS sites (8.3% even year site; 7.3% odd year site), indicating decreasing growth rates over time (ESM Fig. S2). Growth rate changes can be the result of food web perturbations and changing water temperatures. Invasive species, such as round goby and dreissenid mussels can reduce the prey energy density for lake trout (Barbiero et al., 2012; Evans et al., 2011) and the increasing temperatures can limit the growth rate of cold water species such as lake trout (Ng and Gray, 2011).
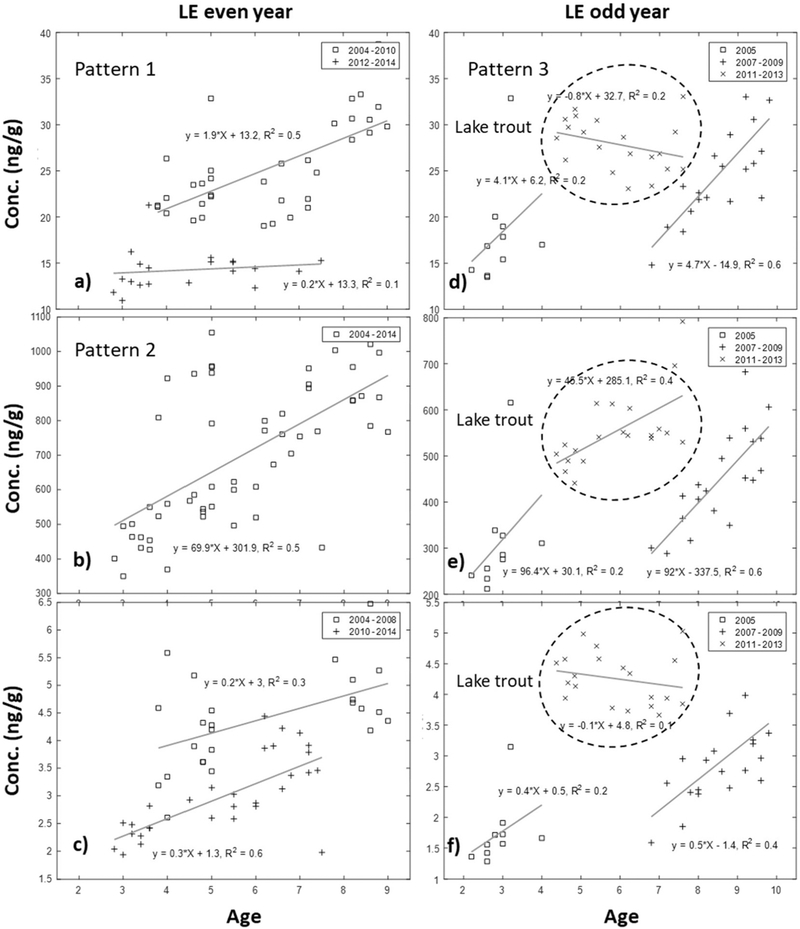
Three bioaccumulation patterns were found: 1) multiple clusters where the earlier years have higher concentrations and steeper slopes, and the recent years have lower concentrations with shallower slopes (Fig. 3a, c, and all the results in ESM Figs. S4 to S6); 2) single clusters where all the years have the same bioaccumulation rates (Fig. 3b) indicating that the bioaccumulation rate (age vs. conc.) did not change during the sampling period; and 3) negative correlation clusters with negatively correlated fish age and concentration (Fig. 3d and f). The bio-accumulation rates generally changed from greater bioaccumulation in earlier years to relatively lower bioaccumulation in later years indicating there was decreased transmission of contaminants from prey sources or a decrease in fish trophic status over this time period.(El-Shaarawi et al., 2011; Ng and Gray, 2011; Simoneau et al., 2005). When multiple bioaccumulation cluster patterns occurred, most clusters fall into three time-periods (ESM Figs. S4 to S6), an earlier period (2004–2005), a middle-period (2006–2010), and a current period (after 2011). Also, a negative correlation was found only for the LE odd year site implying growth dilution may be important. Note that the sampled fish species was changed from walleye to lake trout after 2010, so only limited data were available for each species.
Fig. 3.
Bioaccumulation patterns for the three clusters for the two LE sites (other sites are shown in the ESM Figs. S4, S5, S6). Three bioaccumulation patterns were found 1) the earlier years have higher concentrations and steeper slopes, while the current years have lower concentrations and flatter slopes; 2) all the years have the same bioaccumulation pattern; 3) a negative correlation between age and fish concentration. The equations shown were obtained from corresponding year groups and used for age normalization.
For fish species, dietary uptake of legacy contaminants remains the predominant route of exposure (Drouillard et al., 2009; Russell et al., 1999). The elimination of legacy contaminants through fecal egestion, respiratory, and metabolic biotransformation is negligible (Buckman et al., 2006; Drouillard et al., 2009; Paterson et al., 2010). For example in lake trout, PCB half-lives (around 15–30 years, and increasing in the recent years) generally exceed the trout’s lifespan (Carlson et al., 2010; Simoneau et al., 2005). In this case, a slower growth rate as seen in more recent years will lead to a lower bioaccumulation rate (Ng and Gray, 2011). Detailed age normalization clustering results and the equations used for age normalization including r2 values for each lake are presented in ESM Tables S4 and S5.
Overall concentration trends
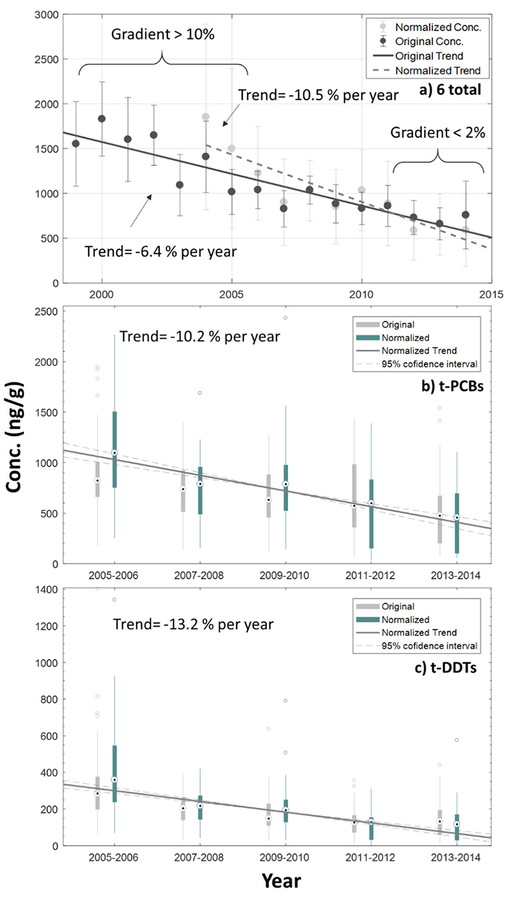
The overall trend in the GL region for the sum of the six legacy contaminants with long-term measurements (t-PCBs, t-DDTs, dieldrin, chlordane, oxychlordane, and nonachlor) is −6.4% per year (from 1999 to 2014) without age normalization, and −10.5% per year (from 2004 to 2014) with age normalization (Fig. 4a). However, smaller negative slopes were found in the current years (<2% after 2010 both in age normalized and non-normalized trends) compared to the earlier years (>10%).
Fig. 4.
(a) Long-term (1999–2014) trend for six primarily contaminants combined (t-PCBs, t-DDTs, dieldrin, chlordane, oxychlordane, and nonachlor) in GL lake trout (excluding walleye samples in Lake Erie) determined by fitting a linear equation. The upper and lower 95% confidence interval is −4% and −8.8% per year for the complete timeline, −5.1% and −15.9% per year for the trend after 2004. (b) t-PCBs and (c) t-DDTs. Overall Kendall-Theil trends after age normalization for t-PCBs and t-DDTs from 2004 to 2014. The upper and lower 95% confidence interval is −8.5% and −12.1% per year for t-PCB, and −11.3% and −15.1% per year for t-DDT.
Significant regional overall decreasing trends from 2005 to 2014 were detected for all the legacy contaminant concentrations after age normalization when examined individually (Table 1 and Fig. 4). LE data were not included in this analysis because walleye are collected in the western basin and lake trout became the primary collection species in the eastern basin in 2011. An overall trend was not reported for mirex as the overall percentage would be significantly impacted by the extreme LO mirex concentrations; 20 times higher than other sites. However, the mirex trends for individual sites are presented in the following section. DDTs had the fastest annual overall decreasing trend with a Sen’s slope showing an annual decrease of −13.2% per year (Kendall’s tau = −0.48, p < 0.01). For the other major contaminants, PCBs and nonachlor, the decreasing trend was −10.2% (Kendall’s tau = −0.40, p < 0.01), and −11.5% per year (Kendall’s tau = −0.52, p < 0.01), respectively. HCB has the smallest decreasing trends possibly due to HCB being near equilibrium in the environment, and/or its continued release as a byproduct from chemical manufacturing processes and waste incineration (Leger, 1991; Luscombe and Costner, 2001).
Table 1.
Overall Kendall’s tau coefficients and Kendall-Theil trends before and after age normalization for legacy contaminants in GL lake trout (excluding walleye samples in Lake Erie) from 2005 to 2014. A number in bold means the results are statistically significant.
| Legacy contaminants | Kendall’s tau coefficient before normalizationa | Kendall-Theil trend before normalization (percentage/year)b | Kendall’s tau coefficient after normalizationa | Kendall-Theil trend after normalization (percentage/year)b |
|---|---|---|---|---|
| PCBs | −0.39 | −7.9 (−6.4, −9.4) | −0.40 | −10.2 (−8.5, −12.1) |
| DDTs | −0.39 | −8.1 (−6.7, −9.6) | −0.48 | −13.2 (−113, −15.1) |
| Dieldrin | −0.36 | −7.6 (−6.1, −9.1) | −0.47 | −11.7 (−9.9, −13.6) |
| Endrin | −0.36 | −9.2 (−7.4, −11) | −0.46 | −10.5 (−8.6, −12.4) |
| Chlordane | −0.41 | −7.7 (−63, −9.1) | −0.48 | −11.4 (−9.8, −13.2) |
| Oxychlordane | −0.31 | −6.6 (−5, −8.2) | −0.53 | −12 (−10.5, −13.5) |
| Nonachlor | 0.01 | 0.1 (1.2, −0.9) | −0.52 | −11.5 (−9.9, −13.2) |
| HCB | −0.22 | −2 (−1.4, −2.7) | −0.21 | −3.7 (−2.4, −4.9) |
Kendall’s tau coefficient (from −1 to 1) indicating the slope of the trend.
Two numbers in parentheses indicate lower 95% and upper 95% slopes.
To present temporal trends independent of age bias, concentrations were normalized to a consistent age. This normalization will increase the concentration in younger fish and decrease the concentration in older fish. Therefore, as shown in Table 1, age normalization results in trends being detected now even though no trends were found in previous studies when age correction was not applied. These decreases in PBT concentrations could be attributed in part to successful management actions. For PCBs in particular, these include the remediation to AOCs via the Great Lakes Legacy Act (GLLA, since 2004) and GLRI (since 2010); completed and ongoing remediation efforts in 31 AOCs within the United States equate to over 3.5 million cubic yards of sediment remediated in the GL basin (USEPA, 2008, 2016).
Individual lake concentration trends
ANOVA analysis, which was used to determine if there was a difference between concentrations for the two sites in the same lake, indicates that only the two sites in LO can be combined together as they are not statistically different (ESM Table S4). For the other lakes, the sites were analyzed separately, which indicates the significant concentration difference between industry influenced and non-industry influenced areas. Only current trends (trends after the breakpoint if a breakpoint existed) are discussed here although for most contaminants for most sites (>90%) a breakpoint was not detected. Complete results are presented in ESM Table S6. Note that the LE odd year site was not included in the individual trend analysis, as the sampled fish species changed for this site from walleye 2005–2009 to lake trout after 2011–2013. A statistically significant contaminant concentration difference exists between these two species because the small sample size for eastern basin lake trout (<3 years of data) is not sufficient for trend analysis.
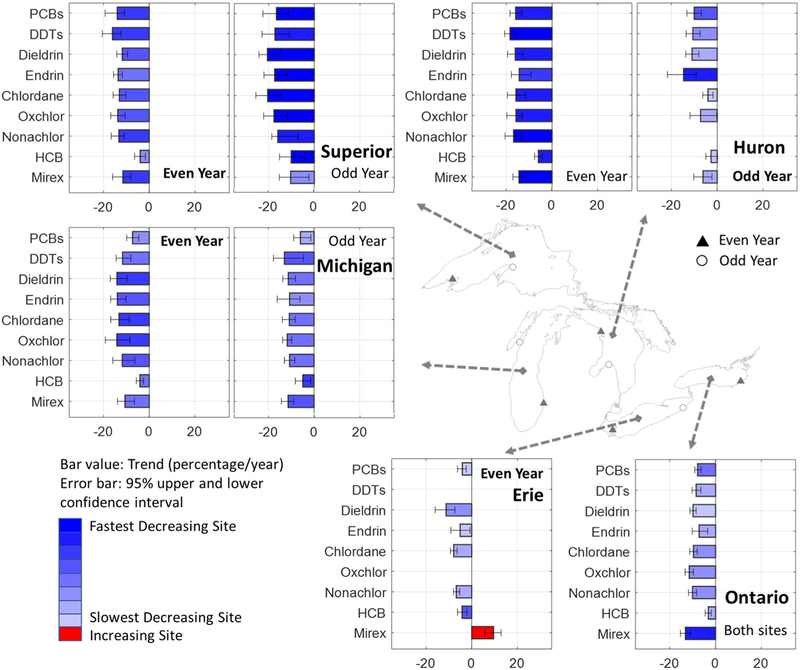
The current trends for all the PBTs in each site are shown in Fig. 5 and ESM Table S6. The sites sorted based on the rate of PCB decrease (fastest to slowest) is: LS non-industry site, LH non-industry influenced site, LS industry site, LH industry site, LO both sites, LM industry site, LM non-industry site, and LE industry site. The trend ranges for PBT declines for each site are: LS non-industry site (ranging from −10.1 to −20.5%), LH non-industry influenced site (ranging from −6.0 to −18.4%), LS industry site (ranging from −4.0 to −16.1%), LH industry site (ranging from −2.7 to −14.7%, no trend was found for nonachlor), LO both sites (ranging from −3.2 to −13.2%), LM industry site (ranging from −4.1 to −14.2%), LM non-industry site (ranging from −6.0 to −13.0%), and LE industry site (ranging from −4.1 to −11.2%, no trend was found for Oxchlor, +9.5% was found for mirex). Overall significant decreasing trends were found for most contaminants at most sites in the most recent period (after the breakpoint, if detected) except a significant increasing trend was detected for mirex at the LE even year (western basin, industry influenced) site where walleye were collected.
Fig. 5.
Kendall-Theil trends (bar) and confidence interval (error bar) after age normalization for legacy contaminants in GL region from 2004 (or after the break point) to 2014. A missing value indicates the most current trends are not significant. The bar values (x-axis) represent the Kendall-Theil trends (% per year), and the bar colors indicates the trends rank among the 10 sites. Note that a site name in bold font indicates the site with a more industrial influence for that lake. No trends are reported for LE odd year as not enough data were available (see text).
Similar to the contaminant concentrations, spatial gradients were also found in the trends results. Contaminants were decreasing faster in the west/north (sites in LS and even year site in LH) than in the east/south (sites in LO and LE). The east/south sites are generally closer to, and more often downwind of, urban areas (Evans and Muir, 2016; Khairy et al., 2014; Shunthirasingham et al., 2016) and impacted by direct effluent and non-point source inputs (Melymuk et al., 2014; Peverly et al., 2015). These spatial gradients imply that the decreasing rate is related to the distance from the population/industrial centers. LE and LO also have smaller surface areas, shallower depths, and shorter water residence times (Wang et al., 2012; Warner and Lesht, 2015), which makes them more sensitive to changing contaminant inputs and changes in lake trophic status (Hornbuckle, 2004; Liu et al., 2016). Our previous research on fish mercury concentrations found that LE and LO have increasing mercury trends even after fish age normalization, likely due to increasing local mercury inputs around LE and LO (Zhou et al., 2017). For the PBTs examined in this study, LO and LE had smaller decreasing trends than the other lakes suggesting that sources such as atmospheric deposition, watershed derived inputs (primarily from the Niagara river), increased sediment resuspension due to less ice cover and increasing storm intensity, and emissions from existing products and unintentional sources (for example electric arc furnace, cement kilns, or medical waste incineration) may be responsible (Gallistl et al., 2017; Liu et al., 2013; Marvin et al., 2004a; Marvin et al., 2002; Marvin et al., 2004b).
Trends of t-PCBs ratio
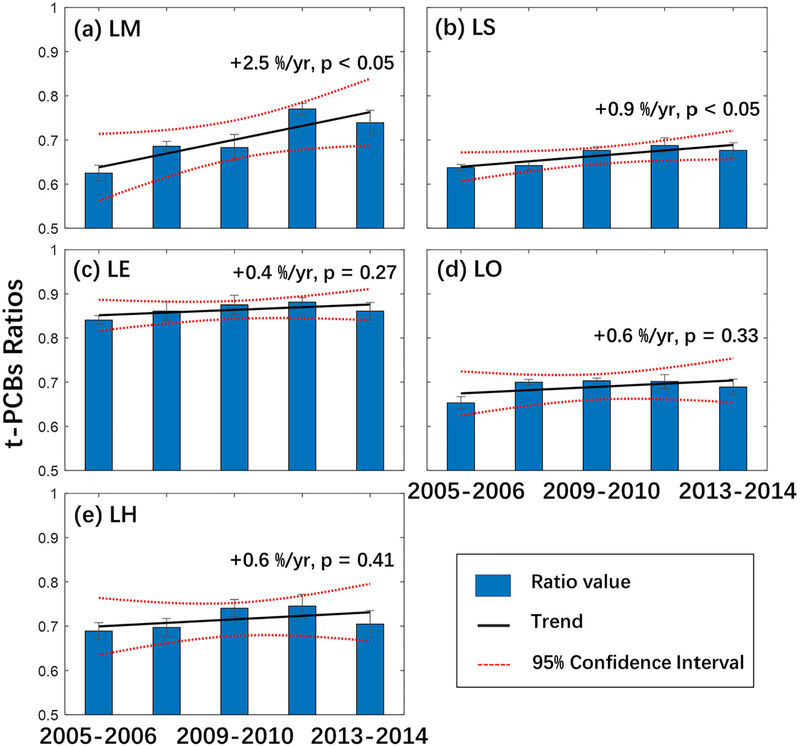
As t-PCBs had the highest concentrations and the second slowest decreasing trends among the PBTs in this study, trends of the ratio of t-PCBs to total legacy contaminants concentrations were also examined. Although the concentrations in even and odd year sites were different (ESM Table S4), sites in the same lake had similar patterns (Fig. 2b) therefore, the two sites in the same lake were combined before trends analysis (Zhou et al., 2017). As shown in Fig. 6, increasing trend ratios were found in all GLs ranging from +0.4%/yr to +2.5%/yr, and significant increasing trends were found in LM (+2.5%/yr) and LS (+0.9%/yr). t-PBCs have the slowest decreasing trend among these legacy contaminants except for HCB. The order of decreasing trends for the legacy contaminants (ESM Table S6, fastest to slowest based on median %/yr for each site, except for the LE odd year site) are endrin (−13.8% (−5.1% to −17.3%)), oxychlordane (−12.8% (no trend to −17.6%)), DDTs (−12.3% (no trend to −18.4%)), chlordane (−12% (−4.1% to −20.4%)), dieldrin (−11.6% (−9.9% to −20.5%)), nonachlor (−11.3% (no trend to −16.9%)), mirex (−11% (+9.5% to −10.1%)), t-PCBs (−9.0% (−4.1% to −16.6%)), and HCB (−4.2% (−2.7% to −10.1%)). t-PCBs currently contribute the most mass of all the PBTs (70–75%); this finding combined with the trend ratio results suggest that the importance of PCBs in the GLs relative to the other legacy contaminants will keep increasing into the future.
Fig. 6.
Trends of t-PCBs ratio (t-PCBs/total legacy contaminants) for each lake: a) LM with trend +2.5%/yr (p < 0.05), b) LS with trend +0.9%/yr (p < 0.05), c) LE with trend +0.4%/yr (p =0.27), d) LO with trend +0.6%/yr (p = 0.33), e) LH with trend +0.6%/yr (p = 0.41).
Conclusions
Significant decreasing concentration trends, ranging from −4.1% to −21.6% per year, were found for PBT contaminants, including polychlorinated biphenyls (PCBs), dichloro-diphenyl-trichlorethane (DDTs), dieldrin, endrin, chlordane, oxychlordane, nonachlor, hexachlorobenzene (HCB) and mirex, for most Great Lake (GL) sites after fish age normalization. The only exception to this finding was for mirex in Lake Erie walleye. These decreasing trends reflecting the successful historical and ongoing reduction of fugitive releases and remediation efforts in U.S. and Canada including physical removal (dredging) coupled with sediment sequestration. Spatial gradients are important both in the legacy contaminant concentrations and temporal trends, with contaminants decreasing faster and having lower concentrations in the west/north than in the east/south. This finding can be explained by the relative distance from population/industry influenced centers. The contaminants t-PCBs and HCB, which may be released from ongoing sources, including emission from existing products, byproducts from other chemical manufacture, wastes incineration, and unintentional sources, generally have slower decreasing trends. Total PCBs will continue to be of the greatest concern into the future since t-PCBs have largest mass and the second smallest decreasing trend for these legacy contaminants.
Supplementary Material
Acknowledgements
Funding for this work was provided by the Great Lakes National Program Office under the United States Environmental Protection Agency, grant nos. GL96594201 and GL00E00454. The views expressed in this publication are those of the author (s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. GLFMSP data is publicly available at https://www.epa.gov/great-lakes-legacy-act/great-lakes-environmental-database-glenda.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jglr.2018.03.007.
References
- ATSDR, A.f.T.S.a.D.R, September 2002. Public Health Statement for Hexachlorobenzene; (Accessed 2010.11.15). [Google Scholar]
- Barbiero RP, Lesht BM, Warren GJ, 2012. Convergence of trophic state and the lower food web in lakes Huron, Michigan and Superior. J. Great Lakes Res. 38, 368–380. [Google Scholar]
- Bhavsar SP, Jackson DA, Hayton A, Reiner EJ, Chen T, Bodnar J, 2007. Are PCB levels in fish from the Canadian Great Lakes still declining? J. Great Lakes Res. 33, 592–605. [Google Scholar]
- Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT, 2006. Bio-transformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol 78, 176–185. [DOI] [PubMed] [Google Scholar]
- Carlson DL, Vault DSD, Swackhamer DL, 2010. On the rate of decline of persistent organic contaminants in lake trout (Salvelinus namaycush) from the Great Lakes, 1970–2003. Environ. Sci. Technol 44, 2004–2010. [DOI] [PubMed] [Google Scholar]
- Chang F, Pagano JJ, Crimmins BS, Milligan MS, Xia X, Hopke PK, Holsen TM, 2012. Temporal trends of polychlorinated biphenyls and organochlorine pesticides in Great Lakes fish, 1999–2009. Sci. Total Environ. 439, 284–290. [DOI] [PubMed] [Google Scholar]
- Clement RE, Reiner EJ, Bhavsar SP, 2012. Organohalogen contaminants of emerging concern in Great Lakes fish: a review. Anal. Bioanal. Chem 404, 2639–2658. [DOI] [PubMed] [Google Scholar]
- Comba ME, Norstrom RJ, Macdonald CR, Kaiser KL, 1993. A Lake Ontario-Gulf of St. Lawrence dynamic mass budget for mirex. Environ. Sci. Technol 27, 2198–2206. [Google Scholar]
- Cornwell ER, Goyette J-O, Sorichetti RJ, Allan DJ, Kashian DR, Sibley PK, Taylor WD, Trick CG, 2015. Biological and chemical contaminants as drivers of change in the Great Lakes–St. Lawrence river basin. J. Great Lakes Res. 41, 119–130. [Google Scholar]
- Crane DP, Einhouse DW, 2016. Changes in growth and diet of smallmouth bass following invasion of Lake Erie by the round goby. J. Great Lakes Res. 42, 405–412. [Google Scholar]
- Dellinger JA, Moths MD, Dellinger MJ, Ripley MP, 2014. Contaminant trends in freshwater fish from the Laurentian Great Lakes: a 20-year analysis. Hum. Ecol. Risk Assess. Int. J 20, 461–478. [Google Scholar]
- Diamond ML, Melymuk L, Csiszar SA, Robson M, 2010. Estimation of PCB Stocks,Emissions, and Urban Fate: Will our Policies Reduce Concentrations and Exposure? ACS Publications [DOI] [PubMed] [Google Scholar]
- Drouillard KG, Paterson G, Haffner GD, 2009. A combined food web toxicokinetic and species bioenergetic model for predicting seasonal PCB elimination by yellow perch (Perca flavescens). Environ. Sci. Technol 43, 2858–2864. [DOI] [PubMed] [Google Scholar]
- El-Shaarawi AH, Backus S, Zhu R, Chen Y, 2011. Modelling temporal and spatial changes of PCBs in fish tissue from Lake Huron. Environ. Monit. Assess 173, 611–623. [DOI] [PubMed] [Google Scholar]
- Evans MS, Muir DC, 2016. Persistent organic contaminants in sediments and biota of Great Slave Lake, Canada: Slave River and long-range atmospheric source influences.J. Great Lakes Res. 42, 233–247. [Google Scholar]
- Evans MA, Fahnenstiel G, Scavia D, 2011. Incidental oligotrophication of North American Great Lakes. Environ. Sci. Technol 45, 3297–3303. [DOI] [PubMed] [Google Scholar]
- French TD, Petro S, Reiner EJ, Bhavsar SP, Jackson DA, 2011. Thirty-year time series of PCB concentrations in a small invertivorous fish (Notropis hudsonius): an examination of post-1990 trajectory shifts in the Lower Great Lakes. Ecosystems 14, 415–429. [Google Scholar]
- Gallistl C, Lok B, Schlienz A, Vetter W, 2017. Polyhalogenated compounds (chlorinated paraffins, novel and classic flame retardants, POPs) in dishcloths after their regular use in households. Sci. Total Environ. 595, 303–314. [DOI] [PubMed] [Google Scholar]
- Gewurtz SB, Shen L, Helm PA, Waltho J, Reiner EJ, Painter S, Brindle ID, Marvin CH, 2008. Spatial distributions of legacy contaminants in sediments of lakes Huron and Superior. J. Great Lakes Res. 34, 153–168. [Google Scholar]
- GLFMSP, 2004. Quality Assurance Project Plan for Sample Collection Activities. [Google Scholar]
- GLWQA, 2015. Public Comment Completed: Canada and the United States Release Draft Binational Summary Reports for Candidate Chemicals of Mutual Concern. [Google Scholar]
- Granato GE, 2006. Kendall–Theil Robust Line (KTRLine — version 1.0), a visual basic program for calculating and graphing robust nonparametric estimates of linear regression coefficients between two continuous variables. Techniques and Methods of the U.S. Geological Survey (book 4, Chap. A7, 31 pp.). [Google Scholar]
- Hornbuckle KC, 2004. Magnitude and origin of polychlorinated biphenyl (PCB) and dichlorodiphenyltrichloroethane (DDT) compounds resuspended in southern Lake Michigan. J. Geophys. Res 109. [Google Scholar]
- Huang YF, Puah YJ, Chua KC, Lee TS, 2015. Analysis of monthly and seasonal rainfall trends using the Holt’s test. Int. J. Climatol 35, 1500–1509. [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FA, 2007. Food web–specific biomagnification of persistent organic pollutants. Science 317, 236–239. [DOI] [PubMed] [Google Scholar]
- Kendall MG, 1948. Rank Correlation Methods. Griffin, Oxford, England. [Google Scholar]
- Khairy M, Muir D, Teixeira C, Lohmann R, 2014. Spatial trends, sources, and air-water exchange of organochlorine pesticides in the Great Lakes basin using low density polyethylene passive samplers. Environ. Sci. Technol 48, 9315–9324. [DOI] [PubMed] [Google Scholar]
- Leger D, 1991. Environmental Concentrations of Hexachlorobenzene in Atlantic Canada. Environment Canada, Conservation and Protection, Inland Waters Directorate, Atlantic Region, Water Quality Branch. [Google Scholar]
- Lepak RF, Krabbenhoft DP, Ogorek JM, Tate MT, Bootsma HA, Hurley JP, 2015. In-fluence of Cladophora-Quagga mussel assemblages on nearshore methylmercury production in Lake Michigan. Environ. Sci. Technol 49, 7606–7613. [DOI] [PubMed] [Google Scholar]
- Litaor MI, Barnea I, Reichmann O, Zohar I, 2016. Evaluation of the ornithogenic influence on the trophic state of East Mediterranean wetland ecosystem using trend analysis. Sci. Total Environ. 539, 231–240. [DOI] [PubMed] [Google Scholar]
- Liu G, Zheng M, Cai M, Nie Z, Zhang B, Liu W, Du B, Dong S, Hu J, Xiao K, 2013. Atmospheric emission of polychlorinated biphenyls from multiple industrial thermal processes. Chemosphere 90, 2453–2460. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang S, McDonough CA, Khairy M, Muir DC, Helm PA, Lohmann R, 2016. Gaseous and freely-dissolved PCBs in the Lower Great Lakes based on passive sampling: spatial trends and air-water exchange. Environ. Sci. Technol 50, 4932–4939. [DOI] [PubMed] [Google Scholar]
- Luscombe D, Costner P, 2001. Zero Toxics: Sources of By-product POP’s and Their Elimination. Stichting Greenpeace Council. [Google Scholar]
- Macksasitorn S, Janssen J, Gray K, 2015. PCBs refocused: correlation of PCB concentrations in Green Bay legacy sediments with adjacent lithophilic, invasive biota. J. Great Lakes Res. 41, 215–221. [Google Scholar]
- Mahmood M, Bhavsar SP, Arhonditsis GB, 2013. Fish contamination in Lake Erie: an examination of temporal trends of organochlorine contaminants and a Bayesian approach to consumption advisories. Ecol. Inf 18, 131–148. [Google Scholar]
- Marvin CH, Charlton MN, Reiner EJ, Kolic T, MacPherson K, Stern GA, Braekevelt E, Estenik J, Thiessen L, Painter S, 2002. Surficial sediment contamination in lakes Erie and Ontario: a comparative analysis. J. Great Lakes Res. 28, 437–450. [Google Scholar]
- Marvin CH, Painter S, Williams D, Richardson V, Rossmann R, Van Hoof P, 2004aSpatial and temporal trends in surface water and sediment contamination in the Laurentian Great Lakes. Environ. Pollut 129, 131–144. [DOI] [PubMed] [Google Scholar]
- Marvin CH, Sverko E, Charlton MN, Thiessen PL, Painter S, 2004b. Contaminants associated with suspended sediments in lakes Erie and Ontario, 1997–2000. J. Great Lakes Res. 30, 277–286. [Google Scholar]
- McGoldrick DJ, Murphy EW, 2016. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008–2012). Environ. Pollut 217, 85–96. [DOI] [PubMed] [Google Scholar]
- Melymuk L, Robson M, Helm PA, Diamond ML, 2013. Application of land use regression to identify sources and assess spatial variation in urban SVOC concentrations. Environ. Sci. Technol 47, 1887–1895. [DOI] [PubMed] [Google Scholar]
- Melymuk L, Robson M, Csiszar SA, Helm PA, Kaltenecker G, Backus S, Bradley L, Gilbert B, Blanchard P, Jantunen L, Diamond ML, 2014. From the city to the Lake: loadings of PCBs, PBDEs, PAHs and PCMs from Toronto to Lake Ontario. Environ. Sci. Technol 48, 3732–3741. [DOI] [PubMed] [Google Scholar]
- Ng CA, Gray KA, 2011. Forecasting the effects of global change scenarios on bioaccumulation patterns in great lakes species. Glob. Chang. Biol 17, 720–733. [Google Scholar]
- Paterson G, Liu J, Haffner GD, Drouillard KG, 2010. Contribution of fecal egestion to the whole body elimination of polychlorinated biphenyls by Japanese koi (Cyprinus carpio). Environ. Sci. Technol 44, 5769–5774. [DOI] [PubMed] [Google Scholar]
- Paterson G, Ryder M, Drouillard KG, Haffner GD, 2016. Contrasting PCB bioaccumulation patterns among Lake Huron lake trout reflect basin-specific ecology. Environ. Toxicol. Chem 35, 65–73. [DOI] [PubMed] [Google Scholar]
- Peverly AA, O’Sullivan C, Liu LY, Venier M, Martinez A, Hornbuckle KC, Hites RA, 2015. Chicago’s sanitary and ship canal sediment: polycyclic aromatic hydrocarbons, polychlorinated biphenyls, brominated flame retardants, and organophosphate esters. Chemosphere 134, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M, Melymuk L, Csiszar SA, Giang A, Diamond ML, Helm PA, 2010. Continuing sources of PCBs: the significance of building sealants. Environ. Int 36, 506–513. [DOI] [PubMed] [Google Scholar]
- Russell RW, Gobas FA, Haffner GD, 1999. Role of chemical and ecological factors in trophic transfer of organic chemicals in aquatic food webs. Environ. Toxicol. Chem 18, 1250–1257. [Google Scholar]
- Sadraddini S, Ekram Azim M, Shimoda Y, Mahmood M, Bhavsar SP, Backus SM, Arhonditsis GB, 2011. Temporal PCB and mercury trends in Lake Erie fish communities: a dynamic linear modeling analysis. Ecotoxicol. Environ. Saf 74, 2203–2214. [DOI] [PubMed] [Google Scholar]
- Shunthirasingham C, Gawor A, Hung H, Brice KA, Su K, Alexandrou N, Dryfhout-Clark H, Backus S, Sverko E, Shin C, Park R, Noronha R, 2016. Atmospheric concentrations and loadings of organochlorine pesticides and polychlorinated biphenyls in the Canadian Great Lakes Basin (GLB): spatial and temporal analysis (1992–2012). Environ. Pollut 217, 124–133. [DOI] [PubMed] [Google Scholar]
- Simoneau M, Lucotte M, Garceau S, Laliberté D, 2005. Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res 98, 73–82. [DOI] [PubMed] [Google Scholar]
- Stonevičius E, Valiuškevičius G, Rimkus E, Kažys J, 2014. Climate induced changes of Lithuanian rivers runoff in 1960–2009. Water Resour. 41, 592–603. [Google Scholar]
- Stow CA, Lamon EC, Qian SS, Schrank CS, 2004. Will Lake Michigan Lake Trout Meet the Great Lakes Strategy 2002 PCB Reduction Goal? ACS Publications [DOI] [PubMed] [Google Scholar]
- Sun P, Basu I, Blanchard P, Brice KA, Hites RA, 2007. Temporal and spatial trends of atmospheric polychlorinated biphenyl concentrations near the Great Lakes. Environ. Sci. Technol 41, 1131–1136. [DOI] [PubMed] [Google Scholar]
- USATSDR, Aug. 2015. Toxicological Profile FOR Hexachlorobenzene. [PubMed] [Google Scholar]
- USEPA, 2002. Great Lakes Strategy 2002. http://www.epa.gov/glnpo/gls/glstoc.html.
- USEPA, 2003. Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Endrin. [Google Scholar]
- USEPA, 2004. Quality Assurance Project Plan for Sample Collection Activities (Great Lakes Fish Monitoring Program). [Google Scholar]
- USEPA, 2008. Great Lakes Legacy Act. [Google Scholar]
- USEPA, 2010. Great Lakes Open Lakes Trend Monitoring Program: Mirex. https://www.epa.gov/great-lakes-monitoring/great-lakes-open-lakes-trend-monitoring-program-mirex.
- USEPA, 2012. Quality Assurance Project Plan for Sample Collection Activities (Great Lakes Fish Monitoring Program). [Google Scholar]
- USEPA, October, 2014. TSCA Work Plan for Chemical Assessments: 2014 Update. [Google Scholar]
- USEPA, 2016. Justification of Appropriation Estimates for the Committee on Appropriations. [Google Scholar]
- Wang J, Bai X, Hu H, Clites A, Colton M, Lofgren B, 2012. Temporal and spatial variability of Great Lakes ice cover, 1973–2010*. J. Clim 25, 1318–1329. [Google Scholar]
- Warner DM, Lesht BM, 2015. Relative importance of phosphorus, invasive mussels and climate for patterns in chlorophyll a and primary production in lakes Michigan and Huron. Freshw. Biol 60, 1029–1043. [Google Scholar]
- Wellenkamp W, He JX, Vercnocke D, 2015. Using maxillae to estimate ages of Lake Trout. N. Am. J. Fish Manag. 35, 296–301. [Google Scholar]
- Xia X, Hopke PK, Crimmins BS, Pagano JJ, Milligan MS, Holsen TM, 2012. Toxaphene trends in the Great Lakes fish. J. Great Lakes Res. 38, 31–38. [Google Scholar]
- Zananski TJ, Holsen TM, Hopke PK, Crimmins BS, 2011. Mercury temporal trends in top predator fish of the Laurentian Great Lakes. Ecotoxicology 20, 1568–1576. [DOI] [PubMed] [Google Scholar]
- Zhou C, Cohen MD, Crimmins BA, Zhou H, Johnson TA, Hopke PK, Holsen TM, 2017. Mercury temporal trends in top predator fish of the Laurentian Great Lakes from 2004 to 2015: are concentrations still decreasing? Environ. Sci. Technol 51, 7386–7394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.