Abstract
Until recently, most of energy and industrially produced chemicals were derived from fossil fuel-based resources. This along with the continued depletion of finite fossil resources and their attributed adverse environmental impacts, alternatively sourced and more sustainable resources are being pursued as feedstock replacements. Thus, biomass has been identified as an alternate renewable and more sustainable resource as a means to reduce this sector’s dependence on fossil fuel-based resources and to alleviate their environmental impacts. As such, lignocellulosic biomass has been further identified and demonstrated as an abundant renewable resource for the production of biofuels, platform chemicals, and their respective value-added products. This review article provides an overview of the techniques developed for the valorization of biomass in the production of platform chemicals within a biorefinery, and the status for commercialization.
Keywords: Biorefinery, biomass, platform chemicals, lignocellulose, gasification, fermentation
Summary:
As demonstrated and discussed, various platform chemicals can and are being produced in biorefineries and are utilizing a renewable biomass as a resource. This is exemplified by the industrial production of platform chemicals such as ethanol, lactic acid, succinic acid, levulinic acid, sorbitol, and 2,5-furandicarboxylic acid from biomass. In addition, several value-added chemical products and polymers produced in a biorefinery have been commercialized. However, still there are challenges in the valorization of lignocellulosic biomass to produce chemicals with high selectivity and yields. Various large chemical companies such as DuPont, BASF, and the Dow Chemical Company are actively pursuing valorization of lignocellulosic biomass. The production of renewable chemicals from biorefineries is expected to increase substantially in the coming years with an increased market share. Although chemicals and materials can be produced in a single product process in a biorefinery, the production in an integrated biorefinery producing both the bio-based products and energy such as fuel, power and heat will be a superior approach for the sustainable valorization of biomass. The biobased products produced in a biorefinery should be market competitive for their economic sustainability. Thus, to develop a sustainable biorefinery, it is critical to produce high value-added bioproducts along with bio-energies in an integrated biorefinery. The production of biobased chemicals in a biorefinery can significantly reduce the greenhouse gas emissions, thus stimulating innovative advancements in the area of biorefining processes and expanding the bio-based economy will lead to a more environmentally benign chemical manufacturing industrial sector.
Introduction:
Currently, the majority of chemicals and energy are produced from finite fossil fuel-based resources. The world’s societies and commercial markets are dependent on these depleting fossil fuels for the production of approximately 80% of its energy and 90% of its chemicals (Fernando et al. 2006). The large-scale production and use of these fossil fuels have contributed to negatively impacting the environment due to the emission of harmful greenhouse gases and toxic materials. With a growing world population, the demand for energy and chemicals is increasing considerably. Along with a growing world population, the corresponding amount of waste generated is also gradually increasing. In 2014, approximately 258 million tons of municipal solid waste (MSW) was generated in the United States alone. Approximately 35% of this MSW is recycled/composted, 13% of it is utilized for generation of energy, through incineration processes, and about 53% of MSW is discarded into landfills (U.S. EPA 2016). Because of the increasing demands for energy and chemicals and to overcome the issues associated with depleting fossil fuels and its related environmental impact, it is imperative to identify alternate resources to produce energy and chemicals. In this process, biomass has been identified as a renewable resource for the production of chemicals and energy, along with addressing the generation of waste issue. Although biomass is available from natural resources, alternatively it can be obtained from various waste sources such as agricultural waste (corn stover, rice husk, and sugar cane bagasse), municipal solid waste (MSW), and industrial waste, such as pulp and paper mills. Biomass waste valorization also has a very high importance in resource recovery within an integrated waste management approach. A biorefinery, which utilizes a renewable biomass as a feedstock resource, may offer a more sustainable solution for the conversion of harvested and waste biomass into platform chemicals (Esposito and Antonietti 2015). A biorefinery could also bring about sustainable growth, along with environmental advantages in the reduction of overall greenhouse gas emissions (NREL) and air toxics.
Biorefinery:
The concept of the biorefinery evolved during the late 1990’s. The US Department of Energy defines a biorefinery as an overall concept of a processing plant where biomass feedstocks are converted and/or extracted into a spectrum of valuable products (Kamm et al. 2006). According to National Renewable Energy Laboratory (NERL), a biorefinery integrates biomass conversion processes and equipment to produce power, chemicals, and fuels (NREL). The International Energy Agency (IEA) Bioenergy Task 42 has defined a biorefinery as “the sustainable processing of biomass into a spectrum of marketable products (food, feed, materials, chemicals) and energy (fuels, power, heat)”. Thus, a biorefinery can be a facility, a process, a plant, or a cluster of facilities for the conversion of biomass (de Jong et al. 2013). Furthermore, a biorefinery can be assimilated to a petroleum-based refinery. In petroleum-based refinery fossil-based resources such as oil and natural gas are used to produce energy and chemicals, whereas, in a biorefinery, biomass is used as the feedstock to produce energy and chemicals (Kamm and Kamm 2004; de Jong and Jungmeier 2015). To expand on this concept, biorefineries are further classified based on the type of feedstocks used, the type of intermediates generated (syngas or sugar), conversion processes (thermochemical, biochemical, two platform) and the status of technology execution (conventional, advanced, etc.). Thus, biorefineries are classified into three different types (Kamm and Kamm 2004; Clark and Deswarte 2008).
Phase I Biorefinery:
This type of biorefinery utilizes only one feedstock material, has fixed processing capability and produces a single primary product. Examples of this type of biorefinery are biodiesel from vegetable oil, pulp and paper mills, and the production of ethanol from corn grain (Naik et al. 2010).
Phase II Biorefinery:
Is similar to a Phase I Biorefinery and uses only one feedstock, but is capable of producing various products. Examples of Phase II Biorefineries are the production of various chemicals from starch and the production of multiple carbohydrate derivatives and bioethanol from cereal grains.
Phase III Biorefinery:
Phase III Biorefineries are advanced biorefineries and can utilize various types of feedstocks, processing technologies and produce multiple types of products. There are four classes of Phase III Biorefinery systems (Clark and Deswarte 2008). These are identified as: 1. Whole-crop biorefinery, 2. Green biorefinery, 3. Lignocellulosic biorefinery, 4. Two-platform concept biorefinery.
A whole-crop biorefinery uses an entire crop, such as cereal grains, as raw materials. In this class of biorefinery, a cereal grain crop is first separated into corn and straw, and the corn subsequently converted into starch. The starch upon hydrolysis provides glucose, which serves as a substrate (feedstock) for the production of various chemicals. The cellulosic straw can be processed in a lignocellulosic biorefinery. In a green biorefinery, natural biomass such as grass, green crops or plants are utilized as feedstocks. Whereas, in a lignocellulosic biorefinery the main feedstock is a dry biomass, such as cellulose-based biomass. Recently NREL has introduced the two-platform concept biorefinery. This type of biorefinery consists of two different types of platforms, i.e. sugar platform and syngas platform. The sugar platform employs biochemical conversion methodologies (fermentation of sugars obtained from biomass), whereas the syngas platform depends on thermochemical methods (gasification of biomass to produce syngas). Both of these platforms can produce energy and chemicals as the end products.
A biorefinery can be further classified, based on the chemical nature (composition) of feedstock, into three categories (Octave and Thomas 2009).
1. Triglyceride Biorefinery (TGB):
This biorefinery utilizes vegetable oils, animal fats, oil from algae, and waste cooking oil as feedstocks, and has received considerable attention due to the scientific advancements made in the production of biofuels. Triglycerides are converted into a biofuel via a transesterification reaction with methanol in the presence of an acid, base, or enzyme acting as a catalyst. In this reaction, glycerol is formed as a byproduct, which is also considered as a platform chemical, and has the potential of adding commercial value to this process.
2. Sugar and Starchy Biorefinery (SSB):
This biorefinery for sugar and starch is also well recognized due to the well-developed and executed fermentation process for sugar and starch into the production of ethanol (Serrano-Ruiz and Dumesic 2011). Feedstocks for a SSB are sugar beet, sugar cane, wheat, corn, and maize. In the fermentation process, the starch is first enzymatically degraded into its respective monomer sugars and then the resulting mixture is fermented by Saccharomyces cerevisiae at ambient temperature to produce ethanol (Naik et al. 2010). Currently, ethanol accounts for 94% of global biofuel production (Demirbas 2011).
3. Lignocellulosic Biorefinery (LCB):
The LCB utilizes a lignocellulosic-based feedstock (LCF) and can produce a wide spectrum of products through various processing approaches. Lignocellulosic feedstocks (LCF) include wood, straw, grasses, etc. and LCFs are composed of 40-50% of cellulose, 25-30% hemicellulose and 15-20% lignin (Alonso 2010). The chemical composition of LCFs also varies considerably, depending on its source (Jorgensen et al. 2007). Cellulose is a high molecular weight linear polymer composed of β-glucose (5000-10000 units) and linked by β-1,4-glycosidic bonds. It is a highly crystalline material, and because of this crystallinity, not soluble in water. This attribute makes it challenging to convert into monomer sugars through hydrolysis (Brethauer and Studer 2015). When hydrolyzed cellulose yields D-glucose, which acts as a substrate for the production of chemicals. Hemicellulose is an amorphous branched polymer and contains both C5 (xylose, arabinose, and rhamnose) and C6 sugars (glucose, mannose, and galactose), as well as uronic acids components. Hemicellulose contains approximately 150 repeating monosaccharide units, and the type of monomer sugars present varies depending on the type of material. For example, in hardwood and agricultural plants, hemicellulose contains mainly xylan, (Maki-Arvela et al. 2011), a polymer of xylose, whereas in softwood hemicellulose its main building block is glucomannan, a polymer of D-mannose and D-glucose linked by β-1,4-glycosidic bonds. Hemicellulose is amorphous in nature, highly soluble in water, and thus readily hydrolyzes to the corresponding monomer sugars as opposed to cellulose.
International Energy Agency (IEA) Biorefinery Classification:
In 2008, the IEA Bioenergy Task 42 developed a biorefinery classification system based on feedstocks, biorefinery platform, products, and processes (Cherubini et al. 2009, de Jong et al. 2013). The biorefinery feedstocks include: grasses, starch crops (wheat and maize), sugar crops (beet and cane), lignocellulosic crops, lignocellulosic residues (stover and straw), oil crops, aquatic biomass (algae and seaweeds) and organic residues (industrial, commercial and post-consumer waste). The IEA classified the processes used in a biorefinery into four groups. 1. Mechanical/Physical, such as pre-treatment, milling, pressing, separation and distillation, which perform size reduction or a separation of feedstock components without effecting the nature of chemical components of the biomass. 2. Biochemical, those processes carried out by enzymes or microorganisms, such as fermentation, anaerobic digestion, etc. 3. Chemical, hydrolysis, synthesis, hydrogenation, oxidation, etc. and 4. Thermochemical, where feedstocks are subjected to very high temperature and/or pressure, such as gasification, hydrothermal upgrading, and pyrolysis.
These biomass feedstocks can be processed into a variety of biorefinery platforms, which are the key intermediates linking the feedstocks and their respective final product(s). Examples of important platforms in the energy sector are syngas from gasification, biogas from anaerobic digestion, C5 and C6 sugars from starch, cellulose and hemicellulose, lignin from lignocellulosic biomass, pyrolysis liquid from pyrolysis, oil from oilseed crops and algae, organic juice from wet biomass, and electricity and heat. These platforms are then further transformed into a variety of products using a thermal, biological or chemical process, or a combination of these processes. Based on the type of products produced, biorefineries are classified into energy-driven or material-driven biorefinery systems. In an energy-driven biorefinery system, biomass is used mainly for the production of biofuels, power and heat. Whereas, in a material-driven biorefinery system biobased products such as food, feed, chemicals, biomaterials, etc are produced. The process residues, in both systems, can be further utilized to produce energy, thus minimizing waste generation
A few examples of this IEA biorefinery classification system are:
One-platform C6 sugar biorefinery for the production of bioethanol and animal feed from corn crops.
One-platform syngas biorefinery for biofuels and chemicals generation from lignocellulosic residues.
Two-platform (biogas and organic juice) biorefinery for biomethane, chemicals, biomaterials (fiber products) and fertilizer from grasses.
Four-platform (lignin/syngas, C5/C6 sugar) biorefinery for liquid biofuel, bioethanol, and animal feed from lignocellulosic crop such as switchgrass.
Valorization of Lignocellulosic Biomass in a Biorefinery
In the valorization of a lignocellulosic biomass, the first step is pretreatment, which aids in the isolation of the cellulose, hemicellulose and lignin components. Biorefineries, which utilize polysaccharides from cellulose/hemicellulose portion of lignocellulosic biomass to produce platform chemicals and materials, generate a substantial amount of lignin as a by-product. Many of the traditional biorefineries, which use polysaccharides, have utilized the resulting byproduct lignin for generation of the power that is needed to achieve the transformation of the biomass. However, there are efforts underway for the valorization of lignin for the production of potential high-value products such as carbon fiber, engineered plastics, thermoplastics elastomers, polymeric foams, membranes and a variety of aromatic chemicals (Ragauskas et al. 2014). These achievements are focused on the valorization of the cellulose and hemicellulose portions of lignocellulosic biomass in a biorefinery to produce platform chemicals and their high value-added products.
Pretreatment of Lignocellulosic Biomass
Conversion of biomass into higher-value chemicals in a biorefinery involves the hydrolysis of lignocellulose to fermentable sugars. However, before this can occur, a biomass pretreatment step is needed to facilitate the efficient hydrolysis of cellulose into its respective monomer sugars (Agbor et al. 2011). The hydrolysis step is carried out using acids or enzymes, followed by the fermentation process being done by either a bacteria or yeast. Various factors can influence the hydrolysis of cellulose, which include the porosity of the biomass material, crystallinity of the cellulose and the content of hemicellulose and lignin (McMillan 1994). The presence of hemicellulose and lignin hinders accessibility of the enzyme and/or acid in reaching the cellulose and thus limits the extent of the hydrolysis process. Pretreatment will help alter the size, structure and chemical composition of biomass, which enhances the hydrolysis process produce monomeric sugars in high yields. There are several types of pretreatment methods. These include physical (milling, grinding etc.), chemical (steam explosion and ammonia fiber explosion), physicochemical (acid pretreatment, alkali pretreatment, oxidative methods and organosolve methods), biological, or a combination of these methods. These pretreatment methods were reviewed by Kumar et al. (2009), and Singh et al. (2015) and provide insight into the different types of lignocellulosic biomass and the requirements of each with varying pretreatment processes.
Platform Chemicals:
A platform chemical is defined as a chemical that can serve as a substrate for the production of various other higher value-added products. In 2004, the Department of Energy (DOE) identified 12 chemical building blocks, that can be obtained from biomass, as potential platform chemicals (Werpy et al. 2004). In 2010, the DOE updated the Platform Chemical List (Bozell and Petersen 2010) which includes ethanol, furfural, hydroxymethylfurfural, 2,5-furandicarboxylic acid, glycerol, isoprene, succinic acid, 3-hydroxypropionic acid/aldehyde, levulinic acid, lactic acid, sorbitol, and xylitol. All these identified platform chemicals, except glycerol and isoprene, can be produced from biomass-derived carbohydrate sources. According to McKinsey & Co. (BIO 2016) estimates, bio-based product sales in 2012 were $252 billion and renewable-based chemical sales were approximately 9% of worldwide chemical sales and are expected to grow by 4% annually. The bio-based product sales are expected to increase with an annual growth rate of 8% to $375 to $441 billion by 2020.
Ethanol:
There are two different types of processes employed to produce ethanol from lignocellulosic biomass. The first utilizes a thermal gasification process to generate syngas, which is then converted to ethanol by either chemical catalytic methods or biochemical fermentative methods. The second is based on the biochemical fermentation process.
Gasification:
Gasification is the process of converting an organic mass into a high-energy gas via partial oxidation at a temperature ranging from 500-800°C (Arena 2012). There are three steps in the conversion of biomass to syngas, pre-treatment of feedstock, gasification, and syngas cleaning/conditioning. Syngas consists of a mixture of carbon monoxide (CO) and hydrogen (H2). Raw syngas also contains some other impurities such as CO2, tars and a small amount of other gases such as methane, ethane, oxygen, ammonia, HCl (hydrochloric acid) and H2S (hydrogen sulfide) (McKendry 2002). Since the impurities present in the syngas can affect process efficiency, it is necessary to purify prior to its conversion into ethanol. Initially, particulates from syngas are removed by cyclone separators. If any tars are present in the raw syngas, they can be decomposed by catalytic steam reforming utilizing Ni-based catalysts (Magrini-Bair et al. 2012). The purified syngas can then be converted into ethanol via two different methods. The first is based on a catalytic chemical process (Subramani and Gangwal 2008) and the second based on a biological fermentation process (Abubackar et al. 2011; Mohammadi et al. 2011). The catalytic chemical conversion of syngas into ethanol has been carried out utilizing various homogeneous (Co, Ru, and Rh) metal complexes and heterogeneous catalysts (Rh, Cu, Mo, Ni-based, Hayes 2009). For the biological-based conversion of syngas into ethanol microbial fermentation is used. The gas fermentation process is a hybrid thermochemical/biochemical process where syngas can be generated by the gasification process, which is then converted into ethanol via a microbial reaction (Mohammadi et al. 2011). This fermentation of syngas takes place at ambient temperatures and results with very high selectivity to ethanol. Although this process is slow when compared to chemical catalytic process, it’s very high specificity results in a higher yield of the desired product.
Biochemical Fermentation:
This process is comprised of three steps. 1. Pretreatment, 2. Saccharification and 3. Fermentation (Sarkar et al. 2012).
Pretreatment:
This is an important aspect in the processing of biomass for the production of ethanol. As described earlier, the pretreatment process aids in increased susceptibility of the biomass to hydrolysis and results in generating higher yields of monomer sugars.
Saccharification:
In this second step, complex carbohydrates are converted into simple monomers by hydrolysis using cellulase and hemicellulase enzymes (Naik et al. 2010; Talebnia et al. 2010). The hydrolysis of the cellulose starting material yields glucose, whereas the hydrolysis of hemicellulose generates several isomers of pentoses and hexoses.
Fermentation:
The biomass sugars obtained upon saccharification are then used for fermentation by various microorganisms. An ideal microorganism for the commercial production of ethanol should be capable of utilizing various types of sugars which can be used to produce ethanol in high yields (Talebnia et al. 2010). Since naturally occurring microorganisms cannot ferment both pentose and hexose sugars, genetically modified microorganisms are needed to ferment these sugars completely, thus providing higher yields of ethanol. There are two types of processes normally employed in this fermentation. In the first type, simultaneous saccharification and fermentation (SSF) occurs and in the second type being the separate hydrolysis and fermentation (SHF) processes. Although the SHF process is traditionally utilized, the SSF process is found to be superior for producing ethanol in higher yields, as well as eliminating the need for separate reactors. To date, several companies are adopting the biochemical method for production of bioethanol (Schwab et al. 2016). Although most of the bioethanol produced in the US is used as a fuel additive, a small percentage is also used as a platform chemical for production of higher value-added products. Bioethanol can serve as a renewable source for production of ethylene, propylene, and butadiene, which are critical building blocks for polymer synthesis. Dow, Solvay, and Braskem have built plants to convert bioethanol to ethylene. With Dow and Braskem then converting the ethylene into green polyethylene (320,000 tons/year, 180,000 tons/year respectively); whereas Solvay uses bio-based ethylene for the production of polyvinyl chloride, 55,000 tonnes/year. Additionally, bioethanol can be readily converted into other commodity chemicals such as acetaldehyde, and acetic acid.
Furfural:
Furfural is produced in a lignocellulosic biorefinery from various renewable agricultural resources (Dutta et al. 2012). Worldwide production of furfural is estimated to be approximately 300,000 tonnes/year. Bio-based furfural is commercially produced via the acid catalyzed dehydration of xylose, (Fig.1, Gravitis et al. 2001). First, C5 polysaccharides present in biomass hemicellulose are hydrolyzed by mineral acids to produce monosaccharides, primarily xylose, which is then converted into furfural by an acid catalyzed dehydration (Machado et al. 2016, Verma et al. 2017). Furfural production from corn stalk, sugarcane bagasse and eucalyptus wood using varying concentrations of mineral acids has also been reported (Barbosa et al. 2014). Several solvents, such as ionic liquids, organic solvents and supercritical fluids were investigated in the monophasic system, whereas water/organic solvent mixtures were tested in a biphasic system (Mariscal et al. 2016). Among the organic solvents tested in a biphasic system, a renewable solvent 2-methyltetrahydrofuran, was found to increase the selectivity and yield of furfural.
Fig. 1.
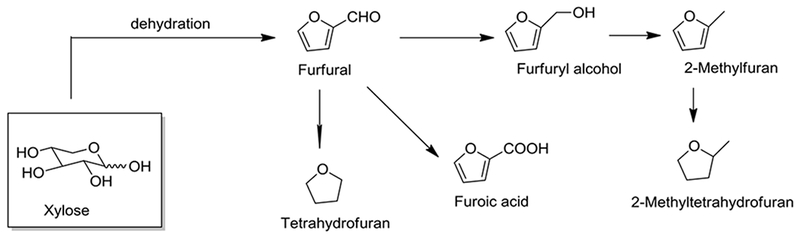
Synthesis of Furfural and its derivatives
Approximately 70% of furfural manufactured is a chemical feedstock for the production of furfuryl alcohol (Yan et al. 2014). In addition, furfural can also be transformed into other 5-membered oxygen-heterocycles, succinic acid and levulinic acid (Mariscal et al. 2016). Furfural has been extensively used in plastics, pharmaceutical and agrochemical industries, adhesives and flavor enhancers.
Hydroxymethylfurfural (HMF):
The production of HMF from mono- and polysaccharides and pretreated biomass is reported (Rout 2016) using various acid catalysts (Fig. 2, Dutta et al. 2012). HMF is produced in high yields by the acid catalyzed dehydration of fructose (Verma et al. 2017). Various organic solvents have been evaluated for production of HMF, and among the solvents tested dimethyl sulfoxide (DMSO) was found to be most suitable for conversion of sugars into HMF (Esposito and Antonietti 2015). However, the high boiling point of dimethyl sulfoxide poses a challenge in the separation of the product.
Fig. 2.
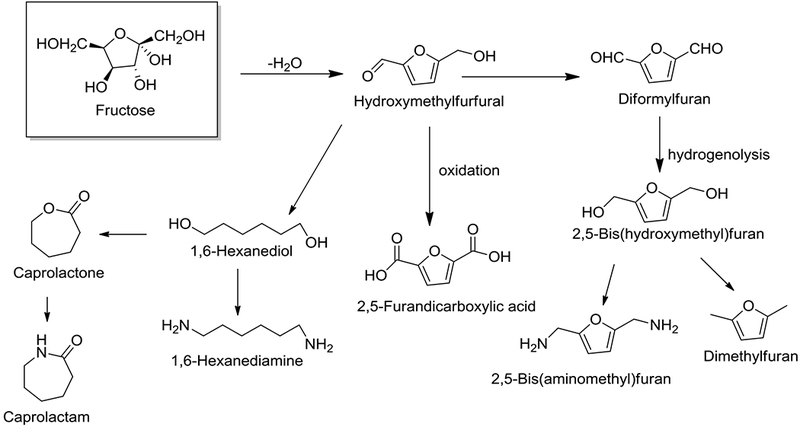
Synthesis of Hydroxymethylfurfural and its derivatives
Water has been tested as a reaction medium in the presence of a variety of acid catalysts. When water was used as a solvent, the product yield was found to be only about 50% (Wang et al. 2014). This is attributed to problems associated with the degradation of the product in water monophasic solvent system. To overcome this effect, biphasic solvent systems have been explored. In a biphasic system, the product formed is continuously extracted into the organic layer, thus avoiding any degradation of the desired HMF product (Saha and Abu-Omar 2014). Additionally, the biphasic solvent system helps in facilitating separation of the product from the reaction medium, improving HMF selectivity and the yield of the product. HMF can then be converted into other value-added polyester building blocks (Fig. 2) (Dutta et al. 2012; Isikgor and Becer 2015) such as 2,5-furandicarboxylic acid (FDCA), and 2,5-bis(hydroxymethyl)furan, and potential biofuels 2,5-dimethylfuran, 5-ethoxymethylfurfural, ethyl levulinate, and γ-valerolactone (Li et al. 2016; van Putten et al. 2013). HMF can also be converted to 1,6-hexanediol (1,6-HDO), which is used in the preparation of polycarbonatediols for production of polyurethanes for use in coatings, elastomers, and adhesives. In addition, 1,6-HDO can be converted to 1,6-heaxanediamine and ε-caprolactone, which are used in the synthesis of various polymers (Isikgor and Becer 2015).
2,5-Furandicarboxylic acid (FDCA):
FDCA has been extensively investigated due to its potential as a substitute for petrochemical-based adipic acid and terephthalic acid. FDCA is produced by the oxidation of HMF in high yields (Fig. 2, Rass et al. 2013) utilizing various homogeneous and heterogeneous systems (van Putten et al. 2013; Chatterjee et al. 2015). The synthesis of FDCA is also accomplished by starting from fructose in a two-step dehydration followed by oxidation, without needing to isolate the HMF intermediate. FDCA has many potential applications in polyesters, polyamides, and plasticizers. It has also been demonstrated polyethylene furanoate (PEF) polymers produced from FDCA and ethylene glycol have similar physical, chemical and mechanical properties of the petroleum-based polymers polyethylene and terephthalate (Isikgor and Becer 2015). Avantium is operating a pilot plant for production of FDCA and polyethylene furanoate polymers, and has formed a joint venture with BASF to build a commercial production plant with an annual capacity of up to 50,000 metric tons/year (Avantium 2016). Dupont and Archer Daniels Midland (ADM) have developed a high yielding process for production of FDCA methyl ester directly from fructose (DuPont press release 2016).
Glycerol:
Although microbial production of glycerol has been known for more than 100 years, recent developments in the biodiesel industry have led to the production of vast amounts of glycerol. Approximately 10% (w/w) of glycerol is formed during the biodiesel manufacturing process, this has led to about 90% of total glycerol produced. (Okoye and Hameed 2016). In 2012, glycerol production was estimated to be at more than 2 × 106 tons and expected to increase its growth by approximately 6% a year between 2012 and 2018 (Transparency Market Research). In terms of revenue, its demand is predicted to reach $2.1B by 2018. Because of its ready availability and its potential to act as a primary building block in a biorefinery, glycerol has received significant attention in its conversion to the higher value-added chemicals (Schultz 2014; Zheng et al. 2008). One such method is the catalytic hydrogenation of glycerol in the presence of Ru/C or Pt/C to provide ethylene glycol, propylene glycol (Dasari et al. 2005; Nakagawa et al. 2014), and acetol (Chiu et al. 2006). When the corresponding reduction of glycerol was carried out, in the presence of hydroxide bases, lactic acid is produced as the primary product (Maris et al. 2007).
Archer Daniels Midland (ADM), Dow Chemical Company (Dow press release 2007), Huntsman (Huntsman news 2006), and Cargill (Cargill 2006) are producing renewable propylene glycol from glycerin. The use of glycerol as a feedstock (Fig. 3) in biochemical transformations for production of 1,3-propanediol (1,3-PDO) is being explored by various research groups (Yang et al. 2012). Fermentation of glycerol with genetically modified Clostridium acetobutylicum provides a higher yield of 1,3-PDO (Gonzalez-Pajuelo et al. 2006). Glycerol is also utilized in the production of 1,2-propanediol by an Escherichia coli fermentation process (Altaras and Cameron 1999). Another derivative of glycerol is glycerol carbonate which is produced either by reacting glycerol with urea or ethylene or propylene carbonate (Okoye 2016) or carbon dioxide (Ma et al. 2012). Glycerol carbonate has a wide range of applications in the synthesis of industrially important chemicals such as glycidol, and in polymers, coatings, adhesives and lubricants. Glycerol has also been utilized in the commercial production of epichlorohydrin. Glycerol is reacted with 2 equivalents of HCl in the presence of acetic acid and primarily forms 1,3-dichloro-2-propanol, which on a further base-catalyzed cyclization provides the product with the elimination of NaCl (Santacesaria et al. 2010). Solvay and Dow Chemical Company (Bell et al. 2008) are manufacturing epichlorohydrin utilizing a similar process. This process is found to be superior when compared to traditional manufacturing methods and produces less chlorinated waste and uses 90% less water overall. When glycerol is subjected to the selective oxidation of the primary hydroxyl groups, the commercially useful compounds glyceraldehyde (Kim et al. 2014), glyceric acid (Kondamudi et al. 2012) and tartronic acid (Behr et al. 2008) are obtained. Oxidation of secondary hydroxyl group provides dihydroxyacetone (DHA), while oxidation of all three hydroxyl groups yields ketomalonic acid (Ciriminna and Pagliaro 2003; Pagliaro et al. 2007; Gil et al. 2014). Glycidol, another glycerol derivative, has the enormous potential for production of other industrially valuable chemicals, epoxy resins, polyurethanes and polyglycerol esters. A bio-based process for the synthesis of glycidol from glycerol has been recently reported (Bai et al. 2013). Glycerol can be converted into polyols and various organic acids by yeast and filamentous fungi fermentation. The anaerobic fermentation of glycerol to various alcohols and acids has been reviewed by Clomburg and Gonzalez (2013). Similarly, 1-butanol, 2,3-butanediol, 1,3-propanediol, ethanol, lactic acid, succinic acid, propionic acid, and dihydroxyacetone can be produced utilizing various microorganisms (Almeida et al. 2012; Abad and Turon 2012).
Fig. 3.

Glycerol as a Platform chemical
Succinic acid:
The synthesis of succinic acid is traditionally accomplished by the hydrogenation of petroleum-based maleic acid or via the oxidation of butanediol. However, recently some companies have started manufacturing succinic acid via the biochemical fermentation of biorefinery sugars. Roquette/DSM (Press release 2008), as well as Bioamber (Press release 2015) are producing succinic acid at the commercial scale by the E. coli fermentation of glucose. Succinic acid, as a platform chemical (Fig. 4), can be converted into succinate esters, which are precursors for 1,4-butanediol, tetrahydrofuran, and γ-butyrolactone (Delhomme et al. 2009; Luque et al. 2009). Dehydrogenative cyclization of succinic acid provides succinic anhydride, which acts as a key starting material for production of fumaric acid and maleic acid (Delhomme et al. 2009). NatureWorks and Bioamber formed an alliance to explore the production of completely bio-renewable polyester copolymers of succinic acid and 1,4-butanediol (Adkins et al. 2012). Among various polyesters that can be prepared, poly(ethylene succinate) (PES), poly(propylene succinate) (PPS), poly(butylene succinate) (PBS) are widely studied, with PES and PBS having been successfully commercialized ((Isikgor and Becer 2015). The market value of succinic acid in 2013 was approximately $115 million and it is expected to reach to $1.1 billion by the year 2020.
Fig. 4.
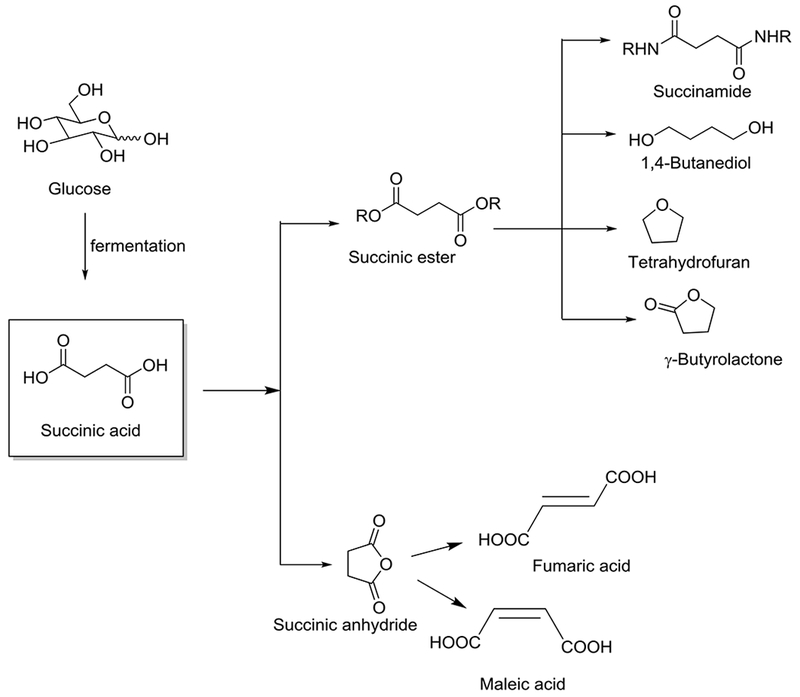
Synthesis of Succinic acid and its value-added products
Lactic acid:
Lactic acid (LA) is commercially produced via the fermentation of various sugars such as glucose, sucrose, or lactose (Datta and Henry 2006; John et al. 2007; Ghaffar et al. 2014). Corbion manufactures L-lactic acid by fermenting carbohydrates (Corbion). The yield of calcium lactate produced in the fermentation process is approximately 90%, which upon neutralization provides lactic acid. The neutralization process produces one equivalent of CaSO4, which poses a disposal problem. To overcome the issue of neutralization other technologies such as nanofiltration and ion-exchange resins have been evaluated (Ghaffar et al. 2014; Datta and Henry 2006). Recently other processes have been developed to convert xylose to lactate using engineered yeast (Ilmen et al. 2007). In addition, Direvo industrial biotechnology has recently produced lactic acid on a pilot scale directly from lignocellulose in a single-step utilizing a consolidated bioprocessing technology (Direvo press release 2013). Lactic acid global production is approximately 350,000 tons/year and is expected to grow considerably in the next decade. Lactic acid (Fig. 5) upon esterification provides lactate esters, which can be used as green solvents (Pereira et al. 2011). Reduction of lactic acid provides propylene glycol, which on dehydration yields propylene oxide. Dehydration of lactic acid affords acrylic acid and esters; whereas hydrogenolysis of the secondary hydroxyl group yields propanoic acid (Maki-Arvela et al. 2014). Lactic acid can also be converted into a biodegradable polylactic acid either by polymerization of lactic acid or its cyclic dimer lactide (Maki-Arvela et al. 2014, Yao and Tang 2013). The polylactic acid polymer exhibits performance properties similar to polystyrene or polyethyleneterephthalate with excellent barrier properties for flavors and good heat stability. Due to its low price and availability, polylactic acid has an extremely high potential among the biodegradable polymers (Erickson et al. 2012). There are several companies that are currently manufacturing polylactic acid at commercial scale, with most of the polylactic acid produced being used in the packaging market and textiles (Isikgor and Becer 2015).
Fig. 5.
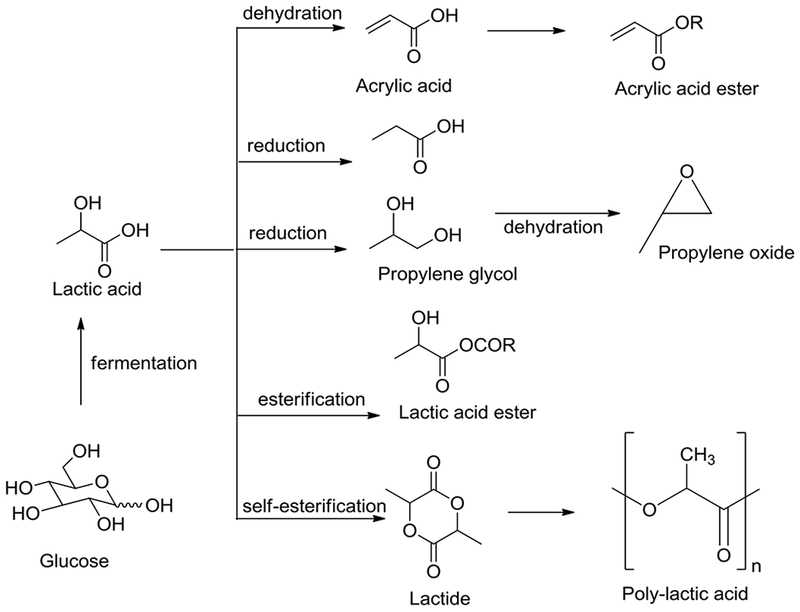
Synthesis of lactic acid and its value-added products
Levulinic acid:
Levulinic acid is produced in high yield by the acid-catalyzed hydrolysis of C6 sugars. Recently, the large-scale production of levulinic acid from lignocellulosic feedstock has been demonstrated (Pileidis and Titirici 2016). Maine Bioproducts utilizes an acid catalyzed dehydration of lignocellulosic feedstock (Rose and Palkovits 2011) using a two-stage process for production of levulinic acid. GF Biochemicals has started commercial production of levulinic acid from cellulosic biomass in 2015 using their proprietary technology (GF Biochemicals press release 2015).
Levulinic acid acts as a building block in many applications such as pharmaceuticals, plasticizers, fragrances and cosmetics. Avantium has developed a process for the synthesis of levulinic acid methyl ester starting from plant carbohydrates (Avantium). Several other companies have also demonstrated the production of levulinic acid based bio-polymers. For example, Segetis (acquired by GF Biochemicals in 2016) investigated the use of levulinic acid based ketals in polyurethane and thermoplastic applications (Rose and Palkovits 2011). Levulinic acid also has the potential for substituting petroleum-based chemicals. For example, levulinic acid derived diphenolic acid (DPA) (Guo et al. 2008) can serve as a substitute for bisphenol-A (BPA) in food containers and consumer products. In addition, levulinic acid can also be converted into various higher value-added products (Fig. 6, Pileidis and Titirici 2016) such as levulinic acid esters, 5-aminolevulinic acid, valeric acid, γ-valerolactone, and 2-methyltetrahydrofuran.
Fig. 6.
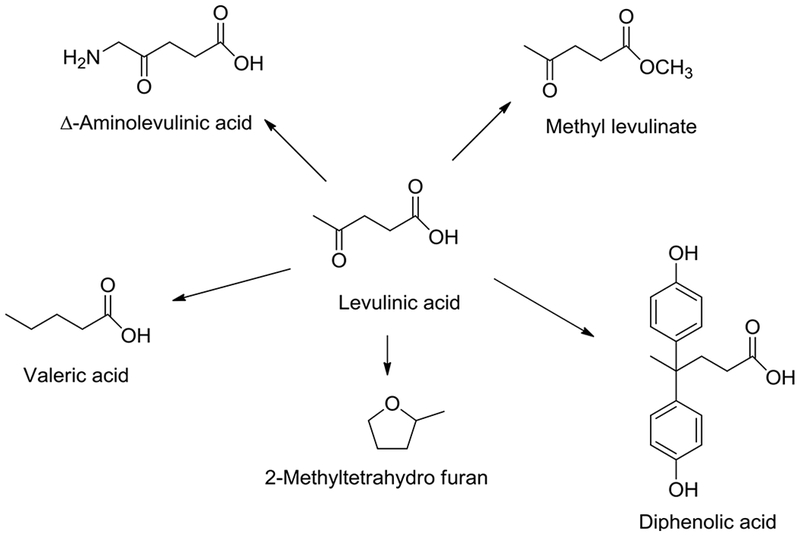
Levulinic acid value-added products
3-Hydroxypropionic acid (3-HP) and 3-hydroxypropionaldehyde (3-HPA):
3-HP and 3-HPA are important platform chemicals which can be converted to various higher value-added chemicals. Although considerable research is in progress, currently there are not any commercial production processes for 3-hydroxypropionaldehye and 3-hydroxypropionic acid production from renewable sources. One of the challenges associated with the biological production of 3-hydroxypropionaldehyde is the toxicity of 3-HPA itself (Zheng et al. 2006). Currently, 3-hydroxypropionaldehyde is produced by the fermentation of glycerol, which upon oxidation yields 3-hydroxypropionic acid. 3-Hydroxypropionic acid is produced directly from glycerol by using the Klebsiella pneumoniae and E. coli strains. It is also produced from glucose at neutral pH by E. coli (Kumar et al. 2013), or at lower pH by yeast S. cerevisiae. Since 3-HP has a pKa of 4.51, carrying out the fermentation process at low pH improves the economics and reduces the amount of waste generated in the process (Kildegaard et al. 2015), thus the yeast-based process is preferable.
As a platform chemical 3-HP and 3-HPA can be converted to various value-added chemicals (Fig. 7) (Corma et al. 2007) such as acrolein, acrylic acid, acrylic acid esters and amides (Craciun et al. 2009), 1,3-propanediol, malonic acid and 3-hydroxypropionic esters. Acrylic monomers are used in the synthesis of various polymers. Polyacrylic acid is commercially produced by The Dow Chemical Company, AkzoNobel, Lubrizol and BASF. Acrylamides are produced by the treatment of 3-HPA with an amine. Although the acrylamide monomer is toxic, its polymer polyacrylamide is not toxic and is used in various applications (Huang et al. 2001) such as in water treatment, paper manufacture, mining, oil recovery, absorbents and as electrophoresis gels. Acrolein and acrylonitrile are two other industrially essential derivatives which are used in the synthesis of various polymers.
Fig. 7.
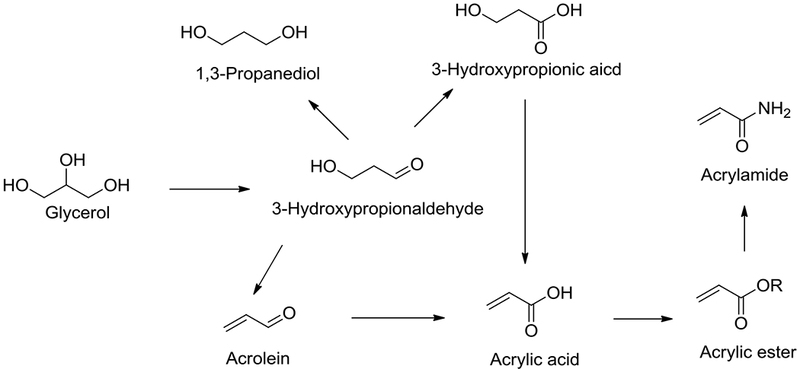
3-Hydroxypropionaldehyde and 3-hydroxypropionic acid value-added chemicals
Sorbitol:
Sorbitol is the most commonly used sugar substitute with an approximate annual global production of 800,000 tons, and it is widely used in food, beverages, drugs, cosmetics and to produce value-added chemicals (Banu et al. 2012; Zhang et al. 2013). Sorbitol is commercially produced by a transition metal catalyzed hydrogenation of D-glucose (Kamm 2007; Ortiz et al. 2013). In the industrial process, raw materials such as corn, cassava and wheat are converted into glucose by enzymatic hydrolysis, and in a second step the glucose is subjected to hydrogenation to yield the desired sorbitol. Although Ni catalysts have been used in the earlier industrial processes, Ru catalysts have been found to be superior at converting glucose into sorbitol with very high selectivity (Zhang et al. 2013). Currently, Roquette Freres is the most prominent producer of sorbitol, and shares approximately 70% of the market volume with Cargill and SPI Polyols. Recently, there have been reports on the conversion of cellulose and cellobiose to sorbitol by hydrolysis followed by hydrogenation (Chen et al. 2013; Ribeiro et al. 2015). The conversion of cellulose to sorbitol has also been reported using catalysts containing an acid or heteropolyacid with supported metal catalysts such as Pt, or Ru (Zhu et al. 2014; Wang et al. 2016; Zhang et al. 2016). Approximately, 15% of the sorbitol produced is utilized in the industrial conversion into ascorbic acid by fermentation (Kobayashi and Fukuoka 2013). Isosorbide (Fig. 8) is produced by dehydration of sorbitol (Rose and Palkovits 2012) in the presence of a Cu catalyst. Hydrogenolysis of sorbitol with multifunctional catalysts produces lower carbon number alcohols (glycerol, propyleneglycol, ethylene glycol, ethanol and methanol) and these lower alcohols can then be further converted to higher value-added products (Isikgor and Becer 2015). Sorbitol can also be utilized in the synthesis of polymers, which have a wide range of applications as biodegradable polymers and in biocomposites and biomedicines. Similarly, the isosorbide polymer, poly(isosorbide carbonate) is considered as a promising alternative to the petroleum-based BPA polycarbonate due to its superior properties (Roquette). Roquette is also manufacturing polymer grade isosorbide and isosorbide diesters as phthalate-free plasticizers (Roquette).
Fig. 8.

Synthesis of Sorbitol and its value-added products
Xylitol:
Xylitol is a naturally occurring five-carbon sugar alcohol which is 20% sweeter than sucrose, but with 40% the calories. In addition, the metabolism of xylitol is not dependent on insulin, thus it is an ideal sugar substitute for people with diabetes (Lugani et al. 2017).
Industrial production of xylitol (Figure 10) is accomplished via the catalytic reduction of pure D-xylose obtained from hardwood hemicellulosic hydrolysate in the presence of Ni catalyst at high temperature and pressure (Rafiqul and Sakinah 2013). Although it is commercially produced by chemical reductive methods, several reports have appeared describing biochemical reductive methods utilizing either enzymes or microorganisms (Chen et al. 2010). Recently a one-pot procedure for the conversion of hemicellulose to xylitol using an acid in combination with Ru on carbon catalyst has been reported (Dietrich et al. 2017). Similarly, the conversion of corncob derived hemicellulose to xylitol is accomplished using a ruthenium catalyst supported on carbon nanotubes (Ribeiro et al. 2016).
Fig. 10.
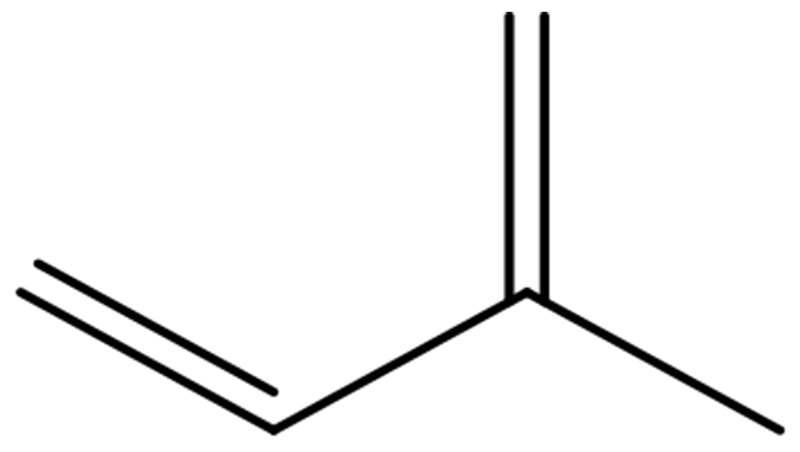
Structure of Isoprene
Isoprene:
Isoprene is a 5- carbon building block for the synthesis of a variety of polymers. The majority of isoprene produced is converted into the polyisoprene polymer, which is used in a variety of products such as footwear, mechanical instruments, medical appliances, sporting goods, and rubber tires.
Although commercial production of isoprene is currently carried out from petroleum-based feedstocks, manufacturing processes based on renewable resources are in development. Dupont-Genencor and the Goodyear Tire Company are co-developing (DuPont) an integrated process to manufacture bio-isoprene from renewable raw materials on an industrial scale (Genencor). Their technology utilizes a genetically engineered E. coli for the fermentation process (Whited et al. 2010). Amyris in partnership with Michelin and Braskem is also exploring the development and commercialization of isoprene from plant sugars (Amyris).
A transition from today’s fossil-based economy towards a more sustainable bio-economy is emphasized by International Energy Agency (IEA), Organization for Economic Co-Operation and Development (OECD), and the World Economic Forum (WEF), as well as several national government entities (e.g. NREL). Each emphasizing the drivers for the transition to a bio-economy being, the need to develop an environmentally, socially and economically more sustainable global economy, the reduction in greenhouse gas emissions, and to reduce the dependence on nonrenewable fossil fuel resources (de Jong et al. 2013). The expansion of a successful bio-economy will depend on the development of robust biorefinery systems each with advanced technologies, which can process biological feedstocks into a variety of bio-based products with efficient, and cost-effective processes. In a bio-economy, the basic building blocks for chemicals, materials and energy are derived from renewable sources and are considered as an integral part of the development toward a more sustainable economy. Lignocellulosic biomass is the most widely available renewable source of biomass with less competition in terms of food and feed production. For the lignocellulosic biorefineries to be successful and to achieve fruitful results, major investment in the development and the application of highly efficient conversion technologies are needed. Advanced biorefineries, which can use lignocellulosic raw materials as the feedstocks, can produce large quantities of biofuels, as well as higher value-added products. These biorefineries can be integrated into the existing industrial infrastructures, such as pulp and paper mills and chemical production facilities. Thus, synergies can be achieved in energy, material flows along with processes, logistics, product and raw material markets (Nanda et al. 2015). Advanced biorefineries when developed at commercial scale will create and drive new business opportunities. However, the most advanced biorefineries are not yet commercialized and the development of these biorefineries depends on the progress made in the thermochemical and biochemical platform technologies (Pandey 2011).
Fig. 9.
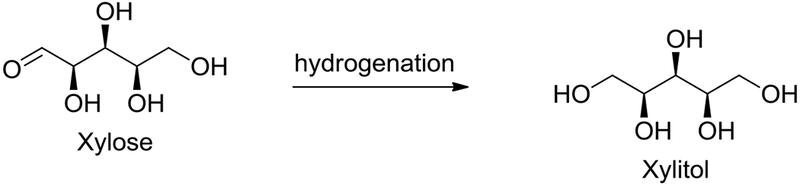
Synthesis of Xylitol from Xylose
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References:
- Abad S, Turon X (2012) Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: focus on polyunsaturated fatty acids. Biotechnol Adv 30:733–741 [DOI] [PubMed] [Google Scholar]
- Abubackar HN, Veiga MC, Kennes C (2011) Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuels Bioprod Bioref 5: 93–114 [Google Scholar]
- Adkins J, Pugh S, McKenna R, Nielsen DR (2012) Engineering microbial chemical factories to produce renewable “biomonomers”. Front Microbiol 3:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: Fundamentals toward application. Biotechnol Adv 29:675–685 [DOI] [PubMed] [Google Scholar]
- Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12: 1493–1513 [Google Scholar]
- Almeida JRM, Favaro LCL, Quirino BF (2012) Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol Biofuels 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaras NE, Cameron DC (1999) Metabolic engineering of a 1,2-propanediol pathway in Escherichia coli. Appl Environ Microbiol 65:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyris. https://amyris.com/products/isoprene/ Accessed 22 June 2017
- Arena U (2012) Process and technological aspects of municipal solid waste gasification. A review. Waste Management 32:625–639 [DOI] [PubMed] [Google Scholar]
- Avantium. https://www.avantium.com/yxy/products-applications/ Accessed 22 June 2017
- Avantium (2016) press release available at https://www.avantium.com/press-releases/synvina-joint-venture-basf-avantium-established/
- Azzam M (1989) Pretreatment of cane bagasse with alkaline hydrogen peroxide for enzymatic hydrolysis of cellulose and ethanol fermentation. J Environ Sci Health B 24:421–433. [Google Scholar]
- Bai R, Zhang H, Mei F, Wang S, Li T, Gu Y, Li G (2013) One-pot synthesis of glycidol from glycerol and dimethyl carbonate over a highly efficient and easily available solid catalyst NaAlO2. Green Chem 15:2929–2934 [Google Scholar]
- Banu M, Venuvanalingam P, Shanmugam R, Viswanathan B, Sivasanker S (2012) Sorbitol hydrogenolysis over Ni, Pt and Ru supported on NaY. Top Catal 55:897–907 [Google Scholar]
- Barbosa BM, Colodette JL, Longue D Jr, Gomes FJB, Martino DC (2014) Preliminary studies on furfural production from lignocellulosics. J Wood Chem Technol 34:178–190 [Google Scholar]
- Behr A, Eilting J, Irawadi K, Leschinski J, Lindner F (2008) Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem 10:13–30. [Google Scholar]
- Bell BM, Briggs JR, Campbell RM, Chambers SM, Gaarenstroom PD, Hippler JG, Hook BD, Kearns H, Kenney JM, Kruper WJ, Schreck DJ, Theriault CN,Wolfe CP (2008) Glycerin as a renewable feedstock for epichlorohydrin production. The GTE process. Clean 36:657–661. [Google Scholar]
- BIO 2016. Advancing the biobased economy: Renewable chemical biorefinery commercialization, progress and market opportunities, 2016 and beyond. Available at: https://www.bio.org/advancing-biobased-economy-renewable-chemical-biorefinery-commercialization-progress-and-market Accessed 22 June 2017
- Bioamber (2015) Press release available at https://www.bio-amber.com/bioamber/en/news/2015/bioamber-now-shipping-bio-succinic-acid-to-customers
- Bjerre AB, Olesen AB, Fernqvist T (1996) Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol Bioeng 49:568–577 [DOI] [PubMed] [Google Scholar]
- Botello JI, Gilarranz MA, Rodriguez F, Oliet M (1999) Preliminary study on products distribution in alcohol pulping of Eucalyptus globulus. J Chem Technol Biotechnol 74:141–148 [Google Scholar]
- Bozell JJ, and Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554 [Google Scholar]
- Brethauer S, Studer MH (2015) Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals – A Review. Chimia 69:572–581 [DOI] [PubMed] [Google Scholar]
- Cargill (2006) available at, http://www.foodingredientsfirst.com/news/Cargill-to-Commercialize-Renewable-Propylene-Glycol-from-Glycerin.html/ Accessed 30 June 2017.
- Carriquiry MA, Du X, Timilsina GR (2011) Second generation biofuels: economics and policies. Energy Policy 39:4222–4234 [Google Scholar]
- Chatterjee C, Pong F, Sen A (2015) Chemical conversion pathways for carbohydrates. Green Chem 17:40–71 [Google Scholar]
- Chen J, Wang S, Huang J, Chen L, Ma L, Huang X (2013) Conversion of Cellulose and Cellobiose into Sorbitol Catalyzed by Ruthenium Supported on a Polyoxometalate/Metal–Organic Framework Hybrid. ChemSusChem 6:1545–1555 [DOI] [PubMed] [Google Scholar]
- Chen X, Jiang Z, Chen S, Qin W (2010) Microbial and Bioconversion Production of D-xylitol and Its Detection and Application. Int J Biol Sci 6:834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini F, Jungmeier G, Wellisch M, Willke T, Skiadas I, Van Ree R, de Jong E (2009) Toward a common classification approach for biorefinery systems. Biofuels Bioprod Bioref 3:534–546 [Google Scholar]
- Chiu CW, Dasari MA, Suppes GJ, Sutterlin WR (2006) Dehydration of glycerol to acetol via catalytic reactive distillation. AIChE J 52:3543–3548 [Google Scholar]
- Ciriminna R, Pagliaro M (2003) One-Pot Homogeneous and Heterogeneous Oxidation of Glycerol to Ketomalonic Acid Mediated by TEMPO. Adv Synth Catal 345:383–388 [Google Scholar]
- Clark JH, Deswarte FEI (2008) The Biorefinery Concept–An Integrated Approach In: Clark JH, Deswarte FEI (eds) Introduction to Chemicals from Biomass, John Wiley & Sons, Ltd, Chichester, UK, pp 1–20. doi: 10.1002/9780470697474 [DOI] [Google Scholar]
- Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends in Biotechnol 31:20–28 [DOI] [PubMed] [Google Scholar]
- Corbion. http://www.corbion.com/biochemicals/pharma/brands/purac Accessed 22 June 2017
- Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502 [DOI] [PubMed] [Google Scholar]
- Craciun L, Benn GP, Dewing J, Schriver GW (2009), US Pat, 7538247
- Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A 281:225–231 [Google Scholar]
- Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies-a review. J Chem Technol Biotechnol 81:1119–1129 [Google Scholar]
- de Jong E, Jungmeier G (2015) Biorefinery concepts in comparison to petrochemical refineries In: Pandey A, Hofer R, Taherzadeh M, Nampoothiri M, Larroche C (eds) Industrial Biorefineries and White Biotechnology, 1st edn Elsevier, pp 3–33. [Google Scholar]
- de Jong E, Higson A, Walsh P, Wellissch M (2013) Bio-based chemicals, value added products from biorefineries. In: IEA Bioenergy Task 42 report 2012. www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Biobased-Chemicals-value-added-products-from-biorefineries.pdf Accessed 12 March 2018
- Delhomme C, Weuster-Botz D , Kuhn FE (2009) Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media. Green Chem 11:13–26 [Google Scholar]
- Demirbas A (2011) Competitive liquid biofuels from biomass. Appl Energy 88:17–28 [Google Scholar]
- Dietrich K, Hernandez-Mejia C, Verschuren P, Rothenberg G, Shiju NR (2017) One-Pot Selective Conversion of Hemicellulose to Xylitol. Org Process Res Dev 21:165−170 [Google Scholar]
- Direvo press release. [Accessed 22 June 2017]; available at, http://www.direvo.com/uploads/media/DIREVO-PressRelease_No6_2013.pdf.
- Dow press release (2007) available at, http://www.dow.com/propyleneglycol/news/20070315b.htm Accessed 30 June 2017
- DuPont. http://biosciences.dupont.com/about-us/collaborations/goodyear/ Accessed 22 June 2017
- DuPont press release. [Accessed 22 June 2017];2016 is available at, http://www.dupont.com/products-and-services/industrial-biotechnology/press-releases/dupont-adm-announce-platform-technology-for-long-sought-after-molecule.html.
- Dutta S, De S, Saha B (2012) A Brief Summary of the Synthesis of Polyester Building-Block Chemicals and Biofuels from 5-Hydroxymethylfurfural. ChemPlusChem 77:259–272 [Google Scholar]
- Dutta S, De S, Saha B, Alam Md.I (2012) Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels. Catal Sci Technol 2:2025–2036 [Google Scholar]
- Erickson B, Nelson JE, Winters P (2012) Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol J 7:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D, Antonietti M (2015) Redefining biorefinery: the search for unconventional building blocks for materials. Chem Soc Rev 44: 5821–5835 [DOI] [PubMed] [Google Scholar]
- Fernando S, Adhikari S, Chandrapal C, Murali N (2006) Biorefineries: Current status, challenges and future direction. Energy Fuels 20:1727–1737 [Google Scholar]
- Genencor. http://www.genencor.com/uploads/tx_tcdaniscofiles/GENC-10053_BioIsoprene_Backgrounder_prt.pdf Accessed 22 June 2017
- GF Biochemicals press release. [Accessed 22 June 2017]; available at http://www.gfbiochemicals.com/news/2015/07/gfbiochemicals-announces-production-start-up/
- Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: A brief review on production to purification. J Radiat Res Appl Sci 7:222–229 [Google Scholar]
- Gil S, Cuenca N, Romero A, Valverde JL, Sáchez-Silva L (2014) Optimization of the synthesis procedure of microparticles containing gold for the selective oxidation of glycerol. Appl Catal A 472:11–20 [Google Scholar]
- Gonzalez-Pajuelo M, Meynial-Salles I, Mendes F, Soucaille P, Vasconcelos I (2006) Microbial Conversion of Glycerol to 1,3-Propanediol: Physiological Comparison of a Natural Producer, Clostridium butyricum VPI 3266, and an Engineered Strain, Clostridium acetobutylicum DG1(pSPD5). Appl Environ Microbiol 72:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravitis J, Vedernikov N, Zandersons J, Kokorevics A (2001) Furfural and levoglucosan production from deciduous wood and agricultural wastes In: Chemicals and Materials from Renewable Resources, vol. 784 American Chemical Society; Washington, DC, pp. 110–122 [Google Scholar]
- Guo Y, Li K, Yu X, Clark JH (2008. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl Catal B 81:182–191 [Google Scholar]
- Hamelinck CN, Hooijdonk GV, Faaji APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass and Bioenergy 28:384–410 [Google Scholar]
- Hayes DJ (2009) An examination of biorefining processes, catalysts and challenges. Catalysis Today 145:138–151 [Google Scholar]
- Hu F, Ragauskas A (2012) Pretreatment and lignocellulosic chemistry. Bioenerg Res 5:1043–1066 [Google Scholar]
- Huang SY, Lipp DW, Farinato RS (2001) Acrylamide Polymers, in Encyclopedia of Polyer Science and Technology, John Wiley & Sons, Inc; DOI: 10.1002/0471440264 [DOI] [Google Scholar]
- Huntsman news (2006) available at, http://www.huntsman.com/performance_products/Applications/itemrenderer?p_item_id=230137714&p_item_caid=1143 Accessed 30 June 2017.
- Ilmen M, Koivuranta K, Ruohonen L, Suominen P, Penttila M (2007) Efficient Production of L-Lactic Acid from Xylose by Pichia stipites. Appl Environ Microbiol 73:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Poly Chem 6:4497–4559 [Google Scholar]
- John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74:524–534 [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Kirstensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod Bioref 1:119–134 [Google Scholar]
- Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145 [DOI] [PubMed] [Google Scholar]
- Kamm B, Gruber PR, Kamm M (2006) Biorefineries-industrial processes and products. Wiley-VCH Verlag GmbH &Co. K GaA, Weinheim. [Google Scholar]
- Kamm B (2007) Production of Platform Chemicals and Synthesis gas from biomass. Angew Chem Int Ed 46:5056–5058 [DOI] [PubMed] [Google Scholar]
- Kildegaard KR, Wang Z, Chen Y, Nielsen J, Borodina I (2015) Production of 3-hydroxypropionic acid from glucose and xylose by metabolically engineered Saccharomyces cerevisiae. Metabolic Engineering Communications 2:132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee J, Green SK, Huber GW, Kim WB (2014) Selective Glycerol Oxidation by Electrocatalytic Dehydrogenation. ChemSusChem 7:1051–1054 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Fukuoka A (2013) Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem 15:1740–1763 [Google Scholar]
- Kondamudi N, Misra M, Banerjee S, Mohapatra S (2012) Simultaneous production of glyceric acid and hydrogen from the photooxidation of crude glycerol using TiSi2. Appl Catal B 126:180–185 [Google Scholar]
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729 [Google Scholar]
- Kumar V, Ashok S, Park S (2013) Recent advances in biological production of 3-hydroxypropionic acid. Biotechnol Adv 31:945–961 [DOI] [PubMed] [Google Scholar]
- Li H, Yang S, Riisager A, Pandey A, Sangwan RS, Saravanamurugan S, Luque R (2016) Zeolite and zeotype-catalysed transformations of biofuranic compounds. Green Chem 18:5701–5735 [Google Scholar]
- Lugani Y, Oberoi S, Sooch BS (2017) Xylitol: A sugar substitute for patients of diabetes mellitus. World J Pharm Pharm Sci 6:741–749 [Google Scholar]
- Luque R, Clark JH, Yoshida K, Gai PL (2009) Efficient aqueous hydrogenation of biomass platform molecules using supported metal nanoparticles on Starbons. Chem Commun 5305–5307 [DOI] [PubMed] [Google Scholar]
- Ma J, Song J, Liu H, Liu J, Zhang Z, Jiang T, Fan H, Han B (2012) One-pot conversion of CO2 and glycerol to value-added products using propylene oxide as the coupling agent. Green Chem 14:1743–1748 [Google Scholar]
- Machado G, Leon S, Santos F, Lourega R, Dellius J, Mollmann ME, Eichler P (2016) Literature review on furfural production from lignocellulosic biomass. Natural Resources 7:115–129 [Google Scholar]
- Magrini-Bair KA, Jablonski WS, Parent YO, Yung MM (2012) Bench and pilot scale studies of reaction and regeneration of Ni-Mg-K/Al2O3 for catalytic conditioning of biomass-derived syngas. Top Catal 55:209–217 [Google Scholar]
- Maki-Arvela P, Salmi T, Holmbom B, Willfor S, Murzin DY (2011) Synthesis of sugars by hydrolysis of hemicelluloses – a review. Chem Rev 111:5638–5666 [DOI] [PubMed] [Google Scholar]
- Maki-Arvela P, Simakova IL, Salmi T, Murzin DY (2014) Production of Lactic Acid/Lactates from Biomass and Their CatalyticTransformations to Commodities. Chem Rev 114:1909–1971 [DOI] [PubMed] [Google Scholar]
- Maris EP, Ketchie WC, Murayama M, Davis RJ (2007) Glycerol hydrogenolysis on carbon-supported PtRu and AuRu bimetallic catalysts. J Catal 251:281–294 [Google Scholar]
- Mariscal R, Maireles-Torres P, Ojeda M, Sadaba I, Lopez Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9:1144–1189 [Google Scholar]
- McKendry P (2002) Energy production from biomass (part 3): gasification technologies. Bioresour Technol 83:55–63 [DOI] [PubMed] [Google Scholar]
- McMillan JD (1994) Pretreatment of lignocellulosic biomass In: Jimmel ME, Baker JO, Overend RP (eds), Enzymatic conversion of biomass for fuels production, American Chemical Society, Washington, DC, pp292–324 [Google Scholar]
- Mohammadi M, Najafpour GD, Younesi H, Lahijani P, Uzir MH, Mohamed AR (2011) Bioconversion of synthesis gas to second generation biofuels. A review. Renew Sustain Energy Rev 15:4255–4273 [Google Scholar]
- Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597 [Google Scholar]
- Nakagawa Y, Tamura M, Tomishige K (2014) Catalytic materials for the hydrogenolysis of glycerol to 1,3-propanediol. J Mater Chem A 2:6688–6702 [Google Scholar]
- Nanda S, Azargohar R, Dalai AK, Kozinski JA (2015) An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew Sustain Energy Rev 50:925–941 [Google Scholar]
- NREL. [Accessed 22 June 2017]; https://www.nrel.gov/workingwithus/re-biomass.html.
- Octave S, Thomas D (2009) Biorefinery: Toward an industrial metabolism. Biochimie 91:659–664 [DOI] [PubMed] [Google Scholar]
- Okoye PU, Hameed BH (2016) Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew Sustain Energy Rev 53:558–574 [Google Scholar]
- Ortiz ME, Bleckwedel J, Raya RR, Mozzi F (2013) Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl Microbiol Biotechnol 97:4713–4726 [DOI] [PubMed] [Google Scholar]
- Pagliaro M, Ciriminna R, Kimura H, Rossi M, Pina CD (2007) From glycerol to value-added product. Angew Chem Int Ed 46:4434–4440 [DOI] [PubMed] [Google Scholar]
- Pandey A (ed.) (2011) Biofuels:Alternative feedstocks and conversion processes. Academic Press, Kidlington, Oxford, ISBN-13: 978-0123850997 [Google Scholar]
- Pereira CSM, Silva VMTM, Rodrigues AE (2011) Ethyl lactate as a solvent: Properties, applications and production processes – a review. Green Chem 13: 2658–2671 [Google Scholar]
- Pileidis FD, Titirici M-M (2016) Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 9:562–582 [DOI] [PubMed] [Google Scholar]
- Rafiqul ISM, Sakinah AMM (2013) Processes for the Production of Xylitol—A Review. Food Rev Int 29:127–156 [Google Scholar]
- Ragauskas AJ, Beckham GT, Biddy MJ Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman C (2014) Lignin valorization: Improving lignin processing in the biorefinery. Science 344:1246843. [DOI] [PubMed] [Google Scholar]
- Rass HA, Essayem N, Besson M (2013) Selective aqueous phase oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Pt/C catalysts: influence of the base and effect of bismuth promotion. Green Chem 15:2240–2251 [Google Scholar]
- Ribeiro LS, Órfão JJM, Pereira MFR (2015) Enhanced direct production of sorbitol by cellulose ball-milling. Green Chem 17:2973–2980 [Google Scholar]
- Ribeiro LS, Delgado JJ, de Melo Orfao JJ, Pereira MFR (2016) A one-pot method for the enhanced production of xylitol directly from hemicellulose (corncob xylan). RSC Adv 6:95320–95327 [Google Scholar]
- Rose M, Palkovits R (2011) Cellulose-Based Sustainable Polymers: State of the Art and Future Trends. Macromol Rapid Commun 32:1299–1311 [DOI] [PubMed] [Google Scholar]
- Rose M, Palkovits R (2012) Isosorbide as a Renewable Platform chemical for Versatile Applications--quo vadis?. ChemSusChem 5:167–176 [DOI] [PubMed] [Google Scholar]
- Rout PK, Nannaware AD, Prakash O, Kalra A, Rajasekharan R (2016) Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem Eng Sci 142:318–346 [Google Scholar]
- Roquette. https://www.roquette.com/industries/performance-materials/polycarbonates/ Accessed 22 June 2017
- Roquette/DSM Press release. https://www.dsm.com/corporate/about/business-entities/dsm-biobased-productsandservices/reverdia.html Accessed 22 June 2017
- Saha B, Abu-Omar MM (2014) Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem 16:24–38 [Google Scholar]
- Santacesaria E, Tesser R, Di Serio M, Casale L, Verde D (2010) New Process for Producing Epichlorohydrin via Glycerol Chlorination. Ind Eng Chem Res 49:964–970 [Google Scholar]
- Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: An overview. Renew Energ 37:19–27 [Google Scholar]
- Schultz EL, de Souza DT, Damaso MCT (2014) The glycerol biorefinery: a purpose for Brazilian biodiesel production. Chem Biol Technol Agric 1: 7 [Google Scholar]
- Schwab A, Warner E, Lewis J (2016) Survey of Non-Starch Ethanol and Renewable Hydrocarbon Biofuels Producers. NREL/TP- 6A10-65519 Available at www.nrel.gov/publications.
- Serrano-Ruiz JC, Dumesic JA (2011) Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Env Sci 4:83–99 [Google Scholar]
- Singh J, Suhag M, Dhaka A (2015) Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydrate polymers 117: 624–631 [DOI] [PubMed] [Google Scholar]
- Subramani V, Gangwal SK (2008) A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy Fuels 22:814–839 [Google Scholar]
- Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101:4744–4753 [DOI] [PubMed] [Google Scholar]
- Teymouri F, Perez LL, Alizadeh H, Dale BE (2004) Ammonia fiber explosion treatment of corn stover. Appl Biochem Biotechnol 113-116:951–963. [DOI] [PubMed] [Google Scholar]
- Transparency Market Research, http://www.transparencymarketresearch.com/glycerol.market.html, Accessed June 6, 2017
- US Department of Energy, Biomass Resources. Available from https://energy.gov/eere/bioenergy/biomass-resources Accessed 22 June 2017
- US EPA (2016) Advancing sustainable material management: Facts and Figures Report. Available from https://www.epa.gov/smm/advancing-sustainable-materials-management-facts-and-figures-report Accessed 22 June 2017
- Van Putten R-J, van der Waal JC, de Jong E, Rasrendra CB, Heeres HJ, de Vries JG (2013) Hydroxymethylfurfural, A versatile platform chemical made from renewable resources. Chem Rev 113:1499–1597 [DOI] [PubMed] [Google Scholar]
- Verma S, Baig RBN, Nadagouda MN, Len C, Varma RS (2017) Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem 19:164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Tan X, Lv H, Zhao M, Wu M, Zhou J, Zhang X, Zhang L (2016) Highly Selective Conversion of Cellobiose and Cellulose to Hexitols by Ru-Based Homogeneous Catalyst under Acidic Conditions. Ind Eng Chem Res 55:5263–5270 [Google Scholar]
- Wang T, Nolte MW, Shanks BH (2014) Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem 16:548–572 [Google Scholar]
- Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, Manheim A, et al. (2004) In: Werpy T, Petersen G (eds) Top Value Added Chemicals from Biomass – Vol. 1: Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Pacific Northwest National Laboratory, National Renewable Energy Laboratory and Department of Energy, Washington, D. C.) [Google Scholar]
- Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK, McAuliffe JC, LaDuca RJ, Ben-Shoshan EA, Sanford KJ (2010) Technology update: Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol 6:152–163 [Google Scholar]
- Yan K, Wu G, Lafleur T, Jarvis C (2014) Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew and Sustain Energy Rev 38: 663–676 [Google Scholar]
- Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol–a byproduct of biodiesel production. Biotechnol Biofuels 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Tang C (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712 [Google Scholar]
- Zhang J, Li J, Wu S, Liu Y (2013) Advances in the catalytic production and utilization of sorbitol. Ind Eng Chem Res 52:11799–11815 [Google Scholar]
- Zhang X, Durndell LJ, Isaacs MA, Parlett CMA, Lee AF, Wilson K (2016) Platinum-Catalyzed Aqueous-Phase Hydrogenation of D‑Glucose to D‑Sorbitol. ACS Catal 6:7409−7417 [Google Scholar]
- Zheng P, Wereath K, Sun JB, van den Heuvel J, Zeng A (2006) Overexpression of genes of the dha regulon and its effects on cell growth, glycerol fermentation to 1,3-propanediol and plasmid stability in Klebsiella pneumoniae. Process Biochem 41:2160–2169 [Google Scholar]
- Zhu W, Yang H, Chen J, Chen C, Guo L, Gan H, Zhao X, Hou Z (2014) Efficient hydrogenolysis of cellulose into sorbitol catalyzed by a bifunctional catalyst. Green Chem 16:1534–1542 [Google Scholar]


