Abstract
Background
CD36 plays a critical role in many sterile inflammatory diseases, including type 2 diabetes mellitus, atherosclerosis, and primary nephrotic syndrome. This study investigated whether CD36 activates the nucleotide-binding domain leucine-rich repeat-containing family, pyrin domain-containing-3 (NLRP3) inflammasome and promotes podocytes apoptosis in primary nephrotic syndrome.
Material/Methods
The mouse podocyte cell line MPC5 was used as a model. mRNA and protein expression of CD36 and NLRP3 was quantified by real-time PCR and Western blotting, respectively. Levels of caspase-1 activity and total cholesterol were determined using commercial kits. Intracellular lipid droplets were detected by Oil Red O staining. CD36 expression was also examined in nephrotic mouse kidney tissue by immunohistochemistry and immunofluorescence. Intracellular lipid droplet was examined by Oil Red O staining.
Results
CD36 expression was increased in nephrotic mouse kidney tissue. Treatment with interleukin-1β increased expression of CD36 and total cholesterol in MPC5 cells. Moreover, this treatment increased expression of NLRP3 and the percentage of apoptotic cells, both of which were inhibited by co-treatment with an anti-CD36 antibody.
Conclusions
CD36 might play an important role in podocyte apoptosis by activating the NLRP3 inflammasome in primary nephrotic syndrome.
MeSH Keywords: Antigens, CD36; Inflammasomes; Inflammation; Nephrotic Syndrome; Podocytes
Background
Primary nephrotic syndrome (PNS) is the most common chronic kidney disease and is an important cause of end-stage renal disease in children. PNS is also thought to underlie many renal diseases in adults [1]. The major pathophysiological feature of PNS is massive proteinuria, which is caused by damage to the glomerular filtration membrane.
Podocytes are terminally differentiated visceral epithelial cells and a crucial component of the glomerular filtration membrane. Podocyte injury leads to proteinuria and even glomerular dysfunction in various human renal diseases and many nephropathic animal models, including PNS [2,3]. PNS is pathologically characterized by the degree of podocyte injury. Effacement of podocyte foot processes induces minimal disease change of PNS, accompanied by a better prognosis. By contrast, increased podocyte apoptosis causes serious non-minimal disease change such as focal segmental glomerulosclerosis.
Recent studies indicate that the pathogenesis of PNS involves immune dysregulation, inflammation, and lipometabolic disorders. Increasing evidence suggests that lipometabolic disorders, especially cholesterol abnormalities, contribute to the process of PNS [3,4]. CD36, which belongs to the class B receptor family, is a receptor for long-chain fatty acids and oxidized low-density lipoprotein (LDL) in several cell types, including adipocytes, renal proximal tubular cells, macrophages, and podocytes. Many studies have demonstrated that CD36 is responsible for the progression of diseases characterized by lipid dysfunction. For example, CD36 is a component of the oxidized LDL intracellular signaling pathway that converts macrophages into foam cells, leading to atherosclerosis [5], is involved in the development of non-alcoholic steatohepatitis by promoting hepatic macrophage infiltration [6], and promotes diabetic nephropathy by triggering oxidative stress and inflammatory responses [7].
The nucleotide-binding domain leucine-rich repeat-containing family, pyrin domain-containing-3 (NLRP3) inflammasome, which consists of the Nod-like receptor NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1, is a member of the cytoplasmic pattern recognition receptor family that plays a critical role in inducing inflammatory factors such as interleukin (IL)-1β. CD36 is thought to activate the NLRP3 inflammasome. However, it is unclear whether CD36 and the NLRP3 inflammasome affect podocyte apoptosis in PNS. This study investigated the role of the NLRP3 inflammasome and CD36 in apoptosis of podocytes.
Material and Methods
Mouse kidney tissue
Male BALB/c mice (6–8 weeks old; mean weight, 20 g) were provided by the Center of Experimental Animals (Chongqing Medical University, China) and raised under a constant temperature and humidity with a 12-h/12-h light/dark cycle and access to adequate feed and water. Mice were randomly divided into the control (CTR) and Adriamycin nephrosis (ADR) groups. The ADR group received a single injection of ADR (10.5 mg/kg body weight; Wanle, China) via the tail vein to simulate nephrotic syndrome, while the CTR group received an equal volume of saline, and both of the groups were housed for a further 12 weeks.
Paraffin-embedded kidney tissue samples were obtained from mice in both groups (CTR=7, ADR=7). CD36 was detected by immunohistochemistry and immunofluorescence.
Cell culture and treatment
The mouse podocyte cell line MPC5 was kindly provided by Professor Ruan (Center for Nephrology, Royal Free and University College Medical School, London,
United Kingdom). Cells were cultured on rat tail type I collagen (Millipore, USA) in RPMI-1640 (Gibco, USA) supplemented with 10% fetal bovine serum (PAN, Germany), 100 U/l penicillin (Gibco, USA), 0.1 mg/ml streptomycin (Sigma, USA), and 100 IU/ml interferon-γ (R&D, USA) at 33°C in 5% CO2 for 14 days. Thereafter, cells were cultured in the same conditions, except that the medium lacked interferon-γ. Cells were treated with human LDL (200 μg/ml, extracted from blood) with or without mouse IL-1β (20 ng/ml; PeproTech, USA) and a monoclonal anti-CD36 antibody (2 μg/ml; ab23680, Abcam, UK). Levels of CD36 and NLRP3 expression, lipidosis, and apoptosis were measured.
Real-time quantitative PCR
Total RNA was extracted from MPC5 cells using an RNA isolation kit (Bioteke, China) according to the manufacturer’s instructions. cDNA was synthesized using a reverse transcription kit (Vazmy, USA). The following forward (f) and reverse (r) primers were used: CD36 (f: TTGAAGGCATTCCCACGTATC; r: CGGACCCGTTGGCAAA). RT-PCR was executed performed using SYBR Premix (Vazmy, USA) with PCR instrument (BioRad, USA), and ΔΔCt values were calculated. mRNA levels were normalized against that of GAPDH and are presented relative to those in the CTR group (non-treated MPC5 cells).
Western blot analysis
Proteins were extracted using a bicinchoninic acid protein kit (Beyotime, China) after lysing cells in RIPA buffer (Beyotime, China). Extracted proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene membranes (Millipore, USA). Membranes were washed with Tris-Buffered Saline Tween (TBST), blocked with 5% non-fat milk for 1 h, and then incubated with a rabbit monoclonal anti-CD36 antibody (1: 1000; ab133625, Abcam, UK) or a rabbit monoclonal anti-NLRP3 antibody (1: 500; ab91413, Abcam, UK) overnight at 4°C. After being washed with TBST, membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1: 20 000; ZSGB, China) for 1 h at room temperature. Finally, signals were detected with an enhanced chemiluminescence kit (Millipore, USA).
Immunofluorescence
Frozen kidney tissues of mice from the CTR and ADR groups were sliced into 3-μm-thick sections. The sections were washed with phosphate-buffered saline (PBS), blocked with 10% bovine serum albumin (BSA) for 30 min at room temperature, and incubated with a mouse anti-CD36 antibody (1: 70; ab23680, Abcam, UK) overnight at 4°C. Thereafter, sections were washed with PBS, incubated with a secondary antibody (1: 100; ZGBD, China) for 1 h, stained with 4,6-diamidino-2-phenylindole (DAPI; 1: 20; Invitrogen, USA) for 25 min, and imaged with a microscope (Nikon, Japan).
Immunohistochemistry
Kidney tissues of mice from the CTR and ADR groups were sliced into 3-μm-thick paraffin sections. The sections were deparaffinized in xylene, dehydrated using various concentrations of ethanol, boiled in 10 mmol/l sodium citrate buffer for 10 min, and incubated with 3% H2O2 for 10 min. After being washed with PBS, the sections were incubated with a mouse monoclonal anti-CD36 antibody (1: 100; Abcam, UK) overnight at 4°C, washed with PBS, labeled with a biotinylated anti-mouse secondary antibody (1: 100; ZGBD, China) for 10 min, and then incubated with 3,3-diaminobenzidine for 2 min. Finally, the sections were stained with hematoxylin (Leagene, China), mounted with neutral gum, and imaged with a microscope (Nikon, Japan).
Oil red O staining
MPC5 cells on glass coverslips were washed with PBS and fixed in 4% paraformaldehyde for 30 min. Fixed cells were incubated with propylene glycol for 2 min, stained with Oil Red O working solution (NJBI, China) for 30 min, labeled with hematoxylin for 1 min, mounted with neutral glycerin, and imaged with a microscope (Nikon, Japan).
Measurement of total cholesterol
Total cholesterol was measured in MPC5 cells using a total cholesterol detection kit (Applygene, China) according to the manufacturer’s instructions. Briefly, cells were lysed and the supernatant was collected and applied to the assay. Absorbance at 550 nm was detected using a microplate reader (Thermo, USA).
Measurement of caspase-1 activity
Caspase-1 activity was measured in MPC5 cells using a caspase-1 detection kit (Applygene, China) according to the manufacturer’s instructions. Briefly, cells were lysed and the supernatant was collected and applied to the assay. Absorbance at 405 nm was detected using a microplate reader (Thermo, USA).
Detection of apoptotic cells
Apoptosis of MPC5 cells was examined using an annexin V-propidium iodide kit (KeyGene, China) according to the manufacturer’s instructions. The percentage of apoptotic cells was determined by flow cytometry (BD, USA).
Statistical analysis
All experiments were repeated at least 3 times. Statistical analysis was performed using SPSS 21.0. Numerical data are presented as mean ±SD. Groups were compared using the t test and one-way analysis of variance. p<0.05 was considered statistically significant.
Results
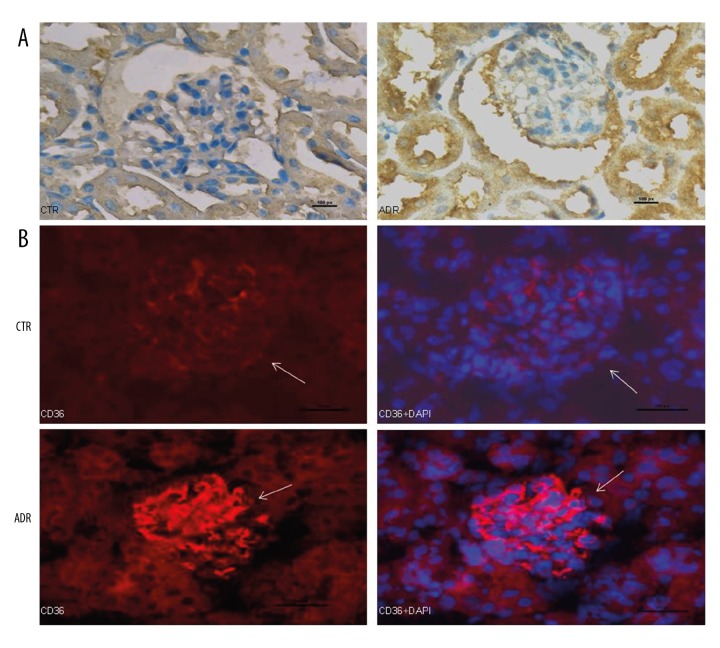
CD36 expression is increased in nephrotic mouse kidney tissue
CD36 expression in kidney tissue of mice from the ADR and CTR groups was investigated by immunohistochemistry and immunofluorescence. Renal expression of CD36 was markedly higher in the ADR group than in the CTR group (Figure 1A, 1B).
Figure 1.
CD36 expression is increased in nephrotic mouse kidney tissue. CD36 expression in kidney tissue of mice from the ADR and CTR groups was investigated by immunohistochemistry (A) and immunofluorescence (B). Scale bars: A, 50 μm; B, 100 μm.
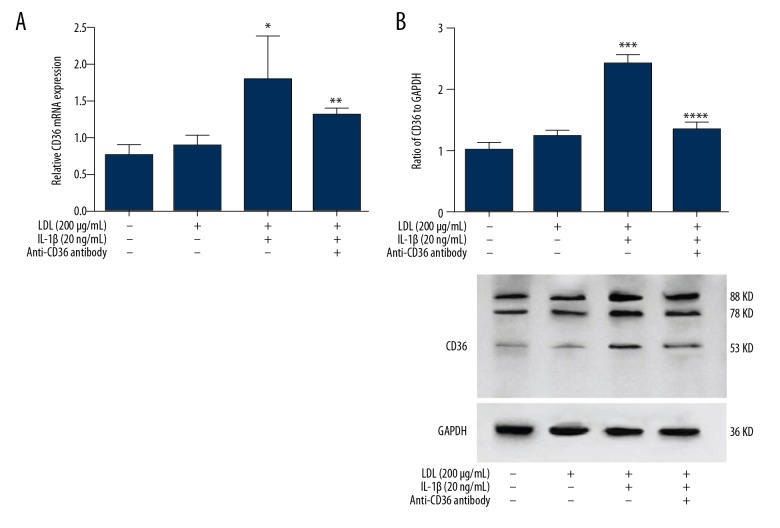
IL-1β treatment increases CD36 expression in MPC5 cells
As a member of a scavenger receptor family, CD36 is reportedly responsible for the degradation of most modified LDL in macrophages [8] and monocytes [9]. IL-1β is considered to play a role in the pathogenesis of many sterile inflammatory diseases, such as atherosclerosis and type 2 diabetes mellitus. To investigate the relationship between CD36 and IL-1β in podocytes, we treated MPC5 cells with 200 μg/ml LDL for 48 h, with or without 20 ng/ml IL-1β. The mRNA and protein levels of CD36 were not significantly changed in cells treated with LDL alone but were increased in cells co-treated with LDL and IL-1β (Figure 2). This indicates that inflammatory factors can upregulate CD36 expression in cells exposed to a high level of lipids.
Figure 2.
Treatment with LDL and IL-1β increases CD36 expression in MPC5 cells. mRNA and protein expression of CD36 in MPC5 cells treated as indicated was investigated by quantitative RT-PCR (A) and Western blotting (B), respectively. *, *** p<0.05 vs. CTR cells; **, **** p<0.05 vs. LDL+IL-1β-treated cells.
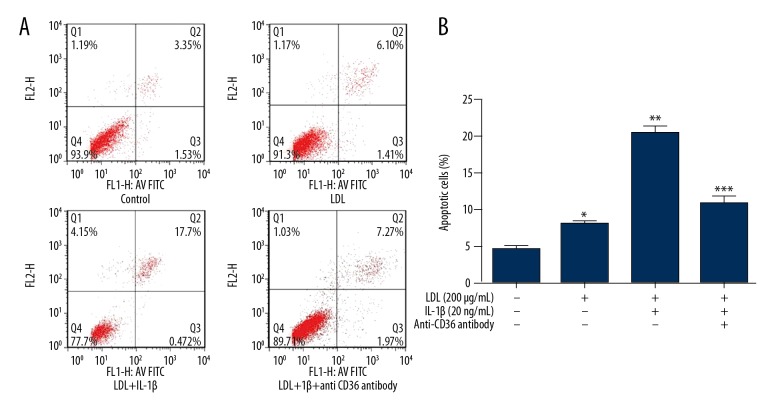
Treatment with LDL and IL-1β induces apoptosis of MPC5 cells via CD36
LDL and IL-1β promote apoptosis of mesangial cells in vitro [10]. To investigate whether they elicit the same effect in podocytes, we treated MPC5 cells with 200 μg/ml LDL for 48 h, with or without 20 ng/ml IL-1β. Flow cytometric analysis of annexin V-propidium iodide staining revealed that the percentage of apoptotic cells was increased by LDL treatment alone and to a greater extent by co-treatment with LDL and IL-1β (Figure 3).
Figure 3.
Treatment with LDL and IL-1β induces apoptosis of MPC5 cells via CD36. MPC5 cells were treated as indicated for 48 h and the percentage of apoptotic cells was determined by flow cytometry. (A) Flow cytometric plots. (B) Percentage of apoptotic cells. * p<0.05 vs. CTR cells; ** p<0.05 vs. CTR cells; *** p<0.05 vs. LDL+IL-1β-treated cells.
To explore whether CD36 is involved in this increase in apoptosis, we co-treated MPC5 cells with LDL, IL-1β, and an anti-CD36 monoclonal antibody for 48 h. The percentage of apoptotic cells was lower among these cells than among those co-treated with LDL and IL-1β (Figure 3).
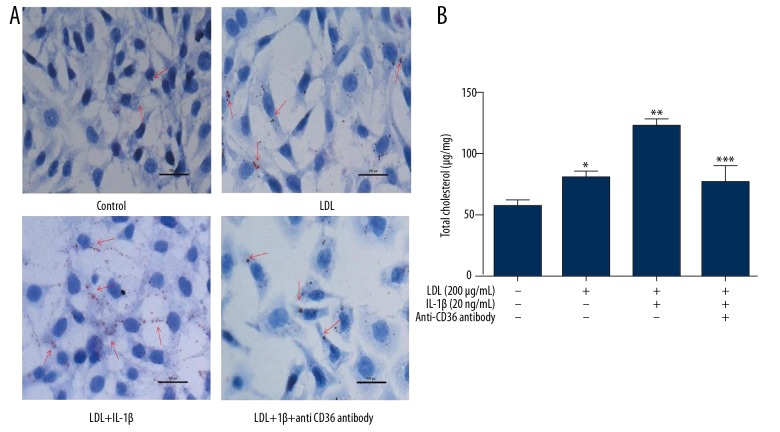
Treatment with LDL and IL-1β promotes lipid accumulation in MPC5 cells via CD36
CD36 is considered to be critical for lipid uptake. Oil Red O staining revealed that lipid accumulation in MPC5 cells was increased by treatment with LDL and to a greater extent by co-treatment with LDL and IL-1β. To determine whether CD36 is involved in this process, we co-treated cells with LDL, IL-1β, and an anti-CD36 antibody. This reduced lipidosis in MPC5 cells (Figure 4A). This treatment elicited similar effects on the total cholesterol level in MPC5 cells (Figure 4B). These results suggest that CD36 promotes lipid transfer into podocytes when the extracellular lipid level is high and that IL-1β can accelerate this process.
Figure 4.
Treatment with LDL and IL-1β promotes lipid accumulation in MPC5 cells via CD36. MPC5 cells were treated as indicated. Lipid accumulation was investigated by Oil Red O staining (A) and the total cholesterol level was determined using a commercial kit (B). *, ** p<0.05 vs. CTR cells; *** p<0.05 vs. LDL+IL-1β-treated cells. Scale bars: 50 μm.
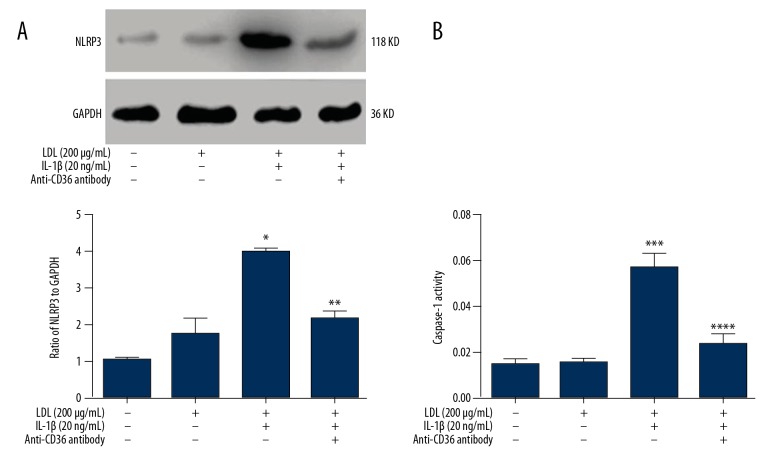
Treatment with LDL and IL-1β activates the NLRP3 inflammasome in MPC5 cells via CD36
To determine whether the NLRP3 inflammasome is involved in podocyte injury, we treated MPC5 cells with 200 μg/ml LDL for 48 h, with or without 20 ng/ml IL-1β. NLRP3 expression was not changed significantly in MPC5 cells treated with LDL alone (Figure 5A). However, co-treatment with LDL and IL-1β increased protein expression of NLRP3 (Figure 5A) as well as caspase-1 activity (Figure 5B). These effects were inhibited by treatment with monoclonal anti-CD36 antibody.
Figure 5.
Treatment with LDL and IL-1β activates the NLRP3 inflammasome in MPC5 cells via CD36. MPC5 cells were treated as indicated. Protein expression of NLRP3 (A) and caspase-1 activity (B) were determined by Western blotting and using a commercial kit, respectively. *, *** p<0.05 vs. CTR cells; **, **** p<0.05 vs. LDL+IL-1β-treated cells.
Discussion
PNS is the most common chronic kidney disease and an important cause of renal failure in childhood. Furthermore, it has been considered to be a source of many kidney diseases in adults [1]. An abnormality in the number or function of podocytes is the primary pathological change in PNS and results in damage to the glomerular filtration membrane, proteinuria, and even glomerulosclerosis. PNS is mainly treated with glucocorticoids and immunosuppressants; however, these drugs have severe adverse effects and are associated with a high rate of relapse. Furthermore, some patients are insensitive to this treatment and develop glomerulosclerosis and even end-stage renal disease. Consequently, the pathogenesis and treatment of PNS must be investigated. In addition to immune disorders [11] and inflammation, lipometabolic disorders are important factors in the pathogenesis of PNS [3,4,12,13].
CD36 is a single-stranded transmembrane protein comprising 472 amino acids that belongs to the class B scavenger receptor family. This protein is expressed on the surface of mononuclear macrophages, adipocytes, cardiomyocytes, renal tubular epithelial cells, and podocytes, where it binds to its ligand. In atherosclerosis, oxidized LDL promotes the formation of foam cells via CD36 and increases the expression of CD36 and cytokines, which leads to intimal hyperplasia and narrowing of arteries [14]. In type 2 diabetes mellitus, abnormal expression of CD36 increases absorption of fatty acids, decreases glucose uptake, and enhances glycogen synthesis, all of which promote insulin resistance [15]. Moreover, CD36 plays an important part in many kidney diseases. Upregulation of CD36 in response to a high level of glucose or fatty acids can promote epithelial-mesenchymal transition in renal tubular epithelial cells and apoptosis of podocytes, which can promote diabetic nephropathy [16,17]. Here, we treated podocytes with LDL and IL-1β to mimic the high level of lipids and inflammation in PNS. This treatment increased expression of CD36, intracellular cholesterol deposit, and apoptosis of MPC5 cells, all of which were inhibited by treatment with an anti-CD36 antibody. These appearances suggest that LDL induces damage to podocyte, which can be aggravated by IL-1β, while blocking CD36 can reduce this damage. Similarly, as we found, Kim et al. also reported that CD36 is upregulated in nephrotic mouse kidney tissue [18]. These findings indicate that CD36 is involved in the development of PNS. However, the mechanism by which CD36 damages podocytes needs to be elucidated.
The NLRP3 inflammasome, which comprises NLRP3, ASC, and caspase-1, is the most extensively studied member of the nucleotide-binding oligomerization domain-like receptor family. Activation of the NLRP3 inflammasome leads to activation of pro-caspase-1, which triggers an inflammatory response via cytokines such as IL-1β and IL-18 [19,20]. The NLRP3 inflammasome can be activated by the generation of reactive oxygen species, an intracellular potassium deficiency, and lysosomal instability. Increasing evidence demonstrates that CD36 can activate the NLRP3 inflammasome in many sterile inflammatory diseases, including type 2 diabetes mellitus, Alzheimer’s disease, and atherosclerosis [21]. In the present study, treatment with IL-1β increased expression of CD36 and NLRP3 in MPC5 cells, the latter of which was suppressed by treatment with anti-CD36 antibody. This suggests that CD36 plays a critical role in podocyte apoptosis by activating the NLRP3 inflammasome.
Conclusions
In conclusion, we demonstrated that expression of CD36 was increased in nephrotic mouse kidney tissue. In a high-fat and sterile environment, CD36 expression was upregulated in response to the inflammatory cytokine IL-1β. This promoted activation of the NLRP3 inflammasome, which increased podocyte apoptosis. Our findings suggest that CD36 increases podocyte apoptosis in PNS by activating the NLRP3 inflammasome. Further studies are required to elucidate the mechanism by which CD36 and the NLRP3 inflammasome promote podocyte apoptosis in PNS.
Footnotes
Source of support: This study was supported by a grant from the National Natural Science Foundation of China awarded to Qiu Li (no. 81270802)
Conflict of interests
None.
References
- 1.Lesley R, Paul AB, Detlef B, Nicholas JA. Pediatric nephrology. 2nd ed. Oxford University Press; UK: 2012. [Google Scholar]
- 2.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189–95. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Li Q, Wang L, Yang X. Simvastatin ameliorates glomerulosclerosis in Adriamycin-induced-nephropathy rats. Pediatric Nephrology. 2008;23:2185–94. doi: 10.1007/s00467-008-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Vaziri ND. Role of PCSK9 and IDOL in the pathogenesis of acquired LDL receptor deficiency and hypercholesterolemia in nephrotic syndrome. Nephrology Dialysis Transplantation. 2014;29:538–43. doi: 10.1093/ndt/gft439. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Ming X. CD36: the common soil for inflammation in obesity and atherosclerosis. Cardiovasc Res. 2011;89:485–86. doi: 10.1093/cvr/cvq406. [DOI] [PubMed] [Google Scholar]
- 6.Bechmann LP, Gieseler RK, Sowa JP, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30:850–59. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 7.Okamura DM, Pennathur S, Pasichnyk K, et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CK. J Am Soc Nephrol. 2009;20:495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–88. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Yin Y, Zhou Z, et al. OxLDL-induced IL-1beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm Res. 2014;63:33–43. doi: 10.1007/s00011-013-0667-3. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Cui J, Shi J, et al. Endoplasmic reticulum stress participates in inflammation-accelerated, lipid-mediated injury of human glomerular mesangial cells. Nephrology. 2017;22:234–42. doi: 10.1111/nep.12748. [DOI] [PubMed] [Google Scholar]
- 11.Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol. 2012;23:381–99. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Li Q, Wang L, et al. The effects of inflammation on lipid accumulation in the kidneys of children with primary nephrotic syndrome. Inflammation. 2011;34:645–52. doi: 10.1007/s10753-010-9274-4. [DOI] [PubMed] [Google Scholar]
- 13.Clement LC, Macé C, Avila-Casado C, et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20:17–28. doi: 10.1111/jcmm.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhou X, Zhang Y, et al. Association of the CD36 gene with impaired glucose tolerance, impaired fasting glucose, type-2 diabetes, and lipid metabolism in essential hypertensive patients. Genet Mol Res. 2012;11:2163–70. doi: 10.4238/2012.July.10.2. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Yao K, Huang D, et al. High glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cells. Cardiovasc Diabetol. 2013;12:80–84. doi: 10.1186/1475-2840-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua W, Huang H, Tan L, et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One. 2015;10:e0127507. doi: 10.1371/journal.pone.0127507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Vaziri ND. Sterol regulatory element-binding proteins, liver X receptor, ABCA1 transporter, CD36, scavenger receptors A1 and B1 in nephrotic kidney. Am J Nephrol. 2009;29:607–14. doi: 10.1159/000193631. [DOI] [PubMed] [Google Scholar]
- 19.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase- 1 adaptors ASC and Ipaf. Nature. 2004;430:213–18. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Yaginuma K, Tsutsui H, et al. ASC is essential for LPS-induced activation of procaspase-1 in dependently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–67. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 21.Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]