Abstract
Background
The aim of this study was to design and test a novel composite scaffold with antibacterial efficacy for treating bone infections using a three-dimensional (3D) printed poly(ɛ-caprolactone) (PCL) scaffold coated with polydopamine (PDA) for the adsorption of polylactic acid-glycolic acid (PLGA) microspheres loaded with vancomycin.
Material/Methods
Vancomycin-loaded PLGA microspheres were produced by the double-emulsion method, and microsphere morphology, drug-loading dosage, encapsulation efficiency, average diameter, and release characteristics were examined. Composite scaffolds were prepared by adsorption of the microspheres on PDA-coated, 3D-printed PCL scaffolds, and scaffold morphology, biocompatibility, vancomycin release, and antibacterial efficacy were evaluated.
Results
The vancomycin-loaded microspheres were smooth, round, uniform in size, and had no adhesion phenomenon, and exhibited sustained release of vancomycin from the microspheres for more than 4 weeks. Upon modification with PDA, the PCL scaffold changed from white to black, and after microsphere adsorption, dot-like white particles were seen. On scanning electron microscopy, PDA particles were observed on the PCL/PDA composite scaffolds, and PLGA microspheres were evenly dispersed over the PDA coating on the PCL/PDA/PLGA composite scaffolds. Cell viability assays showed that the adhesion and proliferation of rabbit bone mesenchymal stem cells were greater on the PCL/PDA scaffolds than on unmodified PCL scaffolds. Microsphere adsorption had no significant effect on cell proliferation. In vitro release of vancomycin from the composite scaffolds was observed for more than 4 weeks, and observation of the inhibition zone on agar plates of Staphylococcus aureus showed that the scaffolds maintained their antibacterial effect for more than 4 weeks.
Conclusions
The 3D-printed, PDA-coated PCL scaffold carrying vancomycin-loaded PLGA microspheres exhibited good biocompatibility and a sustained antibacterial effect in vitro.
MeSH Keywords: Bone Diseases, Infectious; Microspheres; Tissue Scaffolds; Vancomycin
Background
Infection of bone tissue, known as osteomyelitis, is a serious condition that can be difficult to treat. The incidence of osteomyelitis reportedly doubled from the 1960s to the 2000s in the USA alone [1]. While the most common cause of childhood osteomyelitis is blood-borne infection [2], osteomyelitis in adults is more commonly caused by exogenous infection following an open fracture resulting from trauma [3] or orthopedic surgery. Notably, the tibia is the most common site of open fractures and is also the most common site of bone infection [4]. Moreover, an estimated 10% of orthopedic surgery patients will develop bone infection following surgery [5]. The traditional treatment for bone infection is to remove all necrotic tissue, apply antibiotic treatment, and repair the associated bone defect. Early application of large doses of effective antibiotics is the basis of all treatment. Two forms of antibiotic treatment are available: systemic administration and topical administration. Unfortunately, bacterial biofilm formation on the surfaces of implants and sequestra limits the effectiveness of systemic antibiotics, as the drugs are unable to penetrate the biofilm at sufficient concentrations to treat osteomyelitis [6]. Topical application of antibiotics can provide a much greater concentration of antibiotics at the infection site compared with systemic delivery, while also avoids the adverse effects of systemic antibiotic use. However, due to the internal site of bone infection, antibiotics can only be applied surgically; thus, a method for the sustained local delivery of antibiotics at the infection site is required. To address this need, the development of a controllable biodegradable antibiotic carrier has become a research hotspot in the field of orthopedics [7].
An early approach to topical antibiotic delivery for osteomyelitis involved antibiotic loading of bone cements, such as polymethyl methacrylate (PMMA), which can deliver antibiotics to the lesion and maintain effective antibacterial concentrations for a period of time [8,9]. However, these bone cements require a second surgery for their removal, as they are not biodegradable. One material that does offer excellent biodegradability and biocompatibility, polylactic acid-glycolic acid (PLGA), has now been widely used as a drug carrier and scaffold in tissue engineering applications [10]. The ability to control the release of a loaded drug by adjusting the ratio of lactic acid (LA) and glycolic acid (GA), the length of polymers used, and the rate of polymerization makes PLGA scaffolds a good sustained-release carrier [11]. PLGA can also easily be processed into any shape and size [12], making it a suitable material for filling a bone defect during surgery. In addition, PLGA can be used to fabricate drug-releasing microspheres [13]. Based on these properties, and chiefly its non-toxic nature, PLGA has been approved by the U. S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for parenteral administration [14], and with this approval, PLGA copolymers have become widely used in various fields. With its adjustable degradation characteristics and biocompatibility, PLGA microspheres can be fabricated to deliver almost all types of antibiotics. Durations of release varying from 17 and 49 days have been reported, depending on the type of antibiotic loading [15]. Another study reported antibiotic release from PLGA microspheres for more than 8 weeks in vitro and demonstrated significant improvement in the repair of an infected bone defect in an in vivo model [16].
Clinically, staphylococcal infection is common among cases of osteomyelitis, and the corresponding antibiotic for first-line treatment is vancomycin, a broad-spectrum glycopeptide antibiotic to which Gram-positive bacteria are especially sensitive [17]. To date, vancomycin has been loaded into various forms of PLGA, including nanoparticles [18], microspheres [19], scaffolds [20], electrospun membranes [21], and others. Originally, in the 1990s, Atkins et al. developed a vancomycin-loaded PLGA microsphere and tested its antibiotic properties [22]. However, the vancomycin-loaded microspheres were easily washed away within the blood and, thus, did not remain within the site of debridement [23] to maintain an effective antimicrobial concentration over time. Therefore, the need for a carrier of the antibiotic-loaded microspheres was quickly realized. Since then, many studies have tested other materials as carriers for the simple microspheres, such as cement composites and hydrogels [24,25].
The rapid development of digital medical technology has made possible 3D printing of scaffolds of any shape, size, and porosity to precisely fill bone defects [26]. The bone infection area is first characterized by data from computed tomography (CT) scanning, and then the corresponding scaffold structure can be printed by 3D printing technology based on computer-aided design (CAD) and auxiliary manufacturing (CAM). One material that has been used in 3D printing is poly (ɛ-caprolactone) (PCL), an inexpensive polyester. PCL is widely used in the field of tissue engineering [27] because it can be used in a variety of environments without significant loss of its mechanical, physical, and chemical properties. For this reason, it has been mainly used to replace hard tissues, specifically bone tissue. Surface modification is necessary to give the PCL biofunctionality, and polydopamine (PDA), which is formed by the oxidation self-polymerization of dopamine in an alkaline solution [28], can form a tightly adhered coating on the surface of many organic or inorganic materials through Michael addition or Schiff base reaction [29].
In the present study, a PDA coating was applied to the surface of 3D-printed PCL scaffolds, and PLGA microspheres loaded with vancomycin were then adsorbed onto the surface to create a scaffold for treating infections at the site of bone defects. First, vancomycin-loaded PLGA microspheres were produced by a multiple emulsion method. The microsphere morphology, drug-loading dosage, encapsulation efficiency, average particle diameter distribution, and drug release curves were evaluated in vitro. Suitable microspheres were selected for adherence to the surface of PCL/PDA composite scaffolds. Then, the appearance, morphology, and biocompatibility of the composite scaffolds were evaluated by electron microscopy, fluorescence immunostaining, and CCK-8 cell proliferation assay. Finally, we examined the release of vancomycin from the scaffolds in vitro as well as the corresponding antibacterial efficacy.
Material and Methods
Production and characterization of PLGA microspheres
The double-emulsion solvent evaporation method was used to produce vancomycin-loaded PLGA microspheres. Briefly, 400 mg PLGA (75/25/51000, Jinan Bioengineering Co., Ltd.) was dissolved in 5 ml dichloromethane solution by ultrasonic mixing for 30 min. Then, sonication was continued for another 30 min after the addition of 200 μl of 0.5% Span 80. Separately, vancomycin (50, 100, or 200 mg, Dalian Meilun Biotechnology Co., Ltd.) was dissolved in 1 ml distilled water by ultrasonic mixing for 30 min, followed by the addition of 200 μl of 2% polyvinyl alcohol (PVA) solution and another 30 min of sonication. Once the PLGA and vancomycin were fully dissolved in the respective solutions, the aqueous phase was added dropwise to the oil phase. The colloidal solution was formed by application of a shear force using the T18 digital Ultra-Turrax apparatus (German IKA Group) at 3000 rpm for 2 min. Then, the colloidal solution was added dropwise to 20 ml of 50 mg/ml sodium chloride solution containing 2% PVA rapidly and uniformly, with mechanical stirring at 300 rpm for 2 min to obtain the double emulsion. The double emulsion was diluted with 30 ml of distilled water. After stirring at a low speed of 600 rpm for 6 h, the multiple emulsion was centrifuged, washed 3 times, pre-frozen for 24 h, and finally freeze-dried for 24 h (Labconco FreeZone 2.5).
The obtained microspheres were dispersed in distilled water, and the particle diameter was measured with a Malvern particle diameter analyzer (Malvern Instruments, Mastersizer 2000, UK). Samples of microsphere suspension also were dropped onto a glass slide and observed under an optical microscope (Nikon, Japan). Additionally, microspheres in lyophilized powder form were uniformly placed on pieces of double-sided tape and electroplated using a BAL-TECs SCD 500 ion plating apparatus for observation of their morphology by scanning electron microscopy (SEM; Hitachi S4800 SEM).
Determination of drug-loading dosage and encapsulation efficiency of PLGA microspheres
For the evaluation of vancomycin loading in PLGA microspheres, we first constructed a standard curve of the absorbance of vancomycin solutions in dimethyl sulfoxide (DMSO) at concentrations of 0, 0.1, 0.25, 0.5, 1, and 2 mmol/L using a multifunctional microplate reader (TWIN200PRO, Tecan). Then, we dissolved accurately weighted amounts of the microspheres formed with the 3 different loading amounts (50, 100, or 200 mg vancomycin) in 50 ml DMSO by sonication for 30 min. The absorbance of 100-μl aliquots of the resulting solutions were measured by UV spectrophotometry. For absorbance measurements outside of the range of calibration, the sample was diluted proportionally and retested. The wash solutions for the microspheres prepared with the 3 different loading amounts also were retained, and the vancomycin concentration in those solutions was also measured by ultraviolet spectrophotometry. Each experiment was carried out in triplicate. The drug-loading dosage and encapsulation efficiency were calculated using the following formulae:
Vancomycin release from PLGA microspheres in vitro
The dialysis bag method was used to evaluate the release characteristics of the microspheres, and 100 mg of vancomycin standard was used as a control. PLGA microspheres prepared using the 3 amounts of vancomycin were precisely weighed and placed in a dialysis bag. After 5 ml of the release medium (phosphate-buffed saline [PBS], pH 7.4) was added, the dialysis bag was fastened and placed in 50 ml PBS in a measuring cup kept in a 37°C constant-temperature water bath shaker set at a speed of 100 rpm. Samples of 100 μl were taken on days 1, 3, 5, 7, 14, 21, and 28 and each time we replaced the same volume of release medium at the same temperature. The absorbance of collected samples was measured by ultraviolet spectrophotometry, and the drug content was calculated according to the calibration curve. Triplicate samples were evaluated for microspheres loaded with the different amounts of vancomycin, and the mean and standard deviation for vancomycin concentrations were calculated to generate release percentage curves.
Preparation and characterization of PCL scaffolds, PDA-coated PCL scaffolds, and PCL/PDA composite scaffolds loaded with vancomycin-loaded PLGA microspheres
A triangular-shaped scaffold of PCL (Mn 80000, Sigma-Aldrich, USA) was designed using Mimics 17.0 software, with the following parameters: diameter of 5 mm, height of 1.05 mm, triangular pores (0°/60°/120° pattern), and a mesh fill width of 750 μm. The PCL scaffolds were printed using a biological fused deposition 3D printer (Shanghai Fuqifan Electromechanical Technology Co., Ltd.)
For coating with PDA, the PCL scaffolds were placed in a solution of Tris buffer (0.183 g Tris [Sigma-Aldrich] in 150 ml deionized water with pH adjusted to 8.5 using 0.5 mM hydrochloric acid). Then, 0.3 g dopamine (Sigma-Aldrich) was added, and the solution was stirred for 24 h in the dark. Afterward, the scaffolds were removed, washed 3 times with deionized water, and freeze-dried. These PDA-coated PCL scaffolds are denoted as PCL/PDA scaffolds hereafter.
For absorption of vancomycin-loaded PLGA microspheres onto the PCL/PDA composite scaffolds, samples of vancomycin-loaded PLGA microspheres prepared with the highest drug-loading amount were dispersed in distilled water to form a suspension. PCL/PDA scaffolds were placed into this suspension, which was stirred at a low speed (150 rpm) by magnetic stirring for 2 h. Then, the composite scaffolds were freeze-dried. These scaffolds are designated as PCL/PDA/PLGA scaffolds hereafter.
For observation of the scaffold appearance and morphology, scaffold samples were photographed under a shadowless lamp and then fixed on the sample cup with a conductive adhesive. After gold plating of the scaffold surface, the surface morphology of the scaffolds was observed by SEM.
Cytocompatibility of the composite scaffolds
Bone marrow samples were aseptically obtained from the posterior superior iliac spine of the New Zealand White rabbits (aged 4–6 months, weighting 3.5–4.0 kg) via the bone marrow puncture procedure. Rabbit bone marrow-derived mesenchymal stem cells (rBMSCs) were then isolated and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Gibco), 1% HEPES buffer, and 1% antibiotics/antimycotics. rBMSCs from the third generation were seeded at a density of 2×104 cells/ml in wells of a 96-well plate containing scaffold samples and cultured for 7 days. The medium was removed from the 6 wells on days 1, 3, 5, and 7. Then, 100 μl of culture medium and 10 μl of CCK-8 solution (Sigma-Aldrich) were added. After incubation for 2 h in a 37°C incubator, the absorbance at 450 nm of the sample solutions was measured by a microplate reader.
For SEM observation of cells seeded on the scaffolds, rBMSCs at a density of 2×106 cells/ml were added to wells of a 96-well plate containing the scaffolds and cultured for 7 days. On days 1 and 7, scaffold samples were collected, washed 3 times with PBS, and fixed in 4% paraformaldehyde. Then, the specimens were dehydrated using a series of graded ethanol solutions and freeze-dried for 24 h. After gold plating of the surface, the scaffolds and attached cells were viewed by SEM.
The viability of rBMSCs seeded onto the different scaffolds was assessed using a Live/Dead Reduced Biohazard Viability/Cytotoxicity kit (Molecular Probes; Thermo Fisher Scientific, Inc.). As above, rBMSCs at a density of 2×106 cells/ml were added to wells of a 96-well plate containing the scaffolds and cultured for 7 days. On day 7, samples were washed with PBS, incubated in the diluted dye solution for 15 min in the dark at room temperature, and then fixed in 4% glutaraldehyde for 1 h. The samples were imaged using a confocal microscope (Leica TCS SP2 confocal microscope; Leica, Mannheim, Germany). Live cells were stained green and dead cells were stained red.
Vancomycin release and antibacterial activity of the composite scaffolds in vitro
A percent release curve was generated for vancomycin release from the PCL/PDA/PLGA scaffolds by the dialysis bag method described above. The PCL and PCL/PDA scaffold controls were magnetically stirred at low speed (150 rpm) for 2 h in an aqueous solution of vancomycin containing an amount of vancomycin equal to that in the microsphere suspensions used to make the PCL/PDA/PLGA scaffolds. Then, these control scaffolds were freeze-dried to obtain PCL/VAN and PCL/PDA/VAN scaffolds, for which percent release curves were also generated using the dialysis bag method. Samples were taken on days 1, 3, 5, 7, 14, 21, and 28 from triplicate samples for each type of scaffold. From the calculated mean and standard deviation values for vancomycin concentration, we plotted the percent release curves.
Staphylococcus aureus (strain 29213 from the American Type Culture Collection) at a density of 1.5×108 colony forming units (CFU)/mL was supplied by the clinical laboratory of the affiliated Nanjing Hospital of Nanjing Medical University. Aliquots of 0.5 ml of bacterial suspension were inoculated uniformly on Mueller-Hinton plates (Keygene Biotechnology Co., Ltd., Nanjing, China). Next, samples of the PCL/VAN, PCL/PDA/VAN, and PCL/PDA/PLGA scaffolds were placed on agar plates and incubated at 37°C in a constant-temperature bacterial incubator. The antibacterial activity was observed by recording of the size of the inhibition zone on days 0, 1, 3, 5, 7, 14, 21, and 28. The medium was exchanged every 24 h.
Statistical methods
One-way analysis of variance (ANOVA) and the least significant difference (LSD) method were used to determine the differences among groups. All data are expressed as mean ± standard deviation (SD). P<0.05 indicated a statistically significant difference. All statistical analyses were performed using SPSS 18.0 statistical software.
Results
Characterization of vancomycin-loaded PLGA microspheres
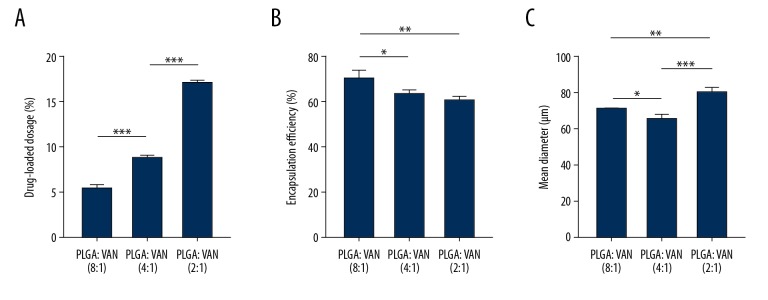
Figure 1 shows the results of the evaluations of drug-loading dosage, encapsulation efficiency, and mean diameter of the vancomycin-loaded PLGA microspheres. According to the initial ratio of PLGA to vancomycin − 8: 1, 4: 1, and 2: 1 – the final formed microspheres were labeled as groups 1, 2, and 3, respectively. With the use of 400 mg of PLGA and either 50, 100, or 200 mg vancomycin to prepare the drug-loaded microspheres, the initial ratios of PLGA to vancomycin were 8: 1, 4: 1, and 2: 1, respectively, for the different formulations of microspheres. As the ratio of PLGA to vancomycin decreased, the drug-loading dosage increased significantly from 5.48±0.36% to 16.98±0.34%, with significantly more vancomycin loaded in the scaffolds prepared with the 2: 1 ratio of materials than in those prepared with the other ratios (P<0.001), and a corresponding slight decrease in the encapsulation rate, from 70.20±3.62% to 60.37±1.77%, was observed with these different ratios (P<0.05). The microspheres prepared with the 2: 1 ratio had the largest diameter compared with the microspheres prepared with the 8: 1 ratio (P<0.01) or the 4: 1 ratio (P<0.001) of polymer to drug.
Figure 1.
Characterization of drug-loaded microspheres. (A) Dosage of vancomycin loaded into microspheres prepared using 3 different formulations. (B) Vancomycin encapsulation efficiency of 3 microsphere formulations. (C) Mean diameters of microspheres prepared using the 3 formulations. * P<0.05.
Vancomycin release from PLGA microspheres in vitro
From the release curves shown in Figure 2, an initial burst release of vancomycin solution was obvious, and the majority of drug was released within 3 days. However, the obvious initial burst release phenomenon within 24 h was followed by slow release for all drug-loaded microsphere formulations, with the total release time exceeding 28 days. Notably, no significant differences were observed among the release curves for the 3 loading conditions (P>0.05). Therefore, we selected the microspheres with the highest drug-loading dosage for the subsequent experiments.
Figure 2.
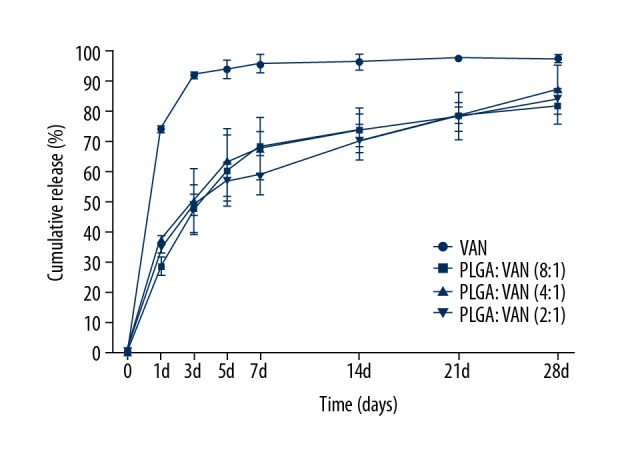
Cumulative release of vancomycin from drug-loaded microspheres prepared with 3 different formulations over 28 days in vitro.
Gross appearance of the PCL, PCL/PDA, and PCL/PDA/PLGA scaffolds
After coating of the PCL scaffolds with PDA, the PCL scaffold color changed from white to black (Figure 3A, 3B). With the adherence of the PLGA microspheres, no further change in the general color occurred, and spot-like white particles were seen on the scaffold surface (Figure 3C). Consistently, optical microscopy observation of the microsphere suspension on a slide revealed that the microspheres were smooth, round, uniform in size, and non-adherent to the slide surface (Figure 3D).
Figure 3.
General appearance of the PCL, PCL/PDA, and PCL/PDA/PLGA scaffolds, as well as the microsphere suspension, under optical microscopy. (A) PCL scaffold. (B) PCL/PDA scaffold. (C) PCL/PDA/PLGA scaffold. (D) Microsphere suspension on a slide.
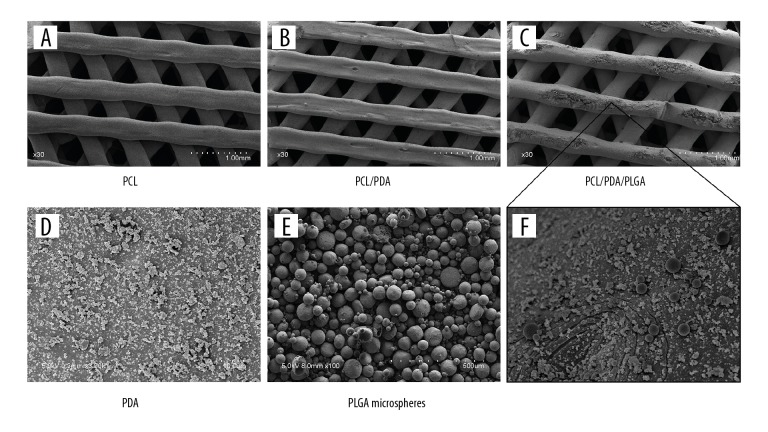
Morphology of the PCL, PCL/PDA, and PCL/PDA/PLGA scaffolds on SEM
Under SEM at low magnification, the prepared scaffolds with pores arranged in the 0°–60°–120° triangular pattern showed no obvious differences in morphology or structure (Figure 4A–4C). At high magnification, PDA particles were observed over the PCL/PDA scaffolds (Figure 4D), and PLGA microspheres (Figure 4E) were evenly dispersed among the PDA particles on the PCL/PDA/PLGA composite scaffold (Figure 4F).
Figure 4.
SEM observation of scaffold morphology. Lower magnification images of the: (A) PCL scaffold; (B) PCL/PDA scaffold and (C) PCL/PDA/PLGA scaffold. Higher magnification images showing: (D) PDA particles on the PCL/PDA scaffold; (E) morphology of vancomycin-loaded PLGA microspheres; and (F) the coexistence of PDA particles and vancomycin-loaded PLGA microspheres on the PCL/PDA/PLGA scaffold surface.
Survival and proliferation of rBMSCs on all prepared scaffold types
The CCK-8 assay was used to detect the viability of rBMSCs cultured on the different scaffold types, and the increase in the number of cells over time with all scaffold samples indicated that all of the prepared scaffolds were non-toxic to the cells (Figure 5). On day 1, the OD values, representing cell number, for the PCL/PDA and PCL/PDA/PLGA groups were higher than that for the PCL group (both P<0.05). However, no significant differences in OD values were observed between the groups on the third day in culture (all P>0.05), and by days 5 and 7 the OD values for the PCL/PDA and PCL/PDA/PLGA groups were again significantly higher than in the PCL group (all P<0.001).
Figure 5.
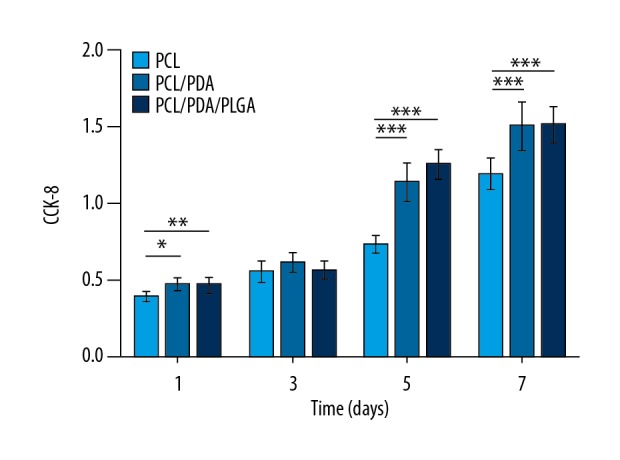
Proliferation of rBMSCs on each scaffold type as measured by CCK-8 assay. * P<0.05.
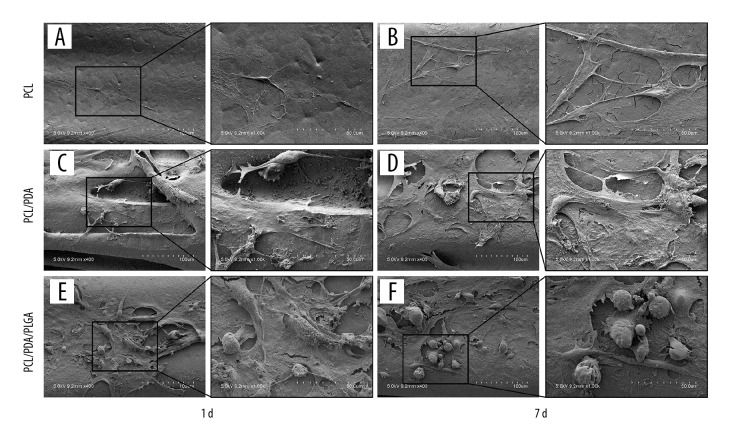
Representative scanning electron micrographs of rBMSCs cultured on the scaffold surfaces are shown in Figure 6. A small amount of cell adhesion and matrix secretion was observed on the surface of the PCL scaffold on day 1, with slight increases in both by day 7 (Figure 6A, 6B). Cell adhesion and matrix secretion were observed on the surface of the PCL/PDA scaffold on day 1, and both were significantly increased by day 7 (Figure 6C, 6D). PLGA microspheres were distributed over the PCL/PDA/PLGA composite scaffold along with many adherent cells, with cellular adhesion and extracellular matrix secretion being similar to those seen on the PCL/PDA scaffold (Figure 6E, 6F).
Figure 6.
SEM observation of rBMSC adhesion and matrix deposition on the 3 scaffold types. (A) PCL scaffold on day 1. (B) PCL scaffold on day 7. (C) PCL/PDA scaffold on day 1. (D) PCL/PDA scaffold on day 7. (E) PCL/PDA/PLGA scaffold on day 1. (F) PCL/PDA/PLGA scaffold on day 7.
Live/dead staining of rBMSCs seeded on the different scaffolds after 7 days in culture revealed that more cells were present on the PCL/PDA and PCL/PDA/PLGA scaffolds than on the PCL scaffold (Figure 7). These results further confirm that the surfaces coated with PDA can effectively promote cell adhesion and that the presence of vancomycin-loaded PLGA microspheres had no effect on rBMSC adherence or proliferation.
Figure 7.
Live/dead staining of rBMSCs seeded on the different scaffold surfaces and cultured for 7 days. Representative individual and merged confocal micrographs show live (green) and dead (red) cells on the: (A) PCL scaffold, (B) PCL/PDA scaffold, and (C) PCL/PDA/PLGA scaffold.
Sustained release of vancomycin and bacterial inhibition by PCL/PDA/PLGA composite scaffolds in vitro
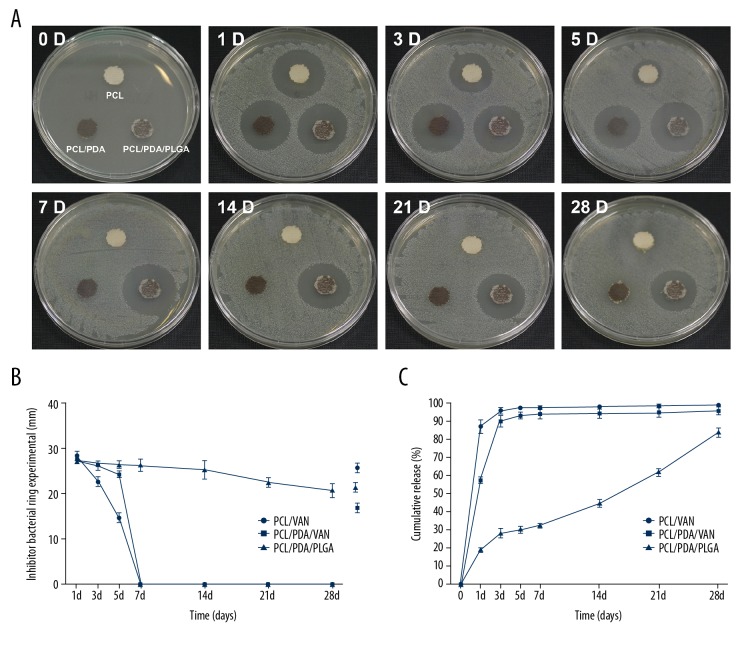
Immediate burst release of vancomycin from the control PCL/VAN and PCL/PDA/VAN scaffolds was obvious, with the maximum release occurring within 3–5 days (Figure 8C). In contrast, the release profile for vancomycin from the PCL/PDA/PLGA scaffolds indicated a slower, more sustained release, with a cumulative release rate of only 82.53% at 28 days.
Figure 8.
(A) Representative photographs of the bacterial inhibition zones created by the different scaffolds on days 0, 1, 3, 5, 7, 14, 21, and 28. (B) Quantitated inhibition zone diameters for each scaffold type over the experimental time course. (C) Cumulative vancomycin release profiles for the 3 scaffold types over 28 days in vitro.
Consistent with the calculated antibiotic release profiles, the PCL/VAN and PCL/PDA/VAN scaffolds had antibacterial effects against S. aureus in the agar medium in the first few days of culture and no antibacterial effects after 5 days as the bacteria grew back within the inhibition zone around these scaffolds (Figure 8A, 8B). The PCL/PDA/PLGA scaffold exhibited a significant inhibitory effect against the bacteria initially, and this effect was sustained over the 28-day culture period, although the zone of inhibition did gradually decrease with time. Overall, the results of the bacterial inhibition experiment were basically consistent with the vancomycin release profiles for the different scaffolds, further demonstrating the dual-phase release mode consisting of an initial burst release followed by continuous controlled release.
Discussion
With the rapid development of societies worldwide, the incidence of traumatic bone injuries in motor vehicle accidents as well as the use orthopedic implants continue to increase, and consequently, the incidence of osteomyelitis also continues to increase. Osteomyelitis has become a common secondary disease in orthopedics [30], and antibiotic use is necessary for its treatment. Vancomycin is often used to prevent and treat infections caused by Staphylococcus bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). In fact, vancomycin has remained the standard drug used to treat orthopedic MRSA infections since the mid-1980s [31]. While the glycopeptide antibiotic vancomycin is the first choice for treating orthopedic infections in general [17], effective delivery of this antibiotic has proved challenging. Oral delivery of such a large hydrophilic molecule that cannot pass through the lipophilic gastrointestinal mucosa leads to very low bioavailability, but intravenous administration of high doses of vancomycin can cause nephrotoxicity, ototoxicity, an allergic reaction, and gastrointestinal problems [32]. To overcome these challenges, a local drug delivery system is needed that can achieve effective drug concentrations at the site of the bone infection, while the drug concentration is blood remains low and adverse effects are avoided. In the present study, we aimed to develop a suitable local delivery strategy based on 3D-printed PCL scaffolds coated with PDA to facilitate of adsorption of vancomycin-loaded PGLA microspheres.
At present, drug-loaded PLGA microspheres can be prepared using many techniques, such as the double-emulsion solvent evaporation method, the spray drying method, ultrasonic atomization, an electrospray method, and others [33]. Among these, the double-emulsion solvent evaporation method [15] involves dispersing the drug in an organic solvent that can dissolve the carrier material. Then, the drug-loaded microspheres are formed by solidification when the organic solvent is removed by extraction, volatilization, or phase separation. Advantages of this method include its simple operation, controllable preparation process, favorable loading of hydrophilic drugs, and high drug-loading dosage capability; therefore, it has been widely used in the pharmaceutical field in recent years. We used this technique to prepare vancomycin-loaded PLGA microspheres in the present study. Multiple studies have demonstrated that drug release from PLGA microspheres is influenced by the ratio of lactic acid to glycolic acid in the co-polymer [34], the size of the microspheres [35], and the stabilizer used [36]. We selected an LA: GA polymerization ratio of 75: 25 to obtain a longer sustained release time. Moreover, the stirring rate of 600 rpm was chosen to increase the diameter of the microspheres, and the stabilizer Span 80 was added to increase both the drug-loading and encapsulation rate, which also prolongs the release time of the drug. Microsphere size is generally designed according to the planned route of administration and desired degradation time. For microspheres that will be implanted, a relatively large mean diameter is needed to prevent easy diffusion, which is not conducive to maintaining an effective local concentration of the drug. Therefore, the mean diameter of such microspheres is commonly in the range of several micrometers to 10 micrometers [15]. In the present study, we tested the loading and release of vancomycin using 3 different ratios of PLGA to vancomycin (8: 1, 4: 1, and 2: 1) to prepare drug-loaded microspheres. With these formulations, the mean diameter of the microspheres ranged from 65.4±2.66 μm to 80.13±2.72 μm, the drug-loading dosage ranged from 5.48±0.36% to 16.98±0.34%, and the encapsulation efficiency ranged from 60.37±1.77% to 70.20±3.62%. As the ratio of PLGA to vancomycin decreased (the relative amount of vancomycin increased), the drug-loading dosage increased significantly and the encapsulation rate decreased slightly. Under SEM, all of the microspheres were smooth and round, with a uniform spherical shape and no adhesion to each other. Moreover, our in vitro drug release study using the dialysis bag method showed that a similar vancomycin release profile was obtained from microspheres fabricated using the different formulations, with an initial burst within the first 24 h followed by sustained release over more than 4 weeks. Therefore, we used the microspheres formed with a 2: 1 ratio of PLGA to vancomycin, which had a relatively larger mean diameter and the largest loading dosage, in our subsequent experiments.
Although many studies have demonstrated that the microspheres can carry antibiotics to the site of a lesion and maintain antibacterial activity for a desired period of time, vancomycin-loaded microspheres are difficult to apply directly to an infected bone cavity in practice, and their loss in vivo diminishes the curative effect. Therefore, an effective carrier for the drug-laden microspheres is needed. Rapid prototyping technology based on 3D printing is currently a research hotspot. 3D printer systems (3DPSs) use digital drivers to direct the accumulation of materials to form 3D solid models with few limits on shape complexity, making it suitable for the fabrication of idealized custom scaffolds with precise shape and size [37,38]. Currently, in the field of orthopedics, the CAD/CAM software Mimics can use 3D imaging data of bone defects obtained by CT scanning to design and synthesize personalized 3D scaffolds for filling bone defect sites [39,40]. PCL, which is approved by the FDA for use in the human body, is a biodegradable semi-crystalline linear aliphatic polyester biomaterial with good biocompatibility and biodegradability. Due to its thermoplastic characteristics [41], as well as its suitability for constructing scaffolds via layer stacking with a 3DPS [42], it has good mechanical properties for use as a scaffold material in hard tissues. Among the most commonly used synthetic polymers [e.g., PLA, PGA, poly(D-L-lactic-co-glycolic acid)], PCL has the slowest degradation rate [43], with complete degradation in vivo generally taking 3–5 years. With these characteristics, PCL degradation is favorably matched to bone tissue growth and complete healing [44]. In addition to fabricating scaffolds with a specific shape, 3D printing can also be used to control the characteristics of interconnected pores with the printed scaffold, which can allow the formation of vascularized tissue to supply the nutrients and oxygen required for cell growth within the scaffold [45]. Studies have shown that macroporosity with interconnected pores of 300 μm or more is critical for the vascularization of implanted scaffolds and beneficial for the associated tissue regeneration [46]. The porous scaffold applied in the present study had a pore diameter of 750 μm, which provides sufficient space within the scaffold for drug release and cell growth, and this structure is similar to the extracellular matrix with a high ratio of surface area to volume.
However, studies have shown that a single polymer may not have all the properties required for an ideal scaffold, and most synthetic polymers have poor cell affinity due to their hydrophobic surface and lack of cell biological recognition [47]. Therefore, a variety of methods have been used to create hybrid scaffolds with carefully tuned surface roughness and hydrophilicity in order to create a good tissue interface conducive to cell ingrowth and osteoblast differentiation [48,49]. PDA coating technology was inspired by the adhesive proteins secreted by mussels. Lee et al. [13] first reported the use of PDA as a surface modification material. Further research attributed the adhesive properties of PDA to its the viscous catecholamine group [50]. It has been suggested that the PDA coating not only changes the hydrophobicity of a 3D-printed PCL scaffold, but also significantly increases cell adhesion to the scaffold [51]. We have manufactured PCL scaffolds using the 3D printing method known as fused deposition modeling (FDM) [52]. In the present study, when these PCL scaffolds were soaked in PDA solution, its color changed from white to black, and the complex internal porous structure was functionalized via a dip-coating method. Two assays of cell viability, the CCK-8 test and LIVE/DEAD test, showed that more cells were adhered to the PCL/PDA scaffolds than to the PCL scaffolds, consistent with previously studies suggesting that PDA improves cell adhesion. SEM showed that the PDA coating not only promoted cell adhesion, but also promoted the secretion of extracellular matrix, which is conducive to the maintenance of cellular activity. Finally, we were able to uniformly adsorb microspheres onto the surface structures of the 3D-printed PCL scaffold through adhesion to the PDA coating. The CCK-8 and LIVE/DEAD assays both indicated that the PCL/PDA/PLGA scaffolds, like the PCL/PDA scaffolds, were conducive to cell attachment and proliferation, indicating that the PCL/PDA/PLGA scaffolds also have good biocompatibility. These findings were confirmed by SEM observations.
We compared the antibacterial effect of the PCL/PDA/PLGA composite scaffold to that of PCL and PCL/PDA scaffolds first soaked in vancomycin solution containing the same amount of vancomycin present in the microsphere suspension used to generate the PCL/PDA/PLGA scaffolds. This allowed us to attribute the observed antimicrobial effects of the PCL/PDA/PLGA scaffolds to vancomycin released from the microspheres in a controlled manner. Evaluations based on drug release curves and zones of bacterial inhibition indicated that the PCL/PDA/PLGA scaffolds maintained a bacteriostatic effect for more than 4 weeks.
Conclusions
In the present study, we present the design of a new composite scaffold for the prevention and treatment of bone infections. In the composite scaffold, vancomycin-loaded PLGA microspheres were absorbed onto 3D-printed PCL scaffolds that had been coated with PDA. To achieve sustained drug release over several weeks and a suitable microsphere diameter, we used PLGA with a 75/25 co-polymer ratio, a dispersive shear force to generate the colloidal solution, and low-speed mechanical agitation to increase the diameter of the microspheres. The addition of the stabilizer Span 80 not only prolonged the duration of drug release, but also increased the encapsulation rate and drug-loading dosage. The vancomycin-loaded PLGA microspheres exhibited good biodegradability over a relatively short period of time, leading to the local release of the drug at effective concentrations. In contrast, the clinically used vancomycin-loaded PMMA carrier requires a second surgery for removal because it does not degrade in vivo. Given that vancomycin-loaded microspheres are easily washed away upon direct injection in vivo and cannot completely fill the empty space create by debridement of the infected bone, directly injected microspheres cannot maintain an effective antimicrobial concentration over time. Thus, a carrier material for the drug-loaded microspheres is needed. The composite scaffold designed for this purpose in the present study has several advantages: 1. The prepared microspheres had a smooth, round surface and uniform spherical shape and exhibited sustained drug release for more than 4 weeks; 2. Due to the adhesive surface properties of the new composite scaffolds achieved via PDA coating, microspheres can be well adsorbed on the scaffold surface, the scaffold properties are stable, and the scaffolds have better cell compatibility than unmodified PCL scaffolds; and 3. Vancomycin was released from the microspheres adsorbed on the composite scaffold with an initial burst followed by long-lasting sustained release and exhibited good antibacterial activity against Staphylococci for more than 4 weeks. At the same time, because the shape of the 3D printed scaffold is controllable, it can be fitted to specific areas of bone infection, and because it is biodegradable, no second removal surgery is needed. Thus, the composite scaffold represents a promising new material for treatment of bone infection.
There are some limitations in the present study that should be considered. We have not yet designed the properties and tested the related efficacy of the composite scaffolds for use in various human bones. In addition, the efficacy of the composite scaffold must be tested further in vivo.
Footnotes
Source of support: This research was sponsored by the National Natural Science Foundation of China (Grant No. 81601612, 81702148, 81771985), the Key Research Program of Science and Technology Support Program of Jiangsu Province (BE 2015613, BE 2016763), and the Orthopedic Clinical Medical Center of Nanjing City of China
Conflict of interest
None.
References
- 1.Kremers HM, Nwojo ME, Ransom JE, et al. Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J Bone Joint Surg Am. 2015;97(10):837–45. doi: 10.2106/JBJS.N.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen E, Lifshitz K, Fruchtman Y, et al. Current data on acute haematogenous osteomyelitis in children in Southern Israel: Epidemiology, microbiology, clinics and therapeutic consequences. Int Orthop. 2016;40(9):1987–94. doi: 10.1007/s00264-016-3211-6. [DOI] [PubMed] [Google Scholar]
- 3.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72(2):299–304. [PubMed] [Google Scholar]
- 4.Agaja SB, Ayorinde RO. Chronic osteomyelitis in Llorin, Nigeria. S Afr J Surg. 2008;46(4):116–18. [PubMed] [Google Scholar]
- 5.Stanley CM, Rutherford GW, Morshed S, et al. Estimating the healthcare burden of osteomyelitis in Uganda. Trans R Soc Trop Med Hyg. 2010;104(2):139–42. doi: 10.1016/j.trstmh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Luo F, Huang K, et al. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res. 2016;5(3):101–5. doi: 10.1302/2046-3758.53.2000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi M, Chen L, Wang Y, et al. Effect of low-frequency pulsed ultrasound on drug delivery, antibacterial efficacy, and bone cement degradation in vancomycin-loaded calcium phosphate cement. Med Sci Monit. 2018;24:797–802. doi: 10.12659/MSM.908776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluin OS, van der Mei HC, Busscher HJ, et al. Biodegradable vs. non-bio-degradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10(3):341–51. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 9.Shinsako K, Okui Y, Matsuda Y, et al. Effects of bead size and polymerization in PMMA bone cement on vancomycin release. Biomed Mater Eng. 2008;18(6):377–85. doi: 10.3233/BME-2008-0554. [DOI] [PubMed] [Google Scholar]
- 10.Martins C, Sousa F, Araújo F, et al. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv Healthc Mater. 2018;7(1) doi: 10.1002/adhm.201701035. [DOI] [PubMed] [Google Scholar]
- 11.Fonte P, Araújo F, Silva C, et al. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol Adv. 2015;33(6 Pt 3):1342–54. doi: 10.1016/j.biotechadv.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Gentile P, Chiono V, Carmagnola I, et al. Int J Mol Sci. 2014;15(3):3640–59. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Ni B, Zhu Z, et al. Intra-discal vancomycin-loaded PLGA microsphere injection for MRSA discitis: An experimental study. Arch Orthop Trauma Surg. 2011;131(1):111–19. doi: 10.1007/s00402-010-1154-8. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor DN, Bhatia A, Kaur R, et al. PLGA: A unique polymer for drug delivery. Ther Deliv. 2015;6(1):41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 15.Shah SR, Henslee AM, Spicer PP, et al. Effects of antibiotic physicochemical properties on their release kinetics from biodegradable polymer microparticles. Pharm Res. 2014;31(12):3379–89. doi: 10.1007/s11095-014-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spicer PP, Shah SR, Henslee AM, et al. Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model. Acta Biomater. 2013;9(11):8832–39. doi: 10.1016/j.actbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Cui X, Zhao C, Gu Y, et al. A novel injectable borate bioactive glass cement for local delivery of vancomycin to cure osteomyelitis and regenerate bone. J Mater Sci Mater Med. 2014;25(3):733–45. doi: 10.1007/s10856-013-5122-z. [DOI] [PubMed] [Google Scholar]
- 18.Posadowska U, Brzychczy-Wloch M, Pamula E. Injectable gellan gum-based nanoparticles-loaded system for the local delivery of vancomycin in osteomyelitis treatment. J Mater Sci Mater Med. 2016;27(1):9. doi: 10.1007/s10856-015-5604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du L, Yang S, Li W, et al. Scaffold composed of porous vancomycin-loaded poly(lactide-co-glycolide) microspheres: A controlled-release drug delivery system with shape-memory effect. Mater Sci Eng C Mater Biol Appl. 2017;78:1172–78. doi: 10.1016/j.msec.2017.04.099. [DOI] [PubMed] [Google Scholar]
- 20.Cheng T, Qu H, Zhang G, et al. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif Cells Nanomed Biotechnol. 2017 doi: 10.1080/21691401.2017.1396997. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Chou YC, Lee D, Chang TM, et al. Combination of a biodegradable three-dimensional (3D) - printed cage for mechanical support and nanofibrous membranes for sustainable release of antimicrobial agents for treating the femoral metaphyseal comminuted fracture. J Mech Behav Biomed Mater. 2017;72:209–18. doi: 10.1016/j.jmbbm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Atkins TW, Peacock SJ, Yates DJ. Incorporation and release of vancomycin from poly(D,L-lactide-co-glycolide) microspheres. J Microencapsul. 1998;15(1):31–44. doi: 10.3109/02652049809006833. [DOI] [PubMed] [Google Scholar]
- 23.Ford CA, Cassat JE. Advances in the local and targeted delivery of anti-infective agents for management of osteomyelitis. Expert Rev Anti Infect Ther. 2017;15(9):851–60. doi: 10.1080/14787210.2017.1372192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnieders J, Gbureck U, Vorndran E, et al. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J Biomed Mater Res B Appl Biomater. 2011;99(2):391–98. doi: 10.1002/jbm.b.31910. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Ren J, Chen G, et al. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater Sci Eng C Mater Biol Appl. 2018;89:213–22. doi: 10.1016/j.msec.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CN, Miller AT, Hollister SJ, et al. Design and structure-function characterization of 3D printed synthetic porous biomaterials for tissue engineering. Adv Healthc Mater. 2018;7(7):e1701095. doi: 10.1002/adhm.201701095. [DOI] [PubMed] [Google Scholar]
- 27.Malikmammadov E, Tanir TE, Kiziltay A, et al. PCL and PCL-Based materials in biomedical applications. J Biomater Sci Polym Ed. 2018;29(7–9):863–93. doi: 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Dellatore SM, Miller WM, et al. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho CC, Ding SJ. Structure, properties and applications of mussel-inspired polydopamine. J Biomed Nanotechnol. 2014;10(10):3063–84. doi: 10.1166/jbn.2014.1888. [DOI] [PubMed] [Google Scholar]
- 30.Hake ME, Oh JK, Kim JW, et al. Difficulties and challenges to diagnose and treat post-traumatic long bone osteomylitis. Eur J Orthop Surg Traumatol. 2015;25(1):1–3. doi: 10.1007/s00590-014-1576-z. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KD, Johnston DW. Orthopedic experience with methicillin-resistant Staphylococcus aureus during a hospital epidemic. Clin Orthop Relat Res. 1986;(212):281–88. [PubMed] [Google Scholar]
- 32.Rao S, Kupfer Y, Pagala M, et al. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scand J Infect Dis. 2011;43(5):386–88. doi: 10.3109/00365548.2010.544671. [DOI] [PubMed] [Google Scholar]
- 33.Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J Control Release. 2010;146(2):241–60. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsionsolvent extraction/evaporation method. Biomaterials. 2001;22(3):231–41. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 35.Siepmann J, Faisant N, Akiki J, et al. Effect of the sizes of biodegradable microparticles of drug release: Experiment and theory. J Control Release. 2004;96(1):123–34. doi: 10.1016/j.jconrel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Blanco-Príeto MJ, Leo E, Delie F, et al. Study of the influence of several stabilizing agents on the entrapment and in vitro release of pBC 264 from poly (lactide-co-glycolide) microspheres prepared by a W/O/W solvent evaporation method. Pharm Res. 1996;13(7):1127–29. doi: 10.1023/a:1016087530812. [DOI] [PubMed] [Google Scholar]
- 37.Kang SW, Bae JH, Park SA, et al. Combination therapy with BMP-2 and BMSCs enhances bone healing efficacy of PCL scaffold fabricated using the 3D plotting system in a large segmental defect model. Biotechnol Lett. 2012;34(7):1375–84. doi: 10.1007/s10529-012-0900-0. [DOI] [PubMed] [Google Scholar]
- 38.Yao Q, Wei B, Guo Y, et al. Design, construction and mechanical testing of digital 3D anatomical data-based PCL–HA bone tissue engineering scaffold. J Mater Sci Mater Med. 2015;26(1):5360. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- 39.Park CH, Rios HF, Taut AD, et al. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20(7):533–42. doi: 10.1089/ten.tec.2013.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YS, Shin YS, Park DY, et al. The application of three-dimensional printing in animal model of augmentation rhinoplasty. Ann Biomed Eng. 2015;43(9):2153–62. doi: 10.1007/s10439-015-1261-3. [DOI] [PubMed] [Google Scholar]
- 41.Mi HY, Jing X, Yu E, et al. Manipulating the structure and mechanical properties of thermoplastic polyurethane/polycaprolactone hybrid small diameter vascular scaffolds fabricated via electrospinning using an assembled rotating collector. J Mech Behav Biomed Mater. 2018;78:433–41. doi: 10.1016/j.jmbbm.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 42.Park SA, Lee SH, Kim WD. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst Eng. 2011;34(4):505–13. doi: 10.1007/s00449-010-0499-2. [DOI] [PubMed] [Google Scholar]
- 43.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 44.Savarino L, Baldini N, Greco M, et al. The performance of poly-epsilon-caprolactone scaffolds in a rabbit femur model with and without autologous stromal cells and BMP4. Biomaterials. 2007;28(20):3101–9. doi: 10.1016/j.biomaterials.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Mercado-Pagán ÁE, Stahl AM, Shanjani Y, et al. Vascularization in bone tissue engineering constructs. Ann Biomed Eng. 2015;43(3):718–29. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng Part B Rev. 2013;19(6):485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tallawi M, Rosellini E, Barbani N, et al. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J R Soc Interface. 2015;12(108):20150254. doi: 10.1098/rsif.2015.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin YH, Chiu YC, Shen YF, et al. Bioactive calcium silicate/poly-ɛ-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J Mater Sci Mater Med. 2017;29(1):11. doi: 10.1007/s10856-017-6020-6. [DOI] [PubMed] [Google Scholar]
- 49.Rasperini G, Pilipchuk SP, Flanagan CL, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94(9 Suppl):153S–57S. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Song J, Zhang Y, et al. Bioinspired design of polycaprolactone composite nanofibers as artificial bone extracellular matrix for bone regeneration application. ACS Appl Mater Interfaces. 2016 doi: 10.1021/acsami.6b10417. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Jo S, Kang SM, Park SA, et al. Enhanced adhesion of preosteoblasts inside 3D PCL scaffolds by polydopamine coating and mineralization. Macromol Biosci. 2013;13(10):1389–95. doi: 10.1002/mabi.201300203. [DOI] [PubMed] [Google Scholar]
- 52.Lin KF, He S, Song Y, et al. Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl Mater Interfaces. 2016;8(11):6905–16. doi: 10.1021/acsami.6b00815. [DOI] [PubMed] [Google Scholar]