Abstract
Background
Pancreatic cancer causes tremendous mortality across the globe mainly due to late diagnosis and unavailability of efficient chemotheruptic agents. In the current study the anticancer potential of a plant derived alkaloid, Mahanimbine, was examined against a panel of pancreatic cancer cells.
Material/Methods
The cell proliferation was determined by MTT assay. Annexin V/PI and DAPI staining were performed to detect apoptosis. Cell cycle distribution was investigated by flow cytometery. Cell migration was detected by wound healing assay and protein expression was checked by western blotting.
Results
The results revealed that Mahanimbine could inhibit the proliferation of the all the pancreatic cancer cells with lower cytoxicity against the normal cells. The IC50 ranged from 3.5 to 64 μM against the pancreatic cancer cell lines. The lowest IC50 of 3.5 μM was observed tor the Capan-2 and SW119 pancreatic cancer cell lines. The anticancer activity of Mahanimbine against the Capan-2 and SW119 cells was found to be due to G0/G1 cell cycle arrest and induction of apoptosis. Mahanimbine prompted apoptosis was also associated with decline in Bcl-2 and enhancement of the Bax expression. Further, it was observed that Mahanimbine could inhibit the AKT/mTOR and STAT3 signalling pathways in the Capan-2 and SW119 pancreatic cancer cells. The effects of the Mahanimbine were also examined on the migration of the Capan-2 and SW119 pancreatic cancer cells. It was found that Mahanimbine could inhibit the motility and migration of both the pancreatic cancer cell lines.
Conclusions
We found that Mahanimbine inhibits the proliferation of pancreatic cancer cells and as such Mahanimbine may prove beneficial in the management of pancreatic cancer.
MeSH Keywords: Apoptosis, Cell Cycle Checkpoints, Cell Migration Assays
Background
Pancreatic cancer is one of the frequent causes of cancer associated mortality worldwide. It is ranked as 7th and 4th major cause of cancer related deaths in China and United states respectively [1,2]. Around 2.5 million people die annually of this brutal type of disease in United States alone [2]. The prognosis of pancreatic cancer patients is poor, probably the poorest of all the cancers, owing to the little advancements made in its early detection and treatment [3,4]. Given this background, there is an urgent need to explore new drug options for the treatment of pancreatic cancer. Natural products especially plants and microbes have served as important sources of anticancer drugs in the past and because of the huge diversity of plant species, it is believed that they can serve as the source of more drugs to combat the deadly diseases such as cancer [5]. Among plant metabolites that have shown potential to inhibit the growth of cancers, alkaloids are considered of utmost importance [6]. Mahanimbine is a carbazole alkaloid that has been reported to been identified and isolated from a number of plant species [7]. Studies carried out previously have shown that alkaloids such as Mahanimbine exhibit the capacity to inhibit the proliferation of the cancer cells [8]. For example, Mahanimbine has been shown to inhibit the growth of human leukemia cancer cells [9]. Further, the plant extracts containing Mahanimbine have been reported to inhibit the growth of different types of cancers [10,11]. However, there is no report on the anticancer activity of Mahanimbine against the pancreatic cancer cells. Therefore, this study was designed to screen Mahanimbine against a panel of pancreatic cancer and normal cell lines for its anticancer activity and to assess the underlying mechanisms. The results showed that Mahanimbine could significantly the growth of pancreatic cancer cells with minimal cytotoxicity on the normal cells. The anticancer effects of Mahanimbine were found to be mainly due to G0/G1 cell cycle arrest and induction of apoptosis. AKT/mTOR and STAT3 are important signal transduction pathways [12,13]. The expression of the main proteins of these pathways has often been found to be dysregulated in cancer cells. In addition, these pathways have also been reported to be involved in the development, progression and tumorigenisis of several types of cancers [14]. In this study we found that Mahanimbine could inhibit both these pathways. Finally, the effects of Mahanimbine were also evaluated on the migration of the human pancreatic cells and it was found that Mahanimbine could inhibit the migration of pancreatic cancer cells. We therefore propose that Mahanimbine may severe as a beneficial alkaloid that can be utilized to develop chemotherapy against pancreatic cancer.
Material and Methods
Cell lines and culture conditions
Pancreatic cancer cell lines (CAPAN-2, BxPC3, CFPAC1, HPAFII, and SW 1190) and a non-cancerous pancreatic cell line (hTRET-HPNE) were procured from the American Type Culture Collection. All of these cell lines were maintained in Dulbecco’s modified Eagle’s medium containing fetal 10% bovine serum, antibodies (100 units/mL penicillin and 100 μg/mL streptomycin), and 2 mM glutamine. The cells were cultured in a CO2 incubator (Thermo Scientific) at 37°C with 98% humidity and 5% CO2.
Cell proliferation assay
The proliferation of the pancreatic cancer and normal cells was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. In brief, the cells were cultured in 96-well flat bottom microtiter culture plates at a density of 5×104 cells per well and subjected to treatment with Mahanimbine (Sigma Aldrich, USA, 98% purity). Afterwards, 20 μL of a fresh MTT (2.5 mg/ml) solution was added, then the cells were incubated for 4 h at 37°C. This was followed by the addition of DMSO to dissolve the formazan. Finally, the absorbance was taken at 570 nm using a microplate reader.
Cell cycle analysis
To carry out the cell cycle analysis, the transfected pancreatic cancer cells were cultured in medium containing FBS (10%) for 24 h. For the preparation of the cell samples for flow cytometry, the cells were subjected to treatment with trypsinization followed by fixation with 70% ethanol. The DNA of the cells was stained by incubation with PBS containing propidium iodide (100 μg/ml) and RNase (40 U/ml) for 35 min at 37°C. The cell samples were finally analyzed using a fluorescence-activated cell sorter (FACS).
Apoptosis assay
Pancreatic cells were grown in 6-well plates at 1×106 cells per well and subjected to treatment with a 7-μM concentration of Mahanimbine followed by incubation for 24 h. This was immediately followed by DAPI (4′,6-diamidino-2-phenylindole) staining. The cells were examined and photomicrographs were taken using a fluorescence microscope. Thereafter, the cells were harvested, washed with FBS, and then treated with Annexin V/FITC and Propidium iodide for 20 min. The percentage of apoptotic cells was checked by flow cytometry.
Wound healing assay
Cell migration capacity of the pancreatic cancer cells was examined by wound healing assay. In brief, 5×104 cells per well were cultured in 96-well plates. The plates were then subjected to incubation at 37°C for 24 h to allow the cells to adhere. A wound was scratched with a sterile pipette tip on the surface when the cells reached confluence. The cells were then subjected to washing with PBS and monitored after 48 h and photographed.
Western blot analysis
The pancreatic cells were lysed using ice-cold hypotonic buffer. After estimating the protein concentrations in each of the cell extracts, the samples containing the proteins were loaded and separated on SDS–PAGE. This was followed by transfer to a nitrocellulose membrane and incubation with the primary antibody (AKT, p-AKT, mTOR, p-mTOR, p-STAT3, and STAT-3) (1: 1000) for 24 h at 4°C. Then, the membrane was incubated with HRP-conjugated secondary antibody (1: 1000) for at 24°C for about 1 h. The visualisation of the proteins was carried out using enhanced chemiluminescence reagent.
Results
Mahanimbine inhibits the growth of pancreatic cancer cells
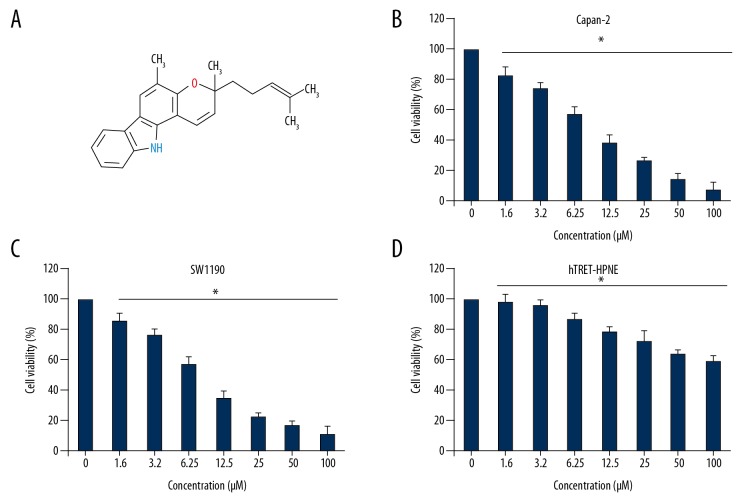
The anti-proliferative effects of Mahanimbine (Figure 1A) were examined on a panel of pancreatic cancer and normal cell lines by MTT assay. The results of the proliferation assay showed that Mahanimbine exerts antiproliferative effects on all the pancreatic cancer cell lines with IC50 ranging from 3.5 to 64 μM (Table 1). However, the IC50 of Mahanimbine was found to be much higher against the normal pancreatic cells (IC50; 110 μM). Further, it was observed that the anticancer effects of Mahanimbine on the Capan-2 and SW1190 pancreatic cancer cells were concentration-dependent (Figure 1B–1D). The highest anticancer activity of Mahanimbine was observed on the Capan-2 and SW1900 cell lines, with an IC50 of 3.5 μM. Based on the IC50 values, these cell lines were selected for further experimentation.
Figure 1.
(A) Chemical structure of Mahanimbine. (B) Effect of Mahanimbine on the proliferation of Pancreatic cancer Capan-2, (C) SW1190, and (D) normal hTRET-HPNE cells. The experiments were repeated 3 times and are presented as mean ±SD (p<0.05).
Table 1.
The antiproliferative effects of Mahanimbine against the pancreatic cancer and normal cell lines as depicted by MTT assay.
| S. No | Cell lines | IC50 (μM) |
|---|---|---|
| 1 | CAPAN-2 | 3.5 |
| 2 | BxPC3 | 16 |
| 3 | CFPAC1 | 64 |
| 4 | HPAFII | 32 |
| 5 | SW 1190 | 3.5 |
| 6 | hTRET-HPNE | 110 |
Mahanimbine induces G0/G1 cell cycle arrest of pancreatic cancer cells
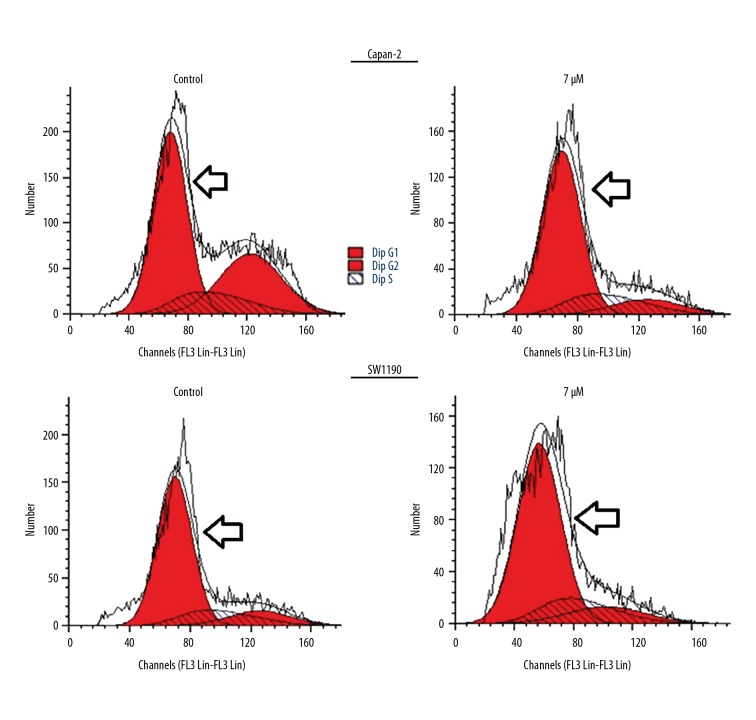
To assess if Mahanimbine causes arrest of cancer cells, the Capan-2 and SW1190 cells were subjected to treatment with Mahanimbine followed by flow cytometery. The results revealed that Mahanimbine caused a remarkable increase in the percentage of Capan-2 and SW1190 cells in G1 phase of the cell cycle (Figure 2). The percentage of Capan-2 and SW1190 cells in G1 phase increased remarkably upon treatment with Mahanimbine. These results clearly indicate that Mahanimbine induces G0/G1 cell cycle arrest of pancreatic cancer cells.
Figure 2.
Mahanimbine at varied concentrations triggers G0/G1 cell cycle arrest of Capan-2 and SW1190 cells, as indicated by flow cytometery. The experiments were repeated 3 times in triplicate.
Mahanimbine triggers apoptotic cell death of pancreatic cancer cells
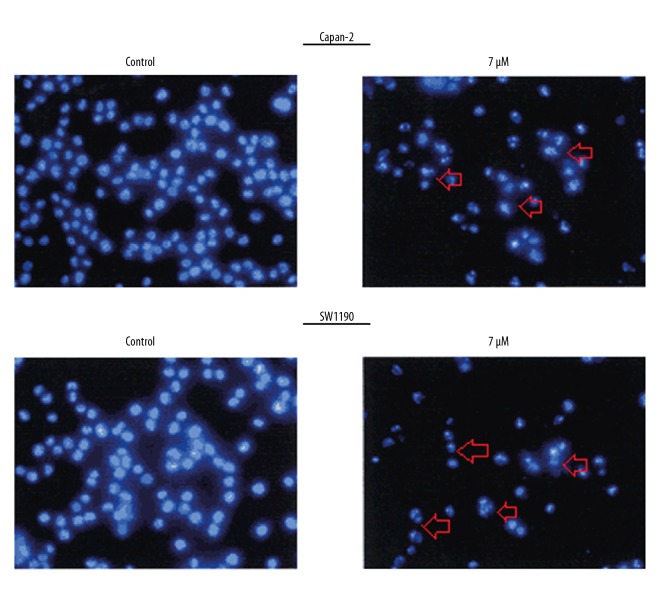
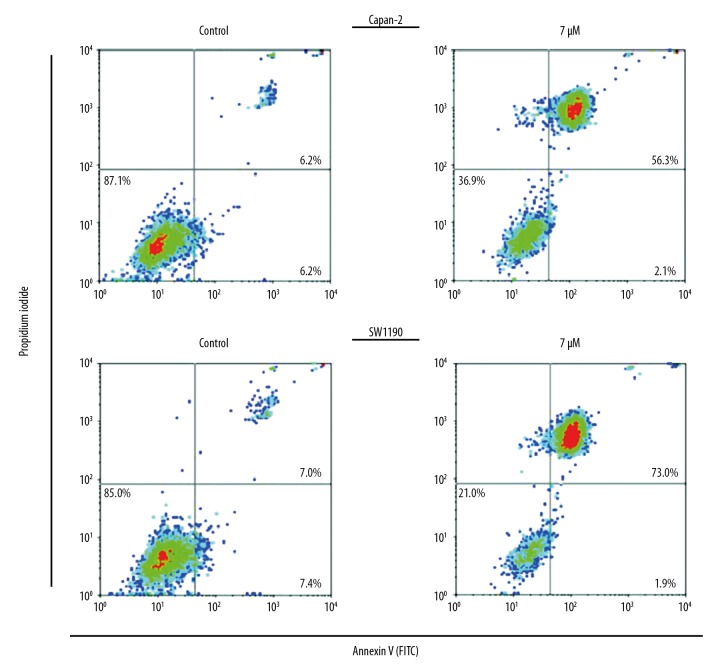
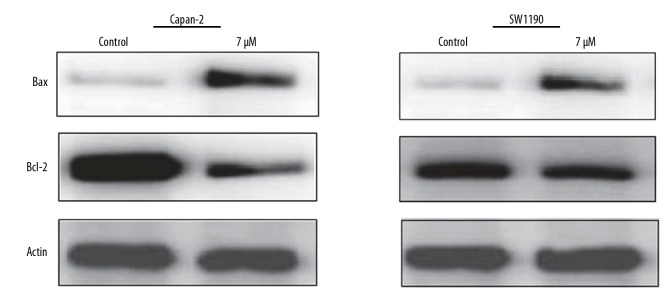
The influence of Mahanimbine on the apoptotic pathway in Capan-2 and SW1190 cells was examined by DAPI and annexin V/PI staining. The results showed that Mahanimbine caused considerable changes in the nuclear morphology of the Capan-2 and SW1190 cancer cells (Figure 3). These changes were found to be characteristic of apoptosis; therefore, annexin V/PI staining was performed to determine the percentage of apoptotic cells. The results showed that the percentage of apoptotic cells increased from 6.2% and 7.0% to 56.3% and 73% in Capan-2 and SW1190 pancreatic cancer cells, respectively (Figure 4). The results of Western blotting further confirmed that Mahanimbine induces apoptosis in Capan-2 and SW1190 pancreatic cancer cells. We observed that Mahanimbine induced the upregulation of Bax and downregulation of Bcl-2 expression in both pancreatic cell lines (Figure 5).
Figure 3.
DAPI staining showing induction of apoptosis in Capan-2 and SW1190 cells upon treatment with Mahanimbine. The experiments were repeated 3 times in triplicate.
Figure 4.
Annexin V/PI staining showing the percentage of apoptosis in Capan-2 and SW1190 cells upon treatment with Mahanimbine. The experiments were repeated 3 times in triplicate.
Figure 5.
Effect of Mahanimbine of the expression of Bax and Bcl-2 in Capan-2 and SW1190 cells, as depicted by Western blotting. The experiments were repeated 3 times in triplicate.
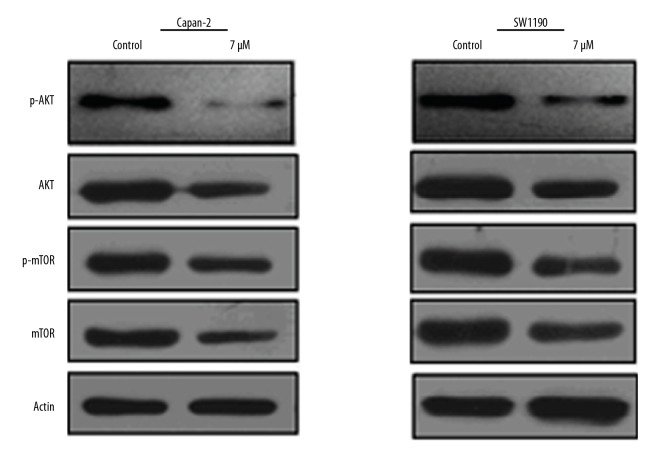
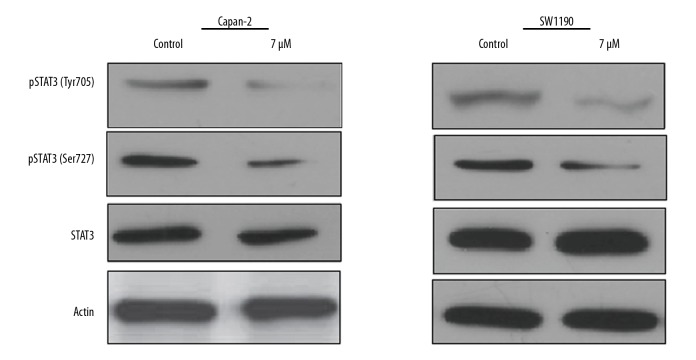
Mahanimbine targets AKT/mTOR and STAT3 pathways in pancreatic cancer cells
The effects of Mahanimbine were also investigated on the AKT/mTOR (Figure 6) and STAT3 (Figure 7) signalling pathways. The results showed that Mahanimbine causes the downregulation of the AKT, p-AKT, mTOR, p-mTOR, and p-STAT3 protein expression. However, the expression of the total STAT3 exhibited no apparent change upon treatment with Mahanimbine.
Figure 6.
Effect of Mahanimbine on the protein expression of AKT/mTOR pathway in Capan-2 and SW1190 cells as indicated by Western blotting. The experiments were repeated 3 times.
Figure 7.
Effect of Mahanimbine on protein expression of the STAT3 pathway in Capan-2 and SW1190 cells, as indicated by Western blotting. The experiments were repeated 3 times.
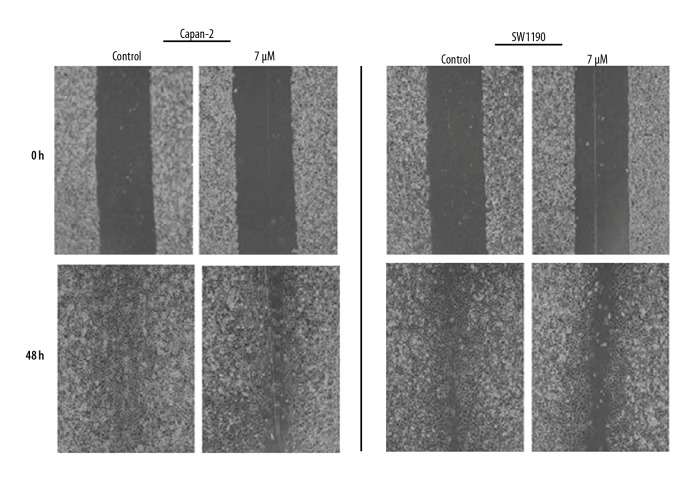
Mahanimbine inhibits the migration of pancreatic cancer cells
The effect of Mahanimbine was also examined on the migration of the SW1900 and capan-2 pancreatic cancer cells by wound-healing assay (Figure 8). The results of this migration revealed that Mahanimbine caused significant inhibition of migration of both Capan-2 and SW1900 pancreatic cancer cell lines.
Figure 8.
Effect of Mahanimbine on the migration of migration of Capan-2 and SW1190 cells, as depicted by wound healing assay. The experiments were repeated 3 times.
Discussion
Pancreatic cancer causes considerable mortality across the world. The unavailability of reliable biomarkers and therapeutic targets, as well as lack of efficient drugs, is one of the major obstacles in the management of pancreatic cancer [1,2]. In the present study, the anticancer effects of the plant-derived alkaloid Mahanimbine were examined against a panel of pancreatic cancer cells. The results revealed that Mahanimbine decreased the viability of pancreatic cancer cells. The highest antiproliferative effects were observed on Capan-2 and SW1190 pancreatic cancer cells. However, the cytotoxicity on the normal pancreatic cells was minimal, indicating that Mahanimbine selectively targets pancreatic cancer cells. These observations are also complemented by previous studies wherein Mahanimbine or other related alkaloids have been found to inhibit growth of cancer cells [7,15]. A previous study reported that Mahanimbine inhibited the proliferation of leukemia cells [9]. Anticancer agents exert their antiproliferative effects through various mechanisms. However, the induction of cell cycle arrest and apoptotic cell death are 2 important mechanisms that inhibit the growth of cancer cells [16]. Several of the plant-derived alkaloids have been shown to induce apoptosis or cell cycle arrest or both [17,18]. In the present study we also examined the effect of the Mahanimbine on the distribution of the Capan-2 and SW1190 cells in various phases of the cell cycle. Interestingly, we found that Mahanimbine caused the arrest of Capan-2 and SW1190 cells in G0/G1 phase of the cell cycle. However, the IC50 of Mahanimbine was very low (7 μM) and cell cycle arrest alone could not be responsible for such a low IC50. Hence, we speculated that Mahanimbine may also induce apoptosis in pancreatic cancer cells, given the fact that many alkaloids derived from plants activate apoptosis in cancer cells. Interestingly, the results of DAPI and annexin V/PI staining revealed that Mahanimbine induces apoptosis in pancreatic cancer cells, concomitant with alteration in the Bax/Bcl-2 ratio. This is also supported by previous investigations reporting that upregulation of Bax and downregulation of Bcl-2 favors apoptotic cell death [19]. Several pathways have been found to be involved in the proliferation and tumorigenesis of cancer cells [20]. AKT/mTOR and STAT3 are 2 such pathways reported to be upregulated in cancer cells [12]. We found that Mahanimbine can inhibit both of these pathways, suggesting that Mahanimbine could be used to treat pancreatic cancer by targeting AKT/mTOR and STAT3 signalling pathways. Metastasis of cancer cells is dependent on their ability to migrate to neighbouring tissues [21]; in the present study, we observed that Mahanimbine suppressed the migration of pancreatic cancer cells, indicating the anticancer potential of Mahanimbine.
Conclusions
We showed that Mahanimbine inhibits the proliferation of pancreatic cancer cells dose-dependently. The anticancer effects are mainly due to induction of cell cycle arrest and apoptosis. Mahanimbine can also inhibit migration of pancreatic cancer cells, indicating the potential of Mahanimbine as an anticancer agent. Given these interesting results, this molecule warrants further studies, including in vivo evaluation.
Footnotes
Source of support: Departmental sources
References
- 1.Zhang Q, Zeng L, Chen Y, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/8962321. 8962321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Cullis J, Siolas D, Avanzi A, et al. Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol Res. 2017;5(3):182–90. doi: 10.1158/2326-6066.CIR-16-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 6.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14(2):111–29. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 7.S Shaikh M, Karpoormath R, Thapliyal N, et al. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anticancer Agents Med Chem. 2015;15(8):1049–65. doi: 10.2174/1871520615666150113105405. [DOI] [PubMed] [Google Scholar]
- 8.Dahiya J, Singh J, Kumar A, et al. Isolation, characterization and quantification of an anxiolytic constituent-mahanimbine, from Murraya koenigii Linn. Spreng Leaves. J Ethnopharmacol. 2016;193:706–11. doi: 10.1016/j.jep.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Nagappan T, Ramasamy P, Wahid ME, et al. Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules. 2011;16(11):9651–64. doi: 10.3390/molecules16119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arun A, Patel OP, Saini D, et al. Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT downregulation and mitochondrial apoptosis. Biomed Pharmacother. 2017;93:510–21. doi: 10.1016/j.biopha.2017.06.065. [DOI] [PubMed] [Google Scholar]
- 11.Sukari MA, Ismail N, Bakar NA, et al. Cytotoxic carbazole alkaloids from Murraya koenigii (rutaceae) Research Journal of Chemistry and Environment. 2014;18(11):8–11. [Google Scholar]
- 12.Li Y, Cui N, Zheng PS, et al. BMX/Etk promotes cell proliferation and tumorigenicity of cervical cancer cells through PI3K/AKT/mTOR and STAT3 pathways. Oncotarget. 2017;8(30):49238–45. doi: 10.18632/oncotarget.17493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5(5):1602–9. [PMC free article] [PubMed] [Google Scholar]
- 14.He SQ, Gao M, Fu YF, Zhang YN. Glycyrrhizic acid inhibits leukemia cell growth and migration via blocking AKT/mTOR/STAT3 signaling. Int J Clin Exp Pathol. 2015;8(5):5175–79. [PMC free article] [PubMed] [Google Scholar]
- 15.Das R, Bhattacharya K, Sarkar S, et al. Mahanine synergistically enhances cytotoxicity of 5-fluorouracil through ROS-mediated activation of PTEN and p53/p73 in colon carcinoma. Apoptosis. 2014;19(1):149–64. doi: 10.1007/s10495-013-0907-6. [DOI] [PubMed] [Google Scholar]
- 16.Queiroz EA, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014;9(5):207–12. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe PB, Power Coombs MR, et al. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog. 2015;54(10):1070–85. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 18.García V, Lara-Chica M, Cantarero I, et al. Galiellalactone induces cell cycle arrest and apoptosis through the ATM/ATR pathway in prostate cancer cells. Oncotarget. 2016;7(4):4490–506. doi: 10.18632/oncotarget.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 20.Kolch W, Halasz M, Granovskaya M, et al. The dynamic control of signal transduction networks in cancer cells. Nat Rev Cancer. 2015;15(9):515–27. doi: 10.1038/nrc3983. [DOI] [PubMed] [Google Scholar]
- 21.Seguin L, Desgrosellier JS, Weis SM, et al. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–40. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]