Summary
Differentiation of astrocytes from human pluripotent stem cells (hPSCs) is a tedious and variable process. This hampers the study of hPSC-generated astrocytes in disease processes and drug development. By using CRISPR/Cas9-mediated inducible expression of NFIA or NFIA plus SOX9 in hPSCs, we developed a method to efficiently generate astrocytes in 4–7 weeks. The astrocytic identity of the induced cells was verified by their characteristic molecular and functional properties as well as after transplantation. Furthermore, we developed a strategy to generate region-specific astrocyte subtypes by combining differentiation of regional progenitors and transgenic induction of astrocytes. This simple and efficient method offers a new opportunity to study the fundamental biology of human astrocytes and their roles in disease processes.
Keywords: fast differentiation from hPSCs, NFIA, SOX9, fast astrocyte differentiation, functional astrocytes, transplantable astrocytes, subtype astrocytes, CRISPR/Cas9 induced differentiation, human astrocytes, engineering hPSCs
Graphical Abstract
Highlights
-
•
Fast differentiation of astrocytes from human pluripotent stem cells (hPSCs)
-
•
NFIA or NFIA plus SOX9 overexpression facilitates astrocyte generation
-
•
Fast generation of subtype-specific astrocytes from hPSCs
-
•
CRISPR/Cas9-engineered hPSCs for fast generation of astrocytes
In this article, Zhang and colleagues show that functional and subtype-specific astrocytes can be fast generated from hPSCs by using CRISPR/Cas9-mediated inducible expression of NFIA or NFIA plus SOX9 in hPSCs. This simple and efficient method offers the opportunity to study the fundamental biology of human astrocytes and their roles in disease processes.
Introduction
Astrocytes are functionally indispensable for normal brain activities (Barres, 2008, Ullian et al., 2001, Zhang, 2001). Astrocytes play critical roles for the establishment and maintenance of functional neural networks through refining synapses, coordinating neuronal firing, maintaining the blood-brain barrier, as well as structural and metabolic support (Iadecola and Nedergaard, 2007, Sofroniew and Vinters, 2010, Ullian et al., 2001). Functional loss or impairment of astrocytes is implicated in a wide range of pathological processes and neural disorders, including Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis, and epilepsy (Molofsky et al., 2012, Scuderi et al., 2013, Sofroniew and Vinters, 2010). In humans, astrocytes display unique hominid features (Oberheim et al., 2009). Human astrocytes are morphologically larger, structurally more complex, and functionally more diverse than those in the rodent brain (Oberheim et al., 2009, Robertson, 2014). The complexity of human astrocytes allows for the increased functional competence of the adult human brain (Oberheim et al., 2009, Robertson, 2014, Vasile et al., 2017). Thus, access to human astrocytes will substantially enable studies on the biology and pathology of astrocytes and facilitate therapeutic development.
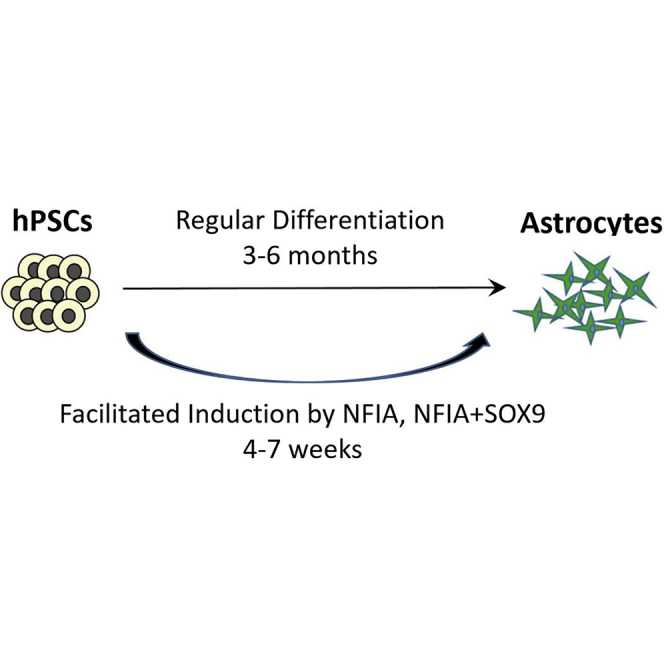
Astrocytes have been successfully generated from human pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Emdad et al., 2012, Hu et al., 2010, Krencik et al., 2011, Mormone et al., 2014, Roybon et al., 2013, Palm et al., 2015, Shaltouki et al., 2013, Tcw et al., 2017, Zhou et al., 2016) (Table S1). These methods are based on developmental principles to expand the neural progenitors until gliogenesis (Steinbeck and Studer, 2015, Tao and Zhang, 2016). Hence, the differentiation process ranges from 3 to 6 months. There are reports on astrocyte generation in a shorter time but the identity and functional properties of the astrocytes are less rigorously examined (Table S1). The main reason for this long process is that we lack effective ways to promote the gliogenic program of the neural progenitors and, as a result, wait for the progenitors to become gliogenic by “default.” Such an approach also results in variations in the differentiated products.
One way to accelerate the differentiation process is to force the expression of cell-type-specific transcription factors in stem cells. This has been demonstrated by fast (2–4 weeks) generation of functional neurons from human ESCs (hESCs) through virus-mediated expression of a proneural gene, Ngn2 (Zhang et al., 2013). In this study, using a similar strategy, we created hESC and iPSC lines with inducible expression of gliogenic transcription factors NF1A or NF1A plus SOX9 via CRISPR/Cas9. Utilizing inducible expression of NF1A or NF1A plus SOX9, we developed a method for fast generation of homogeneous functional and transplantable astrocytes from hESCs and iPSCs in 4–7 weeks. Importantly, by patterning with morphogens during the induction, we developed a method to generate region-specific subtype astrocytes in the same time frame.
Results
Expression of NFIA Facilitates Astrocyte Differentiation from hPSCs
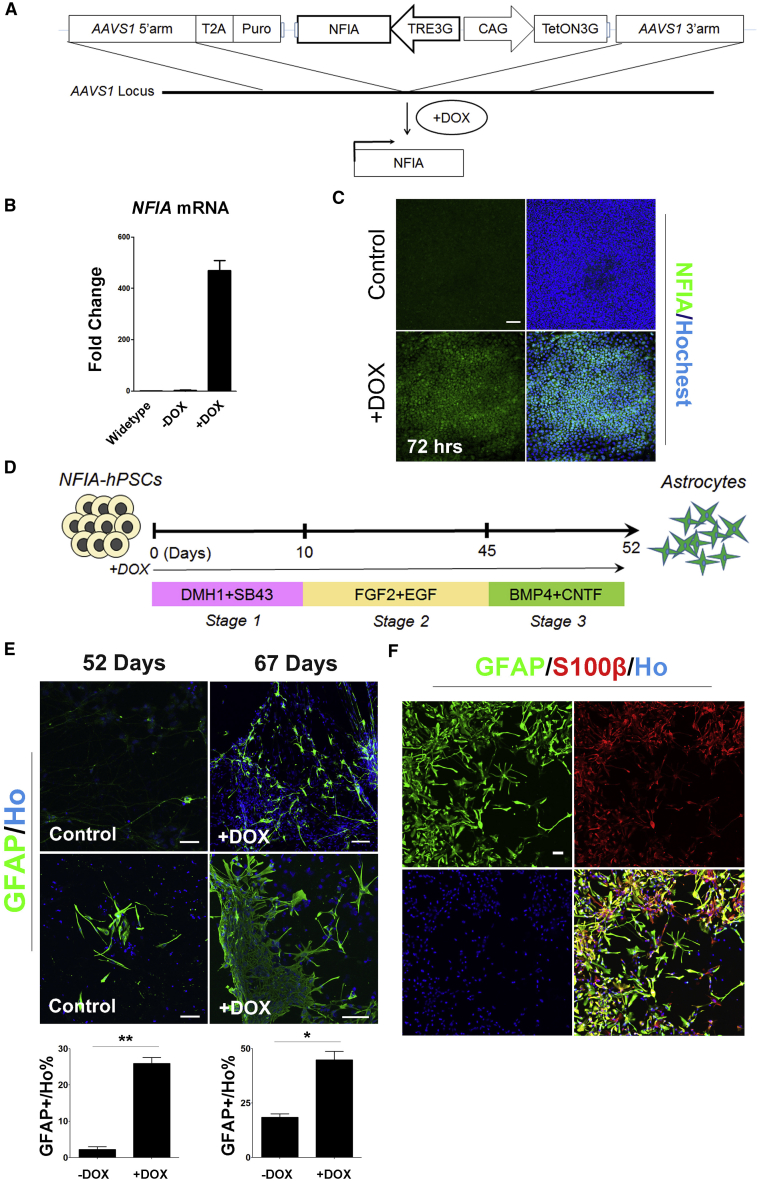
The NFI (nuclear factor I) family transcription factors are crucial for the initiation of gliogenesis and acquisition of gliogenic competence in the developing CNS (Deneen et al., 2006, Kang et al., 2012, Miller and Gauthier, 2007, Tsuyama et al., 2015, Vong et al., 2015). To determine if NFI promotes astrocyte differentiation from human stem cells, we established hESC lines with inducible expression of NFIA. This was achieved by targeting TRE3G-NFIA in the AAVS1 locus by CRISPR/Cas9 (Qian et al., 2014) (Figure 1A). After drug selection and PCR verification, colonies were grown and cultured >3 passages before being subjected to induced expression of NFIA by adding doxycycline (DOX). Upon induction with DOX for 72 hr, NFIA mRNA and protein were robustly expressed, as indicated by qRT-PCR (Figure 1B) and immunocytochemistry (Figure 1C).
Figure 1.
Astrocyte Differentiation Induced by NFIA Expression
(A) Schematic depiction of the strategy for constructing and targeting TRE3G-NFIA into the AAVS1 locus.
(B) qRT-PCR analysis of induced expression of NFIA. n = 3 replicate reactions.
(C) Immunostaining analysis of induced expression of NFIA. Scale bar, 200 μm.
(D) Diagram of the facilitated generation of astrocytes by NFIA induction. Stage 1, neural fate commitment by dual SMAD inhibition; stage 2, astrocyte progenitor induction by a suspension culture; stage 3, astrocyte specification and maturation on a monolayer culture.
(E) Representative images and quantification of induced GFAP+ cells after 52 or 67 days of differentiation. n = 3 independent experiments (with replicate wells) were analyzed. Scale bars, 100 μm.
(F) Induced cells co-expressed GFAP and S100β. Scale bar, 100 μm. Data of this figure are produced by using the TRE3G-NFIA hPSCs (H9).
The data are presented as the means ± SEM. ∗p < 0.05; ∗∗p < 0.01 (Student's t test).
Previously, we developed the method of directed differentiation of hPSCs into astrocytes, in which GFAP-expressing astrocytes begin to appear at around 3 months and reach a peak at 6 months (Krencik et al., 2011). In this method, the hPSCs were committed in monolayer culture into the neural fate by SMAD inhibition as stage 1, then the cells were floated as neural progenitors in culture with fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF) for as long as 5–6 months for the neural-to-glia developmental fate switch as stage 2, and plated down for astrocytic maturation as stage 3. To determine the effect of NFIA, we used the same differentiation scheme but with induced NFIA expression from the start of differentiation by DOX, and then subjected the induced cells to a 7-day maturation (Figure 1D). By weekly examination in stage 2, we found that about 20% GFAP+ cells were generated at 45 days of induction plus a 7-day maturation (Figures 1E and 1F). In contrast, the control group (without DOX) generated less than 3% GFAP+ astrocytes (Figure 1E). By extending the induction to 67 days, the GFAP+ cell population was further increased to 40%–50%, and the cells were larger with more processes than the induced cells at day 52 (Figure 1E). The astrocytic identity was further confirmed by its co-expression with S100β, another astrocyte-associated gene (Figure 1F). These results indicate that NF1A expression substantially speeds up the astrocyte differentiation from hESCs.
Expression of NFIA and SOX9 Further Accelerates Astrocyte Differentiation
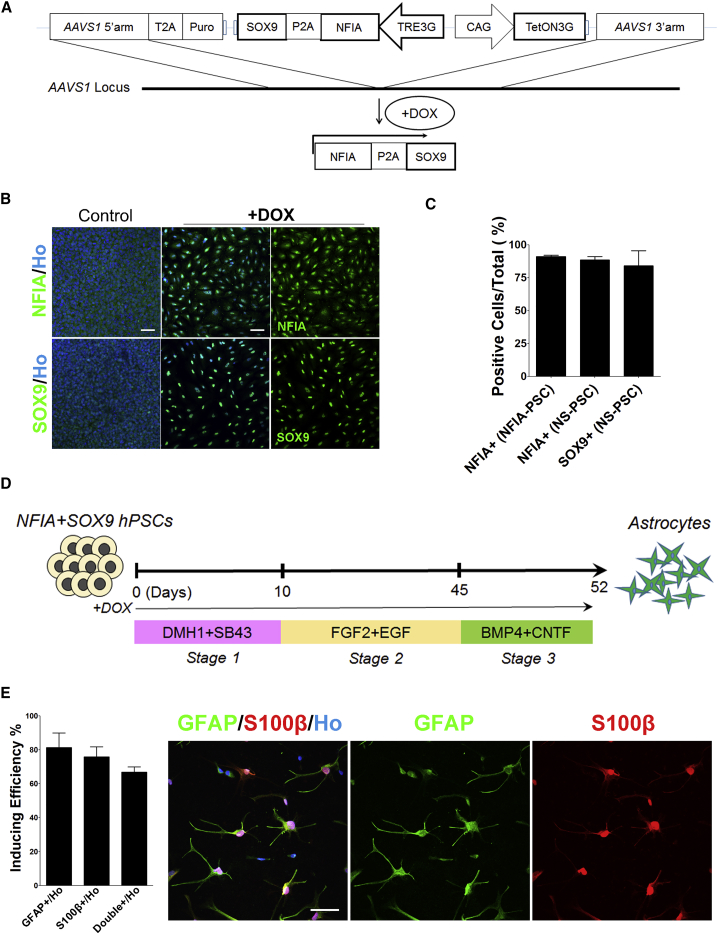
Besides NFIA, SOX9 is another determining factor for the astroglial cell fate (Kang et al., 2012, Stolt et al., 2003, Vong et al., 2015). NFIA and SOX9 form a complex to coordinate a transcriptional regulatory cascade for initiating gliogenesis during neural development (Kang et al., 2012). Astrocytic fates can be induced from neural progenitor cells in vivo or from cultured mouse fibroblasts by overexpressing NFI + SOX9 (Gaiazzo et al., 2015). To determine if the combination of NFIA and SOX9 further accelerates the astrocyte differentiation, we generated hESC lines with inducible expression of NFIA and SOX9 by targeting the Tet3G-NFIA + SOX9 into the AAVS1 locus using the same approach (Figure 2A). Again, the mRNA and protein of both genes were reliably induced by DOX (Figures 2B and S1A). Western blotting indicated that NFIA expression was higher in the NFIA + SOX9 line than the NFIA line (Figure S1C). This result is consistent with the previous report that NFIA expression is regulated and reinforced by SOX9 to coordinate a transcriptional regulatory cascade for the initiation of gliogenesis (Kang et al., 2012). Quantification of the immunostained cells showed that over 90% of the cells expressed either NFIA or SOX9, indicating that the vast majority of the cells co-expressed NFIA and SOX9 (Figures 2C and S1B).
Figure 2.
Astrocyte Differentiation Induced by Expression of NFIA and SOX9
(A) Schematic depiction of the strategy for constructing and targeting TRE3G-NFIA + SOX9 into the AAVS1 locus.
(B) Immunostaining analysis of induced expression of NFIA and SOX9. Scale bar, 200 μm.
(C) Quantification of positive cells with the induced expression of NFIA in NFIA-hPSC line (NFIA+ in NFIA-PSC) and NFIA or SOX9 in NFIA + SOX9 hPSC line (NFIA+ and SOX9+ in NFIA-PSC). PSC colonies from n = 3 replicate cultures were analyzed.
(D) Diagram of the facilitated generation of astrocytes by NFIA and SOX9 induction. Stage 1, neural fate commitment by dual SMAD inhibition; stage 2, astrocyte progenitor induction by a suspension culture; stage 3, astrocyte specification and maturation on a monolayer culture.
(E) Representative images and quantification of induced GFAP+/S100β+ cells after 52 days of differentiation. n = 3 independent experiments (with replicate wells) were analyzed. Scale bar, 50 μm. Data in this figure were produced using the TRE3G-NFIA + SOX9 hPSCs (H9). The data are presented as the means ± SEM.
The astrocyte induction and maturation was performed in the same way as for NFIA lines (Figure 2D). After 37 days’ induction, around 30% of the cells became GFAP+ (Figure S1D). With extended (52 days) induction of NF1A and SOX9, 70%–80% of the cells became GFAP+ (Figure 2E). The astrocytic identity of the induced cells was confirmed by their co-expression of GFAP and S100β (Figure 2E). The efficiency and identity of the induced cells were reproducible using different engineered hESC clones (Figure S1E). Thus, expression of NF1A and SOX9 results in a substantially shorter period for generation of astrocytes than that by regular differentiation, which takes 6 months to generate equivalent percentage of GFAP+ cells (Krencik et al., 2011).
To validate the applicability of this strategy, we established two transgenic iPSC lines (WC50 and IMR90) with inducible expression of NF1A and SOX9 using the same approach described above (Figures S2A, S2B, S3A, and S3B). Again, induced astrocytes were efficiently generated from the two iPSC lines (Figures S2C and S3C).
Our time course analysis indicated that the GFAP-expressing astrocytes increase from day 37 to 52 with induction of NF1A or NF1A + SOX9. When we further extended the induction of NF1A and SOX9 for another 60 days, we found that the population of GFAP-expressing astrocytes increased from 80% to 90% (Figure S4A). We noticed that, even at the extended culture, some cells, with expression of S100β and an astrocyte morphology, remained negative or only weakly positive for GFAP (Figure S4A). It has been reported that not all astrocytes express GFAP (Walz and Lang, 1998).
We then asked if the phenotypes of the induced astrocytes are stable when the transgene expression is stopped. We withdrew DOX at day 52 and continued the culture for another 14 days. The induced cells retained their expression of GFAP and S100β (Figure S4B). Furthermore, these induced astrocytes promoted the growth of neurites, including the length and branching (Figure S4C). Thus, the induced cells retain the identity and function of astrocytes. We refer the induced astrocytes from TRE3G-NFIA + SOX9 hPSC lines after a 52-day induction as iAstro.
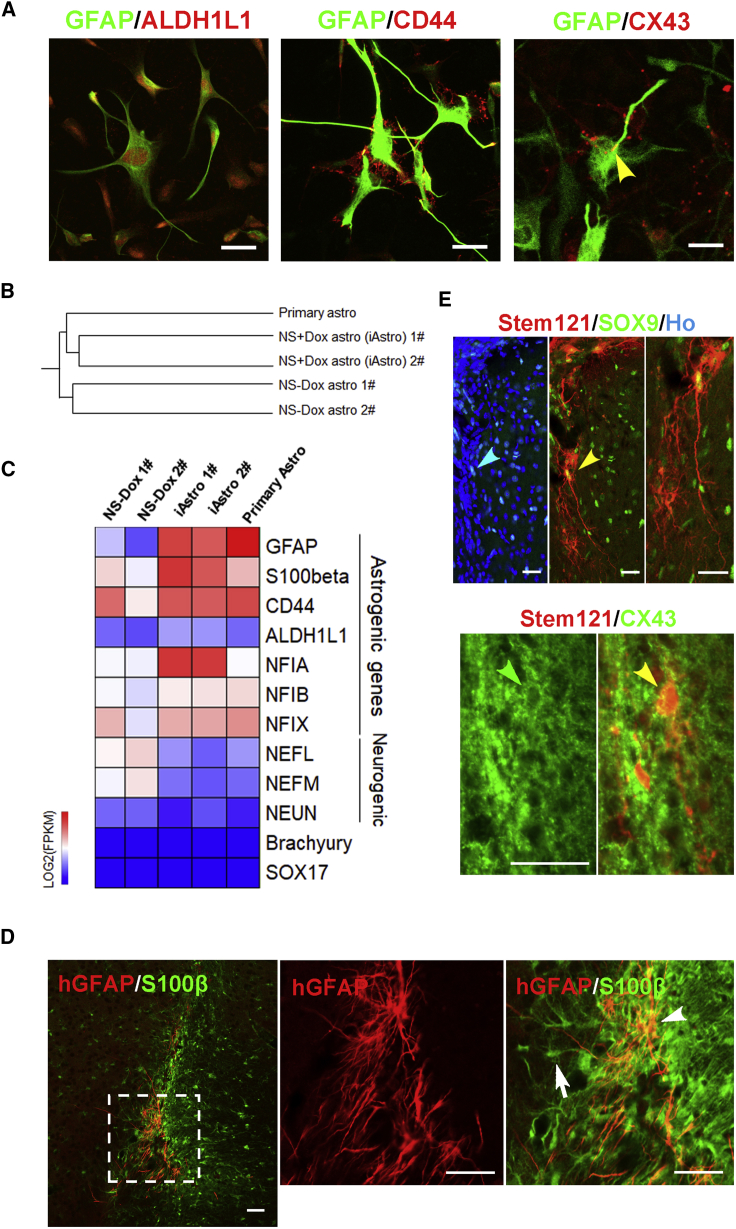
The iAstro Display Molecular Signatures of Astrocytes
Under our culture condition, the iAstro displayed a star-shaped morphology with delicate processes (Figures 2E and 3A). Immunocytochemical analysis showed that the induced astrocytes co-expressed astrocyte markers, including S100β, ALDH1L1, CD44, and Connexin43 (CX43) (Figure 3A).
Figure 3.
Cellular and Molecular Characterization of iAstro
(A) Representative images of iAstro, showing co-expression of GFAP with ALDH1L1, CD44, and CX43.
(B) Hierarchical clustering analysis of RNA sequencing data from the iAstro, non-transgenically induced cells, and primary astrocytes.
(C) Heatmap profiling of astrogenic and neurogenic genes, mesoderm gene (Brachyury) and endoderm gene (SOX17).
(D) Representative images of transplanted iAstro, showing human-specific GFAP+/S100β+ (hGFAP+/S100β+) and hGFAP−/S100β+ host astrocytes. Arrowhead indicates graft and arrow indicates a host astrocyte. Scale bars, 100 μm.
(E) Representative images of transplanted iAstro (indicated by human-specific cytoplasm marker, Stem121, arrowhead) co-expressing SOX9 and CX43. The area indicated by the yellow arrowhead in the upper row is amplified on the right.
Scale bars, 100 μm. Data in this figure were produced using the TRE3G-NFIA + SOX9 hPSCs (H9).
If induced transgene expression contributes to the glial differentiation, it is expected that the methylation status of the GFAP promoter is demethylated. Methylation analysis on the GFAP promoter by bisulfite sequencing indicated that the GFAP promoter was methylated in hPSCs but demethylated in the iAstro after induction with NFIA and SOX9 (Figure S4D). This epigenetic change in the GFAP promoter region coincides with transgene-induced glial differentiation.
To further define the identity of the iAstro, we performed RNA-sequencing (RNA-seq) analysis. Hierarchical clustering analysis or principal-component analysis showed that iAstro were placed close to the primary astrocytes but distant from the non-transgene-induced cells, which are mostly neural progenitors (Figures 3B and S4E). Compared with the cells without transgenic induction, the iAstro enriched the expression of astrocyte signature genes, including GFAP, S100β, ALDH1L1, and others (Figure 3C). In contrast, the expression of neuronal genes (e.g., NEFL, NEFM, and NEUN) was suppressed (Figure 3C). Gene ontology analysis further revealed that the upregulated genes after transgenic induction were mainly enriched in cell-cell signaling, extracellular matrix and cell adhesion, inflammatory response, and K+ ion transmembrane transport, whereas the downregulated genes were mainly involved in neuronal fate differentiation, forebrain cell proliferation, synaptic transmission, peptide signaling pathways, neuronal migration, and long-term synaptic potentiation (Figure S4E). Together, these results further validate that the iAstro are indeed astrocytes but not neurons, oligodendrocytes, or neural progenitors.
To determine whether the iAstro retain the astrocyte identity in vivo, we transplanted the iAstro after a 52-day induction into the corpus callosum and cerebral cortex of adult severe combined immunodeficiency mice. Three months following transplantation, the grafted astrocytes were detected, as indicated by human-specific GFAP (hGFAP) (Figure 3D). The hGFAP+ cells were larger with elaborated processes compared with the endogenous mouse astrocytes that were labeled by S100β (Figure 3D). Furthermore, the transplanted human cells, as indicated by the expression of human-specific cytoplasm protein Stem121 (Chen et al., 2016), were also positive for SOX9 and CX43 (Figure 3E), markers expressed by mature astrocytes (Roybon et al., 2013, Sun et al., 2017, Wiencken-Barger et al., 2007). Thus, the in vitro generated iAstro retain the astrocyte identity in vivo.
The iAstro Display Functional Properties of Astrocytes
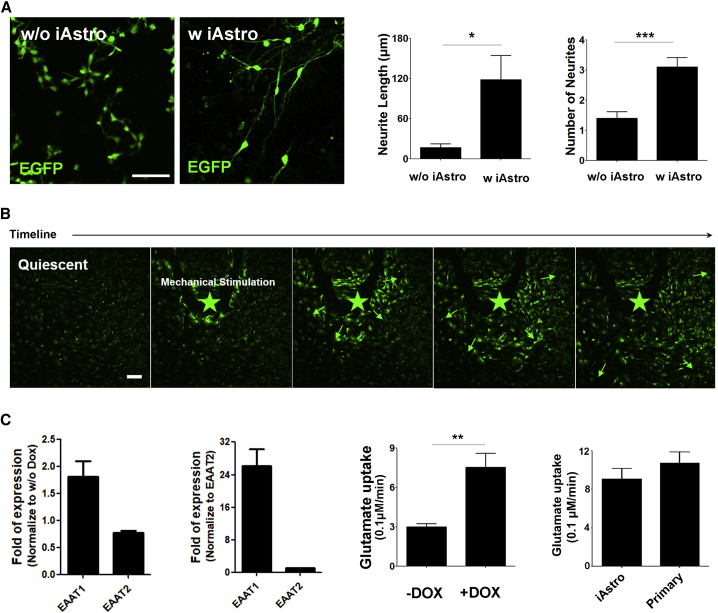
In vitro, astrocytes are known to support neurite growth. We induced the neurons from EGFP hPSCs (Figure S4F). These resulting EGFP neurons were plated on to the iAstro or alone for 5 days. Measurement of the GFP+ neurites by ImageJ revealed that the neurons co-cultured with the iAstro developed significantly more and longer neurites compared with those without astrocyte co-culture (Figure 4A). A similar phenomenon was observed when using the iAstro derived from the transgenic iPSCs (Figure S4G) or primary fetal human astrocytes (Figure S4H).
Figure 4.
Functional Characterization of iAstro
(A) Quantification of neurite outgrowth from neurons alone or neurons co-culturing with iAstro. n = 3 independent experiments (with replicate wells) were analyzed. Scale bar, 100 μm.
(B) Images of calcium wave propagation over the duration of mechanical stimulation. Asterisk indicates the site of stimulation. Scale bar, 50 μm.
(C) Expression of ETTA1 and ETTA2 in iAstro and the kinetics of cellular uptake of glutamate from the medium. The expression of ETTA1 and ETTA2 are normalized to control cells (w/o DOX). n = 2 replicates for fold expression analysis. n = 8 replicate wells for glutamate uptake analysis and the data are presented as the means ± SEM.
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (Student's t test). Data in this figure were produced using the TRE3G-NFIA + SOX9 hPSCs (H9).
Calcium wave propagation across astrocytes is critical for neuron-glia and glia-glia communication (Scemes and Giaume, 2006). When the iAstro derived from TRE3G-NFIA + SOX9 hPSCs (H9) were loaded with Fluor 4 and mechanically stimulated by a pipette, intracellular calcium waves and spikes were instantaneously induced from the stimulation site (Figure 4B). A similar phenomenon was observed when using the primary fetal human astrocytes (Figure S4H). These calcium waves of iAstro traveled outward to adjacent cells at a speed of 10 ± 1.63 μm/s (Figure S4F), which is faster than primary adult mouse astrocytes in vivo (8.6 ± 0.6 μm/s) and slower than primary adult human astrocytes in vivo (43.4 ± 4.7 μm/s) (Oberheim et al., 2009).
Astrocytes play a critical role in maintaining the homeostatic brain environment by taking up neurotransmitters. The mRNA expression of glutamate transporters, especially EAAT1, increased significantly after the transgenic induction by DOX (Figure 4C). To determine if the glutamate transporters are functional, we measured glutamate uptake by the iAstro from the culture medium. The iAstro took up glutamate at a rate of 754 ± 214 nM/min. As a comparison, the glutamate uptake by primary astrocytes was 1,066 ± 235 nM/min (Figure 4C). Thus, the iAstro take up glutamate similarly as primary astrocytes.
Fast Generation of Subtype Astrocytes
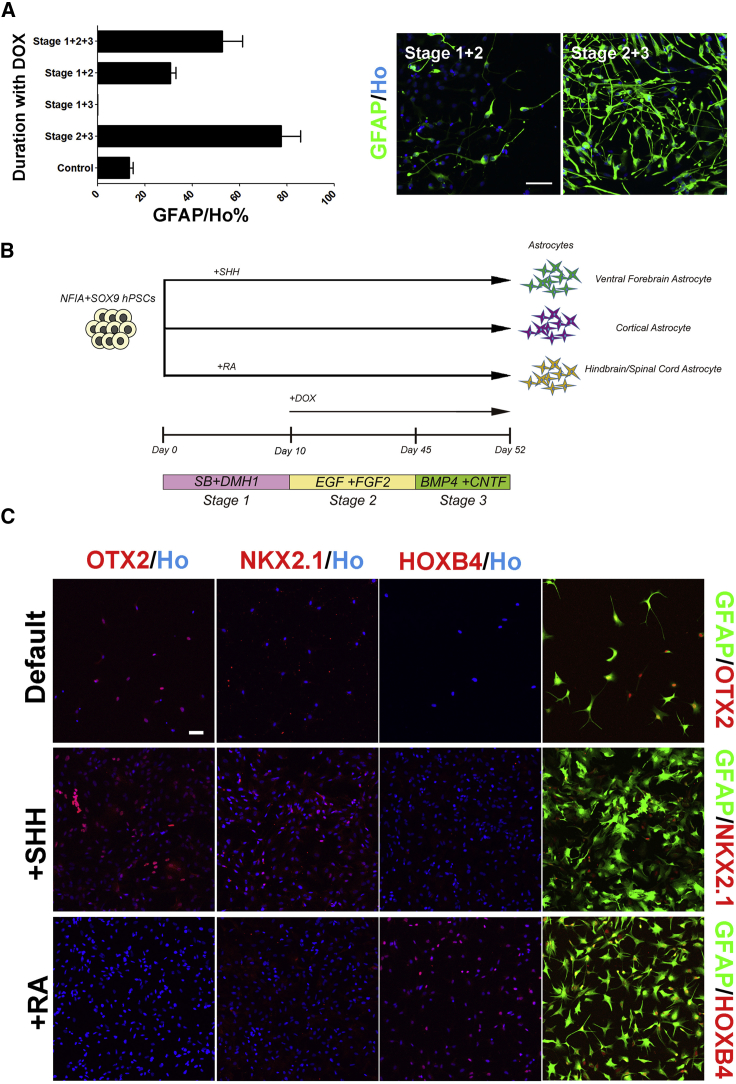
To determine the optimal window of transcription factor-induced astrocyte differentiation, we titrated the duration of DOX application for inducing iAstro from TRE3G-NFIA + SOX9 hPSCs (H9). We found that the induced transgenic expression in stage 2 (day 10 to 45) was indispensable for facilitating astrocyte generation, and the transgenic expression in stage 3 (7-day maturation) further enhanced the efficiency (Figure 5A). In contrast, stage 1 (neuroepithelial induction from day 1 to 10) is dispensable (Figure 5A). This result suggests that NF1A or NF1A plus SOX9 promotes astrocyte differentiation from specified neural progenitors.
Figure 5.
Fast Generation of Region-Specific Astrocyte Subtypes from hPSCs
(A) Optimal window of transcription factor expression for fast induction of astrocytes. n = 3 independent experiments (with replicate wells) were analyzed. Stage 1, neural fate commitment by dual SMAD inhibition; stage 2, astrocyte progenitor induction by suspension culture; stage 3, astrocyte specification and maturation on monolayer culture.
(B) Schematic strategy for fast generation of subtype astrocytes. Examples include fast generation of cortical, ventral forebrain, and hindbrain/spinal cord astrocytes.
(C) Regionally specified cell identity with region-specific markers: OTX2 (forebrain), NKX2.1 (ventral), and HOXB4 (spinal). The astrocyte identity of the induced subtype cells was verified by co-immunostaining with the astrocyte marker GFAP.
Scale bar, 100 μm. Data in this figure were produced using the TRE3G-NFIA + SOX9 hPSCs (H9).
In stage 1 of astrocyte differentiation (day 1–10), hPSCs are specified to neuroepithelia which are readily patterned to regional progenitors by morphogens (Li et al., 2005, Tao and Zhang, 2016). We hence asked if regional astrocyte types may be generated at the same speed when the early neuroepithelia are patterned with respective morphogens in stage 1. We differentiated the hPSCs to neuroepithelia in the presence of morphogens for 10 days before induced expression of transgenes for iAstro generation (Figure 5B). In the absence of morphogens, the resulting iAstro (around 70%) were of mostly dorsal forebrain (cortical) identity, as indicated by expression of the forebrain marker OTX2 (Figures 5C and S5). In the presence of ventral patterning morphogen, sonic hedgehog (Shh), a proportion (around 50%) of GFAP+ cells expressed NKX2.1, a ventral forebrain marker (Figures 5C and S5). In the presence of caudal patterning morphogen retinoic acid (RA), they (>80%) became spinal astrocytes at day 52, as indicated by their co-expression of GFAP and HOXB4 (Figures 5C and S5). Thus, iAstro with different regional identities are generated at the same speed by combining patterning of neuroepithelia and induction of NF1 and SOX9.
Discussion
We have developed a robust method to generate functional astrocytes from hESCs and hiPSCs in 4–7 weeks. This is achieved by CRISPR/Cas9-mediated inducible expression of the gliogenic transcription factors NFIA or NFIA + SOX9 in stem cells. The induced cells (or iAstro) exhibit characteristic cellular and functional properties of astrocytes and retain their identity after withdrawal of transgene expression and following transplantation into the mouse brain. Importantly, our method can be extended to generation of astrocyte subtypes in the same time frame by combining regional patterning of neural progenitors and induction of transgenes. This simple and efficient method is substantially faster than the regular differentiation methods mimicking astrocyte development that take 3–6 months (Krencik et al., 2011) and thus helps facilitate the study on human astrocyte biology.
Gliogenesis is a late event during neural development. In humans, it begins at 3 months into gestation and continues for many months. Accordingly, available methods for differentiating hPSCs to astrocytes takes 3–6 months by mimicking the developmental process (Krencik et al., 2011). This is because we lack a non-genetic means to switch the neurogenic progenitors to the gliogenic phase. From the standpoint of developmental biology, gliogenic transcription factors, including NFIA and SOX9, initiate gliogenesis and maintain the glial identity during neural development (Deneen et al., 2006, Kang et al., 2012, Miller and Gauthier, 2007, Stolt et al., 2003, Tsuyama et al., 2015, Vong et al., 2015). Forced expression of NFI and SOX9 in neuronal progenitor cells in vivo or in cultured mouse fibroblasts promotes astrocyte generation (Gaiazzo et al., 2015). By expressing NFIA during hPSC neural differentiation, we are now able to generate astrocytes substantially earlier. The addition of SOX9 further accelerates the differentiation of astrocytes. This is likely due to the coordinated effect of NFIA and SOX9 in initiating gliogenesis (Kang et al., 2012). Indeed, our RNA-seq analysis shows that NF1A and SOX9 repress neuronal differentiation and facilitate glial differentiation. This is also indicated by the methylation assay, showing that the GFAP promoter is demethylated after the transgenic induction, which enables the cells to express the gliogenic gene GFAP during the iAstro differentiation.
Astrocytes in different brain regions exhibit differential properties (Hewett, 2009, Schitine et al., 2015, Sofroniew and Vinters, 2010, Tabata, 2015, Zhang, 2001). We have shown that the iAstro with regional identities can be generated by patterning the early neural progenitors during regular differentiation (Krencik et al., 2011). However, forced expression of neural transcription factors at the beginning of differentiation skips the neural patterning step, resulting in the generation of mature neurons, as demonstrated by Ngn2-induced neurons (Zhang et al., 2013). Interestingly, we found that, for iAstro generation, the expression of NF1A and SOX9 is dispensable at the early stage of neural differentiation, suggesting that they act on specified neuroepithelia for astrocyte differentiation. This opens a window of opportunity for us to pattern the early neuroepithelia with respective morphogens for generation of region-specific progenitors before driving the progenitors to iAstro through induction of NF1A and SOX9. The combination of neural induction/patterning and expression of NF1A and SOX9 enables fast generation of subtypes of functional astrocytes (iAstro).
The functional properties of astroglial subtypes in the adult CNS remain largely undefined (Barres, 2008, Morel et al., 2017, Oberheim et al., 2012). It has been demonstrated that region-specific astrocytes (e.g., cortical or subcortical) selectively promote neurite growth of neurons from the corresponding region (Morel et al., 2017). The gap junction coupling strength appears higher in astrocytes from hippocampus and hypothalamus than those from cerebral cortex and brain stem (Blomstrand et al., 1999). Calcium waves in gray matter protoplasmic astrocytes rely on gap junction coupling to propagate, while fibrous astrocytes of the white matter in the corpus callosum propagate calcium waves depending on ATP (Oberheim et al., 2012). The ability to generate mature astrocyte subtypes from hPSCs will facilitate the investigation of the functional diversity of region-specific astrocytes.
The generation of clonal hPSC lines through CRISPR-mediated transgenesis enables regulation of transgenes in a consistent and reproducible manner, thus producing homogeneous cell populations. This method is simple and can be readily applied to patient iPSCs. The inducible hPSC lines can also be introduced with disease-related mutations for studying the roles of astrocytes in pathogenesis. Hence, the method is advantageous over virus-mediated approaches that involves random transgene integration.
The iAstro exhibit characteristic astrocyte morphology, express astrocytic markers, and maintain their identity after withdrawal of transgenes and transplantation into the mouse brain. Functionally, they resemble primary astrocytes, propagating calcium waves, taking up glutamate, and supporting neurite growth. Therefore, the iAstro are appropriate for studying the biology of human astrocytes as well as their interactions with surrounding neurons and glia under physiological and pathological conditions. Their ability to survive and integrate into the mouse brain makes it appropriate to analyze the behaviors of the iAstro in vivo.
Experimental Procedures
hPSC Culture
HESCs (line H9, passages 20–40) and iPSCs lines (WC50 and IMR90; see also Supplemental Experimental Procedures) were cultured as described previously (Krencik et al., 2011). In brief, cells were passaged weekly by using dispase (1 mg/mL, Gibco) and plating on a monolayer of irradiated mouse embryonic fibroblasts (WiCell). The hPSC culture medium consisted of DMEM/F12 basal medium (Gibco), 20% KnockOut serum replacement (Gibco), 0.1 mM β-mercaptoethanol (Sigma), 1 mM L-glutamine (Gibco), nonessential amino acids (Gibco), and 4 ng/mL FGF-2 (R&D Systems).
Fast Induction of Subtype Astrocytes from Engineered hPSC Lines
TRE3G-NFIA/TRE3G-NFIA + SOX9 hPSC lines were established (detailed in the Supplemental Information) and first differentiated to neuroepithelia in the presence of DMH1 (2 μM, Tocris) plus SB431542 (2 μM, Tocris) for 10 days as reported (Chambers et al., 2009, Hu et al., 2010). The cells were digested by EDTA (Gibco) and proceeded into stage 2 induction in suspension in T25 flasks as described above: heparin (1 μg/mL, Sigma) is added in the first week of stage 2 with FGF-2 (10 ng/mL, R&D) and then the cells were cultured with FGF-2 (10 ng/mL, R&D) plus EGF (10 ng/mL, R&D) until stage 3 for maturation. For fast differentiation to cortical astrocytes, the culture was continued without additional regionally patterning factors; for fast differentiation to ventral forebrain astrocytes, Shh (500 ng/mL, R&D Systems) was added (day 0–21); for fast differentiation to spinal astrocytes, RA (500 nM, Tocris) was added (day 0–21). All the groups were treated with DOX (1 μg/mL) from day 11 to induce astrocyte differentiation. After 45 days of induction, the cells were dissociated with Accutase (Chemicon) and attached to Matrigel-coated plates at a density of 5,000–10,000/cm2 in the presence of BMP4 (10 ng/mL) and CNTF (10 ng/mL) for another 7 days for maturation. For cell transplantation, all procedures were approved by the Animal Care and Use Committee of University of Wisconsin-Madison.
Author Contributions
S.-C.Z. and X.L. conceptualized the study and designed the experiments together. S.-C.Z. supervised the project. X.L. developed the methods. Z.D. provided suggestions and carried out replications in BrainXell. Y.T. performed cell transplantation. R.B. and others provided assistance. L.K. performed glutamate uptake assay. X.L. wrote the manuscript with S.-C.Z.
Acknowledgments
This work was supported by NIH-NIMH (MH099587 and MH100031), NICHD (HD076892 and U54 HD090256), NIH-NINDS (NS076352, NS086604, and NS096282), the Bleser Family Foundation, the Busta Foundation, and UW2020. X.L. acknowledges a Parkinson's Disease Foundation Postdoctoral Fellowship. S.-C.Z. acknowledges a Steenbock Professorship.
Z.D. and S.-C.Z. are co-founders of BrainXell.
Published: September 27, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.08.019.
Supplemental Information
References
- Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Blomstrand F., Aberg N.D., Eriksson P.S., Hansson E., Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xiong M., Dong Y., Haberman A., Cao J., Liu H., Zhou W., Zhang S.C. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen B., Ho R., Lukaszewicz A., Hochstim C.J., Gronostajski R.M., Anderson D.J. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Emdad L., D'Souza S.L., Kothari H.P., Qadeer Z.A., Germano I.M. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21:404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Gaiazzo M., Giannelli S., Valente P., Lignani G., Carissimo A., Sessa A., Colasante G., Bartolomeo R., Massimino L., Ferroni S. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Reports. 2015;4:25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J.A. Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system. J. Neurochem. 2009;110:1717–1736. doi: 10.1111/j.1471-4159.2009.06288.x. [DOI] [PubMed] [Google Scholar]
- Hu B.Y., Weick J.P., Yu J., Ma L.X., Zhang X.Q., Thomson J.A., Zhang S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kang P., Lee H.K., Glasgow S.M., Finley M., Donti T., Gaber Z.B., Graham B.H., Foster A.E., Novitch B.G., Gronostajski R.M. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74:79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Weick J.P., Liu Y., Zhang Z.J., Zhang S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.J., Du Z.W., Zarnowska E.D., Pankratz M., Hansen L.O., Pearce R.A., Zhang S.C. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Miller F.D., Gauthier A.S. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Molofsky A.V., Krencik R., Ullian E.M., Tsai H.H., Deneen B., Richardson W.D., Barres B.A., Rowitch D.H. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L., Chiang M.S.R., Higashimori H., Shoneye T., Iyer L.K., Yelick J., Tai A., Yang Y. Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci. 2017;37:8706–8717. doi: 10.1523/JNEUROSCI.3956-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormone E., D'Sousa S., Alexeeva V., Bederson M.M., Germano I.M. “Footprint-free” human induced pluripotent stem cell-derived astrocytes for in vivo cell-based therapy. Stem Cells Dev. 2014;23:2626–2636. doi: 10.1089/scd.2014.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim N.A., Goldman S.A., Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm T., Bolognin S., Meiser J., Nickels S., Träger C., Meilenbrock R.L., Brockhaus J., Schreitmüller M., Missler M., Schwamborn J.C. Rapid and robust generation of long-term self-renewing human neural stem cells with the ability to generate mature astroglia. Sci. Rep. 2015;5:16321. doi: 10.1038/srep16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian K., Huang C.T., Chen H., Blackbourn L.W., 4th, Chen Y., Cao J., Yao L., Sauvey C., Du Z., Zhang S.C. A simple and efficient system for regulating gene expression in human pluripotent stem cells and derivatives. Stem Cells. 2014;32:1230–1238. doi: 10.1002/stem.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J.M. Astrocytes and the evolution of the human brain. Med. Hypotheses. 2014;82:236–239. doi: 10.1016/j.mehy.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Roybon L., Lamas N.J., Garcia A.D., Yang E.J., Sattler R., Lewis V.J., Kim Y.A., Kachel C.A., Rothstein J.D., Przedborski S. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E., Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schitine C., Nogaroli L., Costa M.R., Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Front. Cell Neurosci. 2015;9:76. doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C., Stecca C., Iacomino A., Steardo L. Role of astrocytes in major neurological disorders: the evidence and implications. IUBMB Life. 2013;65:957–961. doi: 10.1002/iub.1223. [DOI] [PubMed] [Google Scholar]
- Shaltouki A., Peng J., Liu Q., Rao M.S., Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31:941–952. doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck J.A., Studer L. Moving stem cells to the clinic: potential and limitations for brain repair. Neuron. 2015;86:187–206. doi: 10.1016/j.neuron.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt C.C., Lommes P., Sock E., Chaboissier M.C., Schedl A., Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Cornwell A., Li J., Peng S., Osorio M.J., Aalling N., Wang S., Benraiss A., Lou N., Goldman S.A. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J. Neurosci. 2017;37:4493–4507. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H. Diverse subtypes of astrocytes and their development during corticogenesis. Front. Neurosci. 2015;9:114. doi: 10.3389/fnins.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zhang S.C. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell. 2016;19:573–586. doi: 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcw J., Wang M., Pimenova A.A., Bowles K.R., Hartley B.J., Lacin E., Machlovi S.I., Abdelaal R., Karch C.M., Phatnani H., Slesinger P.A. An efficient platform for astrocyte differentiation from human induced pluripotent stem cells. Stem Cell Reports. 2017;9:600–614. doi: 10.1016/j.stemcr.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyama J., Bunt J., Richards L.J., Iwanari H., Mochizuki Y., Hamakubo T., Shimazaki T., Okano H. MicroRNA-153 regulates the acquisition of gliogenic competence by neural stem cells. Stem Cell Reports. 2015;5:365–377. doi: 10.1016/j.stemcr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian E.M., Sapperstein S.K., Christopherson K.S., Barres B.A. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Vasile F., Dossi E., Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct. Funct. 2017;222:2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong K.I., Leung C.K., Behringer R.R., Kwan K.M. Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum. Mol. Brain. 2015;8:25. doi: 10.1186/s13041-015-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W., Lang M.K. Immunocytochemical evidence for a distinct GFAP-negative subpopulation of astrocytes in the adult rat hippocampus. Neurosci. Lett. 1998;257:127–130. doi: 10.1016/s0304-3940(98)00813-1. [DOI] [PubMed] [Google Scholar]
- Wiencken-Barger A.E., Djukic B., Casper K.B., McCarthy K.D. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–686. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- Zhang S.C. Defining glial cells during CNS development. Nat. Rev. Neurosci. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Szczesna K., Ochalek A., Kobolák J., Varga E., Nemes C., Chandrasekaran A., Rasmussen M., Cirera S. Neurosphere based differentiation of human iPSC improves astrocyte differentiation. Stem Cells Int. 2016;2016:4937689. doi: 10.1155/2016/4937689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.