Abstract
Objective:
To examine early cerebral hermodynamic changes among youth hospitalized with sports related traumatic brain injury (TBI).
Study design:
Youth 0–18 years admitted to a level one trauma center with sports-related TBI were enrolled. Daily measures included clinical symptoms and Glasgow Coma Scale (GCS) score. Using Transcranial Doppler (TCD) ultrasonography and tilt testing, we measured middle cerebral artery flow velocity (Vmca) and cerebral autoregulation index (ARI).
Results:
Six previously healthy males age 14 (IQR 12–16) years with headache and abnormal head CT were admitted with median admission GCS 15. Six patients underwent 12 TCD examinations between hospital days 0–9. Low Vmca occurred in 3/6 patients and on the side of TBI, whereas high Vmca occurred in 2/6 patients. Five patients had at least one measurement of impaired and five patients had absent cerebral autoregulation of at least one hemisphere; all these five patients had GCS 15 and headache during TCD examinations. Three patients were discharged with absent cerebral autoregulation. Five (83%) patients were discharged home and one patient was discharged to a rehabilitation facility.
Conclusion:
Headache, abnormal Vmca, and impaired cerebral autoregulation occur after sports-related TBI, despite normal GCS. Headache may signal underlying neurovascular abnormality in sports-related TBI.
Keywords: sports related concussion, traumatic brain injury
Introduction
While the majority of patients with sports-related traumatic brain injury (TBI) have concussion and are not hospitalized, more than 600,000 hospitalizations occur annually due to sports-related TBI [1]. Pediatric sports-related TBI accounts for approximately 60–80% of sports related hospitalizations [1]. While most sports-related TBI results in sports related concussion and while most recover in one week to 10 days [2–4], sports related TBI can result in the full spectrum of TBI severity. Yet, little is known about the pathophysiology of sports related TBI especially pertaining to cerebral hemodynamics and cerebrovascular physiology which may be altered, impact clinical care and affect outcome.
Autoregulation of cerebral blood flow (CBF) is a normal homeostatic process that maintains nearly constant CBF over a range of mean arterial pressures (MAP). Traumatic brain injury can impair or abolish cerebral autoregulation, leaving the brain vulnerable to cerebral ischemia or cerebral hyperemia [5]. We have previously shown that youth with non-sports related mild, moderate and severe TBI have impaired cerebral autoregulation [6], there are hemispheric differences in cerebral autoregulation [7], impaired cerebral autoregulation is associated with poor 6 month outcome [8], and that young age is a risk factor [9]. In a study of otherwise children who are otherwise healthy with non-sports related mild TBI presenting for orthopedic surgery, the prevalence of impaired cerebral autoregulation was 17% [10]. Under normal conditions, postural changes do not result in impaired cerebral autoregulation [11] and normative data on cerebral autoregulation for children and adolescents provide an opportunity for examining pathophysiological changes after sports related TBI [12,13].
Neuroimaging, such as computed tomography (CT) scanners or magnetic resonance managing, are often used as a non-invasive means to diagnose sports related TBI because the ability to quickly perform the imaging in emergency departments and ease of use [14]. In addition to neuroimaging, the Glasgow Coma Scale (GCS) is a non-invasive clinical assessment and scoring system used to assess the severity of TBI and includes eye opening, verbal response and motor response. These assessments may be utilized to determine the course of care, and prognosticate outcome[15]. Other noninvasive neuroimaging measures such as electroencephalography and magnetic resonance imaging techniques are promising, but their clinical utility remains to be established [16]. Transcranial Doppler (TCD) ultrasonography is an established neurovascular non-invasive and real time bedside tool to estimate cerebral blood flow, and understand changes in cerebral perfusion in clinical care and in research [17–20]. Coupled with tilt testing, TCD can be used to understand changes in cerebral autoregulation. Since low cerebral blood flow can result in cerebral ischemia if cerebral autoregulation is impaired, it is important to understand whether this otherwise homeostatic mechanism is intact. In an outpatient study of 17 athletes with mean age of 20 years and concussion who underwent arterial spin labeling magnetic resonance imaging relative cerebral blood flow was examined at three time points after concussion. Compared to healthy volunteers, patients with concussion had reduced cerebral blood flow at one day and one week, which then recovered at one month [21]. To add to our understanding of early cerebrovascular changes in youth hospitalized with sports related TBI, we examined cerebral autoregulation among youth hospitalized with sports related TBI.
Methods
Study participants and setting
After University of Washington Human Subjects Approval (IRB # 35291), we enrolled youth aged 0–18 years who were admitted to a level one trauma center with a diagnosis of TBI with an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for TBI (ICD 800.1–800.4, 800.6–800.9, 850.0–854.9, 801.1–801.4, 801.6–801.9, 803.1–803.4, 803.6–803.9, 804.1–804.4, and 804.6–804.9). This is an ongoing study of cerebral autoregulation among 75 youth hospitalized with all cause TBI which is expected to continue enrollment through end of calendar year 2017. In this report, we present findings from patients whose mechanism of injury was recorded as sports related. Patients with polytrauma were included. Eligible patients could be recruited from the Harborview Medical Center pediatric intensive care unit (ICU) or hospital ward (Seattle, WA). Informed consent for participation was obtained from parent or guardian, and age-appropriate assent was obtained from each patient.
Harborview Medical Center is a level one adult and pediatric trauma center with an accredited cerebrovascular laboratory. Trained TCD technicians commonly perform bedside TCD examinations in adults and children admitted with neurological and neurosurgical conditions to estimate CBF, and cerebral autoregulation per written protocols. Ordersets for TCD use and cerebral autoregulation testing are included in the electronic health record.
Study design and protocol
This was a single center prospective cohort study of youth meeting eligibility criteria. Clinical characteristics were abstracted from daily physician and nurse records. Physiologic testing with TCD ultrasonography was conducted at the bedside daily for the duration of hospital length of stay (LOS).
Clinical characteristics
We abstracted age, sex, and race. The mechanism of injury that led to hospitalization was categorized using ICD-9 external cause of injury codes. We recorded TBI type from radiology reports (subarachnoid hemorrhage, intracerebral hemorrhage, subdural hemorrhage, epidural hemorrhage, contusion, and concussion). Admission and daily GCS scores were abstracted, along with information on surgery, hospital and intensive care unit LOS, and discharge disposition.
Cerebrovascular testing using TCD ultrasonography and tilt testing
General procedure
Each participant was positioned in a bed with head and back adjustable for elevation. An appropriately sized noninvasive blood pressure cuff was placed on an upper extremity at the arm. Transcranial Doppler ultrasonography (Multidop X; DWL Corp., Sipplingen, Germany) was used to measure bilateral middle cerebral artery flow velocities (Vmca). Two certified TCD technicians (AM and colleague) obtained TCD data blinded to the patient and the research staff member who were abstracting patient data and recording blood pressure during testing (AW and MSV).
Measuring middle cerebral artery flow velocity (Vmca)
A hand-held 2-MHz ultrasound probe was used to insonate each middle cerebral artery and positioned for sufficient time to achieve steady-state measurement, at previously published age appropriate depths [7]. During steady-state conditions, Vmca and corresponding MAP were recorded.
Cerebral autoregulation testing and determining autoregulatory index (ARI)
The change in head and back position proceeded from supine to upright position (13.6 cm / 10 mmHg difference between two positions) served as the stimulus for testing cerebral autoregulation [22]. For the relatively upright position, the vertical distance between the noninvasive blood pressure cuff and the external auditory meatus was used to calculate the estimated MAP (MAPe) at the Circle of Willis. Because MAP decreases by 1 mm Hg for every 1.36-cm increase in vertical height, the change in height from supine to sitting upright was divided by 1.36 cm to calculate the MAPe in the sitting upright position [23].
The ARI for each middle cerebral artery was calculated off-line as previously described [8].Briefly, cerebral autoregulation was quantified using the autoregulatory index (ARI), where ARI = % ΔeCVR/% ΔMAPe; eCVR is the estimated cerebrovascular resistance calculated as the ratio of MAP to Vmca as appropriate [22].
Statistical analysis
Sociodemographic, injury and admission GCS, cerebrovascular data and age were examined using descriptive statistics. Change in Vmca and ARI were examined using Pearson correlation statistics and mean Vmca and ARI were determined using descriptive statistics. Differences between left and right side Vmca and ARI were examined using univariate analysis. Abnormal Vmca was defined as Vmca < 2 SD [low Vmca] or > 2SD [high Vmca] for age and sex) and abnormal cerebral autoregulation was defined as ARI < 0.4 [impaired] and ARI 0 [absent]) [6, 13, 22]. Patients were divided into two outcome groups: those who discharged with normal cerebral autoregulation (ARI > 0.4) and those who discharged with impaired cerebral autoregulation (ARI < 0.4). TCD characteristics, including Vmca, ARI, and MAP change where examined for each group using Fisher exact test, chi square test or student t-test and univariate analysis. Mixed Analysis of Variance analysis was utilized to analyze the differences in ARI and Vmca between the group of patients who discharged with impaired cerebral autoregulation versus those who discharged with normal cerebral autoregulation. Although all patients were male, age-and sex related Vmca were compared with age-related normative data [23]. Significance was set at p < 0.05. All data are presented as mean or median ± SD, except for age, GCS, and LOS which are presented as interquartile range (IQR). SAS software (SAS Institute Inc., Cary, NC, USA) was used for data analysis.
Results
Over the two-month period, six patients were admitted with a diagnosis of sports related TBI. Consent was obtained within 24 hours of admission from all patients.
Clinical characteristics
Baseline characteristics of patients who underwent cerebral autoregulation testing are listed in Table 1. Admission GCS ranged from 5 −15 (median admission GCS 15 (IQR 12–15). Five patients had GCS 15 and one patient had GCS 5. All were male and most (83%) were white. Median age was 14 (IQR 12–16) years and median admission GCS was 15 (IQR 12–15). Sports mechanisms were baseball (N=3), football (N=1), soccer (N=1), and skateboard (N=1). All six patients had isolated TBI. Concussion, which accounted for 25% of moderate TBI, was the most frequently diagnosed TBI type (Table 1). Median ICU length of stay (LOS) was 1 days (1 [IQR 1–2]) days, and hospital LOS was 3 days (3 [IQR 2–5] days. All subjects were admitted from the emergency department to the ICU prior to transfer to the ward.
Table 1.
Clinical characteristics of six children and adolescents hospitalized with sports related traumatic brain injury. GCS = Glasgow Coma Scale, CT = computed tomography, LOC = Loss of consciousness, LOS = length of stay, SDH = subdural hematoma, EDH = Epidural hematoma, SAH = subarachnoid hemorrhage.
| Age (y)/Sex | Sport/Mechanism | Head CT diagnosis | Admit GCS | LOC | Surgery | Hospital LOS (days) |
|---|---|---|---|---|---|---|
| 10 M | Soccer Fall backward |
Left occipital skull fracture Left SDH Left transverse venous sinus narrowing |
14 | - | - | 3 |
| 13 M | Baseball to temple No helmet |
Left EDH with midline shift left frontal lobe SAH and hemorrhagic contusion |
5T | - | Left craniotomy day1 |
9 |
| 13 M | Baseball bat to head No helmet |
Left temporal fracture Left SAH Pneumocephalus |
15 | - | - | 3 |
| 14 M | Flag football Fall backward No helmet |
Left frontal SAH Parafalcine SDH |
14 | 3–5 min | - | 2 |
| 16 M | Baseball Elbow to right eye No helmet |
Right frontal contusion Right orbital fracture |
15 | - | - | 2 |
The one patient admitted with GCS 5T suffered from an epidural hematoma with midline shift. After craniotomy, GCS improved to 15 on hospital day 2. Five of six patients were discharged home and one patient was discharged to a rehabilitation facility.
Clinical symptoms
All six patients reported headache and nausea/emesis on admission. At discharge, all patients remained symptomatic with headache (n=4), or nausea/emesis (n=2). One patient complained of blurry vision which resolved at hospital discharge.
Measures of cerebral blood flow velocity (Vmca) and cerebral autoregulation (ARI)
Test quality
Six children underwent a total of 12 TCD ultrasonography and tilt tests (Table 2). Four (66%) patients underwent more than one TCD and tilt test. All six children experienced at least a 5 mmHg decrease (mean 10±3 mmHg) in MAPe during tilt testing. There was no difference in MAPe between the five patients with impaired cerebral autoregulation and those with intact cerebral autoregulation. Data on Vmca and ARI data for individual participants are given in Tables 1 and 2.
Table 2.
Symptoms, middle cerebral artery flow velocity (Vmca) and cerebral autoregulation (ARI) within 24 Hours after sports related traumatic brain injury. GCS = Glasgow Coma Scale, MAP = mean arterial pressure, ARI = autoregulatory index. * = Supine Vmca
| Age (y) and Sex | Clinical Symptoms | Transcranial Doppler Ultrasonography Examination for Vmca and ARI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge | Hospital Day | GCS at | Vmca L∗ (cm/s) | Vmca R∗ (cm/s) | Supine MAP (mmHg) | Upright MAP (mmHg) | MAP change (mmHg) |
ARI L | ARI R | |
| 10 M | Emesis x 1 Blurry vision x 1d Headache x 2d Nausea x 3d |
Day 3: Nausea Disposition: Home |
2 3 |
15 15 |
78 89 |
75 80 |
89 84 |
84 69 |
5 15 |
1.0 0.81 |
0 1.0 |
| 13 M | Emesis x 4 Nausea x 5d Headache x 9d |
Day 9; Mild headache, cognitive deficit, word finding difficulties, balance impairment Disposition: Inpatient rehabilitation |
2 3 7 8 |
15 15 15 15 |
90 72 72 78 |
94 90 79 85 |
86 83 76 74 |
76 74 67 64 |
10 9 9 9 |
0 0.63 0.53 1.0 |
0 0.80 0.36 1.0 |
| 13 M | Emesis x 1 Headache x 3d |
Day 3: Nausea, mild headache Disposition: Home No symptoms on day 6 by phone follow up |
2 3 |
15 15 |
67 47 |
57 63 |
68 76 |
60 67 |
8 9 |
0.75 0.68 |
1.0 1.0 |
| 14 M | Seizure x 1 min Nausea x 1d Headache x 2d |
Day 2: Headache Disposition: Home |
2 | 15 | 55 | 58 | 72 | 63 | 9 | 0 | 0 |
| 16 M | Emesis x 3 Headache x 2d |
Day 2: Pain localized to eye Disposition: Home |
1 2 |
15 15 |
59 63 |
61 64 |
71 83 |
54 73 |
17 10 |
0.58 0 |
0.73 0.09 |
| 17 M | Emesis x 1 Nausea x 1d Headache x 2d |
Day 2: Headache Disposition: Home |
1 | 15 | 56 | 56 | 74 | 61 | 13 | 1.1 | 0 |
Vmca
Figure 1 shows that mean Vmca increases for the cohort of six patients over eight days after sports related TBI and that there are hemispheric differences in Vmca; mean baseline (supine) Vmca was 68 ± 14 cm/sec and 67 ±15 cm/sec for the left and right sides, respectively. Low Vmca occurred in 3/6 patients and on the side of TBI, whereas high Vmca occurred in 2/6 patients on at-least one TCD examination. Figure 2 shows there is between patient variation in Vmca.
Figure 1.
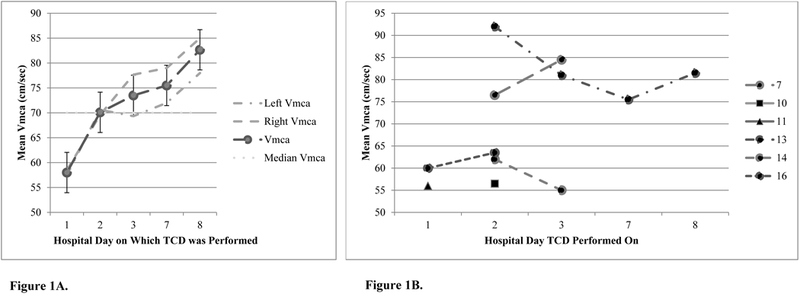
(A) Mean middle cerebral artery flow velocity (Vmca) of all six patients after sports-related traumatic brain injury. (B) Change in mean middle cerebral artery flow velocity (Vmca) after sports-related traumatic brain injury in four of six patients who underwent repeat Transcranial Doppler ultrasonography testing.
Figure 2.
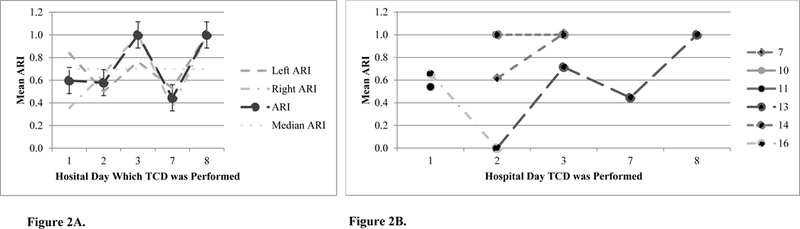
(A) Hemispheric differences in autoregulatory index (ARI) of six patients after sports-related traumatic brain injury. (B) Change in mean autoregulatory index (ARI) in four of the six patients after sports-related traumatic brain injury; TCD = Transcranial Doppler Ultrasonography, ARI < 0.4 = impaired cerebral autoregulation and ARI > 0.4 = intact cerebral autoregulation. ARI 0 = absent cerebral autoregulation and ARI 1 = perfect cerebral autoregulation.
ARI
Figure 2A shows differences in mean ARI over the first 8 days after sports related TBI and Figure 2B shows between patient variation in the trajectory of ARI. Five (83%) of the six patients had impaired cerebral autoregulation (Tables 1 and 2) within 24 hours of admission. One patient with right frontal contusion with right orbital fracture intact cerebral autoregulation on hospital day 1 had impairment on hospital day 2. Of the four patients with more than one test, one had impaired cerebral autoregulation impairment on more than one day.
Five (83%) patients were discharged home and one patient was discharged to a rehabilitation facility. Three (50%) patients were discharged with impaired cerebral autoregulation. These patients had a statistically significant lower left ARI (0.4 ± 0.5) in comparison to patients (N=3) who were discharged with normal cerebral autoregulation (0.7±0.3; p= 0.02). These patients also had a statistically significant lower mean right supine Vmca (60± 4 cm/s) in comparison to the patients (N=3) who discharged with normal cerebral autoregulation (78 ± 13 cm/s; p=0.02). Eighty three percent (5/6) had at least one measurement of impaired and 83% had absent cerebral autoregulation; all these five patients had GCS 15 and headache at time of testing.
Discussion
In this study, we examined cerebral autoregulation among youth hospitalized after sports related TBI. The urgency to disseminate these preliminary research findings primarily stems from the paucity such information from hospitalized children and from the first week after TBI, and to accelerate knowledge regarding cerebral autoregulation after sports related TBI. Moreover, the number of patients with sports related TBI among children hospitalized is relatively small and waiting until the end of the ongoing study is expected to yield two to three additional subjects.
The main findings of this study in hospitalized sports related TBI are that: 1) impaired cerebral autoregulation is common, 2) impaired cerebral autoregulation is associated with headache, and 3) impaired cerebral autoregulation occurs even when GCS is 15. We also observed that Vmca increases and ARI changes variably over the first week after sports related TBI. These findings suggest a potential link between clinical symptoms such as headache, with impaired cerebral autoregulation after sports related TBI. We included the one patient admitted with GCS 5T and hence report our findings as sports related TBI. However, five of the patients had admission GCS 15, representing the group of TBI patients who are phenotypically equivalent to those with sports related concussion but who have more complicated underlying brain pathology.
Postural change as induced by tilt testing results in a decrease in mean arterial pressure to the cerebral circulation. When cerebral autoregulatory mechanisms are intact, arteries and arterioles dilate to preserve cerebral perfusion. Thus, postural change represents a cerebral autroegulatory stimulus. The cerebral autoregulatory index (ARI) a standard measure of how well cerebral blood flow is maintained during changes in mean arterial pressure, as experienced by the artery being insonated by TCD ultrasonography. An ARI of 0 reflects absent autoregulation, an ARI 1 reflects perfect autoregulation. ARI < 0.4 reflects impaired autoregulation and ARI > 0.4 reflects intact cerebral autoregulation [20]. The significance of impaired autoregulation is that the neurovascular unit may not be able to maintain normal cerebral perfusion with changes in mean arterial pressure, induced by posture or other exposures such as general anesthesia.
Our prior work, also using tilt testing and using TCD ultrasonography compared cerebral autoregulation of the anterior and posterior circulations in healthy boys and girls who were 10–16 years of age [21]. Among the 13 boys and 13 girls, middle cerebral artery flow velocities were higher in girls than boys. While all children demonstrated intact autoregulation, boys had higher autroegulatory indices of the middle cerebral artery than girls. This study provided normative data on cerebral autoregulation of the posterior circulation in healthy, awake boys and girls, which we used as a reference to understand ARI of the MCA in this study. While all our subjects were male, further study of female athletes would be important to understand differences between healthy normal females and those with sports related TBI”.
Some changes e.g. reduced CBF, neuronal metabolism and perfusion, impaired axonal function, are described after sports related concussion [24, 25]. These changes may be associated with persistent symptoms after concussion. Findings from this study using TCD ultrasonography corroborate previous results that CBF is low after sports related TBI [21, 26]. These findings also add to existing knowledge on hemispheric differences, and trajectory of change after TBI. Specifically, patients with one sided head CT lesions also demonstrate Vmca and ARI differences in contralateral hemispheres, suggesting that underlying brain pathology is more diffuse than is detected by initial CT imaging. Cerebral hyperemia or abnormally high Vmca was not observed in this series, suggesting that cerebral ischemia rather than cerebral hyperemia is likely the more common pathway after sports related TBI. These findings are consistent with non-sports related TBI.
Impaired cerebral autoregulation has clinical implications for treating clinicians. First, it indicates abnormality in the neurovascular unit where CBF is either relatively or absolutely pressure passive. This means that CBF is dependent on blood pressure. All these patients except one had admission GCS 15. In some cases, Vmca was low despite MAP being above lower limit of cerebral autoregulation for age. Additionally, the vulnerability of the neurovascular unit is evident in the one patient with GCS 5T after baseball injury who underwent craniotomy. In this case, although resting baseline (supine) Vmca was within normal limits, the ARI was low when the stimulus of postural change was applied. While one can argue that this abnormality represents a more serious case of sports related TBI, similar observations were made for those with GCS 15 where four of the six patients with GCS 15 had absent cerebral autoregulation of at-least one cerebral hemisphere at the time of testing. We did not have serial testing in all these patients because patients were discharged from hospital. While hemodynamic management was not required for five of the six patients in this series, one patient underwent craniotomy and exposure to general anesthesia where risks of systemic hypotension translate to cerebral hypoperfusion when autoregulation is impaired [27]. Importantly, while none of the five other patients required surgery either for the TBI or other polytrauma that may happen in athletics, a GCS of 15 may suggest a false sense of neurovascular stability. Early changes in cerebral activity may be important because they indicate susceptibility to second insults at a time when the brain is vulnerable after sports related concussion. For example, youth who have impaired autoregulation and have surgery and anesthesia care may require refinement of choice of medications because of known medication-brain interactions after TBI However, even patients with mild TBI undergoing general anesthesia have impaired cerebral autoregulation which may or may not be related to preoperative autoregulation status. Therefore, knowledge of the status of cerebral autoregulation is important to facilitate tight hemodynamic control during general anesthesia [28]. These findings may be important to longer term symptoms and patient outcomes [8]. Increased awareness that cerebral autoregulation is often impaired after sports related TBI, including concussion, is urgently needed.
Current clinical pediatric TBI practice does not utilize routine neuroimaging to examine cerebral perfusion or cerebral autoregulation either at time of admission, discharge or planned at follow up. However, present findings suggest that neurovascular diagnostics may be needed because normal GCS does not imply a lack of vulnerability for the following reasons. First, despite hospitalization, most are phenotypically normal per GCS, which is an important facilitating clinical criterion for discharge. Second, these patients with GCS 15 have shorter hospital LOS than other youth admitted with TBI. In studies of cerebral autoregulation in moderate-severe TBI, hospital LOS frequently exceeds two to three days, and cerebral autoregulation typically returns to normal within one week after TBI assuming normal recovery course [8]. Third, there is a lack of awareness that clinical symptoms despite normal GCS may represent underlying neurovascular abnormalities, regardless of mechanism of TBI. While it is possible that children with non-sports related TBI similarly discharged with headache and GCS 15 within 48 hours of TBI and have impaired cerebral autoregulation at discharge, these patients typically have longer hospital LOS and are discharged when cerebral autoregulation improves and returns to normal. Further work will need to elucidate whether mechanism of injury is important to this pathophysiology and what cerebrovascular diagnostics are needed prior to hospital discharge specifically in sports related TBI.
Although youth hospitalized with sports related TBI are symptomatic and complain of dizziness, these patients do not typically undergo formal balance testing during hospital course or prior to discharge. In this series, balance impairment was formally recorded in the medical record of one patient and while dizziness was reported by some of these six patients, formal balance testing was not performed in these patients to link dizziness with balance impairment. However, this is an important point because postural abnormalities are reported among athletes after sports related concussion [29]. Since self-reported balance status is not reported to a reliable marker for balance performance in adolescents, we are not able to associate balance abnormalities with impaired cerebral autoregulation. However, this does beg the question as to whether youth hospitalized with sports related TBI should undergo formal balance testing if they are ambulating [30], and whether formal balance testing would be a marker for impaired cerebral autoregulation. This argument seems logical because the tilt test stimulus for cerebral autoregulation testing involves postural change. Whether the balance error scoring system or center of point test more accurately predict impaired cerebral autoregulation is not known but what is suggested is that center of pressure tests demonstrate balance deficits are detected one month post sports related concussion using center of pressure testing [29], rather than the balance error scoring system.
Cerebral autoregulation can be evaluated quantitatively using several different techniques. Although static cerebral autoregulation testing, using TCD, has been validated and can be performed using pharmacologic intervention to increase MAPe, it may be too invasive for awake, healthy children [6]. Tilt testing allows cerebral autoregulation to be studied at the bedside noninvasively, is well tolerated by children, and has been used for many years in the evaluation of children and adults with syncope [31]. It has also been combined with TCD ultrasonography to evaluate changes in cerebral vascular resistance in patients with dysautonomia [32].
There are some limitations to this study. First, the sample size is small. However, we are able to provide detailed cerebral hemodynamic within and between patients data on cerebral autoregulation. First, we measured Vmca, which estimates but does not directly measure CBF. However, the study was conducted during steady state by investigators with experience using TCD technology, and the flow velocities in this study correlate well with the normal values previously reported by Bode as well as by previous studies examining CBF using arterial spin labelling [26, 33]. Additionally, since patients are hospitalized, we were able to conduct serial TCD examinations during the first week, whereas other studies examining cerebral autoregulation examined outpatients. Moreover, this study is devoid of confounders previously present in examination cerebral autoregulation in mild TBI such as sedation or general anesthetic exposure [34, 35]. Although we could estimate the decrease in MAPe with tilt testing, we could not control the blood pressure to ensure a specified drop in MAPe. However, the range of MAPe tested (77–55 mm Hg) was within physiologic norms and sufficiently broad to precipitate an autoregulatory response. We are unable to comment on potential differences in lower limit of cerebral autoregulation between testing. In addition, we evaluated only the MCA. To maintain steady-state conditions, testing was conducted in a quiet setting, and we ensured that flow velocities reached steady state TCD recordings. Finally, we are unable to definitively link clinical symptoms with cerebral autoregulation status. However, we provide provocative new information that may fuel a new line of investigation to detect underlying cerebrovascular abnormalities after sports related TBI.
Conclusion
Impaired cerebral autoregulation commonly occurs after sports related TBI despite normal GCS. Headache symptoms may suggest impaired cerebrovascular homeostasis and may be a marker for a brain at risk, after sports related TBI. Further studies are needed to better understand change in cerebral autoregulation and its relationship with clinical symptoms among the larger number of youth who sustain sports related TBI, including those with concussion who are not admitted to a hospital for post-concussion care.
Acknowledgements
This study was conducted at Harborview Medical Center, University of Washington, Seattle, WA
Financial support: NINDS R21NS095321 (PI: Vavilala) and The Seattle Pediatric Concussion Collaborative (PI: Rivara).
Footnotes
Declaration of Interest Statement
All authors have disclosed no potential conflict of interest.
References
- [1].Yue JK, Winkler EA, Burke JF, Chan AK, Dhall SS, Berger MS, Manley GT, Tarapore PE. Pediatric sports-related traumatic brain injury in United States trauma centers. Neurosurgical focus. 2016;40:E3. [DOI] [PubMed] [Google Scholar]
- [2].Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F, Seattle Sports Concussion Research C. Sports- and recreation-related concussions in US youth. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- [3].Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2011;31:85–93. [DOI] [PubMed] [Google Scholar]
- [4].McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ. et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017. [DOI] [PubMed] [Google Scholar]
- [5].Obrist WD, Gennarelli TA, Segawa H, Dolinskas CA, Langfitt TW. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg. 1979;51:292–300. [DOI] [PubMed] [Google Scholar]
- [6].Vavilala MS, Lee LA, Boddu K, Visco E, Newell DW, Zimmerman JJ, Lam AM. Cerebral autoregulation in pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2004;5:257–63. [DOI] [PubMed] [Google Scholar]
- [7].Vavilala MS, Tontisirin N, Udomphorn Y, Armstead W, Zimmerman JJ, Chesnut R, Lam AM. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9:45–54. [DOI] [PubMed] [Google Scholar]
- [8].Chaiwat O, Sharma D, Udomphorn Y, Armstead WM, Vavilala MS. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. Journal of neurotrauma. 2009;26:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology. 2008;108:588–95. [DOI] [PubMed] [Google Scholar]
- [10].Vavilala MS, Lee LA, Lee M, Graham A, Visco E, Lam AM. Cerebral autoregulation in children during sevoflurane anaesthesia. Br J Anaesth. 2003;90:636–41. [DOI] [PubMed] [Google Scholar]
- [11].Garrett ZK, Pearson J, Subudhi AW. Postural effects on cerebral blood flow and autoregulation. Physiol Rep. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Verlhac S Transcranial Doppler in children. Pediatr Radiol. 2011;41 Suppl 1:S153–65. [DOI] [PubMed] [Google Scholar]
- [13].Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988;63:606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prabhu SP. The role of neuroimaging in sport-related concussion. Clin Sports Med;30(1):103–14. DOI: 10.1016/j.csm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- [15].Zollman FS. Manual of traumatic brain injury assessment and management. 2nd ed. ed. : Springer Publishing Company; 2016. [Google Scholar]
- [16].Kutcher JS, Mccrory P, Davis G, Ptito A, Meeuwisse WH, Broglio SP. What evidence exists for new strategies or technologies in the diagnosis of sports concussion and assessment of recovery? British Journal of Sports Medicine 2013;47(5):299. [DOI] [PubMed] [Google Scholar]
- [17].Adams R, McKie V, Nichols F, Carl E, Zhang DL, McKie K, Figueroa R, Litaker M, Thompson W, Hess D. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–10. [DOI] [PubMed] [Google Scholar]
- [18].Ma L, Roberts JS, Pihoker C, Richards TL, Shaw DW, Marro KI, Vavilala MS. Transcranial Doppler-based assessment of cerebral autoregulation in critically ill children during diabetic ketoacidosis treatment. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15:742–9. [DOI] [PubMed] [Google Scholar]
- [19].Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55:112–23. [DOI] [PubMed] [Google Scholar]
- [21].Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, Mayer AR. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015;72:530–8. [DOI] [PubMed] [Google Scholar]
- [22].Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–9. [DOI] [PubMed] [Google Scholar]
- [23].Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. Journal of neurotrauma. 2014;31:1497–506. [DOI] [PubMed] [Google Scholar]
- [25].Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang Y, Nelson LD, LaRoche AA, Pfaller AY, Nencka AS, Koch KM, McCrea MA. Cerebral blood flow alterations in acute sport-related concussion. Journal of neurotrauma. 2016;33:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Philip S, Udomphorn Y, Kirkham FJ, Vavilala MS. Cerebrovascular pathophysiology in pediatric traumatic brain injury. The Journal of trauma. 2009;67:S128–34. [DOI] [PubMed] [Google Scholar]
- [28].Vavilala MS, Ferrari LR, Herring SA. Perioperative care of the concussed patient: making the case for defining best anesthesia care. Anesth Analg. 2017. [DOI] [PubMed] [Google Scholar]
- [29].Rochefort C, Walters-Stewart C, Aglipay M, Barrowman N, Zemek R, Sveistrup H. Balance markers in adolescents at 1 month postconcussion. Orthop J Sports Med. 2017;5:2325967117695507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rochefort C, Walters-Stewart C, Aglipay M, Barrowman N, Zemek R, Sveistrup H. Self-reported balance status is not a reliable indicator of balance performance in adolescents at one-month post-concussion. J Sci Med Sport. 2017. [DOI] [PubMed] [Google Scholar]
- [31].D’Andrea A, Conte M, Scarafile R, Riegler L, Cocchia R, Pezzullo E, Cavallaro M, Carbone A, Natale F, Russo MG, et al. Transcranial Doppler Ultrasound: physical principles and principal applications in neurocritical care unit. J Cardiovasc Echogr. 2016;26:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fuente Mora C, Palma JA, Kaufmann H, Norcliffe-Kaufmann L. Cerebral autoregulation and symptoms of orthostatic hypotension in familial dysautonomia. J Cereb Blood Flow Metab. 2016:271678X16667524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Militana AR, Donahue MJ, Sills AK, Solomon GS, Gregory AJ, Strother MK, Morgan VL. Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging Behav. 2016;10:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surg Neurol. 1997;47:128–31. [DOI] [PubMed] [Google Scholar]
- [35].Jaffres P, Brun J, Declety P, Bosson JL, Fauvage B, Schleiermacher A, Kaddour A, Anglade D, Jacquot C, Payen JF. Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med. 2005;31:785–90. [DOI] [PubMed] [Google Scholar]


