Abstract
Background and aims
So far, no randomized trial or meta-analysis has been conducted on overall survival (OS) and recurrence-free survival (RFS) factors in patients treated with radiofrequency ablation (RFA) alone. The purpose of this meta-analysis was to evaluate prognostic factors of OS and RFS in patients treated with RFA.
Methods
A primary analysis was planned to evaluate the clinical prognostic factor of OS. RFS was the secondary aim. Thirty-four studies published from 2003 to 2017 were analyzed. They included 11,216 hepatocellular carcinoma patients.
Results
The results showed that Child–Pugh B vs Child–Pugh A (HR =2.32; 95% CI: 2.201–2.69; P<0.0001) and albumin–bilirubin score 1 vs 0 (HR =2.69; 95% CI: 2.10–3.44; P<0.0001) were predictive of poor OS. Tumor size as a continuous variable was not predictive of OS, although it was predictive of OS when we considered the size as a cutoff value (.2 cm vs <2 cm: HR =1.41; 95% CI: 1.23–1.61; P<0.0001; >3 cm vs <3 cm: HR =1.43; 95% CI: 1.17–1.74; P<0.0001) and in presence of >1 nodule (HR =1.59; 95% CI: 1.46–1.74; P<0.0001). Alpha-fetoprotein >20 ng/mL (HR =1.46; 95% CI: 1.25–1.70; P<0.0001) was the only predictive factor of poor prognosis.
Conclusion
Our meta-analysis highlighted that the maximum benefit of RFA in terms of OS and RFS is reached in the presence of Child–Pugh A, albumin–bilirubin score 1, single-nodule tumor sized <2 cm, and alpha-fetoprotein <20 ng/mL.
Keywords: radiofrequency, ALBI score, NLR, outcome, marker, immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-lymphocyte ratio, chilpugh, alpha-fetoprotein
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide.1 Hepatic resection and transplantation are considered the best treatments for early-stage patients with high probability of long-term survival.2 Radiofrequency ablation (RFA) is emerging as an effective local treatment for curative intent in patients with small HCC with a diameter <3 cm.3,4 Several meta-analyses5,6 have shown that RFA and surgical resection have a comparable impact on overall (OS) and recurrence-free survival (RFS). Given the different therapeutic options that occur in patients with HCC in the initial stage, it is absolutely essential to identify prognostic factors that can predict the possibility of relapse. There are several works published by RFA. All these studies have a heterogeneous duration of patient groups, to tell the reason, it is difficult to compare them. Furthermore, to date, neither randomized studies on RFA vs best supportive care nor meta-analyses evaluating OS and RFS have been completed on RFA patients alone.
The purpose of this meta-analysis was to evaluate prognostic factors of OS and RFS in patients treated with RFA, with the aim to identify parameters that can help clinicians in the therapeutic choice, and determine stratification factors for future studies in this subset of patients.
Materials and methods
Study design and inclusion criteria
Clinical trials on the prognostic factors of RFA in HCC patients were considered, excluding randomized controlled trials comparing RFA and surgery, studies with insufficient data to estimate the outcomes, and studies on RFA with microwave and ethanol. A primary analysis was planned to evaluate the clinical prognostic factor of OS. RFS was the secondary aim. OS was defined as the time interval between the day of start of treatment until the day of death or last follow-up visit. The RFS was defined as the observation time during the follow-up period during which the patient developed a intrahepatic distant recurrence, extrahepatic recurrence, or death.
Search strategy
We conducted a bibliographic search of the PubMed, Embase, Cochrane Library. Keywords used included “radiofrequency AND hepatocellular carcinoma”, “radiofrequency AND liver cancer”. Articles published in English until September 2017 and reporting data of studies conducted on human participants were retrieved. Relevant reviews and meta-analyses of loco-regional treatments of unresectable HCC were also examined for potential suitable studies and data. The 2000–2017 proceedings of the Annual Meeting of the American Society of Clinical Oncology (ASCO and ASCO Gastrointerstinal), European Society of Clinical Oncology (ESMO and ESMO Gastrointerstinal), European Association for the Study of the Liver, American Association for the Study of Liver Diseases, and International Liver Cancer Association were systematically reviewed for relevant unpublished data.
The computer search was supplemented with a manual search of the primary studies referenced in all of the retrieved review articles. When the results of a study were reported in multiple subsequent analyses, only the most recent and complete version was considered.
Data extraction and management
Two review authors (ACG and MV) independently screened the titles of all the selected studies, and read the abstracts of potentially eligible papers. Whenever discrepancies in trial search or selection occurred between the 2 review authors, they were discussed with a third review author (FGF) to reach an agreement. All selected trials published as full-text articles in peer-reviewed journals were analyzed and classified using the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies. ACG and MV independently performed the qualitative and quantitative analysis of the selected articles. Whenever discrepancies occurred, they were discussed with FGF to reach an agreement.
Statistical analysis
All analyses were carried out using Stata version 15.0 (Stata Corporation, College Station, TX, USA). HR reported in each study was used as an outcome measure of the prognostic value. The summary estimates were generated using a fixed-effect model (Mantel–Haenszel method) or a random-effect model49 depending on the absence or presence of heterogeneity.
The inter-study heterogeneity was examined by the Cochran’s Q and I-squared statistic with an I-squared >50% representing significant heterogeneity.7
We assessed the potential of publication bias by visually inspecting the funnel plot symmetry and Egger’s test for asymmetry.8
Sensitivity analyses were conducted by excluding 1 study at a time and reanalyzing the remaining to test whether the results had changed substantially by any individual study. A value of P<0.05 was regarded as statistically significant for all statistical analyses. All tests were 2-sided.
Results
Study selection and characteristics
Figure 1 reports the search strategy used in this meta-analysis. Thirty-four9–42 studies published between 2003 and 2017 were analyzed. They included 11,216 HCC patients treated with RFA. The characteristics of the study are gathered in Table 1.
Figure 1.
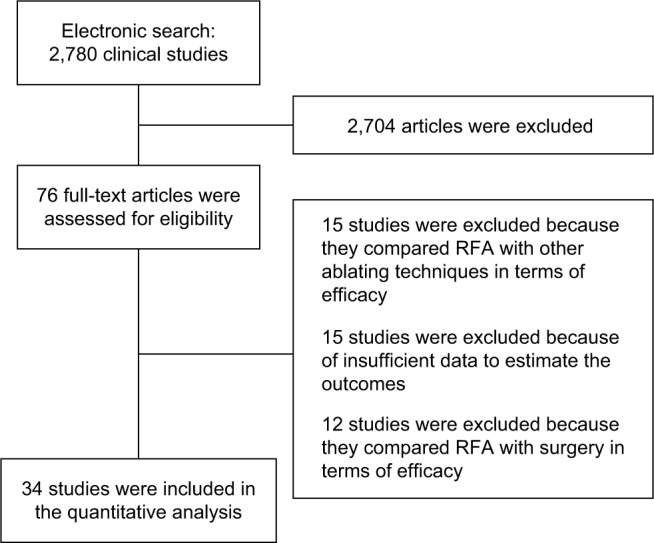
Flow diagram of the included and excluded studies.
Abbreviation: RFA, radiof requency ablation.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Date of publication | Data of collection | Number of patients | Study period | % Child– Pugh A | % of patients with >1 nodule | % liver etiology (HBV; HCV; alcohol; metabolic; other) | Follow-up | The Newcastle– Ottawa scale (NOS); total score |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al9 | 2014 | Retrospective study | 162 | 2006–2007 | 84.6 | 10.5 | (72.9; 21.6; 3.7; NR; NR) | Mean 50.3 ± 19.9 | 8 |
| El-Fattah et al10 | 2016 | SEER registries | 1,981 | 2004–2012 | NR | 26.2 | NR | Median 20; range 9–38 | 7 |
| Zhang et al11 | 2017 | Retrospective study | 410 | 2005–2016 | 97.7 | NR | NR | NR | 7 |
| Kao et al12 | 2017 | Retrospective study | 622 | 2002–2013 | 86.5 | 19.1 | (47.7; 43.2; NR; NR; 9.1) | Median 35.7 | 9 |
| Cho et al13 | 2016 | Retrospective study | 438 | 2006–2009 | 85.9 | NR | (72.4; 16.2; 3.9; NR; 6.4) | Median 68.4 | 8 |
| Kang et al14 | 2017 | Retrospective study | 572 | 2006–2012 | 81.8 | NR | (63.6; 14.4; NR; NR; 10.5) | Median 57.9 | 8 |
| Lin et al15 | 2015 | Retrospective study | 70 | 2009–2011 | 85.7 | 46.9 | (45.7; 44.3; NR; NR; NR) | Median 20.7 ± 10.3 | 9 |
| Yang et al16 | 2016 | Retrospective study | 316 | 2000–2013 | 77 | 21.6 | (86.6; 10.2; 1.9; NR; 1.3) | Mean 20.4 | 9 |
| Dohi et al17 | 2016 | Retrospective study | 357 | 2001–2013 | 84 | 31.4 | (12.1; 81.4; NR; NR; NR) | NR | 8 |
| Gao et al18 | 2015 | Retrospective study | 184 | 2005–2013 | 51 | NR | (87; 9; NR; NR; 2) | Median 65 | 9 |
| Montasser et al20 | 2014 | Retrospective study | 105 | 2007–2011 | NR | 28.6 | (2.8; 94.2; NR; NR; 1.9) | Mean 20.1 ± 10.67 | 8 |
| Facciorusso et al21 | 2014 | Not indicated | 103 | 2005–2010 | 83.4 | NR | (22.3; 60.1; NR; NR; 17.6) | NR | 7 |
| Dan et al22 | 2013 | Retrospective study | 178 | 2005–2008 | 83.9 | NR | (89.2; NR; NR; NR; NR) | Median 52.7 | 8 |
| Lee et al23 | 2014 | Retrospective study | 161 | 2006–2007 | 86.9 | 22.6 | (72; 19; NR; NR; 6) | Mean 45 ± 21 | 8 |
| Moribata et al24 | 2012 | Retrospective study | 97 | 2001–2006 | 63.6 | NR | (NR; 88.6; NR; NR; NR) | NR | 7 |
| Lu et al25 | 2012 | Not indicated | 661 | 2004–2006 | NR | NR | (NR; NR; NR; NR; NR) | Median 41.9 | 8 |
| Kao et al26 | 2012 | Retrospective study | 313 | 2002–2009 | 87.5 | 16 | (44.7; 47.2; NR; NR; NR) | Median 26.7 ± 19.1 | 7 |
| Chen et al27 | 2012 | Retrospective study | 158 | 2003–2010 | 84.8 | 19.7 | (36; NR; NR; NR; NR) | Mean 34 | 8 |
| Giorgio et al28 | 2011 | Not indicated | 143 | 2005–2010 | 50 | NR | (42.9; 57; NR; NR; NR) | Mean 37 | 8 |
| Goto et al29 | 2011 | Retrospective study | 69 | 2000–2007 | 78.2 | NR | (23.1; NR; NR; NR; NR) | Median 17 | 9 |
| Chen et al30 | 2011 | Retrospective study | 135 | 2003–2009 | NR | 16.8 | (34.3; 56.3; NR; NR; NR) | Mean 32.2 | 8 |
| Rossi et al31 | 2011 | Retrospective study | 706 | 1998–2008 | 76.2 | 21.7 | (4.5; 85.9; 4.2; NR; 2.4) | Median 29 | 7 |
| Takahashi et al32 | 2010 | Retrospective study | 461 | 2000–2007 | 77 | 37 | (5.4; 85.2; NR; NR; NR) | NR | 7 |
| Imai et al33 | 2010 | Not indicated | 24 | 2006–2007 | 83.3 | NR | (NR; NR; NR; NR; NR) | Mean 12.3 | 7 |
| dal Bello et al34 | 2010 | Retrospective study | 207 | 2000–2008 | 91.8 | 20.3 | (NR; NR; NR; NR; NR) | Median 36 | 7 |
| N’Kontchou et al35 | 2009 | Retrospective study | 235 | 2001–2007 | 85 | 22 | (7; 50; 37; NR; 4) | Mean 27 | 8 |
| Chinnaratha et al36 | 2015 | Retrospective study | 539 | 2006–2012 | 73 | NR | (18.3; 33.3; 15.1; 8.7; NR) | Mean 13.5 | 8 |
| Kao et al37 | 2012 | Retrospective study | 258 | NR | 87.6 | 19.5 | (42.9; 47.6; NR; NR; NR) | Median 28.5 | 8 |
| Lencioni et al38 | 2003 | Prospective study | 102 | NR | 87 | 23 | (12; 42; 15; NR; 6) | Mean 22.9 | 9 |
| Kao et al39 | 2011 | Retrospective study | 190 | 2002–2007 | 84.2 | 20 | (47.6; 45.7; NR; NR; NR) | Median 30.7 | 8 |
| Tajiri et al40 | 2016 | Retrospective study | 163 | 2003–2014 | 79.1 | NR | (15.9; 68; NR; NR; NR) | NR | 7 |
| Oh et al41 | 2017 | Retrospective study | 368 | 2007–2012 | 100 | NR | (78; NR; NR; NR; NR) | Median 61 | 8 |
| Lo et al42 | 2017 | Retrospective study | 152 | 2007–2015 | 78.3 | NR | (53.3; 30.9; NR; NR; NR) | Median 10 | 8 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NR, not reported; SEER, Surveillance, Epidemiology, and End Results.
Overall survival
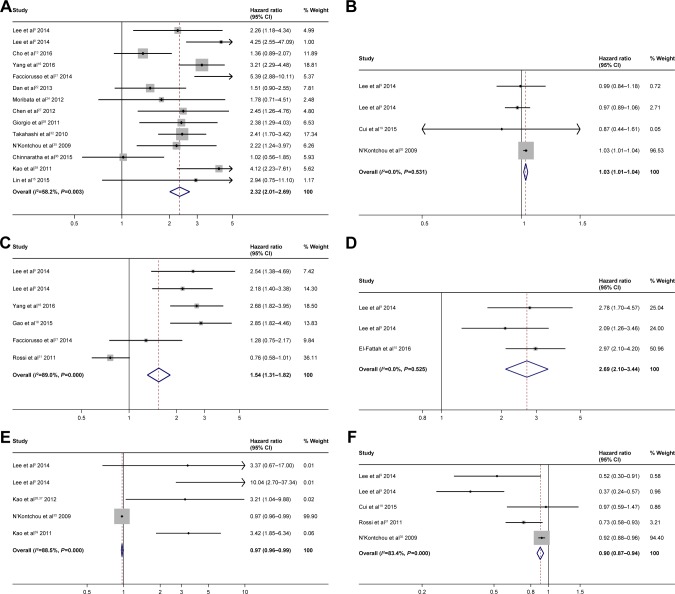
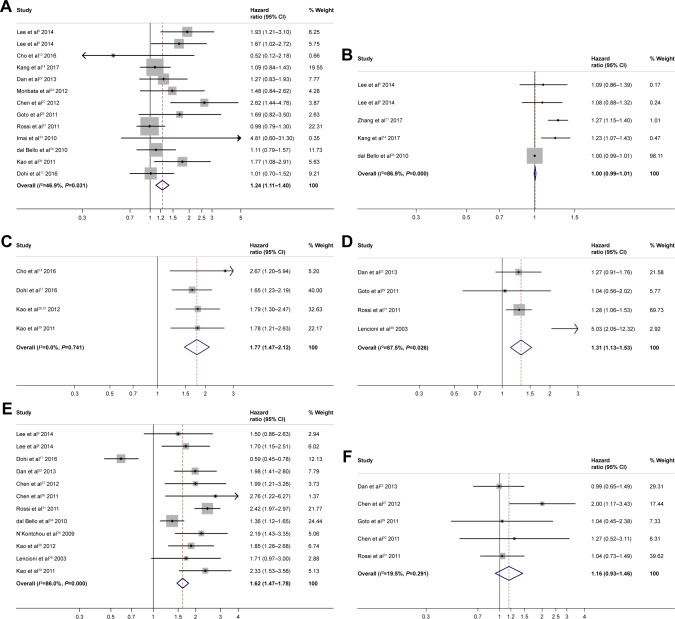
The analysis of liver functionality showed that Child– Pugh B vs Child–Pugh A (HR =2.32; 95% CI: 2.201–2.69; P<0.0001) (Figure 2A), increase in bilirubin (HR =1.03; 95% CI: 1.01–1.04; P<0.0001) (Figure 2B), presence of Portosystemic collaterals (HR =1.54; 95% CI: 1.31–1.82; P<0.0001) (Figure 2C), and albumin-bilirubin (ALBI) score 1 vs 0 (HR =2.69; 95% CI: 2.10–3.44; P<0.0001) (Figure 2D) were predictive of poor OS. Decrease in prothrombin activity (HR =0.97; 95% CI: 0.96–0.99; P<0.0001) (Figure 2E) and increase in albumin (HR =0.90; 95% CI: 0.87–0.94; P<0.0001) (Figure 2F) were predictive of better OS.
Figure 2.
Forest plots for overall survival showing Child–Pugh (A); bilirubin (B); portosystemic collaterals (C); ALBI score (D); prothrombin activity (E); albumin (F).
Abbreviation: ALBI, albumin–bilirubin.
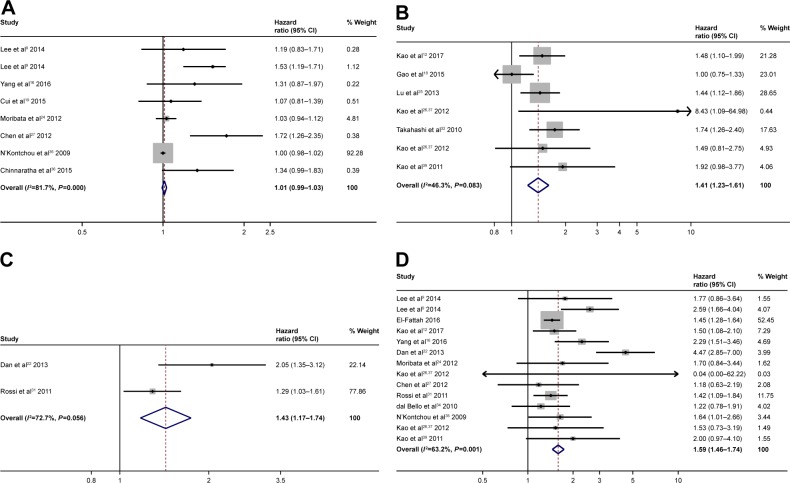
Tumor size was not predictive of OS (HR =1.01; 95% CI: 0.99–1.03; P=0.269) (Figure 3A) when considered as a continuous variable. Yet, it was predictive of OS when considered as a cutoff value. An either size cutoff of 2 or 3 cm was predictive of poor OS (>2 cm, HR =1.41; 95% CI: 1.23–1.61; P<0.0001, Figure 3B; >3 cm, HR =1.43; 95% CI: 1.17–1.74; P<0.0001, Figure 3C). When considering the number of nodules, the presence of >1 nodules (HR =1.59; 95% CI: 1.46–1.74; P<0.0001) (Figure 3D) was predictive of poor OS.
Figure 3.
Forest plots for overall survival showing the tumor size as a continuous variable (A); cutoff of 2 cm (B); cutoff of 3 cm (C); presence of >1 nodules (D).
Gender was not predictive of OS (male vs female HR =1.07; 95% CI: 0.99–1.15; P=0.091) (Figure S1A), while an older age (HR =1.02; 95% CI: 1.01–1.03; P<0.0001) (Figure S1B) and an age >65 years (HR =1.73; 95% CI: 1.40–2.12; P<0.0001) (Figure S1C) were predictive of poor OS.
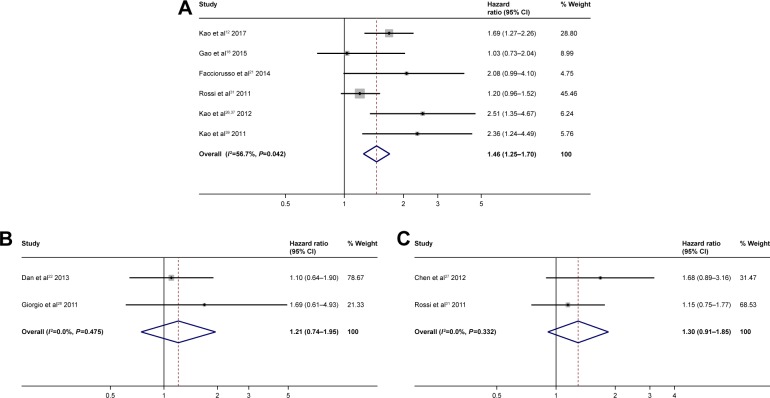
Data showed that an alpha-fetoprotein cutoff of 20 ng/mL (>20 ng/mL vs <20 ng/mL HR =1.46; 95% CI: 1.25–1.70; P<0.0001) (Figure 4A) was predictive of poor prognosis, whereas alpha-fetoprotein cutoffs of 200 ng/mL (>200 ng/mL vs <200 ng/mL HR =1.21; 95% CI: 0.74–1.95; P 0.475) (Figure 4B) and 400 ng/mL (>400 ng/mL vs <400 ng/mL HR =1.30; 95% CI: 0.91–1.85; P 0.332) (Figure 4C) were not predictive of poor prognosis.
Figure 4.
Forest plots for overall survival showing the alpha-fetoprotein with a cutoff of 20 ng/mL (A); cutoff of 200 ng/mL (B); cutoff of 400 ng/mL (C).
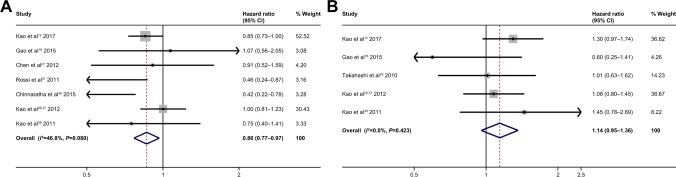
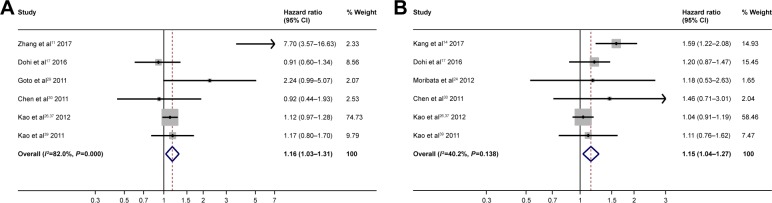
As for etiology, data show that hepatitis B virus (HBV) infection (HBV infection vs no HBV infection HR =0.86; 95% CI: 0.77–0.97; P 0.011) (Figure 5A) was predictive of good prognosis, whereas patients with hepatitis C virus (HCV) infection vs patients without HCV infection showed no statistically significant difference (HR =1.14; 95% CI: 0.95–1.36; P 0.147) (Figure 5B).
Figure 5.
Forest plots for overall survival showing HBV infection (A); HCV infection (B).
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
Finally, neutrophil–lymphocyte ratio (NLR) was predictive of poor prognosis (high vs low HR =1.91; 95% CI: 1.35–2.70; P<0.0001) (Figure S1D).
Recurrence-free survival
The analysis of liver functionality showed that only Child– Pugh B vs Child–Pugh A was predictive of poor RFS (HR =1.24; 95% CI: 1.11–1.40; P<0.0001) (Figure 6A). Bilirubin, albumin, prothrombin activity, and portosystemic collaterals were not predictive of RFS (Figure S2A–D).
Figure 6.
Forest plots for recurrence-free survival showing Child–Pugh (A); tumor size as a continuous variable (B); tumor size with a cutoff of 2 cm (C); tumor size with a cutoff of 3 cm (D); presence of >1 nodules (E); alpha-fetoprotein with a cutoff of 400 ng/mL (F).
Tumor size was not predictive of RFS when the size of the nodule was considered as a continuous variable (HR =1.00; 95% CI: 0.99–1.01; P 0.465) (Figure 6B). Yet, when the cutoff was considered, tumor sizes >2 cm vs <2 cm (HR =1.77; 95% CI: 1.47–2.12; P<0.0001) (Figure 6C) and >3 cm vs <3 cm (HR =1.31; 95% CI: 1.13–1.53; P<0.0001) (Figure 6D) were predictive of poor RFS. When considering the number of nodules, the presence of >1 nodule (HR =1.62; 95% CI: 1.47–1.78; P<0.0001) (Figure 6E) was predictive of poor RFS.
Gender was not predictive of RFS (male vs female HR =1.05; 95% CI: 0.96–1.15; P 0.243) (Figure S2E), whereas an older age (HR =1.01, 95% CI: 1.00–1.01; P 0.021) (Figure S2F) was predictive of poor RFS.
Data showed that an alpha-fetoprotein cutoff of 400 ng/mL (>400 ng/mL vs <400 ng/mL HR =1.16; 95% CI: 0.93–1.46; P 0.186) (Figure 6F) was not predictive of RFS.
As for etiology, HBV infection (HBV infection vs no HBV infection HR =1.16; 95% CI: 1.03–1.31; P 0.012) (Figure 7A) was predictive of poor RFS. The presence of HCV infection vs no HCV infection (HR =1.15, 95% CI: 1.04–1.27; P 0.008) (Figure 7B) was predictive of poor RFS.
Figure 7.
Forest plots for recurrence-free survival showing HBV infection (A); HCV infection (B).
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
Finally, NLR was not predictive of RFS (high vs low HR =1.28; 95% CI: 0.98–1.69; P 0.075).
Publication bias
The funnel plots were evaluated and seemed symmetrical. No publication bias was observed and Egger’s tests for asymmetry were not significant (P-value=0.851 for OS, P=0.806 for RFS and P=0.573).
Sensitivity analysis
Sensitivity analyses were performed in order to examine the stability of the results (data not shown). The pooled HRs suggest that results were statistically reliable because they were not changed substantially omitting 1 study at a time.
Discussion
In this meta-analysis of >10,000 individuals, we evaluated what factors are capable of predicting OS and RFS in HCC patients treated with RFA. As most studies and meta-analyses considered RFA vs surgery, this is the first meta-analysis to have evaluated only clinical or laboratory parameters in this subset of patients without comparing with surgery.
Our study showed that Child–Pugh B was a significant predictor of poor OS (HR =2.32) and RFS (HR =1.24). Our data showed that other liver function parameters are also highly predictive of poor OS (bilirubin, presence of portosystemic circles, prothrombin, and albumin), whereas only Child–Pugh B vs Child–Pugh A was predictive of poor RFS. The severity of the underlying liver disease may also be a risk factor for the development and recurrence of HCC, suggesting the importance of the role of the liver function in these patients. A recent study by Wei-Yu Kao et al12 evaluated ALBI grade and platelet-albumin-bilirubin grade as prognostic and predictive indexes in patients treated with RFA. The data highlighted a significant difference in OS between Child–Pugh A and ALBI grade 1 vs Child– Pugh A and ALBI grade 1 and 2. This study showed for the first time that ALBI grade can better stratify these patients. Their results have also been confirmed by Oh Is et al41 and CH Lo et al.42 Also, our meta-analysis confirms that ALBI grade is currently one of the best indexes for predicting survival in this patient subset. As shown by other works at different disease stages,43–45 ALBI grade is better predictive index than Child–Pugh, as the latter is composed of 5 arbitrary parameters, whereas the former is formed by only 2 non-arbitrary parameters (albumin and bilirubin). Interestingly, this meta-analysis showed that the presence of the portosystemic collateral is a predictive factor of OS. As for liver resection, the presence of portal hypertension is a well-known predictor for survival, regardless of the Child–Pugh class.46,47
Another factor evaluated in this meta-analysis was the pre RFA tumor size. The size of the nodules, taken as a continuous variable, was not predictive of either OS or RFS, because many studies included in the meta-analysis considered only small nodules. Conversely, when we evaluated the size of the nodule as a cutoff value, we observed that the maximum benefit of RFA was reached when nodules were <2 cm, confirming the literature data48 and supporting the choice of RFA as the first treatment option. For tumors >2 cm, other factors must also be considered. As for the number of nodules, our meta-analysis showed that the presence of multiple nodules is a negative prognostic index both in terms of OS (HR =1.59) and RFS (HR =1.62): therefore, in most nodular patients, especially if operable, RFA is not recommended.
In regard to etiology, our results showed that HBV-positive patients have better OS and worse RFS (HR =1.16) when treated with RFA. These data, however, are difficult to explain, particularly for the contrasting data between OS and RFS. In all considered studies, etiology was regarded as presence or absence of HBV or HCV infection. Only in 1 study,31 the different etiologies were directly compared, highlighting our data as a benefit in terms of OS in HBV-positive patients compared with HCV-positive patients with a 56% reduction in death risk.
Concerning the predictive role of alpha fetoprotein, our meta-analysis revealed that only a cutoff of 20 ng/mL can predict OS and RFS outcomes in these patients.
Although NLR might play a role in predicting OS and RFS, data are currently limited and cannot be employed in normal clinical practice.
Limitations
Among the limitations of our meta-analysis are the low number of published studies considered for some subgroup analyses by prognostic factor, and the consideration of studies only reporting HR and 95% CI, thus potentially introducing further bias. Another limitation is that in this is a meta-analysis of aggregate patient data and not of individual patient data.
Conclusion
Our meta-analysis highlighted that the maximum benefit of RFA in terms of OS and RFS is reached when all the following features are present: Child–Pugh A, ALBI score 1, single-nodule tumor sized <2 cm, and alpha-fetoprotein <20 ng/mL. The role of the different etiologies still remains to be clarified. These clinical/laboratory data should also be used to better stratify patients in future RFA randomized trials.
Supplementary materials
Forest plots for overall survival for male vs female (A), age as continue variable (B), age 65 years (C) and neutrophil–lymphocyte ratio (D).
Forest plots for recurrence free survival for bilirubin (A), albumin (B), prothrombin activity (C), portosystemic collaterals (D), male vs female (E), and age (F).
References
- 1.Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Luo E, Gan J, et al. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J Surg Oncol. 2017;15(1):122. doi: 10.1186/s12957-017-1189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao WY, Su CW, Chiou YY, et al. Hepatocellular Carcinoma: Nomo-grams Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology. 2017;285(2):670–680. doi: 10.1148/radiol.2017162382. [DOI] [PubMed] [Google Scholar]
- 4.Cho JY, Choi MS, Lee GS, et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(4):477–486. doi: 10.3350/cmh.2016.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang TW, Rhim H, Song KD, et al. Radiofrequency Ablation of Hepatocellular Carcinoma with a “Nodule-in-Nodule” Appearance: Long-Term Follow-up and Clinical Implications. Cardiovasc Intervent Radiol. 2017;40(3):401–409. doi: 10.1007/s00270-016-1525-9. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol. 2016;22(10):2993–3005. doi: 10.3748/wjg.v22.i10.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohi C, Nouso K, Miyahara K, et al. Potential of alpha-fetoprotein as a prognostic marker after curative radiofrequency ablation of hepatocellular carcinoma. Hepatol Res. 2016;46(9):916–923. doi: 10.1111/hepr.12636. [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Wu Y, Wang Z, Liu X, Wang S, Qin C. MicroRNA-34a expression is predictive of recurrence after radiofrequency ablation in early hepatocellular carcinoma. Tumour Biol. 2015;36(5):3887–3893. doi: 10.1007/s13277-014-3031-5. [DOI] [PubMed] [Google Scholar]
- 9.Montasser MF, Shaker MK, Albreedy AM, Montasser IF, El Dorry A. Risk factors for early intrahepatic distant recurrence after radiofre-quency ablation for hepatocellular carcinoma in Egyptian patients. J Dig Dis. 2014;15(12):676–683. doi: 10.1111/1751-2980.12190. [DOI] [PubMed] [Google Scholar]
- 10.Facciorusso A, del Prete V, Antonino M, et al. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29(11):1905–1910. doi: 10.1111/jgh.12618. [DOI] [PubMed] [Google Scholar]
- 11.Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8(3):e58184. doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao WY, Chiou YY, Hung HH, et al. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy. Clin Radiol. 2012;67(5):429–436. doi: 10.1016/j.crad.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Goto T, Yoshida H, Tateishi R, et al. Influence of serum HBV DNA load on recurrence of hepatocellular carcinoma after treatment with per-cutaneous radiofrequency ablation. Hepatol Int. 2011;5(3):767–773. doi: 10.1007/s12072-011-9255-1. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53(1):136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Mizuta T, Kawazoe S, et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010;40(10):997–1005. doi: 10.1111/j.1872-034X.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Takai K, Nishigaki Y, et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol Res. 2010;40(4):376–382. doi: 10.1111/j.1872-034X.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 17.dal Bello B, Rosa L, Campanini N, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16(7):2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 18.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50(5):1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 19.Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27(3):349–354. doi: 10.1097/MEG.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 20.Kao WY, Chiou YY, Hung HH, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46(1):62–70. doi: 10.1097/MCG.0b013e31822b36cc. [DOI] [PubMed] [Google Scholar]
- 21.Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23(6):528–536. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- 22.Moribata K, Tamai H, Shingaki N, et al. Ultrasonogram of hepatocellular carcinoma is associated with outcome after radiofrequency ablation. World J Hepatol. 2012;4(12):374–381. doi: 10.4254/wjg.v4.i12.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27(3):553–561. doi: 10.1111/j.1440-1746.2011.06910.x. [DOI] [PubMed] [Google Scholar]
- 24.Tajiri K, Baba H, Kawai K, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol. 2016;31(7):1291–1299. doi: 10.1111/jgh.13287. [DOI] [PubMed] [Google Scholar]
- 25.Lu LC, Shao YY, Kuo RN, et al. Hospital volume of percutaneous radiofrequency ablation is closely associated with treatment outcomes for patients with hepatocellular carcinoma. Cancer. 2013;119(6):1210–1216. doi: 10.1002/cncr.27800. [DOI] [PubMed] [Google Scholar]
Acknowledgments
The authors would like to thank Veronica Zanoni and Cristiano Verna for editorial assistance.
The abstract for this paper was presented at the 27th Annual Conference of The Asian Pacific Association for the Study of the Liver (APASL), March 14–18, 2018, New Delhi, India and was published in Hepatology International Volume 12, Supplement 2, 2018.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Faloppi L, Scartozzi M, Maccaroni E, et al. Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer Treat Rev. 2011;37(3):169–177. doi: 10.1016/j.ctrv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010;2(11):417–424. doi: 10.4329/wjr.v2.i11.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucchetti A, Piscaglia F, Cescon M, Ercolani G, Pinna AD. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;19(26):4106–4118. doi: 10.3748/wjg.v19.i26.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287(2):461–472. doi: 10.1148/radiol.2017162756. [DOI] [PubMed] [Google Scholar]
- 6.Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. doi: 10.1038/srep07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 10.El-Fattah MA, Aboelmagd M, Elhamouly M. Prognostic factors of hepatocellular carcinoma survival after radiofrequency ablation: A US population-based study. United European Gastroenterol J. 2017;5(2):227–235. doi: 10.1177/2050640616659024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Luo E, Gan J, et al. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J Surg Oncol. 2017;15(1):122. doi: 10.1186/s12957-017-1189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao WY, Su CW, Chiou YY, et al. Hepatocellular Carcinoma: Nomo-grams Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology. 2017;285(2):670–680. doi: 10.1148/radiol.2017162382. [DOI] [PubMed] [Google Scholar]
- 13.Cho JY, Choi MS, Lee GS, et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(4):477–486. doi: 10.3350/cmh.2016.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang TW, Rhim H, Song KD, et al. Radiofrequency Ablation of Hepatocellular Carcinoma with a “Nodule-in-Nodule” Appearance: Long-Term Follow-up and Clinical Implications. Cardiovasc Intervent Radiol. 2017;40(3):401–409. doi: 10.1007/s00270-016-1525-9. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Cheng YT, Chen M WT, Lin SM. The Effectiveness of Multiple Electrode Radiofrequency Ablation in Patients with Hepatocellular Carcinoma with Lesions More than 3 cm in Size and Barcelona Clinic Liver Cancer Stage A to B2. Liver Cancer. 2016;5(1):8–20. doi: 10.1159/000367755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol. 2016;22(10):2993–3005. doi: 10.3748/wjg.v22.i10.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dohi C, Nouso K, Miyahara K, et al. Potential of alpha-fetoprotein as a prognostic marker after curative radiofrequency ablation of hepatocellular carcinoma. Hepatol Res. 2016;46(9):916–923. doi: 10.1111/hepr.12636. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Wang SH, Ding XM, et al. Radiofrequency ablation for single hepatocellular carcinoma 3 cm or less as first-line treatment. World J Gastroenterol. 2015;21(17):5287–5294. doi: 10.3748/wjg.v21.i17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, Wu Y, Wang Z, Liu X, Wang S, Qin C. MicroRNA-34a expression is predictive of recurrence after radiofrequency ablation in early hepatocellular carcinoma. Tumour Biol. 2015;36(5):3887–3893. doi: 10.1007/s13277-014-3031-5. [DOI] [PubMed] [Google Scholar]
- 20.Montasser MF, Shaker MK, Albreedy AM, Montasser IF, El Dorry A. Risk factors for early intrahepatic distant recurrence after radiofrequency ablation for hepatocellular carcinoma in Egyptian patients. J Dig Dis. 2014;15(12):676–683. doi: 10.1111/1751-2980.12190. [DOI] [PubMed] [Google Scholar]
- 21.Facciorusso A, del Prete V, Antonino M, et al. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29(11):1905–1910. doi: 10.1111/jgh.12618. [DOI] [PubMed] [Google Scholar]
- 22.Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8(3):e58184. doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol. 2014;37(3):705–715. doi: 10.1007/s00270-013-0708-x. [DOI] [PubMed] [Google Scholar]
- 24.Moribata K, Tamai H, Shingaki N, et al. Ultrasonogram of hepatocellular carcinoma is associated with outcome after radiofrequency ablation. World J Hepatol. 2012;4(12):374–381. doi: 10.4254/wjg.v4.i12.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu LC, Shao YY, Kuo RN, et al. Hospital volume of percutaneous radiofrequency ablation is closely associated with treatment outcomes for patients with hepatocellular carcinoma. Cancer. 2013;119(6):1210–1216. doi: 10.1002/cncr.27800. [DOI] [PubMed] [Google Scholar]
- 26.Kao WY, Chiou YY, Hung HH, et al. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy. Clin Radiol. 2012;67(5):429–436. doi: 10.1016/j.crad.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27(3):553–561. doi: 10.1111/j.1440-1746.2011.06910.x. [DOI] [PubMed] [Google Scholar]
- 28.Giorgio A, di Sarno A, de Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res. 2011;31(6):2291–2295. [PubMed] [Google Scholar]
- 29.Goto T, Yoshida H, Tateishi R, et al. Influence of serum HBV DNA load on recurrence of hepatocellular carcinoma after treatment with per-cutaneous radiofrequency ablation. Hepatol Int. 2011;5(3):767–773. doi: 10.1007/s12072-011-9255-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26(5):858–865. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 31.Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53(1):136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi H, Mizuta T, Kawazoe S, et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010;40(10):997–1005. doi: 10.1111/j.1872-034X.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 33.Imai K, Takai K, Nishigaki Y, et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol Res. 2010;40(4):376–382. doi: 10.1111/j.1872-034X.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 34.dal Bello B, Rosa L, Campanini N, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16(7):2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 35.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50(5):1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 36.Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27(3):349–354. doi: 10.1097/MEG.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 37.Kao WY, Chiou YY, Hung HH, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46(1):62–70. doi: 10.1097/MCG.0b013e31822b36cc. [DOI] [PubMed] [Google Scholar]
- 38.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 39.Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23(6):528–536. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- 40.Tajiri K, Baba H, Kawai K, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol. 2016;31(7):1291–1299. doi: 10.1111/jgh.13287. [DOI] [PubMed] [Google Scholar]
- 41.Oh IS, Sinn DH, Kang TW, et al. Liver Function Assessment Using Albumin-Bilirubin Grade for Patients with Very Early-Stage Hepatocellular Carcinoma Treated with Radiofrequency Ablation. Dig Dis Sci. 2017;62(11):3235–3242. doi: 10.1007/s10620-017-4775-8. [DOI] [PubMed] [Google Scholar]
- 42.Lo CH, Liu MY, Lee MS, et al. Comparison Between Child-Turcotte-Pugh and Albumin-Bilirubin Scores in Assessing the Prognosis of Hepatocellular Carcinoma After Stereotactic Ablative Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):145–152. doi: 10.1016/j.ijrobp.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 43.Lee PC, Chen YT, Chao Y, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int. 2018;38(2):321–330. doi: 10.1111/liv.13527. [DOI] [PubMed] [Google Scholar]
- 44.Hansmann J, Evers MJ, Bui JT, et al. Albumin-Bilirubin and Platelet-Albumin-Bilirubin Grades Accurately Predict Overall Survival in High-Risk Patients Undergoing Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28(9):1224–1231. doi: 10.1016/j.jvir.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30(6):1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 47.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111(4):1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 48.de Lope CR, Tremosini S, Forner A, Reig M. Bruix J: Management of HCC. [Accessed August 23, 2018];J Hepatol. 2012 1:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22300468. [DOI] [PubMed] [Google Scholar]
- 49.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plots for overall survival for male vs female (A), age as continue variable (B), age 65 years (C) and neutrophil–lymphocyte ratio (D).
Forest plots for recurrence free survival for bilirubin (A), albumin (B), prothrombin activity (C), portosystemic collaterals (D), male vs female (E), and age (F).