Abstract
Abnormal development of parallel cortical-striatal networks may contribute to abnormal motor, cognitive, and affective behavior prior to the onset of psychosis. Partitioning individuals at clinical high-risk (CHR) using motor behavior may provide a novel perspective on different etiological pathways or patient subtypes. A K-means cluster analysis was conducted in CHR (N=69; 42% female, mean age=18.67 years) young adults using theoretically distinct measures of motor behavior. The resulting subtypes were then compared on positive and negative symptoms at baseline, and 2-year risk of psychosis conversion. CHR participants were followed for 2 years to determine conversion to psychosis. CHR subtypes and healthy controls (N=61; 57% female, mean age=18.58 years) were compared on multiple cognitive domains and cortical-striatal connectivity. Results suggest 3 vulnerability subtypes of CHR individuals with different profiles of motor performance, symptoms, risk for conversion to psychosis, cognition, and thalamocortical connectivity. This approach may reflect a novel strategy for promoting tailored risk assessment as well as future research developing individualized medicine.
Keywords: clinical high-risk, psychosis, cluster, movement abnormalities, vulnerability subtypes
INTRODUCTION
From its earliest conceptions, psychosis has been used to describe a heterogeneous presentation of syndromes (Andreasen, 1999; Dickinson et al., 2017). Today, this heterogeneity continues to create challenges for identifying reliable biomarkers, predicting illness trajectories, and early detection of risk for psychosis (Cuthbert & Insel, 2013). People who are identified as being at clinical high-risk (CHR) for the disorder show subthreshold psychotic symptoms as well as decreased functioning. The trajectory of CHR individuals is also heterogeneous; approximately a third will continue to suffer with psychopathology and decreased functioning, a third will develop psychosis, and a third will achieve full remission within the first 3 years of follow-up (Cornblatt et al., 2015; Fusar-Poli et al., 2012; Schlosser et al., 2012). Long term outcomes for the CHR population suggest that there continues to be substantial risk for psychosis and functional problems even up to 15 years of follow-up (de Wit et al., 2017; Lin et al., 2015; Nelson et al., 2013; Rutigliano et al., 2016). Detection of more homogeneous subtypes within the CHR population may help direct individualized treatment planning and recovery.
Clustering methods using multivariate data may hold promise for finding homogeneous subtypes in heterogeneous clinical samples (Van Dam et al., 2017). A growing body of work implementing this unsupervised machine learning approach has provided insight into potential subtypes of patients with psychosis and has shown promising classification of endophenotypes for the disorder. For example, Clementz and colleagues employed clustering algorithms to find 3 biotypes of psychosis patients and their unaffected relatives that gave a unique perspective beyond traditional diagnostic categories for psychotic disorders (Clementz et al., 2016). Along similar lines, Sponheim and colleagues used 4 biologically defined variables that are consistently found to be abnormal in schizophrenia populations to find 3 clusters of patients. These biologically defined clusters yielded better prediction of ocular motor performance in schizophrenia patients and their relatives compared to traditional diagnostic categories (Sponheim, Iacono, Thuras, & Beiser, 2001). In addition, clustering methods have noted subtypes of patients with schizophrenia based on cognition (Lewandowski, Sperry, Cohen, & Ongur, 2014), negative symptoms (Dickinson et al., 2017; Kirkpatrick, Buchanan, Ross, & Carpenter Jr., 2001), and white matter integrity (Pearlson, Clementz, Sweeney, Keshavan, & Tamminga, 2016). Walther and colleagues have used principal components analysis to find motor performance measures that map on to hyperconnectivity within cortical-striatal networks in schizophrenia patients (Walther et al., 2017).
As previous studies have noted, using behavioral measures tied to endophenotypes and biomarkers for psychosis may yield important insights into diagnostic categories and patient subtypes. Along those lines, the current study looks toward theories regarding abnormal dopamine modulation within cortical-striatal networks as a final common pathway linking the heterogeneous presentation of symptoms and neurocognitive impairments observed in prodromal and active phases of psychotic illness (Howes et al., 2009; Howes & Kapur, 2009). The parallel organization of dopaminergic pathways between basal ganglia and the thalamus, which has multiple afferent and efferent connections with the cortex, governs separate networks that contribute to motor, cognitive, and affective behavior (Obeso, Rodriguez-Oroz, Stamelou, Bhatia, & Burn, 2014). Abnormal development in cortical-striatal networks could potentially lead to diverse behavioral symptoms such as hyperkinetic or hypokinetic movements, language and cognitive impairments, and affective dysfunction that are ultimately related to the expression of positive and negative symptoms observed in psychosis (Dandash et al., 2014; Pantelis, Stuart, Nelson, Robbins, & Barnes, 2001; Strik, Stegmayer, Walther, & Dierks, 2017).
Movement abnormalities have been considered as proximal measures of aberrant cortical-striatal development (Dandash, Pantelis, & Fornito, 2016; Hirjak, Wolf, Wilder-Smith, Kubera, & Thomann, 2013; Mittal & Wakschlag, 2016). Indeed, there is a rich history of longitudinal and archival research suggesting that movement abnormalities occur in a majority of unmedicated schizophrenia patients and may be predictive of eventual transition to psychosis within several years (Callaway, Perkins, Woods, Liu, & Addington, 2014; Mittal et al., 2007; Mittal, Walker, et al., 2010; Pappa & Dazzan, 2009) and decades following the first assessment (Schiffman et al., 2004, 2009; Walker, Savoie, & Davis, 1994). A number of movement abnormalities have been proposed as core features of psychosis risk, including spontaneous dyskinesia, psychomotor slowing, and neurological soft signs (NSS), among others (Hirjak et al., 2015; Hirjak, Meyer-Lindenberg, Kubera, Thomann, & Wolf, 2018; Walther & Mittal, 2017). Multivariate approaches to investigating movement abnormalities in youth at risk for psychosis could potentially identify vulnerability subtypes and provide insight into illness trajectory of youth at risk for psychosis.
In the current study, we investigate whether CHR individuals can be clustered based on multiple domains of motor performance. A total of 69 CHR young adults completed a battery of motor tasks aimed at measuring psychomotor slowing, dyskinesia, and NSS. These motor measures were then subjected to k-means clustering within the CHR group. Based on previous work noting motor clusters in schizophrenia patients, we hypothesized that CHR participants would separate into distinct groups with different performance across the motor indices (Docx et al., 2012; Walther et al., 2017). In order to look at the associations between clusters of motor behavior, symptoms of risk for psychosis, cognitive functioning, and cortical-striatal functioning; the CHR subtypes and a group of healthy controls were compared on multiple cognitive domains and cortical-striatal seed based resting state functional magnetic resonance imaging (rsFMRI). As the associative and affective basal ganglia loops are also associated with movement, we hypothesize that impaired motor performance on one or more indices would be related to more severe symptoms and impaired cognitive function (Mittal, Walker, et al., 2010). Previous cross sectional research investigating motor subtypes in schizophrenia patients suggests that there is aberrant hyperconnectivity within cortical-thalamic-striatal networks (Walther et al., 2017). We hypothesized that there would be differences in functional connectivity between the CHR subtypes and healthy controls for motor cortex, thalamus and striatal seed regions. In order to validate the motor clusters in terms of psychosis conversion, exploratory analysis included a subset of CHR individuals (N=53) who were followed for 24-months after the motor assessment to determine whether they transitioned to psychosis. Each CHR participant was also rated on a recently developed calculation of probability for psychosis conversion (Cannon et al., 2016; Carrion et al., 2016) at the same time point as the motor assessment. The rationale was that exploratory use of comparing subtypes on this metric at baseline, even without the large sample and longer follow-up, would provide a novel perspective regarding risk of conversion to psychosis, which may help to further flesh out the theoretical and clinical utility of the motor clusters.
MATERIALS AND METHODS
About the participants
Adolescent and young adult CHR and matched healthy control participants between 13 and 21 years (M = 18.62, SD = 1.97), were recruited to the Adolescent Development and Preventive Treatment (ADAPT) research program using internet, newspaper, and public transportation advertisements, email postings, and community professional referrals. Exclusion criteria consisted of head injury, the presence of a neurological disorder, lifetime substance dependence, and the presence of any contraindication to the MRI environment. The presence or lifetime history of an Axis I psychotic disorder at baseline was also an exclusion criterion. The study was approved by the Institutional Review Board, and written consent or assent was obtained. See Table 1 for demographic information.
Table 1.
Demographic characteristics of the clinical high risk (CHR), healthy controls and CHR subtypes.
| Control | CHR | Statistic | CHR Clusters | Statistic | |||
|---|---|---|---|---|---|---|---|
| S-PMS | HMP | D-NSS | |||||
| Age | |||||||
| Mean (SD) | 18.67 (2.17) | 18.58 (1.78) | t(128) = 0.27, p = 0.79 | 18.8 (1.29) | 18.81 (1.78) | 17.62 (2.33) | F(3, 126) = 1.32, p = 0.27 |
| Sex | |||||||
| Male | 26 | 40 | 19 | 17 | 4 | ||
| Female | 35 | 29 | 6 | 14 | 9 | ||
|
|
|
||||||
| Total | 61 | 69 | X2(1, N = 130) = 2.47, p = 0.12 | 25 | 31 | 13 | X2(3, N = 130) = 10.27, p = 0.02 |
| Education (years) | |||||||
| Mean (SD) | 12.61 (2.22) | 12.35 (1.80) | t(128) = 0.76, p = 0.45 | 12.64 (1.55) | 12.53 (1.92) | 11.69 (1.93) | F(3, 126) = 0.76, p = 0.52 |
| Parent Education | |||||||
| Mean (SD) | 15.72 (2.80) | 15.35 (3.02) | t(128) = 0.73, p = 0.47 | 14.84 (3.30) | 15.27 (2.95) | 16.5 (2.48) | F(3, 126) = 1.12, p = 0.35 |
| Recruitment Method | |||||||
| Advertisements | 24 | 58 | 7 | 11 | 6 | ||
| Email Postings | 27 | 13 | 13 | 10 | 4 | ||
| Professional Referral | 18 | 0 | X2(2, N = 130) = 30.52, p < 0.001 | 5 | 10 | 3 | X2(4, N = 69) = 3.34, p = 0.50 |
| Symptoms | |||||||
| Positive: Mean (SD) | 0.66 (1.38) | 11.86 (4.55) | t(130.5) = 8.87, p < 0.001 | 12.36 (4.71) | 11.48 (4.79) | 11.77 (3.83) | F(2, 66) = 0.25, p = 0.77 |
| Negative: Mean (SD) | 0.36 (.78) | 10.04 (7.01) | t(130.3) = 6.50, p < 0.001 | 8.88 (7.30) | 8.90 (6.30) | 15.00 (6.30) | F(2, 66) = 4.41, p = 0.01 |
Clinical Interviews
The Structured Interview for Prodromal Syndromes (SIPS) was administered at baseline to diagnose a prodromal syndrome (McGlashan, Walsh, & Woods, 2010). Participants in the present study met SIPS criteria for a prodromal or high-risk syndrome, defined by moderate to severe but not psychotic levels of positive symptoms (rated from 3 to 5 on a six-point scale) and/or a decline in global functioning accompanying the presence of schizotypal personality disorder and/or a family history of schizophrenia. A total sum score for the positive and negative symptom domain is used as an indicator of the respective dimensions of symptomatology. Family history of psychosis was attained by asking participants if any first-degree family members had been diagnosed with a psychotic disorder. In most cases, family history was corroborated with another family member of the participant.
The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 1995) was also administered to rule out a psychotic disorder diagnosis at baseline. Training of advanced doctoral student interviewers was conducted over a 2-month period, and inter-rater reliabilities exceeded the minimum study criterion of Kappa ≥ 0.80.
Cognitive Testing
Participants completed the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery of cognitive tasks (Green & Nuechterlein, 2004). The MATRICS battery includes tests for attention, reasoning and problem solving, social cognition, speed of processing, verbal and visual learning, and working memory. The MATRICS was administered by trained graduate students in a quiet room. Raw scores were transformed to T-scores using established standard scores, which were used in further analyses.
Movement performance variables
Movement performance variables were chosen based on distinct theoretical domains of movement abnormalities in CHR and psychosis populations. These domains include dyskinesia (Mittal et al., 2013; Mittal, Dean, & Pelletier, 2012; Mittal, Dean, Pelletier, & Caligiuri, 2011; Mittal, Daley, et al., 2010), psychomotor slowing (Dean & Mittal, 2015), and neurological soft signs (Barkus, Stirling, Hopkins, & Lewis, 2005; Compton et al., 2007; Mittal et al., 2014). Measurements of force stability have been validated as a measure of dyskinesia in studies of tardive dyskinesia, levodopa-induced dyskinesia, and spontaneous dyskinesia and is highly reliable (Caligiuri & Lohr, 1994; Mittal et al., 2013). The force stability task involves having a subject attempt to apply a constant pressure on a strain gauge, and measuring the amount of variability in the applied force. Three 20-s trials were obtained using the participant’s dominant hand each separated by a 5-s rest. Final analysis of force error involved calculating a coefficient of variation (CV) by dividing the standard deviation by the mean of the force waveform. Larger CV values are thought to be a measure of force instability.
Previous research suggests that velocity scaling (VS) may be used as a measure of psychomotor slowing (Caligiuri, Teulings, Dean, Niculescu, & Lohr, 2010). A measure of VS was calculated for each participant as described in detail previously where lower values are thought to be indicative of impaired ability to scale arm movement (Dean & Mittal, 2015).
The Neurological Evaluation Scale is a 26-item instrument designed to measure neurological soft signs (NSS) in psychotic disorders (Buchanan & Heinrichs, 1989). Trained evaluators administered the scales based on the original scoring instructions. The evaluators were not blind to the group status of the participants. Importantly, there was minimal overlap for NSS evaluators who also conducted either the clinical interview (N = 4) or cognitive assessment (N = 2). Theoretically based subscales including sensory integration, motor coordination, and motor sequencing were calculated and used in the subsequent clustering analysis.
Resting state functional connectivity MRI processing
A blood oxygenation level dependent (BOLD) rsFMRI scan was acquired. Detailed methods for the processing of rsFMRI have been published previously (Bernard, Goen, & Maldonado, 2017; Bernard, Orr, & Mittal, 2016). Data were preprocessed in FSL (v.5; http://fsl.fmrib.ox.ac.uk/fsl), and rsFMRI analysis was performed in the CONN toolbox v.17f (Whitfield-Gabrieli & Ford, 2012). Connectivity between lateral and bilateral anatomical seeds from the Harvard-Oxford cortical and subcortical structural atlases in FSL for the left/right caudate, left/right putamen, left/right thalamus, primary motor and supplementary motor area (SMA) was calculated with all other voxels in the brain. The CONN toolbox uses a GLM approach with connectivity measures calculated as bivariate correlations. The correlations were r-to-z transformed and all comparisons were defined as between-subject T or F contrasts using the z values.
Exploratory psychosis conversion assessment and calculation of risk probability
Diagnoses for psychosis were determined using the SCID up to 24 months following the baseline clinical assessment. Conversion was noted as a yes/no dichotomous variable and specific diagnosis was recorded.
In addition to the SCID evaluation for psychosis diagnoses, each CHR participant was assessed for risk of conversion to psychosis at baseline. As noted above, we were interested in examining dichotomous conversion; however, the sample size at follow-up was limited to 53 returning CHR participants. To address this limitation in analyses and subsequent interpretation of psychosis risk in this study, we also employed an additional continuous measure of psychosis risk using a freely available online calculator (http://riskcalc.org:3838/napls/) recently developed by the North American Prodrome Longitudinal Study (NAPLS; Addington et al., 2012; Cannon et al., 2016). In the initial development, Cannon et al. showed in a well powered study that the calculator had good discriminant ability to classify CHR who did or did not convert to psychosis. Carrion also replicated these results in an independent sample using the same calculation procedure (Carrion et al., 2016). The current study used the same materials as described in initial development of the calculator, collected during the clinical interviews and MATRICS cognitive battery to input information into the online calculator. As noted above, family history of psychosis in a first degree relative was collected during the clinical interview. The SIPS scores for unusual thought content and suspiciousness were readjusted and summed based on the described procedure in Cannon et al. Additional measures included age, Brief Assessment of Cognition in Schizophrenia symbol coding score (Keefe et al., 2004), the Hopkins Verbal Learning Test–Revised sum score for trials 1–3 (Brandt & Benedict, 2001), the total sum score of 31 negative life events aggregated from the Research Interview Life Events Scale (Dohrenwend, Krasnoff, Askenasy, & Dohrenwend, 1978), and the Global Functioning: Social Scale (Cornblatt et al., 2007) were also used. The number of traumas was calculated based on information given in the SCID interview. Because we were interested in a 24-month follow-up window in establishing psychosis diagnoses, we used a 2-year probability of psychosis risk given by the NAPLS online calculator in subsequent exploratory analyses.
Statistical analysis
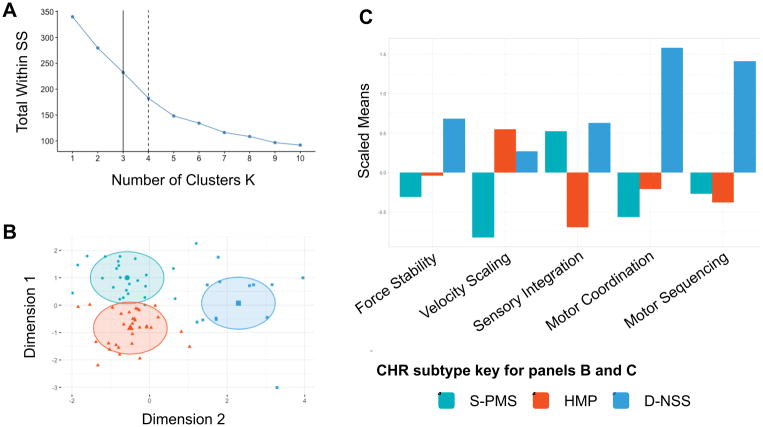
K-means clustering was conducted in R (v.3.3.3; “Another Canoe”) with the “cluster” package using the motor variables for force stability, VS, and NSS sensory integration, motor coordination, and motor sequencing. Because the control group showed few abnormal movements on the NES, k-means clustering was conducted only in the CHR group. Most of the participants were able to complete all of the motor tasks (N = 56), however some participants had missing data for force stability (N = 1), VS (N = 7), sensory integration (N = 4), motor coordination (N = 4) and motor sequencing (N = 5). Missing values create problems for clustering approaches, and omitting entire cases with one missing domain decreases the sample size considerably. Therefore, before performing the k-means clustering, missing values were approximated using a principal components approach for multivariate data with the “missMDA” and “FactoMineR” packages. The complete observations for all motor variables were scaled prior to clustering. A scree plot of the scaled variables was visually inspected in order to determine the optimal number of clusters (see Figure 1).
Figure 1.
Results of the k-means cluster analysis. Panel A shows a scree plot where either a 3 or 4 cluster solution is considered optimal. After visual inspection of cluster groupings using a 3 or 4 cluster solution, it was determined that the 3-cluster solution represented the most parsimonious picture of motor profiles in the CHR group. Panel B shows the 3 clusters based on the first and second principal components (Dimensions 1 and 2). Panel C shows the scaled means with respect to the CHR group of the motor variables for all three clusters. Lower values for velocity scaling and higher values for force stability and NSS suggest impairment. The first cluster showed increased sensory integration and decreased VS, indicative of psychomotor slowing (S-PMS cluster). The second cluster showed healthy motor performance across all domains (HMP cluster). The third cluster contains participants with increased CV on the force stability task and higher NSS scores on sensory integration, motor coordination and motor sequencing domains, suggesting more dyskinesia and problematic NSS (D-NSS cluster).
K-means clustering was initially run with a four-cluster solution, however, this solution showed overlapping regions between clusters and a cluster with three individuals. A three-cluster solution was considered optimal because it provided clusters with sufficient group sizes to conduct further analyses and a parsimonious clustering of movement performance within the CHR group.
Demographic characteristics including age, sex, years of education, parent education, and recruitment method between CHR and healthy control groups were examined using independent samples t-tests and chi-squared tests for continuous and categorical data, respectively. Following the cluster analysis, demographic variables were compared between the CHR cluster groups and healthy controls using one-way analyses of variance (ANOVA) for continuous data and chi-squared tests for categorical data.
Because the controls did not show a psychosis-risk syndrome, comparisons on clinical measures for baseline positive and negative symptoms were conducted using ANOVAs in the CHR cluster groups only.
Transition to psychosis over the 24-month follow-up window was recorded as a dichotomous variable and a chi-squared test was used to test the distribution of participants in each CHR cluster. A one-way ANOVA was used to test CHR cluster group differences on the 2-year probability of risk for psychosis conversion.
CHR cluster and healthy control group differences were tested across the MATRICS cognitive domains in several steps. First, a repeated measures ANOVA was conducted to test for a significant group x cognitive domain interaction. Then, a series of seven one-way ANOVAs were conducted to examine cognitive performance between CHR motor clusters and healthy controls for each individual cognitive domain. Post-hoc testing between each cluster and healthy controls included two-tailed pairwise comparisons with Tukey HSD correction for multiple comparisons.
The rsFMRI analysis included two steps of analysis. In the first step, a series of 8 one-way ANOVAs were conducted to investigate CHR cluster and healthy control group differences in resting state networks for each seed including lateral caudate, putamen, and thalamus, as well as bilateral primary motor and SMA seeds. Then in the second step, independent samples t-tests were conducted to examine directional differences between each of the CHR motor clusters and healthy controls for each seed ROI. For each comparison, the seed-to-voxel results were first thresholded at the voxel-level at puncorr<0.001 and then corrected at the cluster-level to a false-discovery rate (FDR) of p<0.05. A total of 48 independent samples t-tests were conducted to assess directional differences between CHR subtypes and healthy controls, therefore Bonferroni correction was applied for cluster-level results at p(FDR) < 0.001.
RESULTS
K-means cluster results
As noted, the k-means procedure identified 3 clusters of motor subtypes in the CHR group. The first cluster showed increased abnormalities in sensory integration and decreased VS, indicative of psychomotor slowing (S-PMS cluster; N = 25). The second cluster showed healthy motor performance across all domains (HMP cluster; N = 31). The third cluster contains participants with increased CV on the force stability task and higher scores across NSS domains for sensory integration, motor coordination and motor sequencing, suggesting more dyskinesia and problematic NSS (D-NSS cluster; N = 13). See Figure 1.
Demographics and symptom characteristics
There were no differences between CHR and healthy control participants in terms of age, sex, parent education, or years of education. As expected, CHR participants and healthy controls differed in terms of recruitment method. There were no CHR subtype and healthy control group differences in terms of age, parent education, or years of education. There was a significant difference in the distribution of sex among CHR subtypes and healthy controls; pairwise chi-square comparisons showed significant differences between D-NSS and S-PMS (p = 0.02) and between S-PMS and the healthy control group (p = 0.01). See Table 1. The distribution of CHR participants with a positive family history of psychosis was consistent across cluster groups. A total of seven CHR participants were taking antipsychotic medication during the motor assessment and there were no differences in distribution of these participants among CHR subtypes.
There were no differences among CHR subtypes for positive symptoms, F(2, 66) = 0.25, p = 0.77, but there were differences in terms of negative symptoms, F(2, 66) = 4.49, p = 0.014, . D-NSS showed more severe negative symptoms compared to both S-PMS (p = 0.02) and HMP (p = 0.02) clusters. S-PMS and HMP clusters showed a similar severity in terms of negative symptoms (p = 0.9).
Comparisons between cluster groups and cognition
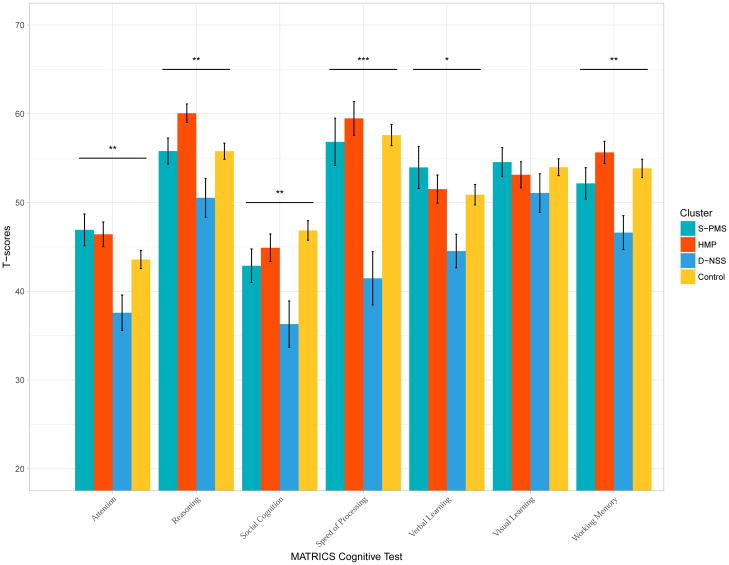
An omnibus repeated measure ANOVA comparing cluster groups including healthy controls was first run in order to test for a cluster group by MATRICS cognitive domain interaction. The omnibus test suggested a significant interaction, F(18, 754) = 2.40, p = 0.0009, . One way ANOVAs within each cognitive domain were then conducted to compare CHR subtypes and healthy controls. There were significant differences among CHR subtypes for attention F(3, 124) = 4.59, p = 0.004, , reasoning and problem solving F(3, 126) = 6.16, p = 0.0006, , social cognition F(3, 126) = 5.38, p = 0.002, ; speed of processing F(3, 126) = 9.71, p ≤ 0.0001, , verbal learning F(3, 126) = 2.91, p = 0.04, and working memory F(3, 126) = 4.27, p = 0.007, . There was similar performance among CHR subtypes and healthy controls for visual learning F(3, 126) = 0.67, p = 0.57. See Figure 2.
Figure 2.
Cognitive domain T scores for CHR subtypes and healthy controls.
Post hoc testing with Tukey HSD correction revealed that S-PMS showed similar cognitive performance on all domains of the MATRICS compared to HMP and healthy controls (all ps > 0.1). S-PMS performed significantly better on attention (p = 0.006), speed of processing (p = 0.0003), and verbal learning (p = 0.02) compared to D-NSS. HMP cluster performed significantly better on attention (p = 0.008), reasoning and problem solving (p = 0.0003), social cognition (p = 0.04), speed of processing (p = 0.001) and working memory (p = 0.006) compared to D-NSS. In addition, HMP performed similarly and in the case of reasoning and problem solving (p = 0.03), better than the healthy controls. The D-NSS cluster performed significantly worse than the healthy controls on social cognition (p = 0.001), speed of processing (p ≤ 0.0001), and working memory (p = 0.004); and worse at a trend level compared to the healthy controls on attention (p = 0.09) and reasoning and problem solving (p = 0.07).
Resting state fMRI results
A series of eight one-way ANOVAs were conducted for each of the seed regions to examine functional connectivity among CHR cluster groups and healthy controls. There were significant differences between the groups for the left and right thalamus seed regions to the insula. The other seed regions did not show differences in connectivity across all CHR cluster groups and healthy controls. Comparisons among cluster groups and healthy controls were also conducted with directional tests for each seed ROI separately. S-PMS showed significant differences in connectivity compared to HMP for left and right thalamus to bilateral areas of the insula, somatosensory cortex, superior parietal cortex and premotor areas. S-PMS showed less connectivity between the right thalamus and occipital cortex compared to D-NSS. Interestingly, HMP cluster did not show significant differences in connectivity for any of the seed ROIs compared to the healthy controls. See Table 2 for a description of brain activation regions. In addition, please see Table S1 and Figure S1 in the Supplemental Material for additional rsFMRI results not surviving Bonferroni correction and an illustration of brain activation regions.
Table 2.
Seed to voxel results for one way ANOVAs and independent samples t-tests comparing the CHR subtypes and healthy controls on functional connectivity for each individual seed. The index of connectivity for each comparison was defined as either a between-subject T or F contrast using r-to-z transformed values. Each of the comparisons in the rsFMRI analysis were first thresholded at the voxel-level at puncorr < 0.001 and then corrected at the cluster-level to a false-discovery rate (FDR) of p < 0.05. Independent samples t-tests were corrected to p(FDR) < 0.001.
| Seed | Comparison | Target peak | MNI Coordinates | Cluster size (voxels) | F/T - value | p ≤ | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Left Thalamus | All cluster comparison | Insula | −42 | −22 | 8 | 355 | 10.68 | 0.001 |
| S-PMS > HMP | Heschl’s gyrus | −40 | −24 | 4 | 1035 | 6.51 | 0.001 | |
|
| ||||||||
| Right Thalamus | All cluster comparison | Insula | −30 | −2 | 14 | 171 | 11.51 | 0.010 |
| S-PMS > HMP | Insula | −30 | −2 | 14 | 810 | 5.5 | 0.001 | |
| Somatosensory cortex | 32 | −6 | 16 | 292 | 5.5 | 0.001 | ||
| Superior parietal cortex | −26 | −50 | 64 | 601 | 4.48 | 0.001 | ||
| Premotor cortex | 0 | −6 | 58 | 258 | 4.17 | 0.001 | ||
| S-PMS < D-NSS | Occipital cortex | 22 | −102 | 12 | 476 | 4.54 | 0.001 | |
|
| ||||||||
| Primary Motor | S-PMS > Control | Supplementary motor area | 14 | 6 | 48 | 426 | 4.56 | 0.001 |
Exploratory psychosis conversion assessment and calculation of risk probability
A subset of the CHR group (N = 53) was assessed for psychosis using the SCID up to 24-months after the baseline assessment. The distribution of individuals who transitioned to psychosis was similar across cluster groups χ2(2, N = 53) = 2.24, p = 0.3. Three of the participants in the S-PMS cluster developed psychotic disorders including bipolar disorder with psychotic features, brief psychosis, and psychosis NOS (12% of cluster). One participant in the HMP cluster met criteria for schizophrenia (3% of cluster) and two participants in the D-NSS cluster group transitioned to psychosis NOS and schizophreniform disorder (15% of cluster).
In regards to the 2-year probability of psychosis conversion risk using the NAPLS risk calculator, there was a marginally significant CHR cluster group difference F(2,66) = 3.12, p = 0.051, . Group comparisons using Tukey HSD correction showed that S-PMS (M = 13.43%, SD = 8.9%) and HMP (12.78%, SD = 7.57%) clusters had similar risk probabilities. There was a trend level group difference (p = 0.09) between the S-PMS and the D-NSS (M = 20.12%, SD = 12.87%) cluster and a significant difference between the HMP and the D-NSS cluster (p = 0.04).
DISCUSSION
To our knowledge, this is the first study to partition young adults at risk for psychosis based on multivariate motor performance. The results revealed three groups with distinct motor performance profiles. The clusters with impaired motor performance, in particular the D-NSS cluster, showed more severe negative symptoms and impaired cognition across multiple domains; there was also some evidence for cluster differences and greater risk for psychosis at baseline based on a risk calculation. These results suggest that these motor clusters may provide meaningful information about psychosis risk. Converging evidence from the behavioral and resting state analysis in this study are consistent with etiological theories suggesting that altered cortical-striatal neurodevelopment is associated with symptom, cognitive, and motor impairment (Obeso et al., 2014; Robbins, 1990). In particular, the resting state results suggest an overall pattern of aberrant thalamocortical connectivity. Most importantly, the multivariate analysis of motor behavior adds to the growing body of work suggesting that motor symptoms should be considered along with other behaviors as markers of risk for psychosis (Mittal & Wakschlag, 2016).
Traditionally, movement abnormalities in psychosis have been described in terms of catatonia, dyskinesia, parkinsonism, psychomotor slowing, and neurological soft signs as different domains (Walther & Strik, 2012). Research in schizophrenia patients using observational measures of movement suggests that motor factor structures may be more complicated than initially proposed (Peralta & Cuesta, 2001). The current cluster analysis suggests that there are subtypes of individuals with psychomotor slowing and others with spontaneous dyskinesia and more neurological soft signs. This is consistent with the factor structure of psychomotor symptoms in a cross section of schizophrenia patients who were grouped in terms of psychomotor poverty and motoric neurological soft signs (Docx et al., 2012).
The finding of different distributions of male and female participants among CHR subtypes suggests that there may be subtle sex differences in regards to motor performance. Putting this finding into context of past work is challenging as there have been few studies in this area and of the work published, findings regarding sex differences and motor performance in CHR individuals and patients with psychosis remain inconclusive. Healthy men tend to show greater deviations in isometric force stability (Endo & Kawahara, 2011); however, sex differences in instrumental force stability or velocity scaling have not been examined in psychosis samples. Cross sectional work in CHR samples has found no sex differences in respect to NSS subdomains (Chan et al., 2018), and meta-analyses examining NSS in patients with psychosis have found a limited number of studies to fully explore this topic, although some research suggests that men with psychosis may show higher total NSS (Chan, Xu, Heinrichs, Yu, & Wang, 2010). In consideration of this past research and theories linking movement abnormalities with altered neurodevelopment in the pathophysiology of psychosis (Mittal, Bernard, & Northoff, 2017), the finding of a higher proportion of female participants in the D-NSS cluster provides initial evidence that clustering by motor performance may highlight different etiologies in CHR individuals. Given the lack of research in this area, further investigation of sex differences across multiple domains of motor performance and replication of this cluster analysis approach in larger samples is needed.
The S-PMS cluster showed average cognitive performance although we might have expected that this cluster would show impairment on cognitive functions such as speed of processing, which also involves a psychomotor component (Docx et al., 2012). These results speak to the idea that instrumental measures of movement pick up on subtle variations in movement that are not otherwise detected in other behavioral paradigms. The results of impaired cognitive performance in D-NSS compared to the other clusters is consistent with previous research noting that the co-occurrence of movement abnormalities and neurocognitive impairment may help discriminate between CHR youth who do or do not transition to psychosis within 2 years (Mittal, Walker, et al., 2010). Moreover, the differences in cognitive functioning, especially for speed of processing, verbal learning and memory domains between the D-NSS and other cluster groups maps on to recent developments in risk calculation suggesting that these are key cognitive domains that are associated with greater probability for psychosis conversion (Cannon et al., 2016).
The resting state FMRI results suggest widespread thalamocortical and cortical motor connectivity differences between the CHR cluster groups and healthy controls. The findings that the motor clusters with greater motor impairment showed increased connectivity between the thalamus and sensory motor regions is consistent with recent studies noting similar patterns of connectivity related to increased risk of conversion to psychosis in other CHR samples (Anticevic et al., 2015). Likewise, increased connectivity between thalamus and motor cortices is in line with results linking this type of aberrant connectivity in early and later stages of psychosis (Woodward & Heckers, 2016). In addition, the current results are consistent with a study in patients with schizophrenia noting that catatonia and dyskinesia symptoms are associated with increased thalamocortical connectivity (Walther et al., 2017). We had initially hypothesized that the CHR cluster groups would show altered connectivity using striatal seed regions—it is worth noting that both S-PMS and D-NSS showed increased connectivity from caudate and putamen seeds to the cortex in comparison to HMP and the healthy control group—but these results did not survive stringent correction for multiple comparisons (see Table S1 and Figure S1). Replication in larger samples is necessary to examine cortical-striatal connectivity in motor clusters. Finally, further work is necessary to assess the unique associations between the motor tasks and functional connectivity in the current study. Importantly, our results argue for distinct patterns of aberrant connectivity between CHR subgroups, which were detected by clustering measures of motor behavior.
Within the context of assessment of risk for psychosis, the current study suggests that there may be vulnerability subtypes in a sample of CHR participants. Importantly, these subtypes were defined by multiple motor behaviors instead of symptom profiles. While the CHR subtypes showed consistent severity in terms of positive symptoms, there were CHR subtype differences in terms of negative symptoms, cognitive functioning, and brain functional connectivity in cortical-thalamic brain regions. Exploratory analysis of risk for psychosis conversion based on a risk calculator suggests that the D-NSS cluster in particular exhibited a higher probability of psychosis conversion at baseline (20%). It is worth noting that overall the CHR participants in this study had an average risk of conversion at baseline of 14.4% using the NAPLS risk calculator. While the distribution of CHR individuals who did transition to psychosis was not significantly different across clusters, the results of an actual 15% transition rate in the D-NSS cluster encourage further research in larger samples using this multivariate approach in conjunction with the development of risk calculators. Future work using the SIPS over time to look at continuous change in positive and negative symptoms will also be informative. Overall, these results are promising for tailored risk assessment for psychosis and suggest different etiological pathways for CHR individuals (Strik et al., 2017).
There are several strengths and limitations to this study. The use of instrumental measures of force stability and velocity scaling is consistent with a growing trend in this line of research, although more work needs to be done in validating instrumental measures based on gold standard assessments (Mentzel et al., 2016; Mittal, 2016). While every effort was made to reduce dependence between measures in the study by using different raters as much as possible for individual tasks, the use of observational methods of NSS motor domains carries some limitations and should be considered with these results. A small group of CHR participants were taking antipsychotic medication, although most were prescribed atypical antipsychotics. The study includes a fairly large sample of CHR individuals but the individual cluster sizes suggest that larger sample sizes are needed. This will be particularly relevant for questions around clinical course and transition to psychotic disorders. Importantly, we were able to ascertain a psychotic diagnosis from a subset of participants (N = 53) within 24-months following the motor assessment. Given the exploratory nature of the diagnostic follow-up and NAPLS risk calculation analyses, replication in larger and more complete samples are needed to test the ability of motor clusters to chart illness trajectories in these youth at risk for psychosis.
Supplementary Material
Acknowledgments
Funding provided by National Institutes of Health Grants R01MH094650, R21/R33MH103231, and R21MH110374 to V.A.M.
Footnotes
Conflict of Interest
Dr. Mittal is a consultant with Takeda Pharmaceuticals. Dr. Walther received honoraria for educational talks from Janssen-Cilag, Otsuka and Lundbeck. No other authors have conflicts to disclose.
Authors Contributions
All authors contributed to developing the study concept and design. V.A.M obtained funding for the study. Testing, data collection as well as data analysis and interpretation were performed by D.J.D under the supervision of S.W, J.A.B, and V.A.M. D.J.D. drafted the paper; S.W., J.A.B., and V.A.M provided the critical revisions. All authors approved the final version of the paper for submission.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, … Cannon TD. North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophrenia Research. 2012;142(1–3):77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56(9):781–787. doi: 10.1001/archpsyc.56.9.781. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12884883. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, … Cannon TD. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry. 2015;72(9):882–891. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus E, Stirling J, Hopkins R, Lewis S. The Presence of Neurological Soft Signs Along the Psychosis Proneness Continuum. Schizophrenia Bulletin. 2005;32(3):573–577. doi: 10.1093/schbul/sbj037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Goen JRM, Maldonado T. A case for motor network contributions to schizophrenia symptoms: Evidence from resting-state connectivity. Human Brain Mapping. 2017;38(9):4535–4545. doi: 10.1002/hbm.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. Differential motor and prefrontal cerebello-cortical network development: Evidence from multimodal neuroimaging. NeuroImage. 2016;124(Pt A):591–601. doi: 10.1016/j.neuroimage.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins verbal learning test--revised: professional manual. Psychological Assessment Resources; 2001. [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=2710870&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biological Psychiatry. 1994;35(2):104–111. doi: 10.1016/0006-3223(94)91199-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7909452. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Research. 2010;177(1–2):77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophrenia Research. 2014;159(2):263–266. doi: 10.1016/j.schres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, … Kattan MW. An Individualized Risk Calculator for Research in Prodromal Psychosis. American Journal of Psychiatry. 2016;173(10) doi: 10.1176/appi.ajp.2016.15070890. appiajp201615070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, Cornblatt BA, Burton CZ, Tso IF, Auther AM, Adelsheim S, … McFarlane WR. Personalized Prediction of Psychosis: External Validation of the NAPLS-2 Psychosis Risk Calculator With the EDIPPP Project. American Journal of Psychiatry. 2016;173(10):989–996. doi: 10.1176/appi.ajp.2016.15121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Cui H, Chu M, Zhang T, Wang Y, Wang Y, … Cheung EFC. Neurological soft signs precede the onset of schizophrenia: a study of individuals with schizotypy, ultra-high-risk individuals, and first-onset schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2018;268(1):49–56. doi: 10.1007/s00406-017-0828-4. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological Soft Signs in Schizophrenia: A Meta-analysis. Schizophrenia Bulletin. 2010;36(6):1089–1104. doi: 10.1093/schbul/sbp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, … Tamminga CA. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. American Journal of Psychiatry. 2016;173(4):373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Bollini AM, Mack LM, Kryda AD, Rutland J, Weiss PS, … Walker EF. Neurological soft signs and minor physical anomalies in patients with schizophrenia and related disorders, their first-degree biological relatives, and non-psychiatric controls. Schizophrenia Research. 2007;94(1):64–73. doi: 10.1016/j.schres.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary Findings for Two New Measures of Social and Role Functioning in the Prodromal Phase of Schizophrenia. Schizophrenia Bulletin. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Carrion RE, Auther A, McLaughlin D, Olsen RH, John M, Correll CU. Psychosis Prevention: A Modified Clinical High Risk Perspective From the Recognition and Prevention (RAP) Program. The American Journal of Psychiatry. 2015;172(10):986–994. doi: 10.1176/appi.ajp.2015.13121686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, … Harrison BJ. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophrenia Bulletin. 2014;40(4):904–913. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Pantelis C, Fornito A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophrenia Research. 2016 doi: 10.1016/j.schres.2016.08.020. [DOI] [PubMed]
- Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. Npj Schizophrenia. 2015:1. doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Ziermans TB, Nieuwenhuis M, Schothorst PF, van Engeland H, Kahn RS, … Schnack HG. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: Applying machine learning techniques to brain imaging data. Human Brain Mapping. 2017;38(2):704–714. doi: 10.1002/hbm.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Pratt DN, Giangrande EJ, Grunnagle M, Orel J, Weinberger DR, … Berman KF. Attacking Heterogeneity in Schizophrenia by Deriving Clinical Subgroups From Widely Available Symptom Data. Schizophrenia Bulletin. 2017 doi: 10.1093/schbul/sbx039. [DOI] [PMC free article] [PubMed]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, … Sabbe B. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatrica Scandinavica. 2012;126(4):256–265. doi: 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the Peri Life Events Scale. Journal of Health and Social Behavior. 1978;19(2):205–229. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/681735. [PubMed] [Google Scholar]
- Endo H, Kawahara K. Gender differences in hand stability of normal young people assessed at low force levels. Ergonomics. 2011;54(3):273–281. doi: 10.1080/00140139.2010.547607. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL. SCID-I/P Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, … McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophrenia Research. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Meyer-Lindenberg A, Kubera KM, Thomann PA, Wolf RC. Motor dysfunction as research domain in the period preceding manifest schizophrenia: A systematic review. Neuroscience and Biobehavioral Reviews. 2018;87:87–105. doi: 10.1016/j.neubiorev.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: A dimensional step towards an underappreciated domain. Schizophrenia Research. 2015;169(1–3):217–233. doi: 10.1016/j.schres.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Wolf RC, Wilder-Smith EP, Kubera KM, Thomann PA. Motor abnormalities and basal ganglia in schizophrenia: evidence from structural magnetic resonance imaging. Brain Topography. 2013;28(1):342. doi: 10.1186/1471-244X-13-342. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia Bulletin. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, … Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of General Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry. 2001;58(2):165–171. doi: 10.1001/archpsyc.58.2.165. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11177118. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongur D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychological Medicine. 2014;44(15):3239–3248. doi: 10.1017/S0033291714000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. The American Journal of Psychiatry. 2015;172(3):249–258. doi: 10.1176/appi.ajp.2014.13030418. [DOI] [PubMed] [Google Scholar]
- McGlashan T, Walsh BC, Woods S. The psychosis-risk syndrome: handbook for diagnosis and follow-up. Oxford University Press; 2010. [Google Scholar]
- Mentzel TQ, Lieverse R, Levens A, Mentzel CL, Tenback DE, Bakker PR, … van Harten PN. Reliability and validity of an instrument for the assessment of bradykinesia. Psychiatry Research. 2016;238:189–195. doi: 10.1016/j.psychres.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Mittal VA. Cross-Cutting Advancements Usher in a New Era for Motor Research in Psychosis. Schizophrenia Bulletin. 2016;42(6):1322–1325. doi: 10.1093/schbul/sbw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Bernard JA, Northoff G. What Can Different Motor Circuits Tell Us About Psychosis? An RDoC Perspective. Schizophrenia Bulletin. 2017;43(5):949–955. doi: 10.1093/schbul/sbx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophrenia Research. 2010;123(1):68–70. doi: 10.1016/j.schres.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Carol EE, … Millman ZB. Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophrenia Bulletin. 2014;40(6):1204–1215. doi: 10.1093/schbul/sbt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A. Dermatoglyphic asymmetries and fronto-striatal dysfunction in young adults reporting non-clinical psychosis. Acta Psychiatrica Scandinavica. 2012;126(4):290–297. doi: 10.1111/j.1600-0447.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophrenia Research. 2011;132(2–3):194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Orr JM, Turner JA, Pelletier AL, Dean DJ, Lunsford-Avery J, Gupta T. Striatal abnormalities and spontaneous dyskinesias in non-clinical psychosis. Schizophrenia Research. 2013;151(1–3):141–147. doi: 10.1016/j.schres.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, … Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. Journal of Abnormal Psychology. 2007;116(2):260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Wakschlag LS. Research Domain Criteria (RDoC) Grows Up: Strengthening Neurodevelopment Investigation within the RDoC Framework. Journal of Affective Disorders. 2016 doi: 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, … Cannon TD. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biological Psychiatry. 2010;68(1):93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, … Yung AR. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70(8):793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384(9942):523–531. doi: 10.1016/S0140-6736(13)62418-6. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Stuart GW, Nelson HE, Robbins TW, Barnes TRE. Spatial Working Memory Deficits in Schizophrenia: Relationship With Tardive Dyskinesia and Negative Symptoms. American Journal of Psychiatry. 2001;158(8):1276–1285. doi: 10.1176/appi.ajp.158.8.1276. [DOI] [PubMed] [Google Scholar]
- Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychological Medicine. 2009;39(7):1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA. Does Biology Transcend the Symptom-based Boundaries of Psychosis? Psychiatric Clinics of North America. 2016;39(2):165–174. doi: 10.1016/j.psc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophrenia Research. 2001;47(2–3):107–116. doi: 10.1016/s0920-9964(00)00013-x. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11278127. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The Case for Frontostriatal Dysfunction in Schizophrenia. Schizophrenia Bulletin. 1990;16(3):391. doi: 10.1093/schbul/16.3.391. Retrieved from http://psycnet.apa.org/journals/szb/16/3/391/ [DOI] [PubMed] [Google Scholar]
- Rutigliano G, Valmaggia L, Landi P, Frascarelli M, Cappucciati M, Sear V, … Fusar-Poli P. Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. Journal of Affective Disorders. 2016;203:101–110. doi: 10.1016/j.jad.2016.05.053. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Sorensen HJ, Maeda J, Mortensen EL, Victoroff J, Hayashi K, … Mednick S. Childhood motor coordination and adult schizophrenia spectrum disorders. American Journal of Psychiatry. 2009;166(9):1041–1047. doi: 10.1176/appi.ajp.2009.08091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. American Journal of Psychiatry. 2004;161(11):2021–2027. doi: 10.1176/appi.ajp.161.11.2021. [DOI] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G, … Cannon TD. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophrenia Bulletin. 2012;38(6):1225–1233. doi: 10.1093/schbul/sbr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Iacono WG, Thuras PD, Beiser M. Using biological indices to classify schizophrenia and other psychotic patients. Schizophrenia Research. 2001;50(3):139–150. doi: 10.1016/s0920-9964(00)00160-2. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11439234. [DOI] [PubMed] [Google Scholar]
- Strik W, Stegmayer K, Walther S, Dierks T. Systems Neuroscience of Psychosis: Mapping Schizophrenia Symptoms onto Brain Systems. Neuropsychobiology. 2017;75(3):100–116. doi: 10.1159/000485221. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, O’Connor D, Marcelle ET, Ho EJ, Cameron Craddock R, Tobe RH, … Milham MP. Data-Driven Phenotypic Categorization for Neurobiological Analyses: Beyond DSM-5 Labels. Biological Psychiatry. 2017;81(6):484–494. doi: 10.1016/j.biopsych.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophrenia Bulletin. 1994;20(3):441–451. doi: 10.1093/schbul/20.3.441. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7526446. [DOI] [PubMed] [Google Scholar]
- Walther S, Mittal VA. Motor System Pathology in Psychosis. Current Psychiatry Reports. 2017;19(12):97. doi: 10.1007/s11920-017-0856-9. [DOI] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant Hyperconnectivity in the Motor System at Rest is Linked to Motor Abnormalities in Schizophrenia Spectrum Disorders. Schizophrenia Bulletin. 2017 doi: 10.1093/schbul/sbx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biological Psychiatry. 2016;79(12):1016–1025. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.