Abstract
Purpose of review
Multiple respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), display significant socioeconomic and racial/ethnic disparities. The objective of this review is to evaluate the evidence supporting a link between disproportionate environmental exposures and these health disparities.
Recent findings
Studies suggest that various co-occurring factors related to the home environment, neighborhood environment, non-modifiable individual factors, and individual behaviors and attributes can increase or modify the risk of adverse respiratory outcomes among socioeconomically-disadvantaged and racially/ethnically diverse populations. Pollutants in the home environment, including particulate matter, nitrogen dioxide, and pesticides, are elevated among lower socioeconomic status populations and have been implicated in the development or exacerbation of respiratory-related conditions. Neighborhood crime and green space are socioeconomically patterned and linked with asthma outcomes through psychosocial pathways. Non-modifiable individual factors such as genetic predisposition cannot explain environmental health disparities but can increase susceptibility to air pollution and other stressors. Individual behaviors and attributes, including obesity and physical activity, contribute to worse outcomes among those with asthma or COPD.
Summary
The root causes of these multifactorial exposures are complex, but many likely stem from economic forces and racial/ethnic and economic segregation that influence the home environment, neighborhood environment, and access to healthy foods and consumer products. Critical research needs include investigations that characterize exposure to and health implications of numerous stressors simultaneously, both to guard against potential confounding in epidemiological investigations and to consider the cumulative impact of multiple elevated environmental exposures and sociodemographic stressors on health disparities.
Keywords: asthma, chronic obstructive pulmonary disease, disparities, environment, housing, neighborhood
Introduction
Significant health disparities exist in multiple respiratory outcomes, with a recent policy statement by the American Thoracic Society and European Respiratory Society concluding that groups with lower socioeconomic status (SES) are up to 14 times more likely to have respiratory diseases than the highest SES groups (1). For example, low-income and racially/ethnically diverse populations in the United States (US) have higher rates of asthma and more frequent use of emergency departments (ED); Puerto Ricans, non-Hispanic blacks, and those below the poverty line have the highest asthma prevalence, and the asthma ED rate is 3.3 times higher for blacks vs. whites (2). Similarly, chronic obstructive pulmonary disease (COPD) is much more prevalent among low-SES populations (3), with far higher rates in the US among individuals with less than a high school education and those with household income < $20,000 (4).
These health disparities are complex and multifactorial, but may be partly explained by environmental exposures and characterized as environmental health disparities, defined as inequities in illnesses that are mediated by disproportionate exposures associated with the physical, chemical, biological, social, natural and built environments (5). The emphasis on inequities rather than inequalities implies that predefined subpopulations, including racially/ethnically diverse populations and those of lower SES, are of particular concern. The empirical evidence for environmental health disparities would therefore be found in two different domains – the evidence that there are disproportionate exposures to environmental stressors by SES or race/ethnicity, and the evidence that these exposures contribute to the underlying inequities in illness (i.e., that there is a causal relationship between the disproportionately distributed exposures and the health outcomes in question). Both lines of evidence are required to conclude that environmental health disparities are present, and there is a growing body of literature that provides empirical evidence for both the underlying exposure patterns and the linkage with specific respiratory outcomes.
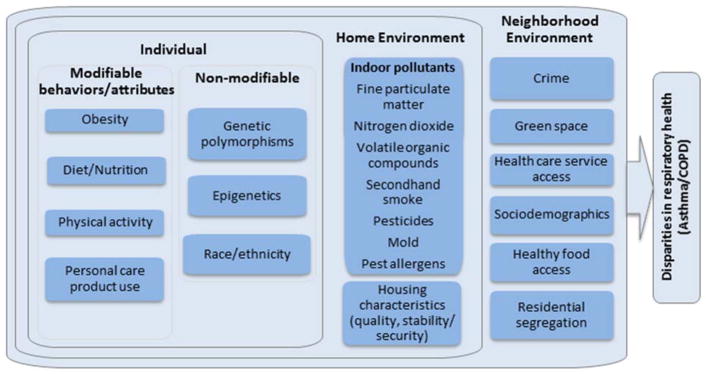
In this article, we focus on four domains hypothesized to contribute to environmental health disparities for respiratory outcomes – the home environment, the neighborhood environment, non-modifiable individual factors, and individual behaviors and attributes (Figure 1). While there are numerous respiratory outcomes with strong evidence for health disparities, we focus on asthma and COPD to illustrate broad trends in the literature. For each domain, we discuss key articles in the recent literature as of February 2018 to evaluate the two lines of evidence required to reach conclusions about environmental health disparities – the evidence for disproportionate exposures for low-SES or racially/ethnically diverse populations, and the evidence that the environmental stressors with evidence for exposure disparities are strongly associated with asthma or COPD. We conclude by determining the implications for environmental health disparities.
Figure 1.
Conceptual diagram indicating the contributions of four domains – the home environment, the neighborhood environment, non-modifiable individual factors, and individual behaviors and attributes – to environmental health disparities for respiratory outcomes.
Home environment
Numerous exposures in the home environment have been shown to be disproportionately distributed by SES or race/ethnicity, either based on first principles or empirical evidence. Indoor residential exposures are driven by indoor sources (e.g., smoking, cooking), outdoor sources (e.g., traffic, industrial activity), physical structure (e.g., size of living space, heating systems), and activity patterns (e.g., smoking behavior) (6). Lower-income populations tend to have smaller and older homes located closer to major pollution sources and with greater prevalence of indoor sources. Empirical evidence showed disparities in exposure to multiple air pollutants (including nitrogen dioxide (NO2), fine particulate matter (PM2.5), and volatile organic compounds (VOCs)); secondhand smoke (SHS) exposure, which refers to exposure to a mixture of exhaled mainstream smoke and side stream smoke released from the burning of cigarettes or other smoking devices (cigar, pipe, etc.) and diluted with ambient air (7); lead; pest allergens; and semivolatile organic compounds in air and dust (6). Another review article (8) emphasized broad-based disparities in housing quality, with 7.5% of non-Hispanic blacks but only 2.8% of non-Hispanic whites living in moderately substandard housing. This has implications for numerous housing-based exposures with linkages to health outcomes, including heat/cold, noise, mold, SHS, lead, air pollution, and pest allergens (9). Studies have also shown that adverse housing conditions (e.g., housing disrepair factors including presence of mold, water damage, peeling paint) prevalent in low-income households in both urban and agricultural settings increase the likelihood of pest infestations and subsequent residential pesticide use (10–15). Pesticide misuse is also common in low-income households due to inadequate knowledge and training (13). Exposure disparities also exist for multiple non-chemical stressors in the home environment. For example, low-income populations may be more challenged by housing instability or financial limitations related to paying for living expenses, which can contribute to psychosocial stress and increased risk of depression, among other impacts (16, 17).
Given this array of stressors, there is significant risk for housing-based environmental health disparities in both asthma and COPD. We conducted a literature search in PubMed using the keywords (housing or nitrogen dioxide or air pollution or mold or environmental tobacco smoke or stress or allergen or temperature or noise or pesticides) and (asthma or COPD). Individual articles in the recent literature were selected that were most directly relevant to the outcomes and exposures in question. We emphasized systematic reviews and articles that considered multiple stressors simultaneously, given both concerns about confounding and the disparities implications of multi-stressor exposures, and we focused on articles since 2013 for stressors with numerous publications but included earlier dates for stressors with more limited publications.
The evidence linking indoor air pollution exposure to asthma is extensive. In particular, there is ample evidence that SHS exposure is linked to higher asthma morbidity, with a recent systematic review finding an odds ratio of 1.85 for asthma hospitalization and 1.66 for ED or urgent care visits (18). Both prenatal and postnatal maternal and paternal smoking have been associated with increased risk of wheeze (19); and SHS exposure has been associated with not only morbidity, but also with incident asthma (20). In a systematic review, findings regarding the impact of domestic fuel combustion were mixed (20), although increases in both indoor particulate matter (PM) and NO2 (primarily associated with stove use) have been associated with pediatric asthma-related outcomes in multiple recent studies, including nighttime inhaler use (21), asthma symptoms, and medication use (22). NO2 was also associated with incident asthma in a population-based birth cohort study (23). In addition to indoor sources, home environments can also be a place of exposure to ambient pollutants infiltrating from the outdoors and lower-income homes are often closer to pollution sources (6). A general consensus is that traffic-related air pollutants are associated with asthma prevalence and exacerbations (20). Other housing-based exposures with evidence for an association with asthma include mold, VOCs and pesticides (20).
Pesticides may increase the risk of asthma through interaction with functional irritant receptors in the respiratory tract that promote neurogenic inflammation, increased bronchial hyper-responsiveness (24), cholinesterase inhibition which may promote bronchoconstriction (25), and through effects of autoinhibitory M2 muscarinic receptors on the parasympathetic nerves in the lung (26). Although epidemiologic studies linking residential pesticide use to respiratory effects are limited and some have relied on self-reported pesticide use rather than biological or environmental measurements of exposure (27–31), these studies have reported positive associations between early life exposures to residential pesticide use and adverse respiratory outcomes in children.
In terms of multi-stressor studies or those considering the influence of housing more generally, a recent analysis from the Urban Environment and Childhood Asthma (URECA) study found that in addition to pollutant exposure, higher maternal stress and depression were associated with increased risk of developing asthma (32). Although the role of psychosocial stress in pediatric asthma has been challenging to define given the bidirectional nature of the relationship (i.e., severe asthma can itself be a stressor), psychosocial stress has been associated with asthma, through modifications to the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis (33) as well as through behavioral pathways (i.e., caregivers who are unable to maintain the medication regimen for their children) (34, 35). Multiple studies have additionally shown interactions between air pollution exposures and psychosocial stress (36, 37). Regardless of potential mechanism or exposure, it has been shown that in general, poor housing quality has been associated with increased asthma diagnosis and morbidity (38), while “green” homes that reduce indoor environmental exposures have been associated with lower risks of asthma morbidity (39).
Although active smoking, which is more prevalent in low income communities (40), is the primary exposure leading to COPD in developed countries, the literature linking SHS exposure with COPD is less extensive. However, it is becoming more evident that residential and/or workplace SHS exposure also leads to impaired lung function and an increased risk of COPD in both never-smokers (41) and those with a heavy smoking history (42). A recent review also confirmed the role of both air pollution and temperature in contributing to COPD morbidity (43). In addition to outdoor air pollution, multiple additional stressors in the indoor environment were also associated with COPD, including PM and NO2 (44). Both hot and cold temperatures were associated with increased risks of COPD exacerbations, and the evidence for synergistic effects with air pollution appears suggestive, thus low-income populations who are more likely to have exposure to both air pollution and temperature extremes (6) would be at greater risk regardless of the nature of the interaction. Furthermore, children with asthma and lower respiratory infections are more likely to have chronic lung function decrements (45), implying that housing-related stressors that increase the risk of pediatric asthma and infections may also increase the risk of COPD later in life. There is also evidence of complex bidirectionality, wherein individuals with chronic lung disease are more likely to be housing insecure, attributable to the financial burden of medication for a chronic disease (46).
Neighborhood environments
Multiple aspects of the neighborhood environment continue to be unequally distributed across racial/ethnic and socioeconomic lines, as documented in the Institute of Medicine’s updated report on Reducing Health Disparities (47). Residential segregation limits access to healthcare services, stores with healthy food choices, and outdoor spaces that encourage physical activity (47, 48). There is a higher density of tobacco outlets in low-income and racially/ethnically diverse neighborhoods (49), which has been associated with higher smoking rates in youth (50). Racially/ethnically diverse populations have long been shown to be exposed to higher rates of community violence (51), and have higher rates of youth group fighting and violence (52). Although total levels of air pollution have decreased in the last decade in the US, studies have shown an increase in ambient air pollution disparities across race/ethnicity, income and education for air pollutants such as PM2.5 (53) and NO2 (53, 54). Public parks located in low-income racially/ethnically diverse neighborhoods are closer to major roadways, with increased exposure to air pollutants (55), and nighttime and daytime noise levels are higher in areas with higher proportions of low-income and racially/ethnically diverse residents (56). Neighborhood green space has also been shown to be unequally patterned, and tied to community revitalization, affordable housing, neighborhood walkability, food security, job creation, and youth engagement (57). Green space disparities are driven by lack of amenities, but also by limitations in access because neighborhood conditions (i.e., high crime) discourage residents from using existing amenities (58, 59). To determine associations between neighborhood-level stressors that have been highlighted more recently and either asthma or COPD, we conducted a literature search in PubMed, using the keywords ((green space, crime, or violence) and (asthma, COPD, or respiratory disease)), limited to articles published since 2013. We also included articles that focused on the mediating impacts of neighborhood characteristics (e.g., green space and community violence) on the effects of air pollution exposures. Lastly, we touch upon the emerging evidence around residential segregation. Very limited COPD literature was available, thus results focus on asthma outcomes only.
Neighborhood crime – whether violent, property or drug abuse – has been associated with multiple asthma-related outcomes through hypothesized pathways including stress and depression (60–62). Children with asthma living in neighborhoods with higher rates of property, violent, and drug abuse crime have higher odds of lifetime asthma, lifetime wheezing, and asthma hospitalization (63). Violent crime and physical disorder are associated with pediatric ED visits for asthma (64) and asthma utilization rates (65), and children who report being afraid to go out to their neighborhood have poorer asthma control (66). In addition, greater neighborhood problems have been associated with detrimental behaviors, including increased smoking and poor adherence to asthma controller medication use (67). Due to the heterogeneity in how exposure to community violence (ECV) is measured, no meta-analysis of ECV and asthma outcomes has been conducted (68).
A recent expert workshop hypothesized that biopsychosocial pathways linking green space to respiratory health fall into three domains: “reducing harm (e.g., reducing exposure to air pollution, noise and heat), restoring capacities (e.g., attention restoration and physiological stress recovery), and building capacities (e.g., encouraging physical activity and facilitating social cohesion)” (69). Exposure to green space is associated with reduced asthma hospitalizations (70), reduced wheezing and bronchitis (71), and reduced risk of asthma development in children living in areas of high pollution (72). Green space has also been shown to exert a protective effect on respiratory death (73) even after accounting for noise and air pollution, although a meta-analysis of related mortality studies did not confirm these results (74). Tree canopy density is associated with lower rates of asthma, even after controlling for green space (75). However, large variability in how greenness is measured across health studies to date may make definitive conclusions with a meta-analysis challenging (76, 77).
Multiple studies have started looking at interactions among neighborhood-level stressors on respiratory health, including between air pollution and neighborhood characteristics (often proxied by measures of SES). A meta-analysis found weak evidence of SES as an effect modifier of air pollution and asthma exacerbations (78); however more recent studies report children with asthma in low-income neighborhoods experience higher impacts when exposed to air pollution (79, 80). Large variability in how SES is measured may contribute to these conflicting results (79). Crowding and poor access to resources modified the association between NO2 and pediatric ED visits for asthma (64). Even in non-asthmatic residents living in neighborhoods that are poorly maintained or experience stress from crime, those who have higher exposure to PM2.5 have decreased lung function (66). Furthermore, exposure to green space may mitigate the impact of air pollution exposures on respiratory health (70, 72).
Residential segregation is hypothesized to contribute to asthma morbidity through multiple mechanisms at the neighborhood level such as neighborhood quality, medical care, and collective efficacy (81). A study looking directly at residential segregation found that neighborhood racial composition predicted asthma burden better than individual-level race/ethnicity, suggesting a strong effect of neighborhood conditions (82).
Non-modifiable individual factors
Environmental health disparities would be magnified if the same populations with elevated exposures to stressors were more susceptible due to individual factors. Several studies suggest that racial or ethnic subgroups have differing susceptibility to air pollution exposure in regards to respiratory health (83). However, it has been difficult to discern whether these differences are due to socioeconomic factors, cultural differences (e.g., differences in dietary habits), and/or non-modifiable risk factors such as genetics. For example, there is evidence that genetic ancestry predicts asthma and chronic lung disease risk (84–87). Additionally, specific genetic polymorphisms have been shown to modify an individual’s susceptibility to active smoking in terms of risk of developing asthma, COPD, and impairments in lung function (88–90). Limited studies investigating interactions between genetics and pollutant exposures also suggest that specific genetic polymorphisms may modify an individual’s susceptibility to environmental exposures, including SHS, endotoxin and ambient air pollution. Specifically, several studies have investigated polymorphisms of the oxidative stress pathway, including glutathione-S-transferase M1 gene (GSTM1) and glutathione S-transfer P1 (GSTP1) gene polymorphisms. For example, children with asthma and the GSTM1 null genotype were more susceptible to ozone exposure (91). Further, findings from a recent genome-wide interaction analysis of air pollution exposure and childhood asthma suggest that several novel genes modify the impact of ambient NO2 exposure on asthma development (92). Despite several studies highlighting the role of gene by environment interactions, differences in genetic risk are not sufficient to explain current environmental disparities observed in respiratory diseases, suggesting that other factors, including individual behaviors and attributes, may also play a role and warrant further examination. A recent study highlighted the complexity of such possibilities, in that children with asthma and genetic susceptibility thought to weaken antioxidant defense, combined with deficient dietary antioxidant intake, showed increased adverse effects of ozone on lung function (93). Therefore, exploration of individual behaviors and attributes and environmental disparities is also warranted.
Individual behaviors and attributes
Multiple individual behaviors and attributes plausibly associated with respiratory health display significant racial/ethnic exposure disparities. In particular, higher obesity rates and increased physical inactivity have been reported among racially/ethnically diverse and low-income populations in the US (94–100). In addition, these same populations disproportionately reside within food deserts and food swamps, areas with limited access to fruits, vegetables, and healthful whole foods in favor of processed, ready-made foods (101, 102). There is also emerging evidence of disparities in exposures to chemicals in consumer products, including personal care products (e.g., perfumes, lotions), and links between exposure to these agents and respiratory health. For example, racial disparities in paraben exposure (an antimicrobial agent present in many personal care products) have been documented with African Americans reported to have higher urinary paraben biomarker concentrations compared to other racial/ethnic groups in the US general population (103). While exposure to phthalates in the US general population is widespread given their presence in many consumer products, including personal care products (hair and skin care products), cleaning agents, PVC flooring, building materials, toys, detergents, and plastic food packaging, phthalate metabolite urinary biomarker concentrations in Hispanic and black populations are generally higher than those reported in non-Hispanic whites. Among women, higher concentrations of urinary phthalate metabolite concentrations have been reported among racially/ethnically diverse pregnant women (104) and women of reproductive age, including those from socioeconomically-disadvantaged backgrounds (105). We therefore conducted a search in PubMed using the keywords (asthma and (personal or individual) behavior), (COPD and (personal or individual) behavior), ((personal care products or phthalates) and asthma), (diet and (asthma or COPD)), (poor diet and asthma), and (obesity and (asthma or COPD)).
Most studies to date support a positive link between obesity and asthma (106, 107), including associations with greater asthma morbidity and severity, and increased asthma prevalence and incidence rates (108). It is also hypothesized that asthma among obese individuals may reflect a unique asthma phenotype, with more severe disease that does not respond well to conventional treatment (106). Similarly, obesity is linked to worse outcomes in patients with COPD (109). The exact mechanisms by which obesity could impact chronic lung disease remain elusive and may be attributed to multiple factors, arising from alterations of the airways and lung parenchyma, to systemic and airway inflammatory and metabolic dysregulation, which negatively affects lung function and/or response to treatment. Notably, obesity has also been identified as a potential determinant of susceptibility to air pollution exposure in both individuals with asthma and COPD, with a heightened adverse response to PM2.5 and NO2 exposure and an increased systemic inflammatory response among overweight individuals (110, 111).
There is also suggestive evidence that diet may play a role on respiratory and allergic diseases (112–117). In the US, increased consumption of fast food and highly processed foods has been coincident with the dramatic rise in asthma. Furthermore, consumption of a “Westernized diet”, characterized by processed foods, high fat intake, less produce consumption, and poor nutrient intake, has been associated with higher asthma prevalence rates in children, while a Mediterranean diet has been associated with reduced asthma-related symptoms (116, 118). Dietary intake has been shown to modify systemic inflammation and the Western diet is believed to promote a pro-inflammatory response resulting from factors such as low antioxidant intake and abundance of saturated fatty acids. It has also been suggested that a poor diet could lead to alterations in the gut microbiota (119). These factors may increase susceptibility to oxidative stress and innate immune activation (120, 121). Thus, it is also reasonable that nutrition may modify air pollution health risks. Limited human studies suggest that adherence to a Mediterranean diet or high fruit and vegetable intake attenuates airway inflammation and changes in lung function in response to ambient air pollution exposure in children with asthma (122).
Studies investigating the role of chemicals in personal care products on respiratory health are limited and, for some chemicals, findings remain inconclusive. However, two recent cross-sectional studies (123, 124) reported an increased risk of allergic sensitization with increasing exposure to antimicrobial agents (triclosan and parabens) present in personal care products. Another study reported sex differences from paraben exposure on allergic sensitization (125). These studies did not identify links between paraben exposure and asthma (123, 125). Still, phthalates, in particular diethyl phthalate (DEP) has been associated with airway inflammation as assessed via exhaled nitric oxide levels among inner-city children (126). DEP is used as a scent retainer in cosmetics, personal care products, and fragrances as well as an excipient in medications and supplements (31, 127). Furthermore, prospective studies (128–134) have reported associations between prenatal phthalate exposure (mostly, butyl benzyl and di-(2-ethylhexyl) phthalate, high molecular weight phthalates commonly found in PVC flooring and food packaging) and an increased risk of developing asthma and allergic outcomes though two studies did not observe any associations with prenatal exposures (135, 136). Phthalates could play a role on respiratory and allergic diseases by altering immune or inflammatory responses (137, 138), including modulating Peroxisome Proliferation Activated Receptors (PPARs) (139) and suppressing cytokine production (140).
Conclusions
There is a substantial literature supporting the contribution of environmental exposures to health disparities in chronic lung diseases including asthma and COPD. Evidence indicates that low-SES and racially/ethnically diverse populations have elevated exposures to multiple stressors of concern, including, but not limited to, air pollution, SHS, pesticides, substandard housing, community violence, lack of green space, unhealthy diet, and chemicals in select consumer products. Genetic and other non-modifiable factors may compound the effects of these elevated environmental exposures. The root causes of these multifactorial exposures are complex, but many likely stem from economic forces and racial/ethnic and economic segregation that influence the home environment, neighborhood environment, and access to healthy foods and consumer products. Critical research needs include investigations that characterize exposure to and health implications of numerous stressors simultaneously, both to guard against potential confounding in epidemiological investigations and to consider the cumulative impact of multiple elevated environmental exposures and non-chemical stressors on health disparities.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Acknowledgments
JL and PF were supported by the National Institute on Minority Health and Health Disparities (NIMHD) and the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) [Award No. P50 MD010428]; the U.S. Environmental Protection Agency (EPA) [Award No. RD-836156]; and the NIEHS/NIH [Award No. R01 ES027816]. KB was supported by the NIEHS/NIH (T32 ES014562). NH was supported by the NIMHD and NIEHS (NIH) [Award No. P50 MD010431 and P50 ES018176]; the U.S. EPA [Award No. 83615001 and No. 83615201]; and the NIEHS/NIH [Award No. R01 ES022607 and R01 ES023500]. LQA was supported by a National Heart Lung and Blood Institute (NHLBI) Career Development Award (K01 HL138124).
This manuscript has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Contributor Information
Jonathan I. Levy, Department of Environmental Health, Boston University School of Public Health.
Lesliam Quirós-Alcalá, Maryland Institute for Applied Environmental Health, University of Maryland School of Public Health; Division of Pulmonary & Critical Care Medicine, Johns Hopkins University.
M. Patricia Fabian, Department of Environmental Health, Boston University School of Public Health.
Komal Basra, Department of Environmental Health, Boston University School of Public Health.
Nadia N. Hansel, Division of Pulmonary & Critical Care Medicine, Johns Hopkins University.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Schraufnagel DE, Blasi F, Kraft M, Gaga M, Finn PW, Rabe KF, et al. An official American Thoracic Society/European Respiratory Society policy statement: disparities in respiratory health. Am J Respir Crit Care Med. 2013;188(7):865–71. doi: 10.1164/rccm.201308-1509ST. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 3.Pleasants RA, Riley IL, Mannino DM. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2475–96. doi: 10.2147/COPD.S79077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosacz NM, Punturieri A, Croxton TL, Ndenecho MN, Kiley JP, Weinmann GG, et al. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention; 2012. Chronic Obstructive Pulmonary Disease Among Adults — United States, 2011; pp. 938–43. [PubMed] [Google Scholar]
- 5.National Institutes of Health. [Accessed February 27, 2018];Centers of Excellence on Environmental Health Disparities Research. 2014 Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-ES-14-010.html.
- 6.Adamkiewicz G, Zota AR, Fabian MP, Chahine T, Julien R, Spengler JD, et al. Moving environmental justice indoors: understanding structural influences on residential exposure patterns in low-income communities. Am J Public Health. 2011;101(Suppl 1):S238–45. doi: 10.2105/AJPH.2011.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(WHO) WHO. Tobacco Free Initiative: Second-hand tobacco smoke. 2018 Available at: http://www.who.int/tobacco/research/secondhand_smoke/en/
- 8.Jacobs DE. Environmental health disparities in housing. Am J Public Health. 2011;101(Suppl 1):S115–22. doi: 10.2105/AJPH.2010.300058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Report on the WHO technical meeting on quantifying disease from inadequate housing. Bonn, Germany: 2005. [Google Scholar]
- 10.Bradman ACJ, Tager I, Lipsett M, Sedgwick J, Macher J, Vargas AB, Cabrera EB, Camacho JM, Weldon R, Kogut K, Jewell NP, Eskenazi B. Association of housing disrepair indicators with cockroach and rodent infestations in a cohort of pregnant Latina women and their children. Environ Health Perspect. 2005;113(12):1795–801. doi: 10.1289/ehp.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Adamkiewicz G, Attfield KR, Kapp M, Spengler JD, Tao L, et al. Household pesticide contamination from indoor pest control applications in urban low-income public housing dwellings: a community-based participatory research. Environ Sci Technol. 2013;47(4):2018–25. doi: 10.1021/es303912n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiros-Alcala L, Bradman A, Nishioka M, Harnly ME, Hubbard A, McKone TE, et al. Pesticides in house dust from urban and farmworker households in California: an observational measurement study. Environ Health. 2011;10:19. doi: 10.1186/1476-069X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Abou El-Nour MM, Bennett GW. Survey of pest infestation, asthma, and allergy in low-income housing. J Community Health. 2008;33(1):31–9. doi: 10.1007/s10900-007-9064-6. [DOI] [PubMed] [Google Scholar]
- 14.Whyatt RMCD, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110:507–14. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reardon AM, Perzanowski MS, Whyatt RM, Chew GL, Perera FP, Miller RL. Associations between prenatal pesticide exposure and cough, wheeze, and IgE in early childhood. J Allergy Clin Immunol. 2009;124(4):852–4. doi: 10.1016/j.jaci.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandel M, Sheward R, Ettinger de Cuba S, Coleman SM, Frank DA, Chilton M, et al. Unstable housing and caregiver and child health in renter families. Pediatrics. 2018;141(2) doi: 10.1542/peds.2017-2199. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez D. Affording housing at the expense of health: Exploring the housing and neighborhood strategies of poor families. J Fam Issues. 2016;37(7):921–46. doi: 10.1177/0192513X14530970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allerg Asthma Im. 2015;115(5):396–U160. doi: 10.1016/j.anai.2015.08.005. Comprehensive systematic review that identified 25 studies linking secondhand smoke with asthma severity in children and determined significant associations with asthma hospitalizations, emergenency department visits, wheeze, and lung function. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedon JC. Risk and protective factors for childhood asthma: What is the evidence? J Allergy Clin Immunol Pract. 2016;4(6):1111–22. doi: 10.1016/j.jaip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4(11):e006554. doi: 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulin LM, Williams DL, Peng R, Diette GB, McCormack MC, Breysse P, et al. 24-h Nitrogen dioxide concentration is associated with cooking behaviors and an increase in rescue medication use in children with asthma. Environ Res. 2017;159:118–23. doi: 10.1016/j.envres.2017.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belanger K, Holford TR, Gent JF, Hill ME, Kezik JM, Leaderer BP. Household levels of nitrogen dioxide and pediatric asthma aeverity. Epidemiology. 2013;24(2):320–30. doi: 10.1097/EDE.0b013e318280e2ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Sbihi H, Koehoorn M, Tamburic L, Brauer M. Asthma trajectories in a population-based birth cohort. Impacts of air pollution and greenness. Am J Respir Crit Care Med. 2017;195(5):607–13. doi: 10.1164/rccm.201601-0164OC. Large birth cohort study with modeled exposure to multiple air pollutants and exposure to green space, with consideration of effects on various asthma phenotypes. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez AF, Parron T, Alarcon R. Pesticides and asthma. Curr Opin Allergy Clin Immunol. 2011;11(2):90–6. doi: 10.1097/ACI.0b013e3283445939. [DOI] [PubMed] [Google Scholar]
- 25.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med. 2002;165(5):683–9. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- 26.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L963–9. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 27.Salam MT, Li YF, Langholz B, Gilliland FD, Children’s Health S. Early-life environmental risk factors for asthma: findings from the Children’s Health Study. Environ Health Perspect. 2004;112(6):760–5. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salameh PBI, Brochard P, Raherison C, Abi Saleh B, Salamon R. Respiratory symptoms in children and exposure to pesticides. Eur Respir J. 2003;22:507–12. doi: 10.1183/09031936.03.00107403a. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Jung KH, Horton MK, Camann DE, Liu X, Reardon AM, et al. Prenatal exposure to pesticide ingredient piperonyl butoxide and childhood cough in an urban cohort. Environ Int. 2012;48:156–61. doi: 10.1016/j.envint.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Nembhard WN, Kan H, Becker A, Talbott EO. Residential pesticide use is associated with children’s respiratory symptoms. J Occup Environ Med. 2012;54(10):1281–7. doi: 10.1097/JOM.0b013e31825cb6ae. [DOI] [PubMed] [Google Scholar]
- 31.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112:751–3. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.06.040. Birth cohort with consideration of multi-stressor exposures prenatally and in early life for high-risk children, which showed increased asthma risk with prenatal tobacco smoke and higher maternal stress and depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SL, Miller GE, Brehm JM, Celedon JC. Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol. 2014;134(5):1009–15. doi: 10.1016/j.jaci.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok T, Kaptein AA, Brand PLP. Non-adherence in children with asthma reviewed: The need for improvement of asthma care and medical education. Ped Allergy Immunol. 2015;26(3):197–205. doi: 10.1111/pai.12362. [DOI] [PubMed] [Google Scholar]
- 35.Bellin MH, Land C, Newsome A, Kub J, Mudd SS, Bollinger ME, et al. Caregiver perception of asthma management of children in the context of poverty. J Asthma. 2017;54(2):162–72. doi: 10.1080/02770903.2016.1198375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, et al. Prenatal particulate matter exposure and wheeze in Mexican children: Effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119(3):232–7e1. doi: 10.1016/j.anai.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–6. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes HK, Matsui EC, Tschudy MM, Pollack CE, Keet CA. Pediatric asthma health disparities: Race, hardship, housing, and asthma in a national survey. Acad Pediatr. 2017;17(2):127–34. doi: 10.1016/j.acap.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colton MD, Laurent JGC, MacNaughton P, Kane J, Bennett-Fripp M, Spengler J, et al. Health benefits of green public housing: Associations with asthma morbidity and building-related symptoms. Am J Public Health. 2015;105(12):2482–9. doi: 10.2105/AJPH.2015.302793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chahine T, Subramanian SV, Levy JI. Sociodemographic and geographic variability in smoking in the U.S.: a multilevel analysis of the 2006–2007 Current Population Survey, Tobacco Use Supplement. Soc Sci Med. 2011;73(5):752–8. doi: 10.1016/j.socscimed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Hagstad S, Bjerg A, Ekerljung L, Backman H, Lindberg A, Ronmark E, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–304. doi: 10.1378/chest.13-1349. [DOI] [PubMed] [Google Scholar]
- 42.van Koeverden I, Blanc PD, Bowler RP, Arjomandi M. Secondhand tobacco smoke and COPD risk in smokers: A COPDGene study cohort subgroup analysis. COPD. 2015;12(2):182–9. doi: 10.3109/15412555.2014.922173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. COPD. 2016;13(3):372–9. doi: 10.3109/15412555.2015.1089846. Review article summarizing the evidence for the effects of air pollution and temperature on COPD, including consideration of COPD development and exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(10):1085–90. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375(9):871–8. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 46.Charkhchi P, Fazeli Dehkordy S, Carlos RC. Housing and food insecurity, care access, and health status among the chronically ill: An analysis of the Behavioral Risk Factor Surveillance System. J Gen Intern Med. 2018 doi: 10.1007/s11606-017-4255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.IOM. How Far Have We Come in Reducing Health Disparities?. Progress Since 2000: Workshop Summary. Institute of Medicine; 2012; [PubMed] [Google Scholar]
- 48.Miller M, Middendorf G, Wood SD. Food availability in the heartland: Exploring the effects of neighborhood racial and income composition. Rural Sociology. 2015;80(3):340–61. [Google Scholar]
- 49.Rodriguez D, Carlos HA, Adachi-Mejia AM, Berke EM, Sargent JD. Predictors of tobacco outlet density nationwide: a geographic analysis. Tobacco Control. 2013;22(5):349–55. doi: 10.1136/tobaccocontrol-2011-050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schleicher NC, Johnson TO, Fortmann SP, Henriksen L. Tobacco outlet density near home and school: Associations with smoking and norms among US teens. Preventive Medicine. 2016;91:287–93. doi: 10.1016/j.ypmed.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampson RJ, Morenoff JD, Raudenbush S. Social anatomy of racial and ethnic disparities in violence. Am J Pub Health. 2005;95(2):224–32. doi: 10.2105/AJPH.2004.037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salas-Wright CP, Nelson EJ, Vaughn MG, Gonzalez JMR, Córdova D. Trends in fighting and violence among adolescents in the United States, 2002–2014. Am J Pub Health. 2017;107(6):977–82. doi: 10.2105/AJPH.2017.303743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosofsky A, Levy JI, Zanobetti A, Janulewicz P, Fabian MP. Temporal trends in air pollution exposure inequality in Massachusetts. Environ Res. 2018;161:76–86. doi: 10.1016/j.envres.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark LP, Millet DB, Marshall JD. Changes in transportation-related air pollution exposures by race-ethnicity and socioeconomic status: Outdoor nitrogen dioxide in the United States in 2000 and 2010. Environ Health Perspect. 2017;125(9):097012. doi: 10.1289/EHP959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su JG, Jerrett M, de Nazelle A, Wolch J. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ Res. 2011;111(3):319–28. doi: 10.1016/j.envres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Casey JA, Morello-Frosch R, Mennitt DJ, Fristrup K, Ogburn EL, James P. Race/Ethnicity, Socioeconomic status, residential segregation, and spatial variation in noise exposure in the contiguous United States. Environ Health Perspect. 2017;125(7):077017. doi: 10.1289/EHP898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jennings V, Baptiste A, Osborne Jelks NT, Skeete R. Urban green space and the pursuit of health equity in parts of the United States. Int J Environ Res Pub Health. 2017;14(11):1432. doi: 10.3390/ijerph14111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogar S, Beyer KM. Green space, violence, and crime: A systematic review. Trauma, Violence, & Abuse. 2016;17(2):160–71. doi: 10.1177/1524838015576412. [DOI] [PubMed] [Google Scholar]
- 59.Han B, Cohen DA, Derose KP, Li J, Williamson S. Violent crime and park use in low-income urban neighborhoods. Am J Preventive Med. 54(3):352–8. doi: 10.1016/j.amepre.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vangeepuram N, Galvez MP, Teitelbaum SL, Brenner B, Wolff MS. The association between parental perception of neighborhood safety and asthma diagnosis in ethnic minority urban children. J Urban Health. 2012;89(5):758–68. doi: 10.1007/s11524-012-9679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramratnam SK, Han YY, Rosas-Salazar C, Forno E, Brehm JM, Rosser F, et al. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109(8):975–81. doi: 10.1016/j.rmed.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas-Salazar C, Han YY, Brehm JM, Forno E, Acosta-Perez E, Cloutier MM, et al. Gun violence, African ancestry, and asthma: A case-control study in Puerto Rican children. Chest. 2016;149(6):1436–44. doi: 10.1016/j.chest.2016.02.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eldeirawi K, Kunzweiler C, Rosenberg N, Riley B, Gao Y, Hebert-Beirne J, et al. Association of neighborhood crime with asthma and asthma morbidity among Mexican American children in Chicago, Illinois. Annals of Allergy, Asthma & Immunology. 117(5):502–7.e1. doi: 10.1016/j.anai.2016.09.429. [DOI] [PubMed] [Google Scholar]
- 64.Shmool JL, Kubzansky LD, Dotson Newman O, Spengler J, Shepard P, Clougherty JE. Social stressors and air pollution across New York City communities: a spatial approach for assessing correlations among multiple exposures. Environ Health. 2014;13(1):91. doi: 10.1186/1476-069X-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck AF, Huang B, Ryan PH, Sandel MT, Chen C, Kahn RS. Areas with high rates of police-reported violent crime have higher rates of childhood asthma morbidity. J Pediatr. 2016;173:175–82e1. doi: 10.1016/j.jpeds.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koinis-Mitchell D, Kopel SJ, Salcedo L, McCue C, McQuaid EL. Asthma indicators and neighborhood and family stressors related to urban living in children. Am J Health Behavior. 2014;38(1):22–30. doi: 10.5993/AJHB.38.1.3. [DOI] [PubMed] [Google Scholar]
- 67.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176(7):644–9. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Wright AW, Austin M, Booth C, Kliewer W. Systematic review: Exposure to community violence and physical health outcomes in youth. Journal of Pediatric Psychology. 2017;42(4):364–78. doi: 10.1093/jpepsy/jsw088. Recent review article summarizing the association between exposure to community violence and asthma or respiratory health in nine studies, of which six found significant associations. [DOI] [PubMed] [Google Scholar]
- 69.Markevych I, Schoierer J, Hartig T, Chudnovsky A, Hystad P, Dzhambov AM, et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ Res. 2017;158:301–17. doi: 10.1016/j.envres.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 70.Alcock I, White M, Cherrie M, Wheeler B, Taylor J, McInnes R, et al. Land cover and air pollution are associated with asthma hospitalisations: A cross-sectional study. Environ Int. 2017;109:29–41. doi: 10.1016/j.envint.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Tischer C, Gascon M, Fernandez-Somoano A, Tardon A, Lertxundi Materola A, Ibarluzea J, et al. Urban green and grey space in relation to respiratory health in children. Eur Respir J. 2017;49(6):1502112. doi: 10.1183/13993003.02112-2015. [DOI] [PubMed] [Google Scholar]
- 72.Feng X, Astell-Burt T. Is neighborhood green space protective against associations between child asthma, neighborhood traffic volume and perceived lack of area safety? Multilevel analysis of 4447 Australian children. Int J Environ Res Pub Health. 2017;14(5):543. doi: 10.3390/ijerph14050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vienneau D, de Hoogh K, Faeh D, Kaufmann M, Wunderli JM, Röösli M. More than clean air and tranquillity: Residential green is independently associated with decreasing mortality. Environ Int. 2017;108:176–84. doi: 10.1016/j.envint.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Gascon M, Triguero-Mas M, Martínez D, Dadvand P, Rojas-Rueda D, Plasència A, et al. Residential green spaces and mortality: A systematic review. Environ Int. 2016;86:60–7. doi: 10.1016/j.envint.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Ulmer JM, Wolf KL, Backman DR, Tretheway RL, Blain CJA, O’Neil-Dunne JPM, et al. Multiple health benefits of urban tree canopy: The mounting evidence for a green prescription. Health & Place. 2016;42:54–62. doi: 10.1016/j.healthplace.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 76•.Lambert KA, Bowatte G, Tham R, Lodge C, Prendergast L, Heinrich J, et al. Residential greenness and allergic respiratory diseases in children and adolescents – A systematic review and meta-analysis. Environ Res. 2017;159:212–21. doi: 10.1016/j.envres.2017.08.002. Recent meta-analysis reporting inconsistent results on the association between residential greenness and allergic respiratory disease in 11 studies. [DOI] [PubMed] [Google Scholar]
- 77•.Fong KC, Hart JE, James P. A review of epidemiologic studies on greenness and health: Updated literature through 2017. Curr Environ Health Rpt. 2018 doi: 10.1007/s40572-018-0179-y. Recent update on epidemiology literature linking greenness to health. Authors concluded that relationships between greenness and asthma were inconsistent, likely due to the variable way in which greenness is characterised. [DOI] [PMC free article] [PubMed]
- 78•.Rodriguez-Villamizar LA, Berney C, Villa-Roel C, Ospina MB, Osornio-Vargas A, Rowe BH. The role of socioeconomic position as an effect-modifier of the association between outdoor air pollution and children’s asthma exacerbations: an equity-focused systematic review. Rev Environ Health. 2016;31(3):297–309. doi: 10.1515/reveh-2016-0005. Recent review article concluding there is weak evidence for socioeconomic position as an effect modifier of the association between air pollution and asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 79.O’Lenick CR, Winquist A, Mulholland JA, Friberg MD, Chang HH, Kramer MR, et al. Assessment of neighbourhood-level socioeconomic status as a modifier of air pollution-asthma associations among children in Atlanta. J Epi Comm Health. 2017;71(2):129–36. doi: 10.1136/jech-2015-206530. [DOI] [PubMed] [Google Scholar]
- 80.O’ Lenick CR, Chang HH, Kramer MR, Winquist A, Mulholland JA, Friberg MD, et al. Ozone and childhood respiratory disease in three US cities: evaluation of effect measure modification by neighborhood socioeconomic status using a Bayesian hierarchical approach. Environ Health. 2017;16(1):36. doi: 10.1186/s12940-017-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(Suppl 3):S174–84. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82••.Alexander D, Currie J. Is it who you are or where you live? Residential segregation and racial gaps in childhood asthma. J Health Econ. 2017;55:186–200. doi: 10.1016/j.jhealeco.2017.07.003. Large cohort study and only study found examining residential segregation, a distal social determinant of health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gwynn RC, Thurston GD. The burden of air pollution: impacts among racial minorities. Environ Health Perspect. 2001;109(Suppl 4):501–6. doi: 10.1289/ehp.01109s4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pino-Yanes M, Thakur N, Gignoux CR, Galanter JM, Roth LA, Eng C, et al. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol. 2015;135(1):228–35. doi: 10.1016/j.jaci.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parker MM, Foreman MG, Abel HJ, Mathias RA, Hetmanski JB, Crapo JD, et al. Admixture mapping identifies a quantitative trait locus associated with FEV1/FVC in the COPDGene Study. Genet Epidemiol. 2014;38(7):652–9. doi: 10.1002/gepi.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–92. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flores C, Ma SF, Pino-Yanes M, Wade MS, Perez-Mendez L, Kittles RA, et al. African ancestry is associated with asthma risk in African Americans. PLoS One. 2012;7(1):e26807. doi: 10.1371/journal.pone.0026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88••.Vonk JM, Scholtens S, Postma DS, Moffatt MF, Jarvis D, Ramasamy A, et al. Adult onset asthma and interaction between genes and active tobacco smoking: The GABRIEL consortium. PLoS One. 2017;12(3):e0172716. doi: 10.1371/journal.pone.0172716. Most recent comprehensive meta-analysis on genome-wide interactions in six large studies; study also identified novel polymorphism, showing suggestive evidence for interaction with active tobacco smoking in the onset of adult asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hancock DB, Soler Artigas M, Gharib SA, Henry A, Manichaikul A, Ramasamy A, et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet. 2012;8(12):e1003098. doi: 10.1371/journal.pgen.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castaldi PJ, Demeo DL, Hersh CP, Lomas DA, Soerheim IC, Gulsvik A, et al. Impact of non-linear smoking effects on the identification of gene-by-smoking interactions in COPD genetics studies. Thorax. 2011;66(10):903–9. doi: 10.1136/thx.2010.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59(1):8–10. [PMC free article] [PubMed] [Google Scholar]
- 92••.Gref A, Merid SK, Gruzieva O, Ballereau S, Becker A, Bellander T, et al. Genome-wide interaction analysis of air pollution exposure and childhood asthma with functional follow-up. Am J Respir Crit Care Med. 2017;195(10):1373–83. doi: 10.1164/rccm.201605-1026OC. Most recent comprehensive meta-analysis on genome-wide interactions of air pollution and pediatric asthma; study results indicate that gene-environment interactions are important for asthma development and provide supporting evidence for interaction with air pollution and select SNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreno-Macias H, Dockery DW, Schwartz J, Gold DR, Laird NM, Sienra-Monge JJ, et al. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: a cohort study. Respir Res. 2013;14:14. doi: 10.1186/1465-9921-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson-Frederick SM, Thorpe RJ, Jr, Bell CN, Bleich SN, Ford JG, LaVeist TA. Examination of race disparities in physical inactivity among adults of similar social context. Ethn Dis. 2014;24(3):363–9. [PMC free article] [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention. Prevalence of regular physical activity among adults--United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56(46):1209–12. [PubMed] [Google Scholar]
- 96.Crespo CJ, Smit E, Andersen RE, Carter-Pokras O, Ainsworth BE. Race/ethnicity, social class and their relation to physical inactivity during leisure time: results from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Prev Med. 2000;18(1):46–53. doi: 10.1016/s0749-3797(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 97.Marshall SJ, Jones DA, Ainsworth BE, Reis JP, Levy SS, Macera CA. Race/ethnicity, social class, and leisure-time physical inactivity. Med Sci Sports Exerc. 2007;39(1):44–51. doi: 10.1249/01.mss.0000239401.16381.37. [DOI] [PubMed] [Google Scholar]
- 98.Kong A, Schiffer L, Antonic M, Braunschweig C, Odoms-Young A, Fitzgibbon M. The relationship between home- and individual-level diet quality among African American and Hispanic/Latino households with young children. Int J Behav Nutr Phys Act. 2018;15(1):5. doi: 10.1186/s12966-018-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99•.Gu X, Tucker KL. Dietary quality of the US child and adolescent population: trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am J Clin Nutr. 2017;105(1):194–202. doi: 10.3945/ajcn.116.135095. This recent article highlights the fact that vulnerable populations, including racial/ethnically diverse populations and low-income individuals, have poorer dietary quality especially compared to other populations. [DOI] [PubMed] [Google Scholar]
- 100.Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112(5):624–35e6. doi: 10.1016/j.jand.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Noia J, Schinke SP, Contento IR. Dietary fat intake among urban, African American adolescents. Eat Behav. 2008;9(2):251–6. doi: 10.1016/j.eatbeh.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Jahns L, Tussing-Humphreys L, Xie B, Rockett H, Liang H, et al. Dietary intake patterns of low-income urban african-american adolescents. J Am Diet Assoc. 2010;110(9):1340–5. doi: 10.1016/j.jada.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104•.James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, et al. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J Expo Sci Environ Epidemiol. 2017;27(2):160–6. doi: 10.1038/jes.2016.2. Article highlights racial/ethnic disparites in exposures to phthlates among pregnant women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res. 2012;115:11–7. doi: 10.1016/j.envres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–35. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 107.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174(2):112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108•.Baffi CW, Winnica DE, Holguin F. Asthma and obesity: mechanisms and clinical implications. Asthma Res Pract. 2015;1:1. doi: 10.1186/s40733-015-0001-7. This review provides a succinct summary of obesity-related mechanisms and the clinical impact on asthma including highlights on recent progress in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. Obesity Is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCormack MC, Belli AJ, Kaji DA, Matsui EC, Brigham EP, Peng RD, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J. 2015;45(5):1248–57. doi: 10.1183/09031936.00081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams DL, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017–23. 23 e1–3. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brigham EP, Kolahdooz F, Hansel N, Breysse PN, Davis M, Sharma S, et al. Association between Western diet pattern and adult asthma: a focused review. Ann Allergy Asthma Immunol. 2015;114(4):273–80. doi: 10.1016/j.anai.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barros R, Moreira A, Fonseca J, de Oliveira JF, Delgado L, Castel-Branco MG, et al. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy. 2008;63(7):917–23. doi: 10.1111/j.1398-9995.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 114.Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–21. doi: 10.2147/JAA.S49960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–33e1. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Garcia-Marcos L, Castro-Rodriguez JA, Weinmayr G, Panagiotakos DB, Priftis KN, Nagel G. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24(4):330–8. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 117.Wickens K, Barry D, Friezema A, Rhodius R, Bone N, Purdie G, et al. Fast foods - are they a risk factor for asthma? Allergy. 2005;60(12):1537–41. doi: 10.1111/j.1398-9995.2005.00945.x. [DOI] [PubMed] [Google Scholar]
- 118.Patel S, Custovic A, Smith JA, Simpson A, Kerry G, Murray CS. Cross-sectional association of dietary patterns with asthma and atopic sensitization in childhood - in a cohort study. Pediatr Allergy Immunol. 2014;25(6):565–71. doi: 10.1111/pai.12276. [DOI] [PubMed] [Google Scholar]
- 119.Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. 2015;45(1):177–83. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wood LG, Gibson PG. Dietary factors lead to innate immune activation in asthma. Pharmacol Ther. 2009;123(1):37–53. doi: 10.1016/j.pharmthera.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 122.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Texcalac-Sangrador JL, Hernandez-Cadena L, Diaz-Sanchez D, et al. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir Res. 2009;10:122. doi: 10.1186/1465-9921-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123••.Spanier AJ, Fausnight T, Camacho TF, Braun JM. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014;35(6):475–81. doi: 10.2500/aap.2014.35.3803. One of the most recent studies linking allergic sensitization and exposure to chemicals in personal care products (triclosan and parabens) using data from a large representative pediatric sample from the US general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130(2):453–60e7. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee-Sarwar K, Hauser R, Calafat AM, Ye X, O’Connor GT, Sandel M, et al. Prenatal and early-life triclosan and paraben exposure and allergic outcomes. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Just AC, Whyatt RM, Miller RL, Rundle AG, Chen Q, Calafat AM, et al. Children’s urinary phthalate metabolites and fractional exhaled nitric oxide in an urban cohort. Am J Respir Crit Care Med. 2012;186(9):830–7. doi: 10.1164/rccm.201203-0398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children’s health. Curr Opin Pediatr. 2013;25(2):247–54. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soomro MH, Baiz N, Philippat C, Vernet C, Siroux V, Nichole Maesano C, et al. Prenatal exposure to phthalates and the development of eczema phenotypes in male children: Results from the EDEN mother-child cohort study. Environ Health Perspect. 2018;126(2):027002. doi: 10.1289/EHP1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jahreis S, Trump S, Bauer M, Bauer T, Thurmann L, Feltens R, et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J Allergy Clin Immunol. 2018;141(2):741–53. doi: 10.1016/j.jaci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 130.Herberth G, Pierzchalski A, Feltens R, Bauer M, Roder S, Olek S, et al. Prenatal phthalate exposure associates with low regulatory T-cell numbers and atopic dermatitis in early childhood: Results from the LINA mother-child study. J Allergy Clin Immunol. 2017;139(4):1376–9. e8. doi: 10.1016/j.jaci.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 131.Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gomez A, Luque N, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol. 2015;135(2):370–8. doi: 10.1016/j.jaci.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 132.Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a Taiwanese birth cohort. PLoS One. 2015;10(4):e0123309. doi: 10.1371/journal.pone.0123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stelmach I, Majak P, Jerzynska J, Podlecka D, Stelmach W, Polanska K, et al. The effect of prenatal exposure to phthalates on food allergy and early eczema in inner-city children. Allergy Asthma Proc. 2015;36(4):72–8. doi: 10.2500/aap.2015.36.3867. [DOI] [PubMed] [Google Scholar]
- 134.Just AC, Whyatt RM, Perzanowski MS, Calafat AM, Perera FP, Goldstein IF, et al. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ Health Perspect. 2012;120(10):1475–80. doi: 10.1289/ehp.1104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vernet C, Pin I, Giorgis-Allemand L, Philippat C, Benmerad M, Quentin J, et al. In utero exposure to select phenols and phthalates and respiratory health in five-year-old boys: A prospective study. Environ Health Perspect. 2017;125(9):097006. doi: 10.1289/EHP1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang IJ, Lin CC, Lin YJ, Hsieh WS, Chen PC. Early life phthalate exposure and atopic disorders in children: a prospective birth cohort study. Environ Int. 2014;62:48–54. doi: 10.1016/j.envint.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 137••.Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep. 2015;2(4):379–87. doi: 10.1007/s40572-015-0066-8. One of the most recent reviews on the role of chemicals in consumer products (BPA and phthalates) on allergy, asthma, and immune function which also includes potential mechanisms of action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kwak ES, Just A, Whyatt R, Miller RL. Phthalates, pesticides, and bisphenol-A exposure and the development of nonoccupational asthma and allergies: How valid are the links? Open Allergy J. 2009;2:45–50. doi: 10.2174/1874838400902010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kocbach Bolling A, Holme JA, Bornehag CG, Nygaard UC, Bertelsen RJ, Nanberg E, et al. Pulmonary phthalate exposure and asthma - is PPAR a plausible mechanistic link? EXCLI J. 2013;12:733–59. [PMC free article] [PubMed] [Google Scholar]
- 140.Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol In Vitro. 2004;18(3):265–9. doi: 10.1016/j.tiv.2003.09.008. [DOI] [PubMed] [Google Scholar]