Abstract
Introduction
Lack of effective pharmacological treatment makes valvular calcification a significant clinical problem in patients with valvular disease and bioprosthetic/mechanical valve replacement therapies. Elevated levels of reactive oxygen species (ROS) in valve tissue have been identified as a prominent hallmark and driving factor for valvular calcification. However, the therapeutic value of ROS-modulating agents for valvular calcification remains elusive. We hypothesized that ROS-modulating shape-specific cerium oxide nanoparticles (CNPs) will inhibit oxidative stress-induced valvular calcification. CNPs are a class of self-regenerative ROS-modulating agents, which can switch between Ce3+ and Ce4+ in response to oxidative microenvironment. In this work, we developed oxidative stress-induced valve calcification model using two patient-derived stenotic valve interstitial cells (hVICs) and investigated the therapeutic effect of shape-specific CNPs to inhibit hVIC calcification.
Methods
Human valvular interstitial cells (hVICs) were obtained from a normal healthy donor and two patients with calcified aortic valves. hVICs were characterized for their phenotypic (mesenchymal, myofibroblast and osteoblast) marker expression by qRT-PCR and antioxidant enzymes activity before and after exposure to hydrogen peroxide (H2O2)-induced oxidative stress. Four shape-specific CNPs (sphere, short rod, long rod, and cube) were synthesized via hydrothermal or ultra-sonication method and characterized for their biocompatibility in hVICs by alamarBlue® assay, and ROS scavenging ability by DCFH-DA assay. H2O2 and inorganic phosphate (Pi) were co-administrated to induce hVIC calcification in vitro as demonstrated by Alizarin Red S staining and calcium quantification. The effect of CNPs on inhibiting H2O2-induced hVIC calcification was evaluated.
Results
hVICs isolated from calcified valves exhibited elevated osteoblast marker expression and decreased antioxidant enzyme activities compared to the normal hVICs. Due to the impaired antioxidant enzyme activities, acute H2O2-induced oxidative stress resulted in higher ROS levels and osteoblast marker expression in both diseased hVICs when compared to the normal hVICs. Shape-specific CNPs exhibited shape-dependent abiotic ROS scavenging ability, and excellent cytocompatibility. Rod and sphere CNPs scavenged H2O2-induced oxidative stress in hVICs in a shape- and dose-dependent manner by lowering intracellular ROS levels and osteoblast marker expression. Further, CNPs also enhanced activity of antioxidant enzymes in hVICs to combat oxidative stress. Cube CNPs were not effective ROS scavengers. The addition of H2O2 in the Pi-induced calcification model further increased calcium deposition in vitro in a time-dependent manner. Co-administration of rod CNPs with Pi and H2O2 mitigated calcification in the diseased hVICs.
Conclusions
We demonstrated that hVICs derived from calcified valves exhibited impaired antioxidant defense mechanisms and were more susceptible to oxidative stress than normal hVICs. CNPs scavenged H2O2-induced oxidative stress in hVICs in a shape-dependent manner. The intrinsic ROS scavenging ability of CNPs and their ability to induce cellular antioxidant enzyme activities may confer protection from oxidative stress-exacerbated calcification. CNPs represent promising antioxidant therapy for treating valvular calcification and deserve further investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s12195-017-0495-6) contains supplementary material, which is available to authorized users.
Keywords: Nanoceria, Reactive oxygen species (ROS), Valve calcification, Patient-derived valvular interstitial cells (hVICs), Cerium oxide nanoparticle, Nanoparticle shape
Introduction
Valve calcification remains a major healthcare burden in developed countries, resulting in ~17,000 deaths per year in the United States and currently affecting a quarter of the senior population older than 65.1 Calcification of the aortic valve, if not treated properly, can impair the normal valve movement and eventually lead to valve stenosis and heart failure.2 Currently, the mainstream treatment option for valve calcification is valve replacement using either mechanical valves or bioprostheses, the former requires life-long anticoagulant therapy and the latter is still unfortunately susceptible to calcification and also need re-operation.3,4
There is a lack of pharmacological treatments for valve calcification.5 Therefore, it is of great significance to elucidate the underlying mechanisms responsible for valve calcification, which can further be targeted for development of new therapies to prevent, halt, or reverse calcification.6 Potential mechanisms contributing to valve calcification include inflammatory cell infiltration,7 lipid accumulation,8 oxidative stress,9,10 and local mechanical stress,11 all of which initiate an active cell-driven process that drives valve interstitial cells (VICs) to acquire myofibroblast phenotype and/or to differentiate into osteoblast phenotype.12 As a result, excessive amounts of extracellular matrix proteins and calcium phosphate are secreted, which stiffen the valve and compromise its opening and closing.13 Of the different mechanisms of valve calcification, oxidative stress has been recognized as a key upstream driving force in the early stages of the disease.10 Increased reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2) levels were found in the calcified regions of human stenotic valve tissues.14 Moreover, instead of being merely a consequence of valve calcification progression, ROS production was found to precede the valve calcification in a mice model.15 ROS have also been shown to induce fibrosis and mineralization by promoting pro-fibrotic and pro-osteogenic signaling pathways such as AKT pathway and TGF-β pathway.16,17 Furthermore, adenoviral transduction of catalase (CAT), a key antioxidant enzyme, decreased oxidative stress-induced differentiation of quiescent or myofibroblastic VICs into osteogenic phenotype and reduced calcific nodule deposition in human valve sclerosis-derived cells.18 However, the benefits of antioxidant therapies, which directly target oxidative stress to treat valve calcification, are yet to be studied.
It should be noted that much of the current understanding of valve calcification is based on in vitro studies using either porcine or ovine models.19 However, these animal models may not fully share the pathobiology of human valve calcification. It was reported that porcine VICs did not form mineral deposits when treated with osteogenic media with TGF-β120 and became contact-inhibited monolayers, which behave unstably during long-term cell culture.21 Other studies have utilized non-valvular cells, such as vascular smooth muscle cells, as a cell model to study the calcification progression and explore potential therapeutics.22 Although oxidative stress is a central component in directing both valvular and vascular calcification,23 there are striking differences in the mechanisms of inducing oxidative stress in these two types of calcifications.9 Specifically, increased expression and activity of NAD(P)H oxidase is a major source of ROS production in vascular, but not valvular, calcification.24 Additionally, when blood vessels are under oxidative stress, there is usually a compensatory increase in the activity of antioxidant enzymes (e.g. catalase, CAT and superoxide dismutase, SOD).25 However, in calcified valves, the activity of these antioxidant enzymes were found to be greatly reduced, which further exacerbate the oxidative stress-induced damage.14 Therefore, use of human (normal or patient-derived) VICs (hVICs) (where calcific deposits initiate26) is of significant importance to delineate the underlying ROS-mediated mechanisms of initiation and progression of human valve calcification. Furthermore, hVICs-based cell model will be more physiologically relevant for screening potential therapeutics in vitro. In this study, the first goal was to determine if the hydrogen peroxide (H2O2)-mediated oxidative stress could induce calcification in hVICs. We isolated hVICs from normal valve donor and patients with aortic valve stenosis. We further characterized normal and diseased hVICs for baseline SOD and CAT activities, mRNA expression levels of myofibroblast and calcification markers, as well as their responses to H2O2-mediated oxidative stress. We also developed an in vitro oxidative stress-induced valve calcification model whereby treatment of inorganic phosphate (Pi) in presence of H2O2 induced calcification in diseased hVICs.
The second goal of the study was to investigate the potential of cerium oxide nanoparticles (CNPs) to mitigate oxidative stress-mediated valve calcification as a novel therapeutic intervention. CNPs act as self-regenerative ROS scavengers due to their catalytic activities by switching between Ce3+ and Ce4+ oxidation states.27 CNPs can scavenge ROS such as H2O2 and superoxide by acting as CAT and SOD-mimetics, respectively.28–30 CNPs are also effective scavengers for hydroxyl radical, nitric oxide radical, and peroxynitrite, making them broad spectrum antioxidants.27,28 CNPs have already been explored for several different cardiovascular applications as demonstrated in several studies in vitro 31,32 and in vivo.33,34 Thus, we hypothesized that ROS scavenging ability of CNPs can be exploited for preventing or treating oxidative stress-induced valve calcification.
Metal oxide nanoparticles like CNPs can be synthesized in various shapes and sizes using controlled synthesis conditions.35 However, most of the studies that use CNPs as antioxidants have been carried out without specifying nanoparticle shape. It is well known that nanoparticle shapes and sizes can have significant effect on their biological activities, such as cellular uptake, intracellular localization,36 and therapeutic activity.37 However, the effect of CNP shape on their ROS-scavenging activity has not been elucidated. Hence, we synthesized CNPs with controlled shapes such as spheres, short rods (SR), long rods (LR) and cubes. We compared the ROS modulating effect of shape-specific CNPs in diseased hVICs and investigated their potential therapeutic benefits in oxidative stress-induced valve calcification model in vitro.
Experimental
Collection of Human Tissue Samples and Isolation and Culture of Human Valvular Interstitial Cells (hVICs)
We used de-identified human valvular tissues recovered at University of Pittsburgh Medical Center (UPMC) as per protocols approved by the University of Pittsburgh Institutional Review Board, PRO16050264 (St. Hilaire) and PRO0702120 with informed consent (Dr. Gleason). The subjects or their designates have given permission for the use of this tissue for medical research. Tissue and cell specimens do not have identifiers associated with them in order to protect human subjects’ identifiable information, privacy and confidentiality. Tissues and cells were collected regardless of gender, race or ethnic composition. To collect control tissue samples from autopsy, we have a Committee for Oversight of Research and Clinical Training Involving Decedents (CORID) protocol #729.
Human aortic valve cells were isolated from a healthy donor with normal valve (referred to as ‘normal’) and two patients with pre-operative diagnosis of valve stenosis (referred to as ‘Diseased-1’ and ‘Diseased-2’). These hVICs were a kind gift from Dr. Thomas Gleason lab. Valve tissues were digested in Collagenase IV (2.5 mg/mL) with DMEM (+1% Penicillin/Streptomycin +1% Fungizone) for 30 min at 37 °C with gentle rocking. Digested tissue was passed through 70 µm filter and saved at 37 °C. Undigested tissue was further digested with Collagenase IV (0.8 mg/mL) for 1 h at 37 °C with gentle rocking. Digested tissue was again passed through 70 µm filter and pooled with the previously digested tissue. The pooled digested tissue was centrifuged at 2000 rpm for 5 min at 4 °C and then the supernatant were discarded. CD31-negative cells (VICs) were separated from the total cell population by bead separation (Miltenyi, USA). Specifically, the entire population of VICs were mixed with Fc Blocking Reagent (Miltenyi 130-091-935) followed by addition of anti-CD31 beads (Miltenyi 130-091-935). After 15 min incubation at 4 °C, the mixture was passed through a 30 µm pre-separation filter (Miltenyi 130-041-407) into a 25 MS MACS Separation Column (Miltenyi 130-042-201) and washed three times with culture medium. The resulting flow-through was retained as the CD31-negative portion of the cell population. The normal hVICs were propagated and cultured using DMEM supplemented with 10% fetal bovine serum according to established protocol.38 Both diseased hVICs were propagated and cultured using Endothelial Cell Growth Media Kit (referred to as ‘hVIC culture media’) (Cell Applications, San Diego, CA) unless mentioned otherwise. All the experiments were carried out by using cells between passage 4 and 8.
Von Kossa Histological Stain
Valves were excised from a healthy control (normal) and a patient with calcified valves (diseased). Tissue sections were fixed in freshly prepared paraformaldehyde (PFA, 4% w/v) in PBS for 2–4 h then washed with PBS for 1–2 h. Tissues were then serially washed in 30, 50, and 70% ethanol for 1–2 h each wash, then submitted to the University of Pittsburgh pathology core for embedding and sectioning onto glass slides. For Von Kossa staining, slides were heated to 65 °C for 1 h, then deparaffinized by two 30 min washes in 100% xylene, followed by 3 min washes in 100% (twice), then one each of 90, 80, 70, and 50% ethanol, followed by deionized water. Slides were then immersed in silver nitrate (3%, w/v) and exposed to UV light for 30 min, rinsed 3× in distilled water, then immersed in sodium thiosulfate (5%, w/v), rinsed in 3x in distilled water, and finally, counter stained with nuclear fast red for 5 min. Images were acquired using the EVOS® XL Imaging system.
Immunostaining of CD31 and α-Smooth Muscle Actin (α-SMA)
hVICs (50,000 cells/well) were cultured in 24-well plates for 2 days and fixed with PFA (4% w/v) for 20 min at room temperature. Subsequently, cell monolayers were permeabilized with Triton X-100 (0.1%, v/v) followed by blocking with bovine serum albumin (BSA, 3%, w/v) to avoid non-specific binding. Cell monolayers were incubated with primary antibodies against CD31 (Dako, pre-diluted) and α-smooth muscle actin (α-SMA; Dako, 1:200) diluted in blocking buffer for 2 h at room temperature along with a negative control (without primary antibody). After washing thrice with PBS, hVICs were incubated with Alexa Fluor 488-conjugated secondary antibodies (1:100) for 1 h at room temperature. Nuclei were counterstained with Hoechst (1:200). Images were acquired on a confocal microscope (Olympus Fluoview, Olympus) as a z-stack containing a series of 5 µm slices using 20X or 40X objectives.
Superoxide Dismutase (SOD) and Catalase (CAT) Activity
SOD and CAT activity of normal and diseased hVICs were determined by SOD activity assay kit (Epigentek) and CAT activity assay kit (Cayman Chemical), respectively. Briefly, cell lysates were prepared by addition of radioimmunoprecipitation (RIPA) assay buffer containing Tris (50 mM, pH 7.4), NaCl (150 mM), sodium deoxycholate (0.5% w/v), NP-40 (1% w/w), phenylmethane sulfonyl fluoride (PMSF, 0.05 mM) and protease inhibitor cocktail to the harvested cells on ice. The enzyme activity was measured in the extracted cell lysates according to the manufacturers’ protocols. The enzyme activity was normalized to the total protein content, which was quantified by microbicinchoninic acid (BCA) protein assay (Pierce BCA, Thermo Scientific) according to the manufacturer’s protocol.
Analysis of mRNA Expression in hVICs by Quantitative Polymerase Chain Reaction (qPCR)
mRNA expression of Vimentin (VIM), α-SMA and Runt-related transcription factor 2 (RUNX-2) and Osteopontin (OPN) were measured using real time quantitative polymerase chain reaction (qPCR). RNA was isolated using GeneJET RNA purification kit (Thermo Scientific, Lithuania, EU) according to the manufacturer’s protocol. RNA quality was measured by absorbance ratio at 260/280 nm. Expression of VIM, α-SMA, OPN and RUNX-2 were analyzed by qRT-PCR using iTaq Universal SYBR one-step RT-PCR kit (BioRad Laboratories Inc., USA). Briefly, reaction mixtures containing 20 ng mRNA, 5 µL of 2X SYBR green master mix and respective primers (0.2 µM) were amplified in triplicate samples using an amplification protocol of 40 cycles of denaturation for 15 s at 95 °C, annealing for 30 s at 55 °C and extension for 30 s at 72 °C (7500 Fast Real-Time PCR System, Applied Biosystems, USA). Gene expression was quantified using the ∆∆CT algorithm. Gene expressions in normal hVICs or respective untreated control of each patient cells served as a control for calculating fold change in mRNA expression as specified in each figure. Primer sequences are provided in Table S1.
Intracellular ROS Detection in hVICs by DCFH-DA Assay
The intracellular ROS levels in hVICs were measured by 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) assay (Cayman chemical, USA). For DCFH-DA detection, media was removed and DCFH-DA (20 μM, 100 μL) in Hank’s Balanced Salt Solution (HBSS) were added to each well and incubated for 30 min at 37 °C. DCFH-DA was then removed and cells were washed with HBSS. Subsequently, different treatments were given as described below and fluorescence intensity was measured at excitation/emission wavelength of 485/528 nm using a microplate reader (Synergy HT, BioTek instruments, USA). To ensure that the change in the fluorescence intensity was not due to the differences in the cell density or cell death, we adopted uniform cell seeding density and have selected the dose of H2O2 and CNPs that didn’t induce significant cell death.
Acute and Long-Term Exposure of hVICs to H2O2
To determine the acute effect of H2O2 on hVIC behaviors, hVICs were seeded in a 96-well plate (9000 cells/well) or a 24-well plate (100,000 cells/well) and allowed to attach for 24 h. Subsequently, hVICs were exposed to media containing H2O2 (100 μM, 100 μL) for 24 h. Cells were then subjected to ROS detection by DCFH-DA assay as described above. % ROS level was calculated using the equation below. Cell lysates and mRNA were analyzed for SOD/CAT activity and expression of VIM, α-SMA, OPN and RUNX-2, respectively. Cells without H2O2 treatment were used as negative control.
To determine the long-term effect of H2O2 on hVIC behavior, hVICs were seeded in a 24-well plate (100,000 cells/well) and allowed to attach for 24 h. Subsequently, hVICs were exposed to media containing H2O2 (100 μM, 100 μL) for up to 14 days with change of media (containing H2O2) every day. Calcium content of cell monolayers were analyzed at day 3, 7 and 14. Cell lysates were analyzed for SOD/CAT activity on day 14. Cells without H2O2 treatment were used as negative control.
Synthesis of Shape-Specific CNPs
CNPs prepared in this study are denoted based on their shapes as observed in Transmission Electron Microscopy (TEM), namely cube, short rod (SR) and long rod (LR) and sphere. Spherical CNPs were prepared by ultra-sonication method.39 Briefly, cerium nitrate hexahydrate (5 g, Sigma Aldrich, USA) and methoxy polyethylene glycol (mPEG, 5000 Da, 1 g, Acros Organics, USA) were dissolved in deionized water (100 mL). Subsequently, sodium hydroxide solution (5 mg/mL in deionized water, Sigma Aldrich, USA) was added at a rate of 5 mL/min under constant sonication and stirring until the pH of the solution became 10. CNPs were washed with deionized water and ethanol several times until the pH of supernatant became neutral. CNPs were obtained by centrifugation followed by overnight drying at 100 °C. Cube, SR, and LR CNPs were synthesized by hydrothermal method.35 Briefly, cerium nitrate hexahydrate (0.868 g) was dissolved in deionized water (5 mL) and added in a drop-wise manner to sodium hydroxide solution (35 mL, 6 M for cube and SR while 9 M for LR) with stirring for 30 min at room temperature. The solution was then sealed in a hydrothermal reactor and allowed to react at 100 °C (SR and LR) or 180 °C (cube) for 24 h. Subsequently, CNPs were washed with deionized water and ethanol for several times until the pH of the supernatant became neutral. CNPs were obtained by centrifugation followed by drying overnight at 60 °C.
Physicochemical Characterization of CNPs
The shapes of different CNPs were examined by TEM. Low concentration dispersions of CNPs were imaged on copper grids using electron microscope (JEOL 1011, Joel, Tokyo, Japan) operated at an accelerating voltage of 80 kV. Images were acquired without any staining. For characterization of particle size (hydrodynamic diameter), stock CNP dispersions (1 mg/mL) in HEPES buffer (pH = 4, GE Healthcare, USA) were diluted to 25 µg/mL in hVIC culture media prior to measurements. Particle sizes were measured by dynamic light scattering (DLS) using a Malvern Zetasizer (Zetasizer 3000, Malvern, USA). To determine the effect of CNP shape on oxidation state of cerium (Ce3+ or Ce4+), the UV–Vis spectra (wavelengths 200–400 nm) were recorded using CNP dispersion (25 µg/mL) in deionized water using a SpectraMax M5e plate reader (Molecular Devices LLC, USA).
Abiotic (Non-cellular) H2O2 Scavenging Assay
The H2O2 scavenging ability of CNPs was measured using Amplex® Red Hydrogen Peroxide Assay according to the manufacturer’s protocol (ThermoFisher Scientific, USA). CNPs (5, 25 and 100 µg/mL) dispersed in HEPES buffer (0.01 M, 60 µL) were allowed to react with H2O2 solution (10 µM, 60 µL) at room temperature for 30 min. Subsequently, equal volume (50 µL) of the reactants and working solution of Amplex® Red reagent (100 µM) and Horseradish Peroxidase (HRP, 0.2 U/mL) were allowed to react at room temperature for 30 min (n = 4). The fluorescence intensity was then measured using microplate reader (Synergy HT, BioTek instruments) at excitation/emission wavelengths of 540/590 nm. HEPES buffer (0.01 M) and butylated hydroxytoluene (BHT; 25 µM in 0.01 M HEPES buffer) served as negative and positive control, respectively. Scavenged H2O2 was calculated using equation below.
Cytotoxicity of CNPs by alamarBlue® Assay
The dose-dependent cytotoxicity of CNPs were determined by alamarBlue® assay (Thermo Scientific, USA) according to the manufacturer’s protocol. Briefly, hVICs (9000 cells/well) were seeded in a 96-well plate and allowed to attach for 24 h. Subsequently, hVICs were incubated with shape-specific CNPs (0.01–100 µg/mL) in hVIC culture media (200 µL) for 24 h. The cells were then incubated with alamarBlue® solution (10% v/v) prepared in hVIC culture media for 3 h at 37 °C. Fifty-microliter solution from each well was transferred to a 96-well plate and the fluorescence intensity was measured at excitation/emission wavelength of 530/590 nm using a microplate reader (Synergy HT, BioTek instruments, USA). The cells that received media without CNPs (0 µg/mL CNPs, media only) were used as a negative control. Viability of CNP-treated cells was calculated using those without CNP treatment as 100% viability as per equation below.
Shape-Dependent Effect of CNPs to Scavenge or Prevent H2O2-Mediated Acute Oxidative Stress
To determine shape-dependent effect of CNPs on scavenging H2O2-mediated pre-existing oxidative stress, the diseased hVICs were seeded in a 96-well plate (9000 cells/well) or a 24-well plate (100,000 cells/well) and allowed to attach for 24 h. Subsequently, hVICs were exposed to media containing H2O2 (100 μM, 100 μL). After 4 h incubation, CNPs were added at different doses (from 0.1 to 100 μg/mL, 100 μL) without removal of H2O2. The incubation was continued for another 24 h. Cells were then subjected to ROS detection by DCFH-DA assay. Cells treated with 100 µM H2O2 and 0 µg/mL CNPs served as positive control (100% ROS generation) and cells without H2O2 and CNP treatment served as negative control (0% ROS generation).
Alternatively, to investigate shape-dependent effect of CNPs on preventing acute ROS generation, hVICs were first exposed to media containing CNPs (25 μg/mL, 100 μL). After 24 h incubation, H2O2 (100 μM, 100 μL) were added without removal of CNPs. The incubation was continued for another 4 h. Cells were then subjected to ROS detection by DCFH-DA assay. Cells that received hVIC culture media without CNPs and H2O2 were used as negative control to calculate percentage ROS level as per the equation:
To further determine the effect of CNP treatment on antioxidant enzyme activity as well as calcification potential, cell lysates and mRNA of LR CNP-treated groups (25 μg/mL) were analyzed for SOD/CAT activity and expression of VIM, α-SMA, OPN, and RUNX-2, respectively. Cells without any treatment (neither H2O2 nor LR CNPs) were used as negative control while those exposed to only H2O2 without CNP treatment were used as positive control.
Alizarin Red S Staining
The calcified nodule formation in hVIC cultures were determined by Alizarin Red S (ARS) staining.40 At specific time points, media was removed and cells were fixed using PFA (4%, w/v) for 15 min and incubated with ARS solution (40 mM, pH 4.3) for 30 min. Samples were washed with deionized water four times before imaging.
Calcium Content Quantification
Intracellular calcium was quantified as reported previously.41 Calcium was extracted from cell monolayers by incubation with nitric acid (1 M) for 1 h. The supernatant (35 μL) was mixed with O-cresolphthalein (0.25 mg/mL, 35 µL) and 2-amino-2-methyl-1-propanol (150 mg/mL, 87.5 µL) at room temperature for 20 min and the absorbance was measured at 575 nm. The amounts of proteins were then quantified by micro BCA protein assay (Pierce BCA, Thermo Scientific) as mentioned earlier and data is presented as amount of calcium normalized to the amount of protein.
ROS-Mediated VIC Calcification Model Induced by Treatment with Inorganic Phosphate (Pi) in the Presence of H2O2
To establish the Pi-induced calcification model in the presence of H2O2, diseased hVICs were cultured in 24-well plate (100,000 cells/well) in hVIC culture media for 48 h followed by calcification media (MEM-alpha supplemented with 10% fetal bovine serum) containing an additional 1 mM sodium phosphate monobasic (Pi) alone or in presence of 100 μM H2O2. Media was replenished every other day. ROS levels were measured after 2 h of each treatment by DCFH-DA assay. Calcification in hVICs cultures was determined using ARS staining and calcium content quantification at day 1, 4, and 7. To confirm that observed calcification was indeed mediated by oxidative stress, cells were also co-treated in parallel with an antioxidant (Vitamin E, 25 μM).
To test the potential of CNPs in preventing calcification, hVICs were treated with LR CNPs (25 μg/mL) in the presence of Pi and H2O2 containing media. ROS were measured 2 h post-treatment while ARS staining and calcium content quantification were carried out 7 days after treatments.
Statistical Analysis
Experimental data are presented either as mean ± standard error of the mean (SEM) or as mean ± standard deviation (SD) as specified in each figure legend. Multiple comparisons were analyzed for significance using one-way ANOVA or two-way ANOVA followed by Tukey’s post hoc analysis. P-values less than 0.05 were considered as significant.
Results and Discussion
Diseased hVICs Exhibit Higher Expression of Calcification Markers and Impaired Antioxidant Mechanisms
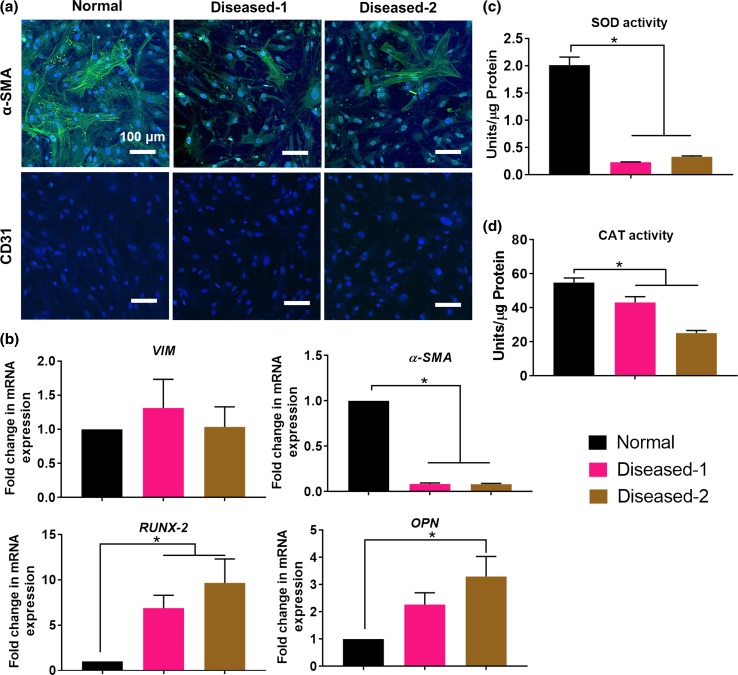
Human heart valve consists of two major cell types, namely VICs and valvular endothelial cells (VECs).12 In this study, we isolated hVIC population from three different subjects, a normal healthy control (referred to as ‘normal’) and two patients with diseased (stenotic) aortic valves. The presence or absence of calcification in the valves from which hVICs were isolated was confirmed via histological analysis of neighboring tissue using Von Kossa stain (Fig. S1). Phenotype of hVICs was evaluated by immunofluorescence staining for α-SMA (VIC marker) and CD31 (VEC marker). All three cell populations stained positively for α-SMA but did not express CD31 (Fig. 1a), confirming that the cells used are predominantly interstitial cell population with no presence of an endothelial cell contaminant. Also, normal hVICs showed higher expression of α-SMA as compared to the diseased hVICs, which suggests that these diseased cells had less myofibroblastic VIC population. VICs are widely recognized as a heterogeneous and plastic cell population with diverse phenotypes including quiescent fibroblast, activated myofibroblast, diseased osteoblast and endothelial/mesenchymal progenitor cells.12 The expression of α-SMA in hVICs is in accordance with earlier reports that VICs are spontaneously activated into myofibroblast phenotype, expressing α-SMA, in two-dimensional (2D) culture.42
Figure 1.
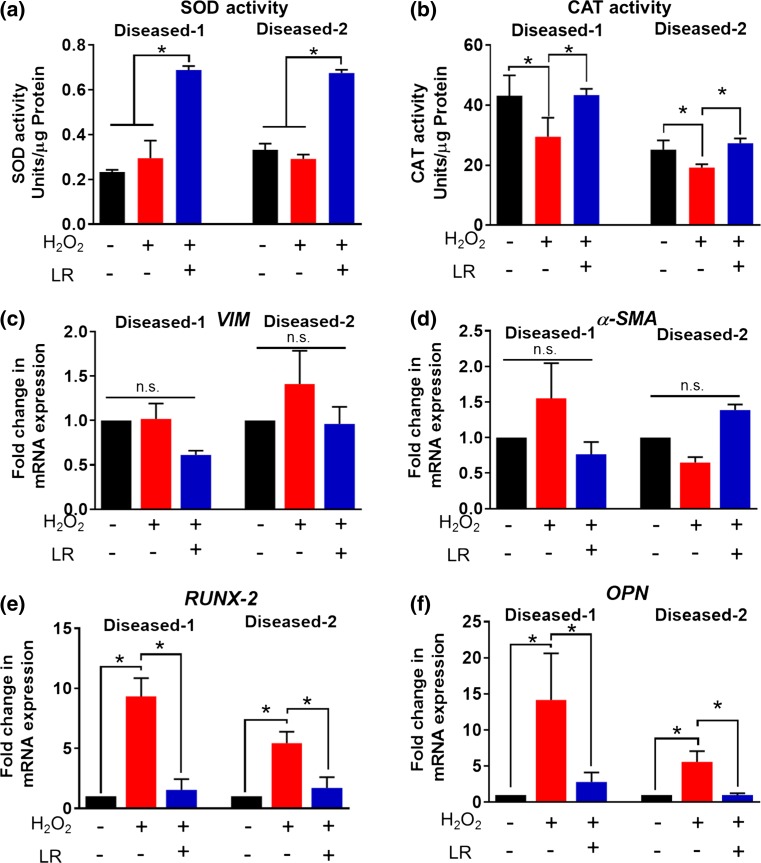
Patient-derived diseased hVICs exhibited higher expression of calcification markers and impaired antioxidant mechanisms. (a) Immunofluorescence staining of patient-derived hVICs revealed α–SMA positive but CD31 negative cell population and similar morphology among the three populations in 2D culture. Scale bar = 100 µM; (b) Patient-derived diseased hVICs exhibited no change in expression of VIM, downregulated α-SMA expression (VIC activation marker) and upregulated RUNX-2 and OPN (calcification markers) when compared with normal hVICs (n = 4); Patient-derived diseased hVICs exhibited decreased (c) SOD and (d) CAT activity when compared with normal hVICs (n = 4); *indicates p < 0.05 (One-way ANOVA). Data are presented as mean ± SEM.
We further characterized the differences between normal and diseased hVICs by quantifying mRNA expression of key VIC markers including VIM (quiescent VIC marker43), α-SMA (activated VIC marker43), RUNX-2 and OPN (osteoblastic VIC marker44). Compared to the normal hVICs, both diseased hVICs demonstrated higher RUNX-2 and OPN levels, while showing similar mRNA levels of VIM (Fig. 1b). These results suggest that osteoblastic markers were upregulated in diseased hVICs isolated from patients with valve stenosis (a late stage of valve calcification). These results are in agreement with other reports that proposed involvement of osteoblastic differentiation of VICs during valve calcification process.10 Diseased hVICs also exhibited reduced α-SMA levels compared to normal hVICs consistent with reduced α-SMA protein expression observed by immunostaining (Fig. 1a). Taken together, reduced expression levels of myofibroblast marker (α-SMA) coupled with increased levels of osteoblast (calcification) markers (RUNX-2 and OPN) in diseased hVICs suggest that diseased valves may have less myofibroblastic VIC population and more osteoblastic VIC population. This is highly plausible given that diseased VICs used in this study were isolated from patients with valve calcification. Similar results were also observed in an in vitro VIC calcification model where α-SMA expression peaked and preceded the calcification and was then reduced in the later stage of calcification process.45
Impaired oxidative stress defense mechanisms due to reduced antioxidant enzyme expression and activity have been found in valve calcification.9,10 SOD and CAT are two key functional antioxidant enzymes in resolving oxidative stress.46 Therefore, we measured the enzyme activities of SOD and CAT in normal and diseased hVICs (Figs. 1c, 1d). Diseased hVICs showed significantly lower SOD and CAT activities compared to normal hVICs similar to what was reported in human valve tissues.14 Impairment of antioxidant enzyme activity may contribute to increased accumulation of H2O2. Indeed, Miller et al.14 demonstrated that calcified human aortic valves generate excessive amounts of superoxide and peroxide due to the impaired antioxidant defense mechanisms, namely, valvular CAT deficiency and reduced nitric oxide production.
Diseased hVICs are More Susceptible to H2O2-Mediated Oxidative Stress and Stress-Induced Upregulation of Calcification Markers
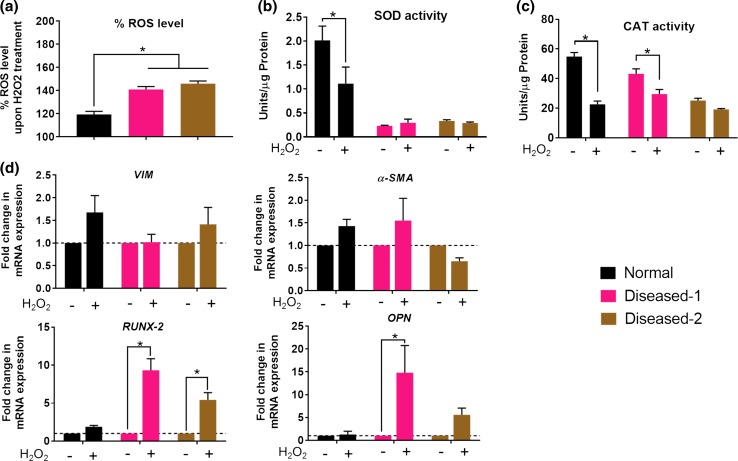
We compared the behaviors of normal and diseased hVICs in response to exogenous oxidative stress. H2O2 is a major type of peroxide found in a wide range of tissues and a major driving factor for ROS-mediated diseases.47 Recent studies have highlighted the involvement of H2O2 in driving oxidative stress and exacerbating valve calcification.18,22 Therefore, H2O2 was selected to induce oxidative stress in the current study. It was found that acute H2O2 treatment (24 h) induced higher levels of ROS measured by DCFH-DA assay in the diseased hVICs than in the normal hVICs (Fig. 2a). Compromised or deficient defense mechanisms that are responsible for removal of ROS may have resulted in higher amount of (un-scavenged) ROS in the diseased hVICs. Indeed, we observed reduced antioxidant enzyme activities in both the diseased hVICs compared to normal hVICs (Figs. 1c, 1d). Furthermore, normal hVICs showed significantly decreased SOD and CAT activities after acute H2O2 treatment (Figs. 2b, 2c). We offer two explanations for this outcome. First, it is possible that these enzymes were utilized to scavenge H2O2-mediated oxidative stress. This explanation aligns with a recent report showing that the increased levels of ROS in the valve tissues were associated with the decreased local antioxidant enzyme activities rather than the induction of compensatory antioxidant enzymes activity, as seen in other tissues such as blood vessels.14 Second, since both diseased hVICs showed lower antioxidant enzyme activities compared to those of normal hVICs (Figs. 1c, 1d), it is possible that their inherent antioxidant capacities were already deficient and could not adequately neutralize the oxidative stress assault.
Figure 2.
Patient-derived diseased hVICs were more susceptible to H2O2-mediated oxidative stress and stress-induced upregulation of calcification markers. (a) Acute treatment with exogenous H2O2 (100 µM, 24 h) resulted in higher intracellular ROS levels in the diseased hVICs than the normal hVICs (n = 4). *indicates p < 0.05 (one-way ANOVA); (b, c) Normal hVICs, but not diseased hVICs demonstrated reduction in the SOD/CAT activities after H2O2 treatment. (n = 4); (d) H2O2-mediated oxidative stress did not affect VIM and α-SMA expression in normal and diseased hVICs. Diseased hVICs exhibited elevated expression of calcification markers compared to the untreated controls while normal hVICs maintained basal expression levels of calcification makers after treatment with H2O2. *indicates p < 0.05 (Two-way ANOVA). Data are presented as mean ± SEM.
To further elucidate the effect of acute H2O2 treatment on the phenotypic changes in hVICs, mRNA expression of VIM, α-SMA, RUNX-2 and OPN were compared before and after acute H2O2 treatment in normal and diseased hVICs (Fig. 2d). In all three hVICs, mRNA expression of VIM and α-SMA were unaffected, indicating H2O2 may not have a direct impact on the VIC activation in 2D cell culture. Importantly, H2O2-induced RUNX-2 and OPN mRNA expression levels were upregulated in both the diseased hVICs but not in normal hVICs. The differential behaviors of diseased and normal hVICs could be due to the ability of normal hVICs to better resolve H2O2-induced acute oxidative stress than that of diseased VICs (Fig. 2a). These data indicate that acute unresolved H2O2-induced oxidative stress promoted the differentiation of quiescent or myofibroblastic hVICs towards the osteoblast-like phenotype, which may contribute to the valve calcification process. Previously, acute H2O2 treatment has been shown to upregulate gene expression of RUNX-2 in vascular smooth muscle cells,48 and the osteogenic transcription factors MSX2 in mouse aorta adventitial myofibroblasts.49 The current study supports prior studies demonstrating that oxidative stress is one of the critical factors contributing to ectopic calcification. Taken together, the diseased hVICs showed impaired abilities to scavenge ROS and respond to oxidative stress-induced changes by promoting gene expression of upstream markers of calcification. Hence, diseased VICs were chosen as the cell model for studying the oxidative stress-induced calcification and exploring potential calcification prevention agents.
Shape-Specific CNPs are Biocompatible Nanoparticles with ROS Scavenging Capability
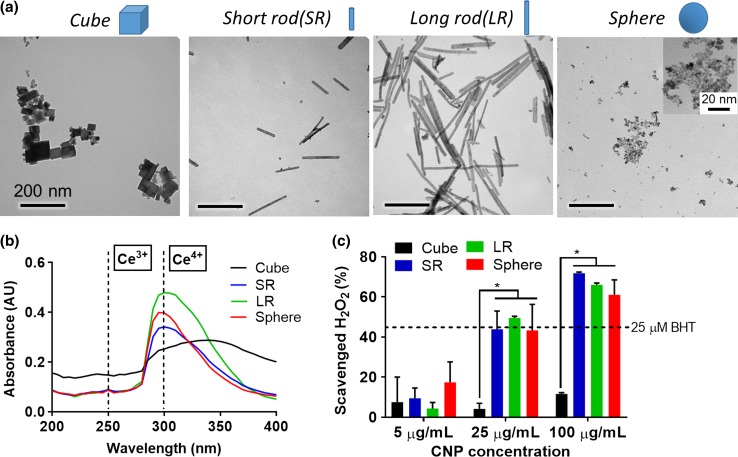
In light of a lack of pharmacological treatments for valve calcification, we explored shape-specific CNPs as a novel antioxidant therapy for the treatment of oxidative stress-induced valve calcification. Furthermore, we investigated the effect of CNP shape on their antioxidant properties. Four shape-specific CNPs were synthesized via hydrothermal method (cube, short rod (SR) and long rod (LR) CNPs) or ultra-sonication method (sphere CNPs) between cerium nitrate and sodium hydroxide and characterized for their physico-chemical properties. TEM revealed uniform shapes in each batch of the nanoparticles (Fig. 3a), thus enabling investigation on the effect of nanoparticle shapes on their antioxidant properties. Synthesized shape-specific CNPs showed aspect ratio ranging from 1:1 to 21:1 (measured by TEM image analysis) and hydrodynamic sizes ranging from 120 to 220 nm (Table 1). The oxidation state of cerium is a key characteristic of CNPs as their antioxidant activity is highly dependent on their ability to switch reversibly between Ce3+ and Ce4+ oxidation state.27,28 UV–Vis absorbance is a facile method to determine the oxidation state of CNPs in aqueous solution.50 None of the CNPs demonstrated significant peak at 250 nm (Fig. 3b), suggesting low or negligible percentage of Ce3+ population.50 SR, LR and sphere CNPs showed strong absorbance peaks at 300 nm (Fig. 3b), suggesting predominant presence of Ce4+ population.50 Cube CNPs showed less intense absorbance peak at 300 nm, indicating lower percentage of Ce4+ population in cube CNPs compared to SR, LR and sphere CNPs.
Figure 3.
Physico-chemical characterization of shape-specific CNPs. (a) Transmission electron microscopy (TEM) images revealed shape-specific CNPs with defined shapes. Scale bar = 200 nm; (b) UV–Vis spectra of shape-specific CNPs showing presence of predominantly Ce4+ oxidation state; (c) Hydrogen peroxide (H2O2) scavenging ability of shape-specific CNPs measured by AmplexRed assay (n = 4). * indicates p < 0.05 (Two-way ANOVA). Data are presented as mean ± SD.
Table 1.
Aspect ratios and hydrodynamic sizes of four shape-specific CNPs.
| Cube | Short rod (SR) | Long rod (LR) | Sphere | |
|---|---|---|---|---|
| Aspect ratioa | 1:1 | 8:1 | 21:1 | 1:1 |
| Hydrodynamic size (nm)b | 220.1 ± 3.6 | 121.8 ± 0.8 | 119.7 ± 1.5 | 121.1 ± 1.2 |
aAspect ratio is defined as the ratio of length to width of a nanoparticle as measured by TEM image analysis
bHydrodynamic size is determined by using CNP suspension (25 µg/mL) in hVIC culture media by dynamic light scattering
The intrinsic ability of CNPs to scavenge H2O2 (non-cellular) was measured by Amplex® Red assay. SR, LR and sphere CNPs exhibited significantly higher dose-dependent H2O2 scavenging ability (5-100 µg/mL) than cube CNPs (Fig. 3c). This observation is in accordance with a previous report that cube CNPs synthesized by hydrothermal method had lower ROS scavenging ability than that of other shapes such as rod and sphere.51 As suggested earlier, this may be attributed to differences in the morphology, agglomeration, and Ce3+/Ce4+ ratio of different shape-specific CNPs.51 Additionally, the lower ROS scavenging ability of cube CNPs may also be associated with its lower Ce4+ population as shown in Fig. 3b since catalase (CAT)-mimetic activity of CNPs requires predominantly Ce4+ oxidation state to scavenge H2O2.52
To measure the acute cytotoxicity of CNPs, the diseased hVICs were exposed to different doses of CNPs (0.01 μg/mL-100 μg/mL in hVIC culture media) for 24 h. None of the CNPs posed significant acute cytotoxicity (Fig. S2), demonstrating their good cytocompatibility. Of note, low dose of CNP treatment (0.01 μg/mL) showed increased cell viability when compared with untreated control. CNPs have been shown to enhance primary mouse embryonic fibroblast proliferation at similar low dose range, which was attributed to its ability to maintain redox balance for enhanced intracellular homeostasis and metabolism.53 Our results indicate that shape-specific CNPs do not exhibit acute cytotoxicity in the dose range of 0.01–100 μg/mL.
Shape-Specific CNPs Scavenge Acute H2O2-Induced Oxidative Stress in the Diseased hVICs
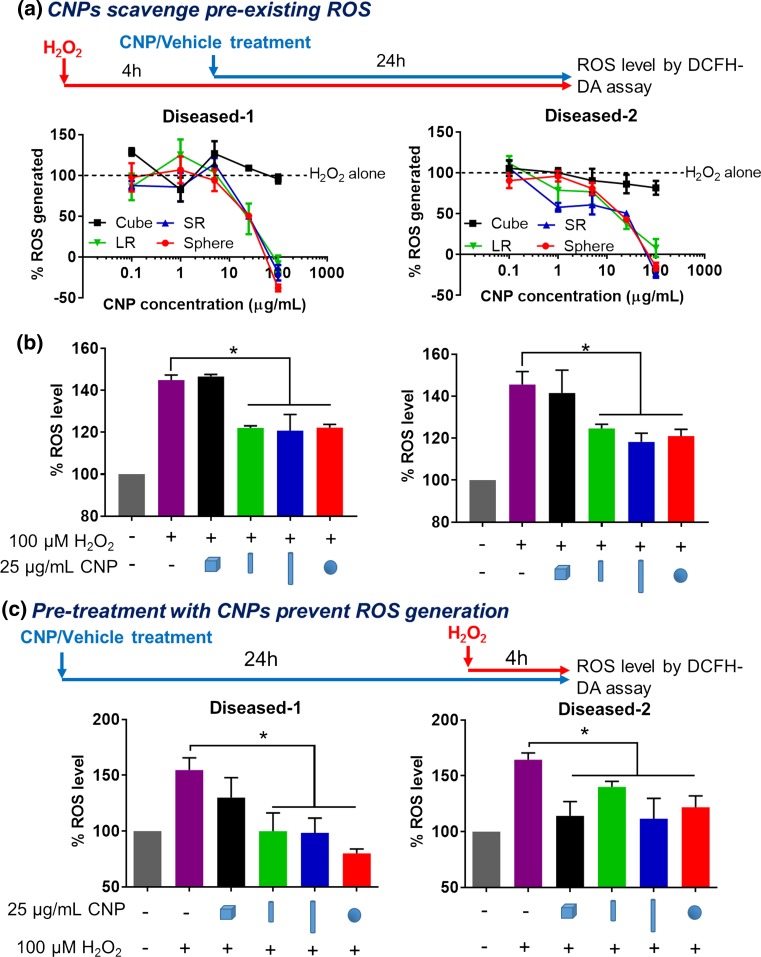
The ROS scavenging ability of CNPs in the diseased hVICs were first determined by measuring the intracellular ROS levels in response to 4 h pre-treatment of H2O2 (100 µM) to induce acute oxidative stress followed by 24 h CNP treatment (0.1–100 μg/mL). This experimental design allows us to explore the dose- and shape-dependent activity of CNPs to scavenge pre-existing ROS induced by H2O2. Consistent with the results from the non-cellular H2O2 scavenging assay (Fig. 3c), SR, LR and sphere CNPs exhibited dose-dependent ROS scavenging ability in both populations of diseased hVICs (Fig. 4a). It is noted that at 100 μg/mL, SR, LR and sphere CNPs were able to scavenge all the ROS generated by H2O2. On the other hand, cube CNPs were unable to scavenge pre-existing ROS even at a high dose of 100 μg/mL. Although antioxidant activity of CNPs has already been reported in a variety of in vitro and in vivo studies,31–34 shape-dependent effect on the ROS scavenging activity has only been studied in a non-cellular system51 and remains unexplored in cellular systems. Here, for the first time, we demonstrated that CNPs exhibited shape-dependent ROS scavenging activity in the diseased hVICs under acute oxidative stress. Since the IC50 values of SR, LR and sphere CNPs in scavenging ROS in the diseased hVICs were less than 25 µg/mL (Table 2), we chose 25 µg/mL as an effective dose for exploring the shape-dependent effects of CNPs in the following studies. We compared therapeutic activity of different shapes at a single dose of 25 µg/mL (Fig. 4b). SR, LR and sphere CNPs but not cube CNPs exhibited ROS scavenging ability in both diseased hVICs.
Figure 4.
Shape-specific CNPs scavenged acute H2O2-mediated oxidative stress in the diseased hVICs. (a) As illustrated in the experimental workflow, SR, LR and sphere but not cube CNPs scavenged in a dose-dependent manner, pre-existing intracellular ROS generated by H2O2 (100 µM, 4 h) in both diseased hVICs as measured by DCFH-DA assay (n = 4). *indicates p < 0.05 (Two-way ANOVA); (b) At the dose of 25 µg/mL, SR, LR and sphere but not cube CNPs scavenged pre-existing intracellular ROS generated by H2O2 (100 µM, 4 h) in diseased hVICs as measured by DCFH-DA assay (n = 4). *indicates p < 0.05 (one-way ANOVA); (c) As illustrated in the experimental workflow, pre-treatment with CNPs (25 µg/mL, 24 h) prevented intracellular ROS accumulation following treatment with H2O2 (100 µM, 4 h) in diseased hVICs in a shape-dependent manner (n = 4). * indicates p < 0.05 compared to H2O2 only treatment (one-way ANOVA). Data are presented as mean ± SEM.
Table 2.
Summary of IC50 values of shape-specific CNPs in scavenging ROS in the diseased hVICs.
| IC50 (µM) | Cube | SR | LR | Sphere |
|---|---|---|---|---|
| Diseased-1 | N/A | 21.7 ± 1.34 | 22.8 ± 1.36 | 16.45 ± 1.22 |
| Diseased-2 | N/A | 12.55 ± 0.88 | 13.46 ± 1.13 | 17.18 ± 1.15 |
The ability of CNPs to prevent ROS generation in the diseased hVICs was evaluated by measuring the intracellular ROS levels after 24 h CNP pre-treatment (25 μg/mL) followed by 4 h treatment of H2O2 (100 µM) to induce oxidative stress. Diseased-1 hVICs pre-treated with SR, LR and sphere CNPs but not cube CNPs showed significant reduction in ROS levels compared to 0 μg/mL CNP pre-treated group (Fig. 4c). Diseased-2 hVICs pre-treated with all CNPs showed significant reduction in ROS levels compared to cells without CNP treatment (Fig. 4c). These results indicated the ability of CNPs to protect hVICs from exogenous oxidative stress.
Together, these data show that LR CNPs are effective in scavenging pre-existing ROS and in preventing ROS generation in both diseased hVICs. Therefore, we chose to focus on LR CNPs and study their therapeutic effects in combating oxidative stress-induced VIC calcification in the following studies.
LR CNPs Enhance Activity of Antioxidant Enzymes and Inhibit Oxidative Stress-Induced Expression of Calcification Markers
To elucidate the mechanisms by which LR CNPs are able to reduce the intracellular ROS, we further measured the SOD/CAT activities of the diseased hVICs after H2O2 pre-treatment followed by LR CNP treatment. LR CNP treatment significantly increased SOD and CAT activities in both diseased hVIC populations (Figs. 5a, 5b). In addition to its intrinsic ability to scavenge ROS (Fig. 3c), LR CNPs may have exerted their ROS scavenging effect by enhancing the cellular antioxidant enzyme activities as means of maintaining redox balance. CNPs are known for their broad spectrum antioxidant mechanisms28,29; however, to the best our knowledge, the ability of CNPs to enhance cellular antioxidant enzyme activity in vitro has not been demonstrated. Due to limited studies in the field, the exact antioxidant mechanisms (scavenging of radicals, superoxides or peroxides) required for inhibiting valve calcification remain elusive. Previously, antioxidants such as tempol and lipoic acid,22 as well as adenovirus-mediated over-expression of SOD or CAT,18 have been explored for treating valve calcification. In these previous studies, supplement of antioxidant (either in the form of small molecule drug or antioxidant enzyme) did not demonstrate consistent therapeutic effects on mitigating the progression of valve calcification. Therefore, CNPs with broad intrinsic ROS scavenging mechanisms as well as the ability to enhance cellular antioxidant enzyme activities (SOD/CAT-mimetic) may be advantageous in mitigating oxidative stress-induced valve calcification.
Figure 5.
LR CNPs enhanced antioxidant enzyme activities and inhibited oxidative stress-induced expression of calcification markers. LR CNP treatment upregulated SOD (a) and CAT (b) activity after H2O2-mediated oxidative stress (100 µM, 24 h) in the diseased hVICs (n = 3); LR CNPs didn’t change expression of fibroblast and myofibroblast markers in the diseased hVICs (c) and (d); LR CNPs decreased H2O2-induced expression of calcification markers in the diseased hVICs (e) and (f) (n = 3). *indicates p < 0.05 (one-way ANOVA). Data are presented as mean ± SEM.
Given the excellent ROS scavenging ability of CNPs in hVICs (Fig. 4), we further evaluated if CNP treatment can inhibit oxidative stress-induced upregulation of markers that precede calcification. As shown in Fig. 5c, LR CNP treatment after oxidative stress induction by H2O2 had no significant effect on the mRNA expression levels of VIM and α-SMA but reduced the expression of upstream calcification markers (RUNX-2 and OPN) in the diseased hVICs. Since we also observed that oxidative stress upregulated calcification marker expression (Fig. 2d), this noted reduction in the presence of LR CNPs suggests that LR CNPs are able to scavenge ROS (Fig. 4) and upregulate SOD/CAT activities (Figs. 5a, 5b). These results suggest that LR CNPs may be able to mitigate oxidative stress-induced calcification in hVIC cultures.
H2O2-Induced Oxidative Stress Enhances Calcification in the Inorganic Phosphate (Pi)-Induced in vitro Calcification Model
Although we observed increased calcium content in the Diseased-1 hVICs cultured with long term (14-day) H2O2 treatment (Fig. S4), H2O2 treatment alone did not induce ARS-positive calcific nodule formation in the Diseased-1 hVICs (data not shown). Therefore, we further explored to enhance oxidative stress (H2O2)-induced calcification by combining with the inorganic phosphate (Pi)-induced in vitro calcification model. Although Pi-induced in vitro calcification model has been widely used in studying ectopic vascular and breast calcification,41,54 we did not find any report on Pi-induced valve calcification in vitro. Nonetheless, hyperphosphatemia is suggested to be one of the major driving factors for in vivo calcification,13 further highlighting the significance of studying Pi-induced valve calcification in vitro. To optimize the experimental conditions for Pi-induced hVIC calcification, Diseased-1 hVICs were treated with different concentrations of Pi (1–10 mM) for 14 days and calcification was evaluated by ARS staining and calcium content quantification. Treatment with exogenous Pi alone induced ARS-positive calcific nodule formation in Diseased-1 hVICs in a dose-dependent manner (Fig. S5) and 1 mM Pi was sufficient to induce a substantial amount of calcification and therefore, chosen as the dose for further study.
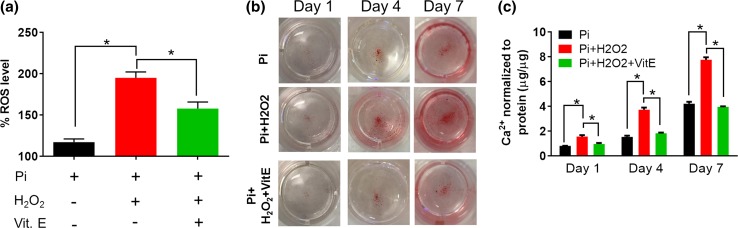
To investigate the role of oxidative stress in promoting hVIC calcification, H2O2 (100 µM) was supplemented with the Pi-containing (1 mM) calcification media. The presence of Pi and oxidative stress (H2O2) recapitulated two major microenvironmental cues that co-exist in the calcified valves6,9 to serve as a physiologically relevant in vitro diseased model. The acute ROS generation was measured by DCFH-DA assay 2 h post-treatment with the calcification media. In accordance with a previous study that showed Pi-induced acute ROS generation in vitro (within 1–2 h),55 we also observed Pi-induced ROS generation in the Diseased-1 hVICs, which was enhanced by the addition of H2O2 and reduced by concurrent administration of antioxidant Vitamin E (Fig. 6a). Similar results of ROS generation were also observed in the Diseased-2 hVICs when treated with Pi alone, Pi + H2O2, and Pi + H2O2 + Vitamin E (Fig. S6). Diseased-1 hVICs were chosen as the cell model for further study. The addition of H2O2 increased Pi-induced positive ARS staining and calcium content in a time-dependent manner compared to Pi alone treatment in a 7-day study. Such increased calcification was diminished by the concurrent administration of vitamin E (Figs. 6b, 6c), thus validating the role of exogenous oxidative stress in promoting VIC calcification. This observation is in agreement with the previous finding that ROS generation precede and mediate valve calcification.15 Overall, these data demonstrate that oxidative stress could further exacerbate calcification progression in hVICs in a Pi-induced calcification model.
Figure 6.
H2O2-mediated oxidative stress further enhanced calcification in the inorganic phosphate (Pi)-induced in vitro calcification model. Diseased-1 hVICs were cultured in the presence of additional Pi (1 mM) alone, Pi + H2O2 (100 µM) or Pi + H2O2 + Vitamin E (50 µM) for 2 h, 1, 4 and 7 days. (a) Addition of H2O2 for 2 h increased intracellular ROS levels compared to Pi alone treatment and addition of Vitamin E prevented such H2O2-induced ROS (n = 4). *indicates p < 0.05 (One-way ANOVA); (b) Positive alizarin red S (ARS) in Pi-treated cultures exhibited a time-dependent increase that was enhanced by addition of H2O2. Co-treatment with antioxidant Vitamin E inhibited H2O2-induced ARS positive staining; (c) Diseased-1 hVICs showed increased calcium content when treated with Pi + H2O2 when compared with Pi treatment alone as measured by o-cresolphthalein assay. Co-treatment of hVICs with Vitamin E inhibited the calcium secretion in the presence of Pi and H2O2 (n = 3). *indicates p < 0.05 (Two-way ANOVA). Data are presented as mean ± SEM.
LR CNPs Reduce Calcium Content in Pi and H2O2-Induced Calcification Model
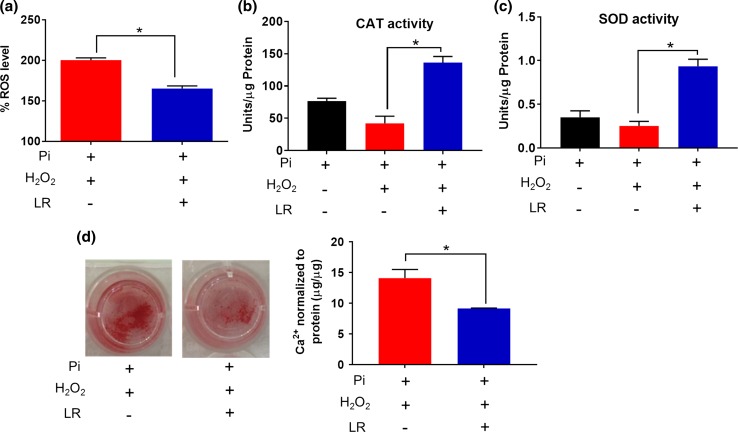
We further tested the hypothesis that CNPs will be able to mitigate the oxidative stress-induced hVIC calcification in vitro. As expected, the concurrent administration of LR CNPs decreased the H2O2-induced ROS generation (2 h) (Fig. 7a). We further revealed that long term (7-day) co-treatment of LR CNPs with Pi and H2O2 significantly increased the SOD and CAT activities in the Diseased-1 hVICs (Figs. 7b, 7c), suggesting the beneficial role of CNPs in modulating cellular antioxidant enzymes to combat oxidative stress. These results agree with the observation of increased SOD and CAT activities in diseased hVICs after short-term (24 h) co-treatment of LR CNPs with H2O2 (Figs. 5a, 5b).
Figure 7.
LR CNPs decreased calcium content in Pi and H2O2-induced calcification model. (a) LR CNPs reduced Pi + H2O2-induced acute intracellular ROS levels after 2 h treatment measured by DCFH-DA assay (n = 4); LR CNPs enhanced SOD (b) and CAT (c) activities of diseased hVICs after 7-day treatment (n = 3). (d) After 7-day treatment, LR CNPs reduced Pi + H2O2-induced calcification as revealed by decreased Alizarin Red S staining and calcium content by O-cresolphthalein assay (n = 3); *indicates p < 0.05 (one-way ANOVA). Data are presented as mean ± SEM.
Finally, the therapeutic benefit of LR CNPs in mitigating the Pi and H2O2-induced calcification was demonstrated by ARS staining and calcium quantification. Compared to the control group treated only with Pi and H2O2 without CNP treatment, the addition of LR CNPs reduced calcified nodule formation as revealed by less ARS staining and decreased total calcium content (Fig. 7d), which may be attributed to the ROS scavenging ability of LR CNPs. Previously, CNPs have been shown to inhibit osteogenic differentiation of primary mouse bone marrow stromal cells as revealed by the inhibition of mineralized nodules formation and alkaline phosphatase activity.56 However, the underlying mechanisms were not explored. Our results strongly suggest that SOD/CAT-mimetic activity coupled with their ROS scavenging ability allow CNPs to efficiently mitigate H2O2-induced oxidative stress and hence, inhibit the in vitro calcification in hVIC cultures.
Study Limitations and Outlook
The current study investigated the role of oxidative stress in mediating valve calcification by developing novel ROS-induced hVIC calcification model in vitro and explored CNPs as a novel potential treatment option. Although the presence of both Pi and oxidative stress were taken into consideration in our study, there are some limitations that we would like to acknowledge. First, the current studies were conducted in 2D cell culture, which may not directly reflect the VIC activities in vivo since VICs are known to undergo phenotypic changes in 2D cultures.45 Future study investigating the role of oxidative stress and potential therapeutics in a 3D VIC culture system using suitable matrix will be informative. Second, in the current study, LR CNPs were co-administrated with Pi and H2O2. However, to further facilitate the translation of CNP-based therapy, it is worthwhile to explore the effect of treatment schedule (time of administration) and duration on preventing and possibly, reversing the progression of pre-existing calcification caused by Pi and H2O2. Last but not least, we studied only two different calcified VIC populations and one normal VIC population. Therefore, it might not be appropriate to generalize our observations for all stenotic and normal VICs based on such small sample size. In order to make general conclusions for each group (normal vs. diseased) on the whole, future studies are required to include calcified and normal VIC populations from larger number of patients to fully understand gene regulation across normal physiology and various pathological states.
In order to facilitate the potential translation of CNP treatment for valve calcification therapy, we envision that the local delivery of CNPs via a tissue-engineered construct4 or polymeric drug carrier57 will minimize the systemic exposure and prevent the calcification of valve replacement.
Conclusion
In conclusion, we demonstrated that hVICs derived from calcified valves exhibited impaired antioxidant defense mechanisms and were more susceptible to oxidative stress than normal hVICs. CNPs scavenged H2O2-induced oxidative stress in hVICs in a shape- and dose-dependent manner. The intrinsic ROS scavenging ability of CNPs and their ability to induce cellular antioxidant enzyme activities may confer protection to hVICs from oxidative stress-exacerbated calcification. In a nutshell, our results suggest CNPs to be a promising antioxidant therapy for treating valvular calcification and deserve further investigations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
We acknowledge funding support from the School of Pharmacy, University of Pittsburgh (SS) and HL117917, NHLBI (CSH). YX acknowledges Graduate Student Research Scholarship from the School of Pharmacy, University of Pittsburgh. We thank Dr. Thomas Gleason, Center for Thoracic Aortic Disease, University of Pittsburgh for providing us with valve cusp tissue to collect the valve cells and Jennifer Hill for isolating valve cells. We thank Dr. Donna Stolz, Center for Biologic Imaging, University of Pittsburgh for access to TEM facility and Akhil Patel, School of Pharmacy, University of Pittsburgh for acquiring the TEM images. We thank Dr. Paul Johnston, School of Pharmacy, University of Pittsburgh for access to the spectrophotometer.
Conflict of interest
Shilpa Sant has an invention disclosure filed as “shape-specific CNPs as ROS and immune-modulating agents”. Yingfei Xue, Cynthia St. Hilaire, Luis Hortells, Julie A. Phillippi, and Vinayak Sant declare that they have no conflicts of interest.
Ethical standards
All human subjects researches were carried out in accordance with the ethical standards approved by the University of Pittsburgh Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No animal studies were carried out by the authors for this article.
Footnotes
Shilpa Sant, PhD, is an Assistant Professor at University of Pittsburgh in the Department of Pharmaceutical Sciences with secondary appointment in Bioengineering. She is also a member faculty at the McGowan Institute for Regenerative Medicine and UPMC Hillman Cancer Center. Dr. Sant received a PhD in Pharmaceutical Technology from University of Montreal, Canada; MS in Pharmacology and B.Pharm. in Pharmaceutical Sciences from University of Mumbai. Before joining Pitt, she was a Ruth Kirschstein fellow with Drs. Ali Khademhosseini and Richard Maas at the Wyss Institute for Biologically Inspired Engineering and the Center for Bioengineering at Brigham and Women’s Hospital. Her major research interests include bioinspired approaches for regenerative therapies and development of biomimetic microenvironments for in vitro three-dimensional disease progression models. Her lab uses interdisciplinary approaches to study role of microenvironments on disease progression in the same cells without any genetic manipulations or artificial culture conditions. Dr. Sant has contributed more than 50 papers, book chapters and patents, including reports in Cancer Research, Advanced Materials, Journal of Controlled Release, and Advanced Drug Delivery Reviews. Her work is highlighted on PNAS journal club, MaterialsToday news, and Women in Nanoscience blog. She has also edited a book entitled “Nanomaterials in Tissue Engineering: Fabrication and Applications” and a journal issue “Stem Cells: Microenvironment, Micro/Nanotechnology, and Application”, in Stem Cells International. Dr. Sant’s exemplary achievements in research have been recognized by prestigious fellowships: Ruth L. Kirschstein National Research Service Award (NIH, USA), Post-doctoral Fellowship (Le Fonds de Recherche du Quebec Nature et Technologies (FRQNT), Canada), Post-graduate Scholarship (Natural Sciences and Engineering Research Council of Canada (NSERC), Canada). She has also received several awards including “2016 CMBE-BMES Rising Star Early Career Faculty Award” “2013 CMBE-BMES Rising Star/Fellow Award”, “2010 Society For Biomaterials – STAR Award” to name a few. She serves as a reviewer on several NIH and NSF grant review panels. She also serves as an Associate Editor for IEEE Transactions on NanoBioScience, and editorial board member for Scientific Reports and In Silico Pharmacology.
This article is part of the 2017 CMBE Young Innovators special issue.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive summary: heart disease and stroke statistics—2016 update. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM. Calcific aortic stenosis—time to look more closely at the valve. N. Engl. J. Med. 2008;359:1395–1398. doi: 10.1056/NEJMe0807001. [DOI] [PubMed] [Google Scholar]
- 3.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann. Thorac. Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Xue Y, Sant V, Phillippi J, Sant S. Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves. Acta Biomater. 2017;48:2–19. doi: 10.1016/j.actbio.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nat. Rev. Cardiol. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathieu P, Boulanger M-C. Basic mechanisms of calcific aortic valve disease. Can. J. Cardiol. 2014;30:982–993. doi: 10.1016/j.cjca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Sverdlov AL, Ngo DT, Chapman MJ, Ali OA, Chirkov YY, Horowitz JD. Pathogenesis of aortic stenosis: not just a matter of wear and tear. Am. J. Cardiovasc. Dis. 2011;1:185–199. [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: the need for naturally engineered solutions. Adv. Drug Deliv. Rev. 2011;63:242–268. doi: 10.1016/j.addr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R. Novel aspects of oxidative stress in cardiovascular diseases. Circ. J. 2009;73:201–207. doi: 10.1253/circj.CJ-08-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ. Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip CYY, Simmons CA. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc. Pathol. 2011;20:177–182. doi: 10.1016/j.carpath.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Schoen FJ. Mechanisms of function and disease of natural and replacement heart valves. Annu. Rev. Pathol.: Mech. Dis. 2012;7:161–183. doi: 10.1146/annurev-pathol-011110-130257. [DOI] [PubMed] [Google Scholar]
- 13.Hutcheson JD, Blaser MC, Aikawa E. Giving calcification its due: recognition of a diverse disease. Circ. Res. 2017;120:270–273. doi: 10.1161/CIRCRESAHA.116.310060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JD, Weiss RM, Serrano KM, Brooks RM, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández Esmerats J, Heath J, Jo H. Shear-sensitive genes in aortic valve endothelium. Antioxid. Redox Signal. 2016;25:401–414. doi: 10.1089/ars.2015.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowler MA, Merryman WD. in vitro models of aortic valve calcification: solidifying a system. Cardiovasc. Pathol. 2015;24:1–10. doi: 10.1016/j.carpath.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aikawa E, Cloyd KL, El-Hamamsy I, Boonrungsiman S, Hedegaard M, Gentleman E, Sarathchandra P, Colazzo F, Gentleman MM, Yacoub MH, Chester AH, Stevens MM. Characterization of porcine aortic valvular interstitial cell ‘calcified’ nodules. PLoS ONE. 2012;7:e48154. doi: 10.1371/journal.pone.0048154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can. J. Cardiol. 1996;12:231–236. [PubMed] [Google Scholar]
- 22.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Pomerantzeff PMA, Laurindo FRM. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2007;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 23.Shao JS. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Inoue N, Azumi H, Seno T, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yokozaki H, Yokoyama M. Expressional changes of the vascular antioxidant system in atherosclerotic coronary arteries. J. Atheroscler. Thromb. 2002;9:184–190. doi: 10.5551/jat.9.184. [DOI] [PubMed] [Google Scholar]
- 26.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed. Proc. 1976;35:156–162. [PubMed] [Google Scholar]
- 27.Walkey C, Das S, Seal S, Erlichman J, Heckman K, Ghibelli L, Traversa E, McGinnis JF, Self WT. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ. Sci.: Nano. 2015;2:33–53. doi: 10.1039/C4EN00138A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson B, Johnson M, Walker M, Riley K, Sims C. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5:15. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rzigalinski BA, Carfagna CS, Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. WIREs Nanomed. Nanobiotechnol. 2017;9:e1444. doi: 10.1002/wnan.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue, Y., S. R. Balmuri, A. Patel, V. Sant, and S. Sant. Synthesis, physico-chemical characterization, and antioxidant effect of PEGylated cerium oxide nanoparticles. Drug Deliv. Transl. Res. 2017. doi:10.1007/s13346-017-0396-1. [DOI] [PubMed]
- 31.Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor- B activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011;338:53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagliari F, Mandoli C, Forte G, Magnani E, Pagliari S, Nardone G, Licoccia S, Minieri M, Di Nardo P, Traversa E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6:3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 33.Kolli MB, Manne NDPK, Para R, Nalabotu SK, Nandyala G, Shokuhfar T, He K, Hamlekhan A, Ma JY, Wehner PS, Dornon L, Arvapalli R, Rice KM, Blough ER. Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomaterials. 2014;35:9951–9962. doi: 10.1016/j.biomaterials.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu J, Azfer A, Rogers L, Wang X, Kolattukudy P. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai H-X, Sun L-D, Zhang Y-W, Si R, Feng W, Zhang H-P, Liu H-C, Yan C-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B. 2005;109:24380–24385. doi: 10.1021/jp055584b. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Kröger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7:16631–16646. doi: 10.1039/C5NR02970H. [DOI] [PubMed] [Google Scholar]
- 37.Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed.: Nanotechnol. Biol. Med. 2015;11:1603–1611. doi: 10.1016/j.nano.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Gould RA, Butcher JT. Isolation of valvular endothelial cells. J. Vis. Exp. 2010;23:12. doi: 10.3791/2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Fu H, Shi L, Pan C, Li Q, Chu Y, Yu W. Synthesis of CeO2 nanorods via ultrasonication assisted by polyethylene glycol. Inorg. Chem. 2007;46:2446–2451. doi: 10.1021/ic061697d. [DOI] [PubMed] [Google Scholar]
- 40.Gaharwar AK, Mihaila SM, Swami A, Patel A, Sant S, Reis RL, Marques AP, Gomes ME, Khademhosseini A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013;25:3329–3336. doi: 10.1002/adma.201300584. [DOI] [PubMed] [Google Scholar]
- 41.Cox RF, Hernandez-Santana A, Ramdass S, McMahon G, Harmey JH, Morgan MP. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br. J. Cancer. 2012;106:525–537. doi: 10.1038/bjc.2011.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pesce M, Latif N, Quillon A, Sarathchandra P, McCormack A, Lozanoski A, Yacoub MH, Chester AH. Modulation of human valve interstitial cell phenotype and function using a fibroblast growth factor 2 formulation. PLoS ONE. 2015;10:e0127844. doi: 10.1371/journal.pone.0138570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J. Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 45.Hjortnaes J, Goettsch C, Hutcheson JD, Camci-Unal G, Lax L, Scherer K, Body S, Schoen FJ, Kluin J, Khademhosseini A, Aikawa E. Simulation of early calcific aortic valve disease in a 3D platform: a role for myofibroblast differentiation. J. Mol. Cell. Cardiol. 2016;94:13–20. doi: 10.1016/j.yjmcc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MatÉs JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 47.Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai C-F, Shao J-S, Behrmann A, Krchma K, Cheng S-L, Towler DA. TNFR1-activated reactive oxidative species signals up-regulate osteogenic Msx2 programs in aortic myofibroblasts. Endocrinology. 2012;153:3897–3910. doi: 10.1210/en.2012-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulido-Reyes G, Rodea-Palomares I, Das S, Sakthivel TS, Leganes F, Rosal R, Seal S, Fernández-Piñas F. Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Sci. Rep. 2015;5:15613. doi: 10.1038/srep15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakthivel T, Das S, Kumar A, Reid DL, Gupta A, Sayle DC, Seal S. Morphological phase diagram of biocatalytically active ceria nanostructures as a function of processing variables and their properties. ChemPlusChem. 2013;78:1446–1455. doi: 10.1002/cplu.201300302. [DOI] [PubMed] [Google Scholar]
- 52.Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JES, Seal S, Self WT. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010;46:2736. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popov AL, Popova NR, Selezneva II, Akkizov AY, Ivanov VK. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng.: C. 2016;68:406–413. doi: 10.1016/j.msec.2016.05.103. [DOI] [PubMed] [Google Scholar]
- 54.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazière C, Savitsky V, Galmiche A, Gomila C, Massy Z, Mazière J-C. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2010;1802:1013–1019. doi: 10.1016/j.bbadis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, Ge K, Ren H, Zhang C, Zhang J. Effects of cerium oxide nanoparticles on the proliferation, osteogenic differentiation and adipogenic differentiation of primary mouse bone marrow stromal cells in vitro. J. Nanosci. Nanotechnol. 2015;15:6444–6451. doi: 10.1166/jnn.2015.10709. [DOI] [PubMed] [Google Scholar]
- 57.Mason D, Chen Y-Z, Krishnan HV, Sant S. Cardiac gene therapy: recent advances and future directions. J. Control. Release. 2015;215:101–111. doi: 10.1016/j.jconrel.2015.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.