Abstract
Background and aim of the work: Revision total knee arthroplasty (TKA) is usually made more complex by the presence of bone defects, which may be caused by periprosthethic infection, polyethylene wear, implant loosening or fractures. The main aim of the present work is to review the available literature to understand the current options to manage with the bone loss during knee revisions. Methods: Available English literature for bone defects in revision TKAs has been evaluated looking at treatment options and their results in terms of clinical and radiological outcomes and failure rates. Results: Anderson Orthopaedic Research Institute (AORI) classification is the most frequently used because it helps in the choice of the most suitable treatment. Several options are available in the management of metaphyseal bone loss in revision knee arthroplasty. For small and contained defects (AORI type 1) cement with or without screws and auto- or allograft morcellized bone are available. In uncontained but mild defects (AORI type 2A) metal augments should be use while large and uncontained defects (AORI type 2B and 3) are best addressed with structural allograft or metal filling devices (cones and sleeves). Stemmed components, either cemented or cementless, are recommended to reduce the strain at the interface implant-host. Conclusions: The treatment of bone defects in revision TKAs has evolved during the last years providing different options with good results at a short/medium term follow up. With the increasing revision burden, further scientific evidence is requested to identify the best approach for each patient. Long-term clinical outcome as well as implant survival after revision TKA are still sub-optimal and depend upon many factors including cause for revision, surgical approach, type of implants used and various patient factors. (www.actabiomedica.it)
Keywords: revision knee arthroplasty, metaphyseal bone loss, treatment options, cone, sleeve, allograft, stem
Introduction
The number of revision total knee arthroplasties (TKA) is rising worldwide following the increased demand for primary knee arthroplasty, especially because of patients’ higher functional requests and longer life expectation (1-3). Whilst performing a revision TKA, several factors should be considered, including the pre-operative planning, the surgical approach, type of primary component and ease or difficulty associated with their removal, the joint line restoration, degree of constraint needed to provide a stable construct and ability to provide durable component fixation. Additionally, these operations become usually more complex because of the presence of bone defects, which may be determined by periprosthethic infection, implant loosening, wear and osteolysis and fractures of either tibial plateau or distal femur (4). Revision TKAs require an accurate analysis of the host bone quality and of the type and location of the defects.
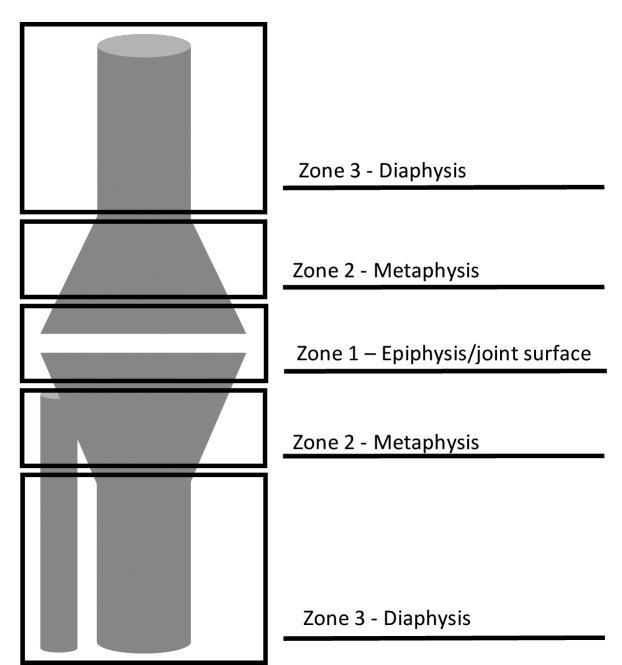
A proper planning is mandatory but the final evaluation of the bone loss is determined intra-operatively after the components’ removal and thorough debridement of fibrous and necrotic tissues. It is important not to underestimate the defect and having different treatments options available. Invariably radiographs underestimate the extent of the defect. Cement, morcellized or structural autografts and allografts, metal augments and filling implants may be useful in this set-up to fill the gaps and stems are needed to better distribute the loads, but currently the optimal method has not yet been established. According to the zonal fixation concepts (Fig. 1) (5), these options help in promoting an additional fixation in the metaphysis (zone 2) and diaphysis (zone 3), as joint surface and epiphysis (zone 1) are usually inadequate.
Figure 1.
Zonal fixation concept
The surgical challenge is to obtain a stable platform to implant the revision components with an optimal joint line level (6).
Classification
A correct classification of the bone defect is mandatory to be fully prepared to face a revision TKA and to predict and compare outcomes. The potential need of bone allografts, long stems and new generation fixation devices should be predicted before the operation time to avoid intra-operative problems. The most useful radiological studies to define the defects are the orthogonal tibial radiographs and a lateral distal femoral view (7). CT scan may help but it is not mandatory. Several classifications have been proposed but actually no one fully meets the clinical demands (see table 1) (8-15). Subjectivity and underestimation of the defects are the most common faults among the classifications during the pre-operative assessment, especially for the femur. On the other hand, intra-operative evaluation improves the accuracy, being more factual after the potential bone loss due to the components removal.
Table 1.
Bone loss classifications
| Classification | Year | Joint side | Assessment | Informations on implant stability | Guide to treatment | Defects features |
|---|---|---|---|---|---|---|
| Dorr | 1989 | Tibia | Intraoperative | No | No | Morphology |
| Rand | 1991 | Femur | Intraoperative | No | No | Dimensions |
| Bargar & Gross | 1992 | Tibia/femur | Pre-/Intraoperative | Yes | Yes | Morphology |
| Elia & Lotke | 1991 | Tibia/femur | Intraoperative | No | No | Dimensions |
| Insall | 1993 | Tibia/Femur | Intraoperative | No | Yes | Morphology |
| Dimensions | ||||||
| Slooff & Malefijt | 1995 | Tibia/Femur | Intraoperative | No | No | Dimensions |
| Anderson Orthopaedic | 1997 | Tibia/Femur | Pre-/Intraoperative | Yes | Yes | Morphology |
| Research Institute (AORI) | Dimensions | |||||
| Massachusetts General Hospital | 2000 | Femur | Intraoperative | No | No | Dimensions |
| SOFCOT | 2000 | Tibia/Femur | Pre-/Intraoperative | Yes | Yes | Morphology |
| Dimensions | ||||||
| University of Pennsylvania | 2003 | Tibia/Femur | Preoperatory | No | Yes | Morphology |
| Clatworthy and Gross | 2003 | Tibia/Femur | Intraoperative | No | Yes | Dimensions |
| Huff and Sculco | 2007 | Tibia/Femur | Pre-/Intraoperative | No | Yes | Morphology |
The assessed criteria include severity, location, size, containment of the defect and implant stability.
Nowadays the most frequently used classification is the Anderson Orthopaedic Research Institute (AORI), first proposed in 1997 (11). It describes the lesions according to the size, localization and soft tissues involvement and it is divided into three ranks. Type 1 includes contained defects limited to the cancellous bone close to the original joint line with intact metaphyseal bone. Type 2A and 2B damages involve metaphyseal bone of one or both condyles or hemi-plateaus respectively. In type 3 the metaphyseal bone loss involves also collateral ligaments and patellar tendon attachments involvement.
Small/contained defects (AORI type 1)
Several options of treatment should be evaluated: cement, cement with screws, autografts or allografts bone (16-18).
Cement
Bone cement is the best surgical choice to fill the bone loss for bone defect less than 5 mm in width and depth, for peripheral deficiency up to 10% of the condylar area, for small central defects, for cystic defects and contained bone defects (9,13,19,20). On the other hand, cement is not a biological scaffold and it is not the recommended procedure in case of uncontained defects mainly for three reasons: first, large amount of cement may lead to potential osseous thermal necrosis secondary to the heat polymerization with blood supply impairment; secondly pressurizing cement in the setting of sclerotic uncontained defects may be difficult; finally cement can lose up to 2% of its volume during its polymerization, leading to a decreased mechanical stability (21).
Cement versatility gives the opportunity to readily fit and fill the size and shape of the defect (21-23).
Indeed, cement is usually the treatment of choice for small defects in elderly patients, being its osteoinductive and osteoconductive capacities not significant in comparison to bone grafts, which are usually preferred in younger patient to preserve and possibly improve the residual bone stock in function of further revisions (24, 25).
Caution is suggested if host bone has sclerotic features because less penetration is allowed into the cancellous bone. These cases should benefit of bone drilling (using drill bits of about 3,5 mm in diameter) to enhance cement penetration and increase the surface contact. Although cement has inferior load transfer properties compared to custom implants or metal augments, its utilization has led to favourable clinical results in selected cases.
Different authors describe good clinical results in long term follow up. Lotke et al reported of 59 knees with a defect of 10 to 20 mm (33 knees) or >20 mm (23 knees) treated with cement and followed up for a mean of 7,1 years. No radiolucent lines were reported in 43 knees, with only one failure needing revision. There was no correlation between radiolucent lines and symptoms (26). Dorr et al reported of 54 patients with AORI type 1 defect treated with cement and followed up for 7 years, reporting good outcomes in all of them, except for one who had loosening (27).
Cement with screws
Some authors also suggest the use of cement in combination with screws in case of contained or uncontained defects between 5 mm and 10 mm both for proximal tibia and distal femur (28).
Therefore, this treatment option should be taken into consideration for both AORI type 1 and AORI type 2A bone defects involving less than 50% of condylar width and up to 10 mm in depth. Screws are used to distribute the load away from the joint line and cement bone interface (29, 30).
This type of fixation results in 30% less loosening of the prosthesis than cement alone in tibial defects reconstruction. It may be used with titanium screw for distal femoral condyle defects: the screws reinforce the cement supporting the deficient condyle, especially when the femoral chamfer cuts did not provide enough rotational stability. Screws should be sunk into the cement enough to avoid contact between their heads and the implant. This solution is suitable also for AORI type 1 defects when used with standard non-stemmed implants. It is reliable, reproducible, easily performed and inexpensive (30, 31).
Different authors have described good clinical results in midterm follow up. Ritter et al reported a study with 57 patients with tibial defects followed up for 3 years. 25% had non-progressive radiolucency at the bone cement interface, but none of the components failed; after 7 years, there was no progression of radiolucency lines either at the bone-cement or at the cement-prosthesis interface (29, 32).
Autografts or allografts bone
These types of grafts are generally used to manage moderate sized contained defects, in the form of impaction grafting with morcellized cancellous bone 18,33-(35). Bone chips used should be as large as practical (about 3-to-5 mm in diameter) to ensure early stability. Adequate impaction force makes morcellized bone grafts strong enough to carry the load while excessive impaction reduces host bone ingrowth (36, 37). Impaction grafting is unique in its osteoconductive ability allowing a more rapid revascularization of the bone graft compared to structural allografts with progressive incorporation and remodelling of the bone graft, shown also radiographically (38). Incorporating autograft with the morcellized allograft bone may add osteoinductive properties to the construct (39).
Bone grafts are usually preferred in younger patients in whom further revisions are predictable and a potential bone stock restoration is desirable (27). Donor site options for autograft include the resected condyles, intercondylar notch and iliac crest with limited availability especially in revision TKA; while there are several sources for allograft such as the distal end of the femur, the proximal part of the tibia and the femoral head, with the latter being the preferred one for morcellized bone (40). Bone allografts are cost-effective compared with metal augments with autologous bone grafts further breaking down this cost. Both solutions are able to increase physiologic load transfer compared to cement (41).
Allograft may be ordered as fresh-frozen, frozen with radiation, freeze-dried, mineralized or demineralized. Bone allograft processing, preservation and sterilization can alter both its initial physical and chemical properties and immune response hence compromising the mechanical stability of the surgical reconstruction. Cryopreserved cancellous graft are superior to freeze-dried cancellous grafts and demineralized cortical grafts in terms of biological efficacy. Mineralized cancellous graft have greater osteoconductive but weaker mechanical properties than demineralized cortical grafts. Long term stability and outcome are affected by the quality of the bone graft, post-harvesting treatment and vascularization of the host cavity (42).
Use of allograft raises the risk of non-union, mal-union or late collapse, and there is a minimal risk of disease transmission compared to autograft. The latter risk is further reduced by 25 kGy radiation treatment without affecting the solidity of a frozen allograft (43, 44).
Midterm results are available for impaction allograft reconstruction of bone loss in revision TKA.
Lotke et al reported 48 revision TKA cases with bone loss treated with impaction allograft (38). All radiographs demonstrated incorporation and remodelling of the bone graft with no mechanical failures at an average of 3.8 years of follow up. Hanna et al demonstrated a cumulative prosthesis survival of 98% at 10 years: 5 patients (9%) had re-operations for complications unrelated to bone graft and 3 patients (5%) developed progressive radiolucencies (18)
While several studies support the versatility and durability of impaction grafting, Hilgen et al highlighted the potential limitation of impaction grafting for more severe defects. Indeed, they reported a survival rate of 50% at 10 years of follow up in revision of rotational and hinged TKAs with AORI type 2 and 3 defects. Failures were related to mechanical breakdown and aseptic loosening of the components showing a lack of incorporation with bone graft in the femur or tibia during the re-revision procedure in all failed cases (45).
Small/uncontained defects (AORI type 2A-2B)
In the last twenty years, many modular prosthetic design have been developed to provide support to orthopaedic surgeon in revision TKAs.
According to the dimensions and the location of these defects there are different surgical solutions.
Uncontained, 5 to 20 mm deep defects, with cortical rim breached, may be ideally managed with modular metal augments that selectively fill bone deficiencies, for example at the distal and posterior femoral condyles or at the proximal tibia (46). Augments are available in wedge or block shape, from 5 mm to 25 mm of size to fit a wide range of defects of one or both condyles (47). They are usually bonded to the implant out of the surgical field, then cemented to the prepared bone. Contrarily to cement, which fits the gap, augments require a reshaping of the defects with some bone sacrifice, especially if blocks are used. Wedge augments, which may be useful in unicompartmental-to-total knee revision in case of tibial plateau collapse, allow more bone preservation, being on the other side subject to shear stress because of their oblique nature (48, 49). Symmetric blocks help in restoring the joint line while asymmetric augments contribute in filling the defect and to rotational stability, as it often happens for the postero-lateral femoral condyle (50). Failure commonly happens when the surgeon faces severe bone loss with impairment of cancellous bone structures. In these situations, the device-host bone interface is compromised, thus structural allograft or porous metaphyseal implants should be used in addition to reach a stable construct.
Large/uncontained defects (AORI type 3)
AORI type 2B and 3 defects have been usually treated with large allograft or with custom made implants. The evolution in materials have brought to the development of porous coated metal devices to improve metaphyseal fixation and achieve primary mechanical stability. Available options include cones made of tantalum or porous titanium and metaphyseal sleeves.
Available literature about these options since 2007 is reported in Table 2 (for allografts (7,40,51-57)), Table 3 (for cones (6,57-73)) and Table 4 (for sleeves (16,74-82)).
Table 2.
Literature review about allografts
| Authors | Year | Journal | Patients/Knees | Classification | Joint side | Allografts | Age (range) years | FU (range) years | Failure for loosening | Failure for infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Engh et al | 2007 | J Bone Joint Surg Am | 47/49 | AORI | T | 45 FH 3 PT 1 DF |
67 (39-86) | 8.1 (5.1-15.9) | 0 | 1 |
| Burnett et al | 2009 | Iowa Orthop J | 8/8 | AORI | B | 6 FH 1 PT 1 DF |
67 (52-78) | 4.0 (2.0-8.0) | 1 | 0 |
| Bauman et al | 2009 | Clin Orhop Rel Res | 74/79 | AORI | B | 63 FH | 68 (34-87) 7 PT 17 DF |
7.5 (5.0-14.8) | 8 | 5 |
| Lyall et al | 2009 | Knee | 15/15 | AORI | T | 15 FH | 59 (38-69) | 5.4 (2.8-9.6) | 1 | 1 |
| Richards et al | 2011 | J Arthrop | 24/24 | AORI | B | 29 FH | 73 (NR) | 4.0 (2.0-8.2) | 0 | 0 |
| Franke et al | 2013 | Acta Orthop Belg | 9/9 | AORI | B | NR | 72 (60-85) | 5.0 (1.0-14.0) | 3 | NR |
| Wang et al | 2013 | Bone Joint J | 28/30 | AORI | B | 50 FH | 70 (53-79) | 6.3 (3.2-11.3) | 0 | 0 |
| Chun et al | 2014 | Knee | 27/27 | AORI | B | 27 FH | 68 (55-76) | 8.9 (8.0-13.1) | 0 | 1 |
| Sandiford et al | 2017 | Clin Orthop Relat Res | 30/30 | AORI | B | 30 FH | 66 (30-85) | 9.1 (6.0-12.0) | 0 | 0 |
| Total | 262/271 |
265 FH 11 PT 19 DF |
13 | 8 |
T: tibia; B: both; FH: femoral head allograft; PT: proximal tibia allograft; DF: distal femur allograft; FU: follow up; NR: not reported
Table 3.
Literature review about cones
| Authors | Year | Journal | Patients/Knees | Classification | Joint side | Cones | Age (range) years | FU (range) years | Failure for loosening | Failure for infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Meneghini et al | 2008 | J Bone Joint Surg Am | 15/15 | AORI | T | 15 | 68 (41-81) | 2.8 (2.0-3.9) | 0 | 2 |
| Long et al | 2009 | J Arthrop | 15/16 | AORI | T | 16 | 66 (48-83) | 2.6 (2.0-3.2) | 0 | 2 |
| Howard et al | 2011 | J Bone Joint Surg Am | 24/24 | AORI | F | 24 | 64 (46-79) | 2.8 (2.0-4.2) | 0 | 0 |
| Lachiewicz et al | 2012 | Clin Orhop Relat Res | 27/27 | AORI | B | 33 | 65 (49-84) | 3.3 (2.0-5.7) | 1 | 1 |
| Panni et al | 2013 | Knee Sports Surg Traumatol Arthrosc | 9/9 | AORI | B | 9 | 75 (65-84) | 7.0 (4.5-9.0) | 0 | 0 |
| Rao et al | 2013 | Bone Joint J | 26/26 | AORI | B | 30 | 72 (62-84) | 3.0 (2.0-4.1) | 0 | 2 |
| Schmitz et al | 2013 | J Arthrop | 38/38 | AORI | B | 54 | 72 (44-85) | 3.1 (2.7-4.0) | 1 | 0 |
| Villanueva- Martinez et al | 2013 | J Arthrop | 21/21 | AORI | B | 29 | 73 (62-86) | 3.0 (0.5-4.6) | 1 | 2 |
| Mozella Ade et al | 2014 | Rev Bras Orthop | 10/10 | AORI | B | 21 | 71 (59-80) | 2.9 (1.0-3.8) | 0 | 1 |
| Derome et al | 2014 | J Arthrop | 29/29 | AORI | B | 33 | 70 (36.84) | 2.8 (1.1-6.1) | 0 | 2 |
| Jensen et al | 2014 | Knee | 36/36 | AORI | T | 36 | 69 (51-84) | 3.9 (0.3-7) | 1 | 2 |
| Bedard et al | 2015 | J Arthrop | 21/21 | AORI | B | 25 | 75 (58-91) | NR | 0 | 0 |
| Boureau et al | 2015 | Orthop Traumatol Surg Res | 7/7 | SOFCOT | F | 14 | 65 (51-79) | 1.4 (1.0-2.1) | 0 | 0 |
| Brown et al | 2015 | J Arthrop | 79/79 | AORI | B | NR | 69 (32-91) | 3.3 (2.0-7.0) | 1 | 8 |
| De Martino et al | 2015 | Clin Orthop Relat Res | 18/18 | AORI | B | 26 | 73 (55-84) | 6.0 (5.0-8.0) | 0 | 2 |
| Kamath et al | 2015 | J Bone Joint Surg Am | 63/63 | AORI | T | 66 | 67 (41-83) | 5.8 (5.0-8.8) | 1 | 1 |
| Girerd et al | 2016 | Orthop Traumatol Surg Res | 51/52 | AORI | B | 71 | 68 (42-89) | 2.8 (2.0-4.3) | 0 | 4 |
| Potter et al | 2016 | J Bone Joint Surg Am | 157/157 | AORI | B | 159 | 64 (24-85) | 5.0 (2.0-10.0) | 6 | 14 |
| Sandiford et al | 2017 | Clin Orthop Relat Res | 14/14 | AORI | B | NR | 71 (44-84) | 7.2 (5.0-9.0) | 1 | 0 |
| Total | 660/662 | 13 | 43 |
T: tibia; F: femur B: both; FU: follow up; NR: not reported.
Table 4.
Literature review about sleeves
| Authors | Year | Journal | Patients/Knees | Classification | Joint side | Sleeves | Age (range) years | FU (range) years | Failure for loosening | Failure for infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Alexander et al | 2013 | J Arthrop | 28/30 | AORI | T | 30 | 71 (48-83) | 2.8 (2.0-4.3) | 0 | 1 |
| Agarwal et al | 2013 | Bone Joint J | 103/104 | AORI | B | 164 | 69 (48-92) | 3.6 (2.5-5.4) | 2 | 0 |
| Barnett et al | 2014 | J Arthrop | 40/40 | AORI | T | 40 | 66 (49-88) | 3.2 (2.0-5.2) | 0 | 1 |
| Huang et al | 2014 | Orthopaedics | 79/83 | AORI | B | 119 | 64 (NR) | 2.4 (2.0-3.7) | 2 | 6 |
| Bugler et al | 2015 | J Arthrop | 34/35 | AORI | B | 59 | 72 (55-86) | 3.3 (2.0-5.2) | 0 | 0 |
| Graichen et al | 2015 | J Arthrop | 121/121 | AORI | B | 193 | 74 (NR) | 3.6 (2.0-6.1) | 4 | 4 |
| Chalmers et al | 2016 | J Arthrop | 227/227 | AORI | B | 322 | 66 (31-90) | 3.2 (2.0-8.0) | 2 | 12 |
| Dalury et al | 2016 | Knee | 40/40 | NR | B | NR | 73 (50-80) | 4.8 (4.0-12.0) | 1 | 0 |
| Gottsche et al | 2016 | Arch Orthop Trauma Surg | 71/71 | AORI | B | NR | NR | NR | 1 | 1 |
| Martin- Hernandez et al | 2016 | Knee Sports Surg Traumatol Arthrosc | 150/150 | AORI | B | NR | 75 (51-88) | 6.0 (1.0-8.9) | 0 | 2 |
| Total | 893/901 | 12 | 27 |
T: tibia; B: both; FU: follow up; NR: not reported
Allograft
Structural allograft is an attractive biological option in the treatment of bone defects, especially in young patient, aiming to potential bone restoration in prevision of eventual further revisions. Alternatives include femoral head, bulk distal femur or proximal tibia.
The possibility of shaping the allograft, especially femoral heads, according to each case is one of the main advantages of this technique. The defect must be cleared of all soft tissues, osteolytic membranes and residual cement. At this point the graft is prepared removing with a burr or a female reamer the sclerotic peripheral bone at the interface with the host to fit into the defect (54). If a femoral head allograft is used, the diameter of the male reamer to prepare the host bone should be 2 mm narrower to obtain a primary press-fit fixation. Temporary fixation of the graft is improved with 2 or 3 K-wires, usually parallel to the expected joint line, which do not interfere with the implant stem. A burr is used to remove excess bone graft, then proceeding with canal preparation and usual cuts to receive the trial implant.
Stemmed components, either cemented or press-fit, need to be used to bypass the defect and to reduce stresses on the allograft, host bone and fixation interface (53, 83). Additional plates and screws may help to achieve primary stability, especially in the larger uncontained defects.
Weaknesses of the allograft comprise of a limited availability, a higher susceptibility to infection (51), non-union, fracture and periprosthethic reabsorption resulting in implant loosening (53). According to Buck et al, the risk of diseases transmission, although present, is very low if strict donor selection criteria and screening are performed (39). Baumann et al found in 65 knees a greater than 20% rate of complications and failures mainly related directly or indirectly to the allografts. In their series allograft size seems to have a role in the failure mechanism, as smaller allografts like femoral heads tend to fail because of resorption with secondary implant loosening. On the other side, larger allografts were more frequently affected by infection or non-union leading to failure. Other factors affecting the bony union are the immune response of the host and the graft type.
Although good clinical results are found in successful implants, the unpredictability of the incorporation process with very limited revascularization and remodelling have been already described by Stevenson et al (84, 85). As found by Parks et al after an average of 41 months, only the peripheral portions of the grafts were infiltrated by some new bone without evidence of remodelling and revascularization. This explains an incidence of non-union of 11% for large frozen allografts, even if it does not always mean that the graft is not retained (85, 86).
More experience and longer follow up studies than porous metal augments are available for allografts in the literature since the first nineties’.
For uncontained defects the use of impaction grafting is not recommended, except in combination with metal augments ormeshes (87).
Highly porous tantalum cones
Highly porous cones have been using for many years in a variety of reconstructive procedure, especially in hip replacement to treat severe acetabular bone loss (60, 88-92). They are usually made of tantalum but more recently porous titanium devices have been introduced. Available literature is focused mainly on the former as the latter does not have any long-term published data at present.
An average porosity of 80% with modulus of elasticity (3 GPa) close to cancellous bone allows for a more physiological load transfer reducing stress shielding and improving osteoconductive properties with better potential osteointegration (60, 93, 94). The low modulus of elasticity and the high coefficient of friction contribute in providing a stable scaffold aimed to joint reconstruction (85). Furthermore, histological studies have shown low potential for bacterial adherence with greater leucocyte activation, reducing the risk of infections (95, 96).
Nowadays several sizes and shapes of cones are available for both knees with symmetrical and asymmetrical options to fit most of the defects (97). The surgical technique for cone insertion include host bone sculpturing with a broach or free-hand high speed burr to optimise cone contact and enhance bone ingrowth. The cone is press-fitted into position and cement is used only to fix the implant to the porous device, allowing a wider range of implant rotation and alignment, independently from cone location. Eventual gaps between the porous surface and the host bone should be filled with morcellized bone allograft, autograft or bone substitutes (60). Axial stability is provided by stems, either cemented or cementless, while rotational stability is improved by keel and box together with the cone for tibial and femoral component respectively.
Cones did not affect the use of uncemented stems allowing to reach a canal fill ratio >85% in most patients, as Bedard et al showed (58). In selected cases of severe bone loss, using two overlapped cones has been described by Boureau et al in 2015 to manage massive femoral defects (59).
Immediate metaphyseal stability allows an early weight bearing.
Re-operation need is usually determined by an infection relapse while device aseptic loosening at the bone-cone interface is very rarely reported (less than 1% according to the available literature) (60), indeed a secure fixation at 5 years of follow up has been confirmed also using radiostereometric analysis (RSA) (97-99).
The main disadvantage of cones similarly to sleeves, is the difficult extraction in case of further revisions, showing solid osteointegration also in situations of re-infection (6, 61, 100), thus making cones not the first-line option for bone loss in young patients. A careful surgical technique is recommended to reduce the risk of patellar tendon avulsion and of intra-operative fractures during broaching or cone impaction in consideration of the low-quality residual bone stock (70, 97).
Available literature, mainly about tantalum, confirms that metal cones represent a viable option in term of surgical efficacy, clinical and radiological results, being at least as effective as other strategies.
Titanum sleeves
Metal sleeves are available both for tibial and femoral component. Unlike cones, sleeves are bonded to the implant with a Morse taper junction instead of cement, removing a possible source of failure at the cement-implant interface (16).
Primary stability, either axial and /or rotational, is achieved press-fit by an instrumented broach which help in preparing the host bone (16, 75, 78). On the other hand, the porous surface is aimed to obtain a long-term bone ingrowth to improve the secondary stability.
Various size and lengths of sleeves are available to fill the defects. Usually the Morse junction allows some degree of rotation of the tibial component to fit each case.
The first step of tibial preparation is sequential medullary canal reaming until a stable endosteal fit is reached to achieve a satisfactory rotational stability (16, 75). During broaching the metaphyseal area, it is relevant to check the proper orientation according to surgeon experience and usual reference points as the final stepwise pattern of the endosteal metaphyseal bone will force the component rotation.
The final step is the proximal resection using the final broach as a tibial cutting guide. Tibia is now ready to hold the trial implant.
If there is significant bone loss also on the femoral side, a sleeve may be used, eventually with augments. It is important to establish the distal cut which determines the joint line. The medullary canal is reamed in the same way of the tibia, being aware of the femoral bowing, which may force the component in the wrong position if a too long stem is chosen (78). Once the surgeon is satisfied with the trials, final components are assembled to the sleeves through the Morse junction on the instrument table and then finally implanted onto the broached area (74).
The most frequent intra-operative complication related to sleeves is fracture during broaching or when impacting the final components, as it may happen in total hip arthroplasty on the femoral side (75, 76). End-of-stem tibia pain is one of the most frequent long term complications (16). It is usually due to the stem length, which should be sufficient to help intra-operative alignment and to contribute to early stability, not forgetting that the main fixation relies mainly on the metaphyseal press fit of the sleeves (78)
In case of re-revision, removal of a well-fixed sleeve may become a problem, determining a further and more severe bone loss. Special instruments are available but a tibial tuberosity osteotomy is often necessary to remove the sleeve.
As the final stability is reached with secondary osteointegration of the sleeves, weight bearing may need to be protected at the beginning especially when the sleeve is used on the femoral side where rotational stability may be more compromised.
According to various short-to-medium term results, these cementless metaphyseal sleeves seem to offer a proper option to manage AORI type 2B and 3 deficits, in terms of subjective, functional and radiological outcomes.
Mega-prosthesis and modular endoprosthesis
Distal femoral and proximal tibia replacement are usually indicated for knee reconstruction after tumour resection. In selected elderly patients with severe bone loss, articular deformities and extreme ligamentous instability, they may represent an appropriate limb-saving procedure to reach an immediate stability also in these non-oncological conditions (101).
Original designs, consisting in hinged implant without any degree of rotation, were affected by high rate of mechanical failure because of implant loosening. The introduction of rotating hinge platform has lowered the failure rate, allowing a more physiological load transmission (102).
Modular endoprosthesis have progressively replaced custom-made mega-prosthesis because of the cost, ductility and prompt availability in the operation theatre (103). Furthermore, in case of mechanical failure of the implant, it is possible to replace only the failed component.
Functional outcomes, especially in elderly low-demand patients (104), appear to be satisfactory in terms of quality of life.
Main issues with this kind of replacement are the restoration of the correct length and rotation of the limb, the reconstruction of the extensor mechanism, the primary and secondary stability and the wound healing (105). Because of the associated poor soft-tissue envelope, the extended approach and prolonged operative time, infection represents one of the most common and disastrous complications, which usually lead to above-the-knee amputation (106).
Modular stems
Stemmed components are mandatory in revision TKA to bypass the metaphyseal bone defect and to reduce the strain at the implant-host bone interface, providing additional surface for implant fixation. Their length is important but the cornerstone is the bone/stem engagement level at which a stable fixation is achieved.
Cementless stems are used with a hybrid fixation, engaging the cortical bone of the diaphysis but with cement at the implant-host bone interface close to the joint. They allow a good primary stability and are easier to remove (58). On the other hand, longer stems may determine end-of-stem tibial pain (up to 10% of patients) and may require off-setting if diaphyseal engagement results in mal-alignment or increased risk of fracture (38, 78, 107-109). This is the reason why cementless stems are preferred if good diaphyseal bone of adequate geometry is available.
Cemented stems become more useful for patients with large, osteopenic endomidollary canals or in presence of axial deformities. They are metaphyseal engaging with cement filling the gaps between stem and cancellous bone. They are usually shorter than press-fit stems because they do not need to engage the endomidollary cortical bone, not influencing the final implant positioning but with higher risk of mal-alignement (58, 110). In case of re-revision smooth and tapered stems are usually easy to remove but the residual cement may determine further bone loss during its removal.
Both cemented and press-fit stems have good outcome and may be useful in specific patients but the choice remains controversial (78, 111). In a recent level IV systematic review by Wang et al, no significant difference was found in terms of in failure for any reason, reoperation, aseptic loosening and infection between revision TKA with cemented or cementless stem fixation (112). Overall bone quality and bone defects, together with the surgeon’s preference seem to lead the choice.
Conclusions
Several options are available in addressing metaphyseal bone loss in revision TKA. The choice depends on the type, size and location of the defect and on the quality of the host bone.
Classifications are useful to quantify the defects and to plan the operation, but the final evaluation should be intra-operative once components have been removed.
“Fill and fix” is the cornerstone concept to pursue during revision TKAs resulting in filling the defects with impaction grafting, cement or metaphyseal porous devices to achieve a satisfactory fixation.
Literature does not provide any evidence based approach. Data are hardly pooled for the differences among studies in terms of classification, techniques, follow up and definition of failure. Available systematic review and meta-analysis are usually of low quality mainly because examined studies are case series without control groups. The wide variety of intra-operative scenarios and the confidence of the surgeon in one technique rather than another make difficult to conduct a controlled trial comparing different options. Further studies with long term results are desirable to draw firm conclusions.
Acknowledgments
The authors would like to thank Prof. Hemant Pandit for his kind support.
References
- 1.Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthro. 2009;24:195–203. doi: 10.1016/j.arth.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–12. doi: 10.1007/s11999-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Causero A, Benedetto P, Beltrame A, Gisonni R, Cainero V, Pagano M. Design evolution in total knee replacement: which is the future? Acta Biomed. 2014;85(Suppl 2):5–19. [PubMed] [Google Scholar]
- 4.Ponzio DY, Austin MS. Metaphyseal bone loss in revision knee arthroplasty. Curr Rev Musculoskelet Med. 2015;8:361–7. doi: 10.1007/s12178-015-9291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan-Jones R, Oussedik SI, Graichen H, Haddad FS. Zonal fixation in revision total knee arthroplasty. Bone Joint J. 2015;97-B:147–9. doi: 10.1302/0301-620X.97B2.34144. [DOI] [PubMed] [Google Scholar]
- 6.Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21:1233–7. doi: 10.1016/j.knee.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthro. 2011;26:1299–304. doi: 10.1016/j.arth.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Waal Malefijt MC, Kampen A, Slooff TJ. Bone grafting in cemented knee replacement. 45 primary and secondary cases followed for 2-5 years. Acta Orthop Scand. 1995;66:325–8. doi: 10.3109/17453679508995554. [DOI] [PubMed] [Google Scholar]
- 9.Dorr LD. Bone grafts for bone loss with total knee replacement. Orthop Clin North Am. 1989;20:179–87. [PubMed] [Google Scholar]
- 10.Elia EA, Lotke PA. Results of revision total knee arthroplasty associated with significant bone loss. Clin Orthop Relat Res. 1991:114–21. [PubMed] [Google Scholar]
- 11.Engh GA, Parks NL. The management of bone defects in revision total knee arthroplasty. Instr Course Lect. 1997;46:227–36. [PubMed] [Google Scholar]
- 12.Huff TW, Sculco TP. Management of bone loss in revision total knee arthroplasty. J Arthro. 2007;22:32–6. doi: 10.1016/j.arth.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Rand JA. Bone deficiency in total knee arthroplasty. Use of metal wedge augmentation. Clin Orthop Relat Res. 1991:63–71. [PubMed] [Google Scholar]
- 14.Hoeffel DP, Rubash HE. Revision total knee arthroplasty: current rationale and techniques for femoral component revision. Clin Orthop Relat Res. 2000:116–32. [PubMed] [Google Scholar]
- 15.Mulhall KJ, Ghomrawi HM, Engh GA, Clark CR, Lotke P, Saleh KJ. Radiographic prediction of intraoperative bone loss in knee arthroplasty revision. Clin Orthop Relat Res. 2006;446:51–8. doi: 10.1097/01.blo.0000214438.57151.a5. [DOI] [PubMed] [Google Scholar]
- 16.Alexander GE, Bernasek TL, Crank RL, Haidukewych GJ. Cementless metaphyseal sleeves used for large tibial defects in revision total knee arthroplasty. J Arthro. 2013;28:604–7. doi: 10.1016/j.arth.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Daines BK, Dennis DA. Management of bone defects in revision total knee arthroplasty. Instr Course Lect. 2013;62:341–8. [PubMed] [Google Scholar]
- 18.Hanna SA, Aston WJ, Roeck NJ, Gough-Palmer A, Powles DP. Cementless revision TKA with bone grafting of osseous defects restores bone stock with a low revision rate at 4 to 10 years. Clin Orthop Relat Res. 2011;469:3164–71. doi: 10.1007/s11999-011-1938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter MA, Keating EM, Faris PM. Screw and cement fixation of large defects in total knee arthroplasty. A sequel. J Arthro. 1993;8:63–5. doi: 10.1016/s0883-5403(06)80109-9. [DOI] [PubMed] [Google Scholar]
- 20.Toms AD, Barker RL, McClelland D, Chua L, Spencer-Jones R, Kuiper JH. Repair of defects and containment in revision total knee replacement: a comparative biomechanical analysis. J Bone Joint Surg Br. 2009;91:271–7. doi: 10.1302/0301-620X.91B2.21415. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PJ, Walker PS, Scott RD. Tibial component fixation in deficient tibial bone stock. Clin Orthop Relat Res. 1984:302–8. [PubMed] [Google Scholar]
- 22.Persson BM, Ekelund L, Lovdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand. 1984;55:209–14. doi: 10.3109/17453678408992339. [DOI] [PubMed] [Google Scholar]
- 23.Persson BM, Wouters HW. Curettage and acrylic cementation in surgery of giant cell tumors of bone. Clin Orthop Relat Res. 1976:125–33. [PubMed] [Google Scholar]
- 24.Lachiewicz PF, Soileau ES. A 30-mm cemented stem extension provides adequate fixation of the tibial component in revision knee arthroplasty. Clin Orthop Relat Res. 2015;473:185–9. doi: 10.1007/s11999-014-3529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson CL, Vanushkina M, Irgit K, Strohecker K, Bowen TR. Stemmed femoral implants show lower failure rates in revision total knee arthroplasty. Knee. 2015;22:429–34. doi: 10.1016/j.knee.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Lotke PA, Wong RY, Ecker ML. The use of methylmethacrylate in primary total knee replacements with large tibial defects. Clin Orthop Relat Res. 1991:288–94. [PubMed] [Google Scholar]
- 27.Dorr LD, Ranawat CS, Sculco TA, McKaskill B, Orisek BS. Bone graft for tibial defects in total knee arthroplasty. Clin Orthop Relat Res. 1986:153–65. [PubMed] [Google Scholar]
- 28.Daines BK, Dennis DA. Management of bone defects in revision total knee arthroplasty. J Bone Joint Surg Am. 2012;94:1131–9. doi: 10.2106/JBJS.L00143. [DOI] [PubMed] [Google Scholar]
- 29.Ritter MA. Screw and cement fixation of large defects in total knee arthroplasty. J Arthro. 1986;1:125–9. doi: 10.1016/s0883-5403(86)80050-x. [DOI] [PubMed] [Google Scholar]
- 30.Scott RD. Bone loss: prosthetic and augmentation method. Orthopedics. 1995;18:923–6. doi: 10.3928/0147-7447-19950901-42. [DOI] [PubMed] [Google Scholar]
- 31.Gross AE. Revision total knee arthroplasty of bone grafts versus implant supplementation. Orthopedics. 1997;20:843–4. doi: 10.3928/0147-7447-19970901-27. [DOI] [PubMed] [Google Scholar]
- 32.Ritter MA, Harty LD. Medial screws and cement: a possible mechanical augmentation in total knee arthroplasty. J Arthro. 2004;19:587–9. doi: 10.1016/j.arth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Lonner JH, Lotke PA, Kim J, Nelson C. Impaction grafting and wire mesh for uncontained defects in revision knee arthroplasty. Clin Orthop Relat Res. 2002:145–51. doi: 10.1097/00003086-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Lotke PA, Carolan GF, Puri N. Technique for impaction bone grafting of large bone defects in revision total knee arthroplasty. J Arthro. 2006;21:57–60. doi: 10.1016/j.arth.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Lotke PA, Carolan GF, Puri N. Impaction grafting for bone defects in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:99–103. doi: 10.1097/01.blo.0000214414.06464.00. [DOI] [PubMed] [Google Scholar]
- 36.Bolder SB, Schreurs BW, Verdonschot N, Unen JM, Gardeniers JW, Slooff TJ. Particle size of bone graft and method of impaction affect initial stability of cemented cups: human cadaveric and synthetic pelvic specimen studies. Acta Orthop Scand. 2003;74:652–7. doi: 10.1080/00016470310018144. [DOI] [PubMed] [Google Scholar]
- 37.Tagil M, Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop Relat Res. 1998:231–8. [PubMed] [Google Scholar]
- 38.Mabry TM, Hanssen AD. The role of stems and augments for bone loss in revision knee arthroplasty. J Arthro. 2007;22:56–60. doi: 10.1016/j.arth.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS) Clin Orthop Relat Res. 1989:129–36. [PubMed] [Google Scholar]
- 40.Lyall HS, Sanghrajka A, Scott G. Severe tibial bone loss in revision total knee replacement managed with structural femoral head allograft: a prospective case series from the Royal London Hospital. Knee. 2009;16:326–31. doi: 10.1016/j.knee.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Shrivastava SC, Ahmed AM, Shirazi-Adl A, Burke DL. Effect of a cement-bone composite layer and prosthesis geometry on stresses in a prosthetically resurfaced tibia. J Biomed Mater Res. 1982;16:929–49. doi: 10.1002/jbm.820160616. [DOI] [PubMed] [Google Scholar]
- 42.Tomford WW, Mankin HJ. Bone banking. Update on methods and materials. Orthop Clin North Am. 1999;30:565–70. doi: 10.1016/s0030-5898(05)70109-7. [DOI] [PubMed] [Google Scholar]
- 43.Huten D. Femorotibial bone loss during revision total knee arthroplasty. Orthop Traumatol Surg Res. 2013;99:S22–33. doi: 10.1016/j.otsr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: What they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–9. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 45.Hilgen V, Citak M, Vettorazzi E, et al. 10-year results following impaction bone grafting of major bone defects in 29 rotational and hinged knee revision arthroplasties: a follow-up of a previous report. Acta Orthop. 2013;84:387–91. doi: 10.3109/17453674.2013.814012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucey SD, Scuderi GR, Kelly MA, Insall JN. A practical approach to dealing with bone loss in revision total knee arthroplasty. Orthopedics. 2000;23:1036–41. doi: 10.3928/0147-7447-20001001-14. [DOI] [PubMed] [Google Scholar]
- 47.Lee JK, Choi CH. Management of tibial bone defects with metal augmentation in primary total knee replacement: a minimum five-year review. J Bone Joint Surg Br. 2011;93:1493–6. doi: 10.1302/0301-620x.93b10.27136. [DOI] [PubMed] [Google Scholar]
- 48.Fehring TK, Peindl RD, Humble RS, Harrow ME, Frick SL. Modular tibial augmentations in total knee arthroplasty. Clin Orthop Relat Res. 1996:207–17. doi: 10.1097/00003086-199606000-00026. [DOI] [PubMed] [Google Scholar]
- 49.Chen F, Krackow KA. Management of tibial defects in total knee arthroplasty. A biomechanical study. Clin Orthop Relat Res. 1994:249–57. [PubMed] [Google Scholar]
- 50.Sculco PK, Abdel MP, Hanssen AD, Lewallen DG. The management of bone loss in revision total knee arthroplasty: rebuild, reinforce, and augment. Bone Joint J. 2016;98-B:120–4. doi: 10.1302/0301-620X.98B1.36345. [DOI] [PubMed] [Google Scholar]
- 51.Bauman RD, Lewallen DG, Hanssen AD. Limitations of structural allograft in revision total knee arthroplasty. Clin Orthop Relat Res. 2009;467:818–24. doi: 10.1007/s11999-008-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burnett RS, Keeney JA, Maloney WJ, Clohisy JC. Revision total knee arthroplasty for major osteolysis. Iowa Orthop J. 2009;29:28–37. [PMC free article] [PubMed] [Google Scholar]
- 53.Chun CH, Kim JW, Kim SH, Kim BG, Chun KC, Kim KM. Clinical and radiological results of femoral head structural allograft for severe bone defects in revision TKA--a minimum 8-year follow-up. Knee. 2014;21:420–3. doi: 10.1016/j.knee.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Engh GA, Ammeen DJ. Use of structural allograft in revision total knee arthroplasty in knees with severe tibial bone loss. J Bone Joint Surg Am. 2007;89:2640–7. doi: 10.2106/JBJS.F.00865. [DOI] [PubMed] [Google Scholar]
- 55.Franke KF, Nusem I, Gamboa G, Morgan DA. Outcome of revision total knee arthroplasty with bone allograft in 30 cases. Acta Orthop Belg. 2013;79:427–34. [PubMed] [Google Scholar]
- 56.Wang JW, Hsu CH, Huang CC, Lin PC, Chen WS. Reconstruction using femoral head allograft in revision total knee replacement: an experience in Asian patients. Bone Joint J. 2013;95-B:643–8. doi: 10.1302/0301-620X.95B5.29915. [DOI] [PubMed] [Google Scholar]
- 57.Sandiford NA, Misur P, Garbuz DS, Greidanus NV, Masri BA. No Difference Between Trabecular Metal Cones and Femoral Head Allografts in Revision TKA: Minimum 5-year Followup. Clin Orthop Relat Res. 2017;475:118–24. doi: 10.1007/s11999-016-4898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bedard M, Cabrejo-Jones K, Angers M, Pelletier-Roy R, Pelet S. The Effect of Porous Tantalum Cones on Mechanical Alignment and Canal-Fill Ratio in Revision Total Knee Arthroplasty Performed with Uncemented Stems. J Arthro. 2015;30:1995–8. doi: 10.1016/j.arth.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Boureau F, Putman S, Arnould A, Dereudre G, Migaud H, Pasquier G. Tantalum cones and bone defects in revision total knee arthroplasty. Orthop Traumatol Surg Res. 2015;101:251–5. doi: 10.1016/j.otsr.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Martino I, Santis V, Sculco PK, D’Apolito R, Assini JB, Gasparini G. Tantalum Cones Provide Durable Mid-term Fixation in Revision TKA. Clin Orthop Relat Res. 2015;473:3176–82. doi: 10.1007/s11999-015-4338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derome P, Sternheim A, Backstein D, Malo M. Treatment of large bone defects with trabecular metal cones in revision total knee arthroplasty: short term clinical and radiographic outcomes. J Arthro. 2014;29:122–6. doi: 10.1016/j.arth.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 62.Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93:478–84. doi: 10.2106/JBJS.I.01322. [DOI] [PubMed] [Google Scholar]
- 63.Kamath AF, Lewallen DG, Hanssen AD. Porous tantalum metaphyseal cones for severe tibial bone loss in revision knee arthroplasty: a five to nine-year follow-up. J Bone Joint Surg Am. 2015;97:216–23. doi: 10.2106/JBJS.N.00540. [DOI] [PubMed] [Google Scholar]
- 64.Lachiewicz PF, Bolognesi MP, Henderson RA, Soileau ES, Vail TP. Can tantalum cones provide fixation in complex revision knee arthroplasty? Clin Orthop Relat Res. 2012;470:199–204. doi: 10.1007/s11999-011-1888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long WJ, Scuderi GR. Porous tantalum cones for large metaphyseal tibial defects in revision total knee arthroplasty: a minimum 2-year follow-up. J Arthro. 2009;24:1086–92. doi: 10.1016/j.arth.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Meneghini RM, Lewallen DG, Hanssen AD. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. J Bone Joint Surg Am. 2008;90:78–84. doi: 10.2106/JBJS.F.01495. [DOI] [PubMed] [Google Scholar]
- 67.Panni AS, Vasso M, Cerciello S. Modular augmentation in revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2837–43. doi: 10.1007/s00167-012-2258-1. [DOI] [PubMed] [Google Scholar]
- 68.Rao BM, Kamal TT, Vafaye J, Moss M. Tantalum cones for major osteolysis in revision knee replacement. Bone Joint J. 2013;95-B:1069–74. doi: 10.1302/0301-620X.95B8.29194. [DOI] [PubMed] [Google Scholar]
- 69.Schmitz HC, Klauser W, Citak M, Al-Khateeb H, Gehrke T, Kendoff D. Three-year follow up utilizing tantal cones in revision total knee arthroplasty. J Arthro. 2013;28:1556–60. doi: 10.1016/j.arth.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 70.Villanueva-Martinez M, Torre-Escudero B, Rojo-Manaute JM, Rios-Luna A, Chana-Rodriguez F. Tantalum cones in revision total knee arthroplasty. A promising short-term result with 29 cones in 21 patients. J Arthro. 2013;28:988–93. doi: 10.1016/j.arth.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Brown NM, Bell JA, Jung EK, Sporer SM, Paprosky WG, Levine BR. The Use of Trabecular Metal Cones in Complex Primary and Revision Total Knee Arthroplasty. J Arthro. 2015;30:90–3. doi: 10.1016/j.arth.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 72.Girerd D, Parratte S, Lunebourg A, et al. Total knee arthroplasty revision with trabecular tantalum cones: Preliminary retrospective study of 51 patients from two centres with a minimal 2-year follow-up. Orthop Traumatol Surg Res. 2016;102:429–33. doi: 10.1016/j.otsr.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Potter GD, 3rd, Abdel MP, Lewallen DG, Hanssen AD. Midterm Results of Porous Tantalum Femoral Cones in Revision Total Knee Arthroplasty. J Bone Joint Surg Am. 2016;98:1286–91. doi: 10.2106/JBJS.15.00874. [DOI] [PubMed] [Google Scholar]
- 74.Agarwal S, Azam A, Morgan-Jones R. Metal metaphyseal sleeves in revision total knee replacement. Bone Joint J. 2013;95-B:1640–4. doi: 10.1302/0301-620X.95B12.31190. [DOI] [PubMed] [Google Scholar]
- 75.Barnett SL, Mayer RR, Gondusky JS, Choi L, Patel JJ, Gorab RS. Use of stepped porous titanium metaphyseal sleeves for tibial defects in revision total knee arthroplasty: short term results. J Arthro. 2014;29:1219–24. doi: 10.1016/j.arth.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 76.Bugler KE, Maheshwari R, Ahmed I, Brenkel IJ, Walmsley PJ. Metaphyseal Sleeves for Revision Total Knee Arthroplasty: Good Short-Term Outcomes. J Arthro. 2015;30:1990–4. doi: 10.1016/j.arth.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Chalmers BP, Desy NM, Pagnano MW, Trousdale RT, Taunton MJ. Survivorship of Metaphyseal Sleeves in Revision Total Knee Arthroplasty. J Arthro. 2016 doi: 10.1016/j.arth.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Graichen H, Scior W, Strauch M. Direct, Cementless, Metaphyseal Fixation in Knee Revision Arthroplasty With Sleeves-Short-Term Results. J Arthro. 2015;30:2256–9. doi: 10.1016/j.arth.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 79.Huang R, Barrazueta G, Ong A, et al. Revision total knee arthroplasty using metaphyseal sleeves at short-term follow-up. Orthopedics. 2014;37:e804–9. doi: 10.3928/01477447-20140825-57. [DOI] [PubMed] [Google Scholar]
- 80.Martin-Hernandez C, Floria-Arnal LJ, Muniesa-Herrero MP, et al. Mid-term results for metaphyseal sleeves in revision knee surgery. Knee Surg Sports Traumatol Arthrosc. 2016 doi: 10.1007/s00167-016-4298-4. [DOI] [PubMed] [Google Scholar]
- 81.Gottsche D, Lind T, Christiansen T, Schroder HM. Cementless metaphyseal sleeves without stem in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2016;136:1761–6. doi: 10.1007/s00402-016-2583-9. [DOI] [PubMed] [Google Scholar]
- 82.Dalury DF, Barrett WP. The use of metaphyseal sleeves in revision total knee arthroplasty. Knee. 2016;23:545–8. doi: 10.1016/j.knee.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Engh GA, Herzwurm PJ, Parks NL. Treatment of major defects of bone with bulk allografts and stemmed components during total knee arthroplasty. J Bone Joint Surg Am. 1997;79:1030–9. doi: 10.2106/00004623-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Stevenson S, Li XQ, Davy DT, Klein L, Goldberg VM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J Bone Joint Surg Am. 1997;79:1–16. [PubMed] [Google Scholar]
- 85.Beckmann NA, Mueller S, Gondan M, Jaeger S, Reiner T, Bitsch RG. Treatment of severe bone defects during revision total knee arthroplasty with structural allografts and porous metal cones-a systematic review. J Arthro. 2015;30:249–53. doi: 10.1016/j.arth.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation. The first ten years. Clin Orthop Relat Res. 1983:69–86. [PubMed] [Google Scholar]
- 87.Rudert M, Holzapfel BM, Rottkay E, Holzapfel DE, Noeth U. Impaction bone grafting for the reconstruction of large bone defects in revision knee arthroplasty. Oper Orthop Traumatol. 2015;27:35–46. doi: 10.1007/s00064-014-0330-3. [DOI] [PubMed] [Google Scholar]
- 88.Unger AS, Lewis RJ, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthro. 2005;20:1002–9. doi: 10.1016/j.arth.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 89.Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthro. 2006;21:83–6. doi: 10.1016/j.arth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 90.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004:201–8. doi: 10.1097/01.blo.0000150133.88271.80. [DOI] [PubMed] [Google Scholar]
- 91.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907–14. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 92.Bobyn JD, Poggie RA, Krygier JJ, et al. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86-A(Suppl 2):123–9. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 93.Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop. 2002;31:216–7. [PubMed] [Google Scholar]
- 94.Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res. 1980:263–70. [PubMed] [Google Scholar]
- 95.Schildhauer TA, Robie B, Muhr G, Koller M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma. 2006;20:476–84. doi: 10.1097/00005131-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Schildhauer TA, Peter E, Muhr G, Koller M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J Biomed Mater Res Part A. 2009;88:332–41. doi: 10.1002/jbm.a.31850. [DOI] [PubMed] [Google Scholar]
- 97.Lachiewicz PF, Watters TS. Porous metal metaphyseal cones for severe bone loss: when only metal will do. Bone Joint J. 2014;96-B:118–21. doi: 10.1302/0301-620X.96B11.34197. [DOI] [PubMed] [Google Scholar]
- 98.Jensen CL, Petersen MM, Schroder HM, Flivik G, Lund B. Revision total knee arthroplasty with the use of trabecular metal cones: a randomized radiostereometric analysis with 2 years of follow-up. J Arthro. 2012;27:1820–6 e2. doi: 10.1016/j.arth.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 99.Henricson A, Rosmark D, Nilsson KG. Trabecular metal tibia still stable at 5 years: an RSA study of 36 patients aged less than 60 years. Acta Orthop. 2013;84:398–405. doi: 10.3109/17453674.2013.799418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Radnay CS, Scuderi GR. Management of bone loss: augments, cones, offset stems. Clin Orthop Relat Res. 2006;446:83–92. doi: 10.1097/01.blo.0000214437.57151.41. [DOI] [PubMed] [Google Scholar]
- 101.Holl S, Schlomberg A, Gosheger G, et al. Distal femur and proximal tibia replacement with megaprosthesis in revision knee arthroplasty: a limb-saving procedure. Knee Surg Sports Traumatol Arthrosc. 2012;20:2513–8. doi: 10.1007/s00167-012-1945-2. [DOI] [PubMed] [Google Scholar]
- 102.Choong PF, Sim FH, Pritchard DJ, Rock MG, Chao EY. Megaprostheses after resection of distal femoral tumors. A rotating hinge design in 30 patients followed for 2-7 years. Acta Orthop Scand. 1996;67:345–51. doi: 10.3109/17453679609002328. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198–210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berend KR, Lombardi AV., Jr Distal femoral replacement in nontumor cases with severe bone loss and instability. Clin Orthop Relat Res. 2009;467:485–92. doi: 10.1007/s11999-008-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calori GM, Colombo M, Malagoli E, Mazzola S, Bucci M, Mazza E. Megaprosthesis in post-traumatic and periprosthetic large bone defects: Issues to consider. Injury. 2014;45(Suppl 6):S105–10. doi: 10.1016/j.injury.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 106.Springer BD, Sim FH, Hanssen AD, Lewallen DG. The modular segmental kinematic rotating hinge for nonneoplastic limb salvage. Clin Orthop Relat Res. 2004:181–7. doi: 10.1097/01.blo.0000126306.87452.59. [DOI] [PubMed] [Google Scholar]
- 107.Kimpton CI, Crocombe AD, Bradley WN, Gavin Huw Owen B. Analysis of stem tip pain in revision total knee arthroplasty. J Arthro. 2013;28:971–7. doi: 10.1016/j.arth.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Barrack RL, Stanley T, Burt M, Hopkins S. The effect of stem design on end-of-stem pain in revision total knee arthroplasty. J Arthro. 2004;19:119–24. doi: 10.1016/j.arth.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 109.Barrack RL, Rorabeck C, Burt M, Sawhney J. Pain at the end of the stem after revision total knee arthroplasty. Clin Orthop Relat Res. 1999:216–25. [PubMed] [Google Scholar]
- 110.Haidukewych GJ, Hanssen A, Jones RD. Metaphyseal fixation in revision total knee arthroplasty: indications and techniques. J Am Acad Orthop Surg. 2011;19:311–8. doi: 10.5435/00124635-201106000-00001. [DOI] [PubMed] [Google Scholar]
- 111.Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003:217–24. doi: 10.1097/01.blo.0000093032.56370.4b. [DOI] [PubMed] [Google Scholar]
- 112.Wang C, Pfitzner T, Roth P, Mayr HO, Sostheim M, Hube R. Fixation of stem in revision of total knee arthroplasty: cemented versus cementless-a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24:3200–11. doi: 10.1007/s00167-015-3820-4. [DOI] [PubMed] [Google Scholar]