Abstract
The knee is the intermediate joint of the lower limb and it allows the movement between the femur, tibia and patella. Under normal conditions there is a normal distribution of the load forces on these three articular components in both the static load and during ambulation. The understanding of anatomy and knee biomechanics is important for the gait analysis, the diagnosis of joint diseases and the design and development of prosthetic implants. In the last decades comprehension of knee physiology and kinematics has led to the introduction of a wide range of enhanced prosthetic implant designs for a variety of indications. There are a number of types of total knee arthroplasty implant designs, which are intended to offer the surgeon options for individual patients. The various choices imply that each specific problem has a corresponding implant that provides a reliable solution. However, until the current date, it has not been possible to produce a prosthetic design fully restoring the complex kinematics of the normal knee joint. (www.actabiomedica.it)
Keywords: knee, arthroplasty, biomechanics, prosthesis
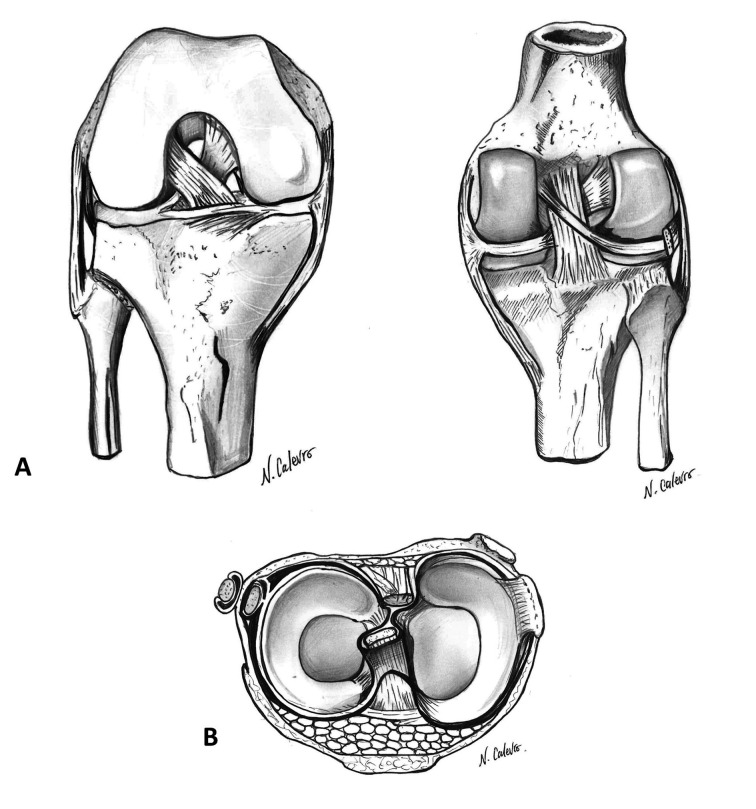
The human knee (Figure 1) (1-5)
Figure 1.
Anatomy of the knee. A; ligaments between femur and tibia. B; menisci (superior view)
Bone articular components
The articular surfaces of the knee are curved and represented by the medial and lateral femoral condyles, which are connected with the corresponding tibial plateaus. The medial tibial plateau is biconcave, unlike the lateral that is concave on the front plane and convex in the sagittal plane. However, both femoral condyles are convex in the frontal and sagittal plane. Therefore, the two tibial surfaces, which present a double concave curvature in the frontal aspect, are divided by the intercondylar eminence. This structure contains two tubercles on which the cruciate ligaments have their origin, thus contributing to fix the femur on the tibia. The geometric shape of the condyles is the most important factor for the stability of the knee under loads.
The articular components of the femur, tibia and patella, which articulates with the femoral trochlea, are covered with hyaline cartilage that allows skeletal elements to slide and rotate one above the other with a low friction.
The superficial layers of cartilage function like deformable bearings and thanks to their visco-elastic properties better distribute the loads across the joint. In the normal cartilage a close correlation between structure and biochemical composition, orientation of the fibrils and its biomechanical properties is present.
The chondrocytes are the most represented cells of the cartilage and are specialized in the synthesis, organization and turnover of extracellular matrix proteins.
Most of the extracellular matrix proteins are composed of type II collagen and aggrecan, a proteoglycan that binds glycosaminoglycans.
A disruption of this equilibrium determines modifications of biochemical and biomechanical cartilage properties with consequent alterations of its tensile capacities and less possibility of loads absorption.
The menisci and their functions
The menisci are fibro-cartilaginous structures that are interposed between the femoral condyles and the tibial plateaus. The lateral meniscus is more circular, while the medial is semicircular. Both are thicker at the periphery, becoming progressively thinner towards the centre of the tibial plateau. The medial is closely connected to the medial collateral ligament (MCL), while the lateral meniscus has greater freedom of movement during flexion and extension. The menisci act as joint shock absorbers by distributing evenly the load between the medial and lateral compartment. In the absence of the menisci the stress per unit area unavoidably increases. Furthermore, these two structures increase joint congruity and diffusion of synovial fluid along the articular surfaces.
The ligaments and their functions
Collateral ligaments
The MCL is composed of two layers: superficial and deep. During flexion of the knee, the superficial vertical fibres are taut, while the posterior and deep are unstretched. The opposite happens during the extension. The primary function of the MCL is to prevent a deviation of the knee during valgus stress. The dissection of its superficial component determines a significant increase of the deviation in valgus between 0° and 45° of flexion, as well as external rotation. On the other hand, the dissection of deep fibres or deep oblique ligaments or the posterior capsule do not increase joint instability during these movements. This means that the superficial component of the MCL is the main structure that counteracts to the stresses in valgus and external rotation and it offers a weak resistance to anterior translation of the tibia. The posterior oblique and deep fibres seem to play a secondary role as stabilizers.
The lateral collateral ligament (LCL) origin on the lateral femoral epicondyle anteriorly to the origin of the gastrocnemius muscle. It has a structure similar to a cord that passes under the lateral retinaculum to insert on the head of the fibula, merging at this level with the insertion of the biceps femoris tendon. It prevents the deviation in varus as well as the excessive internal rotation of the knee. The LCL is taut when the knee is extended; as consequence varus laxity increases when this joint is flexed.
The popliteus muscle function is still controversial, but it appears to act together with the meniscus-femoral ligaments in the control of the external meniscus movement during flexion of the knee and at the same time it facilitates the external rotation of the femur during load. This popliteal-arcuate complex also plays a role, together with the LCL and the posterior cruciate ligament (PCL), in stabilizing the postero-lateral corner against varus movements and tibial external rotation.
Cruciate ligaments
The cruciate ligaments of the knee act as articular stabilizers, preventing the antero-posterior translation of the tibia. They also have an important proprioceptive function, due to the presence inside them of mechanoreceptors and free nervous terminations.
The anterior cruciate ligament (ACL) is characterized by having a straight anterior conformation while it is convex in its posterior surface. Its average length is 38 mm and its average thickness is 11 mm. ACL primary function is to prevent anterior translation of the tibia on the femur with the knee in flexion; it provides up to 85% of this anterior stability.
A secondary function is that of resistance to varus-valgus deviations and to internal rotation of the tibia, especially between 10° and 30° of flexion. Over 30° it becomes taut and the internal rotation is limited by the antero-lateral and postero-medial capsule.
The PCL has an average length of 38 mm and an average thickness of 13 mm. Its main function is to prevent posterior translation of the tibia on the femur. It is stretched at extremes degrees of flexion and internal rotation of the tibia. It is composed of two components: one anterior, which is the most represented portion, which tightens in flexion, and the other posterior, which tightens in extension.
Knee stability and its movements
The overall stability of the knee depends on the interaction of the capsule, menisci, ligaments and muscles, the geometry of the articular surfaces and the femoro-tibial modifications during loading. These are all interdependent between them, thus allowing a normal motility and, at the same time, an effective stability.
The knee is a modified hinge joint where the lack of congruence between the bone surfaces permits six degrees of movement, three translational (anterior-posterior, medial-lateral, and inferior-superior) and three rotational (flexion-extension, intra- external rotation, adduction-abduction). The movements are determined by the sliding of the articular surfaces of the tibia and femur and the orientation of the four major ligaments of the knee.
In particular, the movement of flexion and extension is the broadest and more important. The first is defined as a posterior approaching movement of the leg to the thigh, which can be active or passive and dependent on the hip position. During the active flexion, the knee can reach 120°-140° with the hip flexed, while passively reach up to 160°.
The medial compartment has a contact 1.6 times greater than the lateral. Flexion is ensured by a combination of rotation (“roll-back”) and sliding of the femur over the tibia. The movements of the articular surfaces mainly depend on the conformation and orientation of the articular surfaces and of ACL, PCL, MCL and LCL.
The lateral femoral condyle rotates more than medial in the first 15°-20° of flexion, because of its greater radius of curvature. This different parameter of the two condyles, determines a movement of tibial internal rotation during flexion. Beyond 20° of flexion, slippage on both condyles becomes predominant. On the contrary extension is associated to an external rotation of the tibia relative to the femur; this rotation has been called “the screw-home movement” and is purely passive and dependent on the articular geometry. The menisci, crushed between the articular surfaces in extension, move posteriorly together with the femur in flexion (the lateral more than the medial).
Patellofemoral joint
The patella is a triangular bone in the frontal plane, wider at the top and narrower at the bottom. The articular surface of the patella has seven facets, which are almost divided vertically in third equal parts medially and laterally. The articular surfaces of the femur and the patella are not perfectly congruent.
The patellofemoral joint has four functions:
increase the lever arm of the quadriceps
ensure the stability under load
allow the transmission of the force of the quadriceps on the tibia
provide a bone protection to the trochlea and femoral condyles with the knee flexed.
During flexion of the knee the patella moves distally on the femur, while it rotates on itself about a transverse axis.
The patellofemoral joint contact areas determine the capacity of the patella to transmit the load to the trochlear surface, that depends on the quadriceps muscle activity. The contact between the patella and trochlea begins between 10° and 20° of flexion along a portion of the medial and lateral facets of the lower margin of the patella. With the increase of knee flexion, the contact area increases and moves progressively proximally. At about 90° of flexion, the articular surface of the patella come in contact with the lower part of the trochlea. After 120° the patella is in contact with the femoral condyles.
The patellofemoral joint stability is ensured by anatomical and biomechanical factors, such as the trochlear and patellar articular surfaces, as well as the muscles around the knee and alar ligaments. The quadriceps muscle does not act on a straight line from the femoral head to the centre of the patella. The angle formed between the line connecting the anterior superior iliac spine and the centre of the patella and the line between its centre and the tibial tuberosity is defined as the angle Q. When the knee is totally extended, it is only present a small contact between the patella and femoral trochlea.
During the first part of the flexion (0°-30°), additional stability is provided by the fibres of the vastus medialis muscle. Q angle increases with increasing of the knee flexion, as consequence of the internal rotation of the tibia and in addition the patella tends to lateralize.
The knee in static load and during ambulation (6-10)
The knee joint is subjected to forces both in static load both during ambulation. In the first case on the femoral and tibial articular surfaces are concentrated the reaction forces determined by the lower limb contact with the ground. The optimal condition in which there is a uniform distribution of these forces occurs when the mechanical and anatomical axis fall within normal limits.
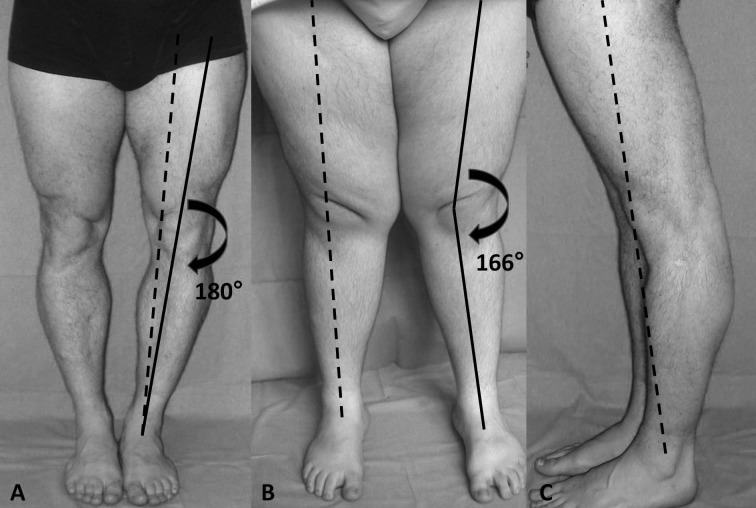
The first is defined as the line traced between the centre of the femoral head and of the ankle and normally passes through the centre of the knee in both anterior-posterior and latero-lateral views. Instead, the anatomical axis originates in the centre of the knee from the union between the axis of the femoral and the tibial shaft. This line delimitates an obtuse angle externally orientated of 172-177° (physiological valgus knee angle) (Figure 2).
Figure 2.
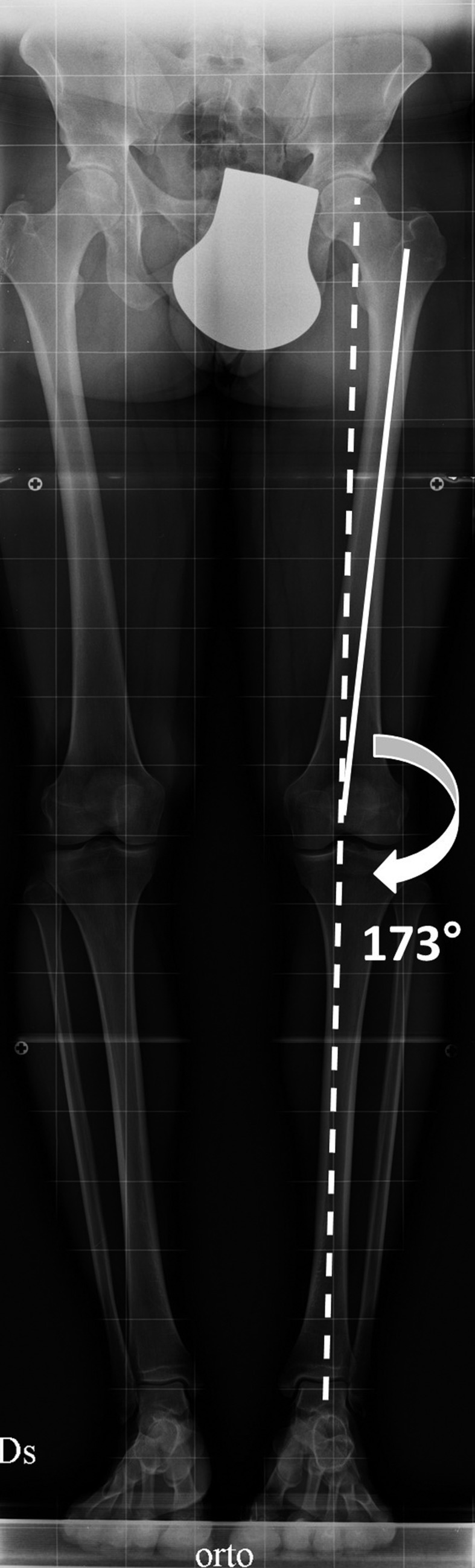
Weightbearing full-length hip-to-ankle X-ray of the lower limbs with normal mechanical (dotted line) and anatomical axis (solid line)
When this angle increases the knee has a varus conformation (Figure 3A); in this situation the lower limb mechanical axis moves medially.
Figure 3.
A; genu varus with mechanical axis (dotted line) medialized and anatomical axis (solid line) that determines a valgus knee angle >172-175°. B; genu valgus with mechanical axis lateralized and anatomical axis that determines a valgus knee angle <172-175°. C: genu recurvatum with mechanical axis anteriorized
On the other hand, when this angle decreases the knee has a valgus conformation (Figure 3B) and the mechanical axis moves laterally. Finally, in the knee recurvatum (Figure 3C) the mechanical axis is located anteriorly with respect to the joint centre.
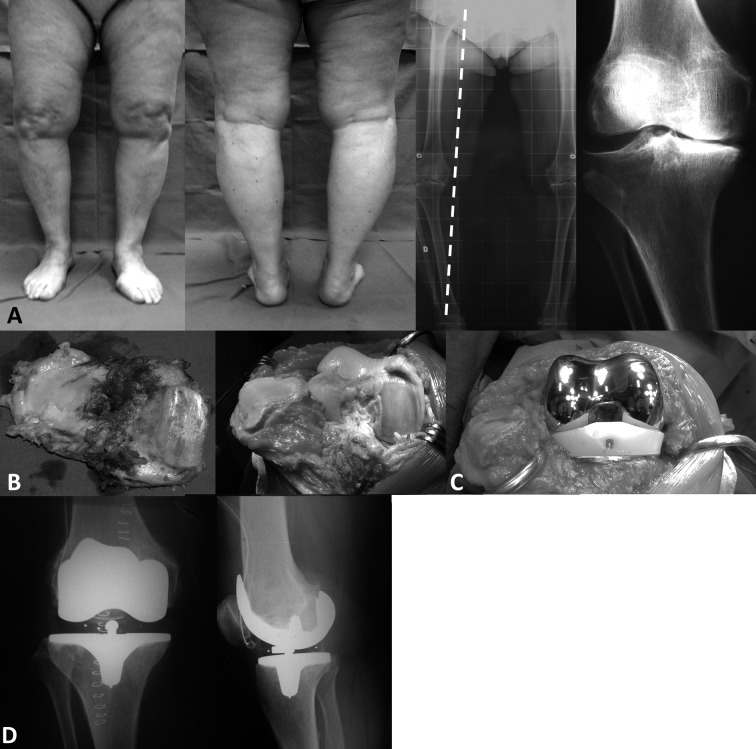
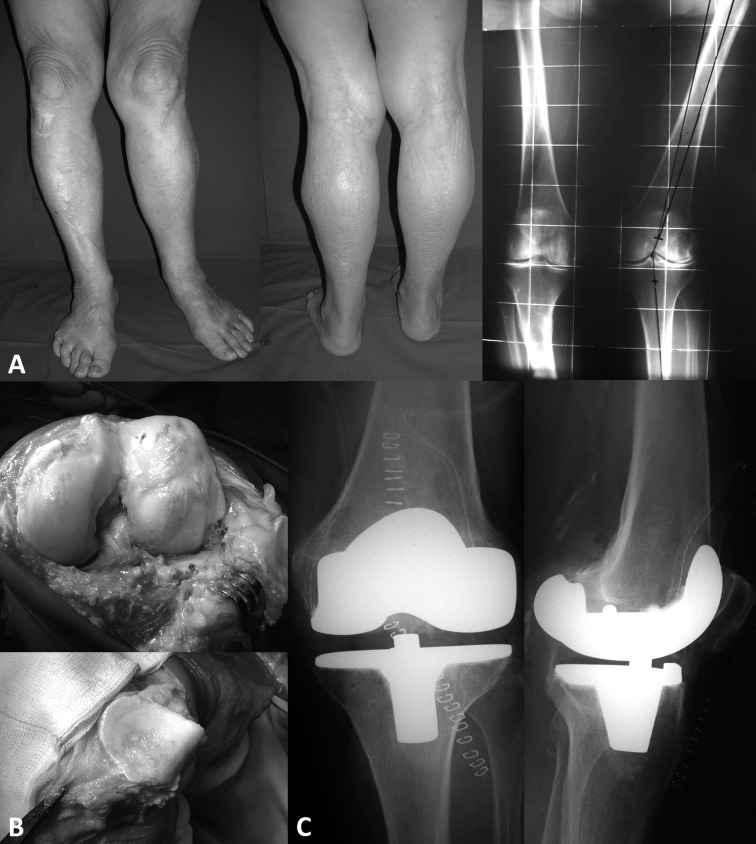
These alterations of the axes causes a stress increase per unit area and consequently facilitate the development of osteoarthritis in the corresponding overloaded compartments (Figure 4 and 5).
Figure 4.
Bilateral varus knee. A; preoperative clinical and x-ray views with medialization of the mechanical axis (dotted line). B; intraoperative images with deterioration of the cartilage. C; intraoperative view after positioning of the prosthesis. D; postoperative radiographs of the right knee
Figure 5.
Left valgus knee. A; preoperative clinical and x-ray views. B; intraoperative images with deterioration of the cartilage. C; postoperative radiographs
Even the femoral trochlea and the patella are subjected to load forces in a standing position but much more, as previously described, during the movements of flexion and extension.
Actually, this articulation is mainly stressed during ambulation that begins when the foot touches the ground and ends when the same foot touch again the ground.
The bipedal gait produces rotational movements of each limb segment around the hip, knee, ankle and foot. These are the result of the interaction of muscle strength and weight around each joint. Through the lever arms, the muscles of each limb create a twisting moment. The active muscles at the same time operate synergistically to produce an internal torque within the joint. In static stance, along with the capsule and ligaments, muscles oppose to the joint torque produced by the weight and, during the swing phase, it creates the rotary joint movement that overcomes the weight and inertia of the leg.
The knee has to withstand loads developed during heel contact with the ground and to provide the forces necessary to overcome the inertia of the leg during the swing phase. During walking these loads change varying in amount and direction. During the contact phase of the heel, the force is directed postero-superiorly, for which the load induces a moment around the articulation that must be countered by the muscles.
It has been calculated that the maximal forces on the knee during walking are comprised between three and seven times the body weight. These are highest at the beginning of the ambulation and are associated with the maximum activity of the quadriceps muscle. During this phase, there are two other peaks: they correspond to the contractile activity of the hamstrings at the beginning of the load and of the gastrocnemius muscle just before the contact of the opposite foot.
Design criteria of knee arthroplasty
The history of total knee arthroplasty (TKA) began in the 1860s, when Fergusson reported the first resection for knee osteoarthritis (11, 12). Interpositional arthroplasties, using materials such as joint capsules, muscle, fascia and free fascial grafts, were attempted over subsequent years, but were ultimately unsuccessful (13). The first reports of total joint replacement were made by Thermestocles Gluck in the 1880s, who used an ivory hinged design fixed with a cement made from plaster of Paris, pumice and colophony (14, 15). Condylar knee designs, in which the femoral and tibial load-bearing surfaces are replaced with unconnected artificial components, were first investigated in the late 1960s (10).
The majority of the knee prosthesis that are currently used are based on the evolution of these systems developed in the 1960’s.
The objective of the knee replacement is twofold:
1) function and kinematics as much as possible equal to the normal knee
2) long-term survival of the implant.
Also the prothesized knee is subjected to compression and shear forces in static load and during every movement and walking.
The comprehension of the entity and direction of these forces is very important. The choice of adequate prosthetic surfaces, a precise capsular-ligamentous balance, also through accurate bone resections, have the aim of minimizing them, thus preserving the bone-prosthesis interface.
The final long-term result should be a stable, well-functioning and asymptomatic prosthetic implant.
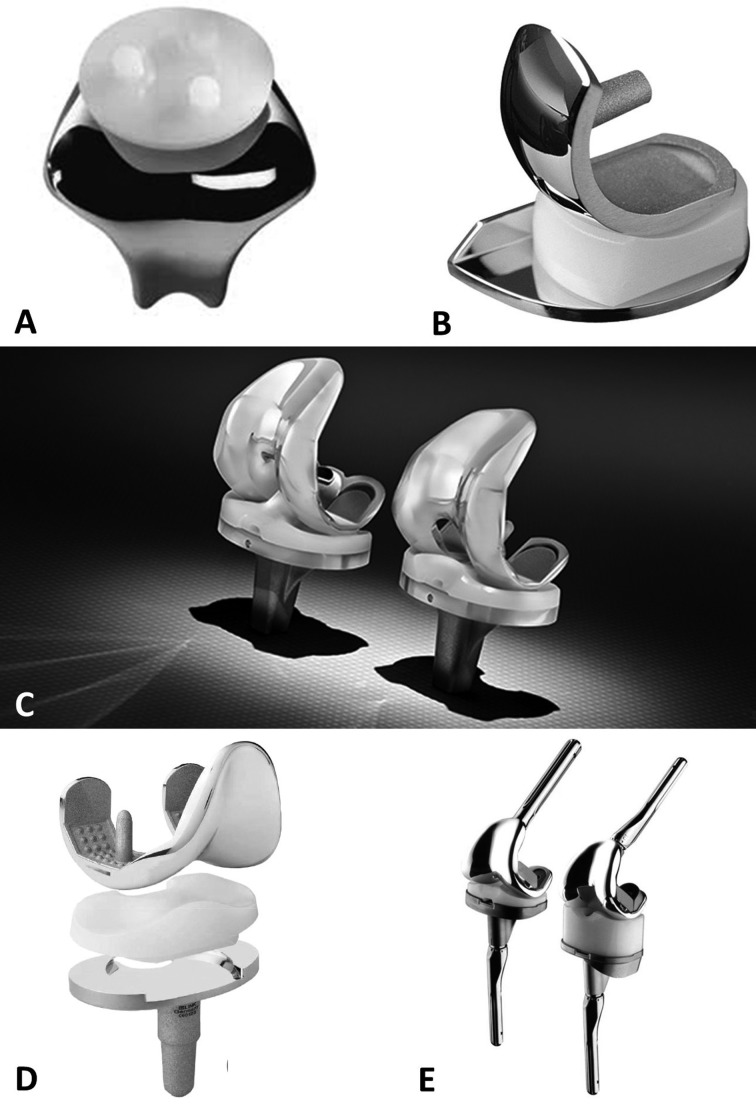
Depending on the type, location and severity of the diseases within the knee joint, several options may be considered, including partial and total joint arthroplasty (Figure 6).
Figure 6.
Different prosthetic knee implants. A; patellofemoral joint design. B; UKA implant. C; PCL retaining and substituting prosthesis. D; mobile-bearing model. E; constrained implants
Partial knee arthroplasty of the patellofemoral joint
Isolated patellofemoral joint (PFJ) pathologies accounts for approximately 10% of knee problems, and is most common in younger females (16). A variety of surgical treatment options are available (chondrocyte implantation, microfracture or partial lateral facetectomy) if nonoperative measures fail to achieve satisfying results (17, 18), with the aim of delaying the necessity for arthroplasty. Joint replacement procedures are considered once both the patellar and femoral sides of the PFJ are involved (19, 20).
The first patellar prosthesis was described by McKeever in 1955, followed by the first femoral component 24 years later, as described separately by Lubinus and Blazina (21, 22). Enhanced second-generation prostheses with a broad symmetrical trochlear flange evolved in the 1990s, and the design has been refined and updated continually in the intervening years.
Partial knee arthroplasty: unicompartmental prosthesis
Unicompartmental, or unicondylar, knee arthroplasty (UKA) is the preferred choice when the intention is to preserve the intrinsic joint stabilizing structures, as well as healthy joint compartments (23, 24). The general indication for consideration of a UKA procedure is based an isolated involvement of either the lateral or medial tibial-femoral compartment, identified upon clinical and radiographic examination (25, 26), without signs of patellofemoral joint disease.
An intact anterior cruciate ligament (ACL) is an important prerequisite for UKA, as the altered knee kinematics and contact stresses would otherwise increase failure rates (27).
Technique associated factors, mainly the achievement of correct alignment during surgery, have been proven predictive for increased polyethylene wear and contralateral progression of arthritis due to undesirable peak loads (28, 29). If correctly indicated and performed, UKA can provide long-lasting successful results (30, 31).
Total knee arthroplasty
The first prosthesis designed to replace all three knee’s compartments was introduced in 1972 by John Insall (the “total condylar prosthesis”) (32, 33). Since then, there has been a wide increase in the number of TKAs performed annually, even if the results of comparative studies into many of the designs have not demonstrated the marked improvements in outcomes that were expected. The most common types of TKA designs are the following.
TKA: cruciate ligament retention versus substitution
Bicruciate retaining prosthesis, since its development by Gunston in the middle 1960s, was designed as a model to allow for more physiological movement of the knee, and throughout the years it evolved in materials and conception; it may still be a satisfying solution in younger patients with more demanding activities, as it preserves normal biomechanics (34, 35). Saving both cruciate ligaments leads to improved performance, as documented by gait analysis and stair climbing (36, 37). However, the difficult surgical technique and concerns regarding prosthetic this design and fixation have made the choice for this implant less attractive. For this reason, the ACL is routinely sacrificed during TKA while the posterior cruciate ligament (PCL) can be sacrificed or preserved.
A corresponding prosthetic design, with or without a tibial intercondular prominence, is implanted accordingly (38). There is no evidence favouring preservation of the PCL over its substitution (39).
Nowadays almost two-thirds of total knee implants that are used preserves PCL, although there is an increasing trend towards the PCL substitution.
The reasons are numerous: the need for a roll-back in order to increase the lever arm of the quadriceps muscle, the preservation of the PCL in combination with shallow tibial surfaces favors the arc of flexion, the better fixation of the tibial component which is obtained and the simpler surgical technique.
TKA: fixed- versus mobile-bearing
Originating from Fred Buechel’s “floating socket” philosophy, mobile bearing in TKA was introduced by the two Johns - Goodfellow and O’Connor - who described the theoretical principle of decreasing polyethylene wear by increasing implant conformity, and surface area for distribution of forces thereby reducing unidirectional stresses (40, 41). The polyethylene components are similar to the menisci. The purpose of this project is to minimize the wear and deformation of the polyethylene and allow a more physiological kinematics, both for the internal-external rotation and the anterior-posterior translation; such movements are helped by the presence of a tibiofemoral cam, which functions as a rear stabilizer and produces a posterior translation in flexion.
These theoretical advantages could not, however, be substantiated by evidence, since a recent meta-analysis showed no difference in incidence of aseptic loosening or revision rates between fixed or mobile bearing designs (42).
TKA: constrained implants
In patients with fixed valgus or varus deformity, constrained devices, such as constrained condylar knee and hinge types, may achieve a satisfactory balance. The level of constraint can be adapted to the individual, with higher constraints reserved for more severe cases (43). The choice of the correct degree of constraint is based on the ligamentous and bony condition. Hinged prosthesis should be preserved for cases of severe ligament disruption and bone loss (44, 45). It is important to consider augmentation techniques using cement, bone grafts or augments to compensate for bone loss, before deciding on further constraint (46).
TKA: gender related designs
There has been a great deal of debate as to the effect of gender, due to gender-related anatomic variability, on the results of TKA (47, 48). The distal femur tends to be narrower in females for any given anteroposterior dimension (49), and a female-specific system was released (Gender- Solutions™, Zimmer Inc., Warsaw, IN, USA). There are, however, larger differences in femur dimensions between races than between genders, which complicate the matter (50, 51). Studies have not yet demonstrated any benefits with gender-specific implants.
Conclusions
TKA is an established surgical procedure and its efficacy is well described by literature. However, the results of comparative studies into many of the designs have not demonstrated, aside from a few instances, the marked improvements in outcomes that were expected. Spatial considerations such as soft-tissue balancing, alignment of the leg and restoration of the joint line are crucial for achieving good long-term outcomes. Surgical options, such as computer-assisted surgery and patients-specific instrumentation, which can help in component positioning, are available and have to be considered.
The considerable advances in the understanding of knee anatomy, kinematics and biomechanics during the past few decades has led to improvements in the design of prosthetic knee implants. The perfect knee arthroplasty does not exist but the key of the success of this type of surgery depends on the correct balance between anatomical design and kinematic functionality.
References
- 1.Flandry F, Hommel G. Normal anatomy and biomechanics of the knee. Sports Med. Arthrosc. 2011 Jun;19(2):82–92. doi: 10.1097/JSA.0b013e318210c0aa. [DOI] [PubMed] [Google Scholar]
- 2.Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: a review of anatomy, function and advances in treatment. Clin Anat. 2015 Mar;28(2):269–87. doi: 10.1002/ca.22456. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani JR, Kilcoyne KG, Rue JP. Anterior cruciate ligament anatomy: a review of the anteromedial and posterolateral bundles. J Knee Surg. 2009 Apr;22(2):148–54. doi: 10.1055/s-0030-1247742. [DOI] [PubMed] [Google Scholar]
- 4.Voos JE, Mauro CS, Wente T, Warren RF, Wickiewicz TL. Posterior cruciate ligament: anatomy, biomechanics, and outcomes. Am J Sports Med. 2012 Jan;40(1):222–31. doi: 10.1177/0363546511416316. [DOI] [PubMed] [Google Scholar]
- 5.Sherman SL, Plackis AC, Nuelle CW. Patellofemoral anatomy and biomechanics. Clin Sports Med. 2014;33(3):389–401. doi: 10.1016/j.csm.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Manjunath KS, Gopalakrishna KG, Vineeth G. Evaluation of alignment in total knee arthroplasty: a prospective study. Eur J Orthop Surg Traumatol. 2015;25:895–903. doi: 10.1007/s00590-015-1638-x. [DOI] [PubMed] [Google Scholar]
- 7.Loudon JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Ther. 2016 Dec;11(6):820–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Kozanek M, Hosseini A, Liu F, Van de Velde SK, Gill TJ, Rubash HE, Li G. Tibiofemoral Kinematics and condylar motion during the stance phase of gait. J Biomech. 2009 Aug 25;42(12):1877–84. doi: 10.1016/j.jbiomech.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates NA, Myer GD, Shearn JT, Hewett TE. Anterior cruciate ligament biomechanics during robotic and mechanical simulations of physiologic and clinical motion tasks: a systematic review and meta-analysis. Clin Biomech (Bristol. Avon) 2015 Jan;30(1):1–13. doi: 10.1016/j.clinbiomech.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Filippo M, Rovani C, Sudberry JJ, Rossi F, Pogliacomi F, Zompatori M. Magnetic resonance imaging comparison of intra-articular cavernous synovial hemangioma and cystic synovial hyperplasia of the knee. Acta Radiol. 2006 Jul;47(6):581–4. doi: 10.1080/02841850600767724. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad SS, Gantenbein B, Evangelopoulos DS, Schär MO, Schwienbacher S, Kohlhof H, Kohl S. Arthroplasty - current strategies for the management of knee osteoarthritis. Swiss Med Wkly. 2015 Feb 9;145:w14096. doi: 10.4414/smw.2015.14096. doi: 10.4414/smw.2015.14096. eCollection 2015. Review. [DOI] [PubMed] [Google Scholar]
- 12.Gordon-Taylor G. Sir William Fergusson, 1808–1877. Medical history. 1961;5:1–14. doi: 10.1017/s0025727300025886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranawat CS. History of total knee replacement. J South Orthop Assoc. 2002;11(4):218–26. [PubMed] [Google Scholar]
- 14.Amendola L, Tigani D, Fosco M, Dallari D. History of Condylar Total Knee Arthroplasty. In: Fokter S, editor. Recent Advances in Hip and Knee Arthroplasty. Rijeka: InTech; 2012. [Google Scholar]
- 15.Williams D, Garbuz D, Masri B. Total knee arthroplasty: Techniques and results. BC Medical Journal. 2010;52(9):447–54. [Google Scholar]
- 16.Davies AP, Vince AS, Shepstone L, Donell ST, Glasgow MM. The radiologic prevalence of patellofemoral osteoarthritis. Clin Orthop Relat Res. 2002;402:206–12. doi: 10.1097/00003086-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson JP. Alternatives to patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;436):76–80. doi: 10.1097/01.blo.0000172305.20156.ba. [DOI] [PubMed] [Google Scholar]
- 18.Saleh KJ, Arendt EA, Eldridge J, Fulkerson JP, Minas T, Mulhall KJ. Symposium. Operative treatment of patellofemoral arthritis. J Bone Joint Surg Am. 2005;87(3):659–71. doi: 10.2106/JBJS.D.03035. [DOI] [PubMed] [Google Scholar]
- 19.Leadbetter WB. Patellofemoral arthroplasty in the treatment of patellofemoral arthritis: rationale and outcomes in younger patients. Orthop Clin North Am. 2008;39(3):363–80. doi: 10.1016/j.ocl.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(Suppl 4):122–37. doi: 10.2106/JBJS.F.00856. [DOI] [PubMed] [Google Scholar]
- 21.Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119–7. doi: 10.3928/0147-7447-19790301-03. [DOI] [PubMed] [Google Scholar]
- 22.Blazina ME, Fox JM, Del Pizzo W, Broukhim B, Ivey FM. Patellofemoral replacement. Clin Orthop Relat Res. 1979;144:98–102. [PubMed] [Google Scholar]
- 23.Scott RD, Santore RF. Unicondylar unicompartmental replacement for osteoarthritis of the knee. J Bone Joint Surg Am. 1981;63(4):536–44. [PubMed] [Google Scholar]
- 24.Tinius M, Hepp P, Becker R. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy. official journal of the ESSKA. 2012;20(1):81–7. doi: 10.1007/s00167-011-1528-7. [DOI] [PubMed] [Google Scholar]
- 25.Berger RA, Nedeff DD, Barden RM, Sheinkop MM, Jacobs JJ, Rosenberg AG, et al. Unicompartmental knee arthroplasty. Clinical experience at 6– to 10–year followup. Clin Orthop Relat Res. 1999;367:50–60. [PubMed] [Google Scholar]
- 26.Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84(8):1126–30. doi: 10.1302/0301-620x.84b8.13447. [DOI] [PubMed] [Google Scholar]
- 27.Suero EM, Citak M, Cross MB, Bosscher MR, Ranawat AS, Pearle AD. Effects of tibial slope changes in the stability of fixed bearing medial unicompartmental arthroplasty in anterior cruciate ligament deficient knees. The Knee. 2012;19(4):365–9. doi: 10.1016/j.knee.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Argenson JN, Parratte S. The unicompartmental knee: design and technical considerations in minimizing wear. Clin Orthop Relat Res. 2006;452:137–42. doi: 10.1097/01.blo.0000229358.19867.60. [DOI] [PubMed] [Google Scholar]
- 29.Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;423):161–5. doi: 10.1097/01.blo.0000128285.90459.12. [DOI] [PubMed] [Google Scholar]
- 30.Bruni D, Gagliardi M, Akkawi I, Raspugli GF, Bignozzi S, Marko T, et al. Good survivorship of all-polyethylene tibial component UKA at long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2016 Jan;24(1):182–7. doi: 10.1007/s00167-014-3361-2. [DOI] [PubMed] [Google Scholar]
- 31.Schlueter-Brust K, Kugland K, Stein G, Henckel J, Christ H, Eysel P, et al. Ten year survivorship after cemented and uncemented medial Uniglide(R) unicompartmental knee arthroplasties. Knee. 2014;21(5):964–70. doi: 10.1016/j.knee.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Insall JN, Hood RW, Flawn LB, Sullivan DJ. The total condylar knee prosthesis in gonarthrosis. A five to nine-year follow-up of the first one hundred consecutive replacements. J Bone Joint Surg Am. 1983;65(5):619–28. [PubMed] [Google Scholar]
- 33.Insall JN, Kelly M. The total condylar prosthesis. Clin Orthop Relat Res. 1986;205:43–8. [PubMed] [Google Scholar]
- 34.Dennis DA, Komistek RD, Colwell CE Jr, et al. In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clin Orthop Relat Res. 1998;356:47–57. doi: 10.1097/00003086-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Stiehl JB, Komistek RD, Cloutier JM, et al. The cruciate ligaments in total knee arthroplasty: a kinematic analysis of 2 total knee arthroplasties. J Arthroplasty. 2000;15:545–50. doi: 10.1054/arth.2000.4638. [DOI] [PubMed] [Google Scholar]
- 36.Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stairclimbing. J Bone Joint Surg Am. 1982;64:1328–35. [PubMed] [Google Scholar]
- 37.Sabatini L, Giachino M, Risitano S, Atzori F. Bicompartmental knee arthroplasty. Ann Transl Med. 2016 Jan;4(1):5. doi: 10.3978/j.issn.2305-5839.2015.12.24. doi: 10.3978/j.issn.2305-5839.2015.12.24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks SA, Markovich GD, Hodge WA. In vivo kinematics of cruciateretaining and -substituting knee arthroplasties. J Arthroplasty. 1997;12(3):297–304. doi: 10.1016/s0883-5403(97)90026-7. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs WC, Clement DJ, Wymenga AB. Retention versus removal of the posterior cruciate ligament in total knee replacement: a systematic literature review within the Cochrane framework. Acta Orthop. 2005;76(6):757–68. doi: 10.1080/17453670510045345. [DOI] [PubMed] [Google Scholar]
- 40.Buechel FF, Pappas MJ, DePalma AF. “Floating-socket” total shoulder replacement: anatomical, biomechanical, and surgical rationale. J Biomed Mater Res. 1978;12(1):89–114. doi: 10.1002/jbm.820120109. [DOI] [PubMed] [Google Scholar]
- 41.Goodfellow J, O’Connor J. The mechanics of the knee and prosthesis design. J Bone Joint Surg Br. 1978;60–b(3):358–69. doi: 10.1302/0301-620X.60B3.581081. [DOI] [PubMed] [Google Scholar]
- 42.Voort P, Pijls BG, Nouta KA, Valstar ER, Jacobs WC, Nelissen RG. A systematic review and meta-regression of mobile-bearing versus fixed-bearing total knee replacement in 41 studies. Bone Joint J. 2013;95–b(9):1209–16. doi: 10.1302/0301-620X.95B9.30386. [DOI] [PubMed] [Google Scholar]
- 43.Girard J, Amzallag M, Pasquier G, Mulliez A, Brosset T, Gougeon F, et al. Total knee arthroplasty in valgus knees: predictive preoperative parameters influencing a constrained design selection. Orthop Traumatol Surg Res. 2009;95(4):260–6. doi: 10.1016/j.otsr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415–9. doi: 10.1007/s00264-012-1739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radnay CS, Scuderi GR. Management of bone loss: augments, cones, offset stems. Clin Orthop Relat Res. 2006;446:83–92. doi: 10.1097/01.blo.0000214437.57151.41. [DOI] [PubMed] [Google Scholar]
- 46.Yang JH, Yoon JR, Oh CH, Kim TS. Primary total knee arthroplasty using rotating-hinge prosthesis in severely affected knees. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):517–23. doi: 10.1007/s00167-011-1590-1. [DOI] [PubMed] [Google Scholar]
- 47.Barrett WP. The need for gender-specific prostheses in TKA: does size make a difference? Orthopedics. 2006;29(9 Suppl):S53–5. 47. [PubMed] [Google Scholar]
- 48.Booth RE., Jr Sex and the total knee: gender-sensitive designs. Orthopedics. 2006;29(9):836–8. doi: 10.3928/01477447-20060901-24. [DOI] [PubMed] [Google Scholar]
- 49.Hitt K, Shurman JR, 2nd, Greene K, McCarthy J, Moskal J, Hoeman T, et al. Anthropometric measurements of the human knee: correlation to the sizing of current knee arthroplasty systems. J Bone Joint Surg Am. 2003;85–A(Suppl 4):115–22. [PubMed] [Google Scholar]
- 50.Ho WP, Cheng CK, Liau JJ. Morphometrical measurements of resected surface of femurs in Chinese knees: correlation to the sizing of current femoral implants. Knee. 2006;13(1):12–4. doi: 10.1016/j.knee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Karlson EW, Daltroy LH, Liang MH, Eaton HE, Katz JN. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102(6):524–30. doi: 10.1016/s0002-9343(97)00050-8. [DOI] [PubMed] [Google Scholar]