Abstract
Despite regular blood transfusion and iron chelation therapy, growth impairment and pubertal delay are commonly seen in children and adolescents with transfusion-dependent Beta thalassaemia major (BTM) and sickle cell disease (SCD). We evaluated growth parameters and endocrine disorders in relation to the liver iron concentration (LIC) assessed by the Ferriscan® method in a cohort of adults with SCD (n=40) and BTM (n=52) receiving blood transfusions and iron chelation therapy since early childhood. Before transfusion, hemoglobin concentration had not been less than 9 g/dl in the past 12 years; subcutaneous daily desferrioxamine was administered for all of them since early childhood (2-5 years of age). All patients were shifted to oral therapy with deferasirox iron chelation, 20 mg/daily for the past 5 years. BTM patients with higher LIC (>15 mg Fe/g dry weight) had significantly shorter stature, lower insulin-like growth factor-I SDS (IGF-I SDS), higher alanine transferase (ALT) and serum ferritin concentrations compared to thalassemic patients with lower LIC. Patients with SCD with LIC >8 mg Fe/g dry weight had significantly shorter stature, lower IGF-I SDS and higher ALT compared to SCD patients with lower LIC. Patients with BTM had significantly shorted final height (Ht-SDS), IGF-I SDS and FT4 level compared to patients with SCD. LIC and mean fasting blood glucose (FBG) were significantly higher in patients with BTM compared to those with SCD. The linear regression analysis showed a significant correlation between LIC and serum ferritin level in SCD and BTM. LIC and serum ferritin level were also correlated significantly with IGF-I level in patients with BTM. LIC was correlated significantly with ALT in patients with BTM. In conclusion, the prevalence of endocrinopathies especially hypothyroidism, DM, and hypogonadism were significantly higher in BTM patients versus SCD patients and higher in patients with higher LIC versus those with lower LIC. These complications occurred less frequently, but still considerable, in chronically transfused patients with SCD. (www.actabiomedica.it)
Keywords: β-thalassemia major (BTM), sickle cell disease (SCD), final adult height (FA-Ht), insulin-like growth factor-1 (IGF-1), endocrine complications, liver iron concentrations (LIC)
Introduction
Iron overload is a common clinical problem, arising from iron hyperabsorption (such as hereditary hemochromatosis or thalassemia intermedia syndromes) or through regular blood transfusion therapy for conditions such as thalassemia major, sickle cell disease (SCD) and myelodysplastic syndrome (1, 2). Under normal iron conditions, apotransferrin (transferrin without bound iron) exists in excess, with only 25% of the binding sites carrying iron to the bone marrow, liver, and other tissues. As the liver is the dominant storage organ for excess iron, hepatocellular iron uptake of transferrin-bound iron is preserved even in iron overload conditions. In severe iron overload, however, transferrin binding capacity becomes saturated and low molecular weight non-transferrin-bound iron (NTBI) or “free” iron species appear. The heart and endocrine glands are sensitive to chronic exposure to NTBI species (1-3).
In SCD iron overload occurs later than in thalassemia major and endocrine disorders are less common (2). Recent reports, however, have documented that endocrine dysfunctions are commonly encountered in children and adolescents with SCD (4-6), and vitamin D deficiency is the most commonly documented endocrine disorder (6).
The biochemical markers of the iron metabolism disorders include an elevated concentration of iron and serum ferritin (SF) and transferrin saturation in plasma. However, these parameters are not always specific for body iron load (7). Furthermore, SF can be unreliable in SCD due to the inflammatory nature of the condition, even in the steady state (8).
In a cross-sectional study of 27 children (10.9±3.3 years) with SCD who had received chronic transfusion therapy without chelation, transfusion volume provided more insight on liver iron content (LIC) than serum iron markers (9). Therefore, treatment with iron chelation and monitoring of transfusional iron overload in SCD aim principally at controlling liver iron, thereby reducing the risk of cirrhosis and hepatocellular carcinoma (2).
Monitoring of liver iron concentration pretreatment and in response to iron chelation therapy by a noninvasive measurement of liver iron concentration (LIC) using validated and widely available magnetic resonance imaging (MRI) techniques reduces the risk of under- or overtreatment (2). A standardized and validated MRI method is now registered in Europe and the United States (Ferriscan®), with reproducible relationship between the value (R2) by MRI and LIC by biopsy over a clinically useful range.
Ferriscan® enables locally acquired data to be analyzed at a central facility and is potentially available in any hospital with an MRI scanner and with minimal training of local staff (10-12).
The aim of present study was to study the growth parameters, IGF-I and endocrine functions in patients with BTM and SCD receiving blood transfusion and iron oral chelation therapy (OIC) with deferasirox in the last 5 years. Iron overload was assessed in both groups of patients by serum ferritin and Ferriscan®.
Patients and Methods
We performed a cross-sectional analysis of linear growth (height and height SDS (Ht-SDS), weight, body mass index (BMI), and endocrine testing in 2 groups of transfusion dependent adult patients with BTM (n=52) and SCD (n=40) who completed their puberty and attained their final adult height (FA-Ht). Before transfusion, hemoglobin concentration had not been less than 9 g/dl in the past 12 years; subcutaneous daily desferrioxamine was administered for all of them since early childhood (2-5 years of age). All patients were shifted to oral therapy with deferasirox iron chelation, 20 mg/daily for the past 5 years. Blood transfusion therapy in SCD was initiated during childhood either for therapy (typically for life-threatening SCD related complication and anemia) or for prophylaxis, to decrease the incidence of specific SCD related complications. In BTM, all patients had started their blood transfusion during their first 2 years of life for correction of severe anemia.
Lab. investigations, using commercial radioimmunoassay included the measurement of fasting serum concentration of free thyroxine (FT4), thyrotropin (TSH), IGF-I, and gonadotrophins (LH and FSH), and serum cortisol.
Fasting blood glucose and hepatic function (ALT, AST and ALP) were also assessed. Liver iron content was measured using the FerriScan® R2-MRI method (7). The severity of LIC was graded as following: severe >15 mg Fe/g dry weight (d/wt), moderate 8-14.9 mg Fe/g d/wt, and mild <8 mg Fe/g d/wt. All patients with hypogonadism and/or hypothyroidism were on hormonal replacement therapy.
Definitions of growth and endocrine disorders
Evidence for growth failure: Height SDS less than −2 (patients receiving GH therapy were excluded)
Evidence for diabetes mellitus: Fasting glucose >6·9 mmol/l and/or non-fasting glucose >11·1 mmol/l and/or exogenous insulin administration and/or use of oral hypoglycemic medications.
Evidence for hypothyroidism (low FT4 and/or high TSH) or ongoing thyroid hormone replacement therapy.
Evidence for hypogonadism: Females: >14 years requiring estrogen replacement therapy or >15 years with primary amenorrhea. Males: >14 years, not yet in Tanner’s stage 2 (i.e. prepubertal genital development) or on androgen replacement therapy or >17 years, not yet in Tanner’s stage 4 (i.e. mid-pubertal genital development).
For the diagnosis oh hypocortisolim and hypoparathyroidism we used the criteria reported in the reference 3.
Ethical approval for the study was obtained by Ethical Committee of Hamad General Hospital, which were in accordance, by the Declaration of Helsinki.
Student t test and ANOVA test were used to compare the growth and lab. Data among the different groups when the data was normally distributed and Wilcoxon rank test was used when the data was not normally distributed. Linear regression equation was used to study possible relations between different factors and FA-Ht.
Results
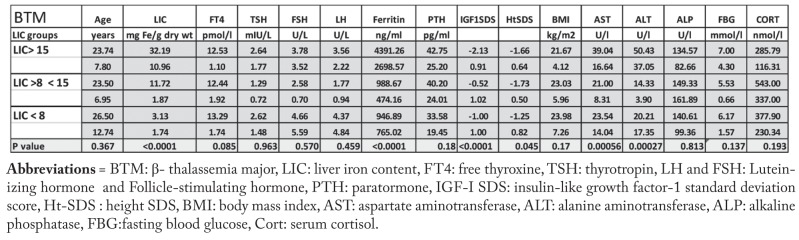
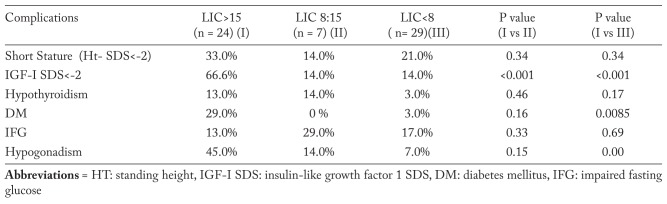
Patients with BTM were categorized in 3 groups according to their LIC (Table 1). Thalassemic patients with severe hepatic iron overload had significantly shorter stature, lower IGF-I SDS and higher ALT, AST and serum ferritin concentrations compared to BMT with mild-moderate LIC. The prevalence of short stature and low IGF-I SDS, diabetes mellitus insulin dependent (DM), and hypogonadism was significantly higher in the BTM group with severe hepatic iron overload compared to the other groups with lower LIC (Table 2).
Table 1.
Growth, biochemical and endocrine parameters in adult patients with BTM according to their degree of LIC.
Table 2.
Prevalence endocrinopathies in adult patients with BTM in relation to LIC
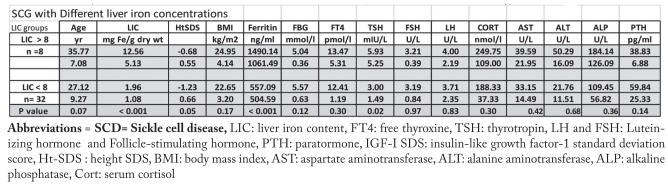
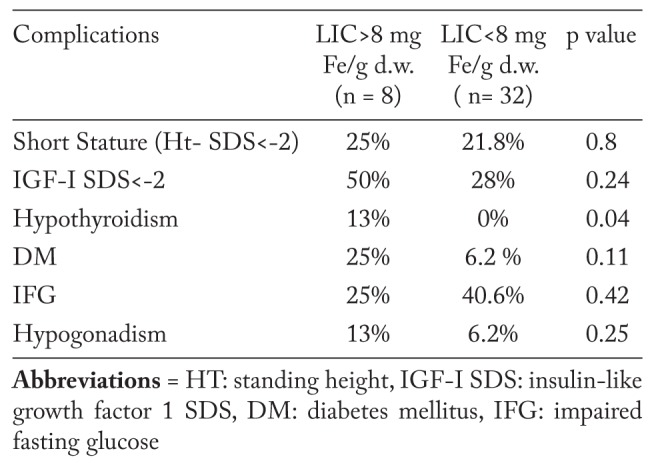
Patients with SCD were categorized in 2 groups based on their LIC values (Table 3 and 4). Patients with moderate LIC (>8 mg Fe/g dry weight) had significantly shorter stature, lower IGF-I SDS and basal TSH, and higher ALT compared to SCD patients with mild LIC (<8 mg Fe/g dry weight). The prevalence hypothyroidism was significantly higher in SCD patients with higher LIC. The prevalence of short stature and low IGF-I SDS, DM, impaired fasting glucose (IFG) and hypogonadism was non-significantly higher in the SCD group with higher LIC compared to the group with lower LIC (Table 4).
Table 3.
Growth, biochemical and hendocrine parameters data in adult patients with SCD according to their degree of LIC
Table 4.
Prevalence endocrinopathies in adult patients with SCD in relation to LIC
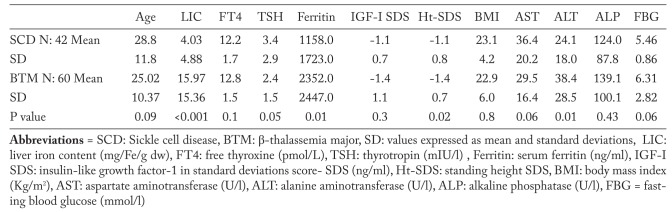
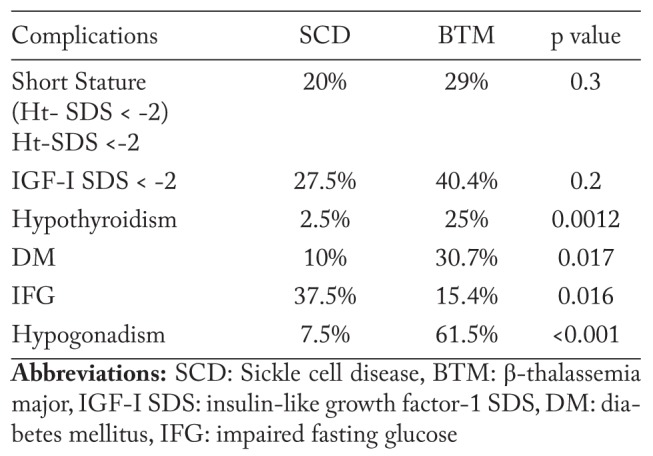
Patients with BTM had significantly lower Ht- SDS level compared to patients with SCD. LIC, serum ferritin and ALT were significantly higher in patients with BTM compared to those with SCD (Table 5). The prevalence of endocrinopathies namely hypothyroidism, DM, and hypogonadism was significantly higher in BTM patients versus SCD patients (Table 6). No cases of hypocortisolim or hypoparathyroidism were documented in both groups.
Table 5.
Comparison between adult patients with SCD versus BTM
Table 6.
Prevalence of endocrinopathies in adult patients with SCD and BTM
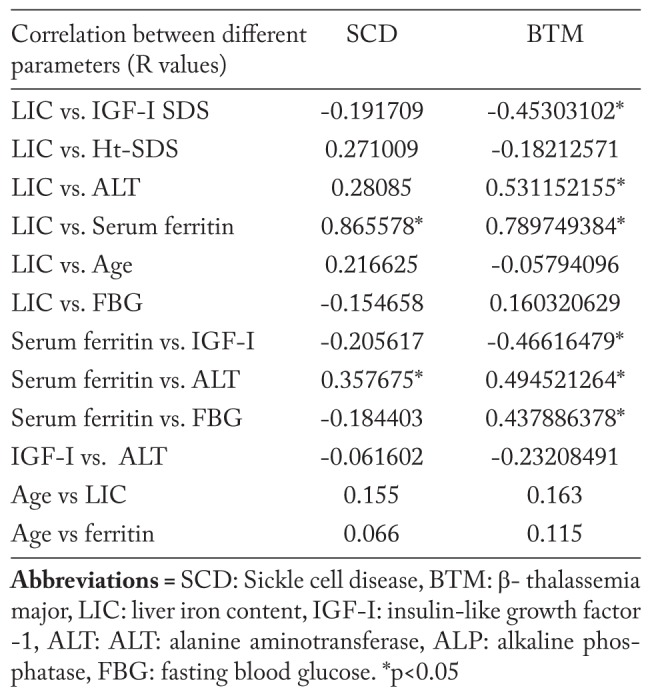
The linear regression analysis showed a significant correlation between LIC and serum ferritin level in SCD and BTM. LIC and serum ferritin level were also correlated significantly with IGF-I level in patients with BTM. LIC was correlated significantly with ALT in patients with BTM. Age of the patients with SCD and BTM was not correlated significantly with LIC or ferritin levels (Table 7). The duration of blood transfusions was not statistically different in patients with BTM (23.5±5.4 years) versus SCD (26.5±8 years) (p> 0.05).
Table 7.
Correlations between different parameters in adult patients with BTM and SCD
Discussion
BTM and SCD are important causes of morbidity and mortality worldwide, causing damage and dysfunction in multiple organs. The complications of these diseases are numerous, affect almost every organ and/or tissue in the body and vary considerably among patients over the time (2-6, 13-15).
Longitudinal studies have reported that the prevalence of endocrine complications has declined in the last few decades thanks to more effective iron chelation and earlier age of first exposure to chelation treatment (16). However, a cross-sectional, analytic study was carried out on 43 TM patients aged 45-60 years. The data indicated that 88.4% of adult BTM patients of this advanced age group suffered from at least one endocrine complication. The majority of patients developed endocrine complications in the second decade of life when serum ferritin level was very high (above 5,000 ng/ml).
The very high peak level of serum ferritin registered in these patients was probably due to inadequate doses of desferrioxamine in the 1st years of life, combined with poor compliance to treatment during the peripubertal or pubertal age and increased amount of transfused blood not balanced by increased chelating treatment (17).
Overall, the prevalence of endocrine disorders in TM patients is affected by various factors such as: genotype, age, hemoglobin level attained before blood transfusion, degree, type and age at the beginning of chelation therapy, compliance to treatment, presence of associated complications, and zinc deficiency (3, 5, 13-16). Therefore, physicians’ strategies to optimize chelation therapy include identifying patients who are at risk for developing organ damage, developing chelation plans, promoting compliance, and educating patients (17).
Our study revealed high prevalence of short stature, hypogonadism, hypothyroidism, DM and IGF-I deficiency in adult patients with BTM. These complications occurred less frequently, but still considerable, in chronically transfused patients with SCD. Because endocrine complications are mostly attributed to iron overload and suboptimal chelation therapy, we compared the final growth and endocrine dysfunctions of BTM (n=52) and SCD (n=40) patients to their LIC, as measured by Ferriscan® method.
Patients with BTM had significantly lower Ht- SDS level compared to patients with SCD. LIC, serum ferritin and ALT concentrations were significantly higher in patients with BTM compared to those with SCD. The prevalence of endocrinopathies namely hypothyroidism, DM, and hypogonadism were significantly higher in BTM patients versus SCD patients. However, the occurrence of significant endocrinopathies in our patients with SCD as well as in other studies proved that SCD patients are unlikely to be completely protected from the extrahepatic effects of iron overload.
The higher prevalence of hypogonadism, DM and IGF-I deficiency in BTM with higher LIC compared to those with lower LIC proves the significant contribution of the degree of iron overload in the pathogenesis of endocrine dysfunction. In accordance with our results, Chirico et al. (18) reported that patients with ferritin values above 1,800 μg/L experienced a significantly faster evolution to hypothyroidism, hypogonadism and multiple endocrinopathies. The intensification of chelation therapy in patients with BTM led to an amelioration of many endocrinopathies including hypothyroidism and hypogonadism (19, 20).
In general, patients with SCD are not routinely screened for iron overload and receive less iron chelation compared to patients with BTM (21). In support of these findings, iron overload was present in approximately one-third of 141 adult SCD patients at post mortem (mean age, 36 y), and 7% of deaths were judged to be related to iron overload (21). In another cohort of 387 young adults with SCD from Atlanta, there were 22 deaths, 45% related to iron overload (22).
Our understanding of iron overload-related organ damage in patients with SCD is still developing. 25% of our adult patients with SCD had LIC >8 mg Fe/g d/wt. Therefore, it appears necessary to routinely monitor iron status in all patients who have been previously transfused with multiple RBC units over a protracted period of time or are currently undergoing regular transfusions.
Serial measurement of steady-state serum ferritin levels is a relatively robust, convenient, and inexpensive marker of body iron burden. Our study found a significant correlation between serum ferritin level and LIC in adult patients with SCD and BTM denoting that serum ferritin is still a good marker of iron overload in patients with BTM and SCD. In support to our view, in a cohort of adults with non-transfusion dependent SCD, LIC correlated significantly with serum ferritin (23). In a cross-sectional study of 27 children (10.9±3.3 years) with SCD who had received chronic transfusion therapy without chelation, serum ferritin and total volume of transfusions were correlated significantly with LIC (24).
Nevertheless, a number of studies have failed to demonstrate a positive correlation of serum ferritin with LIC in patients with SCD (25-29).
Although the liver is the primary site of excess iron storage in both patients with SCD and BMT, there were some disparities in the occurrence of endocrine glands injury. In fact, these complications occurred less frequently, but still considerable, in chronically transfused patients with SCD. There are several hypotheses to explain these distinct differences in iron loading and organ toxicity, including differences in intestinal iron absorption and the rate and duration of transfusions as well as the possibility that the chronic inflammatory state associated with SCD is protective against tissue damage (30). Lower levels of nontransferrin-bound iron (NTBI) in patients with SCD, relative to those seen in patients with BMT, also indicate that there may be intrinsic differences in iron transport and storage between these two populations (31).
In conclusion, the prevalence of endocrinopathies especially hypothyroidism, DM, and hypogonadism were significantly higher in chronically transfused patients with BTM patients versus SCD patients and were more prevalent in patients with higher LIC versus those with lower LIC. This study confirm that iron overload is an inevitable consequence of long-term transfusion therapy, for which iron chelation therapy is indicated and recommended. All patients who receive RBC transfusions and iron chelation therapy should be regularly monitored and given practical and educational support in order to improve compliance with therapy.
References
- 1.Koren A, Fink D, Admoni O, Tennenbaum-Rakover Y, Levin C. Non-transferrin-bound labile plasma iron and iron overload in sickle-cell disease: a comparative study between sickle-cell disease and beta-thalassemic patients. Eur J Haematol. 2010;84:72–78. doi: 10.1111/j.1600-0609.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 2.Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013;2013:447–456. doi: 10.1182/asheducation-2013.1.447. [DOI] [PubMed] [Google Scholar]
- 3.De Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC, Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in thalassemia: The international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J Endocrinol Metab. 2013;17:8–18. doi: 10.4103/2230-8210.107808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagag AA, El-Farargy MS, Elrefaey S, Abo El-enein AM. Study of gonadal hormones in Egyptian female children with sickle cell anemia in correlation with iron overload: Single center study. Hematol Oncol Stem Cell Ther. 2016;9:1–7. doi: 10.1016/j.hemonc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP. Multi-Centre Study of Iron Overload Research Group. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 6.Ozen S, Unal S, Erçetin N, Taşdelen B. Frequency and risk factors of endocrine complications in Turkish children and adolescents with sickle cell anemia. Turk J Haematol. 2013;30:25–31. doi: 10.4274/tjh.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen P, Engelhardt R, Düllmann J, Fischer R. Non-Invasive Liver Iron Quantification by SQUID-Biosusceptometry and Serum Ferritin Iron as New Diagnostic Parameters in Hereditary Hemochromatosis. Blood Cells Mol Dis. 2002;29:451–458. doi: 10.1006/bcmd.2002.0583. [DOI] [PubMed] [Google Scholar]
- 8.Adamkiewicz TV, Abboud MR, Paley C, Olivieri N, Kirby-Allen M, Vichinsky E, Casella JF, Alvarez OA, Barredo JC, Lee MT, Iyer RV, Kutlar A, McKie KM, McKie V, Odo N, Gee B, Kwiatkowski JL, Woods GM, Coates T, Wang W, Adams RJ. Serum ferritin level changes in children with sickle cell disease on chronic blood transfusion are nonlinear and are associated with iron load and liver injury. Blood. 2009;114:4632–4638. doi: 10.1182/blood-2009-02-203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K, Subramony C, May W, Megason G, Liu H, Bishop P, Walker T, Nowicki MJ. Hepatic iron overload in children with sickle cell anemia on chronic transfusion therapy. J Pediatr Hematol Oncol. 2009;31:309–312. doi: 10.1097/MPH.0b013e3181a1c143. [DOI] [PubMed] [Google Scholar]
- 10.St Pierre TG, Clark PR, Chua-Anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 11.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yassin M, Soliman AT, De Sanctis V, Moustafa A, Samaan SA, Nashwan A. A young adult with unintend acute intravenous iron intoxication treated with oral chelation: the use of liver ferriscan for diagnosing and monitoring tissue iron load. Mediterr J Hematol Infect Dis. 2017 Jun 20;9(1):e2017037. doi: 10.4084/MJHID.2017.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman AT, Darwish A, Mohammed SH, Bassiony MR, el Banna N, Asfour M. Circulating growth hormone (GH), insulin-like growth factor-I (IGF-I) and free thyroxine, GH response to clonidine provocation and CT scanning of the hypothalamic-pituitary area in children with sickle cell disease. J Trop Pediatr. 1995;41:285–289. doi: 10.1093/tropej/41.5.285. [DOI] [PubMed] [Google Scholar]
- 14.Soliman AT, el Banna N, alSalmi I, De Silva V, Craig A, Asfour M. Growth hormone secretion and circulating insulin-like growth factor-I (IGF-I) and IGF binding protein-3 concentrations in children with sickle cell disease. Metabolism. 1997;46:1241–1245. doi: 10.1016/s0026-0495(97)90224-9. [DOI] [PubMed] [Google Scholar]
- 15.Soliman AT, elZalabany MM, Mazloum Y, Bedair SM, Ragab MS, Rogol AD, Ansari BM. Spontaneous and provoked growth hormone (GH) secretion and insulin-like growth factor I (IGF-I) concentration in patients with beta thalassaemia and delayed growth. J Trop Pediatr. 1999;45:327–337. doi: 10.1093/tropej/45.6.327. [DOI] [PubMed] [Google Scholar]
- 16.De Sanctis V, Roos M, Gasser T, Fortini M, Raiola G, Galati MC. Italian Working Group on Endocrine Complications in Non-Endocrine Diseases. Impact of long-term iron chelation therapy on growth and endocrine functions in thalassaemia. J Pediatr Endocrinol Metab. 2006;19:471–480. [PubMed] [Google Scholar]
- 17.De Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Soliman NA, Elalaily R, Kattamis C. Endocrine profile of β-thalassemia major patients followed from childhood to advanced adulthood in a tertiary care center. Indian J Endocrinol Metab. 2016;20:451–459. doi: 10.4103/2230-8210.183456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirico V, Rigoli L, Lacquaniti A, Salpietro V, Piraino B, Amorini M, Salpietro C, Arrigo T. Endocrinopathies, metabolic disorders, and iron overload in major and intermedia thalassemia: serum ferritin as diagnostic and predictive marker associated with liver and cardiac T2* MRI assessment. Eur J Haematol. 2015;94:404–412. doi: 10.1111/ejh.12444. [DOI] [PubMed] [Google Scholar]
- 19.Farmaki K, Angelopoulos N, Anagnostopoulos G, Gotsis E, Rombopoulos G, Tolis G. Effect of enhanced iron chelation therapy on glucose metabolism in patients with beta-thalassaemia major. Br J Haematol. 2006;134:438–444. doi: 10.1111/j.1365-2141.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- 20.Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–475. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- 21.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP. Multi-Centre Study of Iron Overload Research Group. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 22.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O (2006) Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 23.Yassin M, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, Kohla S, Soliman D. Liver Iron Content (LIC) in Adults with Sickle Cell Disease (SCD): Correlation with Serum Ferritin and Liver Enzymes Concentrations in Trasfusion Dependent (TD-SCD) and Non-Transfusion Dependent (NT-SCD) Patients. Mediterr J Hematol Infect Dis. 2017 Jun 20;9(1):e2017037. doi: 10.4084/MJHID.2017.037. doi: 10.4084/MJHID.2017.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown K, Subramony C, May W, Megason G, Liu H, Bishop P, Walker T, Nowicki MJ. Hepatic iron overload in children with sickle cell anemia on chronic transfusion therapy. J Pediatr Hematol Oncol. 2009;31:309–312. doi: 10.1097/MPH.0b013e3181a1c143. [DOI] [PubMed] [Google Scholar]
- 25.Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–79. [PubMed] [Google Scholar]
- 26.Karam LB, Disco D, Jackson SM, et al. Liver biopsy results in patients with sickle cell disease on chronic transfusions: Poor correlation with ferritin levels. Pediatr Blood Cancer. 2008;50:62–65. doi: 10.1002/pbc.21215. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol. 2001;38(Suppl 1):57–62. doi: 10.1016/s0037-1963(01)90060-5. [DOI] [PubMed] [Google Scholar]
- 28.Brown K, Subramony C, May W, et al. Hepatic iron overload in children with sickle cell anemia on chronic transfusion therapy. J Pediatr Hematol Oncol. 2009;31:309–312. doi: 10.1097/MPH.0b013e3181a1c143. [DOI] [PubMed] [Google Scholar]
- 29.Pakbaz Z, Fischer R, Fung E, et al. Serum ferritin underestimates liver iron concentration in transfusion independent thalassemia patients as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer. 2007;49:329–332. doi: 10.1002/pbc.21275. [DOI] [PubMed] [Google Scholar]
- 30.Fillet G, Beguin Y, Baldelli L. Model of reticuloendothelial iron metabolism in humans: Abnormal behavior in idiopathic hemochromatosis and in inflammation. Blood. 1989;74:844–851. [PubMed] [Google Scholar]
- 31.Inati A, Musallam KM, Wood JC, et al. Absence of cardiac siderosis by MRI T2* despite transfusion burden, hepatic and serum iron overload in Lebanese patients with sickle cell disease. Eur J Haematol. 2009;83:565–571. doi: 10.1111/j.1600-0609.2009.01345.x. [DOI] [PubMed] [Google Scholar]