Abstract
Osteoporosis is the most important metabolic bone disease, with a wide distribution among the elderly. It is characterized by low bone mass and micro architectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk. Identify bone weakening with an appropriate and accurate use of diagnostic imaging is of critical importance in the diagnosis and follow-up of osteoporotic patients. The aim of this review is to evaluate the detection rates of the different imaging modalities in the evaluation of bone strength, in the assessment of fracture risk and in the management of fragility fractures. (www.actabiomedica.it)
Keywords: bone densitometry, osteoporosis, aging, high resolution imaging, bone
Introduction
Osteoporosis is recognized as a serious and increasingly public health issue due to its high prevalence in aging people, in which it contribute to reduce physical performance and increase the risk of fall-related injury, disability, and mortality (1-5).
Osteoporosis is the most important metabolic bone disease and is characterized by low bone mass and microarchitecture deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk (6-10).
The most common type of osteoporosis is involutional osteoporosis, which is classified into type I or postmenopausal osteoporosis and type II or senile osteoporosis (11-20). Postmenopausal osteoporosis usually occurs in women between ages 50 and 65 years, affecting those within 15 to 20 years of menopause. The estrogenic deficiency is linked to an accelerated trabecular bone resorption, which may lead to fragility fractures that typically involve spine and wrist. Senile osteoporosis occurs in women or men more than 70 years of age and the main feature is that the bone loss pattern involves the cortex and the trabeculae, leading to fragility fractures usually located at the hip, pelvis, and proximal humerus. Despite the well-recognized role of estrogenic deficiency in type I osteoporosis and the consequent higher prevalence of fragility fractures in 40-50 y.o. women, multiple investigations have confirmed an age-related significant prevalence of senile osteoporosis in men as well (21-25).
Although several studies have already highlighted higher mortality rates in women who experienced a vertebral fracture, the social and economic burden of osteoporosis still remains partially underestimated (26-30).
Identify bone weakening with an appropriate and accurate use of diagnostic imaging is of critical importance in the diagnosis of osteoporosis at an early and in the management of the complications that often implicate differential diagnosis issues, most of all in a geriatric patient. It also allows to predict fracture risk, to determine the treatment approach and to help monitor disease progression and response to therapy.
Therefore, the aim of this review is to evaluate the detection rates of the different imaging modalities in the evaluation of bone strength, in the fracture risk’s assessment and in the fragility fractures management.
Imaging techniques
Besides conventional radiography, imaging techniques developed to diagnose the loss of bone mass and the micro architectural deterioration of bone tissue are dual-energy x-ray absorptiometry (DXA), quantitative computed tomography (QCT), and quantitative ultrasound (QUS) (31-35). Generations of DXA systems provide not only accurate and reproducible measurements of BMD but also the opportunity to use high-quality DXA scans in place of standard X-rays to identify vertebral fractures. Semiquantitative and fully quantitative methods to determine the presence of vertebral fracture (36), as well as indices related to hip geometry (35, 37-40), can be derived from high-quality DXA images. Bone stiffness assessed by finite element analysis of X-ray images (FEXI), a technique that uses a finite element analysis model applied to 2D gray-level images, can also be extracted from DXA images (41-45).
Finally, the evaluation of bone mineral distribution at the proximal femur in hip DXA scans may be well suited to enhance standard densitometric evaluations as a predictor of hip fracture risk. Taking advantage of high-quality DXA images, and based upon previous studies using 2D X-ray images to estimate bone microarchitecture, the trabecular bone score (TBS) was developed as another approach for assessing skeletal microstructure noninvasively from 2D DXA projection images.
Dual Energy X-Ray Absorptiometry (DXA)
Dual energy X-ray Absorptiometry (DXA) is the most widely used quantitative technique for bone mineral density (BMD) assessment in clinical practice and represents the “gold standard” for a non-invasive diagnosis of osteoporosis, according to the World Health Organization (WHO) guidelines (46).
BMD, measured in mg/cm3, is determined by peak bone mass and amount of bone loss, whereas bone quality refers to architecture, turnover, damage accumulation (eg, micro fractures), and mineralization (47).
BMD is defined using the T-score, which is the number of standard deviations (SD) above or below the mean for a healthy 30 y.o. adult of the same ethnicity and sex (which refers to the peak bone mass). The World Health Organization (WHO) has defined T-score threshold levels for BMD assessment: ≥-1.0 is considered as normal, values between ≤-1.0 and ≥-2.5 refer to osteopenia, and a T-score ≤-2.5 is classified as osteoporosis.
Z-score is the number of SD above or below the normal values of a healthy subject of the same age, sex, weight and ethnicity; this parameter is mostly used in the assessment of metabolic bone status of children and people aged over 75, but it should be also considered in women prior to menopause and men younger than 50 y.o. (7). A Z-score of −2.0 or lower is defined as “below the expected range for age” and a Z-score above −2.0 is “within the expected range for age” (1).
This definitions of osteopenia and osteoporosis only refer to DXA measurements at the lumbar spine (from L1 to L4, in antero-posterior projection), the proximal femur (neck and total femur as Region Of Interest -ROI) or the distal radius (1/3 distal radius as ROI).
Lumbar spine is the primary site for BMD measurement. The BMD of proximal femur is the best predictor of hip fracture. ROIs include femoral neck, trochanter, Ward’s area, intertrochanteric region, and total hip.
The forearm is a third site used for BMD measurement, useful when spine and hip are not measurable or interpretable due to severe degenerative processes, and implantable devices.
The advantages of DXA are short scan times, low radiation dose, good reproducibility, low cost, and wide availability.
This technique has some limitations, especially in the elderly, because spinal degeneration (most of all marginal osteophytes), spinal deformity, extreme obesity and abdominal aortic calcification may overestimate BMD and reduce the sensitivity of DXA for assessing osteoporosis. Furthermore DXA, being a two-dimensional measurement, cannot distinguish between cortical and trabecular bone (48).
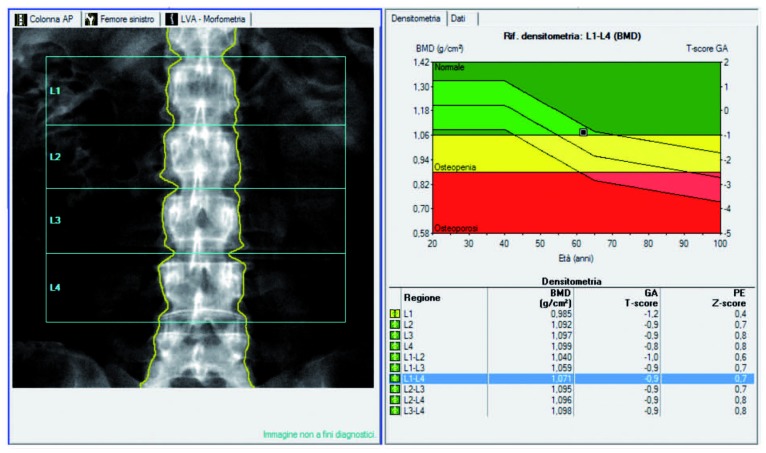
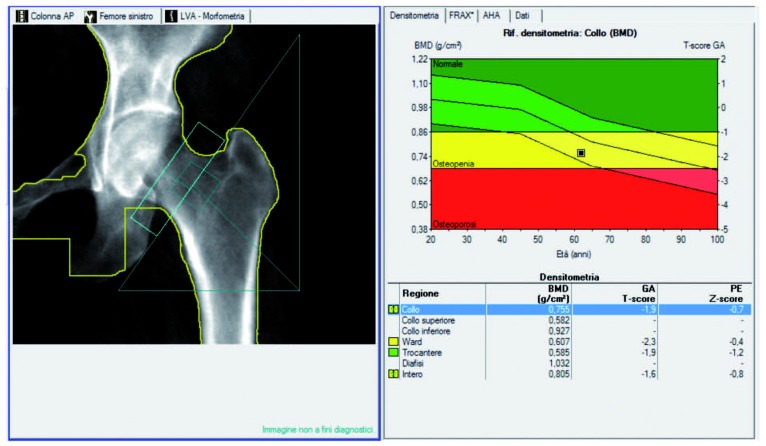
Schneider et al. recommended the use of DXA of the hip for identification of osteoporosis in women aged 65 years and older who are likely to have spinal osteoarthritis (49) (Figure 1a - b).
Figure 1a.
Example of lumbar spine DXA of a 65 y.o. woman showing normal BMD values in mg/cm3 with the corresponding T-score and Z-score
Figure 1b.
Example of hip DXA of the same 65 y.o. woman showing osteopenic BMD values in mg/cm3 with the corresponding T-score and Z-score
The recent implementation of software for advanced hip assessment into DXA systems have provided a noninvasive description of the structural geometry of the proximal femur, depicting several parameters such as cortical thickness with bone mapping, areal BMD, hip axis length, cross-sectional area, cross-sectional moment of inertia, and the femoral strength index (32, 50-53).
Trabecular Bone Score (TBS)
Although BMD measured by DXA is a major determinant of bone strength and fracture risk, most individuals with a fragility fracture will have BMD values in the osteopenic or even normal range (50). In addition to low bone mass, micro architectural deterioration of bone tissue can lead to increased bone fragility and consequent increased risk of fracture.
The evolution of DXA technology has allowed more advanced tools in the assessment of the bone status with the aim to provide bone quality properties unrelated from BMD (54). The trabecular bone score (TBS) evaluates in DXA images of the lumbar spine (L1-L4) pixel grey-level variations, which have been associated to bone micro-architecture (55). An elevated TBS value correlates with better skeletal microstructure; a low TBS value correlates with weaker skeletal microstructure.
TBS has the potential to discern differences between DXA scans that show similar BMD measurements. Since most individuals with fragility fractures may have BMD values in the range of normality or osteopenia, TBS could be useful to select patients to be screened and managed for osteoporosis (34, 56, 57).
Several preliminary studies in patients affected by metabolic bone diseases have suggested that lumbar spine TBS, in addition to BMD and clinical risk factors, could be an important tool in the diagnosis of osteoporosis and especially in fracture risk assessment.
Despite these promising results, opinions in literature are still controversial and further normative data, validation and prospective studies are required (31).
Quantitative Computed Tomography (QCT)
Unlike the DXA, which only measures density/area in g/cm2, Quantitative Computed Tomography (QCT) allows true volumetric mineral density measurements in mg/cm3. QCT also provides separate estimation of trabecular and cortical BMD.
The major advantage of QCT over DXA is the selective measure of trabecular tissue, because trabecular bone is the main determinant of compressive strength in the vertebrae and purely trabecular bone measure is more sensitive to monitoring changes with disease and therapy (58, 59).
To perform quantitative CT is used a standard CT scanner and phantom which acts as bone mineral reference standard to calibrate each scan. Density values measured in Hounsfield units are transformed into BMD measured in milligrams hydroxyapatite per cubic centimeter by using a phantom.
Typically QCT is performed at the spinal vertebrae (T12 to L4): ROIs are positioned in the trabecular portion of the vertebral body, compared to the calibration phantom. The obtained vertebral densities are averaged and compared to those of a gender- and race-specific normal population (51, 60). The results are usually expressed in absolute values and as Z-scores and T-scores.
Despite QCT has shown an excellent ability to predict vertebral fractures and a good sensitivity for BMD changes during the follow-up (61), it has some limitations that have narrowed its clinical diffusion: marrow change processes can affect trabecular measurements, such as myelofibrosis, and the technique has higher radiation doses and costs compared to DXA.
Currently, to obviate the limitations of DXA and axial QCT, is more frequently used a volumetric QCT (vQCT), which provides separate assessment of cortical and trabecular bone at appendicular sites (62). The evolution of post-processing software allowed further analysis on bone geometrical and torsional stability, which correlates to bone strength and consequent susceptibility to fracture (63, 64).
Recommendations in clinical routine to characterize fracture risk with the absolute measurements of volumetric BMD are: 110-80 mg/cm3 = mild increase in fracture risk; 80-50 mg/cm3 = moderate increase in fracture risk; <50 mg/cm3 = severe increase in fracture risk (65, 66).
High resolution quantitative computed tomography (HR-QCT) has been performed on metabolic bone disease patients with the aim of providing a detailed assessment of both cortical and trabecular architecture (67). With an 80-100 μmresolution, HR-QCT can measure (in addition to the parameters classically measured by QCT) bone volume fraction as well as cortical and trabecular parameters including thickness, separation, and number of trabeculae (68-71). Nevertheless, high costs and the expertise level required to handle these techniques has limited their application to few research centers.
Vertebral Morphometry
Vertebral body fractures (VBF) are the most frequent type of osteoporotic fractures, and they occur significantly earlier compared with wrist and hip fractures (72-75). After sustaining an osteoporotic fracture, patients are at a 50-100% greater risk of suffering another osteoporotic fracture. For this reason identify and treat patients with newly developed VBF is essential to prevent further and more severe osteoporotic fractures, such as the hip fractures that leads to persisting disabilities, hospitalization and operation costs (44, 76-78).
Current DXA systems allow to use high quality DXA scans instead of standard X-rays to identify vertebral fractures. Radiographic diagnosis with conventional lateral radiographs of the thoracolumbar spine is considered to be the best way to identify and confirm the presence of osteoporotic vertebral fractures in clinical practice. The two most widely used methods to determine the severity of such fractures in clinical research are the semi quantitative assessment of vertebral deformities, which is based on visual evaluation, and the quantitative approach, which is based on different morphometric criteria (79).
The morphological classification (wedge, biconcave, crush) of VBF results from more than 20% loss in anterior, middle or posterior heights of vertebral bodies. VBFs are also classified as mild (20–25%), moderate (26-40%), and severe (>40%) reductions in any height.
The visual semi-quantitative approach proposed by Genant et al. (79) has been integrated with morphometric methods based on vertebral height measurements. The quantitative vertebral morphometry can be applied on spinal radiographs (MXR: Morphometric X-ray Radiography) or on DXA images (MXA: Morphometric X-ray Absorptiometry).
Several semi-automated software have been introduced with the aim of digitize and automatize MRX, improving its reproducibility (80). The operator has to manually identify the vertebral levels (from T5 to L4) then a semi-automated six-points segmentation of the vertebrae calculates the vertebral heights (posterior - Hp, middle - Hm and anterior Ha) and the ratio between heights (Ha/Hp, Hm/Hp) of each vertebra. The last step of the analysis includes the report of fracture assessment based on normative data and models (81).
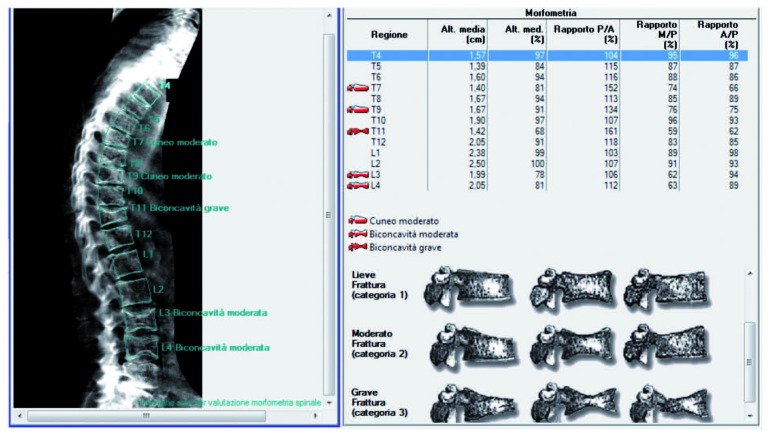
The widespread diffusion of DXA and the technical improvements have allowed the application of quantitative morphometry on lateral DXA images of the spine. Thanks to its lower radiation exposure, MXA nowadays represents the most widely adopted solution for quantitative assessment of fracture status and has been fully integrated into DXA-based BMD assessment of osteoporosis in clinical routine (82) (Figure 2).
Figure 2.
Example of quantitative morphometry on lateral DXA images of the spine, showing several vertebral body fractures (VBF), with the corresponding fracture classification in mild (type1), moderate (type2), and severe (type 3)
However, the radiologist’s role still remains critical in order to distinguish osteoporotic vertebral fractures from malignancies and other congenital or acquired deformities.
Quantitative Ultrasound (QUS)
Quantitative Ultrasound (QUS) is a portable, radiation-free and low cost technique performed with dedicated scanners. It provides measurements of quantitative parameters related to bone quality properties through the analysis of interactions between ultrasound and bone. It is usually performed to peripheral sites such as calcaneus (primary site), metaphysis of the phalanx, radius and tibia. Transit time velocity and ultrasound attenuation represent the most widely adopted parameters: velocity decreases in osteoporotic bone, whereas ultrasound attenuation increases in osteoporotic bone. QUS results can be expressed in absolute values or in T-score and Z-score linked to normative reference data (66, 83-86).
Several studies have shown that QUS parameters can differentiate individuals with from those without fragility fractures and are predictive of osteoporotic fractures (87-89). However, despite quantitative US can be useful as screening tool for the estimation of fracture risk, the WHO has stated that it cannot be used as stand-alone tool for the diagnosis of osteoporosis and and to monitor treatment response (90).
RM Imaging
The concept of bone strength as result of bone quantity and bone quality have induced the scientific community to explore other imaging modalities capable of obtaining micro-architectural data of trabecular bone with the aim to understand the relationship between bone turnover, density and architecture (47).
Several studies have explored MR’s ability to correlate trabecular content and architecture with bone turnover (45, 91-94). The technique has been mainly established for peripheral imaging of the distal radius, tibia, and calcaneus.
Specific high resolution sequences and imaging analysis algorithms have been developed to obtain a non-invasive assessment of bone strength and turnover (95).
Bone marrow is critical to the viability and strength of trabecular and cortical bone.
Dynamic contrast-enhanced MR imaging (DCE-MRI) studies across different age groups have revealed that vertebral marrow perfusion is reduced in elderly and in patients with osteoporosis compared to subjects with osteopenia (96, 97).
Subjects with osteoporosis or osteopenia revealed a significantly increased marrow fat content compared with the fat content in subjects with normal bone density. The concomitant observation that both adipocytes and osteoblasts arise from common precursor cells has suggested the hypothesis that preferential differentiation of mesenchymal stem cells towards the adipocyte lineage may negatively influence osteoblast differentiation (98, 99).
Proton RM spectroscopy (MRS) has been proposed as a promising candidate into routine clinical procedures for quantifying marrow adiposity non-invasively. MRS-based studies have revealed an age-dependent linear increase in vertebral marrow fat content at various skeletal sites (100, 101). In contrast to the qualitative evaluation of red marrow versus yellow marrow provided by conventional MR imaging, MR spectroscopy provides quantitative assessment of water and fat content in bone marrow (102).
Conclusions
Osteoporosis is a major worldwide health problem, which contribute to reduce physical performance and increase the risk of fall-related injury, disability, and mortality in aging people (103). Without prevention and screening, osteoporosis may be clinically silent with an increased incidence of osteoporotic fractures and consequently with an exponentially grow of the socio-economic costs associated to osteoporotic fracture-related morbidity and mortality.
Therefore, early diagnosis of osteoporosis and adequate management of its complications are becoming a particularly relevant concern in the context of guarantee a true “healthy aging”.
References
- 1.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. (3rd) 2008;42:467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Splendiani A, Perri M, Marsecano C, Vellucci V, Michelini G, Barile A, Di Cesare E. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med. 2017 doi: 10.1007/s11547-017-0816-9. [DOI] [PubMed] [Google Scholar]
- 3.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, Ruscitti P, Cipriani P, Giacomelli R, Brunese L, Masciocchi C. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017 doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bruno F, Smaldone F, Varrassi M, Arrigoni F, Barile A, Di Cesare E, Masciocchi C, Splendiani A. MRI findings in lumbar spine following O2-O3 chemiodiscolysis: A long-term follow-up. Interv Neuroradiol. 2017;23:444–450. doi: 10.1177/1591019917703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barile A, Bruno F, Arrigoni F, Splendiani A, Di Cesare E, Zappia M, Guglielmi G, Masciocchi C. Emergency and Trauma of the Ankle. Semin Musculoskelet Radiol. 2017;21:282–289. doi: 10.1055/s-0037-1602408. [DOI] [PubMed] [Google Scholar]
- 6.Compston J. Osteoporosis: social and economic impact. Radiol Clin North Am. 2010;48:477–82. doi: 10.1016/j.rcl.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Barile A, Arrigoni F, Zugaro L, Zappia M, Cazzato RL, Garnon J, Ramamurthy N, Brunese L, Gangi A, Masciocchi C. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34 doi: 10.1007/s12032-017-0909-2. [DOI] [PubMed] [Google Scholar]
- 8.Arrigoni F, Barile A, Zugaro L, Splendiani A, Di Cesare E, Caranci F, Ierardi AM, Floridi C, Angileri AS, Reginelli A, Brunese L, Masciocchi C. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34 doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 9.Reginelli A, Zappia M, Barile A, Brunese L. Strategies of imaging after orthopedic surgery. Musculoskeletal Surg. 2017;101 doi: 10.1007/s12306-017-0458-z. [DOI] [PubMed] [Google Scholar]
- 10.Splendiani A, D’Orazio F, Patriarca L, Arrigoni F, Caranci F, Fonio P, Brunese L, Barile A, Di Cesare E, Masciocchi C. Imaging of post-operative spine in intervertebral disc pathology. Musculoskelet Surg. 2017;101:75–84. doi: 10.1007/s12306-017-0453-4. [DOI] [PubMed] [Google Scholar]
- 11.Anil G, Guglielmi G, Peh WC. Radiology of osteoporosis. Radiol Clin North Am. 2010;48:497–518. doi: 10.1016/j.rcl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Splendiani A, Bruno F, Patriarca L, Barile A, Di Cesare E, Masciocchi C, Gallucci M. Thoracic spine trauma: advanced imaging modality. Radiol Med. 2016;121:780–792. doi: 10.1007/s11547-016-0657-y. [DOI] [PubMed] [Google Scholar]
- 13.Masciocchi C, Arrigoni F, Marra AL, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Splendiani A, Perri M, Grattacaso G, Di Tunno V, Marsecano C, Panebianco L, Gennarelli A, Felli V, Varrassi M, Barile A, Di Cesare E, Masciocchi C, Gallucci M. Magnetic resonance imaging (MRI) of the lumbar spine with dedicated G-scan machine in the upright position: a retrospective study and our experience in 10 years with 4305 patients. Radiol Med. 2016;121:38–44. doi: 10.1007/s11547-015-0570-9. [DOI] [PubMed] [Google Scholar]
- 15.Patriarca L, Letteriello M, Di Cesare E, Barile A, Gallucci M, Splendiani A. Does evaluator experience have an impact on the diagnosis of lumbar spine instability in dynamic MRI? Interobserver agreement study. Neuroradiol J. 2015;28:341–346. doi: 10.1177/1971400915594508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Splendiani A, Ferrari F, Barile A, Masciocchi C, Gallucci M. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: Demonstration with dedicated upright MRI system. Radiol Med. 2014;119:164–174. doi: 10.1007/s11547-013-0330-7. [DOI] [PubMed] [Google Scholar]
- 17.Masciocchi C, Conchiglia A, Conti L, Barile A. Imaging of insufficiency fractures, Geriatric Imaging. Springer-Verlag Berlin Heidelberg. 2013:83–91. [Google Scholar]
- 18.Masciocchi C, Conti L, D’Orazio F, Conchiglia A, Lanni G, Barile A. Errors in musculoskeletal MRI,Errors in Radiology. Springer-Verlag Milan. 2012:209–217. [Google Scholar]
- 19.Barile A, Limbucci N, Splendiani A, Gallucci M, Masciocchi C. Spinal injury in sport. Eur J Radiol. 2007;62:68–78. doi: 10.1016/j.ejrad.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Nurzynska D, Di Meglio F, Castaldo C, Latino F, Romano V, Miraglia R, Guerra G, Brunese L, Montagnani S. Flatfoot in children: anatomy of decision making. Ital J Anat Embryol. 2012;117:98–106. [PubMed] [Google Scholar]
- 21.Kenny A, Taxel P. Osteoporosis in older men. Clin Cornerstone. 2000;2:45–51. doi: 10.1016/s1098-3597(00)90005-x. [DOI] [PubMed] [Google Scholar]
- 22.Masciocchi C, Arrigoni F, Barile A. Role of conventional RX, CT, and MRI in the evaluation of prosthetic joints, Imaging of Prosthetic Joints: A Combined Radiological and Clinical Perspective. Springer-Verlag Milan. 2014:63–69. [Google Scholar]
- 23.De Divitiis O, Elefante A. Cervical spinal brucellosis: A diagnostic and surgical challenge. World Neurosurgery. 2012;78(3-4):257–259. doi: 10.1016/j.wneu.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Dragoni S, Turin I, Laforenza U, Potenza DM, Bottino C, Glasnov TN, Prestia M, Ferulli F, Saitta A, Mosca A, Guerra G, Rosti V, Luinetti O, Ganini C, Porta C, Pedrazzoli P, Tanzi F, Montagna D, Moccia F. Store-operated Ca2+ entry does not control proliferation in primary cultures of human metastatic renal cellular carcinoma. Biomed Res Int. 2014;2014:739494. doi: 10.1155/2014/739494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Zazzo E, Porcile C, Bartollino S, Moncharmont B. Critical Function of PRDM2 in the Neoplastic Growth of Testicular Germ Cell Tumors. Biology (Basel) 2016;5 doi: 10.3390/biology5040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 27.Cappabianca S, Scuotto A, Iaselli F, Pignatelli di Spinazzola N, Urraro F, Sarti G, Montemarano M, Grassi R, Rotondo A. Computed tomography and magnetic resonance angiography in the evaluation of aberrant origin of the external carotid artery branches. Surg Radiol Anat. 2012;34:393–9. doi: 10.1007/s00276-011-0926-3. [DOI] [PubMed] [Google Scholar]
- 28.Iudici M, Cuomo G, Vettori S, Bocchino M, Sanduzzi Zamparelli A, Cappabianca S, Valentini G. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum. 2015;44:437–44. doi: 10.1016/j.semarthrit.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Mandato Y, Reginelli A, Galasso R, Iacobellis F, Berritto D, Cappabianca S. Errors in the radiological evaluation of the alimentary tract: part I. Semin Ultrasound CT MR. 2012;33:300–7. doi: 10.1053/j.sult.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 30.D’Apuzzo F, Cappabianca S, Ciavarella D, Monsurro A, Silvestrini-Biavati A, Perillo L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: overview and clinical relevance. ScientificWorldJournal. 2013;2013:105873. doi: 10.1155/2013/105873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazzocchi A, Ponti F, Diano D, Amadori M, Albisinni U, Battista G, Guglielmi G. Trabecular bone score in healthy ageing. Br J Radiol. 2015;88:20140865. doi: 10.1259/bjr.20140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muto M, Perrotta V, Guarnieri G, Lavanga A, Vassallo P, Reginelli R, Rotondo A. Vertebroplasty and kyphoplasty: Friends or foes. Radiol Med. 2008;113:1171–1184. doi: 10.1007/s11547-008-0301-6. [DOI] [PubMed] [Google Scholar]
- 33.Bandirali M, Di Leo G, Papini GD, Messina C, Sconfienza LM, Ulivieri FM, Sardanelli F. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur Radiol. 2015;25:2951–9. doi: 10.1007/s00330-015-3699-y. [DOI] [PubMed] [Google Scholar]
- 34.Bandirali M, Poloni A, Sconfienza LM, Messina C, Papini GD, Petrini M, Ulivieri FM, Di Leo G, Sardanelli F. Short-term precision assessment of trabecular bone score and bone mineral density using dual-energy X-ray absorptiometry with different scan modes: an in vivo study. Eur Radiol. 2015;25:2194–8. doi: 10.1007/s00330-015-3606-6. [DOI] [PubMed] [Google Scholar]
- 35.Messina C, Bandirali M, Sconfienza LM, D’Alonzo NK, Di Leo G, Papini GD, Ulivieri FM, Sardanelli F. Prevalence and type of errors in dual-energy x-ray absorptiometry. Eur Radiol. 2015;25:1504–11. doi: 10.1007/s00330-014-3509-y. [DOI] [PubMed] [Google Scholar]
- 36.Messina C, Monaco CG, Ulivieri FM, Sardanelli F, Sconfienza LM. Dual-energy X-ray absorptiometry body composition in patients with secondary osteoporosis. Eur J Radiol. 2016;85:1493–8. doi: 10.1016/j.ejrad.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Bandirali M, Messina C, Di Leo G, Sconfienza LM, Aliprandi A, Ulivieri FM, Sardanelli F. Bone mineral density differences between femurs of scoliotic patients undergoing dual-energy X-ray absorptiometry. Clin Radiol. 2013;68:e511–5. doi: 10.1016/j.crad.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Bandirali M, Lanza E, Messina C, Sconfienza LM, Brambilla R, Maurizio R, Marchelli D, Piodi LP, Di Leo G, Ulivieri FM, Sardanelli F. Dose absorption in lumbar and femoral dual energy X-ray absorptiometry examinations using three different scan modalities: an anthropomorphic phantom study. J Clin Densitom. 2013;16:279–82. doi: 10.1016/j.jocd.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Delnevo A, Bandirali M, Di Leo G, Messina C, Sconfienza LM, Aliprandi A, Ulivieri FM, Sardanelli F. Differences among array, fast array, and high-definition scan modes in bone mineral density measurement at dual-energy x-ray absorptiometry on a phantom. Clin Radiol. 2013;68:616–9. doi: 10.1016/j.crad.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Russo A, Reginelli A, Zappia M, Rossi C, Fabozzi G, Cerrato M, Macarini L, Coppolino F. Ankle fracture: radiographic approach according to the Lauge-Hansen classification. Musculoskelet Surg. 2013;97(2):S155–60. doi: 10.1007/s12306-013-0284-x. [DOI] [PubMed] [Google Scholar]
- 41.Russo A, Zappia M, Reginelli A, Carfora M, D’Agosto GF, La Porta M, Genovese EA, Fonio P. Ankle impingement: a review of multimodality imaging approach. Musculoskelet Surg. 2013;97(2):S161–8. doi: 10.1007/s12306-013-0286-8. [DOI] [PubMed] [Google Scholar]
- 42.Grassi R, Lombardi G, Reginelli A, Capasso F, Romano F, Floriani I, Colacurci N. Coccygeal movement: assessment with dynamic MRI. Eur J Radiol. 2007;61:473–9. doi: 10.1016/j.ejrad.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Pinto A, Reginelli A, Pinto F, Lo Re G, Midiri F, Muzj C, Romano L, Brunese L. Errors in imaging patients in the emergency setting. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L. Errors in neuroradiology. Radiol Med. 2015;120:795–801. doi: 10.1007/s11547-015-0564-7. [DOI] [PubMed] [Google Scholar]
- 45.Russo A, Capasso R, Varelli C, Laporta A, Carbone M, D’Agosto G, Giovine S, Zappia M, Reginelli A. MR imaging evaluation of the postoperative meniscus. Musculoskelet Surg. 2017;101:37–42. doi: 10.1007/s12306-017-0454-3. [DOI] [PubMed] [Google Scholar]
- 46.Damilakis J, Guglielmi G. Quality assurance and dosimetry in bone densitometry. Radiol Clin North Am. 2010;48:629–40. doi: 10.1016/j.rcl.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012;263:3–17. doi: 10.1148/radiol.12110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setiawati R, Di Chio F, Rahardjo P, Nasuto M, Dimpudus FJ, Guglielmi G. Quantitative Assessment of Abdominal Aortic Calcifications Using Lateral Lumbar Radiograph, Dual-Energy X-ray Absorptiometry, and Quantitative Computed Tomography of the Spine. J Clin Densitom. 2016;19:242–9. doi: 10.1016/j.jocd.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Schneider DL, Bettencourt R, Barrett-Connor E. Clinical utility of spine bone density in elderly women. J Clin Densitom. 2006;9:255–60. doi: 10.1016/j.jocd.2006.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takakuwa M, Iwamoto J, Konishi M, Zhou Q, Itabashi K. Risedronate improves proximal femur bone density and geometry in patients with osteoporosis or osteopenia and clinical risk factors of fractures: a practice-based observational study. J Bone Miner Metab. 2011;29:88–95. doi: 10.1007/s00774-010-0196-x. [DOI] [PubMed] [Google Scholar]
- 51.Reginelli A, Silvestro G, Fontanella G, Sangiovanni A, Conte M, Nuzzo I, Calvanese M, Traettino M, Ferraioli P, Grassi R, Manzo R, Cappabianca S. Validation of DWI in assessment of radiotreated bone metastases in elderly patients. Int J Surg. 2016;33(1):S148–53. doi: 10.1016/j.ijsu.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Elefante A, Peca C, Del Basso De Caro ML, Russo C, Formicola F, Mariniello G, Brunetti A, Maiuri F. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurological Sciences. 2012;33(3):609–613. doi: 10.1007/s10072-011-0769-z. [DOI] [PubMed] [Google Scholar]
- 53.Reginelli A, Silvestro G, Fontanella G, Sangiovanni A, Conte M, Nuzzo I, Di Lecce A, Martino A, Grassi R, Murino P, Cappabianca S. Performance status versus anatomical recovery in metastatic disease: The role of palliative radiation treatment. Int J Surg. 2016;33(1):S126–31. doi: 10.1016/j.ijsu.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Silva BC, Bilezikian JP. Trabecular bone score: perspectives of an imaging technology coming of age. Arq Bras Endocrinol Metabol. 2014;58:493–503. doi: 10.1590/0004-2730000003456. [DOI] [PubMed] [Google Scholar]
- 55.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42:775–87. doi: 10.1016/j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 56.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24:77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 57.Krieg MA, Aubry-Rozier B, Hans D, Leslie WD, Manitoba Bone Density P. Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int. 2013;24:1073–8. doi: 10.1007/s00198-012-2155-y. [DOI] [PubMed] [Google Scholar]
- 58.Bergot C, Laval-Jeantet AM, Hutchinson K, Dautraix I, Caulin F, Genant HK. A comparison of spinal quantitative computed tomography with dual energy X-ray absorptiometry in European women with vertebral and nonvertebral fractures. Calcif Tissue Int. 2001;68:74–82. doi: 10.1007/BF02678144. [DOI] [PubMed] [Google Scholar]
- 59.Tamburrini S, Solazzo A, Sagnelli A, Del Vecchio L, Reginelli A, Monsorro M, Grassi R. Amyotrophic lateral sclerosis: sonographic evaluation of dysphagia. Radiol Med. 2010;115:784–93. doi: 10.1007/s11547-010-0523-2. [DOI] [PubMed] [Google Scholar]
- 60.Guglielmi G, van Kuijk C, Li J, Meta MD, Scillitani A, Lang TF. Influence of anthropometric parameters and bone size on bone mineral density using volumetric quantitative computed tomography and dual X-ray absorptiometry at the hip. Acta Radiol. 2006;47:574–80. doi: 10.1080/02841850600690363. [DOI] [PubMed] [Google Scholar]
- 61.Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–62. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Griffith JF, Genant HK. New imaging modalities in bone. Curr Rheumatol Rep. 2011;13:241–50. doi: 10.1007/s11926-011-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller TL, Stauber M, Kohler T, Eckstein F, Muller R, van Lenthe GH. Non-invasive bone competence analysis by high-resolution pQCT: an in vitro reproducibility study on structural and mechanical properties at the human radius. Bone. 2009;44:364–71. doi: 10.1016/j.bone.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 64.Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, McKay HA. Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int. 2006;17:1241–51. doi: 10.1007/s00198-006-0110-5. [DOI] [PubMed] [Google Scholar]
- 65.Bousson V, Le Bras A, Roqueplan F, Kang Y, Mitton D, Kolta S, Bergot C, Skalli W, Vicaut E, Kalender W, Engelke K, Laredo JD. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int. 2006;17:855–64. doi: 10.1007/s00198-006-0074-5. [DOI] [PubMed] [Google Scholar]
- 66.Zappia M, Carfora M, Romano AM, Reginelli A, Brunese L, Rotondo A, Castagna A. Sonography of chondral print on humeral head. Skelet Radiol. 2016;45:35–40. doi: 10.1007/s00256-015-2238-x. [DOI] [PubMed] [Google Scholar]
- 67.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13:263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 68.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 69.De Filippo M, Bertellini A, Sverzellati N, Pogliacomi F, Costantino C, Vitale M, Zappia M, Corradi D, Garlaschi G, Zompatori M. Multidetector computed tomography arthrography of the shoulder: diagnostic accuracy and indications. Acta Radiol. 2008;49:540–9. doi: 10.1080/02841850801935559. [DOI] [PubMed] [Google Scholar]
- 70.Zappia M, Reginelli A, Russo A, D’Agosto GF, Di Pietto F, Genovese EA, Coppolino F, Brunese L. Long head of the biceps tendon and rotator interval. Musculoskeletal Surg. 2013;97:S99–S108. doi: 10.1007/s12306-013-0290-z. [DOI] [PubMed] [Google Scholar]
- 71.Zappia M, Di Pietto F, Aliprandi A, Pozza S, De Petro P, Muda A, Sconfienza LM. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging. 2016;7:365–71. doi: 10.1007/s13244-016-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 73.Pinto A, Brunese L, Pinto F, Reali R, Daniele S, Romano L. The Concept of Error and Malpractice in Radiology. Semin Ultrasound CT MRI. 2012;33:275–279. doi: 10.1053/j.sult.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Pinto A, Brunese L, Pinto F, Acampora C, Romano L. E-learning and education in radiology. Eur J Radiol. 2011;78:368–371. doi: 10.1016/j.ejrad.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 75.Cappabianca S, Colella G, Pezzullo MG, Russo A, Iaselli F, Brunese L, Rotondo A. Lipomatous lesions of the head and neck region: Imaging findings in comparison with histological type. Radiol Med. 2008;113:758–770. doi: 10.1007/s11547-008-0258-5. [DOI] [PubMed] [Google Scholar]
- 76.Grigoryan M, Guermazi A, Roemer FW, Delmas PD, Genant HK. Recognizing and reporting osteoporotic vertebral fractures. Eur Spine J. 2003;12(2):S104–12. doi: 10.1007/s00586-003-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cicala D, Briganti F, Casale L, Rossi C, Cagini L, Cesarano E, Brunese L, Giganti M. Atraumatic vertebral compression fractures: Differential diagnosis between benign osteoporotic and malignant fractures by MRI. Musculoskeletal Surg. 2013;97:S169–S179. doi: 10.1007/s12306-013-0277-9. [DOI] [PubMed] [Google Scholar]
- 78.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36:1579–1583. doi: 10.1007/s00296-016-3562-8. [DOI] [PubMed] [Google Scholar]
- 79.McCloskey EV, Spector TD, Eyres KS, Fern ED, O’Rourke N, Vasikaran S, Kanis JA. The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int. 1993;3:138–47. doi: 10.1007/BF01623275. [DOI] [PubMed] [Google Scholar]
- 80.Guglielmi G, Haslam J, D’Errico F, Steiger P, Nasuto M. Comprehensive vertebral deformity and vertebral fracture assessment in clinical practice: intra- and inter-reader agreement of a clinical workflow tool. Spine (Phila Pa 1976) 2013;38:E1676–83. doi: 10.1097/BRS.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 81.Diacinti D, Guglielmi G. Vertebral morphometry. Radiol Clin North Am. 2010;48:561–75. doi: 10.1016/j.rcl.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 82.Rea JA, Li J, Blake GM, Steiger P, Genant HK, Fogelman I. Visual assessment of vertebral deformity by X-ray absorptiometry: a highly predictive method to exclude vertebral deformity. Osteoporos Int. 2000;11:660–8. doi: 10.1007/s001980070063. [DOI] [PubMed] [Google Scholar]
- 83.Guglielmi G, De Terlizzi F, Nasuto M, Sinibaldi L, Brancati F. Quantitative ultrasound at the phalanges in a cohort of monozygotic twins of different ages. Radiol Med. 2015;120:277–82. doi: 10.1007/s11547-014-0440-x. [DOI] [PubMed] [Google Scholar]
- 84.De Filippo M, Corsi A, Evaristi L, Bertoldi C, Sverzellati N, Averna R, Crotti P, Bini G, Tamburrini O, Zompatori M, Rossi C. Critical issues in radiology requests and reports. Radiol Med. 2011;116:152–62. doi: 10.1007/s11547-010-0587-z. [DOI] [PubMed] [Google Scholar]
- 85.Filippou G, Adinolfi A, Cimmino MA, Scire CA, Carta S, Lorenzini S, Santoro P, Sconfienza LM, Bertoldi I, Picerno V, Di Sabatino V, Ferrata P, Galeazzi M, Frediani B. Diagnostic accuracy of ultrasound, conventional radiography and synovial fluid analysis in the diagnosis of calcium pyrophosphate dihydrate crystal deposition disease. Clin Exp Rheumatol. 2016;34:254–60. [PubMed] [Google Scholar]
- 86.Barile A, Bruno F, Mariani S, Arrigoni F, Reginelli A, De Filippo M, Zappia M, Splendiani A, Di Cesare E, Masciocchi C. What can be seen after rotator cuff repair: a brief review of diagnostic imaging findings. Musculoskeletal Surg. 2017;101:3–14. doi: 10.1007/s12306-017-0455-2. [DOI] [PubMed] [Google Scholar]
- 87.Wuster C, Albanese C, De Aloysio D, Duboeuf F, Gambacciani M, Gonnelli S, Gluer CC, Hans D, Joly J, Reginster JY, De Terlizzi F, Cadossi R. Phalangeal osteosonogrammetry study: age-related changes, diagnostic sensitivity, and discrimination power. The Phalangeal Osteosonogrammetry Study Group. J Bone Miner Res. 2000;15:1603–14. doi: 10.1359/jbmr.2000.15.8.1603. [DOI] [PubMed] [Google Scholar]
- 88.Hartl F, Tyndall A, Kraenzlin M, Bachmeier C, Guckel C, Senn U, Hans D, Theiler R. Discriminatory ability of quantitative ultrasound parameters and bone mineral density in a population-based sample of postmenopausal women with vertebral fractures: results of the Basel Osteoporosis Study. J Bone Miner Res. 2002;17:321–30. doi: 10.1359/jbmr.2002.17.2.321. [DOI] [PubMed] [Google Scholar]
- 89.Phan CM, Guglielmi G. Metabolic Bone Disease in Patients with Malabsorption. Semin Musculoskelet Radiol. 2016;20:369–375. doi: 10.1055/s-0036-1592429. [DOI] [PubMed] [Google Scholar]
- 90.Baroncelli GI, Federico G, Vignolo M, Valerio G, del Puente A, Maghnie M, Baserga M, Farello G, Saggese G. Phalangeal Quantitative Ultrasound G. Cross-sectional reference data for phalangeal quantitative ultrasound from early childhood to young-adulthood according to gender, age, skeletal growth, and pubertal development. Bone. 2006;39:159–73. doi: 10.1016/j.bone.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Guglielmi G, Selby K, Blunt BA, Jergas M, Newitt DC, Genant HK, Majumdar S. Magnetic resonance imaging of the calcaneus: preliminary assessment of trabecular bone-dependent regional variations in marrow relaxation time compared with dual X-ray absorptiometry. Acad Radiol. 1996;3:336–43. doi: 10.1016/s1076-6332(96)80254-6. [DOI] [PubMed] [Google Scholar]
- 92.De Filippo M, Rovani C, Sudberry JJ, Rossi F, Pogliacomi F, Zompatori M. Magnetic resonance imaging comparison of intra-articular cavernous synovial hemangioma and cystic synovial hyperplasia of the knee. Acta Radiol. 2006;47:581–4. doi: 10.1080/02841850600767724. [DOI] [PubMed] [Google Scholar]
- 93.Caranci F, Briganti F, La Porta M, Antinolfi G, Cesarano E, Fonio P, Brunese L, Coppolino F. Magnetic resonance imaging in brachial plexus injury. Musculoskeletal Surg. 2013;97:S181–S190. doi: 10.1007/s12306-013-0281-0. [DOI] [PubMed] [Google Scholar]
- 94.Cappabianca S, Iaselli F, Negro A, Basile A, Reginelli A, Grassi R, Rotondo A. Magnetic resonance imaging in the evaluation of anatomical risk factors for pediatric obstructive sleep apnoea-hypopnoea: a pilot study. Int J Pediatr Otorhinolaryngol. 2013;77:69–75. doi: 10.1016/j.ijporl.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 95.Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, Lane NE, Genant HK. In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res. 1998;13:1175–82. doi: 10.1359/jbmr.1998.13.7.1175. [DOI] [PubMed] [Google Scholar]
- 96.Chen WT, Shih TT, Chen RC, Lo SY, Chou CT, Lee JM, Tu HY. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001;220:213–8. doi: 10.1148/radiology.220.1.r01jl32213. [DOI] [PubMed] [Google Scholar]
- 97.Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal spinal bone marrow in adults: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229:703–9. doi: 10.1148/radiol.2293020747. [DOI] [PubMed] [Google Scholar]
- 98.Nasuto M, Pansini V, Cortet B, Guglielmi G, Cotten A. Renal Failure: A Modern Semiology for an Old Disease. Semin Musculoskelet Radiol. 2016;20:353–368. doi: 10.1055/s-0036-1592432. [DOI] [PubMed] [Google Scholar]
- 99.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor. Prostaglandins Leukot Essent Fatty Acids. 2003;68:373–8. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 100.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 101.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, Fusaro M, Wald R, Weinstein J, Jamal SA. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30:913–9. doi: 10.1002/jbmr.2406. [DOI] [PubMed] [Google Scholar]
- 103.Cirillo M, Caranci F, Tortora F, Corvino F, Pezzullo F, Conforti R, Cirillo S. Structural neuroimaging in dementia. Journal of Alzheimer’s disease. 2012;29(1):16–19. [Google Scholar]