Abstract
Background and Aim: The therapy for low back pain boasts different approaches; one of these is nucleoplasty. We wanted to assess the effectiveness of nucleoplasty both by clinical response both by MR imaging evaluation, including even extrusions larger than one third of the spinal canal. Methods: Fifty-seven patients were treated with nucleoplasty in our hospital, 11 of these patients accepted both clinical and MRI evaluation after six months from treatment. The clinical evaluation was performed with Visual Analogue Scale (VAS) of pain, scored before and after the procedure. MRI evaluation consisted of analysing some imaging parameters of disc protrusions before and after the treatment. Results: In 10 out of 11 (91%) patients, VAS was reduced and only 1 out of 11 (9%) had the same pain after procedure. The mean of decrease of VAS score was 64%. In our population 8/11 (72%) patients had a herniation larger than 1/3 of the sagittal diameter of spinal canal and 100% of them had an improvement with a mean VAS reduction value of 75%. With MRI evaluation, the mean percentage of expulsion before and after treatment was respectively 40% and 34%. The expulsion decreased in 7/13 discs, remained equal in 4/13, and increased in 2/13 discs. Among the 9 larger protrusions, 3 didn’t change, 6 reduced with a decrease mean value of 13%. Other MRI parameters didn’t change significantly. Conclusions: Our preliminary experience supports the success of coblation on pain relief, aiming to show progressively that this treatment is suitable even in case of great extrusions, which are generally treated only with surgical approach. It’s not clear the usefulness of MRI control yet, even if in most of cases we could have found a certain reduction of expulsion degree. (www.actabiomedica.it)
Keywords: disc protrusion, nucleoplasty, Visual Analog Scale (VAS), Magnetic Resonance Imaging (MRI)
Introduction
Spinal pain is one of the most frequently reported symptoms in the industrialized world, in particular low back pain.
According to a study by Schmidt et al. (2007), conducted in the Federal Republic of German, the point-prevalence for back pain was 34.2%, one year prevalence was 75.5% and lifetime prevalence was 85.2% (1-5). Another study by Deyo et al. (2006), conducted in USA, confirmed the high prevalence of back pain with a point-prevalence of 26.4% (6-14).
The intervertebral disc, because of its highly specialized role and relatively susceptible nature, is one of the major sources of low back pain syndrome (15-21).
Aging, stress and traumas cause a disc degeneration phenomenon and the loss of volume of pulp nucleus due to a decrease proteoglycans and water concentration (22-24). Because of the lack of nutrients and oxygen, cells are forced to metabolize anaerobically, generating a large amount of lactic acid; it leads to an increase in acidity resulting in further degradation of the intradiscal matrix (25-30).
Low back pain is treated with various modalities including epidural injections, percutaneous adhesiolysis, intradiscal therapy or annular thermal therapy, and mechanical disc decompression for disc-related pain, either discogenic or secondary to disc herniation, radiculitis, spinal stenosis, or post surgery syndrome (3, 12, 16, 31-35).
Treatment of discogenic low back pain is based on the theory that a small reduction in disc volume, involving removal of part of the nucleus via surgical or minimally invasive methods, can result in a large change in intradiscal pressure (15).
The primary modality of treatment remains either open discectomy or microdiscectomy, but several alternative techniques to open discectomy including automated percutaneous lumbar discectomy (APLD), percutaneous lumbar laser disc decompression, mechanical disc decompression with Dekompressor, and nucleoplasty have been described (8, 36-40).
In recent years, the general trend in spinal surgery is shifting toward minimally invasive procedures and lower cost. Nucleoplasty is a relatively new, minimally invasive therapeutic option that has been used for spinal procedures since July 2000 (41-45).
Nucleoplasty uses radiofrequency energy to remove nuclear material and to create small channels within the disc. With Coblation technology, radiofrequency energy is applied to a conductive medium, creating the formation a highly focused plasma field to form around the energized electrodes. The plasma field is composed of highly ionized particles. The created channel is thermally treated, producing a zone of thermal coagulation. Thus, nucleoplasty combines coagulation and tissue ablation (patented Coblation technology) to form channels in the nucleus and decompress the herniated disc (36, 46-50).
Clear inclusion criteria is missing in literature. Most scientific works exclude from nucleoplasty herniations larger than one third than the sagittal diameter of the spinal canal. This criterius was not assumed in our work in order to test the efficacy of coblation treatment even in case of spinal canal reduction greater than 50%.
The aim of our study is to evaluate the outcome of patients with intervertebral disc protrusions and herniations after coblation treatment, both by visual analog scale (VAS) of pain both by magnetic resonance imaging (MRI), performed before and after the treatment.
Materials and Methods
Population
In our Hospital 57 patients were treated with nucleoplasty between September 2016 and May 2017, 10 patients for a cervical protrusion, 47 for a lumbar.
Criteria of inclusion for treatment were the presence of spinal radicular pain due to disc protrusions and herniation.
Exclusion criteria for this procedure included severe spinal stenosis due to osteophytosis, presence of secondary pain issues, gait disorders depending on different neurological or orthopaedic pathology.
We selected the first 30 patients, whose treatment was done six months before: 19 refused the post coblation clinical evaluation with VAS scale and MRI examination, 11 accepted the control. Finally, these 11 cases were selected and considered in the present study (8 male, 3 female, mean age 57 years).
Nucleoplasty tecnique
Percutaneous disc decompression (PDD) using Coblation technology was performed under local anesthesia in a prone position; different radiologists used a uniportal approach under fluoroscopic guidance, entering the disc from the left side or from the predominant pain side. A 17-gauge six-inch long Crawford type spinal access cannula was introduced into the disc using a posterolateral extrapedicular approach. The access cannula was positioned at the junction of the anulus and nucleus . The exact position of the needle tip was confirmed on anteroposterior and lateral views. Discography was performed via the spinal needle to evaluate the configuration of the disc and the integrity of the fibrosus annulus, and a pain provocation test was performed by injection of contrast medium to determine whether the pain was discogenic in origin (Fig. 1a-b). The Perc-DLE tissue ablation and coagulation spinal wand (ArthroCare, Inc. - Sunnyvale, CA) was placed into the access cannula and was advanced until the tip of the wand was approximately 5 mm beyond the tip of the cannula, assuring that the active portion of the wand was beyond the inner layer of the annulus and was placed in the nucleus. A circumferential reference mark on the shaft of the spine wand was placed adjacent to the needle hub at the entry site, marking the proximal channel limit. The wand was advanced until it came into contact with the annulus on the opposite side. The depth stop marker on shaft of the Perc-DLE spine wand was advanced close to the needle hub to designate the distal channeling limit. Each channel was created by advancement of the wand in the ablation mode for 6-8 s followed by retraction in the coagulation mode for 10-15 s. A total of six channels were created at the twelve, two, four, six, eight, and ten o’clock positions to ensure adequate decompression of the disc space. We observed patients at least 6 hour after procedure and patients were advised to stay in bed for the 1st day following the procedure. No lifting of weights, bending, or stooping was permitted for 2 weeks following percutaneous disc decompression. Patients were returned to sedentary or light work after two weeks and were provided with home exercise instructions by a qualified physical therapist.
Figure 1.
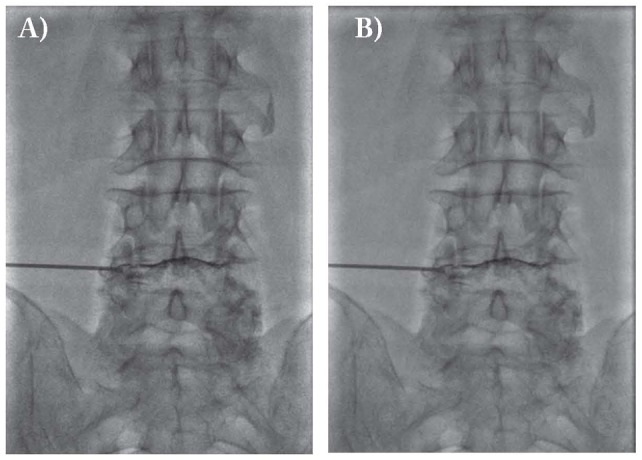
Discography after position of the cannula, during the procedure of coblation of a lumbar herniation at L4-L5 in a 74-year-old man. A. latero-lateral view; B. antero-posterior view
Pain evaluation
We considered VAS as analogic scale pre and post coblation: the VAS is a standardized instrument for measuring pain.
Patients rated the intensity of their subjectively experienced pain on a 10cm scale from 0 (no pain) to 10 (greatest imaginable pain) with a space of one centimetre between the individual values (51).
MR technique
The MRI examinations pre coblation were performed in different medical centres.
All MRI examinations post coblation were performed with a 1.5 T scanner (Optima MR450w GEMSGEMS, GE medical systems). The examination protocol applied consisted of sagittal and transverse sequences with a slice thickness of 4 mm, FSE T1 (TE 12, TR 680), FSE T2 (TE 100, TR 3300) and STIR (TE 54, TR 4370) weighted.
Imaging analysis
Two radiologists in consensus, with ten and five years of experience in spine imaging retrospectively, evaluated all the MRI examinations in order to determine the following parameters of disc protrusion pre and post coblation:
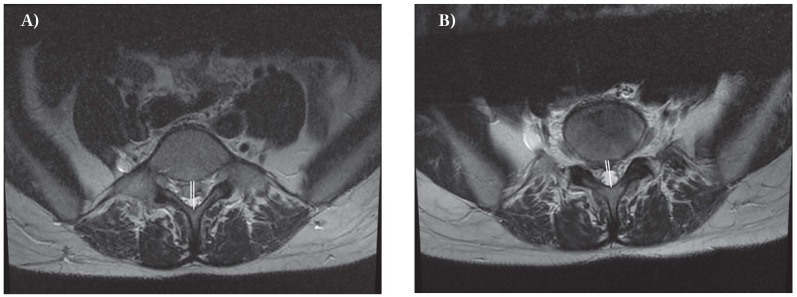
- Percentage of expulsion: percentage of the spinal canal antero-posterior diameter, occupied by herniated disc (fig. 2 a-b).
- Maximum thickness of disc (fig. 3a-b).
- Angle of disc herniation (following the classification of NASS) (52).
- Presence of arthrosic degeneration signs.
- Intensity change of disc.
Figure 2.
Axial T2 image at MRI evaluation of percentage of expulsion pre (A) and post coblation (B) in a 39-year-old woman with a large migrated herniation at L5
Figure 3.
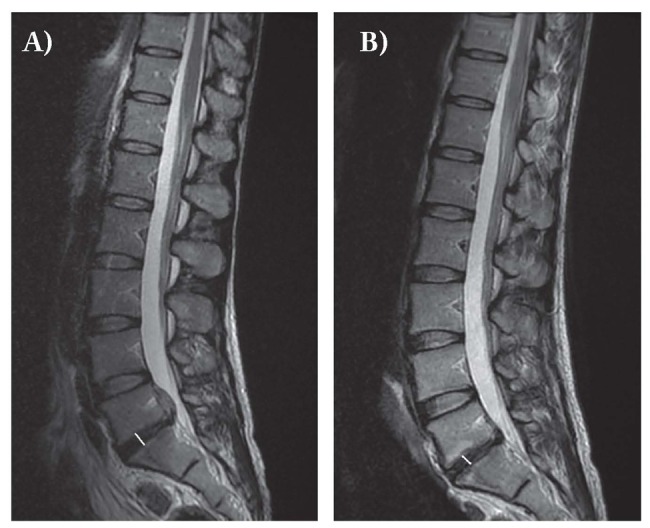
Sagittal T2 image at MRI evaluation of maximum thickness of disc in a 39-year-old woman with a large migrated herniation at L5-S1 (A. pre coblation; B. post coblation)
Results
Among 30 patients selected in our study, who performed nucleoplasty in a period of six months before, 11 accepted to come to our hospital both for a clinical evaluation of pain and for an instrumental evaluation. The work is still in progress and we are going to recruit further patients with the same follow up time.
Pain evaluation
In 10 out of 11 (91%) patients, VAS was reduced and only 1 out of 11 (9%) had the same pain. (Tab. 1)
Table 1.
Pain VAS in our 10 selected patients, pre and post coblation

Among these 10, in 8 (80%) patients the extent of decrease of VAS score was equal or greater than 50%, and in 2 (20%) patients it was less than 50% (respectively of 37% and 16%). The mean VAS reduction value for all patients is 64%.
In 8 out of 11(72%) patients we found herniation larger than 1/3 the sagittal diameter of spinal canal and 100% of them had a clinical improvement (Tab. 2) with a main VAS reduction value of 75%; specifically 7/8 patients reported a decrease of VAS pain >50%, 1/8 patient reported a decrease of 37%.
Table 2.
MRI parameters of the 13 selected discs pre and post coblation
Imaging analysis
All the 11 patients underwent to coblation for lumbar protrusion, of whom 2/11 performed a double coblation, due to the presence of 2 significant concomitant protrusions, so we finally considered 13 discs; 9/13 disc protrusions were larger than one third of spinal channel.
Of each disc, we reported the following parameters (Tab. 2):
- Percentage of expulsion: mean percentage of expulsion before and after treatment was respectively 40% and 34%. The expulsion decreased in 7/13, remained equal in 4/13 discs, and increased in 2/13. Among 9 larger protrusions, 3 didn’t change, 6 reduced with a decrease mean value of 13%.
- Maximum thickness of disc: we reported a decrease mean value of 0,2 mm.
- Angle of disc herniation: 5/13 discs with >180° circumference protrusion didn’t change after treatment; 8/13 discs with <180° circumference protrusion had variable behavior (5 didn’t change, 2 increased and 1 decreased).
- Presence of arthrosic degeneration signs: 11/13 intervertebral levels were affected by arthrosis, 2/13 wasn’t.
- Intensity change of disc: 1/11 disc showed sign of dehydration.
Discussion
Chronic back pain is one of the most frequently occurring types of pain in modern industrial societies. Probably it is generated by a combination of mechanical and neural mechanisms (40, 45, 53-57).
Hydrostatic pressure, between the disc and vertebral endplates, plays a very important role in the regulation of nutrient supply to the disc and in removal of waste from cells of the nucleus pulposus, which is an avascular structure. With aging, disease or injury the disc degeneration progresses causes a drop in the hydrostatic pressure mechanism of regulation (58-60).
Treatment is based on the theory that a small reduction in disc volume, involving removal of part of the nucleus via surgical or minimally invasive methods, can result in a large change in intradiscal pressure (25).
Coblation technology involves the use of radiofrequency energy to determinate a gentle removal effect on target tissue with minimum dissolution effects on surrounding vital structures.
Our first assessment was about clinical trend: 10/11 patients had a pain relief; 1/11 didn’t have any change, either positive or negative. In one successful case the improvement was only one point of VAS.
This datus agrees with the meta-analysis of Institute of Medical Statisics, Informatics and Epidemiology (IMSIE), University Hospital of Cologne, which confirms the positive outcome of coblation in at least 17studies by VAS (61).
No strict inclusion criteria are present in literature, even if it’s recommended to treat little contained erniation (15, 61-65). Nevertheless we chose to include also patients with herniation that was larger than 1/3 the sagittal diameter of spinal canal and whom were asking a not invasive procedure to reduce the unbearable pain, in particular 8/11 patients (72%) had these features. All patients with this type of herniation reported a decrease of VAS pain >50%, except one patient who reported a decrease of 37%. As result, we found that also these patients can benefit by treatment with a significant reduction of pain VAS and we are going to recruit other similar patients to have stronger evidence.
We have though to consider that the patients disappointed from the treatment were less available to the control we proposed six months later; that caused necessarily a bias in our population.
As second assessment we considered the response on MRI examinations.
An innovative perspective of our work, not present in literature, consists in the using of an instrumental evaluation of anatomic evidence by six-months post-operative MRI analysis; so we assumed this period of time suitable in order to consider accomplished every post-treatment tissue modification (66-70).
In 2007 T. Calisaneller et Al. lead an MRI evaluation after 24 hours from the procedure and only a clinical assessment after 3 and 6 months, aiming for further studies with longer follow up (62, 71).
The most remarkable result of our MRI analysis was the reduction of the percentage of disc expulsion (7/13 discs): 6 of these reducing protrusions were larger than one third of spinal canal with a decrease mean value of 13%. Therefore, there was not only a success in pain reduction but also in MRI features. Despite of small number of cases, this result is encouraging for the extrusions, which were excluded from most of approved coblation criteria.
However, both patients, whose six months post-operative MRI showed an increase of disc protrusion, detected at the same time an improvement of their clinical conditions and a reduction of pain. In the other 4 cases, the decrease of painful symptoms measured by VAS, wasn’t linked with reduction of the disc protrusion, which remain equal.
Moreover we didn’t found worthy changing of other anatomical MRI parameters we evaluated: the thickness of disc, the angle of circumferential protrusion or the hydration state.
As a matter of fact, it is already known that nucleoplasty induces only a little volume loss of nucleus pulposus and Masala et al. in their work reported approximately a removal of 1 cm3 of tissue volume (15). However, this relatively small tissue reduction is connected to a significant improvement of clinical conditions. Percutaneous disc decompression, irrespective of the technique, is based on the principle that a small volume loss in a closed hydraulic space, like an intact disc, results in a disproportionately large drop of pressure (25, 72-75). For this reason, nucleoplasty can cause an improvement of clinical conditions and a pain relief even if clear disc modifications aren’t evident. Coblation of the nucleus pulposus causes disc shrinkage with a vacuum effect, able to relieve pressure from the roots. This decompression leads to higher axonal, liquoral, and hematic flow rates, bringing about a resolution of the periradicular inflammatory mechanism and a better endorphin diffusion (15). Also T. Calisaneller et Al. report in their work that, the poor radiological findings after nucleoplasty suggest that the pain relieving effect can be due just to the reduction in the intra-discal pressure and/or nociceptive ablative effect of coblation on the nerve fiber network (62).
Fangan and co-workers described in details discal innervation. They identified areas where innervation is most concentrated as the perianular connective tissue and the central endplate; some of the nerves identify in this area may function as nociceptors (76-80). Thus it is likely that coblation nucleoplasty has an effect on discogenic pain since it denervates in a concentrated manner the central endplate area (72).
Another considered MRI parameter was the presence/absence of arthrosis, but it didn’t represent a discriminant factor for the outcome of treatment.
About the increase/decrease of disc intensity, we have to affirm that it is not reliable, because some pre-treatment MR exams were made in other medical center, using different machine from ours and that involve a remarkable interpretative difficulty.
Moreover, in MRI examination after nucleoplasty we never observed scar or adhesions tissue between the posterior annulus and the nerve root (failed back syndrome), which are instead common complications in traditional surgical treatments (34, 81-85).
In conclusion, with our preliminary experience we can support the success of coblation on pain relief, aiming to show progressively that this treatment is suitable even in case of extrusions, which are generally treated only with surgical approach; it’s not clear the usefulness of MRI control yet, even if in most of cases we have found a certain reduction of expulsion degree.
Limits of our work were the absence of a double-blinded analysis of MRI evidence and, of course, the small numbers of our case group. However we don’t consider accomplished this study yet, sure enough we are still recruiting more patients in our hospital to perform both clinical and MRI controls after six months from treatment and we are confident we can increase our records to obtain stronger results.
References
- 1.Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa 1976) 2007;32:2005–11. doi: 10.1097/BRS.0b013e318133fad8. [DOI] [PubMed] [Google Scholar]
- 2.Ripani M, Continenza MA, Cacchio A, Barile A, Parisi A, De Paulis F. The ischiatic region: normal and MRI anatomy. J Sports Med Phys Fitness. 2006;46:468–75. [PubMed] [Google Scholar]
- 3.Splendiani A, Perri M, Grattacaso G, Di Tunno V, Marsecano C, Panebianco L, Gennarelli A, Felli V, Varrassi M, Barile A, Di Cesare E, Masciocchi C, Gallucci M. Magnetic resonance imaging (MRI) of the lumbar spine with dedicated G-scan machine in the upright position: a retrospective study and our experience in 10 years with 4305 patients. Radiol Med. 2016;121:38–44. doi: 10.1007/s11547-015-0570-9. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Rossi B, Zoccali G, Barbarino E, Gregori L, Barile A, Masciocchi C. A new technique for biopsy of soft tissue neoplasms: A preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–120. [PubMed] [Google Scholar]
- 5.Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, Arrigoni F, Zugaro L, Barile A, Masciocchi C, Gangi A. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33 doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31:2724–7. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 7.Arrigoni F, Barile A, Zugaro L, Fascetti E, Zappia M, Brunese L, Masciocchi C. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia. 2017:1–7. doi: 10.1080/02656736.2017.1334168. [DOI] [PubMed] [Google Scholar]
- 8.Bruno F, Smaldone F, Varrassi M, Arrigoni F, Barile A, Di Cesare E, Masciocchi C, Splendiani A. MRI findings in lumbar spine following O2-O3 chemiodiscolysis: A long-term follow-up. Interv Neuroradiol. 2017;23:444–450. doi: 10.1177/1591019917703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrigoni F, Barile A, Zugaro L, Splendiani A, Di Cesare E, Caranci F, Ierardi AM, Floridi C, Angileri AS, Reginelli A, Brunese L, Masciocchi C. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34 doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 10.Reginelli A, Zappia M, Barile A, Brunese L. Strategies of imaging after orthopedic surgery. Musculoskeletal Surg. 2017;101 doi: 10.1007/s12306-017-0458-z. [DOI] [PubMed] [Google Scholar]
- 11.Barile A, Arrigoni F, Zugaro L, Zappia M, Cazzato RL, Garnon J, Ramamurthy N, Brunese L, Gangi A, Masciocchi C. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34 doi: 10.1007/s12032-017-0909-2. [DOI] [PubMed] [Google Scholar]
- 12.Splendiani A, D’Orazio F, Patriarca L, Arrigoni F, Caranci F, Fonio P, Brunese L, Barile A, Di Cesare E, Masciocchi C. Imaging of post-operative spine in intervertebral disc pathology. Musculoskeletal Surg. 2017;101:75–84. doi: 10.1007/s12306-017-0453-4. [DOI] [PubMed] [Google Scholar]
- 13.Giordano AV, Arrigoni F, Bruno F, Carducci S, Varrassi M, Zugaro L, Barile A, Masciocchi C. Interventional Radiology Management of a Ruptured Lumbar Artery Pseudoaneurysm after Cryoablation and Vertebroplasty of a Lumbar Metastasis. Cardiovasc Intervent Radiol. 2017;40:776–779. doi: 10.1007/s00270-016-1551-7. [DOI] [PubMed] [Google Scholar]
- 14.Barile A, La Marra A, Arrigoni F, Mariani S, Zugaro L, Splendiani A, Di Cesare E, Reginelli A, Zappia M, Brunese L, Duka E, Carrafiello G, Masciocchi C. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masala S, Massari F, Fabiano S, Ursone A, Fiori R, Pastore F, Simonetti G. Nucleoplasty in the treatment of lumbar diskogenic back pain: one year follow-up. Cardiovasc Intervent Radiol. 2007;30:426–32. doi: 10.1007/s00270-006-0223-4. [DOI] [PubMed] [Google Scholar]
- 16.Patriarca L, Letteriello M, Di Cesare E, Barile A, Gallucci M, Splendiani A. Does evaluator experience have an impact on the diagnosis of lumbar spine instability in dynamic MRI? Interobserver agreement study. Neuroradiol J. 2015;28:341–346. doi: 10.1177/1971400915594508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccolo CL, Galluzzo M, Ianniello S, Trinci M, Russo A, Rossi E, Zeccolini M, Laporta A, Guglielmi G, Miele V. Pediatric musculoskeletal injuries: role of ultrasound and magnetic resonance imaging. Musculoskelet Surg. 2017;101:85–102. doi: 10.1007/s12306-017-0452-5. [DOI] [PubMed] [Google Scholar]
- 18.Miele V, Di Giampietro I. Diagnostic imaging in emergency. Salute Soc. 2014:127–141. [Google Scholar]
- 19.Miele V, Andreoli C, Grassi R. The management of emergency radiology: Key facts. Eur J Radiol. 2006;59:311–314. doi: 10.1016/j.ejrad.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L. Errors in neuroradiology. Radiol Med. 2015;120:795–801. doi: 10.1007/s11547-015-0564-7. [DOI] [PubMed] [Google Scholar]
- 21.Pinto A, Brunese L, Pinto F, Reali R, Daniele S, Romano L. The Concept of Error and Malpractice in Radiology. Semin Ultrasound CT MRI. 2012;33:275–279. doi: 10.1053/j.sult.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Muto M, Ambrosanio G, Guarnieri G, Capobianco E, Piccolo G, Annunziata G, Rotondo A. Low back pain and sciatica: Treatment with intradiscal-intraforaminal O2-O3 injection. Our experience. Radiol Med. 2008;113:695–706. doi: 10.1007/s11547-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 23.Muto M, Perrotta V, Guarnieri G, Lavanga A, Vassallo P, Reginelli R, Rotondo A. Vertebroplasty and kyphoplasty: Friends or foes. Radiol Med. 2008;113:1171–1184. doi: 10.1007/s11547-008-0301-6. [DOI] [PubMed] [Google Scholar]
- 24.Grassi R, Lombardi G, Reginelli A, Capasso F, Romano F, Floriani I, Colacurci N. Coccygeal movement: assessment with dynamic MRI. Eur J Radiol. 2007;61:473–9. doi: 10.1016/j.ejrad.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin GE. Advanced concepts in interventional spine care. J Am Osteopath Assoc. 2002;102:S8–11. [PubMed] [Google Scholar]
- 26.Zappia M, Carfora M, Romano AM, Reginelli A, Brunese L, Rotondo A, Castagna A. Sonography of chondral print on humeral head. Skelet Radiol. 2016;45:35–40. doi: 10.1007/s00256-015-2238-x. [DOI] [PubMed] [Google Scholar]
- 27.Zappia M, Di Pietto F, Aliprandi A, Pozza S, De Petro P, Muda A, Sconfienza LM. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging. 2016;7:365–71. doi: 10.1007/s13244-016-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am. 2007;17:87–103. doi: 10.1016/j.nic.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Barile A, Limbucci N, Splendiani A, Gallucci M, Masciocchi C. Spinal injury in sport. Eur J Radiol. 2007;62:68–78. doi: 10.1016/j.ejrad.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Splendiani A, Perri M, Conchiglia A, Fasano F, Di Egidio G, Masciocchi C, Gallucci M. MR assessment of lumbar disk herniation treated with oxygen-ozone diskolysis: The role of DWI and related ADC versus intervertebral disk volumetric analysis for detecting treatment response. Neuroradiol J. 2013;26:347–356. doi: 10.1177/197140091302600316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Splendiani A, Ferrari F, Barile A, Masciocchi C, Gallucci M. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: Demonstration with dedicated upright MRI system. Radiol Med. 2014;119:164–174. doi: 10.1007/s11547-013-0330-7. [DOI] [PubMed] [Google Scholar]
- 32.Perri M, Grattacaso G, di Tunno V, Marsecano C, Gennarelli A, Michelini G, Splendiani A, Di Cesare E, Masciocchi C, Gallucci M. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen–ozone discolysis: a randomized, double-blind trial with steroid and O<inf>2</inf>–O<inf>3</inf> discolysis versus steroid only. Radiol Med. 2015;120:941–950. doi: 10.1007/s11547-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 33.Perri M, Marsecano C, Varrassi M, Giordano AV, Splendiani A, di Cesare E, Masciocchi C, Gallucci M. Indications and efficacy of O2–O3 intradiscal versus steroid intraforaminal injection in different types of disco vertebral pathologies: A prospective randomized double-blind trial with 517 patients. Radiol Med. 2016;121:463–471. doi: 10.1007/s11547-015-0598-x. [DOI] [PubMed] [Google Scholar]
- 34.Caranci F, Leone G, Ugga L, Cesarano E, Capasso R, Schipani S, Bianco A, Fonio P, Briganti F, Brunese L. Imaging of post-surgical treatment and of related complications in spinal trauma. Musculoskeletal Surg. 2017;101:63–73. doi: 10.1007/s12306-017-0457-0. [DOI] [PubMed] [Google Scholar]
- 35.Capasso R, Carbone M, Rossi E, Mamone R, Zeccolini R, Reginelli A, Zeccolini M, Brunese L, Rotondo A. A 4-year-old child presenting morning onset of spontaneous tracheal rupture due to bronchial mucous plug occlusion during the nighttime sleep: A case report. J Med Case Rep. 2016;10 doi: 10.1186/s13256-016-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, Bryce DA, Burks PA, Caraway DL, Calodney AK, Cash KA, Christo PJ, Cohen SP, Colson J, Conn A, Cordner H, Coubarous S, Datta S, Deer TR, Diwan S, Falco FJ, Fellows B, Geffert S, Grider JS, Gupta S, Hameed H, Hameed M, Hansen H, Helm S, Janata JW, Justiz R, Kaye AD, Lee M, Manchikanti KN, McManus CD, Onyewu O, Parr AT, Patel VB, Racz GB, Sehgal N, Sharma ML, Simopoulos TT, Singh V, Smith HS, Snook LT, Swicegood JR, Vallejo R, Ward SP, Wargo BW, Zhu J, Hirsch JA. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. (2nd) 2013;16:S49–283. [PubMed] [Google Scholar]
- 37.Sartoris R, Orlandi D, Corazza A, Sconfienza LM, Arcidiacono A, Bernardi SP, Schiaffino S, Turtulici G, Caruso P, Silvestri E. In vivo feasibility of real-time MR-US fusion imaging lumbar facet joint injections. J Ultrasound. 2017;20:23–31. doi: 10.1007/s40477-016-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandirali M, Di Leo G, Papini GD, Messina C, Sconfienza LM, Ulivieri FM, Sardanelli F. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur Radiol. 2015;25:2951–9. doi: 10.1007/s00330-015-3699-y. [DOI] [PubMed] [Google Scholar]
- 39.Lanza E, Thouvenin Y, Viala P, Sconfienza LM, Poretti D, Cornalba G, Sardanelli F, Cyteval C. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol. 2014;37:1530–9. doi: 10.1007/s00270-013-0815-8. [DOI] [PubMed] [Google Scholar]
- 40.Caranci F, Briganti F, La Porta M, Antinolfi G, Cesarano E, Fonio P, Brunese L, Coppolino F. Magnetic resonance imaging in brachial plexus injury. Musculoskeletal Surg. 2013;97:S181–S190. doi: 10.1007/s12306-013-0281-0. [DOI] [PubMed] [Google Scholar]
- 41.Ren DJ, Liu XM, Du SY, Sun TS, Zhang ZC, Li F. Percutaneous Nucleoplasty Using Coblation Technique for the Treatment of Chronic Nonspecific Low Back Pain: 5-year Follow-up Results. Chin Med J (Engl) 2015;128:1893–7. doi: 10.4103/0366-6999.160518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camperchioli A, Mariani M, Bartollino S, Petrella L, Persico M, Orteca N, Scambia G, Shahabi S, Ferlini C, Fattorusso C. Investigation of the Bcl-2 multimerisation process: structural and functional implications. Biochim Biophys Acta. 2011;1813:850–7. doi: 10.1016/j.bbamcr.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Cappabianca S, Colella G, Pezzullo MG, Russo A, Iaselli F, Brunese L, Rotondo A. Lipomatous lesions of the head and neck region: Imaging findings in comparison with histological type. Radiol Med. 2008;113:758–770. doi: 10.1007/s11547-008-0258-5. [DOI] [PubMed] [Google Scholar]
- 44.Nurzynska D, DiMeglio F, Castaldo C, Latino F, Romano V, Miraglia R, Guerra G, Brunese L, Montagnani S. Flatfoot in children: Anatomy of decision making. Ital J Anat Embryol. 2012;117:98–106. [PubMed] [Google Scholar]
- 45.Briganti F, Tedeschi E, Leone G, Marseglia M, Cicala D, Giamundo M, Napoli M, Caranci F. Endovascular treatment of vertebro-vertebral arteriovenous fistula. Neuroradiol J. 2013;26:339–346. doi: 10.1177/197140091302600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caranci F, Napoli M, Cirillo M, Briganti G, Brunese L, Briganti F. Basilar artery hypoplasia. Neuroradiol J. 2012;25:739–743. doi: 10.1177/197140091202500613. [DOI] [PubMed] [Google Scholar]
- 47.Masciocchi C, Conti L, D’Orazio F, Conchiglia A, Lanni G, Barile A. Errors in musculoskeletal MRI, Errors in Radiology. Springer-Verlag Milan. 2012:209–217. [Google Scholar]
- 48.Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol. 2013;78:66–69. doi: 10.12659/PJR.884011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briganti F, Delehaye L, Leone G, Sicignano C, Buono G, Marseglia M, Caranci F, Tortora F, Maiuri F. Flow diverter device for the treatment of small middle cerebral artery aneurysms. J Neurointervent Surg. 2016;8:287–294. doi: 10.1136/neurintsurg-2014-011460. [DOI] [PubMed] [Google Scholar]
- 50.Muccio CF, Caranci F, D’Arco F, Cerase A, De Lipsis L, Esposito G, Tedeschi E, Andreula C. Magnetic resonance features of pyogenic brain abscesses and differential diagnosis using morphological and functional imaging studies: A pictorial essay. J Neuroradiol. 2014;41:153–167. doi: 10.1016/j.neurad.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. [Development and validation of the Visual Analogue Scale (VAS) Spine Score] Unfallchirurg. 2001;104:488–97. doi: 10.1007/s001130170111. [DOI] [PubMed] [Google Scholar]
- 52.Fardon DF, Milette PC. Combined Task Forces of the North American Spine Society ASoSR, American Society of N. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976) 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 53.Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part I: Spinal stability. Eur J Radiol. 2013;82:118–126. doi: 10.1016/j.ejrad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Masala S, Nano G, Marcia S, Muto M, Fucci FPM, Simonetti G. Osteoporotic vertebral compression fractures augmentation by injectable partly resorbable ceramic bone substitute (Cerament™|SPINE SUPPORT): A prospective nonrandomized study. Neuroradiology. 2012;54:589–596. doi: 10.1007/s00234-011-0940-5. [DOI] [PubMed] [Google Scholar]
- 55.Guarnieri G, Vassallo P, Pezzullo MG, Laghi F, Zeccolini F, Ambrosanio G, Galasso R, Muto M, Izzo R. A comparison of minimally invasive techniques in percutaneous treatment of lumbar herniated discs a review. Neuroradiol J. 2009;22:108–121. doi: 10.1177/197140090902200116. [DOI] [PubMed] [Google Scholar]
- 56.Cellerini M, Mangiafico S, Ammannati F, Ambrosanio G, Muto M, Galasso L, Mennonna P. Ruptured, dissecting posterior inferior cerebellar artery aneurysms: Endovascular treatment without parent vessel occlusion. Neuroradiology. 2008;50:315–320. doi: 10.1007/s00234-007-0333-y. [DOI] [PubMed] [Google Scholar]
- 57.Cicala D, Briganti F, Casale L, Rossi C, Cagini L, Cesarano E, Brunese L, Giganti M. Atraumatic vertebral compression fractures: Differential diagnosis between benign osteoporotic and malignant fractures by MRI. Musculoskeletal Surg. 2013;97:S169–S179. doi: 10.1007/s12306-013-0277-9. [DOI] [PubMed] [Google Scholar]
- 58.Landi A, Gregori F, Mancarella C, Maiola V, Maccari E, Marotta N, Delfini R. Lumbar spinal degenerative “microinstability”: hype or hope? Proposal of a new classification to detect it and to assess surgical treatment. Eur Spine J. 2015;24(7):872–8. doi: 10.1007/s00586-015-4274-6. [DOI] [PubMed] [Google Scholar]
- 59.Saal JS. The role of inflammation in lumbar pain. Spine (Phila Pa 1976) 1995;20:1821–7. doi: 10.1097/00007632-199508150-00013. [DOI] [PubMed] [Google Scholar]
- 60.Rossi C, Reginelli A, D’Amora M, Di Grezia G, Mandato Y, D’Andrea A, Brunese L, Grassi R, Rotondi A. Safety profile and protocol prevention of adverse reactions to uroangiographic contrast media in diagnostic imaging. J Biol Regul Homeost Agents. 2014;28:155–165. [PubMed] [Google Scholar]
- 61.Eichen PM, Achilles N, Konig V, Mosges R, Hellmich M, Himpe B, Kirchner R. Nucleoplasty, a minimally invasive procedure for disc decompression: a systematic review and meta-analysis of published clinical studies. Pain Physician. 2014;17:E149–73. [PubMed] [Google Scholar]
- 62.Calisaneller T, Ozdemir O, Karadeli E, Altinors N. Six months post-operative clinical and 24 hour post-operative MRI examinations after nucleoplasty with radiofrequency energy. Acta Neurochir (Wien) 2007;149:495–500. doi: 10.1007/s00701-007-1146-9. discussion 500. [DOI] [PubMed] [Google Scholar]
- 63.He L, Tang Y, Li X, Li N, Ni J, He L. Efficacy of coblation technology in treating cervical discogenic upper back pain. Medicine (Baltimore) 2015;94:e858. doi: 10.1097/MD.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Genovese L, Anceschi G, Muto M, Cangialosi R. Use of interspinous distractors in symptomatic lumbar degenerative disc osteoarthritis. Riv Ital Neurobiol. 2009;55:43–45. [Google Scholar]
- 65.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, Ruscitti P, Cipriani P, Giacomelli R, Brunese L, Masciocchi C. Computed Tomography and MR Imaging in Rheumatoid Arthritis Radiol Clin North Am. 2017 doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Muto M. MR Imaging of the Spine: Where Are We Now? Magn. Reson. Imaging Clin North Am. 2016;24:xiii–xiv. doi: 10.1016/j.mric.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML) Ann Hematol 91. 2017;(8):1327–1328. doi: 10.1007/s00277-011-1398-6. [DOI] [PubMed] [Google Scholar]
- 68.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, Ruscitti P, Cipriani P, Giacomelli R, Brunese L, Masciocchi C. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017;55:997–1007. doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Pinto A, Reginelli A, Pinto F, Lo Re G, Midiri F, Muzj C, Romano L, Brunese L. Errors in imaging patients in the emergency setting. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carotti M, Salaffi F, Di Carlo M, Giovagnoni A. Relationship between magnetic resonance imaging findings, radiological grading, psychological distress and pain in patients with symptomatic knee osteoarthritis. Radiol Med. 2017 doi: 10.1007/s11547-017-0799-6. [DOI] [PubMed] [Google Scholar]
- 71.Kasch R, Mensel B, Schmidt F, Drescher W, Pfuhl R, Ruetten S, Merk HR, Kayser R. Percutaneous disc decompression with nucleoplasty-volumetry of the nucleus pulposus using ultrahigh-field MRI. PLoS ONE. 2012;7:e41497. doi: 10.1371/journal.pone.0041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexandre A, Coro L, Azuelos A, Pellone M. Percutaneous nucleoplasty for discoradicular conflict. Acta Neurochir Suppl. 2005;92:83–6. doi: 10.1007/3-211-27458-8_18. [DOI] [PubMed] [Google Scholar]
- 73.Zappia M, Cuomo G, Martino MT, Reginelli A, Brunese L. The effect of foot position on Power Doppler Ultrasound grading of Achilles enthesitis. Rheumatol Int. 2016;36:871–874. doi: 10.1007/s00296-016-3461-z. [DOI] [PubMed] [Google Scholar]
- 74.Zappia M, Capasso R, Berritto D, Maggialetti N, Varelli C, D’Agosto G, Martino MT, Carbone M, Brunese L. Anterior cruciate ligament reconstruction: MR imaging findings. Musculoskeletal Surg. 2017;101:23–35. doi: 10.1007/s12306-017-0460-5. [DOI] [PubMed] [Google Scholar]
- 75.Reginelli A, Pinto A, Russo A, Fontanella G, Rossi C, Del Prete A, Zappia M, D’Andrea A, Guglielmi G, Brunese L. Sharp penetrating wounds: spectrum of imaging findings and legal aspects in the emergency setting. Radiol. Med. 2015;120:856–865. doi: 10.1007/s11547-015-0553-x. [DOI] [PubMed] [Google Scholar]
- 76.Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: The innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976) 2003;28:2570–6. doi: 10.1097/01.BRS.0000096942.29660.B1. [DOI] [PubMed] [Google Scholar]
- 77.Belfiore G, Tedeschi E, Ronza FM, Belfiore MP, Borsi E, Ianniello MP, Rotondo A. CT-guided radiofrequency ablation in the treatment of recurrent rectal cancer. American Journal of Roentgenology. 2009;192(1):137–141. doi: 10.2214/AJR.07.2649. [DOI] [PubMed] [Google Scholar]
- 78.Reginelli A, Russo A, Maresca D, Martiniello C, Cappabianca S, Brunese L. Imaging Assessment of Gunshot Wounds. Semin Ultrasound CT MRI. 2015;36:57–66. doi: 10.1053/j.sult.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Miele V, Buquicchio GL, Piccolo CL, Stasolla A, Ianniello S, Brunese L. Injuries of the pleural spaces, Medical Radiology. Springer Verlag. 2017 [Google Scholar]
- 80.Miele V, Giampietro ID, Giannecchini S, Pizzi C, Trinci M. Pediatric polytrauma management, Imaging Trauma and Polytrauma in Pediatric Patients. Springer International Publishing. 2015:1–28. [Google Scholar]
- 81.Anderson PA, Rouleau JP. Intervertebral disc arthroplasty. Spine (Phila Pa 1976) 2004;29:2779–86. doi: 10.1097/01.brs.0000146460.11591.8a. [DOI] [PubMed] [Google Scholar]
- 82.Onesti ST. Failed back syndrome. Neurologist. 2004;10:259–64. doi: 10.1097/01.nrl.0000138733.09406.39. [DOI] [PubMed] [Google Scholar]
- 83.Briganti F, Leone G, Marseglia M, Cicala D, Caranci F, Maiuri F. P64 Flow Modulation Device in the treatment of intracranial aneurysms: initial experience and technical aspects. Journal of NeuroInterventional Surgery. 2016;8(2):173–180. doi: 10.1136/neurintsurg-2015-011743. [DOI] [PubMed] [Google Scholar]
- 84.Pinto A, Pinto F, Faggian A, Rubino G, Caranci F, Macarini L, Genovese EA, Brunese L. Sources of error in emergency ultrasonography. Critical Ultrasound Journal. 2013;5(1):1–5. doi: 10.1186/2036-7902-5-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briganti F, Marseglia M, Leone G, Briganti G, Piccolo D, Napoli M, Caranci F. Endovascular treatment of a small aneurysm of the superior cerebellar artery with a flow-diverter device. Neuroradiol J. 2013;26:327–331. doi: 10.1177/197140091302600313. [DOI] [PMC free article] [PubMed] [Google Scholar]