Abstract
Interventional radiology has known an exponential growth in the last years. Technological advances of the last decades, have made it possible to use new treatments on a larger scale, with safe and effective results. They could be considered as palliative treatments for painful lesions but also curative procedures, as single treatment or specially in combination with other techniques (surgery, radiation and oncology therapies, etc.). The main diffuse techniques are those of thermal ablation that destroy the target lesion through the heat; however there are also endovascular therapies that destroy the target tissue thanks to devascularization. Finally the is also the possibility to stabilize pathological fractures or impending fractures. In this paper all the most diffuse and effective techniques are reviewed and also a discussion of the main indications is done, with an analisys of the success and complications rates. (www.actabiomedica.it)
Keywords: interventional radiology, bone metastasis, tumours
Introduction
Interventional Radiology (IR) is experiencing tremendous development and diffusion due to a variety of factors among which its minimal invasiveness. The interventional procedures, in fact, can be performed on clinically unstable patients, who hardly tolerate surgery, with the advantage of reducing hospitalization times (1-5). The technological advances of the last decades have made it possible to apply highly safe and efficient new treatment techniques on a larger scale, employing them as curative procedures and in combination with other techniques (surgery, radiation and oncology therapies, etc.). One limitation to the diffusion of these procedures, however, is represented by the educational effort needed to prepare the Interventional Radiologists, a specific multitasking category of professionals able to read and interpret images, while possessing the manual and cultural skills of a surgeon. In fact, if diagnostic imaging modalities are proved to be fundamental in the diagnosis of various diseases of the musculoskeletal system (6-15), spine (16-20) and tumors (21-26), on other hand, new technical and surgical skills have to be acquired. Also peculiar skills are required from the health care staff members who co-operate with them (nurses, technicians, etc.). In addition, the interventional radiologists are requested to be part of and interact with interdisciplinary teams, made up of oncologists, surgeons, radiotherapists, anaesthesiologists, etc. Despite all these factors, however, IR is gaining a more and more important role in the oncology field, and provides high quality results in terms of treatment efficacy and patient compliancy, accompanied by astonishingly low complication rates.
Interventional Radiology is currently applied as palliative treatment in patients with painful bone metastases (27-37). On this topic, there is plenty of scientific studies, but more randomized analyses are needed to validate the results obtained so far. Conversely, the curative application of IR is scarcely described in literature. Only in patients affected by oligometastatic diseases this approach was employed, even if limitally (32, 33, 38-41). Before going into details about the clinical indications, we present a brief description of the different techniques employed.
Interventional radiology techniques
Due to the wide choice of techniques available in the IR it is of paramount importance a deep study of the lesion that we are treating: localization, size and morphologic characteristics have to be deeply studied in order to plan also a consolidation (cementoplasty or vertoplasty) and not only the ablation, to spare the sensitive structures around the target lesion and also to choice and to use the more effective technique.
The main goal of the interventional radiologist is to obtain a tissutal damage that can be of double nature: ischemic (arterial embolization) and thermal-ablative. The latter is obtained through delivery of energy (radiofrequencies, microwaves, focused ultrasounds) and/or cold (cryoablation). Occluding the vessels that feed the target lesion, the intra-arterial embolization provokes the necrosis of the tissue (42-51). By selectively cauterizing all branches that feed the lesion, it is possible to obtain an optimal necrosis even of a lesion measuring several centimetres. The limits of this last technique, however, is represented by the impossibility to obtain a surgical sterilization of the lesion and by the fact that poor vascularity can impair the process of target tissue necrosis. The procedure is performed in the angiographic setting starting with an arterial peripheral access without general anesthesia of the patient, who is administered pain killer medicaments to avoid post-embolization syndromes that may occur when large lesions are treated.
Thermal and crio-ablation (28, 52-62) provoke necrosis of the target tissue through the employment of one or more needles delivering energy into the lesions. Under CT, MR and/or US guide (the latter is used in presence of soft tissue lesions) one or more needles are inserted into the lesion while energy is delivered. A necrotic area of varying size and morphology is obtained depending on number and technical features of the needles. Radiofrequencies (RFA) and microwaves (MWA) bring about necrosis through tissutal warming (60° and above), while cryoablation through a decrease in temperature (below 40°). All the procedures produce the same result, but have different characteristics as well as advantages and disadvantages.
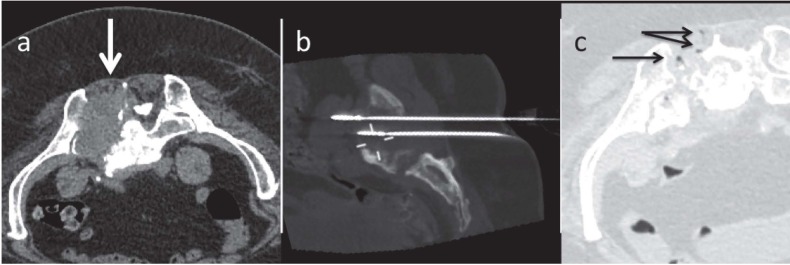
RFA (Fig. 1) is the most widely diffuse procedure being the most cost-effective and the first to find application in several fields (56, 63-68). The needles employed deliver radiofrequencies that induce a temperature increase around the needle tip. The needle size varies from 0.5 to 4 max 5 centimetres, which guarantee spherical ablation areas. The needles cannot be used simultaneously to cover large volumes, but only sequentially. Additionally, the effects of RFA are impaired by the heat sink effect, occurring near the blood vessels. Finally, RFA does not propagate energy as homogeneously and deeply as MWA.
Figure 1.
Osteosclerotic lesion of the sacrum. a. and b. Scintigraphy and CT that detected the lesion (arrows); c. image during the treatment: RFA needle inside the lesion (arrow)
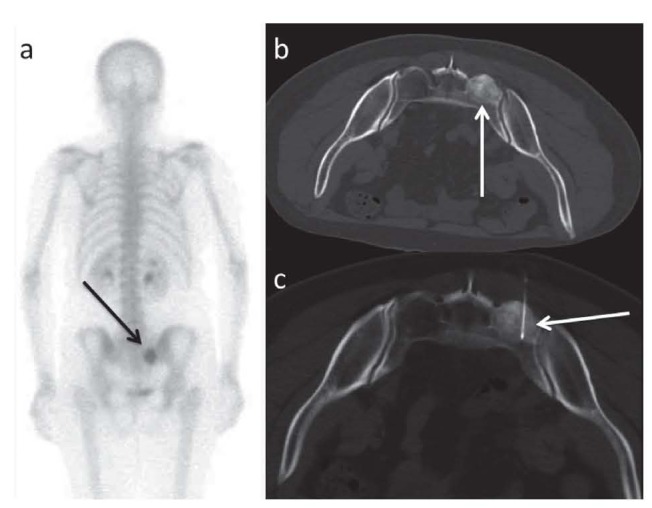
It is widely accepted that MWA (28, 52, 69-74) (Fig. 2) is more powerful than RFA for its ability in delivering immediate and optimal diffusion of energy around the needle (antenna). The heat sink effect is minimal and does not depend on the characteristics of non-conductivity of the tissues. On the other hand, some authors describe MWA energy delivery as less manageable than that delivered by RFA. In addition, MWA produces an oval ablation area considered as a limitation that the researchers are currently directing their efforts to overcome.
Figure 2.
a. Osteolytic lesion of the sacrum treated with two MW antennas (b); c. After treatment it is possible to appreciate air inside the treated lesion: this is an effect of the thermoablation (lung windowing, black arrows)
Crioablation (54, 55, 75-80) (Fig. 3) is extremely more expensive, but also more promising than the techniques described above. First, the ablation area can reach several centimetres (10 cm or even more) and presents an irregular morphology due to the possibility to employ more needles at the same time. The ablated area is imaged with CT where it appears hypodense to the surrounding tissue, not involved in the cooling process. This represents a great advantage to the radiologist who can rely on the possibility to assess in real time both the target area and the procedure. Finally yet importantly, pain is relieved by the antalgic effect of ice both in and around the lesion.
Figure 3.
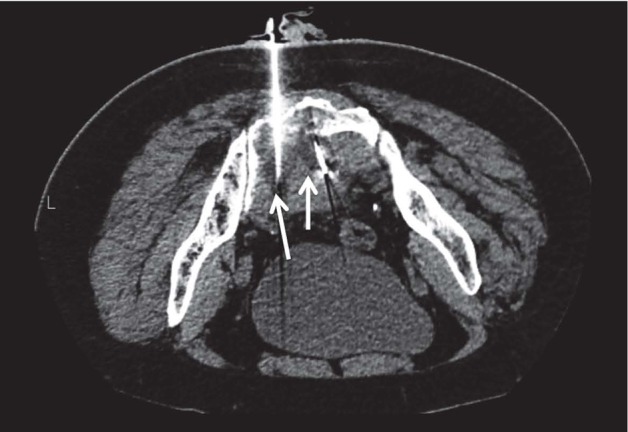
Cryoablation of a sacral osteolytic lesion: two cryoprobes surrounded by the iceballs that appears hypodense compared with the safe tissues
A separate description deserves thermal ablation with MR-guided Focused Ultrasounds (MRgFUS) (29, 40, 63, 81-85) (Fig. 4). With this technique, energy is delivered without the employment of any other device (needles, antennas, etc.), than focused ultrasound beams that pass through the tissues without damaging them. The main advantage is minimal invasiveness. The main disadvantage lies in the impossibility to treat lesions that cannot be reached by the ultrasound beam. For this reason, it is possible to treat only superficial bone lesions that are not hidden by bone cortex and/or other structures, impairing the propagation of the beams (gut or metallic devices). MR guidance allows control of both the lesion and the healthy surrounding structures. Real time control of the temperature in the target lesion is possible by means of specific sequences, which allow evaluation of the efficiency while carrying out the treatment.
Figure 4.
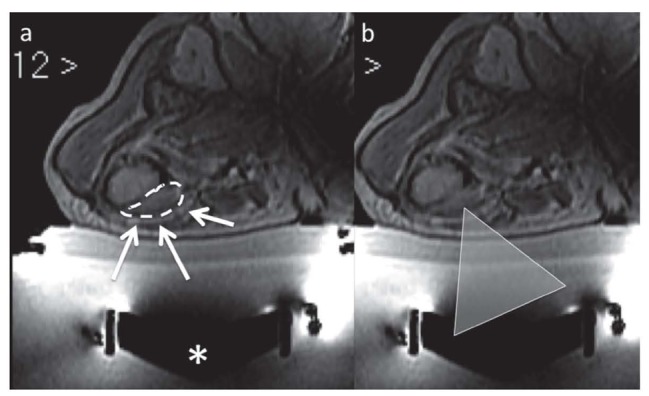
a. Met of femur (dashed line) treated with MRgFUS: * transducer that generates the Ultrasound beam (represented by the triangle, in b)
Another field of application of IR is the use for consolidation and stabilization of pathologic fractures as well as prevention of the latter when the bone segment is replaced by pathologic tissue (impending fractures) (86-90). Due to the intrinsic properties of the material employed in cementoplasty (PMMA), which is quite resistant to compressive forces and less to torsional ones, the stabilization and prevention techniques are more broadly used for treatment of spinal and acetabular lesions than for peripheral extra spinal ones. Suggested indications for percutaneous stabilization of lytic lesions are painful and/or fractured lesions or those at higher risk of fracture according to Mireles’s classification (91-96), in patients unlikely to tolerate a surgical intervention, which remains the therapeutical gold standard. A typical case is represented by vertebroplasty during which cement is injected under fluoroscopic control into the pathologic bone segment. The fractures can also be fixed through percutaneous positioning of screws under CT and fluoroscopy monitoring. Other devices provide a major resistance to torsional forces in combination to cement (metallic nets created by percutaneous injection of thin metallic needles through bioptic needles). The screws are successfully employed for the stabilization of pathologic fractures of the ileo- and ischiopubic tracts, the iliac crest and acetabular region (97-102).
Clinical indications, validation of results and discussion
The main indication for IR treatments in the field of malignant bone lesions is the palliation of painful metastases through direct destruction of neoplastic cells (tumoural mass effect and inflammatory cytokines) and adjacent nervous ends. Despite the presence of several multicentre studies, it is still not possible to intervene radiologically as first choice on painful secondary bone lesions for the lack of randomized studies validating the safety and efficacy of these techniques. Although its limited rate of success (treatment response in 80% of patients; complete response in scarce 30% of treated patients; latency time between treatment and pain relief about 3 weeks; risk of pain recurrence 50%), the gold standard in this field remains radiation therapy (103-107). IR is quite promising in terms of both palliation and stabilization of the bone segment (53, 108-113). There is plenty of scientific literature describing each ablation technique in terms of safety and efficacy. About cryoablation of painful bone metastases, Callstrom et al. (54) describe a 75% response rate in 61 patients with a 24-week follow up and a mean pain reduction by 90%. Pusceddu et al. (28) describe the role of MWA highlighting a 91% rate of improvement in the BPI scale at 12-week follow-up and 72% of patients free from symptomatology. Dupuy et al. (27) strongly suggest the use of RFA for the treatment of painful bone metastases. In their patient series studied over a 3-month period, the authors observed remarkable improvement of symptomatology. The complication rate of these techniques is low, ranging between 0% (Pusceddu) and 5% (Dupuy). Focused ultrasounds present a 1.8% rate of complications and efficacy comparable to radiation therapy, and shorter latency times (pain disappears after only 3 days from the treatment). The use of transarterial embolization for treatment of bone metastases is scarcely described in literature. This technique, in fact, is mainly employed as adjuvant to others. Its main role is to provide devascularization of the target area in order to guarantee safety and efficacy to the procedures carried out subsequently (surgery or percutaneous thermal ablation). The combination of this technique with radiation therapy is described as highly efficient in terms of pain relief in one case of bone metastases secondary to liver cancer (Uemura et al.) (42). The main advantages of Interventional Radiology in the treatment of secondary bone lesions lie in the fact that the procedures allow direct damage of the tumoural tissue; this explains the shorter latency times between treatment and effects compared to radiation therapy and the higher percentage of success in the treated patients. The procedures are repeatable and, apart from complications, can be exclusively focused on the target area, without possibility to damage the surrounding structures. The periprocedural sedation and one night hospitalization after the treatment are considered as major limitations of these techniques.
Another feather in the interventional radiologist’s cap, however, is the possibility to stabilize pathologic fractures or prevent those secondary to metastases. The stabilization by means of cement and/or screws of pathologic fractures provoking pain produces pain relief owing to the antalgic effect of cement. It deserves recognition that preventing fractures and improving quality of life through mini-invasive treatments is better than operating to stabilize pathologic fractures, not to mention the related complications (recovery times, infectious complications, and latency times before starting chemo- and/or radiation therapy).
The technological advances associated to experience of the operators is making these procedures more and more radical and similar to surgery. There is a trend to treat the entire lesion to obtain the most satisfying results in terms of response, pain relief and symptom-free patients. All these factors will certainly pave the way to future, more challenging applications. In the peculiar field of the oligometastatic diseases, for example, the interventional radiologist could operate radically on the low number of metastases, by ablating all the pathologic foci percutaneously. While operating on the lesion, large margins should be maintained in order to ablate also the neighbouring microscopic foci.
Additionally, it has been largely described that radiofrequency and cryoablation prove useful when used in combination with radiation therapy (15, 31, 64, 87, 110, 114, 115). The reason for this favourable synergy is that radiation therapy has low effect on scarcely vascularized tissue, while radiofrequency and cryoablation do not depend on these characteristics of the tissue.
References
- 1.Barile A, Arrigoni F, Zugaro L, Zappia M, Cazzato RL, Garnon J, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34(4) doi: 10.1007/s12032-017-0909-2. [DOI] [PubMed] [Google Scholar]
- 2.Cazzato RL, Buy X, Grasso RF, Luppi G, Faiella E, Quattrocchi CC, et al. Interventional Radiologist’s perspective on the management of bone metastatic disease. Eur J Surg Oncol. 2015;41(8):967–74. doi: 10.1016/j.ejso.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Ringe KI, Panzica M, von Falck C. Thermoablation of Bone Tumors. Rofo. 2016;188(6):539–50. doi: 10.1055/s-0042-100477. [DOI] [PubMed] [Google Scholar]
- 4.Pierot L, Söderman M, Bendszus M, White P, Muto M, Turjman F, et al. Statement of ESMINT and ESNR regarding recent trials evaluating the endovascular treatment at the cute stage of ischemic stroke. Neuroradiology. 2013;55(11):1313–8. doi: 10.1007/s00234-013-1249-3. [DOI] [PubMed] [Google Scholar]
- 5.Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part I: Spinal stability. Eur J Radiol. 2013;82(1):118–26. doi: 10.1016/j.ejrad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Salvati F, Rossi F, Limbucci N, Pistoia ML, Barile A, Masciocchi C. Mucoid metaplastic-degeneration of anterior cruciate ligament. J Sports Med Phys Fitness. 2008;48(4):483–7. [PubMed] [Google Scholar]
- 7.Ripani M, Continenza MA, Cacchio A, Barile A, Parisi A, De Paulis F. The ischiatic region: normal and MRI anatomy. J Sports Med Phys Fitness. 2006;46(3):468–75. [PubMed] [Google Scholar]
- 8.Barile A, Lanni G, Conti L, Mariani S, Calvisi V, Castagna A, et al. Lesions of the biceps pulley as cause of anterosuperior impingement of the shoulder in the athlete: potentials and limits of MR arthrography compared with arthroscopy. Radiol Med. 2013;118(1):112–22. doi: 10.1007/s11547-012-0838-2. [DOI] [PubMed] [Google Scholar]
- 9.Masciocchi C, Barile A, Lelli S, Calvisi V. Magnetic resonance imaging (MRI) and arthro-MRI in the evaluation of the chondral pathology of the knee joint. Radiol Med. 2004;108(3):149–58. [PubMed] [Google Scholar]
- 10.Barile A, Sabatini M, Iannessi F, Di Cesare E, Splendiani A, Calvisi V, et al. Pigmented villonodular synovitis (PVNS) of the knee joint: magnetic resonance imaging (MRI) using standard and dynamic paramagnetic contrast media. Report of 52 cases surgically and histologically controlled. Radiol Med. 2004;107(4):356–66. [PubMed] [Google Scholar]
- 11.Barile A, Conti L, Lanni G, Calvisi V, Masciocchi C. Evaluation of medial meniscus tears and meniscal stability: Weight-bearing MRI vs arthroscopy. Eur J Radiol. 2013;82(4):633–9. doi: 10.1016/j.ejrad.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Limbucci N, Rossi F, Salvati F, Pistoia LM, Barile A, Masciocchi C. Bilateral Suprascapular nerve entrapment by glenoid labral cysts associated with rotator cuff damage and posterior instability in an amateur weightlifter. J Sports Med Phys Fitness. 2010;50(1):64–7. [PubMed] [Google Scholar]
- 13.Masala S, Nano G, Marcia S, Muto M, Fucci FPM, Simonetti G. Osteoporotic vertebral compression fracture augmentation by injectable partly resorbable ceramic bone substitute (Cerament™|SPINESUPPORT): A prospective nonrandomized study. Neuroradiology. 2012;54(11):1245–51. doi: 10.1007/s00234-012-1016-x. [DOI] [PubMed] [Google Scholar]
- 14.Guarnieri G, Vassallo P, Pezzullo MG, Laghi F, Zeccolini F, Ambrosanio G, et al. A comparison of minimally invasive techniques in percutaneous treatment of lumbar herniated discs a review. Neuroradiol J. 2009;22(1):108–21. doi: 10.1177/197140090902200116. [DOI] [PubMed] [Google Scholar]
- 15.Muto M, Perrotta V, Guarnieri G, Lavanga A, Vassallo P, Reginelli R, et al. Vertebroplasty and kyphoplasty: Friends or foes. Radiol Med. 2008;113(8):1171–84. doi: 10.1007/s11547-008-0301-6. [DOI] [PubMed] [Google Scholar]
- 16.Splendiani A, Perri M, Grattacaso G, Di Tunno V, Marsecano C, Panebianco L, et al. Magnetic resonance imaging (MRI) of the lumbar spine with dedicated G-scan machine in the upright position: a retrospective study and our experience in 10 years with 4305 patients. Radiol Med. 2016;121(1):38–44. doi: 10.1007/s11547-015-0570-9. [DOI] [PubMed] [Google Scholar]
- 17.Splendiani A, Ferrari F, Barile A, Masciocchi C, Gallucci M. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: Demonstration with dedicated upright MRI system. Radiol Med. 2014;119(3):164–74. doi: 10.1007/s11547-013-0330-7. [DOI] [PubMed] [Google Scholar]
- 18.Cellerini M, Mangiafico S, Ammannati F, Ambrosanio G, Muto M, Galasso L, et al. Ruptured, dissecting posterior inferior cerebellar artery aneurysms: Endovascular treatment without parent vessel occlusion. Neuroradiology. 2008;50(4):315–20. doi: 10.1007/s00234-007-0333-y. [DOI] [PubMed] [Google Scholar]
- 19.Lanzillo R, Prinster A, Scarano V, Liuzzi R, Coppola G, Florio C, et al. Neuropsychological assessment, quantitative MRI and ApoE gene polymorphisms in a series of MS patients treated with IFN beta-1b. J Neurol Sci. 2006;245(1-2):141–5. doi: 10.1016/j.jns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Briganti F, Napoli M, Leone G, Marseglia M, Mariniello G, Caranci F, et al. Treatment of intracranial aneurysms by flow diverter devices: Long-term results from a single center. Eur J Radiol. 2014;83(9):1683–90. doi: 10.1016/j.ejrad.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Masciocchi C, Lanni G, Conti L, Conchiglia A, Fascetti E, Flamini S, et al. Soft-tissue inflammatory myofibroblastic tumors (IMTs) of the limbs: Potential and limits of diagnostic imaging. Skelet Radiol. 2012;41(6):643–9. doi: 10.1007/s00256-011-1263-7. [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Rossi B, Zoccali G, Barbarino E, Gregori L, Barile A, et al. A new technique for biopsy of soft tissue neoplasms: A preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106(2):117–20. [PubMed] [Google Scholar]
- 23.Barile A, Regis G, Masi R, Maggiori M, Gallo A, Faletti C, et al. Musculoskeletal tumours: Preliminary experience with perfusion MRI. Radiol Med. 2007;112(4):550–61. doi: 10.1007/s11547-007-0161-5. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo M, Caranci F, Tortora F, Corvino F, Pezzullo F, Conforti R, Cirillo S. Structural neuroimaging in dementia. Journal of Alzheimer’s Dis. 2012;29(1):16–19. [Google Scholar]
- 25.Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML) Ann Hematol. 2012;91(8):1327–8. doi: 10.1007/s00277-011-1398-6. [DOI] [PubMed] [Google Scholar]
- 26.Ingegnoli A, Corsi A, Verardo E, De Filippo M, Sverzellati N, Zompatori M. Uncommon causes of tracheobronchial stenosis and wall thickening: MDCT imaging. Radiol Med. 2007;112(8):1132–41. doi: 10.1007/s11547-007-0211-z. [DOI] [PubMed] [Google Scholar]
- 27.Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116(4):989–97. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24(2):229–33. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masciocchi C, Conchiglia A, Gregori LM, Arrigoni F, Zugaro L, Barile A. Critical role of HIFU in musculoskeletal interventions. Radiol Med. 2014;119(7):470–5. doi: 10.1007/s11547-014-0414-z. [DOI] [PubMed] [Google Scholar]
- 31.Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21(9):2004–10. doi: 10.1007/s00330-011-2133-3. [DOI] [PubMed] [Google Scholar]
- 32.Deschamps F, Farouil G, Ternes N, Gaudin A, Hakime A, Tselikas L, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients. Eur Radiol. 2014;24(8):1971–80. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 33.Kurup AN, Callstrom MR. Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol. 2017;72(8):645–56. doi: 10.1016/j.crad.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, et al. Errors in neuroradiology. Radiol Med. 2015;120(9):795–801. doi: 10.1007/s11547-015-0564-7. [DOI] [PubMed] [Google Scholar]
- 35.Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol. 2013;78(3):66–9. doi: 10.12659/PJR.884011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briganti F, Delehaye L, Leone G, Sicignano C, Buono G, Marseglia M, et al. Flow diverter device for the treatment of small middle cerebral artery aneurysms. J Neurointervent Surg. 2016;8(3):287–94. doi: 10.1136/neurintsurg-2014-011460. [DOI] [PubMed] [Google Scholar]
- 37.De Filippo M, Corsi A, Evaristi L, Bertoldi C, Sverzellati N, Averna R, et al. Critical issues in radiology requests and reports. Radiol Med. 2011;116(1):152–62. doi: 10.1007/s11547-010-0587-z. [DOI] [PubMed] [Google Scholar]
- 38.McMenomy BP, Kurup AN, Johnson GB, Carter RE, McWilliams RR, Markovic SN, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24(2):207–13. doi: 10.1016/j.jvir.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378–82. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 40.Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 2013;33(6):1555–68. doi: 10.1148/rg.336125162. [DOI] [PubMed] [Google Scholar]
- 41.Bertolini L, Vaglio A, Bignardi L, Buzio C, De Filippo M, Palmisano A, et al. Subclinical interstitial lung abnormalities in stable renal allograft recipients in the era of modern immunosuppression. Transplant Proc. 2011;43(7):2617–23. doi: 10.1016/j.transproceed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Uemura A, Fujimoto H, Yasuda S, Osaka I, Goto N, Shinozaki M, et al. Transcatheter arterial embolization for bone metastases from hepatocellular carcinoma. Eur Radiol. 2001;11(8):1457–62. doi: 10.1007/s003300000792. [DOI] [PubMed] [Google Scholar]
- 43.Son HY, An SY, Kim EY, Ahn SB, Lee BC. Selective embolization for hypervascular metastasis from differentiated thyroid cancer: a case series. J Med Case Rep. 2014;8:405. doi: 10.1186/1752-1947-8-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marciel AM, Van Zandt BL, Baxter AJ. Transcatheter arterial embolization for the palliation of painful bone lesions. Tech Vasc Interv Radiol. 2011;14(3):141–9. doi: 10.1053/j.tvir.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Owen RJT. Embolization of Musculoskeletal Bone Tumors. Seminars in Interventional Radiology. 2010;27(2):111–23. doi: 10.1055/s-0030-1253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muccio CF, Caranci F, D’Arco F, Cerase A, De Lipsis L, Esposito G, et al. Magnetic resonance features of pyogenic brain abscesses and differential diagnosis using morphological and functional imaging studies: A pictorial essay. J Neuroradiol. 2014;41(3):153–67. doi: 10.1016/j.neurad.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Briganti F, Tedeschi E, Leone G, Marseglia M, Cicala D, Giamundo M, et al. Endovascular treatment of vertebro-vertebral arteriovenous fistula. Neuroradiol J. 2013;26(3):339–46. doi: 10.1177/197140091302600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Zazzo E, Porcile C, Bartollino S, Moncharmont B. Critical Function of PRDM2 in the Neoplastic Growth of Testicular Germ Cell Tumors. Biology (Basel) 2016;5(4) doi: 10.3390/biology5040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Filippo M, Bertellini A, Sverzellati N, Pogliacomi F, Costantino C, Vitale M, et al. Multidetector computed tomography arthrography of the shoulder: diagnostic accuracy and indications. Acta Radiol. 2008;49(5):540–9. doi: 10.1080/02841850801935559. [DOI] [PubMed] [Google Scholar]
- 50.Bernuzzi G, Petraglia F, Pedrini MF, De Filippo M, Pogliacomi F, Verdano MA, et al. Use of platelet-rich plasma in the care of sports injuries: our experience with ultrasound-guided injection. Blood Transfus. 2014;12(1):s229–34. doi: 10.2450/2013.0293-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cataldi V, Laporta T, Sverzellati N, De Filippo M, Zompatori M. Detection of incidental vertebral fractures on routine lateral chest radiographs. Radiol Med. 2008;113(7):968–77. doi: 10.1007/s11547-008-0294-1. [DOI] [PubMed] [Google Scholar]
- 52.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25(9):1470–5. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27(2):124–36. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(5):1033–41. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33(12) doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 56.Palussiere J, Pellerin-Guignard A, Descat E, Cornelis F, Dixmerias F. Radiofrequency ablation of bone tumours. Diagn Interv Imaging. 2012;93(9):660–4. doi: 10.1016/j.diii.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Nurzynska D, DiMeglio F, Castaldo C, Latino F, Romano V, Miraglia R, et al. Flatfoot in children: Anatomy of decision making. Ital J Anat Embryol. 2012;117(2):98–106. [PubMed] [Google Scholar]
- 58.Dragoni S, Turin I, Laforenza U, Potenza DM, Bottino C, Glasnov TN, et al. Store-operated Ca2+ entry does not control proliferation in primary cultures of human metastatic renal cellular carcinoma. Biomed Res Int. 2014;2014:739494. doi: 10.1155/2014/739494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamburrini S, Solazzo A, Sagnelli A, Del Vecchio L, Reginelli A, Monsorro M, et al. Amyotrophic lateral sclerosis: sonographic evaluation of dysphagia. Radiol Med. 2010;115(5):784–93. doi: 10.1007/s11547-010-0523-2. [DOI] [PubMed] [Google Scholar]
- 60.Zappia M, Reginelli A, Russo A, D’Agosto GF, Di Pietto F, Genovese EA, et al. Long head of the biceps tendon and rotator interval. Musculoskeletal Surg. 2013;97(2):S99–S108. doi: 10.1007/s12306-013-0290-z. [DOI] [PubMed] [Google Scholar]
- 61.Reginelli A, Mandato Y, Cavaliere C, Pizza NL, Russo A, Cappabianca S, et al. Three-dimensional anal endosonography in depicting anal-canal anatomy. Radiol Med. 2012;117(5):759–71. doi: 10.1007/s11547-011-0768-4. [DOI] [PubMed] [Google Scholar]
- 62.Floridi C, Radaelli A, Abi-Jaoudeh N, Grass M, Lin MD, Chiaradia M, et al. Erratum: C-arm cone-beam computed tomography in interventional oncology: technical aspects and clinical applications [Radiol med, DOI 10.1007/s11547-014-0429-5] Radiol Med. 2015;120(4):406. doi: 10.1007/s11547-014-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masciocchi C, Zugaro L, Arrigoni F, Gravina GL, Mariani S, La Marra A, et al. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol. 2016;26(8):2472–81. doi: 10.1007/s00330-015-4111-7. [DOI] [PubMed] [Google Scholar]
- 64.Masciocchi C, Arrigoni F, Marra AL, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89(1066) doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arrigoni F, Barile A, Zugaro L, Fascetti E, Zappia M, Brunese L, et al. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia. 2017:1–7. doi: 10.1080/02656736.2017.1334168. [DOI] [PubMed] [Google Scholar]
- 66.Cappabianca S, Iaselli F, Negro A, Basile A, Reginelli A, Grassi R, et al. Magnetic resonance imaging in the evaluation of anatomical risk factors for pediatric obstructive sleep apnoea-hypopnoea: a pilot study. Int J Pediatr Otorhinolaryngol. 2013;77(1):69–75. doi: 10.1016/j.ijporl.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 67.Cappabianca S, Reginelli A, Monaco L, Del Vecchio L, Di Martino N, Grassi R. Combined videofluoroscopy and manometry in the diagnosis of oropharyngeal dysphagia: examination technique and preliminary experience. Radiol Med. 2008;113(6):923–40. doi: 10.1007/s11547-008-0290-5. [DOI] [PubMed] [Google Scholar]
- 68.Floridi C, Radaelli A, Abi-Jaoudeh N, Grass M, De Lin M, Chiaradia M, et al. C-arm cone-beam computed tomography in interventional oncology: Technical aspects and clinical applications. Radiol Med. 2014;119(7):521–32. doi: 10.1007/s11547-014-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cappabianca S, Iaselli F, Reginelli A, D’Andrea A, Urraro F, Grassi R, et al. Value of diffusion-weighted magnetic resonance imaging in the characterization of complex adnexal masses. Tumori. 2013;99(2):210–7. doi: 10.1177/030089161309900215. [DOI] [PubMed] [Google Scholar]
- 70.Iudici M, Cuomo G, Vettori S, Bocchino M, Sanduzzi Zamparelli A, Cappabianca S, et al. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum. 2015;44(4):437–44. doi: 10.1016/j.semarthrit.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Cappabianca S, Scuotto A, Iaselli F, Pignatelli di Spinazzola N, Urraro F, Sarti G, et al. Computed tomography and magnetic resonance angiography in the evaluation of aberrant origin of the external carotid artery branches. Surg Radiol Anat. 2012;34(5):393–9. doi: 10.1007/s00276-011-0926-3. [DOI] [PubMed] [Google Scholar]
- 72.Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, et al. Early systemic sclerosis: marker autoantibodies and videocapillaroscopy patterns are each associated with distinct clinical, functional and cellular activation markers. Arthritis Res Ther. 2013;15(3):R63. doi: 10.1186/ar4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, et al. Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res (Hoboken) 2014;66(10):1520–7. doi: 10.1002/acr.22304. [DOI] [PubMed] [Google Scholar]
- 74.Carotti M, Salaffi F, Di Carlo M, Giovagnoni A. Relationship between magnetic resonance imaging findings, radiological grading, psychological distress and pain in patients with symptomatic knee osteoarthritis. Radiol Med. 2017 doi: 10.1007/s11547-017-0799-6. [DOI] [PubMed] [Google Scholar]
- 75.Lanza E, Thouvenin Y, Viala P, Sconfienza LM, Poretti D, Cornalba G, et al. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol. 2014;37(6):1530–9. doi: 10.1007/s00270-013-0815-8. [DOI] [PubMed] [Google Scholar]
- 76.Sardanelli F. Trends in radiology and experimental research. European Radiology Experimental. 2017;1(1):1. doi: 10.1186/s41747-017-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am. 2007;17(1):87–103. doi: 10.1016/j.nic.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Splendiani A, Perri M, Conchiglia A, Fasano F, Di Egidio G, Masciocchi C, et al. MR assessment of lumbar disk herniation treated with oxygen-ozone diskolysis: The role of DWI and related ADC versus intervertebral disk volumetric analysis for detecting treatment response. Neuroradiol J. 2013;26(3):347–56. doi: 10.1177/197140091302600316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perri M, Grattacaso G, Di Tunno V, Marsecano C, Di Cesare E, Splendiani A, et al. MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2-O3 discolysis. Neuroradiol J. 2015;28(2):198–204. doi: 10.1177/1971400915576658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miele V, Piccolo CL, Sessa B, Trinci M, Galluzzo M. Comparison between MRI and CEUS in the follow-up of patients with blunt abdominal trauma managed conservatively. Radiol Med. 2016;121(1):27–37. doi: 10.1007/s11547-015-0578-1. [DOI] [PubMed] [Google Scholar]
- 81.Arrigoni F, Barile A, Zugaro L, Splendiani A, Di Cesare E, Caranci F, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34(4) doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 82.Arrigoni F, Gregori LM, Zugaro L, Barile A, Masciocchi C. MRgFUS in the treatment of MSK lesions: a review based on the experience of the University of L’Aquila, Italy. Translational Cancer Research. 2014;3(5):442–8. [Google Scholar]
- 83.Perri M, Grattacaso G, di Tunno V, Marsecano C, Gennarelli A, Michelini G, et al. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen-ozone discolysis: a randomized, double-blind trial with steroid and O2-O3 discolysis versus steroid only. Radiol Med. 2015;120(10):941–50. doi: 10.1007/s11547-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 84.Perri M, Marsecano C, Varrassi M, Giordano AV, Splendiani A, di Cesare E, et al. Indications and efficacy of O2–O3 intradiscal versus steroid intraforaminal injection in different types of disco vertebral pathologies: A prospective randomized double-blind trial with 517 patients. Radiol Med. 2016;121(6):463–71. doi: 10.1007/s11547-015-0598-x. [DOI] [PubMed] [Google Scholar]
- 85.Splendiani A, D’Orazio F, Patriarca L, Arrigoni F, Caranci F, Fonio P, et al. Imaging of post-operative spine in intervertebral disc pathology. Musculoskeletal Surg. 2017;101:75–84. doi: 10.1007/s12306-017-0453-4. [DOI] [PubMed] [Google Scholar]
- 86.Cazzato RL, Palussiere J, Buy X, Denaro V, Santini D, Tonini G, et al. Percutaneous Long Bone Cementoplasty for Palliation of Malignant Lesions of the Limbs: A Systematic Review. Cardiovasc Intervent Radiol. 2015;38(6):1563–72. doi: 10.1007/s00270-015-1082-7. [DOI] [PubMed] [Google Scholar]
- 87.Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc Intervent Radiol. 2016;39(1):74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 88.Sun G, Jin P, Liu XW, Li M, Li L. Cementoplasty for managing painful bone metastases outside the spine. Eur Radiol. 2014;24(3):731–7. doi: 10.1007/s00330-013-3071-z. [DOI] [PubMed] [Google Scholar]
- 89.Gallucci M, Puglielli E, Splendiani A, Pistoia F, Spacca G. Degenerative disorders of the spine. Eur Radiol. 2005;15(3):591–8. doi: 10.1007/s00330-004-2618-4. [DOI] [PubMed] [Google Scholar]
- 90.Splendiani A, Puglielli E, De Amicis R, Barile A, Masciocchi C, Gallucci M. Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology. 2004;46(11):916–22. doi: 10.1007/s00234-004-1232-0. [DOI] [PubMed] [Google Scholar]
- 91.Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468(10):2825–7. doi: 10.1007/s11999-010-1326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zappia M, Castagna A, Barile A, Chianca V, Brunese L, Pouliart N. Imaging of the coracoglenoid ligament: a third ligament in the rotator interval of the shoulder. Skelet Radiol. 2017;46(8):1101–11. doi: 10.1007/s00256-017-2667-9. [DOI] [PubMed] [Google Scholar]
- 93.Zappia M, Carfora M, Romano AM, Reginelli A, Brunese L, Rotondo A, et al. Sonography of chondral print on humeral head. Skelet Radiol. 2016;45(1):35–40. doi: 10.1007/s00256-015-2238-x. [DOI] [PubMed] [Google Scholar]
- 94.Zappia M, Di Pietto F, Aliprandi A, Pozza S, De Petro P, Muda A, et al. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging. 2016;7(3):365–71. doi: 10.1007/s13244-016-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russo A, Reginelli A, Zappia M, Rossi C, Fabozzi G, Cerrato M, et al. Ankle fracture: radiographic approach according to the Lauge-Hansen classification. Musculoskelet Surg. 2013;97(2):S155–60. doi: 10.1007/s12306-013-0284-x. [DOI] [PubMed] [Google Scholar]
- 96.Miele V, Piccolo CL, Galluzzo M, Ianniello S, Sessa B, Trinci M. Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol. 2016;89(1061) doi: 10.1259/bjr.20150823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cazzato RL, Koch G, Buy X, Ramamurthy N, Tsoumakidou G, Caudrelier J, et al. Percutaneous Image-Guided Screw Fixation of Bone Lesions in Cancer Patients: Double-Centre Analysis of Outcomes including Local Evolution of the Treated Focus. Cardiovasc Intervent Radiol. 2016;39(10):1455–63. doi: 10.1007/s00270-016-1389-z. [DOI] [PubMed] [Google Scholar]
- 98.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36(11):1579–83. doi: 10.1007/s00296-016-3562-8. [DOI] [PubMed] [Google Scholar]
- 99.Barile A, La Marra A, Arrigoni F, Mariani S, Zugaro L, Splendiani A, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89(1065) doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, et al. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017 doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Reginelli A, Zappia M, Barile A, Brunese L. Strategies of imaging after orthopedic surgery. Musculoskeletal Surg. 2017;101 doi: 10.1007/s12306-017-0458-z. [DOI] [PubMed] [Google Scholar]
- 102.De Cecco CN, Buffa V, Fedeli S, Vallone A, Ruopoli R, Luzietti M, et al. Preliminary experience with abdominal dual-energy CT (DECT): True versus virtual nonenhanced images of the liver. Radiol Med. 2010;115(8):1258–66. doi: 10.1007/s11547-010-0583-3. [DOI] [PubMed] [Google Scholar]
- 103.van der Linden YM, Lok JJ, Steenland E, Martijn H, van Houwelingen H, Marijnen CA, et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys. 2004;59(2):528–37. doi: 10.1016/j.ijrobp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Cappabianca S, Colella G, Pezzullo MG, Russo A, Iaselli F, Brunese L, et al. Lipomatous lesions of the head and neck region: Imaging findings in comparison with histological type. Radiol Med. 2008;113(5):758–70. doi: 10.1007/s11547-008-0258-5. [DOI] [PubMed] [Google Scholar]
- 105.Ierardi AM, Floridi C, Fontana F, Chini C, Giorlando F, Piacentino F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med. 2013;118(6):949–61. doi: 10.1007/s11547-013-0968-1. [DOI] [PubMed] [Google Scholar]
- 106.Cappabianca S, Porto A, Petrillo M, Greco B, Reginelli A, Ronza F, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F] fluorodeoxyglucose standardised uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol. 2011;64(2):114–9. doi: 10.1136/jcp.2010.076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pinto A, Brunese L, Pinto F, Acampora C, Romano L. E-learning and education in radiology. Eur J Radiol. 2011;78(3):368–71. doi: 10.1016/j.ejrad.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 108.Gangi A, Tsoumakidou G, Buy X, Quoix E. Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol. 2010;33(4):706–13. doi: 10.1007/s00270-009-9738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pinto A, Brunese L, Pinto F, Reali R, Daniele S, Romano L. The Concept of Error and Malpractice in Radiology. Semin Ultrasound CT MRI. 2012;33(4):275–9. doi: 10.1053/j.sult.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Carrafiello G, Fontana F, Cotta E, Petullà M, Brunese L, Mangini M, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med. 2011;116(7):1059–66. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 111.Pinto A, Pinto F, Faggian A, Rubino G, Caranci F, Macarini L, et al. Sources of error in emergency ultrasonography. Critical Ultrasound Journal. 2013;5(1):1–5. doi: 10.1186/2036-7902-5-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caranci F, Briganti F, La Porta M, Antinolfi G, Cesarano E, Fonio P, et al. Magnetic resonance imaging in brachial plexus injury. Musculoskelet Surg. 2013;97(2):S181–S190. doi: 10.1007/s12306-013-0281-0. [DOI] [PubMed] [Google Scholar]
- 113.Regine G, Stasolla A, Miele V. Multidetector computed tomography of the renal arteries in vascular emergencies. Eur J Radiol. 2007;64(1):83–91. doi: 10.1016/j.ejrad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 114.Staso MD, Gravina GL, Zugaro L, Bonfili P, Gregori L, Franzese P, et al. Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: Propensity matching analysis in 175 patients. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0129021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Filippo M, Pesce A, Barile A, Borgia D, Zappia M, Romano A, et al. Imaging of postoperative shoulder instability. Musculoskelet Surg. 2017;101(1):15–22. doi: 10.1007/s12306-017-0461-4. [DOI] [PubMed] [Google Scholar]