Abstract
Purpose: To examine both anterior and posterior elements of the lumbar spine in patients with low back pain using MRI T2-weighted sequences with Fat Saturation (FS) and contrast enhanced T1-weighted sequences with FS.Materials and methods: Two thousand eight hundred and twenty (2820) patients (1628 male, 1192 female, mean age 54) presenting low back pain underwent MRI standard examination (Sagittal T1w TSE and T2w TSE, axial T1 SE) with the addition of sagittal and axial T2w Fat Sat (FS) sequences. Among all the patients, 987 (35%) have been studied adding Contrast Enhanced (CE) T1w FS sequences after administration of contrast medium. Results: Among 987 patients studied with contrast medium, we found: active-inflammatory intervertebral osteochondrosis in 646 (65%) patients; degenerative-inflammatory changes in facet joints (facet joint effusion, synovitis, synovial cysts) in 462 (47%); spondylolysis in 69 (7%); degenerative-inflammatory changes of the flava, interspinous and supraspinous ligaments in 245 (25%); inflammatory changes of posterior perispinal muscles in 84 (8%) patients. Conclusions: In patients with suspected no-disc-related low back pain, the implementation of T2w FS and CE T1w FS sequences to the standard MR protocol could allow a better identification of degenerative-inflammatory changes more likely associated to the pain. (www.actabiomedica.it)
Keywords: MRI, Fat Saturation, contrast medium, lumbar spinal degenerative disease
Introduction
Low back pain (LBP) is one of the most common reasons why individuals seek medical care. According to the Global Burden of Disease 2010 Study, LBP causes more global disability than any other condition (1-10).
In most cases, the aetiology of the pain stems from the degenerative state of the intervertebral disc, with subsequent disc-root conflict. However, numerous structural inter-segmental changes of the spine may be the cause of patients’ signs and symptoms.
Degenerative disease of the spine is highly prevalent in the general population, and its incidence increases with age. Among those individuals, pain is the most commonly associated symptom with this pathologic condition; others are neurologic disorders (i.e., sensory, motor, neurovegetative dysfunctions).
Different factors, both individually and concomitantly, can result in degenerative processes of the spine, such as mechanical (e.g., postural anomalies, heavy weight-bearing, sports), anatomic (e.g., malformations, dysplasia), and metabolic factors (e.g., diabetes)(11-20).
The intervertebral disc is generally the main component of the spinal column that undergoes degenerative phenomena: its progressive thickening induces alterations of the bony vertebral endplate and bone marrow of the contiguous vertebral bodies (21-31). The alterations in the disco-vertebral complex also affect the corresponding posterior osteoarticular elements of the spinal column, resulting in narrowing of the vertebral canal and/or the adjacent spinal neural foramina, or instability of the spinal column (21, 32-40).
Computed tomography (CT) and magnetic resonance imaging (MRI) have been widely adopted for the degenerative spine disease. Indeed, MRI represents a very sensitive technique for the assessment of early degenerative changes of the spine (13, 20, 32, 33, 35, 41-45).
However, standard MRI protocol without fat suppression and contrast media injection in patients with low back pain does not always enable clear identification of pain aetiology (46).
In our work we verified if the addition of T2-weighted sequences with Fat Saturation (FS) and, when indicated, T1-weighted sequences with FS after intravenous administration of contrast medium, were able to provide a more sensitive picture of the degenerative changes of the spinal column and occasionally disclose pathologic conditions unsuspected during a “standard” MRI examination (30, 43-45, 47-50).
Materials and methods
From January 2006 to December 2011 we performed MR scans in 2820 patients (1628 male and 1192 female, 14-83 years old, mean age 54) presenting low back pain sometimes radiating to the legs. Physical examination before MR has been performed for all patients aiming to identify those with “mechanical” and radicular pain; other inclusion criteria were: known degenerative disease of lumbar spine previously revealed on X-ray and/or MRI exams. Exclusion criteria were: history of trauma, tumours, known infective spinal disease, vertebral fractures and previous spine surgery.
The study has been conducted according to the declarations of Helsinki, and written informed consent has been obtained from all subjects.
Patients were examined with a 1.5 Tesla MR scanner (Siemens Symphony, Erlangen, Germany) using the study protocol described in table 1.
Table 1.
MR scan protocol (1.5 Tesla). Axial T2-FS and CE T1-FS sequences have been adopted on selective pathological areas previously identified on basic scan
In case of failure to disclose the cause of pain and/or in case of T2-hyperintensity of osteoarticular and muscular-ligamentous structures on sagittal sequence, axial TSE T2-FS sequences on suspected pathologic area have been added; patients who benefitted of sagittal and/or axial TSE T1-w FS (Fat Sat) sequences after contrast medium administration (Dotarem-Guerbet -0.5 mmol/ml, 0.2 ml/kg dose) were selected following these criteria:
presence of osteoarticular or muscular-ligamentous oedema detected on sagittal and axial T2-FS;
presence of clinical findings such as “mechanical” pain, radicular pain, trigger points on palpation, even if the previous sequences failed to disclose oedematous lesions
All non-disc-related findings have been classified as:
Vertebral body changes (active/inflammatory endplate changes)
Facet joints changes (facet joint effusion, synovitis, subchondral cysts, synovial cysts)
Spondylolysis
Ligament changes (Ligamentum Flavum, interspinous and supraspinous ligaments)
Muscular changes (posterior perispinal muscles)
All the MR exams have been reported by consensus by two neuroradiologists with 15 years of experience in spine imaging.
Results
In 1833 out of 2820 patients (65%) no osteoarticular and/or muscular-ligamentous oedema have been detected with standard MR protocol and patients symptoms were variously associated to common causes of low back and sciatic pain: disc disease (1438 cases), degenerative spondylolisthesis (372 cases), and vertebral canal stenosis (567 cases).
987 out of 2820 patients (35%) received intravenous contrast medium because the T2-weighted sequences with FS displayed oedema in the osteo-articular and muscular-ligamentous structures (879 patients) or because the basic scan failed showed no alterations pertinent with the cause of pain (108 cases).
In these 987 cases, CE (Contrast-Enhanced) T1-FS sequences allowed the identification of a single or a combination of these entities:
active/inflammatory endplate changes in 646 (65%) patients (Fig. 1, 3);
facet joints changes in 462 (47%) patients (Fig. 2, 3, 4): facet joint effusion, synovitis (Fig. 2, 4, 7), osteoarthritis, synovial cysts (Fig. 9);
degenerative-inflammatory changes of the flava, interspinous and supraspinous ligaments in 245 (25%) patients (Fig. 4, 5, 7);
changes of the posterior perispinal muscles (oedema, inflammation) in 84 (8%) patients (Fig. 3, 6, 8).
Figure 1.
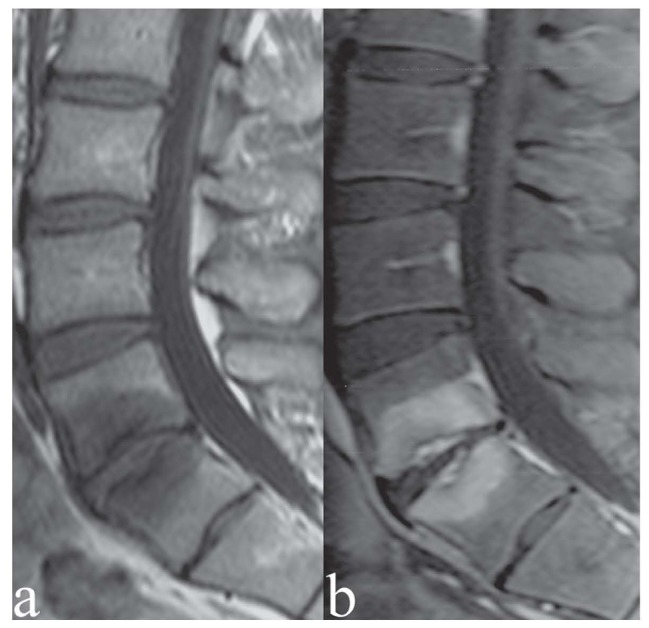
Patient with chronic low back pain. Severe osteochondrosis at L5/S1 with fibro-vascular transformation of bone marrow (Modic I). a) Sagittal T1-weighted image; b) sagittal T1-weighted image with Fat Saturation following contrast medium administration. Contrast enhancement of the endplates and subchondral cancellous bone, indicating active-inflammatory stage of the degenerative process and its extension
Figure 3.
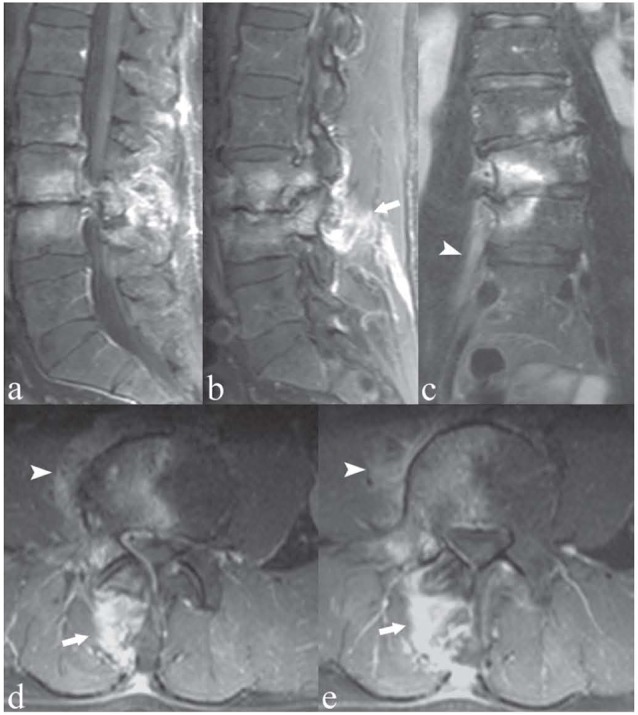
Patient with right-sided low back pain. Sagittal (a, b), coronal (c), and axial (d, e) T1-weighted images with Fat Saturation following contrast medium administration. Right-sided osteochondrosis at L3/L4, with contrast enhancement of the subchondral bone, indicating active inflammatory component of the degenerative process. Note the extension of the inflammation to the adjacent peridiscal soft tissue and psoas muscular fibers (arrowheads). There is also right facet joint osteoarthritis and inflammation of the right perispinal posterior muscular fibres (arrows)
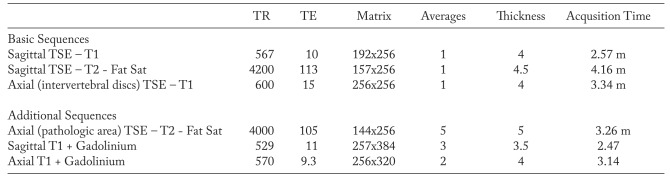
Figure 2.
Patient with bilateral low back pain. a) Sagittal T2-weighted image; b, c) sagittal and axial T2-weighted images with Fat Saturation; d) axial T1-weighted image with Fat Saturation after contrast medium administration. Bilateral facet joint osteoarthritis and synovitis at L4/L5 (arrows). Hyperintensity on T2-weighted images and contrast enhancement of the bone marrow of posterior spinal facet joint articular processes (osteoarthritis) and within the facet joint space (synovitis); the same articular space is enlarged (c). Note the differences between a) and b): “standard” T2-weighted image without fat saturation (a) fails to show the above mentioned pathologic findings that are clearly showed on T2-weighted image with fat saturation (b, c)
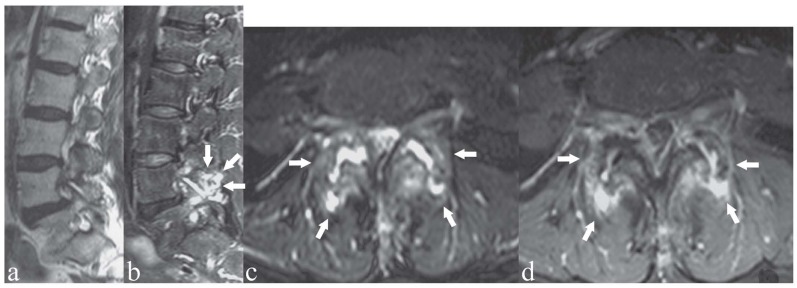
Figure 4.
Patient with right-sided low back pain. a) Axial T2-weighted image with Fat Saturation; b, c) axial T1-weighted images with Fat Saturation following contrast medium administration. Facet joint osteoarthritis and mild synovitis on the right-side, with periapophyseal contrast enhancement indicating soft tissue inflammatory reaction (arrowheads). Note also contrast enhancement of the flava ligaments (c, arrows), indicating ligamentous degenerative-inflammatory changes
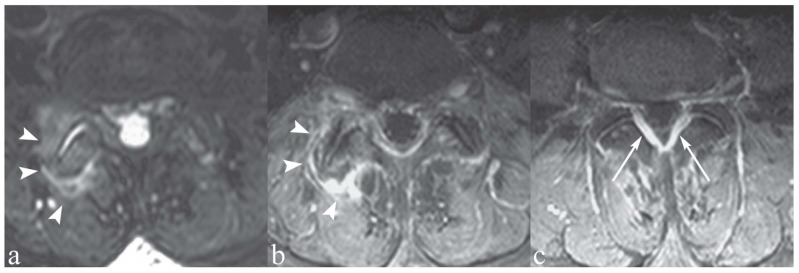
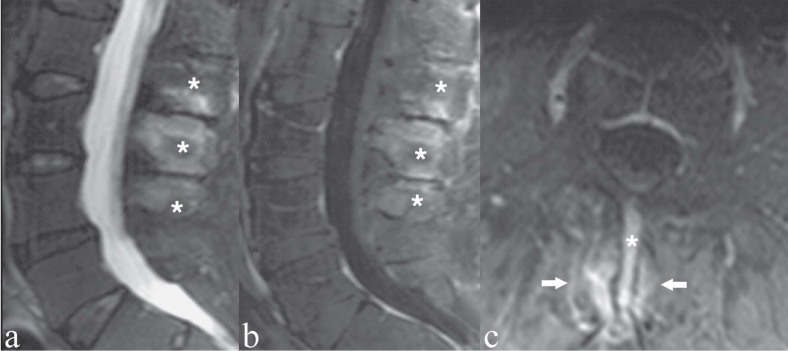
Figure 7.
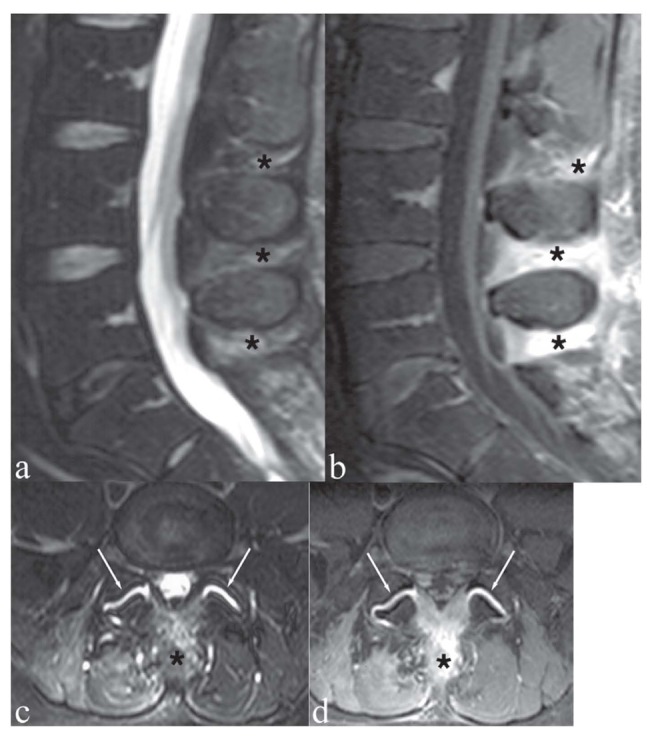
Patient with low back pain and focal tenderness on the midline. a, c) Sagittal and axial T2-weighted images with Fat Saturation; b, d) Sagittal and axial T1-weighted image with Fat Saturation following contrast medium administration. The interspinous ligaments at L3/L4, L4/L5 and L5/S1 (asterisks) show hyperintensity on T2-weighted images (a, c) and marked contrast enhancement (b, d). These findings indicate degenerative-inflammatory ligamentous changes. Note also bilateral facet joint synovitis (c, d, arrows)
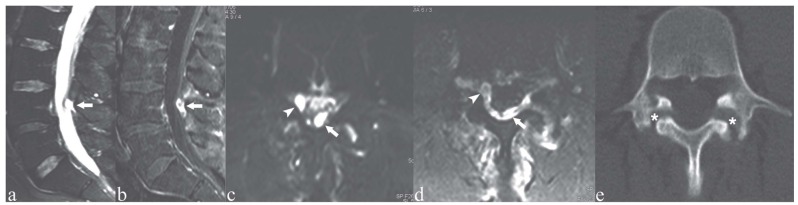
Figure 9.
Patient with low back pain. a, c) Sagittal and axial T2-weighted images with Fat Saturation; b, d) Sagittal and axial T1-weighted images with Fat Saturation following contrast medium administration; e) axial CT image on L4. Mild anterolisthesis of L4 over L5 (a-b), with spondylolysis of L4 (e, asterisks). Note also the intraspinal synovial cyst (c, d arrowheads) and the neo-/pseudocyst in the epidural space of the posterior aspect of the central spinal canal (a-d, arrows)
Figure 5.
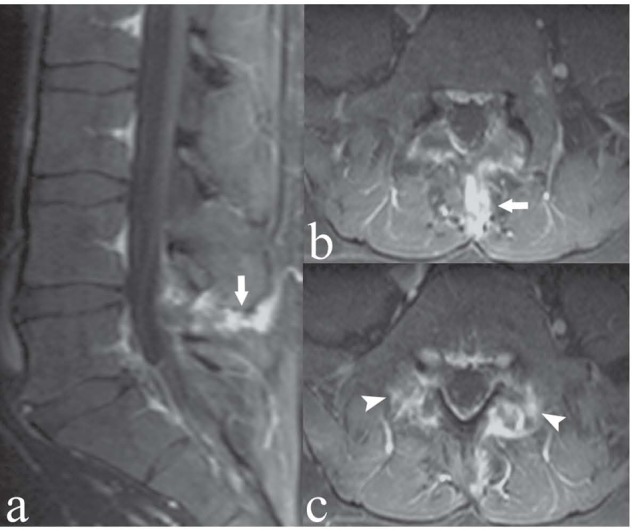
Young patient with low back pain and marked focal tenderness. Sagittal (a) and axial (b, c) T1-weighted images with Fat Saturation following contrast medium administration. The interspinous ligament at L4/L5 enhances following contrast medium administration (arrows), indicating degenerative-inflammatory ligamentous changes. Note also bilateral contrast enhancement in the pars interarticularis of L5 and adjacent soft tissues, indicating bilateral spondylolysis and reactive inflammation of the adjacent soft tissues (arrowheads)
Figure 6.
Patient with persistent low back pain; physical examination showed limited lumbar motion, exacerbation of pain by hyperextension and tenderness on palpation of the interspinous spaces. a) Sagittal T2-weighted image with Fat Saturation; b, c) Sagittal and axial T1-weigthed images with Fat Saturation following contrast medium administration. Lumbar hyperlordosis is present, associated with collision of the spinous processes at L2-L3-L4 (i.e. Baastrup’s phenomenon). The bone marrow of the above-mentioned opposing spinous processes (asterisks) reveals hyperintense areas on T2-weighted image (a) and contrast enhancement (b, c); these findings represent stress-related bony reactive-degenerative changes. The corresponding L2/L3 and L3/L4 interspinous spaces are collapsed. The adjacent muscular fibers (i.e., interspinalis muscles) show contrast enhancement, indicating degenerative-inflammatory muscular alteration (arrows)
Figure 8.
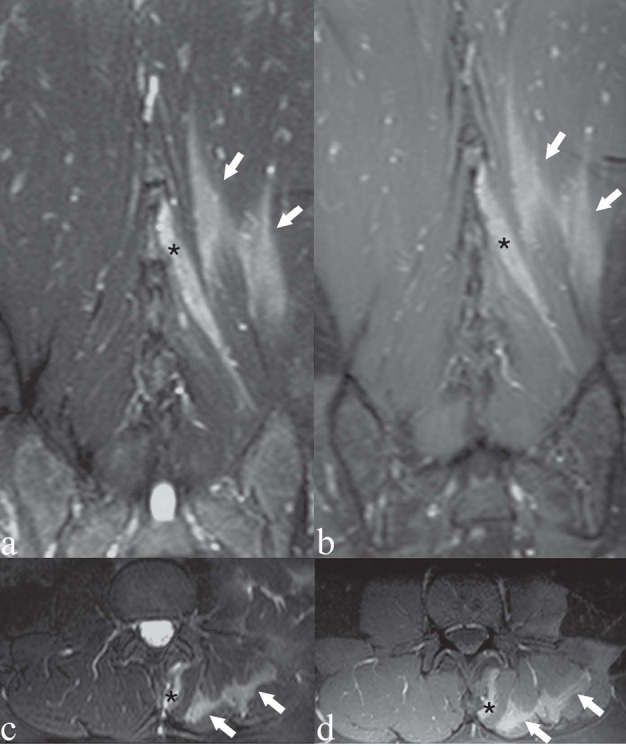
Patient with left-sided low back pain. There was a precise correlation between the MRI findings and the patient’s focus of low back pain. a, c) Coronal and axial T2-weighted images with Fat Saturation; b, d) coronal and axial T1-weighted images with Fat Saturation following contrast medium administration. Hyperintensity on T2-weighted images (a, c) and contrast enhancement (b, d) within the perispinal muscle fibers on the left side: multifidus muscle (asterisk), longissimus and iliocostalis muscles (arrows)
Among the 879 patients with T2-FS findings, CE T1-FS sequences allowed a better definition and extent evaluation of the lesions in 789 patients; in the remaining 90 patients, no substantial benefit have been provided by contrast medium administration.
In 108 out of 987 patients (11%) CE T1-FS allowed the identification of ligamentous lesions (29), synovitis (38), and discal/peridiscal inflammation (41), which have been missed on both standard and additional non-enhanced sequences.
Among all 987 subjects who have shown pathological findings on CE T1-FS images, 908 (92%) revealed a possible association with the clinical examination, 79 (8%) presented no clear agreement between MR findings and clinical examination. All 79 patients showed T2-hyperintensity and contrast enhancement of the lesions.
Discussion
All the patients included in our study protocol suffered of LBP, occasionally radiating to the lower limbs. This work demonstrated that T2 weighted sequence with FS and/or T1 weighted sequence with FS after contrast media injection can improve the diagnostic performances of standard MR examination in patients with LBP. Moreover these sequences in the study presented population, permitted the complete and better definition of some pathological entities causing LBP.
It must be stressed that painful symptomatology can be the result of pathological changes involving several anatomic elements of the spine, with only a limited number of cases related to radicular compression. These various components capable of producing painful sensations are represented by spinal nerves, the osteoarticular elements of the spinal column (i.e., the periphery of the intervertebral disc, the posterior spinal facet joints, spinal ligaments) and the perispinal muscles.
The spinal column can be divided into two anatomic compartments – anterior and posterior – each corresponding to its respective intersomatic joint or disc and joints of the posterior vertebral arch. These regions can be further subdivided according to biomechanical studies and the concept of “anatomic functional unit” of the spinal column (51-54). Morpho-structural changes of one region have repercussions on another entire region or a part of that. These individual regions must be carefully examined by the radiologist.
The dense, complex regional innervation of spinal column structures correlates with the origin of pain, spreading from the spinal nerves via its ventral and dorsal roots which, in turn, generate a smaller series of ramifications. The different type of involved innervation determines the potential nature of the pain (radicular, local, referred) (33, 35, 55-57). Radicular pain depends to a lesser extent on the direct compression of the nerve root than on physiologic factors (altered tropism of the nerve due to compression on arterial afferent vessels and/or from venous stasis) and on neural and perineural inflammatory reactions (4, 20, 39, 42, 58, 59), which produces mechano- and chemo-sensitivity within the neural radicle. We can postulate that similar inflammatory factors are also involved in the pathogenesis of the other types of back pain (19, 24, 36, 49, 60-70).
The majority of the patients in our study protocol presented degenerative phenomena of the intervertebral discs on standard MR scan, consistent with the observation that discs are commonly the primary element of the spinal column that manifest degenerative alterations (29, 33). As with every occurrence of arthrosis, such degenerative phenomena are largely due to genetics, long-term heavy weight-bearing and other complex factors.
From the morphological standpoint, disc degeneration results in loss of height, radial bulging, fissuring of the annulus fibrosus, and disc herniated; moreover, degenerative disc alterations are typically accompanied by morpho-structural changes of the adjacent vertebral bodies (e.g. osteochondrosis and spondylosis).
MRI remarkably documents such degenerative phenomena to a superior degree than other imaging modalities (32, 33, 43-45). Among other findings, the degenerated disc demonstrates a reduction in signal intensity on T2-weighted images (principally due to dehydration processes and variations in proteoglycan composition), and a decrease in height; in the more advanced stages of degeneration, the disc fully collapses and may undergo cystic changes, gasseous degeneration and calcification.
Discal degenerative processes are usually accompanied by bone marrow changes in the supra- and subjacent vertebral bodies, which are currently classified as the following (71, 72):
Type I – fibrovascular replacement of the bone marrow;
Type II – proliferation of the fatty marrow;
Type III – osteosclerosis.
In our study patients, such phenomena, although usually manifested on standard MRI sequences, were more evident on T2-weighted images with FS; in particular, type I alterations were more evident, since the suppression of the fat signal enhances the fibro-vascular transforming phenomena and underlying bone marrow edema. In addition, the use of T1-weighted sequences with FS after administration of contrast medium showed enhancement of this same bone marrow in parallel with the bone oedema (Fig. 1, 3) (this event is normally masked in non-FS T1-weighted sequences after contrast medium administration).
In 16 cases (1,6%) we found contrast enhancement of the intervertebral disc, with or without associated disc changes; in our opinion this finding can be named as “aseptic” or “sterile” discitis, of a degenerative nature (43, 45).
In the study of patients with LBP, it is also necessary to carefully examine the osteoarticular, ligamentous, and muscular elements of the posterior aspect of the spinal column.
The posterior spinal facet joints may undergo degenerative arthrosis, presenting with a narrowing or loss of the joint space (occasionally accompanied by intra-articular vacuum), joint surfaces erosions, subchondral sclerosis, para-articular osteophytosis, calcification of the joint capsule and ligamenta flava, and apophyseal hypertrophy (29, 32, 33, 73). Such events generally occur secondary to degenerative phenomena of the corresponding intervertebral disc that progressively affect the posterior spinal elements, resulting in facet joint subluxation, remodelling of facet joints, and progressive stenosis of the contiguous spinal neural foramen. Phenomena contributing to spinal facet joint degeneration include axial plane facet joint orientation anomalies or lumbosacral hyperlordosis.
From a clinical perspective, the “facet joint syndrome” is characterized by a deep ill defined lumbar pain, which may be referred to the buttocks and lower limbs; the pain is typically induced or increased by hyperextension and rotation of the spinal column, which remains limited in its range of motion (74, 75).
MRI plays an eminent role in the identification of degenerative phenomena of the spinal facet joints, thereby revealing alterations otherwise unsuspected in other imaging techniques (76, 77). T2-weighted sequences, including those with FS, may show hyperintense signal within the joint space, reflecting joint effusion. In our series, 260 patients (26%) showed facet joint effusion. In 161 cases (16%) the same joint space revealed contrast enhancement on CE T1-FS as for facet joint synovitis (Fig. 2, 4, 7). T2-hyperintensity and contrast enhancement of the subchondral bone marrow, in association with the typical degenerative facet alterations, was found in 279 cases (28%). This phenomenon may be termed as degenerative “facet joint osteoarthritis” (Fig. 2, 3, 4). Periapophyseal contrast enhancement, found in 146 cases (15%) in this study, may be defined as sterile degenerative “periapophyseal soft tissue inflammatory reaction” (Fig. 4).
An additional consequence of such degenerative phenomena of the facet joint is represented by the formation of periarticular synovial cysts, resulting from the hydraulic forces during kinetic, weight bearing maneuvers within a closed joint space with an underlying expanded/balooned joint effusion (78). Synovial cysts can extend toward the central spinal canal (resulting in spinal stenosis) or into the contiguous neural foramen (in which case they may cause spinal nerve compression) or toward the retrospinal periapophyseal soft tissues (in which case, pain may be related to degenerative-inflammatory changes of the corresponding facet joint and periarticular soft tissues).
Synovial cysts present a signal similar to cerebrospinal fluid, peaking in hyperintensity on T2-weighted sequences with FS ; in 89 out of 128 cases the wall of the cyst enhanced after contrast medium administration (Fig. 9).
In 69 patients with low back pain we found spondylolysis, with or without spondylolisthesis. Spondylolysis is a fracture of the pars interarticularis (79). The pathogenesis of this alteration seems to be related to repeated microfractures of the pars interarticularis, eventually resulting in a permanent defect. In 19 cases, especially those without spondylolisthesis (13 on 19 cases), visualization of the pars defect may be difficult to detect on MRI. T2-weighted images with FS and contrast medium-enhanced images with FS in these 19 cases allowed clear recognition of these “hidden” or occult fractures (44), otherwise not detectable by standard MR sequences. In particular, in the active-inflammatory phase, areas of T2-hyperintensity and contrast enhancement appeared in the region of the pars interarticularis defect, the contiguous pedicle and occasionally within the immediately adjacent soft tissues (Fig. 5, 9). These changes persist for months to years, presumably a reaction of the bone marrow and related soft tissues to increased local mechanical stresses.
In 245 patients affected by low back pain we found degenerative-inflammatory phenomena of the interspinous and/or supraspinous and/or flava ligaments (Fig. 4, 5, 7). As reported in literature, the fundamental factors responsible for chronic-progressive interspinous soft tissue injury include the following: hyperlordosis of the lumbosacral spine with consequent bony collision of the opposed vertebral spinous processes (i.e. Baastrup’s phenomenon) and injury of the intervening interspinous ligament; collapse of the respective intervertebral discs, with consequent interspinous space narrowing with bony collision and traumatic injury of the corresponding interspinous ligament. Any form of ligamentous stress may, however, cause degenerative-inflammatory alterations in the ligaments themselves, in the absence of disc collapse or interspinous narrowing at the same level. Once again, in 245 cases FS techniques allowed the discernment of ligamentous changes that cannot otherwise be visualised. In 226 patients T2-weighted sequences with FS showed hyperintensity of the above-cited ligaments and contiguous tissues. Following contrast medium administration, in 181 cases, T1-weighted sequences with FS showed areas of enhancement within and surrounding the ligaments themselves. We underline that in 19 cases, we found contrast enhancement of the ligaments without abnormal findings in T2-weighted images. Moreover, in 37 cases we found interspinous ligamentous pseudocysts also presenting as hyperintensity on T2-weighted sequences with FS, with peripheral contrast enhancement.
Degenerative-inflammatory changes may involve not only the interspinous and supraspinous ligaments but also the flavae ligaments (Fig. 4); in many instances several or all of these structures are involved, at one or at multiple levels, on one or both sides.
In 84 patients with low back pain, MRI detected alterations of the intrinsic posterior perispinal lumbosacral muscles (e.g. interspinalis, multifidus muscles) (3-8). It is possible to postulate that three-dimensional lumbar intersegmental hypermobile instability may induce various muscular alterations, including: spasm, inflammation, sprain, rupture and eventual degeneration/atrophy. These changes can either be caused by one or both of two mechanisms related to neuromuscular auto-trauma: rupture/avulsion of the intrinsic spinal muscles or traumatic denervation of the nerves supplying the same muscles (i.e. rupture-avulsion of the medial branch of the dorsal ramus of the spinal nerve .
MRI clearly visualized the above-mentioned muscular changes. In particular, T2-weighted images with FS showed hyperintensity of the posterior perispinal muscular tissues, otherwise not identifiable with standard imaging sequences without FS; typically, these same muscles showed enhancement after intravenous administration of contrast medium (Figure 3, 6, 8).
Such muscular changes may be directly or indirectly (e.g. muscular sprain, rupture, muscular spasm) involved in the pathogenesis of low back pain. In these cases, it was possible to find a close clinical-radiological correlation between the anatomic distribution of pain and the perispinal muscle alterations as visualized by MRI. In 33 patients with low back pain, these changes were the only abnormal findings visualised on MRI.
MRI of patients with low back pain demands careful examination not only of the discal-radicular complex but of the entire osteoarticular apparatus of the spinal column as well as the perispinal soft tissues, to search for clinically relevant degenerative-inflammatory processes. Without a careful imaging analysis of the non-discal and non-radicular tissues of the spine and perispinal tissues, the true clinical source of the patient’s back pain may be overlooked, missed. In addition: 1) the true pain locus will likely go untreated, or 2) the clinician may treat a spinal finding that is not a cause of the dominant pain syndrome leading to a form of the failed therapy syndrome.
We emphasize that the imaging observations sought in this evaluation were intended to stand alone primarily as observational ones (i.e., associated with clinical findings but not unequivocally invoked as causal factors). Additional studies are needed to clearly correlate MRI findings with pain symptomatology and response to treatment.
In conclusion, it has been found that integrating MRI with T2-weighted images with FS and, when indicated, with T1-weighted images with FS after intravenous contrast medium administration, increases diagnostic sensitivity in patients with low back pain, particularly in those cases whose standard MR examination does not disclose a clear cause of pain. In such cases FS MRI enables a focused therapeutic regimen to be generated, and allows critical re-evaluation over time on specific therapy.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part I: Spinal stability. Eur J Radiol. 2013;82:118–126. doi: 10.1016/j.ejrad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Masala S, Nano G, Marcia S, Muto M, Fucci FPM, Simonetti G. Osteoporotic vertebral compression fractures augmentation by injectable partly resorbable ceramic bone substitute (Cerament™|SPINE SUPPORT): A prospective nonrandomized study. Neuroradiology. 2012;54:589–596. doi: 10.1007/s00234-011-0940-5. [DOI] [PubMed] [Google Scholar]
- 4.Muto M, Perrotta V, Guarnieri G, Lavanga A, Vassallo P, Reginelli R, Rotondo A. Vertebroplasty and kyphoplasty: Friends or foes. Radiol Med. 2008;113:1171–1184. doi: 10.1007/s11547-008-0301-6. [DOI] [PubMed] [Google Scholar]
- 5.Guarnieri G, Vassallo P, Pezzullo MG, Laghi F, Zeccolini F, Ambrosanio G, Galasso R, Muto M, Izzo R. A comparison of minimally invasive techniques in percutaneous treatment of lumbar herniated discs a review. Neuroradiol J. 2009;22:108–121. doi: 10.1177/197140090902200116. [DOI] [PubMed] [Google Scholar]
- 6.Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L. Errors in neuroradiology. Radiol Med. 2015;120:795–801. doi: 10.1007/s11547-015-0564-7. [DOI] [PubMed] [Google Scholar]
- 7.Caranci F, Briganti F, La Porta M, Antinolfi G, Cesarano E, Fonio P, Brunese L, Coppolino F. Magnetic resonance imaging in brachial plexus injury. Musculoskeletal Surg. 2013;97:S181–S190. doi: 10.1007/s12306-013-0281-0. [DOI] [PubMed] [Google Scholar]
- 8.Briganti F, Tedeschi E, Leone G, Marseglia M, Cicala D, Giamundo M, Napoli M, Caranci F. Endovascular treatment of vertebro-vertebral arteriovenous fistula. Neuroradiol J. 2013;26:339–346. doi: 10.1177/197140091302600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol. 2013;78:66–69. doi: 10.12659/PJR.884011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurzynska D, DiMeglio F, Castaldo C, Latino F, Romano V, Miraglia R, Guerra G, Brunese L, Montagnani S. Flatfoot in children: Anatomy of decision making. Ital J Anat Embryol. 2012;117:98–106. [PubMed] [Google Scholar]
- 11.Potenza DM, Guerra G, Avanzato D, Poletto V, Pareek S, Guido D, Gallanti A, Rosti V, Munaron L, Tanzi F, Moccia F. Hydrogen sulphide triggers VEGF-induced intracellular Ca(2)(+) signals in human endothelial cells but not in their immature progenitors. Cell Calcium. 2014;56:225–34. doi: 10.1016/j.ceca.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ronco V, Potenza DM, Denti F, Vullo S, Gagliano G, Tognolina M, Guerra G, Pinton P, Genazzani AA, Mapelli L, Lim D, Moccia F. A novel Ca(2)(+)-mediated cross-talk between endoplasmic reticulum and acidic organelles: implications for NAADP-dependent Ca(2)(+) signalling. Cell Calcium. 2015;57:89–100. doi: 10.1016/j.ceca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Cappabianca S, Scuotto A, Iaselli F, Pignatelli di Spinazzola N, Urraro F, Sarti G, Montemarano M, Grassi R, Rotondo A. Computed tomography and magnetic resonance angiography in the evaluation of aberrant origin of the external carotid artery branches. Surg Radiol Anat. 2012;34:393–9. doi: 10.1007/s00276-011-0926-3. [DOI] [PubMed] [Google Scholar]
- 14.Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, Santoriello C, Ciani A, Cozzolino D, De Matteis GM, Cappabianca S, Vitelli F, Spano A. Early systemic sclerosis: marker autoantibodies and videocapillaroscopy patterns are each associated with distinct clinical, functional and cellular activation markers. Arthritis Res Ther. 2013;15:R63. doi: 10.1186/ar4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iudici M, Cuomo G, Vettori S, Bocchino M, Sanduzzi Zamparelli A, Cappabianca S, Valentini G. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum. 2015;44:437–44. doi: 10.1016/j.semarthrit.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, Santoriello C, Ciani A, Cozzolino D, De Matteis GM, Cappabianca S, Vitelli F, Spano A. Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res (Hoboken) 2014;66:1520–7. doi: 10.1002/acr.22304. [DOI] [PubMed] [Google Scholar]
- 17.Di Zazzo E, Porcile C, Bartollino S, Moncharmont B. Critical Function of PRDM2 in the Neoplastic Growth of Testicular Germ Cell Tumors. Biology (Basel) 2016;5 doi: 10.3390/biology5040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappabianca S, Colella G, Pezzullo MG, Russo A, Iaselli F, Brunese L, Rotondo A. Lipomatous lesions of the head and neck region: Imaging findings in comparison with histological type. Radiol Med. 2008;113:758–770. doi: 10.1007/s11547-008-0258-5. [DOI] [PubMed] [Google Scholar]
- 19.Grassi R, Lombardi G, Reginelli A, Capasso F, Romano F, Floriani I, Colacurci N. Coccygeal movement: assessment with dynamic MRI. Eur J Radiol. 2007;61:473–9. doi: 10.1016/j.ejrad.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Cappabianca S, Colella G, Russo A, Pezzullo M, Reginelli A, Iaselli F, Rotondo A. Maxillofacial fibrous dysplasia: personal experience with gadoliniumenhanced magnetic resonance imaging. Radiol Med. 2008;113:1198–210. doi: 10.1007/s11547-008-0329-7. [DOI] [PubMed] [Google Scholar]
- 21.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, Ruscitti P, Cipriani P, Giacomelli R, Brunese L, Masciocchi C. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017 doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Barile A, La Marra A, Arrigoni F, Mariani S, Zugaro L, Splendiani A, Di Cesare E, Reginelli A, Zappia M, Brunese L, Duka E, Carrafiello G, Masciocchi C. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caranci F, Napoli M, Cirillo M, Briganti G, Brunese L, Briganti F. Basilar artery hypoplasia. Neuroradiol J. 2012;25:739–743. doi: 10.1177/197140091202500613. [DOI] [PubMed] [Google Scholar]
- 24.Bandirali M, Di Leo G, Papini GD, Messina C, Sconfienza LM, Ulivieri FM, Sardanelli F. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur Radiol. 2015;25:2951–9. doi: 10.1007/s00330-015-3699-y. [DOI] [PubMed] [Google Scholar]
- 25.Sartoris R, Orlandi D, Corazza A, Sconfienza LM, Arcidiacono A, Bernardi SP, Schiaffino S, Turtulici G, Caruso P, Silvestri E. In vivo feasibility of real-time MR-US fusion imaging lumbar facet joint injections. J Ultrasound. 2017;20:23–31. doi: 10.1007/s40477-016-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am. 2007;17:87–103. doi: 10.1016/j.nic.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Muto M. Degenerative facet joint disease. Neuroradiology. 2011;53(1):S167–168. doi: 10.1007/s00234-011-0934-3. [DOI] [PubMed] [Google Scholar]
- 28.Barile A, Limbucci N, Splendiani A, Gallucci M, Masciocchi C. Spinal injury in sport. Eur J Radiol. 2007;62:68–78. doi: 10.1016/j.ejrad.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Izzo R, Popolizio T, D’Aprile P, Muto M. Spinal pain. Eur J Radiol. 2015;84:746–756. doi: 10.1016/j.ejrad.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Splendiani A, Perri M, Grattacaso G, Di Tunno V, Marsecano C, Panebianco L, Gennarelli A, Felli V, Varrassi M, Barile A, Di Cesare E, Masciocchi C, Gallucci M. Magnetic resonance imaging (MRI) of the lumbar spine with dedicated G-scan machine in the upright position: a retrospective study and our experience in 10 years with 4305 patients. Radiol Med. 2016;121:38–44. doi: 10.1007/s11547-015-0570-9. [DOI] [PubMed] [Google Scholar]
- 31.Patriarca L, Letteriello M, Di Cesare E, Barile A, Gallucci M, Splendiani A. Does evaluator experience have an impact on the diagnosis of lumbar spine instability in dynamic MRI? Interobserver agreement study. Neuroradiol J. 2015;28:341–346. doi: 10.1177/1971400915594508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jinkins JR. Acquired degenerative changes of the intervertebral segments at and suprajacent to the lumbosacral junction. A radioanatomic analysis of the nondiscal structures of the spinal column and perispinal soft tissues. Eur J Radiol. 2004;50:134–58. doi: 10.1016/j.ejrad.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Jinkins JR. Acquired degenerative changes of the intervertebral segments at and suprajacent to the lumbosacral junction. A radioanatomic analysis of the nondiskal structures of the spinal column and perispinal soft tissues. Radiol Clin North Am. 2001;39:73–99. doi: 10.1016/s0033-8389(05)70264-5. [DOI] [PubMed] [Google Scholar]
- 34.Perri M, Marsecano C, Varrassi M, Giordano AV, Splendiani A, di Cesare E, Masciocchi C, Gallucci M. Indications and efficacy of O2–O3 intradiscal versus steroid intraforaminal injection in different types of disco vertebral pathologies: A prospective randomized double-blind trial with 517 patients. Radiol Med. 2016;121:463–471. doi: 10.1007/s11547-015-0598-x. [DOI] [PubMed] [Google Scholar]
- 35.Bruno F, Smaldone F, Varrassi M, Arrigoni F, Barile A, Di Cesare E, Masciocchi C, Splendiani A. MRI findings in lumbar spine following O2-O3 chemiodiscolysis: A long-term follow-up. Interv Neuroradiol. 2017;23:444–450. doi: 10.1177/1591019917703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genovese L, Anceschi G, Muto M, Cangialosi R. Use of interspinous distractors in symptomatic lumbar degenerative disc osteoarthritis. Riv Ital Neurobiol. 2009;55:43–45. [Google Scholar]
- 37.Cicala D, Briganti F, Casale L, Rossi C, Cagini L, Cesarano E, Brunese L, Giganti M. Atraumatic vertebral compression fractures: Differential diagnosis between benign osteoporotic and malignant fractures by MRI. Musculoskeletal Surg. 2013;97:S169–S179. doi: 10.1007/s12306-013-0277-9. [DOI] [PubMed] [Google Scholar]
- 38.Pinto A, Brunese L, Pinto F, Reali R, Daniele S, Romano L. The Concept of Error and Malpractice in Radiology. Semin Ultrasound CT MRI. 2012;33:275–279. doi: 10.1053/j.sult.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Zappia M, Cuomo G, Martino MT, Reginelli A, Brunese L. The effect of foot position on Power Doppler Ultrasound grading of Achilles enthesitis. Rheumatol Int. 2016;36:871–874. doi: 10.1007/s00296-016-3461-z. [DOI] [PubMed] [Google Scholar]
- 40.Versaci F, Trivisonno A, Olivieri C, Caranci F, Brunese L, Prati F. Vascular response after percutaneous sympathectomy: Not all devices are equal. Int J Cardiol. 2014;174:406–407. doi: 10.1016/j.ijcard.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 41.Landi A, Gregori F, Mancarella C, Maiola V, Maccari E, Marotta N, Delfini R. Lumbar spinal degenerative “microinstability”: hype or hope? Proposal of a new classification to detect it and to assess surgical treatment. Eur Spine J. 2015;24(7):872–8. doi: 10.1007/s00586-015-4274-6. [DOI] [PubMed] [Google Scholar]
- 42.Barile A, Arrigoni F, Bruno F, Guglielmi G, Zappia M, Reginelli A, Ruscitti P, Cipriani P, Giacomelli R, Brunese L, Masciocchi C. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017;55:997–1007. doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 43.D’Aprile P, Tarantino A, Lorusso V, Brindicci D. Fat saturation technique and gadolinium in MRI of lumbar spinal degenerative disease. Neuroradiol J. 2006;19:654–71. doi: 10.1177/197140090601900518. [DOI] [PubMed] [Google Scholar]
- 44.D’Aprile P, Tarantino A, Jinkins JR, Brindicci D. The value of fat saturation sequences and contrast medium administration in MRI of degenerative disease of the posterior/perispinal elements of the lumbosacral spine. Eur Radiol. 2007;17:523–31. doi: 10.1007/s00330-006-0324-0. [DOI] [PubMed] [Google Scholar]
- 45.D’Aprile P, Tarantino A, Santoro N, Carella A. Wernicke’s encephalopathy induced by total parenteral nutrition in patient with acute leukaemia: unusual involvement of caudate nuclei and cerebral cortex on MRI. Neuroradiology. 2000;42:781–3. doi: 10.1007/s002340000393. [DOI] [PubMed] [Google Scholar]
- 46.Rossi C, Reginelli A, D’Amora M, Di Grezia G, Mandato Y, D’Andrea A, Brunese L, Grassi R, Rotondi A. Safety profile and protocol prevention of adverse reactions to uroangiographic contrast media in diagnostic imaging. J Biol Regul Homeost Agents. 2014;28:155–165. [PubMed] [Google Scholar]
- 47.Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, Arrigoni F, Zugaro L, Barile A, Masciocchi C, Gangi A. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33 doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 48.Limbucci N, Rossi F, Salvati F, Pistoia LM, Barile A, Masciocchi C. Bilateral Suprascapular nerve entrapment by glenoid labral cysts associated with rotator cuff damage and posterior instability in an amateur weightlifter. J Sports Med Phys Fitness. 2010;50:64–67. [PubMed] [Google Scholar]
- 49.Ripani M, Continenza MA, Cacchio A, Barile A, Parisi A, De Paulis F. The ischiatic region: normal and MRI anatomy. J Sports Med Phys Fitness. 2006;46:468–75. [PubMed] [Google Scholar]
- 50.Masciocchi C, Lanni G, Conti L, Conchiglia A, Fascetti E, Flamini S, Coletti G, Barile A. Soft-tissue inflammatory myofibroblastic tumors (IMTs) of the limbs: Potential and limits of diagnostic imaging. Skelet Radiol. 2012;41:643–649. doi: 10.1007/s00256-011-1263-7. [DOI] [PubMed] [Google Scholar]
- 51.Muto M, Giurazza F, Guarnieri G, Izzo R, Diano A. Neuroimaging of Spinal Instability. Magn Reson Imaging Clin N Am. 2016;24:485–94. doi: 10.1016/j.mric.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Miele V, Di Giampietro I. Diagnostic imaging in emergency. Salute Soc. 2014:127–141. [Google Scholar]
- 53.Piccolo CL, Galluzzo M, Ianniello S, Trinci M, Russo A, Rossi E, Zeccolini M, Laporta A, Guglielmi G, Miele V. Pediatric musculoskeletal injuries: role of ultrasound and magnetic resonance imaging. Musculoskelet Surg. 2017;101:85–102. doi: 10.1007/s12306-017-0452-5. [DOI] [PubMed] [Google Scholar]
- 54.Miele V, Piccolo CL, Trinci M, Galluzzo M, Ianniello S, Brunese L. Diagnostic imaging of blunt abdominal trauma in pediatric patients. Radiol Med. 2016;121:409–430. doi: 10.1007/s11547-016-0637-2. [DOI] [PubMed] [Google Scholar]
- 55.Miele V, Di Giampietro I, Ianniello S, Pinto F, Trinci M. Diagnostic imaging in pediatric polytrauma management. Radiol Med. 2014;120:33–49. doi: 10.1007/s11547-014-0469-x. [DOI] [PubMed] [Google Scholar]
- 56.Arrigoni F, Barile A, Zugaro L, Fascetti E, Zappia M, Brunese L, Masciocchi C. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia. 2017:1–7. doi: 10.1080/02656736.2017.1334168. [DOI] [PubMed] [Google Scholar]
- 57.Reginelli A, Zappia M, Barile A, Brunese L. Strategies of imaging after orthopedic surgery. Musculoskeletal Surg. 2017;101 doi: 10.1007/s12306-017-0458-z. [DOI] [PubMed] [Google Scholar]
- 58.Leonardi M, Simonetti L, Agati R. Neuroradiology of spine degenerative diseases. Best Pract Res Clin Rheumatol. 2002;16:59–87. doi: 10.1053/berh.2001.0207. [DOI] [PubMed] [Google Scholar]
- 59.Muccio CF, Caranci F, D’Arco F, Cerase A, De Lipsis L, Esposito G, Tedeschi E, Andreula C. Magnetic Resonance features of pyogenic brain abscesses and differential diagnosis using morphological and functional imaging studies: a pictorial essay. J Neuroradiol. 2014;41(3):153–167. doi: 10.1016/j.neurad.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36:1579–1583. doi: 10.1007/s00296-016-3562-8. [DOI] [PubMed] [Google Scholar]
- 61.Perrotta FM, Fici M, Guerra G, Brunese L, Salvarani C, Lubrano E. Chronic periaortitis with retroperitoneal fibrosis successfully treated with first line tocilizumab monotherapy: a case report. Clin Exp Rheumatol. 2017;35:226–227. [PubMed] [Google Scholar]
- 62.Lubrano E, Marchesoni A, Olivieri I, D’Angelo S, Palazzi C, Scarpa R, Ferrara N, Parsons WJ, Brunese L, Helliwell PS, Spadaro A. The radiological assessment of axial involvement in psoriatic arthritis. J Rheumatol. 2012;39:54–56. doi: 10.3899/jrheum.120244. [DOI] [PubMed] [Google Scholar]
- 63.Barile A, Arrigoni F, Zugaro L, Zappia M, Cazzato RL, Garnon J, Ramamurthy N, Brunese L, Gangi A, Masciocchi C. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34 doi: 10.1007/s12032-017-0909-2. [DOI] [PubMed] [Google Scholar]
- 64.Carotti M, Salaffi F, Di Carlo M, Giovagnoni A. Relationship between magnetic resonance imaging findings, radiological grading, psychological distress and pain in patients with symptomatic knee osteoarthritis. Radiol Med. 2017 doi: 10.1007/s11547-017-0799-6. [DOI] [PubMed] [Google Scholar]
- 65.Reginelli A, Silvestro G, Fontanella G, Sangiovanni A, Conte M, Nuzzo I, Calvanese M, Traettino M, Ferraioli P, Grassi R, Manzo R, Cappabianca S. Validation of DWI in assessment of radiotreated bone metastases in elderly patients. Int J Surg. 2016;33(1):S148–53. doi: 10.1016/j.ijsu.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Reginelli A, Silvestro G, Fontanella G, Sangiovanni A, Conte M, Nuzzo I, Di Lecce A, Martino A, Grassi R, Murino P, Cappabianca S. Performance status versus anatomical recovery in metastatic disease: The role of palliative radiation treatment. Int J Surg. 2016;33(1):S126–31. doi: 10.1016/j.ijsu.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 67.Splendiani A, Bruno F, Patriarca L, Barile A, Di Cesare E, Masciocchi C, Gallucci M. Thoracic spine trauma: advanced imaging modality. Radiol Med. 2016;121:780–792. doi: 10.1007/s11547-016-0657-y. [DOI] [PubMed] [Google Scholar]
- 68.Gallucci M, Limbucci N, Zugaro L, Barile A, Stavroulis E, Ricci A, Galzio R, Masciocchi C. Sciatica: Treatment with intradiscal and intraforaminal injections of steroid and oxygen-ozone versus steroid only. Radiology. 2007;242:907–913. doi: 10.1148/radiol.2423051934. [DOI] [PubMed] [Google Scholar]
- 69.Splendiani A, Puglielli E, De Amicis R, Barile A, Masciocchi C, Gallucci M. Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology. 2004;46:916–22. doi: 10.1007/s00234-004-1232-0. [DOI] [PubMed] [Google Scholar]
- 70.Guarnieri G, Bonetti M, Muto M, Andreula C, Leonardi M. Percutaneous treatment of cervical and lumbar disks, Interventional Neuroradiology of the Spine: Clinical Features, Diagnosis and Therapy. Springer-Verlag Milan. 2013:69–80. [Google Scholar]
- 71.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–9. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 72.Muto M, Ambrosanio G, Guarnieri G, Capobianco E, Piccolo G, Annunziata G, Rotondo A. Low back pain and sciatica: Treatment with intradiscal-intraforaminal O2-O3 injection. Our experience. Radiol Med. 2008;113:695–706. doi: 10.1007/s11547-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 73.Wybier M. Imaging of lumbar degenerative changes involving structures other than disk space. Radiol Clin North Am. 2001;39:101–14. doi: 10.1016/s0033-8389(05)70265-7. [DOI] [PubMed] [Google Scholar]
- 74.Lippitt AB. The facet joint and its role in spine pain. Management with facet joint injections. Spine (Phila Pa 1976) 1984;9:746–50. doi: 10.1097/00007632-198410000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Jackson RP. The facet syndrome. Myth or reality. Clin Orthop Relat Res. 1992:110–21. [PubMed] [Google Scholar]
- 76.Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML) Ann Hematol 91. 2012;(8):1327–1328. doi: 10.1007/s00277-011-1398-6. [DOI] [PubMed] [Google Scholar]
- 77.Czervionke LF, Fenton DS. Fat-saturated MR imaging in the detection of inflammatory facet arthropathy (facet synovitis) in the lumbar spine. Pain Med. 2008;9:400–6. doi: 10.1111/j.1526-4637.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 78.Doyle AJ, Merrilees M. Synovial cysts of the lumbar facet joints in a symptomatic population: prevalence on magnetic resonance imaging. Spine (Phila Pa 1976) 2004;29:874–8. doi: 10.1097/00007632-200404150-00010. [DOI] [PubMed] [Google Scholar]
- 79.Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40:683–700. doi: 10.1007/s00256-010-0942-0. [DOI] [PubMed] [Google Scholar]