Abstract
Context: Plants of the genus Echinacea (Asteraceae) are among the most popular herbal supplements on the market today. Recent studies indicate there are potential new applications and emerging markets for this natural health product (NHP).
Objective: This review aims to synthesize recent developments in Echinacea biotechnology and to identify promising applications for these advances in the industry.
Methods: A comprehensive survey of peer-reviewed publications was carried out, focusing on Echinacea biotechnology and impacts on phytochemistry. This article primarily covers research findings since 2007 and builds on earlier reviews on the biotechnology of Echinacea.
Results: Bioreactors, genetic engineering and controlled biotic or abiotic elicitation have the potential to significantly improve the yield, consistency and overall quality of Echinacea products. Using these technologies, a variety of new applications for Echinacea can be realized, such as the use of seed oil and antimicrobial and immune boosting feed additives for livestock.
Conclusions: New applications can take advantage of the well-established popularity of Echinacea as a NHP. Echinacea presents a myriad of potential health benefits, including anti-inflammatory, anxiolytic and antibiotic activities that have yet to be fully translated into new applications. The distinct chemistry and bioactivity of different Echinacea species and organs, moreover, can lead to interesting and diverse commercial opportunities.
Keywords: Coneflowers, secondary metabolites, tissue culture, genetic transformation, elicitors
Introduction
Biotechnology centred on species of Echinacea Moench (Asteraceae) has grown substantially in recent decades, owing to the popularity of Echinacea as a natural health product (NHP). Originating in North America and part of the traditional pharmacopeia of Indigenous Peoples (Moerman 1998), Echinacea is now cultivated around the world and has an annual global market value estimated at approximately $1.3 billion (Blumenthal et al. 2003). Despite alternative taxonomies based on molecular, morphometric and phytochemical variation (Binns et al. 2002a), the traditional taxonomy of McGregor (1968) is still widely used and, recently supported by chloroplast genome data (Zhang et al. 2017), recognizes nine species within the genus. Commercial Echinacea preparations contain one or as many as three different species: E. purpurea (L.) Moench, E. angustifolia DC., and less frequently, E. pallida (Nutt.) Nutt., with E. purpurea making up about 80% of commercial production. Other recognized taxa, E. laevigata (C. L. Boynton and Beadle) S. F. Blake, E. atrorubens Nutt., E. paradoxa Norton, E. sanguinea Nutt., E. simulata McGregor and E. tennesseensis (Beadle) J. K. Small, are far less abundant and rarely utilized compared to the commercial species (McKeown 1999). Whereas the commercial species have received extensive research attention, these other Echinacea taxa have received almost none.
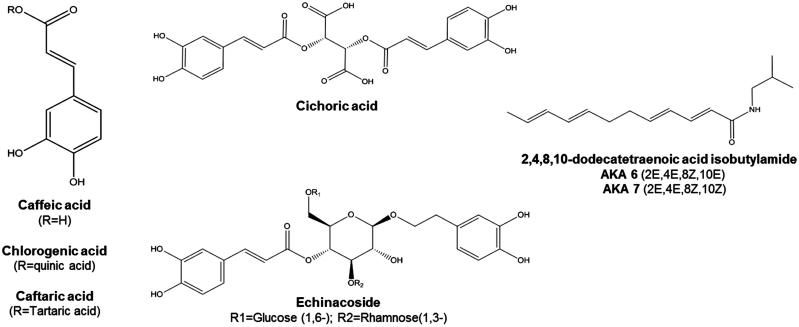
Currently popular as an immune stimulant, Echinacea species were used by North American Indigenous Peoples as a treatment for throat infections, wounds and pain, and was historically used in Eclectic medicine for septic conditions (Shemluck 1982). Related pharmacological activities and therapeutic uses continue to be explored, including anti-inflammatory, analgesic, anxiolytic and antimicrobial activities (Hostettmann 2003; Abbasi et al. 2007a; Haller et al. 2013; Cruz et al. 2014; Shin et al. 2014). The main bioactive compounds present in Echinacea extracts are the phenolics, alkylamides and polysaccharide/glycoproteins (Figure 1). The phenolics include echinacoside, cynarin, cichoric acid, caftaric acid and chlorogenic acids (CADs), and possess antimicrobial and antioxidant activity. The alkylamides are a group of more than 30 lipophilic compounds with anti-inflammatory properties mediated through activation of the endocannabinoid system, exhibit antifungal properties and inhibit cyclooxygenase and lipoygenase enzyme activities. Polysaccharides/glycoproteins include complex carbohydrate moieties such as arabinogalactans that act as immunostimulants. Barnes et al. (2005) give a thorough inventory of bioactive compounds isolated from Echinacea and new activities continue to be reported and reviewed (Cruz et al. 2014; Murthy et al. 2014; Manayi et al. 2015).
Figure 1.
Structures of Echinacea phytochemicals with established bioactivity. Caffeic acid derivatives are represented by caffeic acid, chlorogenic acid, caftaric acid (left) as well as cichoric and echinacoside (middle). Echinacea alkylamides are represented by the isomers of 2,4,8,10-dodecatetraenoic acid isobutylamide; a diversity of alkylamides in Echinacea are similarly isobutylamides with alkyl chains of variable length and saturation.
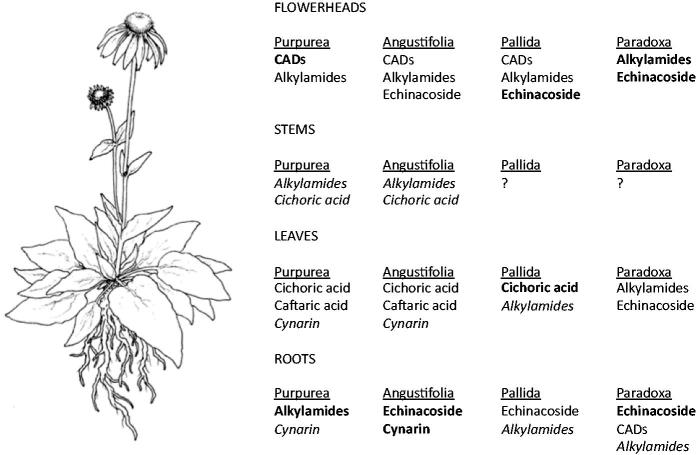
Differences in the composition and content of bioactive phytochemicals are inherent to Echinacea taxa (Figure 2). For example, E. purpurea roots completely lack echinacoside, a common constituent in the roots of other Echinacea species, but have very high levels of certain alkylamides, of which only trace amounts are found in the roots of other species (Sloley et al. 2001; Binns et al. 2002b; Murch et al. 2006). Typically, there can be significant variation in the phytochemistry of populations and/or individuals of the same species as well, particularly in E. angustifolia, for which there are established chemoraces (Kapteyn et al. 2002; Binns et al. 2002c; Liu et al. 2006; Chuang et al. 2010; Abbasi et al. 2012). Additionally, in all Echinacea species studied to date, the localization and content of active metabolites changes over time, both seasonally and with plant age, and varies between plant parts (Figure 2) (Choffe et al. 2000; Binns et al. 2002b). The most recent study on the localization of alkylamides in E. purpurea examined alkylamide content in a total of 36 tissues (Rizhsky et al. 2016), not including the seed. Particularly high concentrations of alkylamides were found in petals and disc flowers, and moderate concentrations were noted in receptacles of mature flower heads. Our group recently observed that the glands on the outer surface of Echinacea seeds (beneath the perianth) are also enriched in alkylamides (Parsons et al. 2018). E. purpurea and E. angustifolia roots and flower heads generally have the highest concentrations of bioactive compounds, whereas the leaves and stems have low concentrations of metabolites, and are rarely used in preparing NHPs (Kabganian et al. 2003; Qu et al. 2005; Chen et al. 2009). However, ascorbic acid (vitamin C) accumulates in the leaves, which could augment immune functions (Zagumennikov et al. 2015). This pattern of localization differs from E. paradoxa, where phytochemicals are concentrated more in the flower heads (Chen et al. 2009), and may differ in other rare species as well. This variation potentially provides a basis for selective breeding programs, selection of useful cultivars, and multiple product streams from different parts of the same plant.
Figure 2.
Localization of bioactive compounds in E. purpurea, E. angustifolia, E. pallida, and E. paradoxa. Compounds in bold are present at high concentrations; italics indicate compounds found in trace amount (Sloley et al. 2001; Binns et al. 2002b; Kabganian et al. 2003; Stuart and Wills 2003).
Despite the success of Echinacea on the market, challenges such as fungal pathogens, seed dormancy, low germination rates and a relatively long maturation time still pose problems for the industry, with the biggest challenge being product standardization (Liu et al. 2006; Zheng et al. 2006; Abbasi et al. 2007a; Kindscher et al. 2008; Maggini et al. 2012). Different commercial brands use diverse genotypes, plant parts and blends as well as different growing conditions, harvesting times and extraction methods, leading to qualitative and quantitative inconsistencies in bioactive compounds composition (Stuart and Wills 2003; Abbasi et al. 2007a; Jones et al. 2009). As industry faces these challenges, research has focused on finding ways to meet a strong demand for consistent, high-quality Echinacea products. In this article, we review the most promising developments and trends in Echinacea biotechnology, focusing on propagation, standardization and optimization of both the production process and the quality of plant material. We will examine advantages and disadvantages of the various technologies, and highlight future applications focusing on diversification and sustainability of the Echinacea industry.
Echinacea production in bioreactors
Traditionally, Echinacea has been propagated by seed, crown division or root cuttings but these processes are time-consuming, have limited propagation potential for producing large numbers of plants and can produce genetically variable plant material. Tissue culture methods – growing plants in vitro – have the potential to solve a number of the problems related to Echinacea propagation, including cultivation bottlenecks, the use of rare species and product standardization. Owing to the rapid development of tissue culture technology, a large – yet fragmented – body of research has accumulated on its application in Echinacea.
In vitro micropropagation techniques, such as adventitious root and shoot culture, and somatic embryogenesis, can produce hundreds of clonal plants from cuttings of a parent plant. Abbasi et al. (2007a) provide a thorough review of micropropagation techniques in Echinacea, all of which allow for more consistent secondary metabolite profiles associated with isogenic lines, year round cultivation and reduction of microbial contamination. Micropropagation can also be used to create plants having unique phytochemical profiles by culturing different parts of a parent plant. For example, shoots regenerated from E. angustifolia flower stalks have proportionately higher content of CADs compared to shoots generated from leaf explants (Lucchesini et al. 2009). Somatic embryogenesis by tissue culture, and to a lesser extent organogenesis, can induce genetic changes (Chuang et al. 2009) – a phenomenon called somaclonal variation. Micropropagation has been accomplished with E. purpurea, E. pallida, E. angustifolia and E. tennesseensis where, as expected, clonal plants have similar phytochemical profiles, showing only minor somaclonal variation (Abbasi et al. 2007a; Moraes et al. 2011; Butiuc-Keul et al. 2012 for additional studies). Although micropropagation provides a rapid way to generate plants, the process is still time consuming and labour intensive. These limitations are likely why, despite the popularity of herbal medicines, commercial production of Echinacea rarely employs cell culture techniques (Baque et al. 2012). In order to make tissue culture methods viable at industrial scales, bioreactors are considered an alternative culturing strategy. In addition to simpler technologies, one of the newer strategies is to use temporary immersion systems (TISs), where tissues are briefly bathed in nutrient medium then drained at specified intervals daily. Such bioreactor systems are modular and can mass produce clonal materials in the range of hundreds to tens of thousands of plants, making them suitable for use in genetic improvement programs.
The simplest bioreactors are used mainly for cell suspension cultures. However, plant cell cultures may not provide a complete phytochemical profile since some compounds are only produced in differentiated cells or following environmental cues. To deal with these limitations, several different bioreactor systems have been developed, including gas-phase, TISs, and hybrid bioreactors, all of which give cultures improved access to air and allow for the growth of differentiated organ cultures (Georgiev et al. 2014). For example, the recently developed “Plantform” bioreactor allowed E. purpurea tissue cultures to produce more adventitious shoots and a greater total biomass compared to cultures grown on solid media (Welander et al. 2014). These bioreactors use automated systems that provide a sterile environment, and produce plants that can be ready for harvest in just a few months. In an airlift bioreactor, both E. purpurea and E. angustifolia adventitious root cultures consistently produce up to 10 times the biomass of field grown plants after 5 weeks, and higher levels of active phytochemicals, including CADs, echinacoside and cynarin (Wu et al. 2007a; Jeong et al. 2009; Baque et al. 2012; Cui et al. 2013). Depending on the species used, co-culturing adventitious roots of different Echinacea species in balloon-type airlift bioreactors can also increase the production of biomass and bioactives, including the synthesis of metabolites absent from single-species cultures (Wu et al. 2017). Whether the increase in phytochemical content is due to stress on the plant cells in the in vitro environment, the availability of excess nutrients in the medium, or to other aspects of the culture environment, remains unclear.
Among the most important limitations of bioreactors is their capacity for scale up and cost. Biomass production often decreases at larger scales, so strategies such as medium replenishment are employed to improve biomass production and phytochemical content in cultured roots (Wu et al. 2007b). Although culture vessels of up to 75,000 L have been used for suspension culture (Ruffoni et al. 2010), scale-up tests have yet to be carried out with new TIS bioreactors. To date, E. purpurea and E. angustifolia adventitious roots have been cultured in balloon-type bubble bioreactors of up to 500 L and in drum-type bioreactors of up to 1000 L without noticeable adverse effects on growth (Wu et al. 2007a; Ruffoni et al. 2010). Nonetheless, commercial use of bioreactors remains costly and is limited to production of cosmetics and high-value pharmaceuticals such as paclitaxel. Although not yet routinely used in the Echinacea industry, bioreactors are becoming the standard when performing tissue culture at an experimental scale. Bioreactors offer a means of producing standardized plant material at a scale unmatched by field production, and the contained nature of bioreactors allows for the use of specialized media, conditions, elicitors, growth enhancers and year-round production. With a viable and consistent propagation method in hand, the focus now shifts to improving production efficiency, economy and the quality of plant material, in terms of both biomass and phytochemical content.
Genetic improvement
Conventional selective breeding techniques have traditionally led to the gradual improvement of many plant species. While industry will undoubtedly continue to develop “improved” varieties, published Echinacea breeding studies (and patents) have focused primarily on ornamentals (Ault 2002; Korlipara 2008) and reducing seed dormancy (Qu and Widrlechner 2012). Traditional selective breeding of Echinacea can make use of the existing genetic and phenotypic variation in commercial and wild collected plants and is widely accepted by the public, including within the organic farming industry. Conversely, direct alteration of the genome of a plant through molecular genetic techniques is the most precise way to modify developmental and biosynthetic processes. Whereas public concerns will likely continue to impede the use of Genetically Modified Organisms (GMOs), several potentially “organically acceptable” biotechnological approaches have been developed to modify Echinacea, including transformation with Agrobacterium and the induction of polyploids.
Hairy root culture utilizes the natural ability of the soil bacterium Rhizobium rhizogenes (formerly Agrobacterium rhizogenes) to infect and transform plant tissue. The bacterial Ri plasmid is transferred into the plant genome causing neoplastic outgrowths, but incorporation of a set of genes, rolA, rolB and rolC, causes roots to grow from the infected site instead of an undifferentiated cell mass (Nilsson and Olsson 1997; Pistelli et al. 2010). Hairy root cultures have several properties that are useful for research and industry, including accelerated growth, spontaneous regeneration of shoots, as well as chemical and morphological similarity to the roots of a wild-type plant (Tepfer 1990; Guillon et al. 2006). Hairy root cultures of all three commercially important Echinacea species produce high levels of secondary metabolites, including polysaccharides, alkylamides, CADs and other phenolics (Trypsteen et al. 1991; Liu et al. 2006; Wang et al. 2006; Romero et al. 2009; Pistelli et al. 2010). Transformed roots are genetically stable, and maintain a constant production of metabolites over a long period of time (Wu et al. 2006). The rapid growth of hairy root cultures on hormone-free media makes them an excellent way to generate biomass quickly, or to clonally propagate plants.
The discovery of R. rhizogenes-based hairy root transformation systems in higher plants provides other opportunities to engineer useful traits in Echinacea. Again, public acceptance of GMOs may limit the application of this useful technology. As an example, glufosinate-resistance and a fungal resistance chitinase gene were simultaneously transferred into E. purpurea using R. tumefaciens (Hanafy et al. 2010). Considering Echinacea plants in the field are particularly susceptible to weed competition and fungal pathogens, this study represents a useful demonstration model. Several factors are noted to influence Rhizabium-based transformation efficiency of Echinacea, and there is room for optimization. For example, the efficacy of the utilized bacterial strain is important; A4 strains were superior for transforming Echinacea leaf explants, whereas R1000 strains worked best with petioles (Wang et al. 2006). Overall, early development stages, such as cotyledon tissue, are more easily transformed and sonication is up to twice as effective for producing transformants compared to the traditional methods of wounding with a sterile needle to enhance R. rhizogenes-mediated gene transfer (Kumar et al. 2006). Addition of inducers to the medium during co-cultivation of agrobacterium with the plant tissue also improves efficacy. For example, indole-3-butyric acid (IBA) increases production of hairy roots in Echinacea by as much as 14 times (Romero et al. 2009). Other inducers of Rhizobium-associated gene transfer in plants (e.g., 6-benzylaminopurine, 2,4-dichlorophenoxyacetic acid) have been applied to Echinacea hairy root cultures to improve transformation but their effectiveness relative to no treatment has not been investigated empirically (Trypsteen et al. 1991; Wang et al. 2006).
Likewise, the manipulation of ploidy can cause changes to morphology and phytochemical content of plants. Naturally occurring polyploids are commonly used in agriculture and widely accepted by the public. Triploid, tetraploid and hexaploid Echinacea plants have been developed, with tetraploids (4× = 44) being the best studied. In comparison to wild-type diploids, the tetraploids studied have altered leaf, root, and flower morphology, reduced seed set and dwarfed phenotypes (Nilanthi et al. 2009; Koul et al. 2010; Abdoli et al. 2013; Xu et al. 2014; Chen et al. 2016). Tetraploid plants have similar phytochemical profiles to wild types, but they consistently yield higher levels of CADs, particularly in the leaves. Increased alkylamide content in the leaves and roots of tetraploids has also been noted (Koul et al. 2010; Abdoli et al. 2013; Xu et al. 2014). The reduced biomass production due to polyploid dwarf phenotypes currently makes ploidy manipulation an impractical way to improve the quality of Echinacea plant material. However, supplementing culture media with 0.3 mg/L IBA can accelerate the emergence of roots and the increased rooting rate of tetraploid shoots in vitro whereas IBA has no positive effect on cultured haploid or diploid shoots (Chen et al. 2016).
Elicitation of secondary metabolites to produce high quality plant material
Bioreactors and micropropagation techniques allow a large number of plants to be produced in a short period of time but developing biomass with a higher concentration of bioactive compounds ultimately makes for a more efficient industrial process. Several methods to enhance the production of secondary metabolites, other than genetic engineering, are currently being studied. In particular, the use of elicitors (e.g., plant hormones, stress signaling molecules and both biotic and abiotic compounds, as well as physical injury) show promise in stimulating the production of bioactive secondary metabolites (reviewed in Abbasi et al. 2007a and updated in Table 1). In general, the basis of elicitation is the activation of a plant’s defence response, which up-regulates the production of many bioactive compounds of commercial and industrial value. Elicitation involves either the direct addition of signal compounds implicated in the stress response or applying compounds that cause tissues to produce stress signals endogenously. For example, nitric oxide (NO) is an important signalling molecule in the plant defence response. In adventitious root cultures of E. purpurea, adding sodium nitroprusside (an exogenous NO producer) to the growth medium increased the accumulation of flavonoids and CADs (Wu et al. 2007c). Natural stress mediators can also be applied as a foliar spray to a mature plant. Salicylic acid elicits a twofold increase in cichoric and caftaric acid in E. purpurea flower heads, and an almost fourfold increase of CAD in the roots when applied as a foliar spray to field-grown plants (Kuzel et al. 2009). Elicitors such as yeast extract stimulate the production of phenolics in Echinacea, presumably by mimicking a pathogenic fungal infection (Li and Barz 2006). From Table 1, it should be noted that the majority of recent elicitor research has been carried out with E. purpurea (15/19 studies), E. angustifolia (4/19) and E. pallida (2/19). Comprehensive screening studies that include other taxa are warranted for biotechnology potential since it is clear that most of the studies report increased metabolite production following administration of elicitors. In particular, CADs, but also phenolics and flavonoids appear to be most responsive to elicitor induction, likely through phenylalanine ammonia lyase (PAL) up-regulation and defence response. Relatively few studies state whether or not other metabolites such as echinacoside, alkylamides or polysaccharides are similarly increased by application of elicitors, so the full potential of inducing secondary metabolite production remains unknown.
Table 1.
Review of elicitor compounds tested in Echinacea to enhance secondary metabolite content.
| Type | Elicitor | Concentration used | Variety | Effects | Reference |
|---|---|---|---|---|---|
| Abiotic | Titanium (IV) ascorbate | Foliar spray – 10 μM | PPA | Increased caftaric, chlorogenic acid in roots, and increased root mass | Kuzel et al. 2009 |
| Biotic | Yeast extract elicitor | 1mg/mL | PPA | Increased production of phenolics, including novel phenolics | Li and Barz 2006 |
| Growth Regulator | Dimethyl amino hexanoate (with 0.3 mg/L IBA) | 0.08 mg/L for petioles, 0.16 mg/L for leaves, 0.01 mg/L for roots | PPA | Enhanced shoot regeneration, increased plant growth and final biomass | Chen et al. 2013 |
| Gibberellic acid | 0.025 μM | PPA | Thicker roots, increased overall biomass, increased levels of anthocyanins and cichoric acid | Abbasi et al. 2012 | |
| 150 μM | PPA | Increase in caftaric acid in early culture stage | Jones et al. 2009 | ||
| Indole-3-butyric acid (IBA) | 1 mg/mL | PLL | Induces root regeneration, increases CAD content | Wu et al. 2012 | |
| 2 mg/L | ANG | Increased final weight of root cultures, increased total phenolics and flavanoids | Wu et al. 2006 | ||
| 15 μM | PPA | increased levels of cichoric and caftaric acid, and detectable levels of echinacoside | Murch et al. 2006 | ||
| Herbicide | Glyphosate treatment + l-tryptophan feeding | 0.5 mM glyphosate, 0.5 mM l-tryptophan | PPA | Higher levels of CADs, phenols, and flavanoids | Mobin et al. 2015 |
| Palcobutrazole + Gibberellic acid | 100 μM Palcobutrazol, 50 μM GA | PPA | Increased caftaric and cichoric acid in roots, slight drop in shoots | Jones et al. 2009 | |
| Stress Response Molecule | Acetylsalicylic acid | Foliar spray – 10 μM | PPA | Increased caftaric acid in tops | Kuzel et al. 2009 |
| Jasmonic acid | 10–40 μM | PPA, ANG, PLL | Several fold increase in alkylamides, but decreased root growth | Romero et al. 2009 | |
| Methyl Jasmonate | 5 μM | PPA | Increased production of phenolics, including novel phenolics | Li and Barz 2006 | |
| Methyl Jasmonate, Methyl salicylate | 200 μM | ANG | Increase of of echinacoside and cichoric acid | Baque et al. 2012 | |
| 100 μM | ANG | Increased polyphenolics, flavanoids, and CADs in root culture | Cui et al. 2013 | ||
| Foliar spray – 10 μM | PPA | Increased caftaric acid in tops, cichoric and chlorogenic acid in roots, and mass of roots | Kuzel et al. 2009 | ||
| Salycilic acid | Foliar spray – 10 μM for tops, 1000 μM for roots | PPA | Increased cichoric and caftaric acid in tops, and cichoric acid, and mass in roots | Kuzel et al. 2009 | |
| Sodium nitroprusside | 100 μM | PPA | Increased phenolics, flavanoids, CADS, and antioxidant potential | Wu et al. 2007c |
E. purpurea: PPA; E. angustifolia: ANG; E. pallida: PLL.
Despite these impressive effects on phytochemical content, elicitors can also have an inhibitory effect on growth (Baque et al. 2012). As such, a two-phased approach – adding elicitors only after the cell culture or plant has had time to grow – may be necessary in order to optimize phytochemical production. For example, by adding methyl jasmonate to the medium on day 28, Cui et al. (2013) increased the echinacoside content in root cultures of E. angustifolia threefold without reducing the biomass. The use of chemical elicitors is particularly important in industrial-scale bioreactors since the production of both biomass and phytochemicals often decreases at large scales (Wu et al. 2007b). Additional tests on the application of elicitors to field grown plants would also be beneficial, since effects may differ under the diversity of field conditions.
Commercial use of elicitors needs to balance secondary product yields against cost and the potential residual toxicity of an elicitor remaining in the harvested tissue. In addition, since Echinacea is sold and regulated as a NHP or supplement (in North America), and since there is a consumer preference for naturally grown products, consideration should be given to the type of elicitor used; growth hormones and stress mediators may be preferred over abiotic elicitors and herbicides.
Other approaches to optimizing secondary metabolite production
In addition to genetic transformation and elicitor treatments, a number of other factors can affect the production of secondary metabolites in Echinacea culture. Aspects of the growing environment, such as growth medium and light regime, as well as abiotic treatments like ultrasound or elevated UV A/B exposure that cause physical cellular damage, have been considered for the optimization of Echinacea products. These alternative elicitation methods have the advantage of leaving no residues but have only been tested in E. purpurea and need to be evaluated in other species for their commercialization potential.
Growth medium is the basis of tissue culture, and numerous studies have assessed what combination of nutrients produce the best growing environment for Echinacea cultures. Depending on the Echinacea species and tissue being cultured, the optimal medium may differ. For instance, Wu et al. (2007a), found that the maximum biomass of adventitious roots of E. purpurea could be obtained on one-quarter strength Murashige Skoog (MS) medium with 50 g/L sucrose and 1 mg/L IBA, but in earlier work with root cultures in E. angustifolia, half strength MS produced roots with more biomass and a higher content of phenolic compounds than one-quarter strength MS (Wu et al. 2006). Focusing on alkylamide production in hairy roots, Romero et al. (2009) reported that half-strength Gamborg’s B5 medium was best for maintaining hairy root production and that the addition of IBA increased growth rate by 14-fold with no impact on alkylamide production, which was further elevated in the presence of the elicitor, jasmonic acid.
Ultrasound treatment is a recently developed method of increasing plant secondary metabolite content, and as such, has not been extensively tested with Echinacea. Two studies with E. purpurea hairy roots grown in bioreactors found that one 6 minute ultrasound treatment at 40 kHz between days 15 and 20 of culture significantly increased both fresh weight and cichoric acid content over 30 days (Abbasi et al. 2009; Liu et al. 2012). Although the exact mechanism is unknown, the effects of ultrasound appear linked to an increase in PAL activity. Both studies noted an increase in cichoric acid, with one also reporting significant increases in anthocyanins and lignins (Abbasi et al. 2009), the biosynthesis of which are all linked to PAL enzyme activity. Liu et al. (2012) observed enhanced CAD biosynthesis related to increases in both rolB-regulated auxin biosynthesis and PAL activity. Accordingly, ultrasound treatment may be less effective at eliciting secondary metabolism using culturing techniques other than hairy roots. Altered secondary metabolism may also result from ultrasound-induced physical damage to the cells, causing a general stress response. Indeed, Liu et al. (2012) noted that stimulating hairy roots with ultrasound caused a significant decrease in biomass accumulation, likely indicating cell damage or stress. This method of elicitation is very simple, requires no chemical input, and leaves no residues, and therefore merits study with other forms of tissue culture as well as with other species of Echinacea.
Light is essential for the growth of plants and for the regeneration of shoots in culture. While callus, root and suspension cultures are generally maintained in the dark, exposing these cultures to light can have beneficial effects on their phytochemical content. Continuous light for 14 days significantly increased CAD levels in cell suspensions of E. angustifolia (Guarnerio et al. 2012). Similarly, hairy root cultures of E. purpurea incubated under continuous light showed not only an increase in CADs but also thicker roots that developed a purple colour, indicating the production of anthocyanins. Increased CAD and anthocyanin production have been linked to the activation of PAL enzymes, although the mechanism of PAL activation by light is unknown (Abbasi et al. 2007b). Enhanced production of CADs and anthocyanins was also observed by Abbasi et al. (2012) with the application of gibberellic acid to E. purpurea hairy roots. If light treatment can produce effects comparable to certain elicitors, then light-induced effects on secondary metabolite production warrants further investigation. In particular, it would be interesting to test different wavelengths, intensities and light/dark regimes to determine if the same or greater effects can be achieved without continuous bright light.
As has been demonstrated with other species, an extension of abiotic light treatment is the recently reported application of elevated ultraviolet light during Echinacea callus and cell culture to alter secondary compound formation. Manaf et al. (2016) tested the effects of UV-B radiation on E. purpurea cultures for short periods in varying exposures. The effects were variable, depending on the dose–time response. All UV-B treatments increased caffeic acid and antioxidant activity of callus cells and growth parameters, total phenols content and antioxidant activity of cell suspensions in a dose-dependent manner. The same group also tested both types of Echinacea cultures with varying doses of UV-C with similar results (Abd El-Aal et al. 2016).
Endophytes, microbial species that colonize plants without causing disease symptoms, are associated with almost all plants on Earth. Endophytes can be isolated from all parts of field-grown Echinacea, including the seeds, leaves, stems and roots. The most common fungal genera in the roots of Echinacea include Glomus, Cladosporium, Alternaria and Fusarium (Lata et al. 2006; Araim et al. 2009; Zubek and Błaszkowski 2009; Rosa et al. 2012; Moszczyńska et al. 2013). Endophytes form symbiotic relationships with the plant, using photosynthesized sugars for nutrition, in turn helping the plant to uptake nutrients (particularly nitrogen), and defend against herbivores and pathogenic microbes (Arnold and Lutzoni 2007; Aly et al. 2011). Plants colonized with endophytic fungi are less often infected by pathogens, show increased growth rates, and have improved stress tolerance (Saikkonen et al. 1998; Lata et al. 2006; Araim et al. 2009; Zubek and Błaszkowski 2009; Gualandi et al. 2014). Notably, colonization with arbuscular mycorrhizal fungi impacts phytochemical content, increasing CADs in the roots of E. purpurea (Araim et al. 2009). The mechanism of this effect in Echinacea is unknown, and could potentially be attributed to increased uptake of nutrients, elicitation of the plant’s defense response (including PAL up-regulation), or production of bioactive compounds by the endophytes, among other explanations. Nevertheless, manipulation of field grown plants to encourage specific endophytes may increase yields of secondary metabolites.
Future directions in Echinacea research and industry
The development of new biotechnologies provides many options for improving Echinacea NHPs and other products. Moving forward, the benefits and drawbacks of each of these approaches should be considered in terms of improvements in yield, optimization and standardization of phytochemistry, propagation efficiency, cost, public perception and ease of use at scale.
As previously noted, bioreactors allow for the rapid growth of cultures and produce cloned propagules, ensuring consistent phytochemical content. In vitro culturing reduces contamination by plant pathogens and other microbes and is less labour intensive than field cultivation. However, individual plant tissues may not produce a full range of phytochemicals, and biomass production may be limited by culture techniques and equipment. Despite reduced labour, the cost of bioreactor culture is high, as it requires specialized materials, facilities and personnel training.
Genetic engineering has the potential to improve plant material in several ways but has multiple drawbacks making it currently impractical for use in the Echinacea industry. Genetic transformation may not improve propagation efficiency, does not necessarily increase yield (except hairy roots), and does not guarantee more standardized plant material. Most importantly, the NHP market may not readily accept the use of GMOs. Despite the fact that ploidy variation and hairy root disease occur naturally, market research should precede employment of such technologies. Genetic transformation is useful to study the growth and biochemistry of plants but, since elicitors and selective breeding can produce similar improvements in yield and quality, genetic engineering may not be the best option for industry.
Elicitors are easy to use, do not change propagation efficiency, and can be applied to either organ cultures or field-grown plants. Elicitors effectively improve phytochemical content and some, such as IBA, can increase yield. Other optimization techniques such as ultrasound, UV and ozone (abiotic elicitors) can similarly produce increases in phytochemical content in tissue culture. Administering ultrasound is inexpensive, simple to use, and will not result in toxic residues, but improper use may lead to decreases in yield and ultrasound technologies have not yet been adapted for use with field cultivation.
In general, bioreactors may have advantages for propagating cultivars through rapid production of cloned propagules. However, a focus on the growth of whole plants for the production of herbal medicines seems the most beneficial overall currently. Whole plants are technically less complicated to maintain and can produce more complete phytochemical profiles. Even though the price of tissue culture has come down, it is still not feasible to grow full plants to maturity at industrial scale using bioreactors. Therefore, it may be best to use tissue culture as a method of propagation followed by growing cloned plantlets in more traditional field, greenhouse or hydroponic systems. Chemical elicitors are most effective for increasing phytochemical content, and can be included in the production process, along with control of light regimes during indoor production. Such models would take advantage of both the benefits of tissue culture and the existing cultivation space.
Growing entire plants would also be worthwhile since each part of the plant has unique properties that can be used for different kinds of products. Echinacea supplements on the market today are most often an extract of the roots, flower heads or both, with the leaves of E. pallida occasionally included. Finding uses for the remaining plant parts and developing alternate applications of Echinacea is the logical next step for research and industry. There is very little information available on the phytochemical and medicinal properties of rare Echinacea species, however in vitro culturing technology may now allow for the growth and study of these species without disturbing natural populations. Non-commercial Echinacea species may yield compounds that are not present in E. purpurea, E. angustifolia or E. pallida (Binns et al. 2002b)
Emerging market opportunities for Echinacea include additives in animal feed. Studies on chickens, pigs, rainbow trout and horses have consistently found that Echinacea feed additives improve immune activity, including increases in lymphocytes, phagocytosis and globulin content (Williams and Lamprecht 2008; Böhmer et al. 2009; Grashorn 2010; Dehkordi et al. 2011; Oskoii et al. 2012). The addition of Echinacea to feed or water also improved the efficacy of vaccines for fowl influenza, swine erysipelas and Newcastle disease virus in fowl, increasing the antibody titers in livestock (Maass et al. 2005; Böhmer et al. 2009; Dehkordi et al. 2011; Najafzadeh et al. 2011). Reducing the incidence of infection in livestock improves growth rate and decreases the chances that pathogens will be transferred between animals or to humans. Restrictions on the addition of synthetic antibiotics to animal feed in several countries may soon expand the market for herbal medicines in the livestock industry. The only negative reports to date on Echinacea feed additives were minor allergic reactions in horses (Williams and Lamprecht 2008). Addition of E. purpurea to feed did not significantly alter growth characteristics of quail (Sahin et al. 2012). Nevertheless, residual Echinacea bi-products from human health applications should be explored as value-added animal feed supplements, including uses for leaves, stems and seed cobs. More research is needed in order to determine the most effective form, delivery method, and dosage for employing Echinacea as a commercial feed additive.
Echinacea seed also may have additional market potential. Only a fraction of the seed produced by Echinacea is required for traditional plant propagation. Expanded use of tissue culture also means that additional seed could be harvested solely for use in NHPs. Indeed, use of seeds may add value to Echinacea crops since seeds are generally not incorporated into commercial products. Seed oils from all three commercial Echinacea species are very nutritious, being high in oleic acid, palmitic acid, linoleic acid, vitamin E (28–85 mg/100 g oil) and other bioactive compounds (Oomah et al. 2006; Vandyshev et al. 2009; Parsons et al. 2018). Seed oil yields range from 13 to 23%, depending on species and seed size, with E. purpurea seeds generating the greatest volume and highest quality of oil. The seeds of E. purpurea and E. angustifolia contain 0.75 and 1.06 mg of bioactive alkylamides per gram, respectively (He et al. 1998). Oils from other members of the Asteraceae family, such as sunflower oil, are commonly used for both dietary and industrial purposes.
Echinacea essential oil also contains a number of medicinal compounds including germacrene-D, a sesquiterpine hydrocarbon with antimicrobial properties, and alkylamides, which can be detected by the tingling sensation caused on the tongue when the oil is tasted (Mirjalili et al. 2006; Oomah et al. 2006). E. purpurea essential oil significantly reduced inflammatory swelling in mice and rats, and decreased the levels of cytokines IL-2, IL-6 and TNF-α in the blood (Yu et al. 2013). These effects are also characteristic after administering alkylamides in animal models. With further research and the development of standard extraction procedures, Echinacea seed oil and essential oils also have the potential to become successful product lines within the Echinacea industry.
Echinacea is almost exclusively sold as an immune-boosting supplement in the contemporary US market. However, the development of micropropagation, bioreactors and elicitors make it possible to take advantage of other bioactivities. Extensive study demonstrates that Echinacea extracts have antibacterial, antiviral and antifungal activities (Merali et al. 2003; Sharma et al. 2008; Mir-Rashed et al. 2010; Hudson 2012). Echinacea alkylamides were shown to have an interesting mode of action through perturbing the fungal cell wall/membrane complex – an ideal antifungal target that is unique to fungi (Cruz et al. 2014). Of those tested, one structural class of alkylamides, the diynoic alkylamides, showed the greatest antifungal and cell wall disruption activities. The natural variability in the phytochemical profiles of Echinacea plants, combined with high-throughput screening and use of elicitors make it possible to quickly select and propagate various cultivars with unique metabolic profiles, tailored to specific functions such as production of antifungals. Alternatively, Echinacea varieties could be selected for large seeds with nutritious seed oil, or modified to produce more CAD in the flowers, which could be used as a dietary supplement. The roots and flowers of cultivars rich in alkylamides and cichoric acid could be used to make antibacterial face washes, shampoos or creams. Like other members of the Asteraceae family, Echinacea contains some polyacetylene compounds that are phototoxic, but these are unstable and could be inactivated by minimal processing (Chen et al. 2010; Chen et al. 2013). Echinacea leaves, high in vitamin C and phenolic metabolites, could also be marketed as a NHP. This potential diversity of uses has yet to be harnessed by the industry.
Moving forward, a combination of tissue culture, chemical treatments and traditional field cultivation will likely be used to generate a new higher standard of production and phytochemical quality. These advances will provide the opportunity to establish a greater variety of Echinacea products to keep up with ever-expanding market opportunities.
Funding Statement
This research was supported by Natural Science and Engineering Council of Canada Discovery Grants to CSH and MLS.
Acknowledgements
We thank Dr. JT Arnason for his editorial assistance and insight.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbasi B, Saxena PK, Murch SJ, Liu C. 2007. Echinacea biotechnology: challenges and opportunities. In Vitro Cell Dev Biol Plant. 43:481–492. [Google Scholar]
- Abbasi BH, Tian C, Murch SJ, Saxena P, Liu C. 2007. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 26:1367–1372. [DOI] [PubMed] [Google Scholar]
- Abbasi BH, Liu R, Saxena PK, Liu C-Z. 2009. Cichoric acid production from hairy root cultures of Echinacea purpurea grown in a modified airlift bioreactor. J Chem Technol Biotechnol. 84:1697–1701. [Google Scholar]
- Abbasi BH, Stiles AR, Saxena PK, Liu C. 2012. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl Biochem Biotechnol. 168:2057–2066. [DOI] [PubMed] [Google Scholar]
- Abd El-Aal MS, Rabie KAE, Manaf HH. 2016. The effect of UV-C on secondary metabolites production of Echinacea purpurea culture in vitro. J Biol Chem Environ Sci. 11:465–483. [Google Scholar]
- Abdoli M, Moieni A, Badi HN. 2013. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant. 35:2075–2083. [Google Scholar]
- Aly AH, Debbab A, Proksch P. 2011. Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol. 90:1829–1845. [DOI] [PubMed] [Google Scholar]
- Araim G, Saleem A, Arnason JT, Charest C. 2009. Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J Agric Food Chem. 57:2255–2258. [DOI] [PubMed] [Google Scholar]
- Arnold AE, Lutzoni F. 2007. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 88:541–549. [DOI] [PubMed] [Google Scholar]
- Ault J. 2002. Breeding and development of new ornamental plants from North American native taxa. Acta Hortic. 624:37–42. [Google Scholar]
- Baque A, Moh S-H, Lee E-J, Zhong J-J, Paek K-Y. 2012. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using a bioreactor. Biotechnol Adv. 30:1255–1267. [DOI] [PubMed] [Google Scholar]
- Barnes J, Anderson LA, Gibbons S, Phillipson JD. 2005. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 57:929–954. [DOI] [PubMed] [Google Scholar]
- Binns SE, Baum BR, Arnason JT. 2002a. A taxonomic revision of Echinacea (Asteraceae: Heliantheae). Syst Bot. 27:610–632. [Google Scholar]
- Binns SE, Livesey JF, Arnason JT, Baum BR. 2002. Phytochemical variation in Echinacea from roots and flowerheads of wild and cultivated populations. J Agric Food Chem. 50:3673–3687. [DOI] [PubMed] [Google Scholar]
- Binns SE, Arnason JT, Baum BR. 2002. Phytochemical variation within populations of Echinacea angustifolia (Asteraceae). Biochem Syst Ecol. 30:837–854. [Google Scholar]
- Blumenthal M, Hall T, Goldberg A, Kunz T, Dinda K. 2003. The ABC clinical guide to herbs. Austin (TX): American Botanical Council. [Google Scholar]
- Böhmer BM, Salisch H, Paulicks BR, Roth FX. 2009. Echinacea purpurea as a potential immunostimulatory feed additive in laying hens and fattening pigs by intermittent application. Livest Sci. 122:81–85. [Google Scholar]
- Butiuc-Keul A-L, Vlase L, Crăciunaş C. 2012. Clonal propagation of cichoric acid in three species of Echinacea. In Vitro Cell Dev Biol Plant. 48:249–258. [Google Scholar]
- Chen CL, Zhang SC, Sung JM. 2009. Caffeoyl phenols and alkamides of cultivated Echinacea purpurea and Echinacea atrorubens var. paradoxa. Pharm Biol. 47:835–840. [Google Scholar]
- Chen LY, Hu A, Chang CJ. 2013. The degradation mechanism of toxic atractyloside in herbal medicines by decoction. Molecules. 18:2018–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yang Y, Wu H. 2016. A comparative study on rooting of in vitro regenerated shoots in haploid, diploid and tetraploid purple coneflower (Echinacea purpurea L.). Biotechnol Biotechnol Equip. 30:44–48. [Google Scholar]
- Chen XL, Zhang JJ, Chen R, Li QL, Yang YS, Wu H. 2013. An uncommon plant growth regulator, Diethyl aminoethyl hexanoate, is highly effective in tissue cultures of the important medicinal plant purple coneflower (Echinacea purpurea L.). BioMed Res Int. 2013:540316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-M, Hu C, Raghubeer E, Kitts DD. 2010. Effect of high pressure pasteurization on bacterial load and bioactivity of Echinacea purpurea. J Food Sci. 75:C613–C618. [DOI] [PubMed] [Google Scholar]
- Choffe KL, Murch SJ, Saxena PK. 2000. Regeneration of Echinacea purpurea: induction of root organogenesis from hypocotyl and cotyledon explants. Plant Cell Tissue Organ Cult. 62:227–234. [Google Scholar]
- Chuang SJ, Chen CL, Chen JJ, Chou WY, Sung JM. 2009. Detection of somaclonal variation in micro-propagated Echinacea purpurea using AFLP marker. Sci Hort. 120:121–126. [Google Scholar]
- Chuang SJ, Chen CL, Chen JJ, Sung JN. 2010. Using bulk AFLP analysis to assess genetic diversity in Echinacea species. Sci Hort. 124:400–404. [Google Scholar]
- Cruz I, Cheetham JJ, Arnason JT, Yack JE, Smith ML. 2014. Alkamides from Echinacea disrupt the fungal cell wall-membrane complex. Phytomedicine. 21:435–442. [DOI] [PubMed] [Google Scholar]
- Cui H-Y, Baque A, Lee E-J, Paek K-Y. 2013. Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep. 7:297–308. [Google Scholar]
- Dehkordi SH, Fallah V, Dehkordi SH. 2011. Enhancement of broiler performance and immune response by Echinacea purpurea supplemented in diet. Afr J Biotechnol. 10:11280–11286. [Google Scholar]
- Georgiev V, Schumann A, Pavlov A, Bley T. 2014. Temporary immersion systems in plant biotechnology. Eng Life Sci. 14:607–621. [Google Scholar]
- Grashorn M. 2010. Use of phytobiotics in broiler nutrition–an alternative to infeed antibiotics. J Anim Feed Sci. 19:338–347. [Google Scholar]
- Guarnerio CF, Fraccaroli M, Gonzo I, Pressi G, Dal Toso R, Guzzo F, Levi M. 2012. Metabolomic analysis reveals that the accumulation of specific secondary metabolites in Echinacea angustifolia cells cultured in vitro can be controlled by light. Plant Cell Rep. 31:361–367. [DOI] [PubMed] [Google Scholar]
- Gualandi RJ Jr Augé RM, Kopsell DA, Ownley BH, Chen F, Toler HD, Dee MM, Gwinn KD. 2014. Fungal mutualists enhance growth and phytochemical content in Echinacea purpurea. Symbiosis. 63:111–121. [Google Scholar]
- Guillon S, Tremouillaux-Guiller J, Pati PK, Rideau M, Gantet P. 2006. Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol. 24:403–409. [DOI] [PubMed] [Google Scholar]
- Haller J, Freund TF, Pelczer KG, Füredi J, Krecsak L, Zámbori J. 2013. The anxiolytic potential and psychotropic side effects of an Echinacea preparation in laboratory animals and healthy volunteers. Phytother Res. 27:54–61. [DOI] [PubMed] [Google Scholar]
- Hanafy M, Aly UI, Matter MA. 2010. Regeneration and transformation via Agrobacterium tumefaciens of Echinacea purpurea L. Plant Tissue Cult Biotechnol. 20:101–111. [Google Scholar]
- He XG, Lin LZ, Bernart MW, Lian LZ. 1998. Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography–electrospray mass spectrometry. J Chromatogr A. 815:205–211. [Google Scholar]
- Hostettmann K. 2003. History of a plant: the example of Echinacea. Forsch Komplementarmed Klass Naturheilkd. 10:9–12. [DOI] [PubMed] [Google Scholar]
- Hudson JB. 2012. Applications of the phytomedicine Echinacea purpurea (purple coneflower) in infectious diseases. BioMed Res Int. 2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JA, Wu CH, Murthy HN, Hahn EJ, Paek KY. 2009. Application of an airlift bioreactor system for the production of adventitious root biomass and caffeic acid derivatives of Echinacea purpurea. Biotechnol Bioprocess Eng. 14:91–98. [Google Scholar]
- Jones AMP, Saxena PK, Murch SJ. 2009. Elicitation of secondary metabolism in Echinacea purpurea L. by gibberellic acid and triazoles. Eng Life Sci. 9:205–210. [Google Scholar]
- Kabganian R, Carrier DJ, Rose PA, Abrams SR, Sokhansanj S. 2003. Localization of alkamides, echinacoside and cynarin with Echinacea angustifolia. J Herbs Spices Med Plants. 10:73–81. [Google Scholar]
- Kapteyn J, Goldsbrough PB, Simon JE. 2002. Genetic relationships and diversity of commercially relevant Echinacea species. Theorl Appl Genet. 105:369–376. [DOI] [PubMed] [Google Scholar]
- Kindscher K, Price DM, Castle L. 2008. Resprouting of Echinacea angustifolia augments sustainability of wild medicinal plant populations. Econ Bot. 62:139–147. [Google Scholar]
- Korlipara H, inventor; Terra Nova Nurseries, Inc., assignee. 2008November 04. Echinacea plant named ‘Tomato Soup’. U.S. Patent No. PP19427. Washington (DC): U.S. Patent and Trademark Office. [Google Scholar]
- Koul S, Sambyal M, Kitchlu SK, Bakshi SK, Kaul MK. 2010. Development, micropropagation and characterization of colchiploid of Echinacea purpurea (L.) Moench. Indian J Biotech. 9:221–224. [Google Scholar]
- Kumar V, Sharma A, Narasimha Prasad BC, Bhaskar Gururaj H, Aswathanarayana Ravishankar G. 2006. Agrobacterium rhizogenes mediated genetic transformation resulting in hairy root formation is enhanced by ultrasonication and acetosyringone treatment. Electron J Biotechnol. 9:349–357. [Google Scholar]
- Kuzel S, Vydra J, Triska J, Vrchotova N, Hruby M, Cigler P. 2009. Elicitation of pharmacologically active substances in an intact medical plant. J Agric Food Chem. 57:7907–7911. [DOI] [PubMed] [Google Scholar]
- Lata H, Li XC, Silva B, Moraes RM, Halda-Alija L. 2006. Identification of IAA-producing endophytic bacteria from micropropagated Echinacea plants using 16S rRNA sequencing. Plant Cell Tissue Organ Cult. 85:353–359. [Google Scholar]
- Li WW, Barz W. 2006. Structure and accumulation of phenolics in elicited Echinacea purpurea cell cultures. Planta Med. 72:248–254. [DOI] [PubMed] [Google Scholar]
- Liu C, Abbasi BH, Gao M, Murch S, Saxena P. 2006. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J Agric Food Chem. 54:8456–8460. [DOI] [PubMed] [Google Scholar]
- Liu R, Li W, Sun LY, Liu CZ. 2012. Improving root growth and cichoric acid derivatives production in hairy root culture of Echinacea purpurea by ultrasound treatment. Biochem Eng J. 60:62–66. [Google Scholar]
- Lucchesini M, Bertoli A, Mensuali-Sodi A, Pistelli L. 2009. Establishment of in vitro tissue cultures from Echinacea angustifolia DC adult plants for the production of phytochemical compounds. Sci Hort. 122:484–490. [Google Scholar]
- Maass N, Bauer J, Paulicks BR, Böhmer BM, Roth, Maier DA. 2005. Efficiency of Echinacea purpurea on performance and immune status in pigs. J Anim Physiol Anim Nutr (Berl). 89:244–252. [DOI] [PubMed] [Google Scholar]
- Maggini R, Tozzini L, Pacifici S, Raffaelli A, Pardossi A. 2012. Growth and accumulation of caffeic acid derivatives in Echinacea angustifolia DC. var. angustifolia grown in hydroponic culture. Ind Crops Prod. 35:269–273. [Google Scholar]
- Manaf HH, Rabie KAE, Abd El-Aal MS. 2016. Impact of UV-B radiation on some biochemical changes and growth parameters in Echinacea purpurea callus and suspension culture. Ann Agric Sci. 61:207–216. [Google Scholar]
- Manayi A, Vazirian M, Saeidnia S. 2015. Echinacea purpurea: pharmacology, phytochemistry and analysis methods. Pharmacogn Rev. 9:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor RL. 1968. The taxonomy of the genus Echinacea (Compositae). The University of Kansas Science Bulletin 6:113–142. [Google Scholar]
- McKeown KA. 1999. A review of the taxonomy of the genus Echinacea In: Janick J, editor. Perspectives on new crops and new uses. Alexandria (VA): ASHS Press; p. 482–489. [Google Scholar]
- Merali S, Binns S, Paulin-Levasseur M, Ficker C, Smith ML, Baum B, Brovelli E, Arnason JT. 2003. Antifungal and anti-inflammatory activity of the genus Echinacea. Pharm Biol. 41:412–420. [Google Scholar]
- Mir-Rashed N, Cruz I, Jessulat M, Dumontier M, Chesnais C, Ng J, Amiguet VT, Golshani A, Arnason JT, Smith ML. 2010. Disruption of fungal cell wall by antifungal Echinacea extracts. Med Mycol. 48:949–958. [DOI] [PubMed] [Google Scholar]
- Mirjalili MH, Salehi P, Badi HN, Sonboli A. 2006. Volatile constituents of the flowerheads of three Echinacea species cultivated in Iran. Flavour Fragr J. 21:355–358. [Google Scholar]
- Mobin M, Wu CH, Tewari RK, Paek KY. 2015. Studies on the glyphosate-induced amino acid starvation and addition of precursors on caffeic acid accumulation and profiles in adventitious roots of Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult 120:291–301. [Google Scholar]
- Moerman DE. 1998. Native American ethnobotany. Portland: Timber Press. [Google Scholar]
- Moraes RM, Lata H, Sumyanto J, Pereira AMS, Bertoni BW, Joshi VC, Pugh ND, Khan IA, Pasco DS. 2011. Characterization and pharmacological properties of in vitro propagated clones of Echinacea tennesseensis (Beadle) Small. Plant Cell Tissue Organ Cult. 106:309–315. [Google Scholar]
- Moszczyńska E, Pląskowska E, Matkowski K, Biesiada A, Weber R. 2013. Fungi assemblages of the phyllosphere of eastern purple coneflower Echinacea purpurea (L.) Moench. depending on the rate of nitrogen. Acta Sci Pol Hortoru. 12:153–162. [Google Scholar]
- Murch SJ, Peiris SE, Shi WL, Zobayed SMA, Saxena PK. 2006. Genetic diversity in seed populations of Echinacea purpurea controls the capacity for regeneration, route of morphogenesis and phytochemical composition. Plant Cell Rep. 25:522–532. [DOI] [PubMed] [Google Scholar]
- Murthy HN, Kim YS, Park SY, Paek KY. 2014. Biotechnological production of caffeic acid derivatives from cell and organ cultures of Echinacea species. Appl Microbiol Biotechnol. 98:7707–7717. [DOI] [PubMed] [Google Scholar]
- Najafzadeh H, Ghorbanpour M, Mayahi M, Gavzan H. 2011. Effect of Echinacea purpurea on antibody production against fowl influenza vaccine. J Appl Anim Res. 39:139–141. [Google Scholar]
- Nilanthi D, Chen XL, Zhao FC, Yang YS, Wu H. 2009. Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. Bio Med Res Int. 2009:343485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Olsson O. 1997. Getting to the root: the role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol Plant. 100:463–473. [Google Scholar]
- Oomah BD, Dumon D, Cardador-Martínez A, Godfrey DV. 2006. Characteristics of Echinacea seed oil. Food Chem. 96:304–312. [Google Scholar]
- Oskoii SB, Kohyani AT, Parseh A, Salati AP, Sadeghi E. 2012. Effects of dietary administration of Echinacea purpurea on growth indices and biochemical and hematological indices in rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Physiol Biochem. 38:1029–1034. [DOI] [PubMed] [Google Scholar]
- Parsons JL, Liu R, Smith ML, Harris CS. 2018. Echinacea fruits: phytochemical localization and germination in four Echinacea species. Botany. 96:461–470. [Google Scholar]
- Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L. 2010. Hairy root cultures for secondary metabolites production In: Giardi MT, Rea G, Berra B, editors. Bio-farms for nutraceuticals. Boston (MA): Springer; p. 167–184. [DOI] [PubMed] [Google Scholar]
- Qu L, Chen Y, Wang X, Scalzo R, Davis JM. 2005. Patterns of variation in alkamides and cichoric acid in roots and aboveground parts of Echinacea purpurea (L.) Moench. HortScience. 40:1239–1242. [PMC free article] [PubMed] [Google Scholar]
- Qu L, Widrlechner MP. 2012. Reduction of seed dormancy in Echinacea pallida (Nutt.) Nutt. by in-dark seed selection and breeding. Ind Crops Prod. 36:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Jin H, Shepard MR, Scott HW, Teitgen AM, Perera MA, Mhaske V, Jose A, Zheng X, Crispin M, et al. 2016. Integrating metabolomics and transcriptomics data to discover a biocatalyst that can generate the amine precursors for alkamide biosynthesis. Plant J. 88:775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero FR, Delate K, Kraus GA, Solco AK, Murphy PA, Hannapel DJ. 2009. Alkamide production from hairy root cultures of Echinacea. In Vitro Cell Dev Biol Plant. 45:599–609. [Google Scholar]
- Rosa LH, Tabanca N, Techen N, Wedge DE, Pan Z, Bernier UR, Becnel JJ, Agramonte NM, Walker LA, Moraes RM. 2012. Diversity and biological activities of endophytic fungi associated with micropropagated medicinal plant Echinacea purpurea (L.) Moench. Am J Plant Sci. 3:1105–1114. [Google Scholar]
- Ruffoni B, Pistelli L, Bertoli A, Pistelli L. 2010. Plant cell cultures: bioreactors for industrial production In: Giardi MT, Rea G, Berra B, editors. Bio-farms for nutraceuticals. Boston (MA): Springer; p. 203–221. [DOI] [PubMed] [Google Scholar]
- Sahin T, Kaya Ö, Sari M. 2012. Effects of ground Echinacea (Echinacea purpurea) supplementation quail diets on growth performance and carcass traits. Kafkas Univ Vet Fak Derg. 18:15–19. [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ. 1998. Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst. 29:319–343. [Google Scholar]
- Sharma M, Vohra S, Arnason JT, Hudson JB. 2008. Echinacea. Extracts contain significant and selective activities against human pathogenic bacteria. Pharm Biol. 46:111–116. [Google Scholar]
- Shemluck M. 1982. Medicinal and other uses of the Compositae by Indians in the United States and Canada. J Ethnopharmacol. 5:303–358. [DOI] [PubMed] [Google Scholar]
- Shin D, Choi K, Lee Y, Kim W, Shin K, Oh S, Jung J, Lee MK, Lee Y, Hong JT, et al. 2014. Echinacea purpurea root extract enhances the adipocyte differentiation of 3T3-L1 cells. Arch Pharm Res. 37:803–812. [DOI] [PubMed] [Google Scholar]
- Sloley BD, Urichuk LJ, Tywin C, Coutts RT, Pang PK, Shan JJ. 2001. Comparison of chemical components and antioxidants capacity of different Echinacea species. J Pharm Pharmacol. 53:849–857. [DOI] [PubMed] [Google Scholar]
- Stuart DL, Wills RBH. 2003. Effect of drying temperature on alkylamide and cichoric acid concentrations of Echinacea purpurea. J Agric Food Chem. 51:1608–1610. [DOI] [PubMed] [Google Scholar]
- Tepfer D. 1990. Genetic transformation using Agrobacterium rhizogenes. Physiol Plant. 79:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trypsteen M, Van Lijsebettens M, Van Severen R, Van Montagu M. 1991. Agrobacterium rhizogenes-mediated transformation of Echinacea purpurea. Plant Cell Rep. 10:85–89. [DOI] [PubMed] [Google Scholar]
- Vandyshev VV, Babaeva EY, Drozdovskaya DD. 2009. Triacylglycerols of the lipid fraction from fruits of two Echinacea species. Pharm Chem J. 43:154–156. [Google Scholar]
- Wang B, Zhang G, Zhu L, Chen L, Zhang Y. 2006. Genetic transformation of Echinacea purpurea with Agrobacterium rhizogenes and bioactive ingredient analysis in transformed cultures. Colloids Surf B Biointerfaces. 53:101–104. [DOI] [PubMed] [Google Scholar]
- Welander M, Persson J, Asp H, Zhu LH. 2014. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci Hort. 179:227–232. [Google Scholar]
- Williams CA, Lamprecht ED. 2008. Some commonly fed herbs and other functional foods in equine nutrition: a review. Vet J. 178:21–31. [DOI] [PubMed] [Google Scholar]
- Wu CH, Dewir YH, Hahn EJ, Paek KY. 2006. Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol. 49:193–199. [Google Scholar]
- Wu CH, Murthy HN, Hahn EJ, Paek KY. 2007. Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnol Lett. 29:1179–1182. [DOI] [PubMed] [Google Scholar]
- Wu CH, Murthy HN, Hahn EJ, Paek KY. 2007. Improved production of caffeic acid derivatives in suspension cultures of Echinacea purpurea by medium replenishment strategy. Arch Pharm Res. 30:945–949. [DOI] [PubMed] [Google Scholar]
- Wu CH, Tewari RK, Hahn EJ, Paek KY. 2007. Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J Plant Biol. 50:636–643. [Google Scholar]
- Wu CH, Huang T, Cui X-H, Paek K. 2012. Induction of adventitious roots of Echinacea pallida and accumulation of caffeic acid derivatives. Zhongguo Zhong Yao Za Zhi. 37:3768–3772. (Abstract only). [PubMed] [Google Scholar]
- Wu CH, An D, Sun LN, Wang M, Chang GN, Zhao CY, Lian ML. 2017. A novel co-culture system of adventitious roots of Echinacea species in bioreactors for high production of bioactive compounds. Plant Cell Tissue Organ Cult. 130:301–311. [Google Scholar]
- Xu CG, Tang TX, Chen R, Liang CH, Liu XY, Wu CL, Yang YS, Yang DP, Wu H. 2014. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult. 116:323–332. [Google Scholar]
- Yu D, Yuan Y, Jiang L, Tai Y, Yang X, Hu F, Xie Z. 2013. Anti-inflammatory effects of essential oil in Echinacea purpurea L. Pak J Pharm Sci. 26:403–408. [PubMed] [Google Scholar]
- Zagumennikov VB, Molchanova AV, Babaeva EY, Petrova AL. 2015. Accumulation of ascorbic acid in fresh Echinacea purpurea plants and their processing products. Pharm Chem J. 48:671–674. [Google Scholar]
- Zhang N, Erickson DL, Ramachandran P, Ottesen AR, Timme RE, Funk VA, Luo Y, Handy SM. 2017. An analysis of Echinacea chloroplast genomes: implications for future botanical identification. Sci Rep. 7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Dixon M, Saxena PK. 2006. Growing environment and nutrient availability affect the content of some phenolic compounds in Echinacea purpurea and Echinacea angustifolia. Planta Med. 72:1407–1414. [DOI] [PubMed] [Google Scholar]
- Zubek S, Błaszkowski J. 2009. Medicinal plants as hosts of arbuscular mycorrhizal fungi and dark septate endophytes. Phytochem Rev. 8:571–580. [Google Scholar]