Abstract
Introduction: Familial type 1 diabetes mellitus (FT1DM) comprises parent-offspring and sib-pair subgroups. The clinical and genetic characteristics of FT1DM cases with and without affected family members have been previously studied with varying results. Some investigators found similarity of presenting features whereas others reported significant differences between the two groups.Objective:To describe the clinical and biochemical characteristics of children with FT1DM in comparison with those with non-familial type 1 diabetes mellitus (NFT1DM). Patients and Methods: We performed a cross-sectional retrospective study in a cohort of children and adolescents with T1DM (n=424) aged between 6 months - 16 years attending to Hamad General Hospital Pediatric Diabetes Center, Doha (Qatar) from 2012-2016. They were divided into 2 groups. Group 1 consisted of 62 children and adolescent with FT1DM (parent-offspring or sib-pair). The other group (Group 2) consisted of 362 patients with NFT1DM. The clinical presentation and prevalence of β-cell autoimmunity (anti-glutamic acid decarboxylase (GAD) antibodies, anti-islet cell and anti-insulin antibodies), thyroid function (Free thyroxine: FT4 and thyroid-stimulating hormone: TSH), anti-thyroid peroxidase antibody (TPO) and anti-tissue transglutaminase (ATT) at their first presentation were recorded, described and analyzed. Results:FT1 DM was more prevalent in boys versus girls (1.4:1, respectively) whereas the prevalence of NFT1DM did not differ between genders (1:1.1, respectively). F1DM occurred relatively early in childhood (40.7% before the age of 4 years and 72% before 9 years of age) versus NFT1DM which occurred relatively later in life (80% after the age of 4 years and 40% after the age of 9 years). 35.2% of FT1DM presented with diabetic ketoacidosis (DKA) versus 32.5% of T1DM patients. Anti-islet antibodies (Ab) were detected more frequently in FT1DM versus NFT1DM. The prevalence of positive anti-insulin and anti- GAD antibodies did not differ between the two groups. Anti TPO were detected in 27.2% of NFT1DM and 35.5% of FT1DM. A primary hypothyroidism, with positive ATPO, was more prevalent in FT1DM versus NFT1DM. ATT IgA was high in 5% of NFT1DM and 19.8% of FT1DM whereas ATT IgG was high in 4.4 % of NFT1DM and 15.4% of FT1DM. Conclusions:FT1DM is more prevalent in boys versus girls and occurs earlier in childhood compared to NFT1DM. Primary hypothyroidism was more prevalent in NFT1DM versus FT1DM. Anti-islet Ab and ATT antibodies were more prevalent in the FT1DM versus NFT1DM. The genetic background may explain some differences between FT1DM and NFT1DM including the age of onset, gender affection, as well as associated autoimmune disorders. (www.actabiomedica.it)
Keywords: familial type 1 DM (FT1DM), non-familial T1DM, prevalence, autoantibodies, thyroid function, diabetic ketoacidosis
Introduction
Familial aggregation accounts for approximately 10% of cases of type 1 diabetes (T1DM), but more than 20% when accounting for the extended family history. However, there is no recognizable pattern of inheritance (1, 2). The risk of diabetes to an identical twin of a patient with T1DM is <40%; for a sibling the risk is approximately 4% by the age of 20 years and 9.6% by the age of 60 years, while for the general population the risk is 0.5%. In some studies, the risk is also higher in siblings of probands diagnosed at younger age, paternal young onset diabetes, male sex, and older parental age (3-12). The reported cumulative risk of T1DM is approximately 4% for offspring of adult onset (15-39 years) T1DM, with a similar recurrence risk in the offspring of mothers and fathers (13). However, data on the possible pathogenetic differences between familial and sporadic type 1 diabetes are still inconsistent. Therefore, we used the registered data from the nationwide Qatar Pediatric Diabetes Register for this cross-sectional observational study (from January 2012 to December 2016) for a better understanding of the characteristics of familial T1DM (FT1DM). We included children and adolescents who had one or more first-degree relatives (parents and siblings) with FT1DM and those with sporadic T1DM (NFT1DM). We compared the clinical characteristics and biochemical data, including the degree of acidosis, the β-cell autoimmunity, the thyroid function and thyroid antibodies at presentation of these two cohorts of T1DM patients.
Patients and Methods
We analyzed all children and adolescents (0.5-16 years of age) with onset of T1DM registered between 2003-2016 in Qatar. The ascertainment of cases was checked through hospital records and outpatient’s diabetes clinic records (62 children and adolescents with FT1DM and 362 children with NFT1DM). Comparisons between the two groups: familial- and sporadic-case patients were performed using chi squared analysis.
In both groups of patients, the clinical presentation and biochemical data including the results of prevalence of β-cell autoimmunity [anti GAD, anti-islet cell (ICA), and anti-insulin antibodies], thyroid function (Free thyroxine: FT4 and thyroid-stimulating hormone: TSH) and anti-thyroid peroxidase antibody (ATPO) and anti-tissue transglutaminase (ATT) at their first presentation were also recorded and compared.
Results
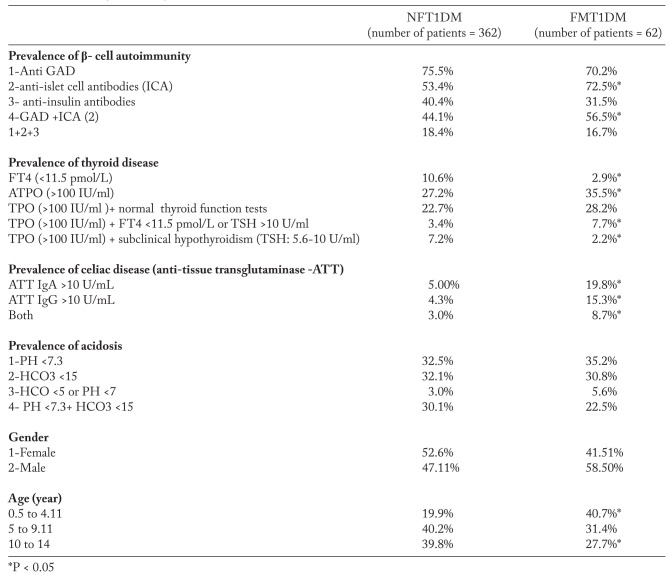
Among a total of 424 patients with T1DM, 62 were diagnosed with FT1DM. Familial cases amounted to 14.62% of all children and adolescents with T1DM. FT1 DM was more prevalent in boys versus girls (58.5: 41.5 respectively) whereas the prevalence of NFT1DM did not differ between genders (1:1.1, respectively). F1DM occurred relatively earlier in childhood (40.7% before the age of 4 years and 72% before 9 years of age) compared to NFT1DM, which occurred relatively later in life (80% after the age of 4 years and 40% after the age of 9 years).
The presence of diabetic ketoacidosis (DKA) was documented in 35.2% of FT1DM and in 32.5% in NFT1DM (P: not significant- NS; Table 1).
Table 1.
Clinical and biochemical comparison in patients with familial type 1 diabetes mellitus (FT1DM) versus non-familial type 1 diabetes mellitus (NFT1DM)
Anti-islet Ab were significantly more prevalent in FT1DM versus NFT1DM (P: <0.05; Table 1). The prevalence of anti-GAD antibodies and anti-insulin AB did not differ between patients in the two groups. Anti TPO were detected in 27.2% of NFT1DM and 35.5% of FT1DM (P: <0.05; Table 1). Primary hypothyroidism (FT4 <11.5 pmol/L) was detected in 10.6% of NFT1DM and 2.9% of FT1DM (P <0.05). Subclinical hypothyroidism was diagnosed in 7.2% of NFT1DM and in 2.2% of FT1DM (P <0.05). 22.7% of NFT1DM and 28.2% of FT1DM had high anti TPO with normal thyroid function (P:NS). ATT IgA was high in 5% of NFT1DM and 19.8% of FT1DM whereas ATT IgG was high in 4.4% of NFT1DM and 15.4% of FT1DM (Table 1).
Discussion
Familial clustering of T1DM is a noticeable feature because the risk of developing T1DM is 8-15- fold higher in first-degree relatives and twofold in second-degree relatives. In other studies, about 10% of children and adolescents with T1DM had a first-degree relative with the disease (14-23).
We analyzed a large set of FT1DM patients at the onset of presentation and compared their age at onset of the disease, the clinical presentation, and other autoimmunity markers with NFT1DM patients. Our finding indicates that the FT1DM constitutes about 14.6% of our children and adolescents with T1DM, a relatively high prevalence compared with the previously published data from 2 large cohort studies from Sweden and Denmark (24, 25). This high prevalence suggests a greater possibility of increased genetic risk factors in our children with T1DM. This finding can be explained partially by the high rate of consanguineous marriage in Qatar (54%), due mainly to socio-cultural factors (26, 27). In support of our data, a study from Kuwait, another Arab Gulf country with high rate of consanguineous marriage, reported a familial form of T1DM in 33% of their patients with T1DM and 17% of children with T1DM presented with DKA (28, 29). Moreover, an increased age- related prevalence of FT1DM has been reported in a Danish study, where the proportion of affected siblings increased from 8% at age 21 to 15.2% at age 50-60 years (30).
In our cohort, the age at onset of T1DM was significantly earlier in the FT1DM group. In addition, FT1 DM was more prevalent in boys versus girls (1.4:1, respectively). This observation is similar to a study reported in the literature, that showed a younger age at onset among familial-case patients (30).
Unlike the findings of a large Finnish study where a positive family history for T1DM was associated with a less severe metabolic decompensation at diagnosis T1DM (31), in our patients the incidence of DKA did not differ in severity or frequency between FT1DM and NFT1DM.
No changes in the severity of metabolic decompensation were also observed in a group of FT1DM children in Kwait (29).
Our results in agreement with some published data (32, 33) showing a higher frequency of β-cell autoantibodies in FT1DM (Table 1). However, this finding was not confirmed in a large cohort of patients in Finland (31).
A young age at diagnosis of T1DM and a high-titer ICA identify a group of T1DM patients at risk for developing rapidly the residual β-cell function (34).
Our children and adolescents with FT1DM showed a significant higher prevalence of anti TPO and ATT antibodies compared to the NFT1DM. In addition, the association between anti TPO positivity and thyroid dysfunction was more prevalent in the FT1DM compared to NFT1DM group. These findings suggest a potential higher autoimmune aggression in FT1DM children and adolescents and could explain in part their earlier presentation compared to NFT1DM patients. Similar findings were reported by Lebenthal et al. (33) where autoimmune diseases were more common in FT1DM (33.5%) versus NFT1DM patients.
A limitation of the present study is the lack of genetic analysis for the entire cohort of T1DM patients.
Conclusions
The prevalence of FT1DM is relatively high in our children with T1DM, and slightly more prevalent in boys versus girls. FT1DM occurred earlier in childhood compared to NFT1DM. The prevalence of children and adolescents with T1DM and hypothyroidism, associated with high ATPO levels was significantly higher in FT1DM versus NFT1DM. ATT antibodies were more prevalent in the FT1DM versus NFT1DM. The genetic background may explain some differences between FT1DM and NFT1DM including the age of onset, gender, and associated autoimmune disorders. Further studies are needed to clarify the genetic susceptibility and the genetic risk factors that could explain the familial clustering of T1D in Qatar.
References
- 1.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009;52:1820–8. doi: 10.1007/s00125-009-1427-3. [DOI] [PubMed] [Google Scholar]
- 2.Parkkola A, Harkonen T, Ryhanen SJ, Ilonen J, Knip M. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care. 2012;36:348–54. doi: 10.2337/dc12-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knip M. Pathogenesis of type 1 diabetes: implications for incidence trends. Horm Res Paediatr. 2011;76(Suppl. 1):57–64. doi: 10.1159/000329169. [DOI] [PubMed] [Google Scholar]
- 4.Olmos P, A’Hern R, Heaton DA, Millward BA, Risley D, Pyke DA, Leslie RD. The significance of the concordance rate for type 1 (insulindependent) diabetes in identical twins. Diabetologia. 1988;31:747–50. doi: 10.1007/BF00274777. [DOI] [PubMed] [Google Scholar]
- 5.Harjutsalo V, Podar T, Tuomilehto J. Cumulative incidence of type 1 diabetes in 10,168 siblings of Finnish young-onset type 1 diabetic patients. Diabetes. 2005;54:563–9. doi: 10.2337/diabetes.54.2.563. [DOI] [PubMed] [Google Scholar]
- 6.Steck AK, Barriga KJ, Emery LM, Fiallo-Scharer RV, Gottlieb PA, Rewers MJ. Secondary attack rate of type 1 diabetes in Colorado families. Diabetes Care. 2005;28:296–300. doi: 10.2337/diacare.28.2.296. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie KM, Gale EA, Bingley PJ. High familial risk and genetic susceptibility in early onset childhood diabetes. Diabetes. 2002;51:210–4. doi: 10.2337/diabetes.51.1.210. [DOI] [PubMed] [Google Scholar]
- 8.No authors listed. Familial risk of type 1 diabetes in European children. Diabetologia. 1998;41:1151–6. doi: 10.1007/s001250051044. [DOI] [PubMed] [Google Scholar]
- 9.Dorman JS, Steenkiste AR, O’Leary LA, McCarthy BJ, Lorenzen T, Foley TP. Type 1 diabetes in offspring of parents with type 1 diabetes: the tip of an autoimmune iceberg? Pediatr Diabetes. 2000;1:17–22. doi: 10.1034/j.1399-5448.2000.010104.x. [DOI] [PubMed] [Google Scholar]
- 10.El Hashimy M, Angelico MC, Martin BC, Krolewski AS, Warram JH. Factors modifying the risk of IDDM in offspring of an IDDM parent. Diabetes. 1995;44:295–99. doi: 10.2337/diab.44.3.295. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzen T, Pociot F, Stilgren L, Kristiansen OP, Johannesen J, Olsen PB, Walmar A, Larsen A, Albrechtsen NC, Eskildsen PC, Andersen OO, Nerup J. Predictors of IDDM recurrence risk in offspring of Danish IDDM patients. Danish IDDM Epidemiology and Genetics Group. Diabetologia. 1998;41:666–73. doi: 10.1007/s001250050966. [DOI] [PubMed] [Google Scholar]
- 12.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–52. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 13.Harjutsalo V, Lammi N, Karvonen M, Groop PH. Age at onset of type 1 diabetes in parents and recurrence risk in offspring. Diabetes. 2010;59:210–4. doi: 10.2337/db09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlquist G, Gustavsson KH, Holmgren G, Hagglof G, Larsson Y, Nilsson KO, Samuelsson G, Sterky G, Thalme B, Wall S. The incidence of diabetes mellitus in Swedish children 0-14 years of age: a prospective study 1977-1980. Acta Paediatr Scand. 1882;71:7–14. doi: 10.1111/j.1651-2227.1982.tb09364.x. [DOI] [PubMed] [Google Scholar]
- 15.Tuomilehto J, Launamaa R, Tuomilehto-Wolf E, Reunanen A, Virtala E, Kaprio EA, Akerblom H the Childhood Diabetes in Finland Study Group. Epidemiology of childhood diabetes mellitus in Finland: background of a nationwide study of type1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:70–74. doi: 10.1007/BF00400854. [DOI] [PubMed] [Google Scholar]
- 16.Pociot F, Norgaard K, Hobolth N, Andersen O, Nerup J the Danish Study Group of Diabetes in Childhood. A nationwide population-based study of familial aggregation of type 1 (insulindependent) diabetes mellitus in Denmark. Diabetologia. 1993;36:870–5. doi: 10.1007/BF00400364. [DOI] [PubMed] [Google Scholar]
- 17.Weires MB, Tausch B, Haug PJ, Edwards CQ, Wetter T, Cannon-Albright LA. Familiarity of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115:634–40. doi: 10.1055/s-2007-984443. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen NM, Westergaard T, Frisch M, Rostgaard K, Wohlfahrt J, Koch-Henriksen N, Melbye M, Hjalgrim H. Type 1 diabetes and multiple sclerosis: a Danish population-based cohort study. Arch Neurol. 2006;63:1001–4. doi: 10.1001/archneur.63.7.1001. [DOI] [PubMed] [Google Scholar]
- 19.Harjutsalo V, Reunanen A, Tuomilehto J. Differential transmission of type 1 diabetes from diabetic fathers and mothers to their offspring. Diabetes. 2006;55:1517–24. doi: 10.2337/db05-1296. [DOI] [PubMed] [Google Scholar]
- 20.Tuomilehto J, Podar T, Tuomilehto-Wolf E, Virtala E. Evidence for importance of gender and birth cohort for risk of IDDM in offspring of IDDM parents. Diabetologia. 1995;38:975–82. doi: 10.1007/BF00400588. [DOI] [PubMed] [Google Scholar]
- 21.Sipetic S, Vlajinac H, Kocev N, Marinkovic J, Radmanovic S, Denic L. Family history and risk of type 1 diabetes mellitus. Acta Diabetol. 2002;39:111–5. doi: 10.1007/s005920200028. [DOI] [PubMed] [Google Scholar]
- 22.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009;52:1820–8. doi: 10.1007/s00125-009-1427-3. [DOI] [PubMed] [Google Scholar]
- 23.Allen C, Palta M, D’Alessio DJ. Risk of diabetes in siblings and other relatives of IDDM subjects. Diabetes. 1991;40:831–6. doi: 10.2337/diab.40.7.831. [DOI] [PubMed] [Google Scholar]
- 24.Dahlquist GG, Mustonen LR. Clinical onset characteristics of familial versus nonfamilial cases in a large population-based cohort of childhood-onset diabetes patients. Diabetes Care. 1995;18:852–4. doi: 10.2337/diacare.18.6.852. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzen T, Pociot F, Hougaard P, Nerup J. Long-term risk of IDDM in first-degree relatives of patients with IDDM. Diabetologia. 1994;37:321–7. doi: 10.1007/BF00398061. [DOI] [PubMed] [Google Scholar]
- 26.Bener A, Alali KA. Consanguineous marriage in a newly developed country: the Qatari population. J Biosoc Sci. 2006;38:239–46. doi: 10.1017/S0021932004007060. [DOI] [PubMed] [Google Scholar]
- 27.Tadmouri GO, Nair P, Obeid T, Al Ali MT, Al Khaja N, Hamamy HA. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moussa MA1, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Prevalence of type 1 diabetes among 6- to 18-year-old Kuwaiti children. Med Princ Pract. 2005;14:87–91. doi: 10.1159/000083917. [DOI] [PubMed] [Google Scholar]
- 29.Shaltout AA, Channanath AM, Thanaraj TA, Omar D, Abdulrasoul M, Zanaty N, Almahdi M, Alkandari H, AlAbdulrazzaq D, d’Mello L, Mandani F, Alanezi A, AlBasiry E, Alkhawari M. Ketoacidosis at first presentation of type 1 diabetes mellitus among children: a study from Kuwait. Scientific Reports. 2016;6:27519. doi: 10.1038/srep27519. doi: 10.1038/srep27519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pociot F, Norgaard K, Hobolth N, Andersen O, Nerup J the Danish Study Group of Diabetes in Childhood. A nationwide population-based study of familial aggregation of type 1 (insulin-dependent) diabetes mellitus in Denmark. Diabetologia. 1993;36:870–5. doi: 10.1007/BF00400364. [DOI] [PubMed] [Google Scholar]
- 31.Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M. Finnish Pediatric Diabetes Register. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care. 2013;36:348–54. doi: 10.2337/dc12-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veijola R, Reijonen H, Vähäsalo P, Sabbah E, Kulmala P, Ilonen J, Akerblom HK, Knip M. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. Childhood Diabetes in Finland (DiMe) Study Group. J Clin Invest. 1996;98:2489–95. doi: 10.1172/JCI119067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebenthal Y, de Vries L, Phillip M, Lazar L. Familial type 1 diabetes mellitus – gender distribution and age at onset of diabetes distinguish between parent-offspring and sib-pair subgroups. Pediatr Diabetes. 2010;11:403–11. doi: 10.1111/j.1399-5448.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decochez K, Keymeulen B, Somers G, Dorchy H, De Leeuw IH, Mathieu C, Rottiers R, Winnock F, ver Elst K, Weets I, Kaufman L, Pipeleers DG, Gorus FK. Belgian Diabetes Registry. Use of an islet cell antibody assay to identify type 1 diabetic patients with rapid decrease in C-peptide levels after clinical onset. Belgian Diabetes Registry. Diabetes Care. 2000;23:1072–8. doi: 10.2337/diacare.23.8.1072. [DOI] [PubMed] [Google Scholar]