Abstract
Significant advances have been made over recent decades in the treatment of childhood malignancies. These advances had an incredible cost, as an increasing number of young survivors suffer subfertility or infertility, because of the high sensitivity of testicular cells, especially the rapidly dividing germ cells, to cytotoxic drugs and irradiation. Therefore, the impact of treatment on future fertility is of significant concern, both to parents and patients. Assessment of fertility damage in childhood remains problematic. For post-pubertal males, semen analysis represents a good indicator of spermatogenesis and testicular function, and allows for sperm cryopreservation. The available method for prepubertal children is only gonadal tissue cryopreservation. This method is still experimental and raises ethical concerns. Ideally, a multidisciplinary team approach needs to be used in addressing the needs of fertility preservation for this population. Precise knowledge of these issues would help pediatric oncologists and endocrinologists to counsel their patients and inform them for factors and resources that may protect or preserve parenthood options in the future. (www.actabiomedica.it)
Keywords: children, adolescents, malignancy, boys, testis, fertility counselling, fertility preservatio
Introduction
Testicular damage can be due to testicular disease, surgery or radiation therapy to the testes and adjacent tissues, as well as to systemic chemotherapy and or radiotherapy. Secondary hypogonadism can be induced by surgery or radiotherapy of tumours of the central nervous system, foremost in the pituitary-hypothalamic region (1).
Approximately half of childhood malignancies are hematologic (leukemia and lymphoma), with an anticipated long-term survival for children of more than 80%. Improvement in prognosis and survival have also been observed for many other childhood malignancies, including Wilms’ tumour, malignant bone tumours, and rhabdomyosarcomas (2, 3). The risks of gonadal toxicity after cancer treatment depend on the type of malignancy and its specific treatment (4, 5).
Although several national organizations support integrating fertility consultation into routine care of these patients (6), fertility preservation is still underestimated.
Numerous studies have shown that oncology providers inconsistently refer adolescent males and females for fertility preservation consultations before the onset of cancer treatment. In one study, 80% of physicians agreed that the threats to fertility are a major concern for them when dealing with adolescent male oncology patients, but only 2/3 of these providers routinely referred patients to a fertility specialist before cancer treatment (7).
This article summarizes reviews on the gonad toxicity of cancer therapies in pediatric and adolescent patients and the current fertility preservation options for these subjects.
Optimizing counselling would help to prevent missed opportunities for fertility preservation and alleviate distress among patients and families (8).
Control of puberty and reproduction
The hypothalamic-pituitary-gonadal axis controls puberty and reproduction and is tightly regulated by a complex network of inducing and inhibitory factors (9, 10).
Gonadotropin-releasing hormone (GnRH) is produced in the preoptic area of the hypothalamus and released from axon terminals in the median eminence in a pulsatile manner, to stimulate the secretion of luteinising hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary, which in turn act on the gonads to promote gametogenesis and production of sex steroids (11-13).
FSH stimulates the seminiferous tubules of testicles to produce sperms. LH stimulates specialized cells in the testes, called Leydig cells, to secrete the male hormone, testosterone. The rate of LH secretion is influenced by the amount of testosterone circulating in the blood. FSH secretion is controlled by inhibin. The rate of inhibin secretion is governed by the amount of sperm produced by the seminiferous tubules (14,15). Besides inducing the male characteristics, testosterone enhances the production of sperm. Two important negative feedback loops exist to regulate the secretion of gonadotropins. The testosterone negative feedback loop is established in fetal life and inhibits hypothalamic and pituitary production of GnRH and LH.
Inhibin-B, produced by the Sertoli cell, exerts inhibitory effects on FSH secretion from the pituitary; this negative feedback loop is established at around puberty (15).
Any disruption of this system or dysfunction of its components may lead to gonadal damage and infertility (16).
Physical changes of puberty
Timing of puberty is the result of both genetic and environmental factors. The age of puberty onset varies between individuals and the timing of puberty initiation is associated with several health outcomes in adult life (17-19).
The most visible changes during puberty are pubertal growth spurt in stature and the development of secondary sexual characteristics. Equally profound are the changes in body composition and the establishment of fertility. The first sign of puberty initiation is typically the thelarche (breast development) in girls and the testicular enlargement (4 ml) in boys (Table 1).
Table 1.
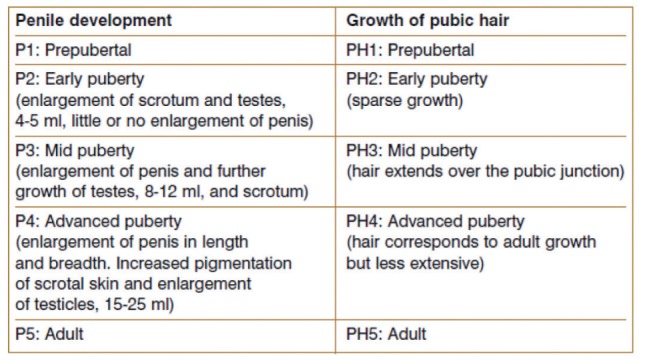
Testes enlargement is mainly due to the increase in volume and tortuosity of seminiferous tubules. Puberty is deemed physiological when it begins between the ages of 8 and 12 years in girls and between 9 and 14 years in boys. The initiation of puberty at younger ages is regarded as precocious puberty and at older ages as delayed puberty (20). Some children reach complete sexual development in less than 2 years, while others require more than 4 years (14).
Physiology of testicular function
The testes fulfil two tasks, namely steroidogenesis and spermatogenesis. Steroidogenesis takes place in the Leydig (interstitial) cells, situated between the seminiferous tubules. Spermatogenesis takes place in the germinal epithelium of the tubules.
The germ cells undergo through various stages of development before spermatozoa (mature sperm) reach maturation. Spermatogenesis commences during puberty and continues throughout life until old age. Spermarche (based on spermaturia, as a marker) may occur when the testicular volume is only slightly increased. Spermaturia is present in 1% to 2% of boys at 11 years of age, 15% to 37% at 12 to 13 years , and 24% to 69% at 14 years of age (21). In brief, the presence of spermatozoa was found in 5% of clinically prepubertal boys and in 50% of boys between Tanner stage II and III for pubic hair pattern (22).
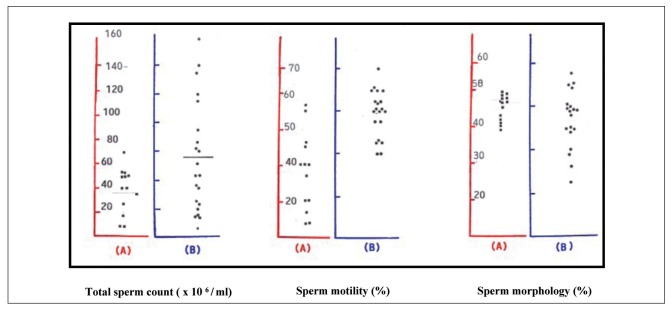
Serum hormone levels are not useful to predict sperm production since at the onset of spermaturia, gonadotropin and testosterone concentrations are low and start to increase after Tanner stage II. Full spermatogenesis process takes about 60 days in the testes and other 10-14 days to pass through the epididymis and vas deferens (21-24). Seminal parameters, collected by masturbation in 35 healthy adolescents and young adults, are presented in figure 1.
Figure 1.
Seminal parameters in 35 healthy adolescents and young adults, aged 14-20 years, with full pubertal development (testicular volume from 15 ml to 25 ml; Tanner stage 5) : (A) 14-16 years; (B) 17-20 years (De Sanctis V; personal observations).
Effects of chemotherapy and radiotherapy on male fertility
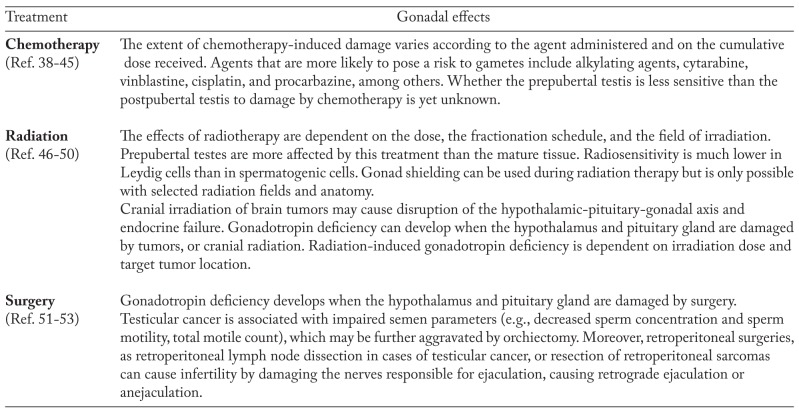
Exposure to chemotherapy and or radiation to gonads or pituitary for treatment of malignancies causes long-term complications on reproductive capacity (25) (Table 2).
Table 2.
Anti-cancer agents that can lead to azoospermia (Modified from: Meistrich ML. The Effects of Chemotherapy and Radiotherapy on Spermatogenesis in Humans. Fertil Steril 2013; 100: 1180-1186).
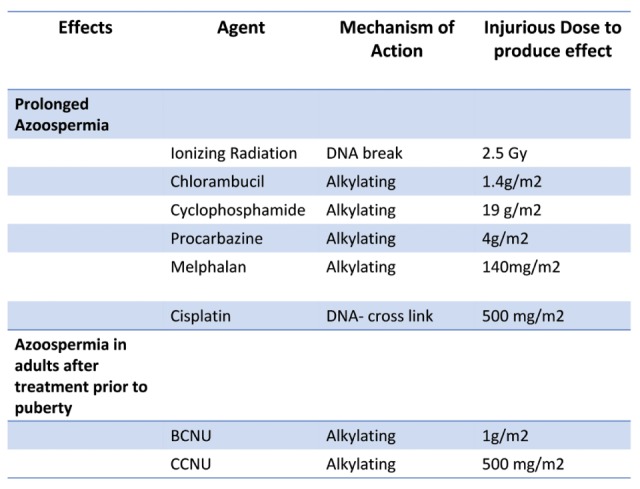
The impact of cancer therapy on fertility is related to the type of malignancy, the age of patient, duration, dose/intensity, and type of treatment. Anticancer treatment, in the form of cytotoxic chemotherapy and radiotherapy may have detrimental effects on the testis at all stages of life (26-30).
Overall, gonad toxic treatment with chemotherapy demonstrated a more significant impact on germ cells than on Leydig cells (Figure 2), and thus cancer survivors who are azoospermic after treatment may maintain adequate testosterone production (31).
Figure 2.
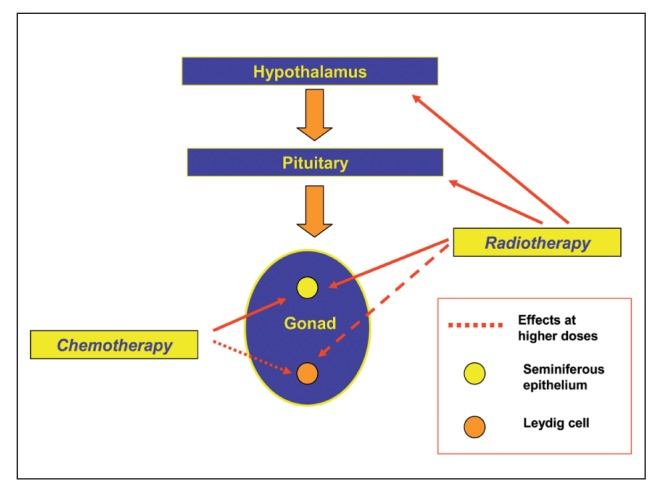
Effects of chemotherapy and radiotherapy on male gonads
Animal studies disclosed that cytotoxic therapy disrupts spermatogenesis by targeting rapidly dividing cells such as the spermatogonial stem cells (SSC) (32).
Gonadal function was evaluated, measuring FSH, LH, inhibin B and total testosterone levels, in 199 male childhood long-term cancer survivors (LTCS). The median follow-up time was 14 years. Spermatogenesis damage (SD) was diagnosed in 68 patients, 16 LTCS had primary hypogonadism (total testosterone <3 ng/dl), and 13 had central hypogonadism. The adjusted risk of gonadal dysfunction was higher in patients treated with radiotherapy (OR = 8.72; 95% CI 3.94-19.30) and in those exposed to both alkylating and platinum-derived agents (OR = 9.22; 95% CI 2.17-39.23). Sarcomas were associated with the higher risk of gonadal dysfunction (OR = 3.69; 95% CI 1.11-12.22). An extremely high rate of gonadal dysfunction was also detected in patients who underwent hematopoietic stem cell transplantation and/or total body irradiation (33).
In general, there are indications that multiple low dose insults with cyclophosphamide are more damaging to the seminiferous epithelium than a single high-dose insult (34). Similar threshold doses for increased incidence of testicular failure have also been reported for other alkylating agents, carboplatin and cisplatin (35, 36). However, it remains difficult to quantify the precise infertility risk posed by each individual drug, as most are administrated as part of multidrug regimens (37).
Table 3 reports the potential negative effects of chemotherapy, radiation and surgery on gonads.
Table 3.
Negative effects of chemotherapy, radiation and surgery on gonads
Management of fertility preservation in prepubertal boys and adolescents
Parents must be informed about fertility preservation options while young patients should be receptive to discussions about fertility preservation, suitable to their age and health status.
a. Prepubertal male patients
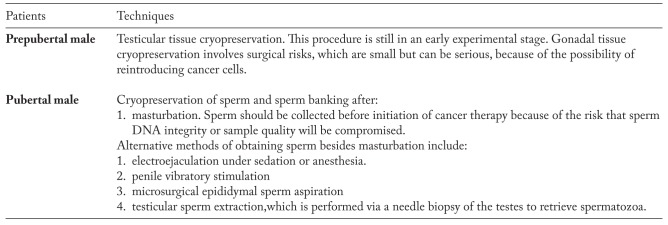
Prepubertal males pose a challenge for fertility maintenance as they cannot produce semen for cryopreservation. Although germ cells of the prepubertal testis include spermatogonial stem cells (SSCs), mature spermatozoa are absent. However, it should be possible to cryopreserve patient’s testicular tissue for eventual restoration of spermatozoa production after completion of cancer treatment (54, 55). To minimize trauma to the patient, the surgical recovery of testicular tissue should be combined with other interventions requiring anaesthesia, such as bone marrow sampling or implantation of venous ports. The main ethical justification for interventions for preservation is the need to safeguard the best interests of the child. A key question that must be addressed in consideration of fertility preservation strategies is to whom storage of sperm and/or testicular tissue should be offered (56).
Although progress in this field is encouraging, fertility preservation in prepubertal boys is still in its infancy and represents a balance between biological, clinical and technical knowledge, ethical and legal questions.
b. Pubertal male patients
Infertility is often a complication for adolescent and young adult males who receive cancer therapy, a problem that might be averted using cryopreserved sperm. Parents and patients desire early information regarding sperm cryopreservation as it plays an important role in the decision to sperm banking.
We recommend sperm banking be offered to all eligible patients. It is important to bank sperm prior to initiating chemotherapy, as even small doses of gonad toxic agents can affect the quality of frozen specimen (57-59). In cases of failure to produce a semen sample by masturbation, assisted ejaculation techniques such as penile vibratory stimulation or electroejaculation under general anaesthesia should be considered as a second-line option (60) (Table 4).
Table 4.
Fertility preservation for prepubertal and pubertal male patients with cancer
Screening for male gonadal function
Screening for problems related to male gonadal function in survivors includes: an annual age-appropriate history with specific attention to growth, pubertal development (standing and sitting height, Tanner staging, assessment of penile size, testicular volume, using the Prader orchidometer, and examination for gynecomastia) and problems with libido, impotence, or fertility. Approximately 85% of the testicular mass consists of germinal tissue, so a reduced germinal cell mass is associated with reduced testicular size and a soft consistency (61).
Spermatogenesis status is evaluated either indirectly by determination of the levels of gonadotropins and testicular volume changes or directly by semen analysis.
Hormonal evaluation can be limited to the determination of serum concentrations of FSH, LH, testosterone (T), TSH and prolactin, as indicated clinically. A low testosterone level is one of the best indicators of hypogonadism of hypothalamic or pituitary origin (62-64). Low LH and FSH values concurrent with low testosterone levels indicate hypogonadotropic hypogonadism. Elevated FSH and LH values help to distinguish primary testicular failure (hypergonadotropic hypogonadism) from secondary testicular failure (hypogonadotropic hypogonadism). An elevated FSH level associated with small, atrophic testes implies severe gonadal damage (62-64). Also the pattern of serum anti-Müllerian hormone (AMH) expression, in combination with other hormones, could delineate testicular damage: serum AMH was correlated with increased FSH and Testosterone and decreased inhibin B in gonadotoxic protocols (cisplatin or busulfan) and remained unchanged in non gonadotoxic protocols (capecitabine) (65). However, large-scale clinical studies are warranted for further define the role of AMH as a biomarker of testicular toxicity.
Although serum inhibin B is known to be a marker of germ cell function, the levels of inhibin B alone or inhibin B in combination with FSH do not reflect normal spermatogenesis in patients who have undergone cancer treatment in childhood (66).
The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer (COG-LTFU Guidelines) guidelines do not recommend screening for inhibin B routinely (67).
An abnormal semen analysis is suggestive of testicular germ cell damage, but azoospermia may also be secondary to ejaculatory dysfunction or hormone deficiencies in survivors at risk for these complications (68).
The World Health Organization Laboratory Manual for Examination of Human Semen and Semen-Cervical Mucous Interactions is highly recommended for evaluation of technical details of macroscopic and microscopic variables of semen (69, 70).
Although semen analysis provides useful descriptive data for sperm count, motility, vitality, and morphology, parameters above the lower reference limits do not guarantee fertility, nor do values outside these limits necessarily imply male infertility or pathology (71).
Azoospermic and severely oligo-asthenozoospermic survivors had significantly smaller mean testicular volume and higher basal FSH levels than the other survivors, but small testicles (sum of both testicular volume ≤20 ml) and/or abnormally high basal FSH (>10 mIU/mL) were present in only half of the azoospermic adult survivors (mean age 20.2 years) (72).
When abnormalities in testicular function are detected, close cooperation with an endocrinologist is needed. When no abnormalities are noted on history and physical examination but sexual maturity has not been completed, the evaluation should be reviewed every 1-2 years. Conversely, in light of the potential recovery of spermatogenesis and interpatient variations in gonadal toxicity, reminding about contraception should be given.
Numerous recent improvements in sperm storage techniques and advances in assisted reproductive technology using intracytoplasmic sperm injection (ICSI) facilitate successful pregnancies using banked sperm, which remain viable for up to 28 years, if stored properly (73).
Treatment of pubertal disorders and reduced fertility
The treatment of delayed or arrested puberty, and hypogonadotrophic hypogonadism depends on factors such as age, associated complications, and the presence of psychological problems resulting from hypogonadism. The severity of radiation induced gonadotropin deficiency, varies from subclinical to severe forms. Clinically significant gonadotropin deficiency is usually a late complication with a cumulative incidence of 20-50% on long-term follow-up, regardless of whether radiation was administered in childhood or during adulthood (74).
For delayed puberty in males, low dosages of intramuscular depot-testosterone esters (25 mg) are given monthly, at a bone age of about 12 years, for 6 months to stimulate growth velocity. In patients with hypogonadism, therapy continues and the dose increased to 100 mg/mo. until the growth rate begins to wane. The fully virilising dose is 75-100 mg of depot-testosterone esters every 10 days administered intramuscularly or 100 mg/m2 twice a month (75). The same effects can be achieved with topical testosterone gel.
In presence of infertility, patients are evaluated to determine the cause and best treatment options (76-81). To-day new assisted reproductive techniques (ART) offer hopes to many couples, e.g. intracytoplasmic sperm injection (ICSI) as a management for severe male factors or for recurrent unexplained failure of in vitro fertilization (IVF) cycles (79). ICSI is a procedure that is performed in conjunction with IVF. With ICSI, a single sperm from the male partner is injected directly into a woman’s egg (oocyte) in the laboratory. The pregnancy rate with ICSI is approximately 20% to 40% per cycle (76,77).
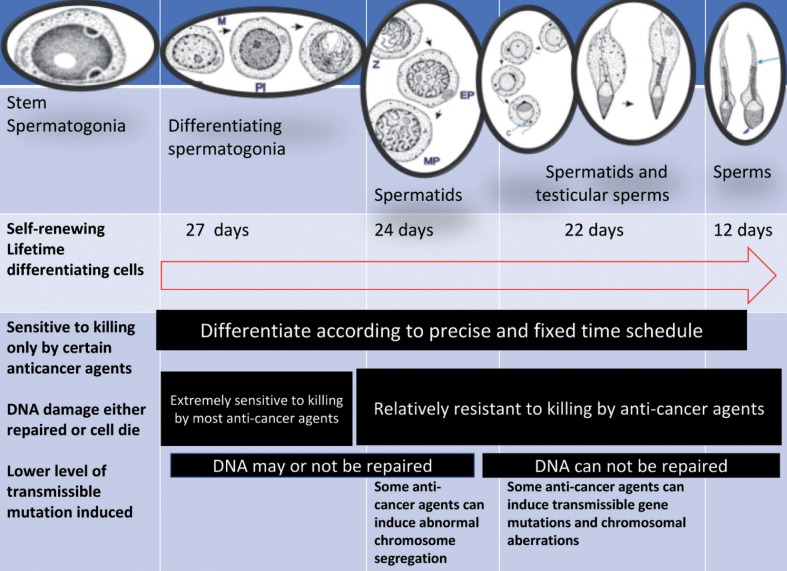
The fertilizing potential of sperm depends not only on the functional competence of spermatozoa but also on sperm DNA integrity (Figure 3). Sperms with compromised DNA integrity, regardless of the degree of DNA damage, appear to have the capacity to fertilize oocytes at the same rate as normal sperm. However, the embryos produced by fertilization of an oocyte with DNA damaged sperm can not develop normally (77). Therefore, the evaluation of sperm DNA integrity, in addition to routine sperm parameters, could add further information on the quality of spermatozoa and improved predictive values could be obtained from validated sperm DNA fragmentation assays. The most commonly used techniques to assess sperm DNA integrity are the TUNEL and sperm chromatin structure assays (SCSA).
Figure 3.
Sequence of spermatogenesis showing relative sensitivity to killing by anticancer agents, ability to accumulate and repair DNA damage, and sensitivity to induction of transmissible mutations (Modified from: Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer 2009; 53: 261-266)
Adoption is offered for cases with unexplained failed IVF cycles and donor insemination for azoospermia.
In 2006, the American Society of Clinical Oncology (ASCO) published a clinical practice guideline on fertility preservation for adults and children with cancer (82). ASCO guidelines are updated periodically by a subset of the original Expert Panel. These guidelines represent a set of comprehensive screening recommendations that can be used to standardize and direct the follow-up care for this group of cancer survivors. The Long-Term Follow-Up Program Resource Guide offers a broad perspective from a variety of long-term follow-up programs within the Children’s Oncology Group and can be downloaded from http://www.survivorshipguidelines.org.
Knowledge about these issues would help pediatric oncologist and endocrinologists to counsel their patients as well as to connect them with the resources that may protect or preserve parenthood options for the future.
Conclusions
In the last years, thanks to the improvement of treatment in the prognosis in prepubertal and pubertal cancer patients, a growing attention has been given to the fertility issues. Chemotherapy and radiotherapy have a direct gonadotoxic effect compromising sperm number, motility, morphology and DNA integrity in male patients. Many factors share in the outcome of fertility in patients treated for cancer (Table 5).
Table 5.
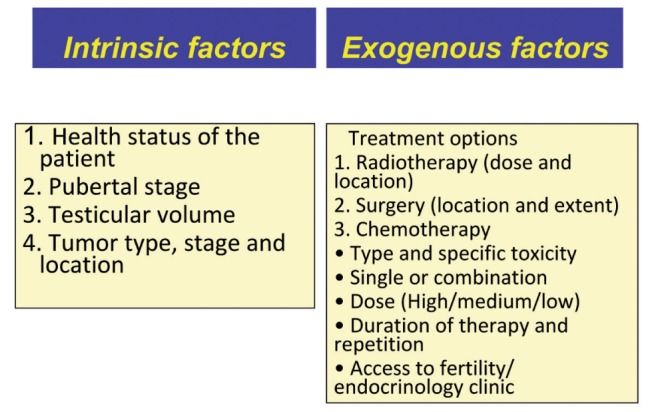
Factors that can affect fertility in adolescents
Fortunately, fertility preservation options exist for both female and male prepubertal and pubertal patients, and discussion of such options with patients and their families prior to the initiation of therapy and/or before further deterioration of gonadal function is crucial.
While the majority of boys aged >12 years considered the information to be clear (72%), complete (80%) and understandable (90.9%), only 33.3% of boys aged <12 years were able to comprehend the information. Pressure from doctors to reduce the delay between diagnosis and cancer treatment increased the number of refusals, while hope for future parenthood favoured acceptance. Family support was considered important for 75% of adolescents and 58% of children, and medical support for 50% of adolescents and 42% of children (83).
In conclusion, special attention should be paid to aspects of future quality of life, in particular: fertility and sexuality. The recent implementation of personalized medicine, in the management of children and adolescents with malignancies is expected to reduce the incidence and severity of gonadal complications related to the high sensitivity of testicular cells, especially the rapidly dividing germ cells, to cytotoxic drugs and irradiation.
References
- 1.Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am. 1998;27:927–943. doi: 10.1016/s0889-8529(05)70048-7. [DOI] [PubMed] [Google Scholar]
- 2.Fallat ME, Hutter J. American Academy of Pediatrics Committee on Bioethics; American Academy of Pediatrics Section on Hematology/Oncology; American Academy of Pediatrics Section on Surgery. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121:e1461–1469. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington D.C: National Academies Press; 2003. [PubMed] [Google Scholar]
- 4.Leroy C, Cortet-Rudelli C, Desailloud R. Endocrine consequences in young adult survivors of childhood cancer treatment. Ann Endocrinol (Paris) 2015;76(6 Suppl 1):S29–38. doi: 10.1016/S0003-4266(16)30005-1. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed September 12, 2007];Infertility. Available at: www.cdc.gov/nchs/fastats/fertile.htm . [Google Scholar]
- 6.Fallat ME, Hutter J. American Academy of Pediatrics Committee on Bioethics; American Academy of Pediatrics Section on Hematology/Oncology; American Academy of Pediatrics Section on Surgery. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008 May;121(5):e1461–9. doi: 10.1542/peds.2008-0593. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 7.Stevens MC, Mahler H, Parkes S. The health status of adult survivors of cancer in childhood. Eur J Cancer. 1998;34:694–698. doi: 10.1016/s0959-8049(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 8.Saraf AJ, Nahata L. Fertility counselling and preservation: considerations for the pediatric endocrinologist. Transl Pediatr. 2017;6:313–322. doi: 10.21037/tp.2017.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4:254–264. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojeda SR, Lomniczi A. Puberty in 2013: Unravelling the mystery of puberty. Nat Rev Endocrinol. 2014;10:67–69. doi: 10.1038/nrendo.2013.233. [DOI] [PubMed] [Google Scholar]
- 11.Bourguignon J, Lebrethon M, Gérard A, Purnell G, Vandersmissen E, Parent A, Yamanaka C. The onset of puberty in perspective. Vol. 119. Amsterdam: Elsevier Science BV; 2000. Amino acid neurotransmission and early ontogeny of pulsatile GnRH secretion from the rat hypothalamus; p. 129. [Google Scholar]
- 13.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(Suppl. 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 14.Sizonenko PC. Normal sexual maturation. Pediatrician. 1987;14:191–201. [PubMed] [Google Scholar]
- 15.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 16.McLachlan RI. The endocrine control of spermatogenesis. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:345–362. doi: 10.1053/beem.2000.0084. [DOI] [PubMed] [Google Scholar]
- 17.Tanner JM. Growth at adolescence. 2nd. Springfield: Charles C Thomas; 1962. [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127:100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 20.Reiter EO, Lee PA. Delayed puberty. Adolesc Med. 2002;13:101–118. [PubMed] [Google Scholar]
- 21.Gerris J. Methods of semen collection not based on masturbation or surgical sperm retrieval. Hum Reprod Update. 1999;5:211–215. doi: 10.1093/humupd/5.3.211. [DOI] [PubMed] [Google Scholar]
- 22.Van Casteren NJ, Dohle GR, Romij JC, de Muinck Keizer-Schrama SMPF, Weber RFA, van den Heuvel-Eibrink MM. Semen cryopreservation in pubertal boys before gonadotoxic treatment and the role of endocrinologic evaluation in predicting sperm yield. Fertil Steril. 2008a;90:119–125. doi: 10.1016/j.fertnstert.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Gerris J. Methods of semen collection not based on masturbation or surgical sperm retrieval. Hum Reprod Update. 1999;5:211–215. doi: 10.1093/humupd/5.3.211. [DOI] [PubMed] [Google Scholar]
- 24.Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- 25.Gertosio C, Magistrali M, Musso P, Meazza C, Bozzola M. Fertility Preservation in Pediatric Oncology Patients: New Perspectives. J Adolesc Young Adult Oncol. 2018 Jan 18 doi: 10.1089/jayao.2017.0117. doi: 10.1089/jayao.2017.0117. [DOI] [PubMed] [Google Scholar]
- 26.Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25:287–302. doi: 10.1016/j.beem.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kenney LB, Laufer MR, Grant FD, Grier H, Diller L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.van Casteren NJ, van der Linden GH, Hakvoort-Cammel FG, Hählen K, Dohle GR, van den Heuvel-Eibrink MM. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer. 2009;52:108–112. doi: 10.1002/pbc.21780. [DOI] [PubMed] [Google Scholar]
- 29.Shalet SM, Horner A, Ahmed SR, Morris-Jones PH. Leydig cell damage after testicular irradiation for lymphoblastic leukaemia. Med Pediatr Oncol. 1985;13:65–68. doi: 10.1002/mpo.2950130204. [DOI] [PubMed] [Google Scholar]
- 30.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Kenney LB, Laufer MR, Grant FD, Grier H, Diller L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 32.Meistrich ML, Finch M, da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–131. [PubMed] [Google Scholar]
- 33.Brignardello E, Felicetti F, Castiglione A, Nervo A, Biasin E, Ciccone G, Fagioli F, Corrias A. Gonadal status in long-term male survivors of childhood cancer. J Cancer Res Clin Oncol. 2016;142:1127–1132. doi: 10.1007/s00432-016-2124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurmio M, Keros V, Lähteenmäki P, Salmi T, Kallajoki M, Jahnukainen K. Effect of childhood acute lymphoblastic leukemia therapy on spermatogonia populations and future fertility. J Clin Endocrinol Metab. 2009;94:2119–2122. doi: 10.1210/jc.2009-0060. [DOI] [PubMed] [Google Scholar]
- 35.Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239–245. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]
- 36.Meistrich ML, Chawla SP, Da Cunha MF, Johnson SL, Plager C, Papadopoulos NE, Lipshultz LI, Benjamin RS. Recovery of sperm production after chemotherapy for osteosarcoma. Cancer. 1989;63:2115–2123. doi: 10.1002/1097-0142(19890601)63:11<2115::aid-cncr2820631108>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 37.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 38.Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara R, Carmen C, Sabine S. Fertility considerations and the pediatric oncology patient. Semin Pediatr Surg. 2016;25:318–322. doi: 10.1053/j.sempedsurg.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin’s disease. Med Pediatr Oncol. 1996;27:74–78. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Papadakis V, Vlachopapadopoulou E, Van Syckle K, Ganshaw L, Kalmanti M, Tan C, Sklar C. Gonadal function in young patients successfully treated for odgkin disease. Med Pediatr Oncol. 1999;32:366–372. doi: 10.1002/(sici)1096-911x(199905)32:5<366::aid-mpo10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Longhi A, Macchiagodena M, Vitali G, Bacci G. Fertility in male patients treated with neoadjuvant chemotherapy for osteosarcoma. J Pediatr Hematol Oncol. 2003;25:292–296. doi: 10.1097/00043426-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Ridola V, Fawaz O, Aubier F, Bergeron C, de Vathaire F, Pichon F, Orbach D, Gentet JC, Schmitt C, Dufour C, Oberlin O. Testicular function of survivors of childhood cancer: a comparative study between ifosfamide-and cyclophosphamide-based regimens. Eur J Cancer. 2009;45:814–818. doi: 10.1016/j.ejca.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Meistrich ML, Wilson G, Brown BW, da Cunha MF, Lipshultz LI. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer. 1992;70:2703–2712. doi: 10.1002/1097-0142(19921201)70:11<2703::aid-cncr2820701123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259:2123–2125. [PubMed] [Google Scholar]
- 46.Ash P. The influence of radiation on fertility in man. Br J Radiol. 1980;53:271–278. doi: 10.1259/0007-1285-53-628-271. [DOI] [PubMed] [Google Scholar]
- 47.Shalet SM, Tsatsoulis A, Whitehead E, Read G. Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol. 1989;120:161–165. doi: 10.1677/joe.0.1200161. [DOI] [PubMed] [Google Scholar]
- 48.Sklar CA, Robison LL, Nesbit ME, Sather HN, Meadows AT, Ortega JA, Kim TH, Hammond GD. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: a report from the Children Cancer Study Group. J Clin Oncol. 1990;8:1981–1987. doi: 10.1200/JCO.1990.8.12.1981. [DOI] [PubMed] [Google Scholar]
- 49.Ogilvy-Stuart AL, Clark DJ, Wallace WH, Gibson BE, Stevens RF, Shalet SM, Donaldson MD. Endocrine deficit after fractionated total body irradiation. Arch Dis Child. 1992;67:1107–1110. doi: 10.1136/adc.67.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Littley MD, Shalet SM, Beardwell CG, Robinson EL, Sutton ML. Radiation induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 1989;31:363–373. doi: 10.1111/j.1365-2265.1989.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 51.Williams DH 4th, Karpman E, Sander JC, Spiess PE, Pisters LL, Lipshultz LI. Pretreatment semen parameters in men with cancer. J Urol. 2009;181:736–740. doi: 10.1016/j.juro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Kort JD, Eisenberg ML, Millheiser LS, Westphal LM. Fertility issues in cancer survivorship. CA Cancer J Clin. 2014;64:118–134. doi: 10.3322/caac.21205. [DOI] [PubMed] [Google Scholar]
- 53.Pearce S, Steinberg Z, Eggener S. Critical evaluation of modified templates and current trends in retroperitoneal lymph node dissection. Curr Urol Rep. 2013;14:511–517. doi: 10.1007/s11934-013-0366-1. [DOI] [PubMed] [Google Scholar]
- 54.Bahadur G, Chatterjee R, Ralph D. Testicular tissue cryopreservation in boys. Ethical and legal issues: case report. Hum Reprod. 2000;15:1416–1420. doi: 10.1093/humrep/15.6.1416. [DOI] [PubMed] [Google Scholar]
- 55.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 56.Murphy T. Parents’ choices in banking boys’ testicular tissue. J Med Ethics. 2010;36:806–809. doi: 10.1136/jme.2010.037192. [DOI] [PubMed] [Google Scholar]
- 57.Ginsberg JP, Ogle SK, Tuchman LK, Carlson CA, Reilly MM, Hobbie WL, Rourke M, Zhao H, Meadows AT. Sperm banking for adolescent and young adult cancer patients: sperm quality, patient, and parent perspectives. Pediatr Blood Cancer. 2008;50:594–598. doi: 10.1002/pbc.21257. [DOI] [PubMed] [Google Scholar]
- 58.Lass A, Akagbosu F, Abusheikha N, Hassouneh M, Blayney M, Avery S, Brinsden P. A programme of semen cryopreservation for patients with malignant disease in a tertiary infertility centre: lessons from 8 years’ experience. Hum Reprod. 1998;13:3256–3261. doi: 10.1093/humrep/13.11.3256. [DOI] [PubMed] [Google Scholar]
- 59.Chung K, Irani J, Knee G, Efymow B, Blasco L, Patrizio P. Sperm cryopreservation for male patients with cancer: an epidemiological analysis at the University of Pennsylvania. Eur J Obstet Gynecol Reprod Biol. 2004;113:S7–11. doi: 10.1016/j.ejogrb.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 60.Levine J, Canada A, Stern CJ. Fertility Preservation in Adolescents and Young Adults With Cancer. J Clin Oncol. 2010 May 10 doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 61.Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ. American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients--2002 update. Endocr Pract. 2002;8:440–456. [PubMed] [Google Scholar]
- 62.Schlegel PN. Male infertility: evaluation and sperm retrieval. Clin Obstet Gynecol. 2006;49:55–72. doi: 10.1097/01.grf.0000197267.02541.ae. [DOI] [PubMed] [Google Scholar]
- 63.Schlegel PN. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–283. [PubMed] [Google Scholar]
- 64.Spitz A, Kim ED, Lipshultz LI. Contemporary approach to the male infertility evaluation. Obstet Gynecol Clin North Am. 2000;27:487–516. doi: 10.1016/s0889-8545(05)70151-0. [DOI] [PubMed] [Google Scholar]
- 65.Levi M, Hasky N, Stemmer SM, Shalgi R, Ben-Aharon I. Anti-Müllerian hormone is a marker for chemotherapy-induced testicular toxicity. Endocrinology. 2015;156:3818–3827. doi: 10.1210/en.2015-1310. [DOI] [PubMed] [Google Scholar]
- 66.Rendtorff R, Beyer M, Muller A, Dittrich R, Hohmann C, Keil T, Henze G, Borgmann A. Low inhibin B levels alone are not a reliable marker of dysfunctional spermatogenesis in childhood cancer survivors. Andrologia. 2012;44(Suppl 1):219–225. doi: 10.1111/j.1439-0272.2011.01167.x. [DOI] [PubMed] [Google Scholar]
- 67.Kenney LB, Cohen LE, Shnorhavorian M, Metzger ML, Lockart B, Hijiya N, Duffey-Lind E, Constine L, Green D, Meacham L. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:3408–3416. doi: 10.1200/JCO.2011.38.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell CR, Sibley GN. A 7-year audit in a single centre of the results of the modified template retroperitoneal lymph node dissection for testicular teratoma. BJU Int. 1999;84:667–670. doi: 10.1046/j.1464-410x.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 70.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–2345. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 71.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu D, Vogel DL. National Cooperative Reproductive Medicine Network. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 72.Lopez Andreu JA, Fernandez PJ, Ferris i Tortajada J, Navarro I, Rodriguez-Ineba A, Antonio P, Muro MD, Romeu A. Persistent altered spermatogenesis in long-term childhood cancer survivors. Pediatr Hematol Oncol. 2000;17:21–30. doi: 10.1080/088800100276631. [DOI] [PubMed] [Google Scholar]
- 73.Feldschuh J, Brassel J, Durso N, Levine A. Successful sperm storage for 28 years. Fertil Steril. 2005;84:1017. doi: 10.1016/j.fertnstert.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy revisited. Endocr Dev. 2009;15:1–24. doi: 10.1159/000207607. [DOI] [PubMed] [Google Scholar]
- 75.De Sanctis V, Soliman AT, Yassin MA, Di Maio S, Daar S, Elsedfy H, Soliman N, Kattamis C. Hypogonadism in male thalassemia major patients: pathophysiology, diagnosis and treatment. Acta Biomed. 2018;89(2-S):6–15. doi: 10.23750/abm.v89i2-S.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McLachlan RI, Krausz C. Clinical evaluation of the infertile male: new options, new challenges. Asian J Androl. 2012;14:3–5. doi: 10.1038/aja.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loughlin KR. Changes in male fertility in the last two decades. Urol Clin North Am. 2012;39:33–36. doi: 10.1016/j.ucl.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Ståhl O, Eberhard J, Cavallin-Ståhl E, Jepson K, Friberg B, Tingsmark C, Spanò M, Giwercman A. Sperm DNA integrity in cancer patients: the effect of disease and treatment. Int J Androl. 2009;32:695–703. doi: 10.1111/j.1365-2605.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- 79.Kim ED. An overview of male infertility in the era of intracytoplasmic sperm injection. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64:71–83. [PubMed] [Google Scholar]
- 80.Schlegel PN. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–283. [PubMed] [Google Scholar]
- 81.Barratt CLR, Björndahl L, De Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, Oates RD, van der Poel S, St John B, Sigman M, Sokol R, Tournaye H. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update. 2017;23:660–680. doi: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.No Authors listed. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol. 1996;14:671–679. doi: 10.1200/JCO.1996.14.2.671. [DOI] [PubMed] [Google Scholar]
- 83.Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod. 2015;30:2022–2030. doi: 10.1093/humrep/dev161. [DOI] [PubMed] [Google Scholar]