Abstract
Chronic Myeloid Leukemia (CML) is a clonal myeloproliferative neoplasm (MPN) characterized by the presence of a reciprocal translocation between the long arms of chromosomes 9 and 22, t(9;22)(q34:q11), resulting in fusion of the break point cluster region (BCR) with the ABL gene, which forms an oncogene, the transcript of which is an oncoprotein with a tyrosine kinase function. In the great majority of CML; BCR/ABL1 is cytogenetically visualized as t(9;22); giving rise to the Ph chromosome, harboring the chimeric gene. Cryptic or masked translocations occur in 2-10% patients with no evidence for the BCR/ABL rearrangement by conventional cytogenetics but are positive by Fluorescence in Situ Hybridization (FISH) and/or reverse transcriptase polymerase chain reaction (RT-PCR). These patients are described as Philadelphia negative (Ph negative) BCR/ABL1-positive CML with the chimeric gene present on the derivative chromosome 22, as in most CML cases, or alternatively on the derivative 9 in rare occasions. In the majority of cases, CML is diagnosed in the chronic phase; it is less frequently diagnosed in accelerated crises, and occasionally, its initial presentation is as acute leukemia. The prevalence of extramedullary blast phase (BP) has been reported to be 7-17% in patients with BP. Surprisingly, no extramedullary blast crises of B-lymphoid lineage have been reported before among cases of CML as the initial presentation. We report an adult male diagnosed as CML-chronic phase when he was shortly presented with treatment-naive extramedullary B-lymphoid blast crises involving multiple lymph nodes, with no features of acceleration or blast crises in the peripheral blood (PB) and bone marrow (BM). In addition the patient had variant/cryptic Philadelphia translocation. This is the first report of CML, on the best of our knowledge, with extramedullary B-lymphoid blast phase, as initial presentation, that showed a cryptic Ph translocation. (www.actabiomedica.it)
Keywords: Chronic Myeloid Leukemia, cryptic Ph translocation, extramedullary B-lymphoid blast phase
Case Report
We report a 45 year-old Egyptian male, heavy smoker with no chronic illness, presenting one month history of swelling of medial canthus of his left eye, and small left neck swelling in the last 3 days. No history of fever, night sweats or weight loss was reported. His initial physical examination showed bilateral cervical lymphadenopathy with left medial canthal growth and splenomegaly (4 cm below the costal margin).
Complete blood count, (CBC) showed a marked leukocytosis with total white blood count (WBC): 127x10^3/ul (4.0-10.0), a hemoglobin level of 12.2 gm/dl (13.0-17.0) and a platelets count of 150x10^3/ul (150-400).
Renal function, liver function and serum electrolytes were normal.
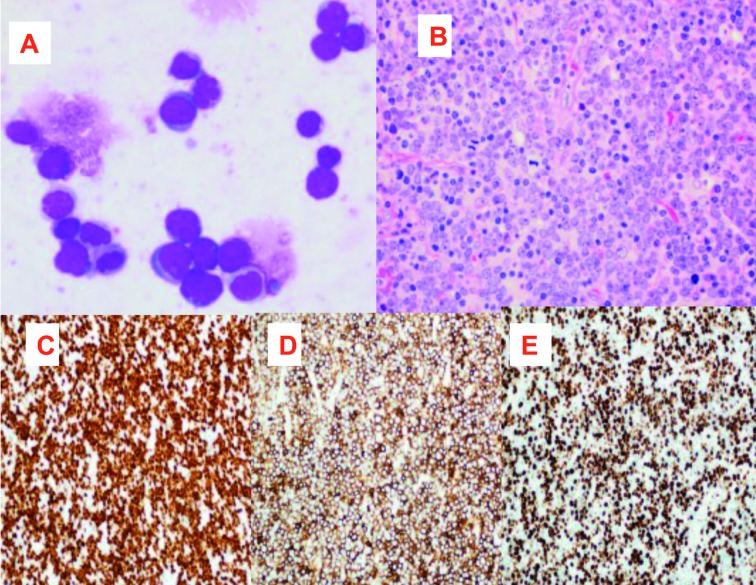
Peripheral blood smear showed marked leukocytosis with shift to the left and basophilia (2%). Bone marrow (BM) aspirate smears showed trilineage hematopoiesis with granulocytic hyperplasia, increased basophils and eosinophils and 2% blasts (Figure 1A). BM biopsy showed marked hypercellularity with marked granulocytic proliferation, prominent eosinophils (Figure 1B), increased vascularity and reticulin fibrosis. No increase in blasts or precursor B-cells as highlighted by CD34 and TdT immunostains (Figures 1C and 1D).
Figure 1.
BM aspirate smear showing granulocytic hyperplasia with increased basophils and no increase in blasts 100x (A). BMB (H&E) showing marked hypercellularity (insert at left upper corner) with marked granulocytic hyperplasia (50x) (B). CD34 immunostain highlighted increased marrow vasculature with no increase in blasts(20x) (C). TdT immunostain shows few scattered positivity (10x) (D)
Flourescence in Situ Hybridization (FISH) analysis was performed on interphase cells directly harvested from BM sample. The probes used were ABL1 (red) and BCR (green) on bands 9q34 and 22q11.2, respectively. The analysis revealed single fusion (yellow) (BCR/ABL1 rearrangement, t(9;22), an extra truncated green signal (BCR, 22q11.2) along with red and green singles emitted by normal chromosomes 9 and 22, in 97% of the cells analyzed. Furthermore, metaphase FISH showed a fusion on chromosome 9 and truncated BCR signal on chromosome 22. These results indicate that translocation between long arms of chromosomes 9 and 22 resulted in BRC/ABL1 rearrangement fusion signal formed on chromosome 9 instead of 22 with deletion of deletion of ABL1 on distal portion of long arm of chromosome 9 (confirmed by metaphase FISH analysis). Variant BCR/ ABL1 rearrangement was detected in 97% of the cells analyzed (der(9)t(9;22), del(9)(q34q34) (ABL1+,BCR+),der(22)t(9;22)(BCR+,ABL1-) (Figure 2 B).
Figure 2.
Ilustrative figure of FISH performed on CML case with classic BCR/ABL rearrangement showing one normal interphase and another two interphases showing the classic BCR/ABL rearrangement with double fusion signals (2 yellow arrows on each abnormal cell), red signal of ABL1 gene on normal chromosome 9q; green signal of BCR on normal chromosome 22q (A). Metaphase and interphase FISH on BM aspirate (our case): red signal of ABL1 gene on normal chromosome 9q; green signal of BCR on normal chromosome 22q; truncated green signal of BCR on chromosome 22q;yellow fusion signal for BCR/ABL chimeric gene on der(9q) (orange arrow) instead of der(22q)(B). Conventional cytogenetics studies on metaphase cells from BM aspirate (C); revealed abnormal karyotype confirming findings obtained by FISH analysis, ISCN nomenclature: 46,XY,?t(9;22)(q34;q11.2).ish der(9)t(9;22)del(9) (q34q34) (ABL1+,BCR+), der (22)t(9;22)(BCR+,ABL1-)
Conventional cytogenetics performed on metaphase cells from cultured BM sample confirmed the presence of cryptic/ variant ph translocation confirming findings obtained by FISH analysis (Figure 2 C). BCR/ABL1 transcript was confirmed by RT-qPCR which showed major BCR-ABL1 transcripts encoding the classic P210 oncoprotein.
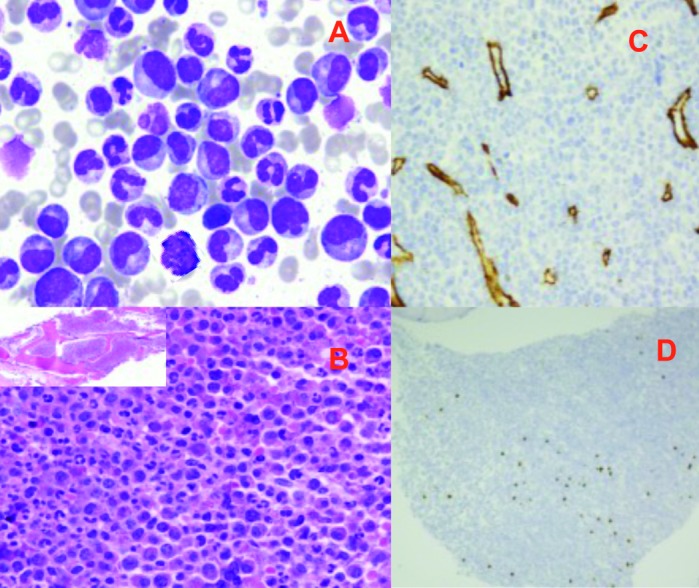
A diagnosis of Chronic Myelogenous Leukemia (CML), Chronic Phase “Cryptic/variant BCR/ABL1 gene rearrangement was made. Meanwhile, the patient’s left neck swelling increased progressively, computerized tomography (CT) scan revealed multiple significantly enlarged cervical lymph nodes (LNs). LN touch preparation showed infiltration with many blasts of high nucleocytoplasmic ratio, open nuclear chromatin and few nucleoli (fig. 3A). Histopathologic examination of the excised LN showed an effacement of architecture with diffuse infiltration by medium to large homogenous population of cells with finely granular chromatin and scanty cytoplasm and associated with many mitotic figures (Figure 3B).
Figure 3.
Lymph node touch prep. (50x) showing many blasts of small to medium with high nucleocytoplasmic ratio (A). LN biopsy (Hematoxylin & Eosin stain) shows medium to large homogenous population of cells with finely granular chromatin, inconspicuous nucleoli and scanty cytoplasm associated with many mitotic figures (B). Immunohistochemical studies showed positivity for TdT (C), CD20 (partial) (D) and high KI-67 (E)
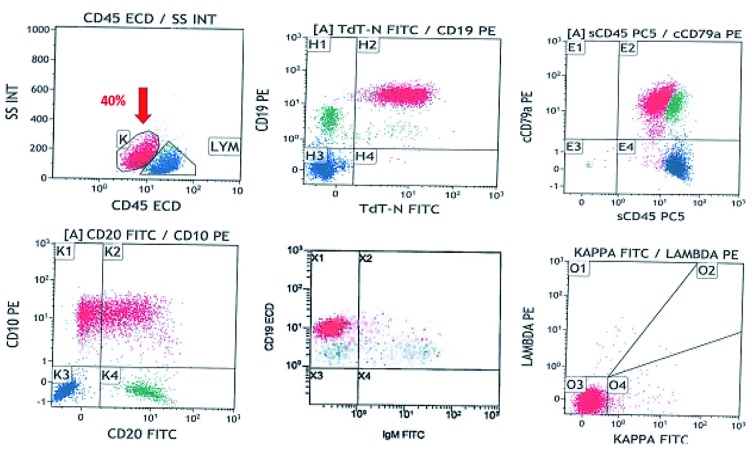
Immunohistochemical studies showed positivity for CD45, CD79a and BCL2 with strong and diffuse positive staining for TdT (Figure 3C) with partial expression of CD20 (Figure 3D) and a high mitotic index as reflected by high Ki67 (~90%) (Fig. 3E). Flow cytometry on excised LN (Figure 4) showed a population of blasts (approximately 40%), expressing CD45 (dim), CD19, CD10, CD9, cytoplasmic CD79a, HLA-DR, CD38, and TdT. There was a partial expression of CD20 on a subpopulation of cells. The blasts were negative for and surface and cytoplasmic IgM.
Figure 4.
Flow cytometry on excised LN shows a population of blasts (~40%), expressing CD45 (dim), CD19, CD10, cytoplasmic CD79a, HLA-DR, CD38, and TdT. There is partial expression of CD20 on a subpopulation of cells. The blasts are negative for and surface and cytoplasmic IgM.
FISH analysis performed on nodal tissue using dual fusion BCR/ABL1 probe revealed two fusions indicate BCR/ABL1 rearrangements on derivative chromosome 9, a truncated BCR (green signal) indicating deletion of 22q and a red and green signal from normal chromosome 9 and 22, respectively (Figure 5), confirming that nodal involvement was a lymphoid blast phase (BP) of CML and excluding the possibility of de novo ALL as second unrelated pathology.
Figure 5.
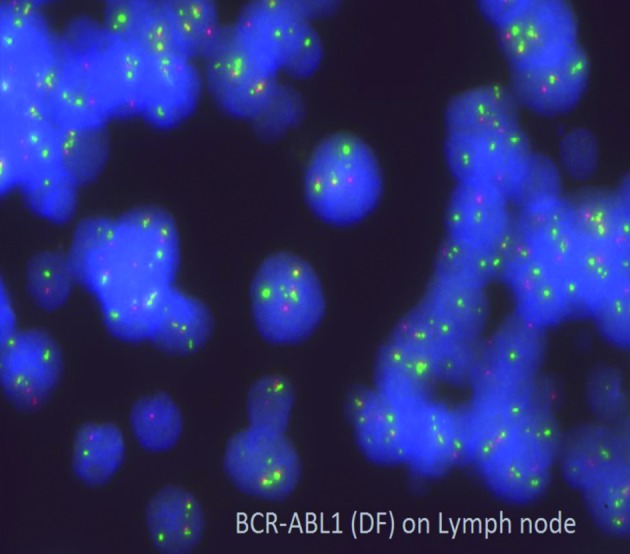
Interphase FISH on LN using dual fusion BCR/ABL1 probe. Two fusions indicate BCR/ABL1 rearrangements on derivative chromosome 9, a truncated BCR (green signal) indicating deletion of 22q and a red and green signal from normal chromosome 9 and 22, respectively
The morphologic, immunophenotypic and immunohistochemical features were consistent with nodal involvement by precursor B-cell lymphoblastic lymphoma, compatible with a diagnosis of Chronic Myeloid Leukaemia (CML), extramedullary B-Lymphoblastic Phase with “cryptic BCR/ABL rearrangement.
Staging PET/CT scan showed evidence of an increased FDG uptake seen in multiple lymph nodes, above and below the diaphragm with increased fluorodeoxyglucose (FDG) uptake of the BM, increased left testicular FDG uptake, suggesting a disease infiltrates and splenomegaly with slightly elevated uptake.
Because of increasing of the patients’ white blood cells count, he started a treatment with hydroxyurea followed by tyrosine kinase inhibitors (TKI; Imatinib). LNs responded very well to treatment with a decrease of their size and metabolic activity.
Hyper CVAD chemotherapy plus Dasatinib was started later. The patient received a total of 4 cycles (8 courses A & B) of Hyper CVAD followed by Dastatinib as maintenance.
FISH for BCR/ABL1 t(9;22)(q34;q11.2) evaluation, after two cycles of HYPECVAD protocol, was negative and molecular monitoring by PCR showed BCR-ABL1 to ABL1 percentage ratio of 2.6% (IS). We observed a significant reduction of hypermetabolic lymph nodes activity without development of any FDG avid lesions.
At end of treatment, after 4 cycles of HYPERCVAD, FISH for BCR/ABL1 was negative, with BCR-ABL1 to ABL1 percentage ratio of 0.06% (IS) assessed by PCR. The patient currently is maintained on Dasatinib 140 mg once daily.
Discussion
In the great majority of CML >90% of patients, BCR/ABL is cytogenetically visualized as t(9;22); giving rise to the Ph chromosome, or derivative 22, harboring the chimeric gene (1, 2). The BCR/ABL dual colour dual fusion (DF) probe set consists of the ABL probe spanning 650 kb from an area centromeric of the ASS (arginosuccinate synthetase) gene to the telomeric end of the ABL gene. The BCR probe target spans about 1.5 Mb, beginning within the variable segments of the immunoglobulin lambda light chain locus (IGLV), and extends through the BCR gene to approximately 900 kb telomeric of BCR. The ABL probe is directly labelled with Spectrum OrangeTM and the BCR probe with Spectrum GreenTM (Figure 2A).
At diagnosis, from 2% to 10% of CML patients lacks the classic Ph by chromosome banding analysis (CBA), despite the detection of the BCR/ABL rearrangement by FISH or by molecular analysis (RT-PCR) (3). These latter patients with variant or masked translocation: are described as CML Ph negative/BCR/ABL-1 positive with the chimeric gene present on the derivative chromosome 22, as in most CML cases, or alternatively on the derivative 9 as seen in our case.
Cryptic BCR/ABL rearrangements can be found in cases with a normal karyotype or in those with complex karyotype (3).
Variant Ph rearrangement can arise by one of two proposed mechanisms: the first one could be a cryptic insertion of chromosome 22, harboring 5’ BCR within the ABL gene or viceversa; the second mechanism could encompass two consecutive translocations, in which a standard t(9;22) translocation is followed by an inverse translocation with different breakpoints, that reconstitutes the normal morphology of the partner chromosomes (3).
The prognostic significance of cryptic Ph translocations has been previously in the literature, while some authors reported an adverse prognosis associated with the presence of these cryptic rearrangements compared to classic translocations in patients treated with conventional chemotherapy and/or α-interferon (α-IFN) therapy; others did not find any differences in terms of outcome (4).
Some reports discussed worse outcome associated with location of BCR/ABL on the der 9 (5).
Data regarding the outcome of Ph-neg CML patients treated with TKIs are limited (6).
Ph-negative CML cases treated with imatinib have been described in 5 previous reports (3, 5, 7). Recently Luatti et al. (6) studied 6 patients with Ph-neg CML patients and found that most patients benefited from TKIs therapy and achieved complete cytogenetics response (CCgR) and major molecular response (MMR), showing outcome similar to that of Ph+ CML patients and concluded that Ph-neg rearrangement does not influence the response to TKIs therapy.
In the majority of cases, CML is diagnosed in the chronic phase; it is less frequently diagnosed in accelerated crises, and occasionally, its initial presentation is as blast phase of CML. Among these patients, approximately 70% have myeloid disease, and the remaining 20% have lymphoid disease or rarely of mixed myeloid and lymphoid origin (4, 8). Extramedullary blast phase of CML is defined as the development of extramedullary BCR/ABL-1 blast proliferation regardless of proliferation of blasts in the bone marrow.
The prevalence of extramedullary blast phase (BP) has been reported to be 7-17% in patients with BP. Extramedullary BP in the newly diagnosed patients is extremely rare as the prevalence of an accelerated phase and BP as initial presentations has been reported to be only 5-10% in CML patients (8). However, the majority of extramedullary involvement occurs in association with peripheral and/or BM blastemia and the patients have features of Blast phase or accelerated phase of CML. It is reported that extramedullary blast crisis is almost always followed by hematological blast crisis, so it is considered to be an early sign of blast crisis in the BM (9).
Although the induction therapy was not consolidated with allogeneic stem cell transplantation (allo-SCT), our patient remained in complete cytogenetic and molecular remission, on single-agent dasatinib, 20 months after the diagnosis with no current evidence of active extramedullary disease.
Since the combination of features, in our case report is so unique and never described before, no standard of care had been published before in such cases.
Conclusions
This is the first case report of CML associated to cryptic Philadelphia (Ph) translocation and extramedullary B-lymphoid blast crisis as an initial presentation with sparing of PB and BM which showed features of CML in chronic phase.
We stress the importance that the absence of hematologic blast proliferation in PB or BM in cases of CML doesn’t by all means exclude the possibility of concomitant extramedullary blast phase as an initial event. Although rare, this possibility should be raised and thoroughly investigated whenever suspected.
The combined cytogenetic, molecular and clinical studies in CML cases can bring to light the frequency of this event and disease outcome.
References
- 1.Deininger MWN, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Goldman JM, Melo JV. Chronic myeloid leukemia: advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 3.Haigh S, Cuthbert G. Fluorescence in situ hybridization characterization study of complex t(9;22) in two chronic myelocytic leukemia cases with a masked Philadelphia chromosome. Cancer Genet Cytogenet. 2004;155:132–137. [Google Scholar]
- 4.Zagaria A, Anelli L, Albano F, Storlazzi CT, Liso A, Roberti MG, Buquicchio C, Liso V, Rochhi M, Specchia G. A fluorescence in situ hybridization study of complex t(9;22) in two chronic myelocytic leukemia cases with a masked Philadelphia chromosome. Cancer Genet Cytogenet. 2004;150:81–85. doi: 10.1016/j.cancergencyto.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Costa D, Espinet B, Queralt R, Carriò A, Solé F, Colomer D, Cervantes F, Hernandez JA, Besses C, Campo E. Chimeric BCR/ABL gene detected by fluorescence in situ hybridization in three new cases of Philadelphia chromosome-negative chronic myelocytic leukemia. Cancer Genet Cytogenet. 2003;141:114–119. doi: 10.1016/s0165-4608(02)00662-3. [DOI] [PubMed] [Google Scholar]
- 6.Fugazza G, Garuti A, Marchelli S, Miglino M, Bruzzone R, Gatti AM, Castello S, Sessarego M. Masked Philadelphia chromosome due to atypical BCR/ABL location in the 9q34 band and duplication on the der(9) in a case of chronic myelogenous leukemia. Cancer Genet Cytogenet. 2005;163:173–175. doi: 10.1016/j.cancergencyto.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Luatti S, Baldazzi C, Marzocchi G, Ameli G, Teresa Bochicchio TM, Soverini S, Testoni N. Cryptic BCR-ABL fusion gene as variant rearrangement in chronic myeloid leukemia: molecular cytogenetic characterization and influence on TKIs therapy. Oncotarget. 2017;8:29906–29913. doi: 10.18632/oncotarget.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennour A, Bellâaj H, Ben Youssef Y, Elloumi M, Khelif A, Saad A, Sennana H. Molecular cytogenetic characterization of Philadelphia-negative rearrangements in chronic myeloid leukemia patients. J Cancer Res and Clin Oncol. 2011;137:1329–1336. doi: 10.1007/s00432-011-1002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Int Med. 1999;131:207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Keating MJ, Talpaz M, Walters RS, Smith TL, Cork A, McCredie KB, Freireich EJ. Chronic myelogenous leukemia in blast crisis. Analysis of 242patients. Am J Med. 1987;83:445–454. doi: 10.1016/0002-9343(87)90754-6. [DOI] [PubMed] [Google Scholar]