Abstract
Context
Somatic mutations have been identified in more than half of aldosterone-producing adenomas (APAs) through mutation hotspot sequencing. The underlying pathogenesis of inappropriate aldosterone synthesis in the remaining population is still unknown.
Objective
To investigate the prevalence and spectrum of somatic mutations in APAs using an aldosterone synthase (CYP11B2) immunohistochemistry (IHC)‒guided next-generation sequencing (NGS) approach.
Methods
Formalin-fixed paraffin-embedded adrenal tissue from white American patients with primary aldosteronism who underwent adrenalectomy at the University of Michigan was used. Genomic DNA was isolated from 75 APAs (identified by CYP11B2 IHC). NGS was performed to identify somatic mutations by sequencing the entire coding region of a panel of genes mutated in APAs.
Results
Somatic mutations were identified in 66 of 75 APAs (88%). Of the APAs with somatic mutations, six were smaller than coexisting CYP11B2-negative adrenocortical adenomas. The most frequently mutated gene was KCNJ5 (43%), followed by CACNA1D (21%), ATP1A1 (17%), ATP2B3 (4%), and CTNNB1 (3%). In addition to identification of previously reported mutations, we identified five previously unreported mutations (two in KCNJ5, one in ATP1A1, one in ATP2B3, and one in CACNA1D genes). KCNJ5 mutations were more frequent in women (70% vs 24% in men).
Conclusion
Comprehensive NGS of CYP11B2-expressing adrenal tumors identified somatic mutations in aldosterone-driving genes in 88% of APAs, a higher rate than in previous studies using conventional approaches.
Our study demonstrates that CYP11B2 immunohistochemistry‒guided next-generation sequencing identified somatic mutations in the vast majority of aldosterone-producing adenomas in white Americans.
Aldosterone-producing adenoma (APA) is a major subtype of primary aldosteronism (PA), the most common cause of endocrine-related hypertension (1). Next-generation sequencing (NGS) enabled the identification of somatic and germline mutations that cause inappropriate aldosterone production in patients with PA. The affected genes described so far include the aldosterone-driving genes KCNJ5 (2), ATP1A1 (3), ATP2B3 (3), CACNA1D (4, 5), CACNA1H (6), and CLCN2 (7, 8). Several studies have investigated the presence of the various aldosterone-driving mutations in APA. The largest mutation prevalence study to date was conducted by the European Network for the Study of Adrenal Tumors investigators. By sequencing directed at previously reported mutational hotspots and selected exons, Fernandes-Rosa et al. (9) assessed 474 APAs obtained through the European Network for the Study of Adrenal Tumors. This large, multicenter collaborative study identified somatic mutations in 54% of APAs, with KCNJ5 being the most frequently altered (9). Although genetic causes have been identified in many APAs, the proportion of tumors without known mutations remains high (9–14). Furthermore, the prevalence of reported somatic mutations in APAs has varied by race. In particular, somatic KCNJ5 mutations are much more common in East Asian patients than in Europeans (12–14). An important caveat to the somatic mutation prevalence studies just described is that nearly all assessed only hotspot regions or selected exons of the previously described genes.
The development of aldosterone synthase (CYP11B2)‒specific antibodies has enabled the identification of aldosterone-producing cells in adrenal tissues. CYP11B2 immunohistochemistry (IHC) provides important insight into the histopathologic and functional landscape of PA (15–19). Importantly, CYP11B2 IHC demonstrated that the aldosterone-producing source(s) may not always correspond with macroscopic adenomas (15, 18, 20) and that CYP11B2 expression may display distinct heterogeneity within a tumor (21). Previous studies that focused on assessing the prevalence of somatic mutations in APAs sequenced tumor DNA without prior demonstration of CYP11B2 expression, raising the possibility of sequence analysis of nonfunctional or nonaldosterone-producing lesions. Hence, in the current study, we investigated the prevalence of somatic mutations in APAs using a CYP11B2 IHC‒guided, comprehensive NGS approach targeting the entire coding regions of a panel of genes frequently mutated in APAs.
Materials and Methods
Patients
Eighty-five white American patients with adrenal tumor(s) who were diagnosed as having PA and who underwent adrenalectomy at the University of Michigan between 1995 and 2016 were studied. The patients were selected on the basis of the availability of formalin-fixed paraffin-embedded (FFPE) adrenal tissue blocks. Race was determined by self-identification as Caucasian or white. The clinical diagnosis of PA was made according to available institutional guidelines at the time or the Endocrine Society’s clinical practice guideline (22). Subtype classification was performed according to the results of cross-sectional imaging (CT or MRI) and/or adrenal venous sampling. Sections from FFPE adrenal tissue blocks were used for IHC and genetic analyses. This study was approved by the institutional review board of the University of Michigan.
IHC
CYP11B2 IHC was performed as described previously (21). Adrenocortical adenomas containing CYP11B2-expressing cells were considered APAs. To assess the tumor’s capacity for cortisol production, IHC for 17α-hydroxylase/17,20 lyase (CYP17A1), an enzyme required for cortisol biosynthesis, was also examined. The following primary antibodies were used for IHC: CYP11B2 (Millipore, MABS1251, mouse monoclonal; RRID:AB_2650562) (23, 24) and CYP17A1 (LifeSpan Biosciences, LS-B14227, rabbit polyclonal; RRID:AB_2088387) (25).
DNA isolation and comprehensive NGS
On the basis of the CYP11B2 IHC results, samples were obtained from APAs by scraping unstained FFPE sections guided by the CYP11B2 IHC slide using a scalpel under the Olympus SZ-40 microscope. DNA from five CYP11B2-negative tumors from five patients with PA were also isolated. These CYP11B2-negative tumors existed as dominant tumors in the adrenal containing small adjacent APAs. Genomic DNA was isolated from scraped FFPE tissue samples using the AllPrep DNA/RNA FFPE kit (Qiagen). For mutational analysis, multiplexed PCR‒based NGS was performed using Ion Torrent AmpliSeq sequencing as described previously (Thermo Fisher Scientific) (21). The panel for library construction contained amplicons targeting the full coding regions of aldosterone-driving genes (KCNJ5, ATP1A1, ATP2B3, CACNA1D, and CACNA1H), genes that are associated with other adrenal diseases (PRKACA, PRKAR1A, and ARMC5), and hotspots in the oncogenes GNAS and CTNNB1. For somatic mutation identification, data analysis was performed as described previously using validated pipelines (26). High-confidence somatic mutations observed herein that were previously reported in APAs were considered somatic mutations after visual inspection of read-level data. For high-confidence somatic mutations observed herein that were not previously reported as somatic mutations in APAs, we performed visual inspection of read-level data and confirmed somatic origin by performing NGS on adjacent normal tissue. Lastly, we also confirmed these previously unreported somatic mutations by Sanger sequencing (Supplemental Methods).
Results
Distribution of somatic mutation spectrum in white American APAs
According to CYP11B2 IHC, of 85 adrenals from patients with PA, 74 had a single APA and 1 had two APAs. Because of low DNA sample quality, one APA was excluded from further analysis, resulting in a total cohort of 75 APAs from 74 patients. Clinical characteristics of these patients are shown in Table 1. Using our CYP11B2 IHC‒guided NGS approach, we achieved an average depth of 1,816× coverage across the 75 APAs, identifying somatic mutations in 66 of 75 APAs (88%). The most frequently altered aldosterone-driving gene was KCNJ5 (32 of 75; 43%), followed by CACNA1D (16 of 75; 21%), ATP1A1 (13 of 75; 17%), and ATP2B3 (3 of 75; 4%) (Supplemental Fig. 1). Most of the identified KCNJ5 mutations occurred at either p.G151R or p.L168R, known aldosterone-dysregulating mutational hotspots in APAs (2) (Table 2). KCNJ5 somatic mutations were more commonly observed in APAs from women than in those from men (70% vs 24%, respectively; P < 0.001 by χ2 test), whereas CACNA1D and ATP1A1 somatic mutations were more frequently found in male patients (Supplemental Fig. 1). No ATP2B3 mutations were identified in women within this cohort. CTNNB1 mutations (both p.S45F) were found in two women.
Table 1.
Clinical Characteristics of Studied Patients With APA
| Characteristic | Value |
|---|---|
| N | 74 |
| Age, y | 52 (42–59) |
| Women, N/% | 30/41% |
| Systolic blood pressure, mm Hga | 157 (137–181) |
| Diastolic blood pressure, mm Hga | 90 (76–106) |
| Number of antihypertensive medicationsb | 4.0 (2.5–5.0) |
| Prevalence of hypokalemia, N/% | 71/96% |
| PAC, ng/dLc | 33.0 (24.0–49.0) |
| PRA, ng/mL/hc | 0.2 (0.1–0.6) |
| ARRd | 140 (63–373) |
Data are expressed as medians with interquartile ranges or as counts and frequencies. Hypokalemia was defined when the lowest serum potassium concentration was <3.5 mEq/L or when potassium supplementation was indicated. Number variation was based on availability of clinical data.
Abbreviations: ARR, aldosterone/renin ratio; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
n= 68.
n = 69.
n = 67.
n = 66.
Table 2.
List of Somatic Mutations Identified in White American APAs
| Amino Acid Substitutions | APAs From Men (n = 45) | APAs From Women (n = 30) |
|---|---|---|
| KCNJ5 gene | 11 (24%) | 21 (70%) |
| p.G151R | 6 | 8 |
| p.L168R | 2 | 11 |
| p.E145Q | 2 | 0 |
| p.[F140L;G151R]a | 0 | 1 |
| p.T149delinsTIa | 1 | 0 |
| p.G151_Y152del | 0 | 1 |
| ATP1A1 gene | 10 (22%) | 3 (10%) |
| p.L104R | 7 | 3 |
| p.M102_L103del | 1 | 0 |
| p.V332G | 1 | 0 |
| p.E960_L964delinsAVa | 1 | 0 |
| ATP2B3 gene | 3 (7%) | 0 |
| p.V424_L425del | 2 | 0 |
| p.V422_L425delinsITa | 1 | 0 |
| CACNA1D gene | 15 (33%) | 1 (3%) |
| p.V259Ga | 2 | 0 |
| p.V259D | 1 | 0 |
| p.G403R | 3 | 0 |
| p.F747V | 1 | 0 |
| p.F747L | 1 | 0 |
| p.I750M | 2 | 0 |
| p.R990H | 1 | 1 |
| p.A998V | 1 | 0 |
| p.V1151F | 2 | 0 |
| p.V1338M | 1 | 0 |
| CTNNB1 gene | 0 | 2 (7%) |
| p.S45F | 0 | 2 |
| Mutation negative | 6 (13%) | 3 (10%) |
Numbers of APA-harboring corresponding mutations are listed.
Indicates previously unreported mutation. The following reference sequences were used for determining amino acid changes: NM_000890 (KCNJ5), NM_000701 (ATP1A1), NM_021949 (ATP2B3), NM_001128839 (CACNA1D), and NM_001904 (CTNNB1).
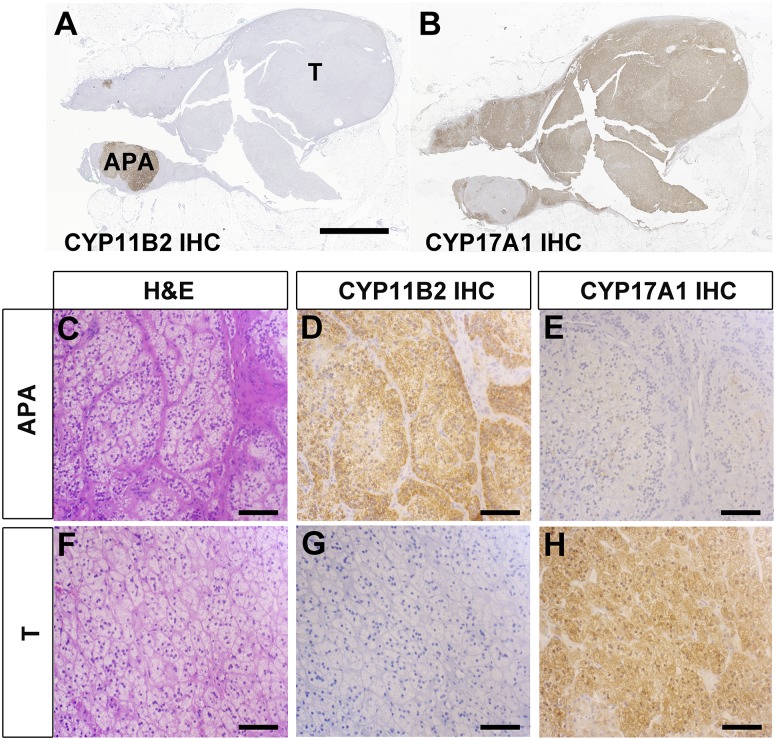
In five patients, IHC identified six CYP11B2-positive satellite tumors adjacent to a dominant CYP11B2-negative adrenocortical tumor (non-APA). The representative IHC images of these adrenals are shown in Fig. 1. In all ancillary small APAs, mutations in aldosterone-driving genes were identified by NGS (KCNJ5 p.G151R in two, CACNA1D p.F747L in one, CACNA1D p.A998V in one, and CACNA1D p.V1151F in two), whereas no aldosterone-driving mutations were identified in any of the CYP11B2-negative adrenocortical tumors. Of note, all five CYP11B2-negative tumors showed positive expression for CYP17A1 by IHC; by NGS, three of them harbored somatic GNAS gain-of-function mutations (p.R201H) that have been reported in cortisol-producing adrenocortical adenomas (27, 28).
Figure 1.
Histologic findings in an adrenal with a small APA and a dominant CYP11B2-negative adrenocortical adenoma. (A and B) Low-magnification views of (A) CYP11B2 IHC and (B) CYP17A1 IHC. Scale bar: 5 mm. (C‒H) High-magnification views of (C‒E) APA and (F‒H) CYP11B2-negative adrenocortical adenoma. (C and F) Hematoxylin and eosin (H&E) staining. (D and G) CYP11B2 IHC. (E and H) CYP17A1 IHC. Scale bar: 100 μm. T, CYP11B2-negative adrenocortical adenoma.
Previously unreported somatic mutations in APAs
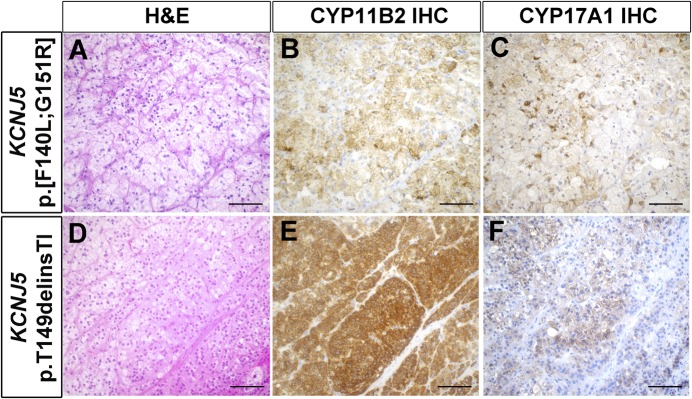
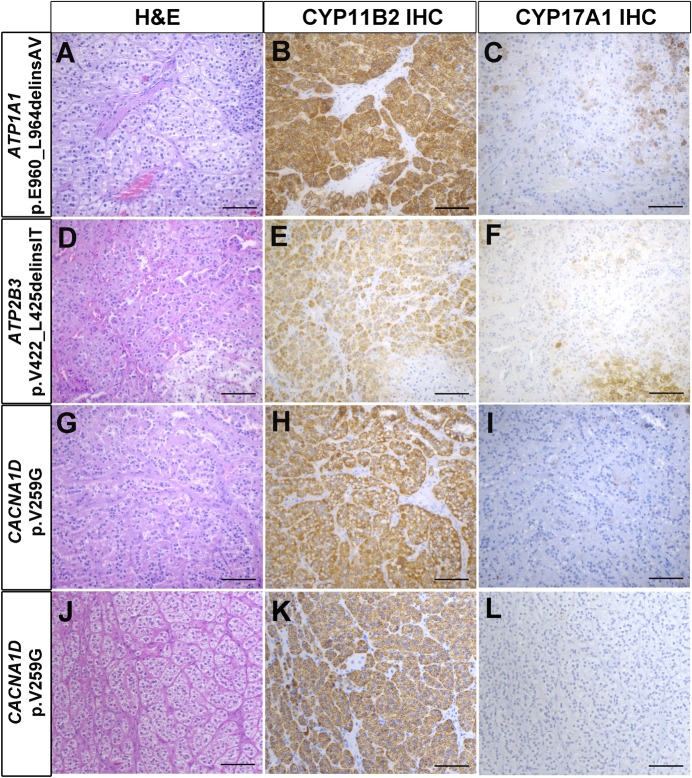
Of the mutations identified, five to our knowledge have not been previously reported, including two mutations in KCNJ5 (p.[F140L;G151R] and p.T149delinsTI), one in ATP1A1 (p.E960_L964delinsAV), one in ATP2B3 (p.V422_L425delinsIT), and one in CACNA1D (p.V259G in two APAs) (Table 2). In all cases, the mutations identified in APAs were not seen in matched, adjacent, normal adrenal tissue, confirming that the mutations were somatic. The NGS findings of these previously unreported mutations are summarized in Supplemental Table 1. Importantly, Sanger sequencing further confirmed the mutations (Supplemental Fig. 2). Read-level data of these mutations, including the in cis KCNJ5 p.[F140L;G151R] mutation (supporting both mutations occurring in the same tumor vs a collision of two tumors), are shown in Supplemental Fig. 3. Histopathologic findings of APAs with previously unreported mutations are shown in Figs. 2 and 3. In agreement with the previous reports (29, 30), APAs harboring KCNJ5 mutations were composed mainly of larger cells with more abundant lipids compared with those with other mutations. All the tumors showed positive CYP11B2 expression, whereas the expression of CYP17A1 varied from mostly negative to moderate expression (Figs. 2 and 3).
Figure 2.
Histopathologic characteristics of APAs harboring KCNJ5 somatic mutations. (A‒C) APA with KCNJ5 p.[F140L;G151R] mutation. (D‒F) APA with KCNJ5 p.T149delinsTI mutation. (A and D) Hematoxylin and eosin (H&E) staining. (B and E) CYP11B2 IHC. (C and F) CYP17A1 IHC. Scale bar: 100 µm.
Figure 3.
Histopathologic characteristics of APAs harboring somatic mutations in ATP1A1, ATP2B3, and CACNA1D. (A‒C) APA with ATP1A1 p.E960_L964delinsAV mutation. (D‒F) APA with ATP2B3 p.V422_L425delinsIT mutation. (G‒I) APA with CACNA1D p.V259G mutation (case 23). (J‒L) APA with p.V259G mutation (case 55). (A, D, G, and J) Hematoxylin and eosin (H&E) staining. (B, E, H, and K) CYP11B2 IHC. (C, F, I, and L) CYP17A1 IHC. Scale bar: 100 µm.
Discussion
The identification of somatic mutations in APAs has greatly improved our understanding of the pathophysiology of the disease and opened several venues for clinical applications in patients with PA. Studies have revealed genotype-phenotype associations of APAs. A recent study identified genotype-specific steroid profiles in APAs; in particular, the plasma hybrid steroid 18-oxocortisol was found to be higher in patients with APAs with KCNJ5 mutations than in those with other mutations (31). The measurement of 18-oxocortisol could be particularly useful as a peripheral biomarker for APAs in East Asian countries, where the prevalence of KCNJ5 mutations is high (32). More recently, Scholl et al. (33) identified macrolides as selective inhibitors of mutant KCNJ5 potassium channels using high-throughput screen models. These studies support the importance of accurate determination of somatic mutations in APAs, which may translate into clinical applicability for streamlined diagnostic workup and personalized treatment of PA.
Previous studies of genetic mutations in patients with unilateral PA have used tissue from macroscopically identified nodules (presumed to be APAs) and mutation hotspot‒based or selected exon-based sequencing approaches. By utilizing our CYP11B2 IHC‒guided, full-gene‒based NGS approach, we identified somatic mutations in 88% of APAs, a much higher detection rate of somatic mutations in APAs than previously reported [54% in the study by Fernandes-Rosa et al. (9); P < 0.001 by χ2 test]. As previously published (18) and observed in 5 of 85 of our PA cases (Fig. 1), some APAs can exist as satellite tumors adjacent to dominant CYP11B2-negative tumors. In addition, the landscape of unilateral PA is even more diverse and includes multiple microscopic aldosterone-producing cell clusters or areas of diffuse zona glomerulosa hyperplasia (17). With this in mind, materials from a dominant CYP11B2-negative tumor could have been used in past studies for DNA sequencing, leading to reduced opportunity to identify somatic mutations in aldosterone-driver genes.
In this white American cohort, KCNJ5 was the most frequently mutated gene in APAs, which is consistent with findings of previous studies from Europe and East Asian countries (9, 12–14, 34, 35). Similar to the previous studies in Europe (9, 36, 37) and a recent meta-analysis (38), KCNJ5 mutations were more frequently observed in women; 70% of APAs from women in our group harbored KCNJ5 mutations, suggesting a possible role for measurement of 18-oxocortisol for PA subtype classification in women. However, considering the relatively small sample size of our study, a multicenter study with a larger sample size using a CYP11B2 IHC‒guided sequencing approach is required to better define the prevalence of somatic mutations in APAs and to avoid potential institutional and/or selection bias.
In the current study, five previously unreported somatic mutations were identified. A full functional analysis of these variants showing an effect on aldosterone production is clearly beyond the focus of our current study. However, we clearly identified these variants as somatic mutations by excluding their presence in the matched, adjacent, normal adrenal DNA, providing evidence for a likely causative involvement of the phenotype of the analyzed APAs. Furthermore, most novel variants carry features shared with well-described pathogenic variants in the respective genes. That said, functional characterization of these mutations, including electrophysiological analysis, will be required in future studies.
Previously unreported KCNJ5 somatic mutations include p.[F140L;G151R] and p.T149delinsTI. The KCNJ5 gene encodes the G protein‒activated inward rectifier potassium channel 4 (GIRK4). The p.T149delinsTI mutation is located near the highly conserved glycine-thyrosine-glycine motif of the ion selectivity filter. Several mutations around these residues, including p.G151R mutation, have been shown to increase adrenal cell CYP11B2 transcription and enhance aldosterone production through cell membrane depolarization due to loss of ion selectivity of the channel (2, 39–42), supporting the pathologic role of the mutations identified in this study.
The previously undescribed ATP1A1 p.E960_L964delinsAV mutation locates at the transmembrane domain 9 of the α1 subunit of the Na+/K+-ATPase. Several APA-related somatic mutations have been identified in the transmembrane domain 9 (4, 34). The E961 residue is thought to contribute to forming a third Na+-binding site and to have an important role in pump function (43, 44). A somatic ATP1A1 mutation in APA involving this residue (p.E960_A963delinsS) was shown to cause inward leak currents in an electrophysiological study using Xenopus laevis oocytes (4). Most of the previously reported mutations in ATP2B3 have been identified in the transmembrane domain 4, where the ATP2B3 p.V422_L425delinsIT mutation locates. The transmembrane domain 4 of the pump is thought to be essential for the binding of Ca2+ ions, and an in vitro study introducing the previously reported L425_V426del mutation into NCI-H295R cells demonstrated increased intracellular Ca2+ activity and enhanced aldosterone production with increased CYP11B2 expression (45). Lastly, a CACNA1D mutation, p.V259G, was recurrently identified in two APAs. Somatic mutations affecting the same residue (CACNA1D p.V259D) were previously reported in APAs and were functionally characterized as causing increased intracellular Ca2+ concentration and elevated aldosterone production (4, 46).
The current study demonstrated that CYP11B2 IHC‒directed tumor selection and a comprehensive, full coding sequence‒based NGS approach results in a higher prevalence of somatic mutations than non‒IHC-directed, hotspot/selected exon sequencing‒based approaches. In agreement with previous studies (21, 37), aldosterone-driving mutations were not found in CYP11B2-negative tumor samples. The heterogeneity of CYP11B2 expression patterns in adrenals from patients with PA has been documented, and this may help explain discrepancies between macroscopic-guided sequencing and the current study’s CYP11B2–guided sequencing results. In summary, the consideration of CYP11B2 expression (by IHC or RNA analysis) and the use of comprehensive NGS-based approaches may more accurately determine the frequency and spectrum of aldosterone-driving gene mutations in APAs, maximizing the opportunity for clinical applications in patients with PA.
Supplementary Material
Acknowledgments
We thank Michelle Vinco and Farah Keyoumarsi for assistance in slide preparation. We also thank Kristina Fields for technical support with IHC.
Financial Support: This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 to W.E.R and S.A.T), the American Heart Association (17SDG33660447 to K.N. and 14SDG17990000 to T.E.), and the National Heart, Lung, and Blood Institute (1R01HL130106 to T.E.). K.O. is supported by an American Heart Association Fellowship (17POST33410759). This research was supported in part by the National Cancer Institute through a University of Michigan Cancer Center Support Grant (P30 CA46592).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- APA
aldosterone-producing adenoma
- CYP11B2
aldosterone synthase
- CYP17A1
17α-hydroxylase/17,20 lyase
- FFPE
formalin-fixed paraffin-embedded
- IHC
immunohistochemistry
- NGS
next-generation sequencing
- PA
primary aldosteronism
References
- 1. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. [DOI] [PubMed] [Google Scholar]
- 2. Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, 444e441–442. [DOI] [PubMed] [Google Scholar]
- 4. Azizan EAB, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AED, Davenport AP, Dekkers T, Tops B, Küsters B, Ceral J, Yeo GSH, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–1060. [DOI] [PubMed] [Google Scholar]
- 5. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholl UI, Stölting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C, Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandes-Rosa FL, Daniil G, Orozco IJ, Göppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, Schwarzmayr T, Strom TM, Jentsch TJ, Zennaro MC. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50(3):355–361. [DOI] [PubMed] [Google Scholar]
- 9. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–361. [DOI] [PubMed] [Google Scholar]
- 10. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6(1):19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, Fan Y, Ouyang J. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94(16):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, Wang JG, Zhu DL, Gao PJ. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65(3):622–628. [DOI] [PubMed] [Google Scholar]
- 14. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191–200. [DOI] [PubMed] [Google Scholar]
- 15. Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A, Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98(4):1567–1574. [DOI] [PubMed] [Google Scholar]
- 16. Volpe C, Hamberger B, Höög A, Mukai K, Calissendorff J, Wahrenberg H, Zedenius J, Thorén M. Primary aldosteronism: functional histopathology and long-term follow-up after unilateral adrenalectomy. Clin Endocrinol (Oxf). 2015;82(5):639–647. [DOI] [PubMed] [Google Scholar]
- 17. Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, Rainey WE, Ito S, Satoh F, Sasano H. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ, Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishimoto K, Koga M, Seki T, Oki K, Gomez-Sanchez EP, Gomez-Sanchez CE, Naruse M, Sakaguchi T, Morita S, Kosaka T, Oya M, Ogishima T, Yasuda M, Suematsu M, Kabe Y, Omura M, Nishikawa T, Mukai K. Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism. Mol Cell Endocrinol. 2017;441:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandes-Rosa FL, Giscos-Douriez I, Amar L, Gomez-Sanchez CE, Meatchi T, Boulkroun S, Zennaro MC. Different somatic mutations in multinodular adrenals with aldosterone-producing adenoma. Hypertension. 2015;66(5):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101(3):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 23. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RRID:AB_2650562.
- 25.RRID:AB_2088387.
- 26. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112(33):E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, Yoshida K, Nagata Y, Sato-Otsubo A, Yoshizato T, Suzuki H, Shiozawa Y, Kataoka K, Kon A, Aoki K, Chiba K, Tanaka H, Kume H, Miyano S, Fukayama M, Nureki O, Homma Y, Ogawa S. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science. 2014;344(6186):917–920. [DOI] [PubMed] [Google Scholar]
- 28. Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, Scholl UI. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015;172(6):677–685. [DOI] [PubMed] [Google Scholar]
- 29. Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97(5):E819–E829. [DOI] [PubMed] [Google Scholar]
- 30. Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67(1):139–145. [DOI] [PubMed] [Google Scholar]
- 32. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, Zhang J, Hoyer D, Merkel JS, Wang W, Lifton RP. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127(7):2739–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Åkerström T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, D Volpe C, Bäckdahl M, Botling J, Stålberg P, Westin G, Walz MK, Lehnert H, Sidhu S, Zedenius J, Björklund P, Hellman P. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22(5):735–744. [DOI] [PubMed] [Google Scholar]
- 35. Zennaro MC, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev. 2017;38(6):516–537. [DOI] [PubMed] [Google Scholar]
- 36. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, Mulatero P, Samson-Couterie B, Hahner S, Quinkler M, Fallo F, Letizia C, Allolio B, Ceolotto G, Cicala MV, Lang K, Lefebvre H, Lenzini L, Maniero C, Monticone S, Perrocheau M, Pilon C, Plouin PF, Rayes N, Seccia TM, Veglio F, Williams TA, Zinnamosca L, Mantero F, Benecke A, Jeunemaitre X, Reincke M, Zennaro MC. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592–598. [DOI] [PubMed] [Google Scholar]
- 37. Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EA, Tops B, Deinum J, Küsters B. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99(7):E1341–E1351. [DOI] [PubMed] [Google Scholar]
- 38. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089–E1095. [DOI] [PubMed] [Google Scholar]
- 39. Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97(8):E1567–E1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, Warth R. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155(4):1353–1362. [DOI] [PubMed] [Google Scholar]
- 41. Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, Fishman V, Zanotti G, Gomez-Sanchez C, Bader M, Warth R, Rossi GP. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab. 2014;99(9):E1765–E1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98(11):E1861–E1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ogawa H, Toyoshima C. Homology modeling of the cation binding sites of Na+K+-ATPase. Proc Natl Acad Sci USA. 2002;99(25):15977–15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li C, Capendeguy O, Geering K, Horisberger JD. A third Na+-binding site in the sodium pump. Proc Natl Acad Sci USA. 2005;102(36):12706–12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157(6):2489–2499. [DOI] [PubMed] [Google Scholar]
- 46. Xie CB, Shaikh LH, Garg S, Tanriver G, Teo AE, Zhou J, Maniero C, Zhao W, Kang S, Silverman RB, Azizan EA, Brown MJ. Regulation of aldosterone secretion by Cav1.3. Sci Rep. 2016;6(1):24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.