Abstract
Context
Many differentiated thyroid cancers (DTC) dedifferentiate and become radioactive iodine (RAI)–refractory (RAIR) with worse outcomes. Targeted therapy (TTx) may downregulate MAPK signaling and sensitize tumors to RAI.
Objective
We describe patients with RAIR DTC receiving TTx with demonstrated RAI uptake allowing for iodine-131 (I131) administration.
Design
Charts of patients with metastatic, progressive, RAIR DTC in whom TTx increased RAI uptake on a diagnostic whole-body scan (WBS), were reviewed. Results of WBS, I131 administration, thyroglobulin (TG) panels, and cross-sectional studies were recorded.
Setting
Thirteen patients [median age (range), 56 (45 to 75) years; seven men] were included; 11 (85%) had DTC, two (15%) had poorly DTC. Nine (69%) had BRAF mutations, three (23%) had RAS mutations, and one (8%) was wild type. Selective BRAF or an MEK inhibitor TTx was continued for a median (range) of 14.3 (1 to 76.4) months before diagnostic WBS.
Results
Nine (69%) patients were treated with I131 [median (range) activity, 204.4 (150 to 253) mCi], after which TTx was discontinued. Median (range) follow-up was 8.3 (0 to 17.4) months after I131 therapy. All nine patients had durable disease control (three had partial response, six had stable disease). TG and TG antibody levels increased in patients who demonstrated uptake before TTx, and declined in eight of the nine patients after I131 treatment. Adverse events included pneumonitis and sialadenitis.
Conclusion
TTx in BRAF−/RAS–mutated RAIR DTC resensitizes tumors to iodine. Subsequent I131 administration results in meaningful responses. Patient selection, adverse events, response duration, and survival impact require additional study.
This is a retrospective review of the effect of targeted therapy on RAI redifferentiation in BRAF- or RAS-mutated DTCs. Long term outcomes using this approach are highlighted.
Differentiated thyroid cancer (DTC) makes up ∼90% of all thyroid cancers (1). A three-pronged treatment approach of surgery with or without radioactive iodine (RAI) therapy and thyrotropin-suppressive thyroid hormone provides an excellent prognosis for most localized, well-differentiated disease. The prognosis for distantly metastatic disease drops significantly, however, with an estimated 10-year survival rate of 42% (2). Radioiodine refractory (RAIR) disease has an even more dismal prognosis, with an estimated 10-year survival of just 10% (3). Management options in that subset of patients include active surveillance, local therapy for metastatic sites, or multikinase inhibitor therapy for rapidly progressing, symptomatic, or life-threatening disease (1, 4–6).
Multiple agents to attempt to restore RAI avidity in patients with RAIR DTC have been studied. The use of retinoids (7–9), romidpesin (10), rosiglitazone (11), and even the multikinase inhibitor sorafenib (12) unfortunately proved to be of little clinical value. Understanding the biological pathways implicated in thyroid cancer has allowed us to define therapeutic targets. Specifically, gain-of-function mutations in the MAPK signaling pathway, in particular the BRAF V600E mutation, have been implicated in loss of the sodium-iodine symporter that mediates iodine uptake (13, 14). In one study, BRAF-mutant tumors had reduced expression of all thyroid-specific genes involved in iodine metabolism (13). In another study, targeted inhibition of the MAPK pathway with a MEK inhibitor restored the expression of iodide-metabolizing genes in normal and malignant thyroid cells (14). In a series of very elegant experiments, Chakravarty et al. (15) showed that activation of BRAF V600E transformed thyroid follicular cells into poorly DTC with inability to incorporate RAI, and that inactivation of this mutation with a MEK or BRAF inhibitor restored normal architecture and RAI sensitivity.
This work laid the foundation for more recent trials that showed promising results with use of the MEK inhibitor selumetinib (16) and the BRAF inhibitor dabrafenib (17). Of 12 patients treated with selumetinib, eight had a clinically meaningful uptake to warrant treatment with RAI, of whom five had objective response and three had durable stable disease (16). Of the 10 patients treated with dabrafenib, six had a clinically meaningful uptake on diagnostic RAI scans to warrant treatment and two had objective response as well (17). Long-term outcomes of these patients, however, are not known. In this article, we describe the experience from a tertiary cancer center of using targeted therapy (TTx) in restoring RAI avidity in patients with previously RAIR, advanced thyroid cancer.
Methods
Study
We describe 13 patients with RAIR disease who were treated with TTx using either single-agent or combination MEK and/or BRAF inhibitors and who underwent a diagnostic whole-body scan (WBS) while receiving therapy. Refractoriness to RAI was defined as disease having at least one of the following: no RAI uptake at known sites of metastases, progressive disease (PD) despite previous RAI treatment with confirmed uptake, or PD within 1 year of RAI therapy. This retrospective study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center.
Design
Demographics, tumor characteristics (i.e., histologic subtype, stage, and mutational status), and details of prior RAI treatments were collected and recorded from charts of the 13 patients identified for this study. Details of TTx, including the name, dose, and duration of treatment, were recorded. If the patients were treated with iodine-131 (I131; after low-iodine diet intake of 1 to 2 weeks) because their physicians interpreted their pretreatment WBS as showing meaningful RAI uptake, subsequent follow-up data were collected, including further TTx, serial cross-sectional imaging, and thyroglobulin (TG) panels.
Response
Radiologic response on cross-sectional imaging over 12 to 14 months was measured for all patients, using Response Evaluation Criteria in Solid Tumors, version 1.1. Target lesions were measured at baseline; at best response on TTx; before RAI therapy, if given; and at best response after RAI therapy while not receiving TTx. TG and TG antibody (TG Ab) levels were also recorded before initiation of TTx, while receiving TTx and after treatment with RAI, when applicable.
Statistical analysis
The primary end point was the additional radiologic response I131 therapy achieved over that seen with TTx alone for patients who were treated with RAI while receiving TTx. Secondary end points were best response to therapy; duration of response, including time off systemic therapy; and time to progression. Radiologic responses were assessed using Response Evaluation Criteria in Solid Tumors, version 1.1, by a single radiologist. Biochemical responses were assessed with serum TG and TG Ab levels measured using the same assay at the same laboratory.
Results
Study population
A total of 13 patients with RAIR, advanced thyroid cancer were included in the study. TTx had been started either for PD (eight of 13 patients; 62%) or with the intent to induce RAI redifferentiation (five of 13 patients; 38%).
Baseline characteristics of the 13 patients are listed in Table 1. The median age was 55.6 (range, 45 to 75) years. Seven (54%) were men. Ten patients (77%) had classic or follicular variant of papillary thyroid cancer, two patients (15%) had poorly DTC, and one patient (8%) had follicular thyroid cancer. Nine patients (70%) had a BRAF V600E mutation, two patients (15%) had an NRAS mutation, one patient (7.5%) had a KRAS mutation, and one patient (7.5%) was wild type for 400 tested genes, including BRAF and RAS. All but one patient had undergone at least one prior RAI therapy, with a median cumulative activity of 163 mCi. One patient had no prior RAI treatments because she was deemed to be RAI refractory on the basis of a negative diagnostic scan at initial presentation to our institution despite biopsy-proven macronodular lung metastases that were fluorodeoxyglucose avid on concomitant fluorodeoxyglucose positron emission–CT (18).
Table 1.
Baseline Characteristics (N = 13)
| Characteristic | No. (%)a |
|---|---|
| Age at diagnosis, mean (range), y | 55.6 (45–75) |
| Male sex | 7 (54) |
| Race | |
| White | 9 (69) |
| Hispanic | 3 (23) |
| Black | 1 (8) |
| Histology | |
| Papillary thyroid cancer | 10 (77) |
| Poorly DTC | 2 (15) |
| Follicular thyroid cancer | 1 (8) |
| Tumor genotype | |
| BRAF V600E | 9 (70) |
| NRAS/KRAS | 3 (23) |
| WT | 1 (7) |
| Prior RAI treatments, median (range), no. | 1 (0–3) |
| Cumulative administered activity, median (range), mCi | 163 (0–470) |
| Prior treatments for thyroid cancer | |
| Radiation therapy | 3 (23) |
| Reoperation | 9 (70) |
| Prior systemic therapyb | 4 (30) |
Abbreviation: WT, wild-type.
Unless otherwise indicated.
Prior systemic therapies include sorafenib, vemurafenib, and lenvatinib.
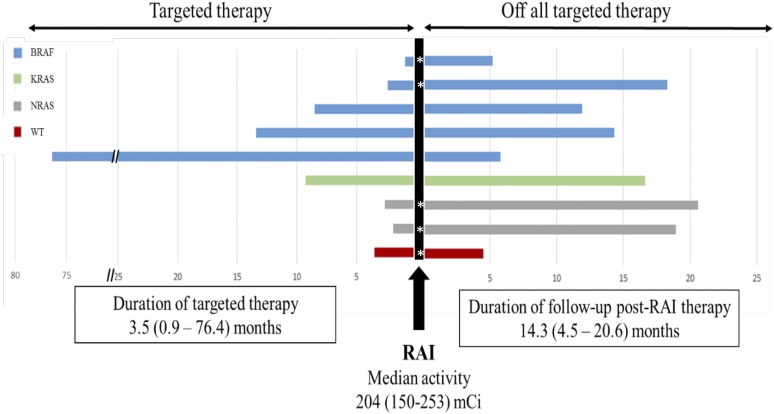
Based on the identified somatic mutation, patients were treated with either a selective BRAF or a MEK inhibitor. Of the nine patients with a BRAF V600E mutation, all were treated with a BRAF inhibitor (seven with dabrafenib, one with vemurafenib, and one with combination dabrafenib and trametinib). The three patients with RAS mutation were all treated with a MEK inhibitor (two with trametinib, one with an investigational MEK inhibitor). The patient who had no identified somatic mutations was treated with trametinib. The median duration of TTx before the diagnostic WBS was 14.3 (range, 0.9 to 76.4) months (Fig. 1).
Figure 1.
TTx, RAI therapy, and follow-up. The left side of the figure represents patients’ data from before RAI therapy while receiving TTx. The right side of the figure shows duration after RAI therapy while not receiving TTx. WT, wild-type.
Efficacy
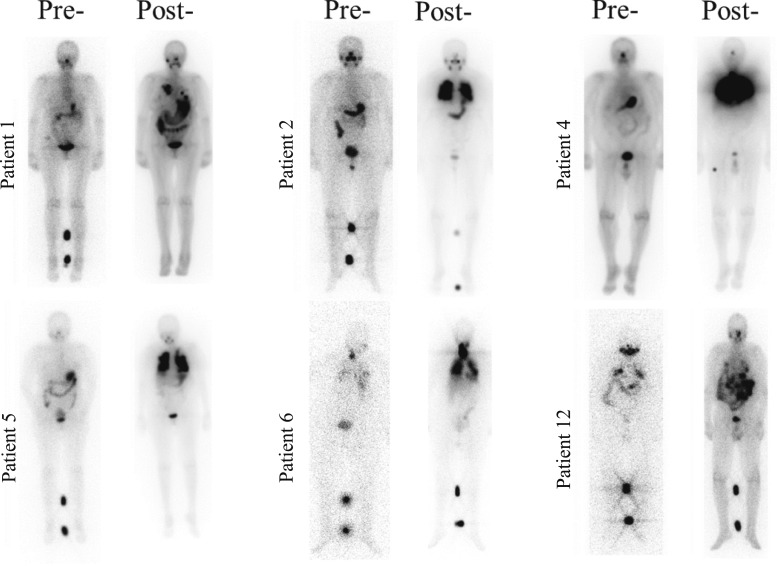
Of the 13 patients, eight (62%) had clinically meaningful uptake to warrant therapy with RAI. Based on the treating physician’s clinical judgment, an additional patient was empirically treated with RAI despite no uptake on pretreatment scan. For the nine patients treated with I131, the median administered activity was 204.4 (range, 150 to 253) mCi. Figure 2 shows posttreatment scans for select patients at the time they were deemed to be RAI refractory and at the time of RAI resensitization while receiving TTx. Of note, all RAS-mutated patients had meaningful uptake to warrant therapy with I131. Blood dosimetry was used to calculate the I131 activity in three patients, and empiric dosing was used in the other patients. TTx was discontinued 2 days after treatment.
Figure 2.
Posttreatment WBSs demonstrating restoration of RAI avidity after TTx. Posttreatment scans demonstrate uptake after RAI resensitization. Post, WBS done while patients were receiving TTx. Pre, before initiation of systemic therapy at the time the patients were deemed to be RAI refractory.
Radiologic response
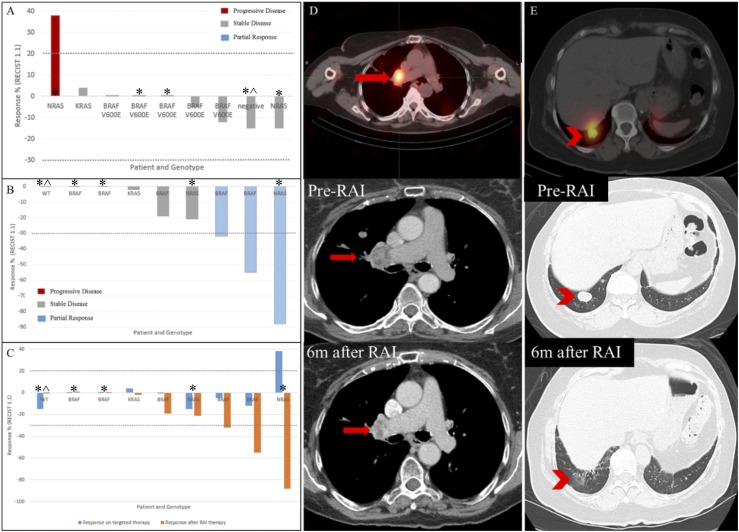
For the nine patients treated with I131, the median duration of follow-up after RAI therapy was 14.3 (range, 4.5 to 20.6) months. While receiving TTx and before RAI therapy, the best overall responses for the nine patients were as follows: Eight patients had stable disease and one patient had PD (Fig. 3A). The patient with PD before RAI therapy had an NRAS mutation, began receiving TTx for the purpose of RAI redifferentiation, and received treatment for 2 months only. All nine patients treated with RAI had an overall response that was in addition to any response they had achieved while receiving TTx alone. While off TTx and compared with their best response while receiving TTx, the best overall responses for the nine patients were as follows: Three patients had a partial response (PR) and five patients had stable disease, of whom three had stable disease with regression (Fig. 3B). The patient with NRAS mutation who had rapidly PD had an additional 88% shrinkage of target lesions after therapeutic RAI administration (Fig. 3C). The patient who was wild type for all mutations tested and who was treated empirically with RAI despite no uptake on scan had stable disease after RAI therapy (Fig. 3). Figure 3C shows side-by-side responses for the nine patients while they were receiving TTx alone and after RAI therapy while off TTx. Figures 3D and 3E show representative patients’ target lesions and their response to RAI after resensitization with TTx.
Figure 3.
Radiologic responses per Response Evaluation Criteria in Solid Tumors, version 1.1, for all nine patients treated with RAI. (A) Best overall response while receiving TTx before RAI therapy. (B) Best overall response after RAI therapy while off TTx. (C) Side-by-side view of additional response achieved after RAI therapy. (D) I131 single-photon emission computed tomography revealed an RAI-avid lymph node in the right hilum (red arrow) in a patient with RAS mutation patient while receiving TTx. Serial CT chest scans demonstrate right hilar adenopathy PR after 208 mCi of I131 (decrease from 3.0 × 2.4 cm to 1.7 × 1.2 cm). (E) I131 single-photon emission computed tomography revealed multiple pulmonary RAI-avid nodules (red arrowhead) in a patient with BRAF mutation while receiving TTx. Serial CT chest scans demonstrated PR after 204 mCi of I131 with almost complete resolution of pulmonary nodule. *Patients who received targeted therapy in an attempt to rensensitize their tumors to RAI. ^The patient who was empirically treated with RAI despite no uptake on scan.
Biochemical response
Before RAI therapy, of the nine patients treated with RAI, six had increasing serum TG levels, one had undetectable TG levels but stable and markedly elevated TG Ab levels, one had undetectable TG and TG Ab levels, and for one, there were insufficient data to trend TG levels before initiation of systemic therapy. Of the four patients who did not end up receiving RAI therapy, one had rising TG levels and three had stable serum TG levels before initiation of systemic therapy despite structurally PD.
While receiving TTx, the TG (or TG Ab) levels of seven patients increased without any evidence of radiologic progression. One patient’s serum TG levels decreased while receiving systemic therapy, and one patient did not have sufficient data to determine TG trends while receiving systemic therapy. Of the four patients who were not treated with RAI therapy, only one had increasing TG levels while receiving systemic therapy (Table 2).
Table 2.
Suppressed Serum TG Values Before TTx, While Receiving TTx, and After RAI Therapy
| Patient | Suppressed Serum TG Values (ng/mL) | |||||
|---|---|---|---|---|---|---|
| Before TTx | While Receiving TTx | Months After RAI Therapy | ||||
| 3 | 6 | 9 | 12 | |||
| 1 | 542 | 2201 | 1143 | 1802 | 1532 | 832 |
| 2 | 1471 | 1549 | 464 | 231 | 201 | 160 |
| 3 | 118 | 141 | 4.8 | — | <0.9 | <0.9 |
| 4a | 3101 | 5301 | 1311 | 864 | 548 | 478 |
| 5 | 1224 | 2041 | 382 | — | 194 | 141 |
| 6 | 1267 | 12,240 | 415 | 131 | 111 | 99.3 |
| 8 | 193 | 15.9 | 6.8 | 3.8 | — | — |
| 10 | 705 | 1054 | 1307 | — | — | — |
| 12 | <0.9 | <0.9 | — | <0.9 | — | — |
Abbreviation: —, no data.
TG Ab levels (IU/mL) were used for patient 4, who always had undetectable antigen levels. All other patients’ TG Ab levels were undetectable.
After RAI therapy, eight of nine patients’ serum TG levels decreased from baseline levels before TTx was started. One patient’s serum TG level became undetectable at nine months after RAI therapy and remained undetectable at 12 months after RAI therapy. She had achieved a PR and her lesions remained stable at subsequent follow-up.
Time off systemic therapy
All nine patients treated with RAI therapy remained off systemic TTx for a median duration of 14.3 (range, 4.5 to 20.6) months.
Safety
RAI-related adverse events occurred in three of the nine treated patients. One patient had pneumonitis and was treated with 204 mCi, with a prior lifetime cumulative activity of >300 mCi (details of one RAI treatment are unknown). She required steroid treatment for symptom resolution. Another patient had a new cough and shortness of breath and was evaluated for pulmonary fibrosis suspected on chest imaging but was then considered to be consistent with pneumonitis. This patient was treated with 253 mCi of I131, with a prior lifetime cumulative activity of 723 mCi. The third patient had bilateral sialadenitis with multifocal parotid ductal stenosis. She was treated with 200.9 mCi of I131, with a prior lifetime cumulative activity of 309 mCi. All three patients’ administered treatments had been selected by blood dosimetry calculations. All three patients’ symptoms resolved within 3 months of symptom onset.
Patients not treated with RAI therapy
At a median duration of follow-up of 6.9 (range, 6.4 to 10.1) months from the diagnostic WBS obtained to determine whether the tumors had resensitized to RAI, all four patients who were not treated had continued to receive systemic therapy. At the time, three of the four had stable disease and one had a mixed response with some lesions remaining stable while others enlarged. All four patients were men (median age, 48 years) with BRAF-mutated papillary thyroid cancer.
Discussion
Although most cases of DTC have an excellent prognosis, survival drastically decreases for patients with metastatic RAIR disease (2, 3, 6). RAI remains the only known cure for metastatic RAI-sensitive disease; there is no curative therapy currently available for metastatic RAIR thyroid cancer. The use of a redifferentiation strategy to permit additional RAI therapy for patients with RAIR disease may offer a cure in appropriately selected patients while minimizing exposure to kinase inhibitor therapy.
Earlier studies that described agents used for resensitization of RAIR thyroid cancer to RAI before the advent of molecular TTx showed little clinical benefit (7, 9, 11). As the understanding of the molecular pathways implicated in thyroid cancer grew and with the knowledge that activated MAPK signaling suppressed uptake of RAI, later studies investigated the use of agents that inhibited this pathway in restoration of RAI sensitivity. Given its weak BRAF inhibition properties, it is thus not surprising that sorafenib did not show any promise using a resensitization approach (12). The use of potent selective BRAF or MEK inhibitors in the two most recent studies by Ho et al. (16) and Rothenberg et al. (17), respectively, further supports the role of the MAPK pathway to regulate iodine uptake in thyroid cells. As in our study, patients enrolled in these two studies also had progressive, metastatic, RAIR DTC. What is interesting to note is that patients with RAS mutations in our study as well as that of Ho et al. (16) had sufficient RAI uptake to allow for a therapeutic I131 therapy. In all three studies, including ours, patients who did not have RAI uptake after TTx had BRAF mutations (16, 17). It is unclear why the response to therapy may be different between these two groups, but potentially RAS mutations may be more amenable to redifferentiation. It is unlikely a factor of the TTx used, because, in our study, one patient with BRAF-mutated disease was treated with combination BRAF/MEK inhibition without sufficient RAI uptake to warrant therapy. The responses seen in the studies by Ho et al. (16) and Rothenberg et al. (17) were evaluated at 6 months, but we offer responses evaluated at >1 year after RAI therapy.
We hypothesize that a sustained increase in TG (or TG Ab) levels while receiving TTx may be a marker of redifferentiation and subsequent response to RAI. In our study and while receiving TTx, seven of the nine patients treated with RAI had increasing TG (or TG Ab) levels without any evidence of radiologic progression. Several other investigators have noted the increase in TG levels in patients treated with BRAF inhibitors (19, 20). From the available published data by Ho et al. (16) and Rothenberg et al. (17), 10 of the 14 patients treated with RAI had increasing TG levels while receiving selumetinib or dabrafenib. Of the four patients who did not show evidence of RAI resensitization, three patients did not mount a TG response while receiving TTx. It thus may be worthwhile to consider obtaining a diagnostic WBS when serum TG levels are increasing while receiving TTx, assuming there is no evidence of radiologic progression.
Although pneumonitis is not a common adverse event of RAI therapy in adults, two of our patients had pneumonitis without progression to fibrosis. Both these patients had BRAF mutations, and they were both treated with dabrafenib (one for ∼8.5 months and one for 10 months) and had been exposed to at least two prior RAI therapies. At time of last follow-up, both had achieved PR and had remained off dabrafenib. Higher activities of RAI may have contributed to their response at the expense of certain toxicities without achieving a cure. We also do not have any data on survival benefit using this approach, and so benefits and risks should be carefully weighed before adopting this management approach.
The disease control rate for patients who converted from RAIR to RAI sensitive in our study was 100% and the responses observed were durable for a median duration of >1 year while not receiving chronic, expensive TTx. This was not a randomized trial; therefore, we do not know the duration of stable disease these patients might have experienced in the absence of RAI therapy. With increasing incidence of cancer diagnoses and use of oral TTx, the financial toxicity as it relates to both the objective and subjective financial consequences that cancer imposes on a patient is also on the rise (21, 22). In regard to patients with RAIR thyroid cancer, existing studies do not adequately portray the disease’s economic burden (23, 24). We do know, however, that use of oral chemotherapeutic agents for various solid tumors is increasing with monthly per-patient payments more than doubling over the last decade, reaching >$7000 per patient per month in 2011 (25). To be able to take patients off these therapies and keep them off for >1 year will also improve other outcomes that have been tied to financial stress, such as quality of life (26, 27), symptom control (27), and even survival (28).
In summary, the use of TTx as a means of redifferentiating RAIR thyroid cancer to RAI treatment appears to be a promising approach in patients with somatic BRAF or RAS mutations. Additional studies are needed to determine the characteristics of patients who are most likely to benefit; to clarify the risks of treatment, such as pneumonitis; and to describe the duration of clinical response and its impact on survival.
Acknowledgments
Financial Support: This work was supported in part by a Cancer Center Support Grant (National Cancer Institute Grant P30 CA016672).
Disclosure Summary: M.E.C. serves on the advisory board of LOXO and Blueprint and has received research funds from Genentech, Eisai, and Exelixis. R.D. serves on the advisory board of Elisai and Bristol Myers-Squibb. N.L.B. has received grant funding from Bayer and consulting fees from Eisai and Sanofi-Genzyme. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- Ab
antibody
- DTC
differentiated thyroid cancer
- I131
iodine-131
- PR
partial response
- RAI
radioactive iodine
- RAIR
radioactive iodine refractory
- TG
thyroglobulin
- TTx
targeted therapy
- WBS
whole-body scan
References
- 1. Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res. 2012;2012:618985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Thyroid Cancer (version 2.2017). www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed 28 August 2017.
- 5. Dadu R, Cabanillas ME. Optimizing therapy for radioactive iodine-refractory differentiated thyroid cancer: current state of the art and future directions. Minerva Endocrinol. 2012;37(4):335–356. [PMC free article] [PubMed] [Google Scholar]
- 6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grüning T, Tiepolt C, Zöphel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer--does it hold its promise? Eur J Endocrinol. 2003;148(4):395–402. [DOI] [PubMed] [Google Scholar]
- 8. Short SC, Suovuori A, Cook G, Vivian G, Harmer C. A phase II study using retinoids as redifferentiation agents to increase iodine uptake in metastatic thyroid cancer. Clin Oncol (R Coll Radiol). 2004;16(8):569–574. [DOI] [PubMed] [Google Scholar]
- 9. Liu YY, Stokkel MP, Pereira AM, Corssmit EP, Morreau HA, Romijn JA, Smit JW. Bexarotene increases uptake of radioiodide in metastases of differentiated thyroid carcinoma. Eur J Endocrinol. 2006;154(4):525–531. [DOI] [PubMed] [Google Scholar]
- 10. Sherman EJ, Su YB, Lyall A, Schöder H, Fury MG, Ghossein RA, Haque S, Lisa D, Shaha AR, Tuttle RM, Pfister DG. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013;23(5):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid. 2009;19(9):953–956. [DOI] [PubMed] [Google Scholar]
- 12. Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, Weijers K, Pereira AM, Huijberts M, Kapiteijn E, Romijn JA, Smit JW. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161(6):923–931. [DOI] [PubMed] [Google Scholar]
- 13. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92(7):2840–2843. [DOI] [PubMed] [Google Scholar]
- 14. Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13(4):1341–1349. [DOI] [PubMed] [Google Scholar]
- 15. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rothenberg SM, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib-response. Clin Cancer Res. 2015;21(24):5640–5641. [DOI] [PubMed] [Google Scholar]
- 18. Tuttle RM, Sabra MM. Defining RAI refractory thyroid cancer: when is RAI therapy unlikely to achieve a therapeutic response? Available at: www.thyroidmanager.org/wp-content/uploads/chapters/s2-defining-rai-refractory-thyroid-cancer-when-is-rai-therapy-unlikely-to-achieve-a-therapeutic-response.pdf. Accessed 24 June 2018.
- 19. Dadu R, Shah K, Busaidy NL, Waguespack SG, Habra MA, Ying AK, Hu MI, Bassett R, Jimenez C, Sherman SI, Cabanillas ME. Efficacy and tolerability of vemurafenib in patients with BRAF(V600E) -positive papillary thyroid cancer: M.D. Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100(1):E77–E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konda B, Nabhan F, Weil L, Busaidy N, Wirth LJ, Daniels GA, DeSouza JA, Timmers CD, Sexton JL, Beshara M, Nichols D, Snyder N, Devine CE, Shah MH. Thyroglobulin does not correlate with response in radioactive iodine refractory (RAIR) BRAF mutated papillary thyroid cancer (PTC) treated with dabrafenib alone or in combination with trametinib [abstract]. Thyroid. 2017;27(S1):P-1-A-156.
- 21. de Souza JA, Yap BJ, Hlubocky FJ, Wroblewski K, Ratain MJ, Cella D, Daugherty CK. The development of a financial toxicity patient-reported outcome in cancer: the COST measure. Cancer. 2014;120(20):3245–3253. [DOI] [PubMed] [Google Scholar]
- 22. de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araújo FS, Hlubocky FJ, Nicholas LH, O’Connor JM, Brockstein B, Ratain MJ, Daugherty CK, Cella D. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson RT, Linnehan JE, Tongbram V, Keating K, Wirth LJ. Clinical, safety, and economic evidence in radioactive iodine-refractory differentiated thyroid cancer: a systematic literature review. Thyroid. 2013;23(4):392–407. [DOI] [PubMed] [Google Scholar]
- 24. Gallop K, Kerr C, Simmons S, McIver B, Cohen EE. A qualitative evaluation of the validity of published health utilities and generic health utility measures for capturing health-related quality of life (HRQL) impact of differentiated thyroid cancer (DTC) at different treatment phases. Qual Life Res. 2015;24(2):325–338. [DOI] [PubMed] [Google Scholar]
- 25. Shih YC, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol. 2015;33(19):2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramsey SD, Bansal A, Fedorenko CR, Blough DK, Overstreet KA, Shankaran V, Newcomb P. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]