Abstract
Context
Adolescents with type 1 diabetes (T1D) have difficulty obtaining optimal glucose control, which may relate to insulin resistance (IR), especially during puberty. Moreover, IR increases the risk for cardiovascular disease, the leading cause of death in T1D. However, the tissue specificity of IR in adolescents with T1D has not been fully phenotyped.
Objective
To assess adipose, hepatic, and peripheral insulin sensitivity in adolescents with and without T1D.
Design and Setting
Thirty-five youth with T1D [median age, 16 (first and third quartiles, 14, 17) years; 53% female; median body mass index (BMI) percentile, 82nd (55th, 96th); late puberty; median hemoglobin A1c, 8.3% (7.3%, 9.4%)] and 22 nondiabetic youth of similar age, BMI, pubertal stage, and level of habitual physical activity were enrolled. Insulin action was measured with a four-phase hyperinsulinemic euglycemic clamp (basal and 10, 16, and 80 mU/m2/min) with glucose and glycerol isotope tracers.
Results
Adolescents with T1D had a significantly higher rate of lipolysis (P < 0.0001) and endogenous glucose production (P < 0.001) and lower peripheral glucose uptake (glucose rate of disappearance, 6.9 ± 2.9 mg/kg/min for patients with T1D vs 11.3 ± 3.3 for controls; P < 0.0001) during hyperinsulinemia compared with controls. In youth with T1D, glucose rate of disappearance correlated with free fatty acid at the 80-mU/m2/min phase (P = 0.005), markers of inflammation (IL-6; P = 0.012), high-sensitivity C-reactive protein (P = 0.001), and leptin (P = 0.008)], but not hemoglobin A1c.
Conclusions
Adolescents with T1D have adipose, hepatic and peripheral IR. This IR occurs regardless of obesity and metabolic syndrome features. Youth with T1D may benefit from interventions directed at improving IR in these tissues, and this area requires further research.
Youth with T1D have worse multitissue insulin sensitivity compared with healthy controls assessed by four-phase hyperinsulinemic euglycemic clamp, and these changes occur regardless of obesity.
Type 1 diabetes (T1D) is characterized not only by insulin insufficiency but also by cardio-metabolic dysfunction and a 10-fold increase in all-cause mortality (1). Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in individuals with T1D, and increasing evidence demonstrates that CVD develops in childhood (1–3). For example, youth with T1D have dyslipidemia, worse myocardial strain, and vascular stiffening compared with normoglycemic peers (2, 4, 5). CVD is associated with insulin resistance (IR) in youths and adults with type 2 diabetes (T2D) (6, 7). Moreover, obesity and metabolic syndrome are traditional risk factors for IR and CVD, and obesity is increasing in T1D (8). However, we have previously demonstrated that even normal-weight adolescents and adults with T1D had more IR than controls without diabetes who had similar body mass index (BMI) (9–11). Therefore, IR in T1D is not due to obesity alone.
A growing body of research implicates IR as a significant feature of T1D-related CVD (5, 11–14). IR in adults with T1D is associated with dyslipidemia, atherosclerosis, and CVD in general (11, 14, 15). Notably, youth with T1D with higher insulin sensitivity relative to other youth with T1D have a CVD risk profile more similar to that of controls without diabetes (5). We previously demonstrated that IR in adolescents with T1D is associated with exercise, cardiac, and vascular dysfunction, as well as a more atherogenic cholesterol subfraction profile (2, 10, 16).
In adults, T2D is well characterized in terms of tissue-specific IR, and similar measures have been performed in adults with T1D (9, 11). Adults with T1D have tissue-specific IR in the adipose, liver, and skeletal muscle (9, 11). Youth-onset T1D increases the risk for complications due to the onset of metabolic dysfunction at a younger age (10). Additionally, metabolism in youth differs from that in adults because of the impact of growth and the profound impact of pubertal IR, a time period where insulin sensitivity is half that seen in prepuberty or adulthood (17). Therefore, studying T1D and IR in young populations is imperative to fully understanding how IR and CVD interact to affect youth with T1D. In 1993, Arslanian et al. (18) published a three-phase hyperinsulinemic euglycemic (HE) clamp study in a smaller cohort of youth with T1D. However, the study did not include a tracer to assess adipose IR, used fewer doses of insulin during the clamp, did not control diet or activity, and used insulin regimens that differ substantially from those currently used in youth, so the mean hemoglobin A1c (HbA1c) was very high.
Given the ability of new therapeutics to target IR in specific organs, elucidating tissue-specific targets for intervention in youth with T1D holds great potential. Therefore, we measured the effects of varied doses of hyperinsulinemia on lipolysis, endogenous glucose release, and stimulated peripheral glucose uptake in a larger group of adolescents with and without T1D on insulin delivery regimens and glycemic control typical of modern youth.
Participants and Methods
Participants
Adolescents with and without T1D were recruited from Children’s Hospital Colorado and the Barbara Davis Center for Childhood Diabetes for one of two prospective, cross-sectional studies: RESistance to InSulin in T1D ANd T2D (RESISTANT) and Androgens and Insulin Resistance Study (AIRS) (2). Inclusion criteria were BMI in the 10th to 99th percentiles and sedentary status to minimize effects of varying physical activity on insulin sensitivity (<3 hours of exercise/week, validated with a 3-day activity recall and 7 days of accelerometer use). Exclusion criteria were alanine aminotransferase (ALT) > 80 IU/mL; blood pressure > 140/90 mm Hg; hemoglobin < 9 mg/dL; serum creatinine > 1.5 mg/dL; smoking; medications affecting IR, blood pressure, or lipids; and, in youths with T1D, HbA1c < 12%. The University of Colorado AMC Institutional Review Board and the Clinical Translational Research Center Scientific Advisory Review Council approved the study. Informed consent was obtained from all participants age ≥ 18 years, and parental consent and participant assent was obtained for all participants age < 18 years.
Physical activity
A 3-day pediatric activity recall assessing metabolic equivalents was completed (19), and participants wore an Actigraph GT3x accelerometer (Actigraph Corp., Pensacola, FL) for 7 days to assess habitual level of physical activity. Data collected were corrected for wear time and were categorized into the following age-appropriate activity levels: sedentary, light, lifestyle, moderate, vigorous, and very vigorous (20).
Insulin sensitivity
To minimize confounding effects on insulin sensitivity, the study day was preceded by 3 days of restricted physical activity and a fixed weight-maintenance diet (55% carbohydrates, 30% fat, and 15% protein), performed in the follicular phase for female participants. Participants were admitted to the inpatient Clinical Translational Research Center for 12 hours of overnight monitored fasting. In participants with T1D, subcutaneous insulin was replaced with a variable-rate overnight intravenous insulin infusion to normalize blood glucose concentrations (100 mg/dL). The following morning, a four-phase HE clamp was performed (7, 10) to determine adipose, hepatic, and peripheral IR. After a 2-hour basal phase, during which the overnight insulin infusion was continued to maintain normoglycemia, insulin doses for each additional 1.5-hour phase were 10, 16, and 80 mU/m2/minute, based on our (7, 10, 21) and others’ (7, 10, 21) experience with the higher insulin requirements in pubertal youth. Twenty percent dextrose (spiked with 6,6-2H2 glucose) was infused to maintain blood glucose at 95 mg/dL, checked every 5 minutes with bedside Yellow Springs Instrument glucose meter (Yellow Springs, OH). Glucose infusion rate was calculated from the steady-state measurements from the last 30 minutes of each phase.
Tracer infusion protocol
At 6 am, blood samples to measure background enrichment and concentrations were obtained. Then, a bolus of 4.5 mg/kg 6,6-2H2-glucose (Isotec, Miamisburg, OH), followed by a continuous infusion at 0.03 mg/kg/minute 6,6-2H2-glucose, was paired with a primed (1.6 µmol/kg), then constant (0.11 µmol/kg/minute), infusion of 2H5-glycerol. During the last 30 minutes of each of the four clamp phases, four samples, each 10 minutes apart, were drawn for glucose, glycerol, free fatty acid (FFA), and insulin concentrations and glucose and glycerol tracer enrichments (22).
Sample analysis
2H5 glycerol and 6,6-2H2 glucose were analyzed by using gas chromatography–mass spectrometry as described (9, 22, 23). Serum insulin, leptin, and adiponectin were analyzed with RIA (Millipore, Billerica, MA); plasma glycerol (R-Biopharm, Marshall, MI) and FFA (Wako Chemicals, Inc., Richmond, VA) were measured enzymatically. HbA1c was measured by Diabetes Control and Complications Trial–calibrated ion-exchange HPLC (Bio-Rad Laboratories, Hercules, CA). Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride assays were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Low-density lipoprotein cholesterol levels were calculated by the Friedewald equation; high-sensitivity C-reactive protein (hs-CRP), via immunoturbidimetric assay (Beckman Coulter, Brea, CA); and C-peptide, via chemiluminescent immunoassay (DiaSorin, Stillwater, MN). Aspartate aminotransferase and ALT were measured via chemical reaction on a Vitros® 5600 chemistry system (Ortho Clinical Diagnostics, Rochester, NY).
Imagine techniques
Body composition was assessed by dual-energy x-ray absorptiometry to determine FFM (24). Abdominal magnetic resonance imaging was performed with a 3-T magnet (GE, Milwaukee, WI). Visceral adiposity was measured by using the gold standard of a magnetic resonance imaging slice at lumbar vertebrae 4 and 5 (25). Hepatic fat fraction was measured by using the modified Dixon method (26).
Tracer calculations
All isotopic enrichments were corrected for background enrichments. The glucose and glycerol rate of appearance (Ra), rate of disappearance (Rd), and metabolic clearance rate (MCR) over the last 30 minutes of each phase of the clamp were calculated by using the Steele non–steady-state equation, accounting for “spiked” glucose in the 20% dextrose infusion (9). Glucose Rd is expressed as both mg/kg/min and mg/kg lean mass/min. Controlling overnight glycemia with an insulin infusion can lead to insulin concentrations, which may partially suppress endogenous glucose production (EGP) and glycerol Ra in the “basal” state. Thus, standard calculations of percentage suppression and IC50 cannot be used because they assume a nonsuppressed basal state. Instead, to describe changes in Ra across the different insulin concentrations of each phase, the intercept and slope of the regression line for each individual’s data were also used to calculate the predicted Ra at the average insulin concentration for all participants during the 10-mU/m2/minute phase for glycerol Ra and the 16-mU/m2/minute phase for EGP (27). Data were log-transformed before regression. However, negative glucose Ra values could not be log-transformed, and thus a constant just larger than the absolute value of the most negative Ra was added to all glucose Ra values. Data were reverse-transformed for presentation of model results.
Statistical analysis
Descriptive statistics are presented as mean ± SD, median (first and third quartiles), or frequencies and percentages, as appropriate. For single-timepoint variables, group comparisons were made by using a χ2 or Fisher exact test and the t test or Kruskal-Wallis test. Associations between tissue-specific IR (10-mU/m2/minute phase glycerol Ra, 16-mU/m2/minute phase EGP, and 80-mU/m2/minute phase MCR), body composition (visceral fat, liver fat, waist-to-hip ratio), inflammation (hs-CRP, IL-6) metabolic markers (adiponectin, leptin, HbA1c, and C-peptide) and activity were examined by using Spearman correlation coefficients.
By modeling the Ra from all clamp phases relative to the insulin concentration, we were able to best describe dynamic physiologic changes in Ra in each individual and overcome problems created by the need to control overnight glycemia. Repeated-measures mixed-effects models were used to compare outcomes measured at multiple time points during the clamp, with data log-transformed prior to modeling. The repeated-measures models for glucose and insulin contained terms for group (T1D or control), clamp phase, and the interaction of group and phase. For repeated-measures outcomes (EGP, glycerol Ra, glycerol, FFA) that could be affected by serum insulin concentrations, insulin was added to the model and the Akaike information criterion was used to test whether a random effect of insulin should be included (28). The three-way interactions of group, phase, and insulin were tested for significance, but all two-way interactions were retained in the models. For each of the repeated-measures models, we examined the significance of all the terms in the models; however, the primary comparison of interest for each clamp variable was defined a priori as the least-squares difference between the patients with T1D and controls at the 10-mU/m2/minute phase and serum insulin concentration equal to the overall average at the 10-mU/m2/minute phase for glycerol Ra, glycerol, and FFA and the 16-mU/m2/minute phase and serum insulin concentration equal to the overall average at the 16-mU/m2/minute phase for EGP. P values < 0.05 were considered to indicate statistically significant differences for main effects; P values < 0.25 were considered to indicate significance for interactions. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Thirty-five youth with T1D and 22 youth without diabetes were enrolled (Table 1). By design, the two groups had similar age, sex, Tanner stage, BMI and BMI percentile distributions, and physical activity data (including metabolic equivalents from the 3-day pediatric activity recall and the accelerometer data). The T1D group consisted of more white youth, reflecting the increased prevalence of the disease in white patients.
Table 1.
Participant Demographic and Physical Characteristics and Lifestyle Measurements
| Characteristic | T1D Group | Control Group | P Value |
|---|---|---|---|
| Participants, n | 35 | 22 | — |
| Age, y | 16 (14, 17) | 14 (12, 17) | 0.08 |
| Female/male, n/n (% female) | 19/16 (53) | 16/6 (73) | 0.16 |
| Height, cm | 168 ± 2 | 162 ± 2 | 0.01 |
| Weight, kg | 71 ± 3 | 64 ± 4 | 0.14 |
| BMI, kg/m2 | 24.0 (21.0, 29.9) | 22.5 (19.0, 30.0) | 0.48 |
| BMI percentile | 82 (55, 96) | 72 (51, 97) | 0.63 |
| Ethnicity | <0.004 | ||
| White | 28 (80) | 9 (41) | |
| Hispanic | 5 (14) | 8 (36) | |
| Black | 1 (3) | 5 (23) | |
| Asian | 1 (3) | 0 (0.00) | |
| Tanner stage | 5.0 (4.0, 5.0) | 4.5 (4.0, 5.0) | 0.11 |
| Waist circumference, cm | 83 (74, 89) | 72 (66, 97) | 0.24 |
| Waist-to-hip ratio | 0.85 ± 0.06 | 0.82 ± 0.09 | 0.19 |
| Lean mass , % | 67.8 ± 9.0 | 64.7 ± 9.1 | 0.02 |
| Total body fat , % | 29.3 ± 9.0 | 32.2 ± 9.3 | 0.26 |
| Liver fat , % | 1.0 (0.0, 1.8) | 1.3 (0.1, 2.3) | 0.51 |
| Visceral fat , % | 15.4 (11.3, 19.4) | 15.8 (12.0, 20.5) | 0.51 |
| Daily METs from 3DPAR | 64 ± 17 | 58 ± 10 | 0.08 |
| Accelerometer data | |||
| Sedentary , % | 0.65 ± 0.18 | 0.61 ± 0.15 | 0.36 |
| Lifestyle + light , % | 0.27 ± 0.12 | 0.32 ± 0.10 | 0.09 |
| Moderate + vigorous + very vigorous, % | 0.05 (0.03, 0.07) | 0.06 (0.03, 0.11) | 0.65 |
Data are shown as mean ± SD or as median (first, third quartiles).
Abbreviations: 3DPAR, 3-day physical activity recall; METs, metabolic units.
Body composition measures (including the waist-to-hip ratio; waist circumference; and percentage of visceral, liver, and total fat) were similar between groups, although lean mass was slightly higher in patients with T1D.
Leptin, adiponectin, HDL, and triglycerides did not differ between groups (Table 2). However, total cholesterol (P = 0.049) and low-density lipoprotein cholesterol (P = 0.03) were lower in youth with T1D. The T1D group had a higher HbA1c (P < 0.0001), and the fasting C-peptide was higher in the control group, as expected (P < 0.0001). There were no differences in any markers of inflammation.
Table 2.
Participant Fasting 6 am Laboratory Measures
| Characteristic | T1D | Control | P Value |
|---|---|---|---|
| Leptin, ng/mL | 14.5 (9.7, 27.0) | 16.8 (7.4, 31.7) | 0.45 |
| Adiponectin, ng/mL | 9.1 (7.20, 13.40) | 8.3 (6.5, 11.1) | 0.37 |
| Cholesterol, mg/dL | 140 (126, 160) | 156 (134, 185) | 0.049 |
| HDL cholesterol, mg/dL | 46 ± 6 | 43 ± 9 | 0.25 |
| LDL cholesterol, mg/dL | 80 (65, 98) | 97 (76, 120) | 0.03 |
| Triglyceride, mg/dL | 73 (56, 102) | 94 (70, 136) | 0.08 |
| C-peptide, ng/mL | 0.05 (0.05, 0.10) | 1.70 (1.48, 2.73) | <0.0001 |
| AST, U/L | 27 (21, 34) | 30 (23, 43) | 0.31 |
| ALT, U/L | 16 (12, 25) | 22 (13, 27) | 0.30 |
| IL-6, mg/dL | 1.3 (1.3, 2.0) | 1.3 (1.3, 3.7) | 0.12 |
| WBC, 109cells/L | 6.4 (5.5, 7.7) | 6.1 (5.5, 7.5) | 0.60 |
| Platelets, 109cells/L | 247 (230, 280) | 243 (239, 274) | 0.82 |
| hs-CRP, mg/dL | 0.6 (0.2, 2.9) | 0.3 (0.2, 1.1) | 0.15 |
| HbA1c, % | 8.3 (7.3, 9.4) | 5.4 (5.2, 5.5) | <0.0001 |
Laboratory measures drawn after a 12-hour monitored inpatient fast are shown below for adolescents with and without T1D. Data are shown as mean ± SD or as median and the first and third quartiles.
Abbreviations: AST, aspartate aminotransferase; LDL, low-density lipoprotein; WBC, white blood cell count.
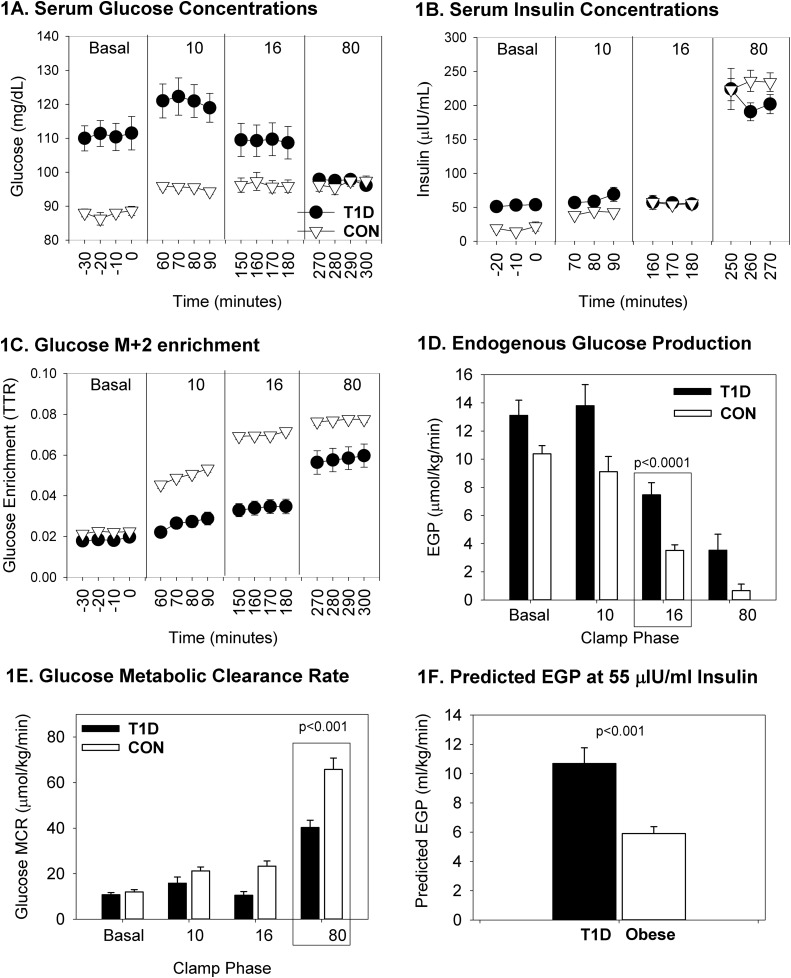
By design, the glucose concentration was steady throughout the phases and was similar between groups (Fig. 1A). The insulin concentration (Fig. 1B) increased in both groups with the administration of increasing doses of insulin. Insulin differed between groups (P = 0.0011) across phases because of the increased insulin dose required during the basal phase in the T1D group to maintain normoglycemia. Glucose enrichment, expressed as the tracer-to-tracee ratio − background enrichment, is shown in Fig. 1C. EGP generally decreased with increases in the insulin dose; however, the observed EGP was higher in T1D during the 16-mU/m2/minute phase (Fig. 1D; P < 0.001), as was the predicted EGP at an insulin concentration of 55 µIU/mL; the values were slightly different than the raw EGP in Fig. 1D because they were calculated at 55 µIU/mL of insulin (Fig. 1F; P < 0.001), with incomplete suppression of glucose Ra in youth with T1D. The slopes of the lines to calculate predicted EGP between the control and T1D groups were different [medians of −0.09 (−0.14, 0.81) in controls vs −0.14 (−0.25, −0.09) in patients with T1D; P = 0.001], as was the intercept [medians of 2.11 (−0.101, 2.30) glucose Ra mg/kg/min in controls vs 2.65 (2.19, 2.98) glucose Ra mg/kg/min in patients with T1D; P < 0.001]. In the repeated-measures model for EGP, the interaction of group and phase of the clamp was not significant (Table 3; P = 0.069), indicating that the groups were not significantly different regarding the change in EGP across all phases of the clamp. Glucose Rd differed between groups during the 80-mU/m2/minute phase, with and without adjustment for lean mass (37±15 µmol/kg/minute for the T1D group vs 61 ± 5 µmol/kg/minute for the control group and 53 ± 21 µmol/kg lean mass/minute for the T1D group vs 92 ± 7 µmol/kg lean mass/minute for the control group; P < 0.001 for both). MCR tended to increase with each increase in insulin dose but was lower in the youths with T1D during the 80-mU/m2/minute phase (Fig. 1E; P < 0.0001);results were similar if MCR was calculated with a lean body mass (P < 0.0001).
Figure 1.
Glucose-related measurements from the four-phase hyperinsulinemic clamp. Data are shown according to T1D and non-T1D status and by phase of the clamp, denoted per insulin dose as nonrepeated measures. Nonrepeated phase-specific measures of hepatic IR are quantified by the 16-mU/m2/min phase of EGP, and measures of peripheral IR are quantified by the 80-mU/m2/min phase of the MCR, enclosed by a rectangle. The remaining phase values were used for modeling the entire curves per group. Data are presented as mean and SEM. (A) Serum glucose concentrations were initially higher in T1D and were similar between groups. (B) Serum insulin concentrations were higher overnight in T1D and similar between groups. (C) Glucose mass+2 enrichment was higher in controls after the basal phase. (D) Endogenous glucose production was higher in T1D during the hepatic phase 16 of the clamp. (E) Glucose metabolic clearance rate was lower in T1D in the peripheral phase 80 of the clamp. (F) Predicted EGP is higher in T1D at 55 μIU/mL insulin. CON, control; TTR, tracer-to-tracee ratio.
Table 3.
Results of Repeated-Measures Models for Clamp Outcomes
| Effect |
Glucose Ra
|
Glycerol Ra
|
Glycerol
|
FFA
|
||||
|---|---|---|---|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | Estimate | P Value | Estimate | P Value | |
| Intercept | 1.5 ± 1.4 | 2.1 ± 1.1 | 40.4 ± 13.3 | 5.6 ± 188.8 | ||||
| T1D | −1.2 ± 1.4 | 0.981 | 1.2 ± 1.2 | 0.083 | 12.8 ± 16.6 | 0.014 | 75.0 ± 206.7 | 0.089 |
| Insulin | −0.006 ± 0.006 | 0.640 | −0.001 ± 0.004 | 0.997 | −0.048 ± 0.045 | 0.134 | 0.14 ± 0.79 | 0.025 |
| Clamp phase | 0.057 | <0.0001 | <0.0001 | <0.0001 | ||||
| Basal phase | 0.5 ± 1.3 | 4.6 ± 1.0 | 44.8 ± 12.6 | 512 ± 177 | ||||
| 10 mU/m2/min phase | 0.4 ± 1.2 | −1.1 ± 1.1 | −18.1 ± 13.8 | 38 ± 165 | ||||
| 16 mU/m2/min phase | −0.5 ± 1.1 | −0.9 ± 1.0 | −8.2 ± 13.3 | −33 ± 164 | ||||
| T1D*phase | 0.069 | <0.001 | <0.0001 | <0.0001 | ||||
| T1D*basal phase | 1.4 ± 1.3 | −2.0 ± 1.1 | −14.3 ± 15.4 | −238 ± 194 | ||||
| T1D*10 mU/m2/min phase | 1.5 ± 1.2 | 1.3 ± 1.1 | 28.1 ± 14.9 | 262 ± 175 | ||||
| T1D*16 mU/m2/min phase | 1.8 ± 1.1 | 0.2 ± 1.0 | 32.9 ± 13.4 | 270 ± 167 | ||||
| Insulin*phase | 0.960 | 0.008 | 0.106 | 0.05 | ||||
| Insulin*basal phase | 0.001 ± 0.003 | −0.033 ± 0.016 | 0.226 ± 0.170 | 1.58 ± 0.63 | ||||
| Insulin*10 mU/m2/min phase | 0.001 ± 0.004 | 0.025 ± 0.014 | 0.461 ± 0.190 | 1.72 ± 0.91 | ||||
| Insulin*16 mU/m2/min phase | −0.001 ± 0.005 | 0.015 ± 0.009 | 0.079 ± 0.139 | 1.53 ± 1.14 | ||||
| Insulin*T1D | 0.008 ± 0.006 | 0.228 | −0.002 ± 0.005 | 0.747 | 0.002 ± 0.053 | 0.971 | −0.11 ± 0.87 | 0.896 |
| Least-squares estimate of group difference at phase of primary interest (T2D − control) | 1.1 ± 0.3 | <0.001 | 2.4 ± 0.7 | <0.001 | 41.0 ± 10.4 | <0.001 | 331 ± 63 | <0.0001 |
Values are estimates ± SEM. The 80-mU/m2/min phase of the clamp was treated as the reference category for phase, and controls were treated as the reference category for group. At the 16-mU/m2/min phase, insulin = 55 IU/mL for EGP; at the 10-mU/m2/min phase, insulin = 53 IU/mL for glycerol Ra, glycerol, and FFA. Phase of interest for EGP is the 16-mU/m2/min phase and for glycerol RA, glycerol, and FFA concentrations the phase of interest is the 10-mU/m2/min phase. The overall comparison of the entire curve from all four phases between groups is the T1D*phase, and the final output of the model at the phase of interest is the least-squares estimate. The remainder of values include all of the parameter estimates from the model.
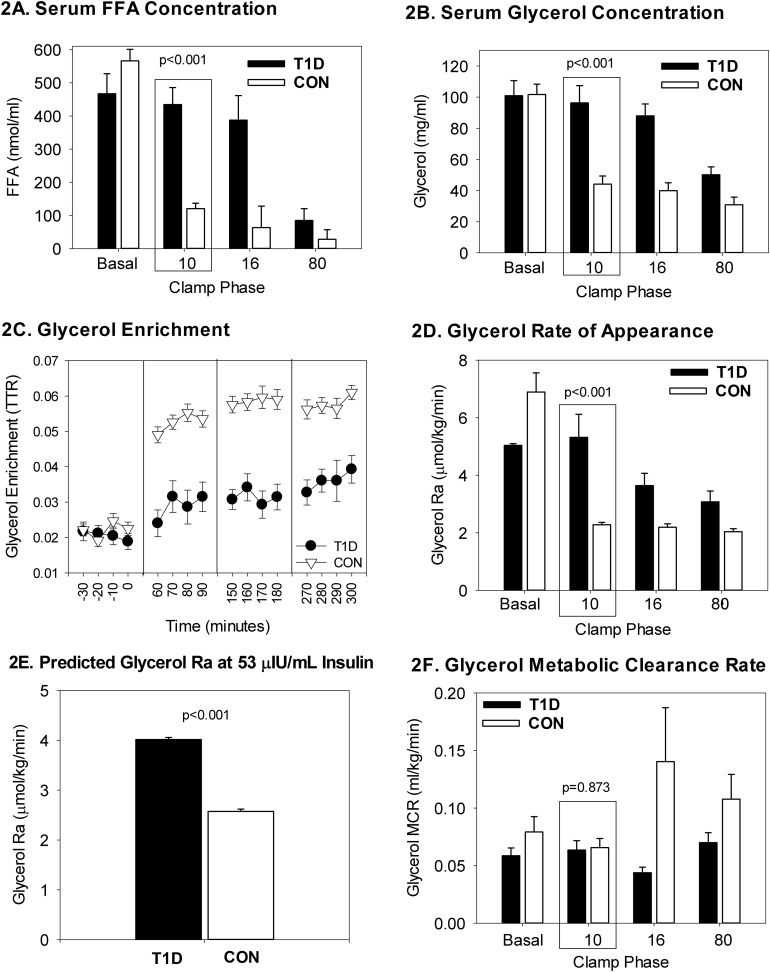
FFA concentrations decreased with increasing insulin doses but were higher in patients with T1D at the 10-mU/m2/minute phase (Fig. 2A; P < 0.0001). Glycerol also decreased per phase, but it was higher in the T1D group at the 10-mU/m2/minute phase (Fig. 2B; P < 0.0001). Glycerol enrichment, expressed as the tracer-to-tracee ratio–background enrichment, is shown in Fig. 2C. Glycerol Ra was more suppressed in the 10-mU/m2/minute phase in controls than the T1D group (Fig. 2D; P < 0.0001); similarly, predicted glycerol Ra was higher in patients with T1D, with the values slightly different than the raw glycerol Ra in Fig. 1D because they were calculated at 53 µIU/mL of insulin (Fig. 2E; P = 0.019). The slopes to calculate predicted glycerol RA between controls and patients with T1D were different [median slopes were −0.33 (−0.43, −0.25) in the control group vs −0.20 (−0.34, −0.04) in the T1D group; P = 0.017], whereas the intercepts were not [medians of 2.36 (1.67, 2.70) in controls vs 1.92 (1.11, 2.45) in patients with T1D; P = 0.112]. In the repeated-measures models for glycerol Ra, glycerol, and FFA, the interactions of group and phase of the clamp were significant (Table 3; P = 0.0005, P < 0.0001, P < 0.0001, respectively), indicating significantly different trajectories between groups across the phases of the clamp. Furthermore, the least-squares means of glycerol Ra, glycerol, and FFA were all significantly higher in the T1D group than in the control group at the 10-mU/m2/minute phase (Table 4; P = 0.0005, P = 0.0002, P < 0.0001, respectively).
Figure 2.
Lipid-related measurements from the four-phase hyperinsulinemic clamp. Data are shown according to T1D and non-T1D status and by phase of the clamp, denoted per insulin dose as nonrepeated measures. Primary lipid endpoints of serum FFA and glycerol concentrations and adipose IR were quantified during the 10-mU/m2/min phase. The remaining phase values were used for modeling the entire curves per group. Data are presented as mean and SEM. (A) Serum FFA were higher in T1D during the adipose phase 10 of the clamp. (B) Serum glycerol were higher in T1D during the adipose phase 10 of the clamp. (C) Glycerol enrichment was higher in controls after the basal phase in controls. (D) Glycerol rate of appearance was higher in T1D during phase 10 of the clamp. (E) Predicted glycerol Ra is higher in T1D at 53 μIU/mL insulin. (F) Glycerol clearance is similar between groups during the adipose phase 10 of the clamp. CON, control; TTR, tracer-to-tracee ratio.
Table 4.
Correlates of Insulin Sensitivity
| Variable |
T1D Group
|
Control Group
|
||
|---|---|---|---|---|
| MCR | r | P Value | r | P Value |
| FFA concentration during 80-mU/m2/min phase | −0.46 | 0.005 | −0.53 | 0.023 |
| Fasting IL-6 | −0.53 | 0.012 | −0.08 | 0.816 |
| Fasting hs-CRP | −0.52 | 0.001 | −0.23 | 0.329 |
| Fasting leptin | −0.44 | 0.008 | −0.42 | 0.063 |
| Habitual activity (3-day METs) | 0.43 | 0.041 | −0.02 | 0.948 |
| EGP | ||||
| HbA1c | 0.18 | 0.466 | 0.49 | 0.0382 |
| Glycerol Ra | ||||
| Fasting IL-6 | 0.79 | 0.035 | 0.04 | 0.904 |
| Fasting WBC | 0.76 | 0.049 | −0.06 | 0.788 |
Correlates of insulin sensitivity per tissue type are shown for youth with and without T1D. Data are shown as correlation coefficients and P values.
Abbreviations: METs, metabolic equivalents; WBC, white blood cell count.
For the entire cohort, glucose Rd had a negative correlation with EGP at the 16-mU/m2/minute phase (P = 0.004). In youth with T1D, we found that MCR correlated with FFA at the 80-mU/m2/minute phase (Table 4; P = 0.005), IL-6 (P = 0.012), hs-CRP (P = 0.001), and leptin (P = 0.008). We also found that glycerol Ra correlated with IL-6 (P = 0.035) and white blood cell count (P = 0.049). In the controls, MCR also correlated with FFA at the 80-mU/m2/minute phase (P = 0.023) and EGP correlated with HbA1c (P = 0.0382).
We modeled EGP, glycerol Ra, glycerol, and FFA concentrations, controlling for the phase and the insulin concentration at each phase (Table 3). The two-way interactions in the model can be interpreted as follows: (1) T1D*phase is the difference in the effect of each phase compared with the reference phase (phase 80) for a participant in the T1D group compared with the control group; (2) insulin*phase is the difference in the effect of insulin at each phase compared with the reference phase (phase 80) for both groups combined; and (3) insulin*T1D is the difference in the effect of insulin for the T1D group compared with the control group. The main effects of T1D, insulin, and phase cannot be interpreted directly in a model with interactions. The least-squares estimate (bottom row of Table 3) is the estimated difference in the outcome between the groups at the phase of interest (16-mU/m2/minute phase for glucose Ra and 10-mU/m2/minute phase for all other outcomes), adjusting for the mean insulin value at that phase. The groups were significantly different for EGP, glycerol Ra, glycerol, and FFA (P < 0.001).
Discussion
By using a four-phase HE clamp, with insulin dosages that specifically target adipose, liver, and peripheral/muscle tissues, in combination with glucose and glycerol isotope tracers, our study comprehensively yet precisely characterized tissue-specific metabolic abnormalities in youth with T1D compared with well-matched controls. Overall, we found that T1D youth had significantly lower peripheral, hepatic, and adipose insulin sensitivity compared with youth without diabetes, despite a lack of traditional markers of the metabolic syndrome and IR, such as low HDL cholesterol or adiponectin or high triglycerides, ALT, or hepatic or visceral fat.
Our findings of peripheral IR in youth with T1D confirm our previous work in another cohort of similarly-aged youth and that by other groups (10, 18). This peripheral IR may in part be due to muscle mitochondrial dysfunction, which we have demonstrated in a similar cohort; the lower rate of muscle oxidative phosphorylation correlated with peripheral IR (29). The relationship between peripheral IR and muscle mitochondrial abnormalities has been well described in other states, such as T2D, aging, and burns, and it appears that this relationship also exists in T1D (17, 20, 30). Likewise, we found that peripheral IR correlated with adipose IR and markers of inflammation (FFA in the 80 mU/m2/minute phase, IL-6, and hs-CRP) in the adolescents with T1D, as has been seen in populations with T2D (7). Elevated FFA levels induce muscle IR and vascular dysfunction. Vascular dysfunction of any kind, which we and others have shown to be present in adolescents with T1D, can also decrease muscle blood flow and secondarily nutrient and/or insulin delivery to muscle and could induce mitochondrial dysfunction and IR (24, 31). In summary, increased FFA levels and vascular and/or mitochondrial defects may all contribute to muscle IR in T1D.
We also found that hepatic EGP did not suppress in response to insulin in youth with T1D, regardless of method of calculation, even during systemic insulin concentrations in excess of 200 µU/mL. Because of the influence of glucose concentrations in the interpretation and measurement of basal EGP, we chose to control overnight blood glucose concentrations (27). This led to significantly greater insulin concentrations during the basal phase in participants with diabetes. Similar findings were observed in a smaller clamp study in youth with T1D (18). Therefore, in this cohort of patients we also applied modeling techniques that are less reliant on measurements obtained during the basal phase, rather than using percentage suppression of basal or IC50 (32). By modeling the EGP from all clamp phases relative to the insulin concentration, we were able to best describe dynamic physiologic changes in EGP in each individual. On the basis of our models for predicted EGP, which use the EGP and insulin concentrations at all phases for each individual, at a serum insulin concentration of 55 µU/mL, the glucose Ra in a participant with diabetes would be more than double that seen in a control. Similar findings have been described in adults with T1D (9, 18), with a single limited study in youth with T1D, suggesting that this hepatic phenomenon occurs early in the pathology of T1D (18).
There are several possible reasons as to why youth with T1D have persistent EGP during hyperinsulinemia, including lack of portal insulin and/or excess glucagon. Interestingly, despite having hepatic IR, akin to people with T2D, our youth with T1D lacked the elevations in hepatic or visceral fat seen in T2D. The most likely explanation is the lack of portal insulin delivery, with a relative hepatic insulin deficiency despite peripheral hyperinsulinemia. Normally, over a third of pancreatic-secreted insulin is cleared from the portal circulation by the liver before release into the periphery (33). Subcutaneous insulin injections, insulin pumps, and our delivery via a peripheral intravenous route did not mimic portal delivery. Formulas to estimate portal insulin concentration and insulin clearance do exist; however, they have not been fully tested in youth with and without diabetes. This would be worthy of further research. Additionally, there may be a failure of suppression of glucagon secretion because of a lack of a paracrine effect of insulin from β cells on the α cells in the pancreas, or other causes of α-cell dysfunction (34). Glucagon secretion can be controlled by adding somatostatin to a clamp, which suppresses pancreatic insulin and glucagon secretion (35). However, we were unable to use this method in youth and did not collect serum for measurement of glucagon. Because there is known α cell dysfunction in T1D, it is possible that not all EGP is due to hepatic IR, and some excess glucagon is contributing to the EGP. Future work is needed to determine the role of glucagon in elevated endogenous glucose release in T1D.
In addition to abnormalities in glucose metabolism, youth with T1D also have evidence of adipose IR. We found that FFA and glycerol concentrations were higher during all phases of the clamp in the group with T1D. The glycerol tracer results indicate that these differences are due to inadequate suppression of lipolysis, and indeed predicted glycerol Ra was suppressed ∼60% more in controls, with no difference in glycerol clearance. Glycerol was selected as a tracer because the majority of glycerol is taken up by the liver and thus serum glycerol falls into a one-pool model. FFA can functionally be used as a fat metabolism marker but can also be re-esterified within the adipose cell and taken up by multiple tissues, leading to the need for more complex modeling with multiple tracers to assess lipolysis. However, the FFA and glycerol results both displayed similar rates of suppression in our study. This suggests that alterations in re-esterification or FFA uptake rates, which would cause FFA results to diverge from glycerol Ra results, are perhaps not different in youth with diabetes; thus, both may reflect adipose IR with increased lipolysis. This confirms previous work with only FFA as a measure of adipose IR in youth with T1D (18).
It is unclear why youth with T1D have adipose IR. Hyperglycemia has been shown to induce inflammation, which is thought to influence IR (36). Although we did not find increased generalized serum markers of inflammation (aspartate aminotransferase, ALT, IL-6, white blood cell count, platelets, or hs-CRP) in T1D, glycerol Ra did positively correlate with IL-6, suggesting a link requiring further investigation, although serum samples may not be truly representative of tissue inflammation. Additionally, other hormones, such as epinephrine, glucagon, and glucagon-like peptide-1, can affect the apparent response of lipolysis to insulin (34, 37, 38). Future work should include the measure of these hormones to determine whether their dysregulation contributes to persistent lipolysis.
Our study had several strengths and weaknesses. Our cohort was larger than in previous studies in youth with T1D and was under better glycemic control; we also added control for diet and activity, additional insulin phases during the clamp, used a glycerol tracer, and avoided hypoglycemic episodes in the early morning, which can induce counter-regulatory hormones, specifically elevated growth hormone (18). Another strength of our study is the use of the gold standard HE clamp and the use of glucose and glycerol tracers to directly measure adipose and hepatic IR. We also carefully controlled for BMI, stage of menstrual cycle, and pubertal stage and admitted the participants overnight to control glycemia and a fasting period, factors known to influence IR. One limitation is that our T1D group consisted of more white patients, but this reflects the T1D population; if anything, it would bias our results to not finding IR. We also could not perform a somatostatin pancreatic clamp because of the pediatric age group, and we did not collect samples for glucagon.
Conclusion
We found that youth with T1D have profound multiorgan IR, including adipose, hepatic, and muscle tissue, without features of the metabolic syndrome. This IR occurs regardless of obesity but may be worsened by the rising rates of obesity in T1D. The IR we demonstrated may contribute to the early onset of CVD in youth with T1D and could be a therapeutic target needing further research.
Acknowledgments
The authors thank the participants and their families as well as the Clinical Translational Research Center nurses and staff.
Financial Support: This work is supported by National Center for Research Resources (NCRR) K23 RR020038-01, National Institutes of Health (NIH)/NCRR Colorado Clinical Translational Science Institute CO-Pilot grant TL1 RR025778, NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 1R56DK088971-01, Juvenile Diabetes Research Foundation—2008-291, American Diabetes Association 7-11-CD-08 (KJ.N.); NIDDK T32 DK063687, Building Interdisciplinary Research Careers in Women's Health K12HD057022, NIDDK K23DK107871 (M.C.G.). This research was also supported by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award grant number UL1 TR001082.
Author Contributions: M.C.G researched and analyzed data and wrote and edited the manuscript; J.J.S. performed statistical analysis and wrote and edited the manuscript; J.T. performed statistical analysis and edited the manuscript; B.C.B, G.V.C., A.D.B., S.B., and A.S. researched data and edited the manuscript; L.P. performed statistical analysis and edited the manuscript; K.J.N. designed the study, researched and analyzed data, contributed to discussion, and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- BMI
body mass index
- CVD
cardiovascular disease
- EGP
endogenous glucose production
- FFA
free fatty acid
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HE
hyperinsulinemic euglycemic
- hs-CRP
high-sensitivity C-reactive protein
- IR
insulin resistance
- MCR
metabolic clearance rate
- Ra
rate of appearance
- Rd
rate of disappearance
- T1D
type 1 diabetes
- T2D
type 2 diabetes
References
- 1. Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984;33(3):271–276. [DOI] [PubMed] [Google Scholar]
- 2. Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JEB, Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications. 2016;30(6):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46(6):760–765. [DOI] [PubMed] [Google Scholar]
- 4. Shah AS, Black S, Wadwa RP, Schmiege SJ, Fino NF, Talton JW, D’Agostino R Jr, Hamman RF, Urbina EM, Dolan LM, Daniels SR, Marcovina SM, Dabelea D. Insulin sensitivity and arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. J Diabetes Complications. 2015;29(4):512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M. Effect of T1D on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in T1D (CACTI) Study. Diabetes. 2003;52:2833–2839. [DOI] [PubMed] [Google Scholar]
- 7. Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, Schwab KO, Holl RW, Hofer SE, Maahs DM. Obesity in youth with T1D in Germany, Austria, and the United States. J Pediatrics. 2015;167(3):627-632.e1-4. [DOI] [PubMed]
- 9. Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, Perreault L, Rewers M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97(5):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes. 2011;60(1):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bacha F, Klinepeter Bartz S. Insulin resistance, role of metformin and other non-insulin therapies in pediatric type 1 diabetes. Pediatr Diabetes. 2016;17(8):545–558. [DOI] [PubMed] [Google Scholar]
- 13. Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, Maahs DM. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 2012;97(4):E642–E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26(5):1374–1379. [DOI] [PubMed] [Google Scholar]
- 15. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, Kinney GL, King M, Eckel RH, Nadeau KJ. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17(4):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cree-Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Curr Diab Rep. 2013;13(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arslanian S, Heil BV, Kalhan SC. Hepatic insulin action in adolescents with insulin-dependent diabetes mellitus: relationship with long-term glycemic control. Metabolism. 1993;42(3):283–290. [DOI] [PubMed] [Google Scholar]
- 19. Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29(1):138–143. [DOI] [PubMed] [Google Scholar]
- 20. Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91(2):401–404. [DOI] [PubMed] [Google Scholar]
- 22. Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem. 1992;205(1):172–178. [DOI] [PubMed] [Google Scholar]
- 23. Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Clement TW, Eckel RH, Perreault L, Rewers M. The importance of palmitoleic acid to adipocyte insulin resistance and whole-body insulin sensitivity in type 1 diabetes. J Clin Endocrinol Metab. 2013;98(1):E40–E50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albertini JP, McMorn SO, Chen H, Mather RA, Valensi P. Effect of rosiglitazone on factors related to endothelial dysfunction in patients with type 2 diabetes mellitus. Atherosclerosis. 2007;195(1):e159–e166. [DOI] [PubMed] [Google Scholar]
- 25. Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362(9388):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dixon W. BMDP Statistical Software Manual. Oakland, CA: University of California Press; 1981.
- 27. Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64(2):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36(1):54–61. [DOI] [PubMed] [Google Scholar]
- 29. Pyle L, Bergman BC, Nadeau KJ, Cree-Green M. Modeling changes in glucose and glycerol rates of appearance when true basal rates of appearance cannot be readily determined. Am J Physiol Endocrinol Metab. 2016;310(5):E323–E331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, Pyle L, Reusch JE, Nadeau KJ. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308(9):E726–E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolfe RR, Shaw JH, Jahoor F, Herndon DN, Wolfe MH. Response to glucose infusion in humans: role of changes in insulin concentration. Am J Physiol. 1986;250(3 Pt 1):E306–E311. [DOI] [PubMed] [Google Scholar]
- 33. Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and Extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 2016;65(6):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW, Crofford OB, Liddle GW. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J Clin Invest. 1974;53(1):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kollind M, Adamson U, Lins PE. Somatostatin reduces posthypoglycemic insulin resistance in insulin-dependent diabetes mellitus. Acta Endocrinol (Copenh). 1988;118(2):173–178. [DOI] [PubMed] [Google Scholar]
- 36. Pozzilli P, Guglielmi C, Caprio S, Buzzetti R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care. 2011;34(Suppl 2):S166–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Voss TS, Vendelbo MH, Kampmann U, Pedersen SB, Nielsen TS, Johannsen M, Svart MV, Jessen N, Møller N. Effects of insulin-induced hypoglycaemia on lipolysis rate, lipid oxidation and adipose tissue signalling in human volunteers: a randomised clinical study. Diabetologia. 2017;60(1):143–152. [DOI] [PubMed] [Google Scholar]
- 38. Gastaldelli A, Gaggini M, Daniele G, Ciociaro D, Cersosimo E, Tripathy D, Triplitt C, Fox P, Musi N, DeFronzo R, Iozzo P. Exenatide improves both hepatic and adipose tissue insulin resistance: A dynamic positron emission tomography study. Hepatology. 2016;64(6):2028–2037. [DOI] [PubMed] [Google Scholar]