Abstract
Context
We hypothesized that TSH-receptor (TSHR) stimulating antibodies (TSAbs) are involved in oxidative stress mechanisms in patients with Graves disease (GD).
Methods
Nicotinamide adenine dinucleotide phosphate oxidase, isoform 2 (NOX2); oxidative parameters; and oxidative burst were measured in serum, urine, and whole blood from patients with GD and control subjects. Superoxide production was investigated in human embryonic kidney (HEK)-293 cells stably overexpressing the TSHR. Lipid peroxidation was determined by immunodot-blot analysis for protein-bound 4-hydroxy-2-nonenal (4-HNE) in human primary thyrocytes and HEK-293–TSHR cells.
Results
Serum NOX2 levels were markedly higher in hyperthyroid untreated vs euthyroid treated patients with GD, hyperthyroid patients with toxic nodular goiter, and euthyroid healthy control subjects (all P < 0.0001). Urine oxidative parameters were increased in patients with GD vs patients with toxic goiter (P < 0.01) and/or control subjects (P < 0.001). The maximum of the zymosan A- and phorbol 12,13-dibutyrate–induced respiratory burst of leukocytes was 1.5-fold higher in whole blood from hyperthyroid patients with GD compared with control subjects (P < 0.001 and P < 0.05). Monoclonal M22 TSAbs stimulated cAMP (HEK cells) in a dose-dependent manner. M22 (P = 0.0082), bovine TSH (P = 0.0028), and sera of hyperthyroid patients with GD (P < 0.05) increased superoxide-specific 2-hydroxyethidium levels in HEK-293 TSHR cells after 48-hour incubation vs control subjects. In contrast, triiodothyronine (T3) did not affect reactive oxygen species (ROS) production. In primary thyrocytes, the 4-HNE marker was higher in patients with GD vs control subjects at 6 and 48 hours (P = 0.02 and P = 0.04, respectively). Further, after 48-hour incubation of HEK-293 TSHR cells with patient sera, 4-HNE was higher in patients with untreated GD compared with control subjects (P < 0.05).
Conclusions
Monoclonal M22 and polyclonal serum TSAbs augment ROS generation and/or induce lipid peroxidation.
Both polyclonal (sera from patients with untreated hyperthyroid Graves disease) and human monoclonal TSHR-stimulating autoantibodies induce oxidative damage and lipid peroxidation.
Increased production of reactive oxygen species (ROS) and uncontrolled stress are associated with the development of cardiovascular and autoimmune diseases, such as Alzheimer’s disease, systemic lupus erythematosus, rheumatoid arthritis, and Graves disease (GD) (1–8). GD is a prevalent organ-specific autoimmune disorder and is the primary cause of hyperthyroidism (9). Increased oxygen consumption, mitochondrial dysfunction, and oxidative stress were reported for patients with hyperthyroidism (4). Increased markers of oxidative stress and decreased antioxidative capacity were also observed in erythrocytes of patients with GD (10).
Under physiological conditions, cells are defended by an antioxidant system (4). ROS are involved in multiple cellular processes, such as cell defense; hormone synthesis and signaling; activation of G protein–coupled receptors, kinases/phosphatases, and transcription factors; and gene expression. However, under pathophysiological conditions, ROS cause inflammation and fibrosis (11). ROS confer, at low concentrations, essential redox signaling in fundamental cellular processes (i.e., differentiation, proliferation, and migration), whereas high levels of ROS are toxic to cells. ROS include the superoxide anion radical (O2–•), peroxy radical (ROO•), hydrogen peroxide (H2O2), singlet oxygen (1O2), per hydroxyl radical (HO2•), and the highly reactive hydroxyl radical (•OH). The respiratory burst in leukocytes is mainly mediated through the phagocytic nicotinamide adenine dinucleotide phosphate oxidase, isoform 2 (NOX2) enzyme via production of ROS during the immune response (12, 13). Increased and chronic ROS production results in oxidative stress, which can lead to protein and DNA damage and lipid peroxidation (14). Lipid peroxidation is the reaction of oxygen or ROS with unsaturated lipids. The primary products of lipid peroxidation are lipid hydroperoxides. Aldehydes, which can be formed as secondary (fragmentation) products during the degradation of lipid hydro peroxides, include 4-hydroxy-2-nonenal (4-HNE), malondialdehyde (MDA), propanal, and hexanal (15).
Circulating TSH-receptor (TSHR)-stimulating autoantibodies (TSAbs) induce the clinical phenotype of GD and are regarded as specific biomarkers for GD. TSHR-Abs act either as an agonist, stimulating unregulated thyroid growth and thyroid hormone production, or as an antagonist, blocking the activity of the natural ligand thyrotropin (16–25). In this study, we hypothesized that TSAbs are involved in oxidative stress mechanisms in GD. Therefore, the generation of ROS and the consequent oxidative damage of lipids, proteins, and nucleic acids were investigated in patients with GD, in patients with toxic nodular goiter, and in control subjects.
Materials and Methods
Study population
The study protocol was approved by the Ethics Committee of the Johannes Gutenberg University Medical Center, Mainz, Germany, and was carried out in accordance with the ethical guidelines of the Helsinki Declaration. Informed consent was obtained from all participants enrolled in the study. Whole blood and urine samples were drawn from hyperthyroid patients with untreated GD (n = 54) and were investigated for the presence of various oxidative stress markers and compared with euthyroid patients with GD on antithyroid drug treatment (n = 19) and hyperthyroid patients with toxic nodular goiter (n = 5) without thyroid antibodies (Abs) and functional autonomy in the thyroid scan. Also included were 42 euthyroid healthy control subjects who were devoid of thyroid, endocrine, and autoimmune disorders and who had negative family history of autoimmune diseases. Diagnosis of GD was based on clinical phenotype, the presence of a diffusely enlarged thyroid gland, thyroid Doppler sonography with enhanced perfusion of the gland, and increased serum levels of thyroid hormones and TSHR-Abs.
Thyroid hormones and related antibodies
Concentrations of serum TSH, free triiodothyronine, free thyroxine, antithyroglobulin and antithyroperoxidase antibodies, and TSHR-binding inhibitory immunoglobulins were measured using electrochemiluminescence immunoassays (Elecsys, Cobas e411; Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
Bioassay for TSHR-stimulating antibodies
Serum TSAb levels were measured with a chimeric TSHR (mutant chimeric, Mc4) bioassay (Thyretain; Quidel, San Diego, CA) according to the manufacturer’s instructions (16, 18, 26). Briefly, Chinese hamster ovary–Mc4 cells were seeded and grown to confluent cell monolayers in 96-well plates for 15 to 18 hours. Patient and control serum samples as well as positive, reference, and normal controls were diluted 1:11 in reaction buffer (RB) (Quidel, San Diego, CA) and added to the cell monolayers, and each plate was incubated for 3 hours at 37°C and 5% CO2. The Chinese hamster ovary–Mc4 cells were lysed, and the relative light unit values were quantified in a luminometer (Infinite M200; Tecan, Crailsheim, Germany). The assay cut-off is at a percentage specimen-to-reference-ratio of 140%.
Measurement of cAMP assay
Intracellular cAMP levels were measured with a homogeneous, fluorescent assay that is based on binding of cAMP to a DNA-binding protein (Bridge-It® cAMP designer fluorescence assay, Cat. #122934M; ediomics LLC, St. Louis, MO) (27).
Oxidative stress kits
Nicotinamide adenine dinucleotide phosphate oxidase, gp91phox (NOX2)-expressed proteins were measured in serum using the ELISA kit (human) (item no. 197769; US Biological, Salem, MA). The lipid peroxidation markers [malondialdehyde (MDA) and 8-isoprostane] were measured in urine by using Thiobarbituric Acid Reactive Substances and enzyme immunoassay kits, respectively (item nos. 700870 and 516360; Cayman Chemical, Ann Arbor, MI). The DNA oxidative damage marker 8-hydroxy-2-deoxy guanosine (8-OH-dG) was detected in urine (sample dilution 1:500) by using an ELISA kit (item no. 589320; Cayman Chemical). All assays were performed according to the manufacturer’s instructions.
Measurement of respiratory burst in whole blood by chemiluminescence
Venous blood samples were drawn from untreated hyperthyroid patients with GD and control subjects. Fresh blood was collected in citrate tubes and kept at room temperature until measurement within 1 hour. The blood samples were diluted 1:50 in PBS containing the luminol analog 8-amino-5-chloro-7-phenylpyridol [3,4-d] pyridazine-1,4-(2H,3H) dione sodium salt (L-012, 100 µM final concentration) (28). Activation of the phagocytic NADPH oxidase was stimulated with phorbol 12,13-dibutyrate (PDBu) (10 µM final concentration from a stock in dimethyl sulfoxide), the fungal endotoxin zymosan A (50 µg/mL final concentration from a stock in PBS), and basal (without stimulators) and was detected with L-012 (8-amino-5-chloro-7-phenyl-pyrido[3,4-d]pyridazine-1,4(2H,3H)dione), a luminol-based chemiluminescent probe. Each sample was tested using eight replicates (200 µL per well) in a 96-well plate and measured in a luminometry plate reader (LB 960; Berthold Technologies, Bad Wildbad, Germany). White blood cells were counted in all samples and analyzed with the automated hematology analyzer (KX-21N; Sysmex Europe GmbH, Norderstedt, Germany).
Measurement of ROS by dihydroethidium
HPLC (Jasco, Groß-Umstadt, Germany) was applied to measure intracellular ROS formation in human embryonic kidney (HEK)-293 cells that stably overexpressed the human TSHR (HEK-293 TSHR). Superoxide was measured by dihydroethidium (DHE), which produces the superoxide-specific product 2-hydroxyethidium (2-HE) and the unspecific oxidation product ethidium (E+) in addition to minor dimerization products (29). E+ and 2-HE were detected by fluorescence (excitation 480 nm/emission 580 nm) (30). In this study we used a modified HPLC protocol (31). Serum samples from patients with untreated GD and control subjects were diluted 1:11 in RB.
The purely stimulatory monoclonal antibody (MAb) M22 (4 μg of IgG with 2.5 mg of BSA per vial, freeze-dried) and the purely TSHR-blocking MAb K1-70 (10 µg of IgG with 2.5 mg of BSA per vial, freeze-dried) were purchased from RSR Ltd. (Cardiff, UK). The final concentration (200 ng/mL) of M22 and K1-70 MAb was diluted in RB and tested in the HPLC. Bovine TSH was purchased from Sigma-Aldrich (St. Louis, MO; Cat. #T8931, 10 IU per vial). For bovine TSH (100 and 1000 µIU/mL) and M22 MAb (0.2 and 1 µg/mL), dose-response measurements were performed under the same conditions as described previously. For testing T3, one tablet containing 20 µg T3 (Thybon 20; Henning, Berlin, Germany) was dissolved in Eagle minimum essential medium to yield a final concentration of 100 and 1000 µg/mL T3, respectively.
The HEK-293 TSHR cells were seeded and grown in 24-well plates at a density of 1.1 × 105 cells per well and incubated with serum for 6, 24, and 48 hours. Subsequently, the cell monolayers were washed with PBS. For the measurement of DHE oxidation products, PBS containing 50 µM DHE was added to the cells. The plates were incubated for 30 minutes at 37°C and 5% CO2. For the measurement of DHE oxidation products in the cell lysate, the supernatant was removed, and a 1:1 mixture of acetonitrile and PBS was added to the cells and then scraped off. After centrifugation for 10 minutes at 10,000 g, the supernatant was measured by HPLC. As an oxidized standard, we used 250 nM 2-HE prepared by reaction of xanthine oxidase and hypoxanthine with DHE as described (31).
Measurement of lipid peroxidation
Primary human thyrocytes and stably transfected cells
For the isolation of primary human thyrocytes, tissue samples were collected from normal thyroid tissue of patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. Patients provided informed consent on an institutional review board–approved protocol, and materials were received anonymously with approval of research activity through the Office of Human Subjects Research, National Institutes of Health. HEK-293 TSHR cells were used as described (32).
Experimental outline
Primary cultures of human thyrocytes or HEK-293 TSHR cells were seeded to a density of 5 × 105 cells per well in 6-well plates or 1.1 × 105 cells per well in 24-well plates in a final volume of 2 or 1 mL DMEM (Biochrom, Berlin, Germany) with high glucose 4.5 g/L and containing l-glutamine and sodium pyruvate, which were supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin, respectively. After cell attachment, DMEM supplemented with 1% FCS was added. The concentration of FCS was reduced from 10% to 1% to avoid nonspecific effects of FCS. The cells were kept in a humidified atmosphere at 37°C and 5% CO2 overnight. Primary human thyrocytes and HEK-293 TSHR cells were incubated with serum samples (diluted 1:11 in RB) from patients with untreated GD and control subjects. For each serum sample one well on the 6-well plate was used. The effects of serum incubation on the lipid peroxidation marker 4-HNE were investigated by performing measurements at 6, 12, 24, and 48 hours.
Cell homogenization
The cell monolayers were washed three times with PBS. After aspiration, the cells were directly homogenized. The cells were scrapped off with ice-cold homogenization solution (150 µL per well). The cell solutions were incubated for 60 minutes on ice. After centrifugation, the protein concentrations were determined by the Bradford assay.
Preparation of the protein samples
A total of 420 µL of each sample was prepared, and 200 µL were applied in duplicate for dot-blot analysis. The tubes were prefilled with 1× PBS containing 0.14% SDS, homogenization buffer, and the corresponding volume of each sample, depending on the measured protein concentration, was added. During analysis, 27 ± 4 µg protein per sample was applied per dot.
Immunodot-blot analysis
Transfer of the protein samples and blocking of the membranes
The filter and nitrocellulose membrane were pre-wet by immersion in PBS. The dot-blot apparatus was assembled from the bottom up, and the dots were pre-washed with PBS. During aspiration, the prepared protein samples were added onto the nitrocellulose membrane by vacuum filtration. After immobilization of the proteins, the nitrocellulose membrane was dried on a glass plate for 60 minutes at 60°C on a heat block. Nonspecific protein binding sites were saturated by soaking the membrane in blocking solution containing 5% milk powder (MP) in PBS for 1 hour at room temperature with gentle swinging.
Primary and secondary antibody binding
The membrane was incubated with the primary Ab goat anti–4-HNE monoclonal antibody (1:500, Millipore, Darmstadt, Germany) and dissolved in PBS with 5% MP at 4°C overnight. The membrane was briefly washed once in PBS with 0.1% Tween-20 and washed four additional times, each for 5 minutes. The secondary Ab donkey anti-goat–IgG–horseradish peroxidase (1:5000; Santa Cruz Biotechnology, Heidelberg, Germany) was dissolved in PBS with 5% MP and added to the membrane for 1 hour at room temperature under gentle swinging. The membrane was washed one time rapidly in PBS with 0.1% Tween-20 and washed four additional times, each for 5 minutes.
Detection and evaluation
The antibody-labeled dots were detected by incubating the membrane with SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL), followed by imaging using a chemiluminescence documentation system (ChemiLux CSX-1400M, INTAS, Göttingen, Germany). The integrated density of the labeled dots was analyzed using Gel-Pro Analyzer software (Media Cybernetics, Bethesda, MD).
Statistical analysis
All data were presented as mean ± SEM and were analyzed with Sigma Stat (Systat, Inc., San Jose, CA) and/or Prism software (version 5.04 and 6; GraphPad, San Diego, CA). One-way analysis of variance was calculated with the Holm-Sidak multiple comparisons test. Student t test for unpaired samples was used for comparison between two independent groups if normally distributed. Statistical comparisons between two independent groups were done by the exact Mann-Whitney U test if not normally distributed. When comparing more than two groups, the Kruskal-Wallis test was used. Correlations between serum TSAb levels and oxidative parameters in serum and urine were quantified with the Spearman test. All P values were two sided, and results were significant at P < 0.05.
Results
The demographic and clinical data of all investigated study groups are shown in Table 1.
Table 1.
Demographic and Clinical Data of the Investigated Study Groups
| Hyperthyroid Untreated Patients with GD | Euthyroid Treated Patients with GD | Toxic Nodular Goiter | Euthyroid Healthy Control Subjects | |
|---|---|---|---|---|
| Number | 54 | 19 | 5 | 42 |
| Sex, F/M | 49/5 | 16/3 | 4/1 | 38/4 |
| Age, y | 38.4 ± 0.9 | 33.1 ± 1.7 | 45 ± 2.5 | 31.1 ± 0.8 |
| TSH, 0.27–4.2 µIU/mL | 0.01 ± 0.01 | 1.1 ± 0.2 | 0.01 ± 0.2 | 1.3 ± 0.1 |
| fT3, 3.1–6.8 pmol/L | 12.5 ± 0.5 | 4.5 ± 0.3 | 9.3 ± 0.3 | 3.6 ± 0.2 |
| fT4, 12–22 pmol/L | 29 ± 0.4 | 17 ± 0.7 | 27 ± 0.1 | 14 ± 0.3 |
| TBII, <1.75 IU/L | 32.3 ± 1 | 3.4 ± 2.7 | 0.5 ± 0.2 | 0.3 ± 0 |
| TSAb, <140 SRR% | 681 ± 10 | 193 ± 21 | 59 ± 7 | 38 ± 1 |
Values are mean ± SEM.
Abbreviations: fT4, free thyroxine; fT3, free triiodothyronine; SRR, specimen-to-reference ratio; TBII TSHR-binding inhibitory immunoglobulins.
Measurement of markers for lipid peroxidation and DNA oxidative damage in urine
The lipid peroxidation markers MDA and 8-isoprostane as well as the DNA damage marker 8-OH-dG were measured in urine samples obtained from patients with untreated hyperthyroid GD, hyperthyroid patients with toxic nodular goiter, and control subjects (Table 2). The markers MDA, 8-isoprostane, and 8-OH-dG were higher in hyperthyroid patients with GD vs hyperthyroid patients with toxic nodular goiter (P = 0.001, P = 0.04, and P = 0.04, respectively) and vs control subjects (P = 0.01, P = 0.02, and P = 0.03, respectively). In patients with untreated Graves hyperthyroidism, serum levels of TSAbs positively correlated with urine concentrations of malondialdehyde (r = 0.79, P < 0.001), 8-isoprostane (r = 0.82, P < 0.001), 8-hydroxy-2-deoxy guanosine (r = 0.69, P < 0.01), and serum NOX2 levels (r = 0.85, P < 0.001).
Table 2.
Measurement of Lipid Peroxidation and DNA Oxidative Damage Markers in Urine Samples
| Hyperthyroid Untreated GD (Group A) (n = 54) | Toxic Nodular Goiter (Group B) (n = 5) | Euthyroid Healthy Control Subjects (Group C) (n = 42) |
P Value
|
||
|---|---|---|---|---|---|
| A vs B | A vs C | ||||
| Lipid peroxidation | |||||
| MDA, µM MDA/g creatinine | 7.4 ± 0.6 | 3.0 ± 0.4 | 3.2 ± 0.2 | 0.001 | 0.01 |
| 8-isoprostane, pg/mL | 959 ± 121 | 450 ± 19 | 419 ± 16 | 0.04 | 0.02 |
| DNA oxidative damage | |||||
| 8-OH-dG, ng/mL | 110 ± 16 | 41 ± 13 | 37 ± 10 | 0.04 | 0.03 |
Values are mean ± SEM.
NOX2 ELISA assay
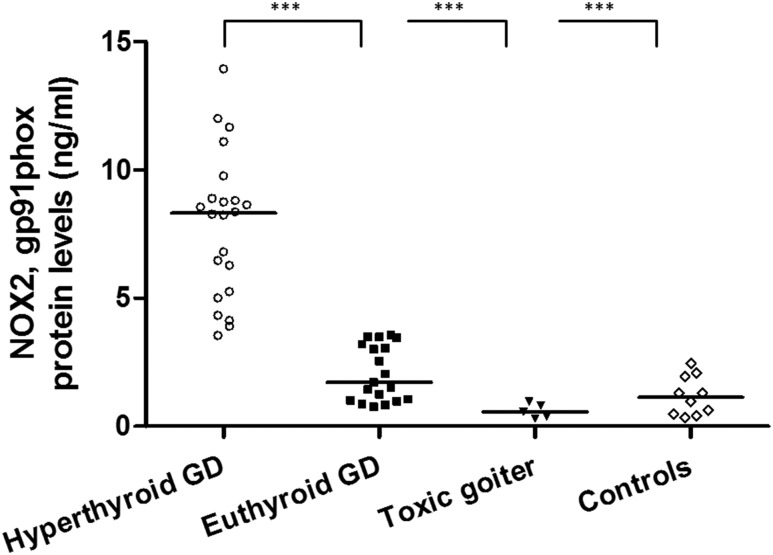
NOX2-expressed proteins were measured in serum of untreated hyperthyroid and treated euthyroid patients with GD, patients with toxic nodular goiter, and control subjects (Fig. 1). Markedly higher levels were noted in hyperthyroid vs euthyroid patients, in hyperthyroid untreated GD vs hyperthyroid patients with toxic nodular goiter, and in euthyroid control subjects (all P < 0.0001).
Figure 1.
Measurement of NOX2 in serum samples of patients with GD and healthy controls. Serum was used from untreated hyperthyroid patients with GD (n = 22), treated euthyroid patients with GD (n = 19), patients with toxic nodular goiter (n = 5), and euthyroid healthy control subjects (n = 10). The black horizontal line indicates the median. Marked differences were noted between hyperthyroid untreated patients with GD vs euthyroid treated patients with GD, hyperthyroid untreated patients with toxic nodular goiter, and healthy control subjects. ***P < 0.0001.
Measurement of the phagocytic NOX2 activity
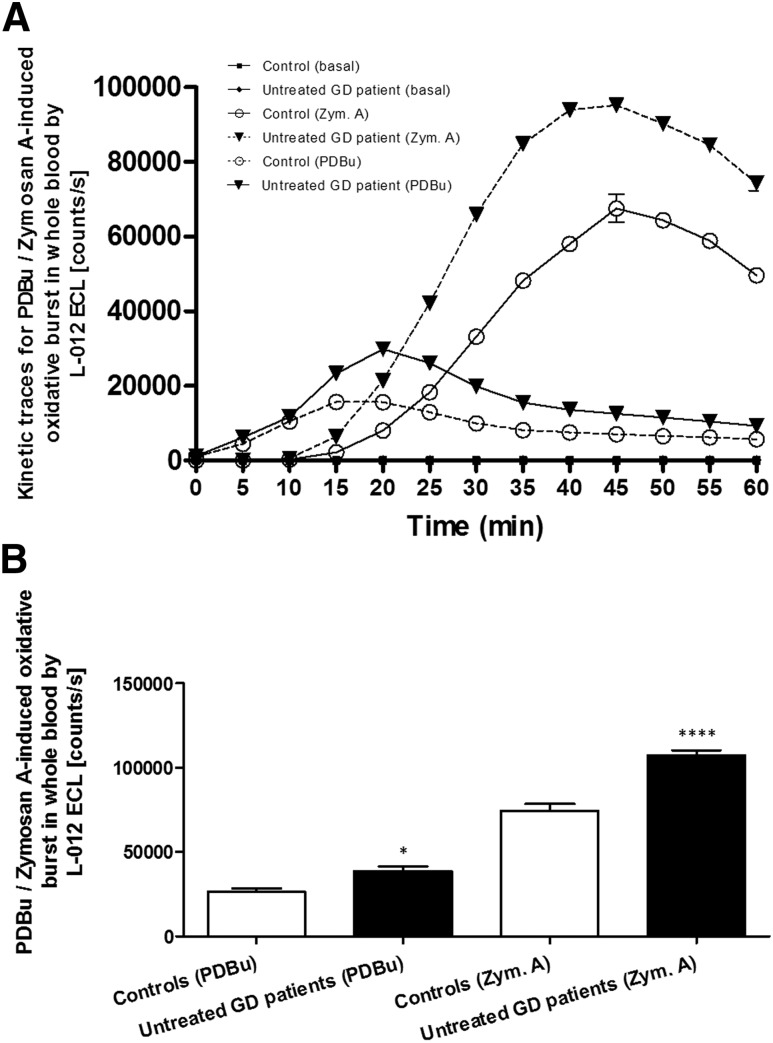
The individual kinetics of the respiratory burst of leukocytes in whole blood of an untreated hyperthyroid GD patient and a control subject after stimulation with PDBu and zymosan A, as well as without stimulation (basal), are shown in Fig. 2A. The maximum stimulation peaks for PDBu and zymosan A were observed after 20 minutes and 45 minutes, respectively. The apparent magnitude of counts per second was increased 1.5-fold in hyperthyroid untreated patients with GD vs control subjects (Fig. 2B) for both PDBu and zymosan A (P < 0.05 and P < 0.00001), respectively. The white blood cell count (number of cells × 103/µL) was comparable in the two patient groups (mean ± SEM: 6.1 ± 0.3 and 6.2 ± 0.4 in hyperthyroid untreated patients with GD and control subjects, respectively).
Figure 2.
(A) The respiratory burst of leukocytes was triggered with the stimulants zymosan A and PDBu in whole blood from a patient with untreated hyperthyroid GD and a healthy control subject. Zymosan A showed a maximum ROS formation after 45 min and PDBu after 20 min as measured by enhanced chemiluminescence. Data are reported as mean ± SEM. (B) Respiratory burst of several patients with untreated hyperthyroid GD and healthy control subjects. Results from patients with GD (n = 8) and control subjects (n = 8) are shown as black and white bars, respectively. PDBu and zymosan A elicited maximal ROS formation, which was significantly different in control subjects vs patients with GD. *P < 0.05; ****P < 0.00001.
Measurement of ROS by DHE
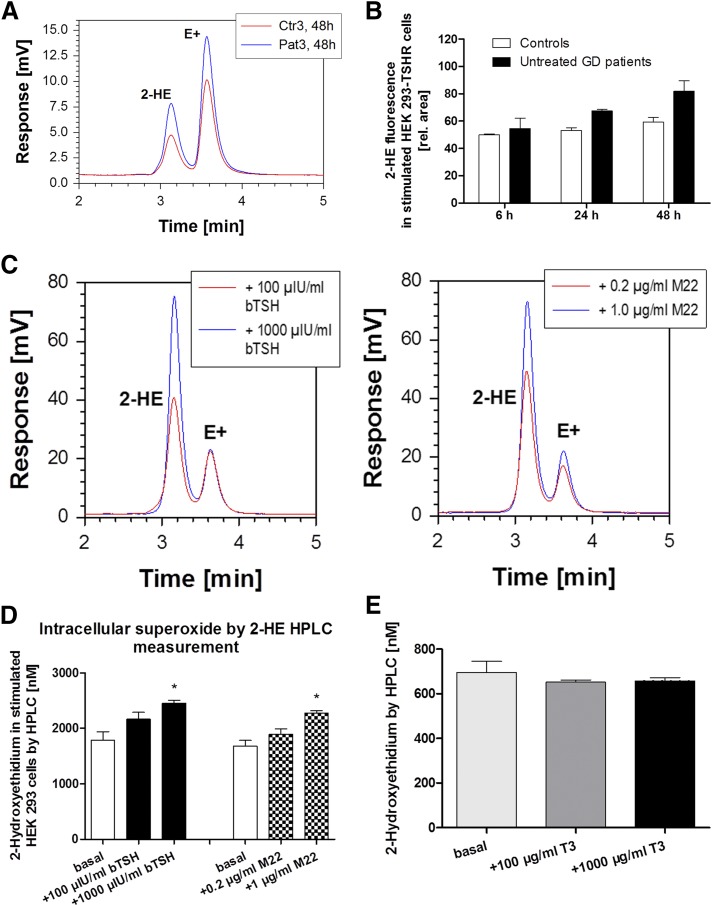
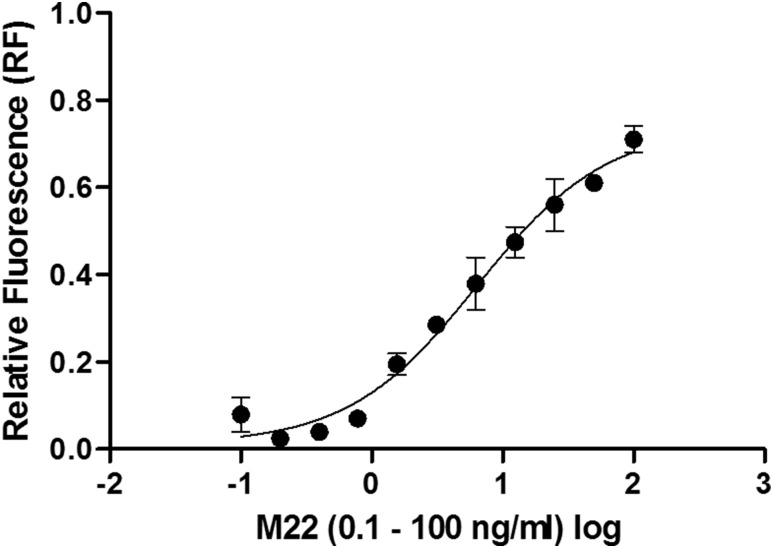
The HPLC chromatograms of the fluorescence detection of the superoxide-specific 2-HE and unspecific E+ DHE product are representative of an untreated hyperthyroid GD patient vs a euthyroid control subject after 48-hour stimulation of HEK-293 TSHR cells (Fig. 3A). HPLC-based analysis revealed increased levels of the 2-HE product in HEK-293 TSHR cells stimulated with serum from patients with untreated hyperthyroid GD (after 48 hours) vs control (after 6, 24, and 48 hours) and vs the 6-hour patient group (P < 0.05) (Fig. 3B). Bovine TSH and M22 showed a dose-dependent increase in intracellular superoxide formation as determined by HPLC analysis in HEK-293 TSHR cells (Fig. 3C and 3D). Also, M22 stimulated, in a dose-dependent manner, cAMP release from HEK-293 TSHR cells using a Bridge-It cAMP assay (Fig. 4).
Figure 3.
(A) Chromatograms of the fluorescence detection of the superoxide-specific 2-HE and unspecific E+ oxidation products of DHE. The peaks for 2-HE and E+ are examples for a patient with untreated GD (in red) vs a serum sample from a healthy control subject (Ctr) (in blue) after 48-hincubation in HEK-293 TSHR cells. (B) Measurement of superoxide-specific 2-HE by HPLC. The graph shows a time course of 6, 24, and 48 h after incubation of HEK-293 TSHR cells with human sera. Untreated hyperthyroid patients with GD (black bars, n = 8) and euthyroid healthy control subjects (white bars, n = 8) are shown with the corresponding 2-HE fluorescence signal. Levels of 2-HE in HEK-293 TSHR cells incubated with serum from patients with untreated hyperthyroid GD (after 48 h) were markedly different vs control subjects after 6, 24, and 48 h and vs the 6-h patient group (P < 0.05), respectively. (C) Chromatograms of the fluorescence detection of the superoxide-specific 2-HE and unspecific E+ oxidation products of DHE. The peaks for 2-HE and E+ are examples for HEK-293 TSHR cells upon stimulation with bovine TSH (100 vs 1000 µIU/mL) or with the M22 MAb (0.2 vs 1.0 µg/mL). (D) Measurement of superoxide-specific 2-HE by HPLC. The graph shows the effects of stimulation of HEK-293 TSHR cells with bovine TSH (100 and 1000 µIU/mL, black bars) or with M22 MAb (0.2 vs 1.0 µg/mL, checkered bars) on intracellular superoxide formation as measured by HPLC-based quantification of 2-HE (n = 4 independent experiments). Basal levels are also shown (incubation of cells without bovine TSH and M22 MAb, white bars). The highest concentrations of both stimulators caused a significant increase in superoxide formation. *P = 0.0028 and P = 0.0082, respectively. (E) Intracellular superoxide by 2-HE HPLC measurement. The graph shows the effects of stimulation of HEK-293 TSHR cells with T3 (100 µg/mL, dark gray bar) and 1000 µg/mL (black bar) on intracellular superoxide formation as measured by HPLC-based quantification of 2-HE (n = 4 independent experiments). Basal levels are also shown (incubation of cells without T3, light gray bar).
Figure 4.
Measurement of cAMP levels with a homogeneous, fluorescent assay. The HEK-293 TSHR cells were seeded to 3 × 104 cells per well and grown overnight. On the x-axis the M22 MAb (0.1 to 100 ng/mL) concentrations were shown in a logarithmic scale and on the y-axis was the relative fluorescence (RF) presented.
The TSHR blocking MAb K1-70 did not show a 2-HE response (data not shown). Finally, T3 was tested in HEK-293 TSHR cells at two different concentrations (100 and 1000 µg/mL) and did not affect superoxide production as measured by HPLC (Fig. 3E).
Measurement of lipid peroxidation in cultured cells
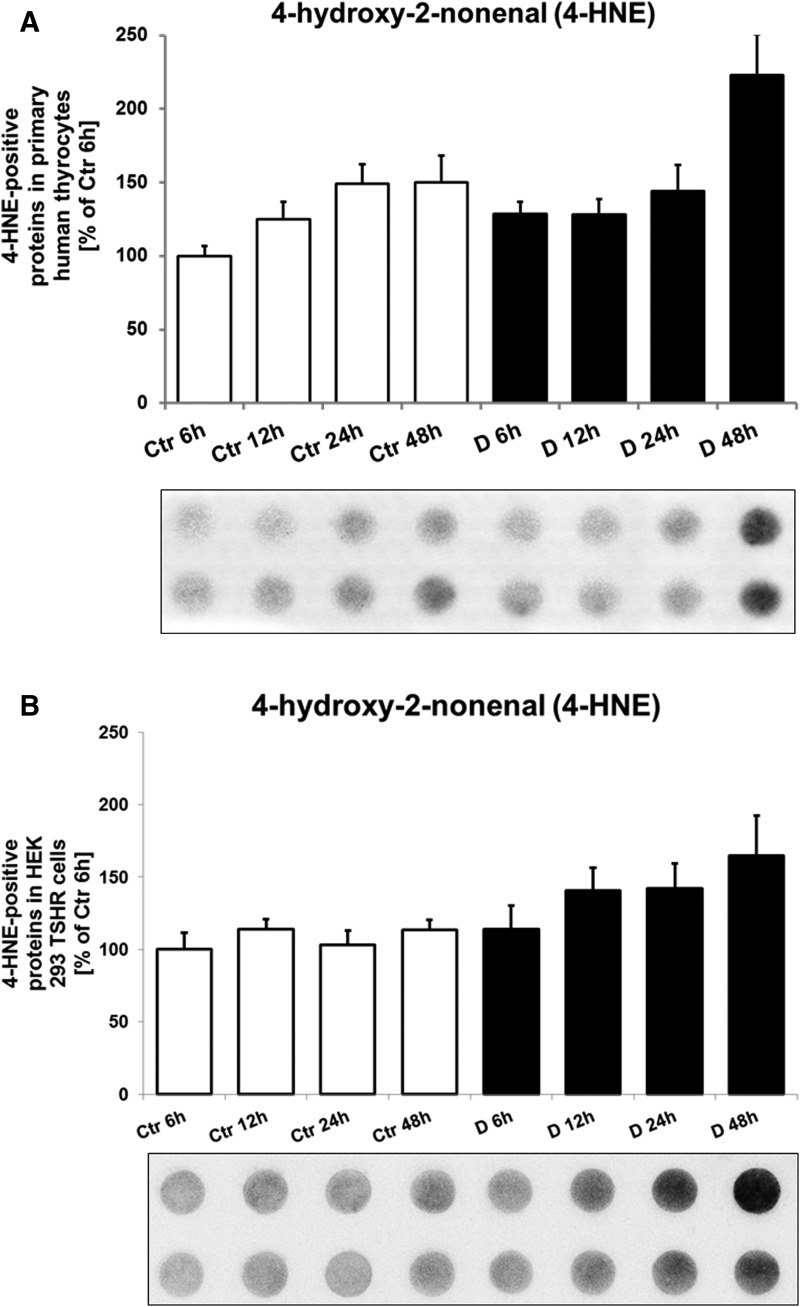
Levels of 4-HNE, as measured by immunodot-blot, in primary human thyrocytes and in HEK-293 TSHR cells are shown in Fig. 5A and 5B. In human thyrocytes, the 4-HNE marker was markedly higher in patients with GD at 48 hours (P = 0.04) in comparison with all other groups. In the HEK-293 TSHR cells, 4-HNE was markedly higher in patients with GD at 48 hours vs control subjects at 6 and 48 hours (both P < 0.05).
Figure 5.
(A) Representative immunodot-blot of the oxidative stress marker 4-HNE in primary human thyrocyte after incubation with serum from untreated hyperthyroid patients with GD (D for disease, n = 8) and euthyroid healthy control subjects (Ctr, n = 8). The cells were incubated with serum in a time series for 6, 12, 24, and 48 h. In primary human thyrocytes, the 4-HNE marker was significantly higher in the GD 48-h group in comparison with all other groups. The 4-HNE levels were higher in patients with GD vs control subjects at 6 and 48 h (P = 0.02 and P = 0.04, respectively) and in patients with GD at 48-h incubation vs 12 h (P = 0.02) and 6 h (P = 0.01). For Ctr and GD groups, a time-dependent increase was observed. (B) Representative immunodot-blot of the oxidative stress marker 4-HNE in HEK-293 TSHR cells after incubation with serum from untreated hyperthyroid patients with GD (n = 8) and euthyroid healthy control subjects (n = 8). The cells were incubated with serum in a time series for 6, 12, 24, and 48 h. Regarding the HEK-293 TSHR cells, 4-HNE was markedly higher in GD at 48 h vs control subjects at 6 and 48 h (both P < 0.05).
Discussion
The current study demonstrates that functional thyrotropin receptor–stimulating antibodies are involved in oxidative stress mechanisms in patients with Graves hyperthyroidism. The excessive generation of ROS may cause oxidative damage, as seen foremost by lipid peroxidation in untreated hyperthyroid patients with GD compared with treated, euthyroid GD, and healthy euthyroid control subjects. These findings were observed in samples of patients with untreated GD containing polyclonal TSAbs and after stimulation with the monoclonal purely stimulatory antibody TSAb M22.
Abnormally high oxidative stress potentially affects the pathogenesis of GD by inducing and augmenting inflammation in the thyroid, leading to apoptosis and breakdown of the normal balance between self-tolerance and immunity (4). In hyperthyroidism, increased oxygen consumption, dysfunction in the mitochondrial respiratory chain, elevated intracellular adenosine triphosphate consumption, and increased ROS production were observed (4). Increasing ROS levels affect membrane lipids, and the consequent oxidative damage leads to cell and tissue damage. Thus, ROS molecules can be regarded as global indicators of oxidative stress.
The DNA damage marker 8-hydroxy-2-deoxy guanosine was lower in urine samples of patients with toxic nodular goiter compared with patients with GD. Our data on 8-OH-dG are in line with a previous study reporting higher expression levels of DNA repair proteins (ATM and γH2AX) in thyroid cancer tissues than in benign nodular goiter and normal adjacent tissues (33). ATM and γH2AX play an important role in the detection and repair of DNA double-strand breaks and the cellular response to DNA damage, which can be observed under oxidative stress conditions (e.g., upon treatment with artesunate) (34).
NOX2 generates ROS (superoxide anions) when it is activated (35). In cellular homeostasis, ROS generated by NOX2 acts as an effector signaling molecule for cell growth, migration, and several other physiological responses (35). NOX2 enzymatic complex reduces oxygen; subsequently, superoxide anions are produced and transformed to the highly reactive hydroxyl radical (•OH) and hydrogen peroxide (H2O2). Increased markers of oxidative stress and decreased antioxidative capacity were also observed in erythrocytes of patients with GD (10). After antithyroid treatment, all analyzed parameters were normalized. The euthyroid state was achieved more rapidly with administration of additional selenium (36), which is an essential factor for glutathione peroxidase as antioxidant enzyme. TSHR-Abs directly induce GD and, through thyroid dysfunction, lead to an imbalance between antioxidants and ROS (i.e., superoxide) (37). In our study and in line with the above statements is the observed increased activation of NOX2, which was measured after zymosan A and PDBu stimulation in whole blood obtained from hyperthyroid patients with GD vs control subjects, leading to oxidative damage. The protein kinase C is activated by PDBu and leads to an increase of superoxide formation by NOX2. No activity of NOX2 was detectable in patients with nonautoimmune hyperthyroidism, suggesting that the activation of NOX2 is induced by TSAbs only in hyperthyroid patients with GD. Thus, ROS formation seems to be a consequence of the chronic activation of leukocytes by functional TSHR-Abs.
The human TSHR-stimulating MAb M22 was able to cause an increased generation of the specific superoxide product 2-HE as measured by HPLC. In contrast, the TSHR blocking human MAb, K1-70 caused no measurable signal. These data are in line with the hypothesis that TSAbs, either monoclonal or polyclonal, are directly involved in the oxidative damage in patients with GD, whereas the functional TSHR blocking Abs showed no such effect. Further, no increase in superoxide-specific 2-HE was observed when testing two different T3 concentrations compared with basal levels (cells incubated without T3) in HPLC, suggesting that ROS formation is not directly affected by the active thyroid hormone T3.
Lipid peroxidation is the reaction of oxygen with polyunsaturated lipids (15), and the lipid peroxidation product 4-HNE was used in the current study as a marker of oxidative stress. For the investigation of lipid peroxidation, human primary thyrocytes (physiological TSHR expression) and HEK-293 TSHR cells stably overexpressing the human TSHR were used as model systems. In comparison with the HEK-293 TSHR cells, the TSHR expression level in primary thyrocytes was 100-fold lower, and TSHR expression may vary from donor to donor. This explains why the HEK-293 TSHR cells are more sensitive in responding to TSAbs in patient serum and M22 MAbs. In both cell types, the detection of the lipid peroxidation marker 4-HNE was confirmed via immunodot-blot analysis. Both polyclonal and monoclonal TSAbs directly increased in vitro superoxide release and the lipid peroxidation marker 4-HNE, which was confirmed by the in vivo measurements showing higher oxidative stress markers (MDA, 8-isoprostane, and 8-OH-dG) in urine of patients with untreated GD.
The investigation of the respiratory burst of whole blood leukocyte-dependent ROS formation and the detection of the oxidation products of DHE in HPLC and the 4-HNE immunodot-blot, which did not include hyperthyroid patients with nonautoimmune hyperthyroidism or toxic nodular goiter, cannot be considered as definitive proof of a direct TSHR-Ab effect. This is a potential limitation of the study, although the HPLC data clearly demonstrated a missing effect of T3 on ROS generation.
In summary, TSAbs are involved in several oxidative stress mechanisms in patients with Graves hyperthyroidism, enhancing ROS production and inducing lipid peroxidation.
Acknowledgments
We thank Elisa Schulze (study nurse, Molecular Thyroid Laboratory, Johannes Gutenberg University Medical Center) for blood specimen collection as well as Elisabeth Ullmann and Ylan Tran (both Molecular Cardiology, Johannes Gutenberg University) for technical assistance.
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Z01 DK011006) (to S.N.).
Disclosure Summary: G.J.K. and P.D.O. consult for Quidel. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 2-HE
2-hydroxyethidium
- 4-HNE
4-hydroxy-2-nonenal
- 8-OH-dG
8-hydroxy-2-deoxy guanosine
- Ab
antibody
- DHE
dihydroethidium
- E+
ethidium
- FCS
fetal calf serum
- GD
Graves disease
- HEK
human embryonic kidney
- MAb
monoclonal antibody
- MDA
malondialdehyde
- MP
milk powder
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- NOX2
nicotinamide adenine dinucleotide phosphate oxidase, isoform 2
- PDBu
phorbol 12,13-dibutyrate
- RB
reaction buffer
- ROS
reactive oxygen species
- T3
triiodothyronine
- TSAb
TSH-receptor stimulating autoantibody
- TSHR
TSH receptor
References
- 1. Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23(1):134–147. [DOI] [PubMed] [Google Scholar]
- 2. Shah D, Mahajan N, Sah S, Nath SK, Paudyal B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci. 2014;21(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gelderman KA, Hultqvist M, Olsson LM, Bauer K, Pizzolla A, Olofsson P, Holmdahl R. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 2007;9(10):1541–1567. [DOI] [PubMed] [Google Scholar]
- 4. Marcocci C, Leo M, Altea MA. Oxidative stress in graves’ disease. Eur Thyroid J. 2012;1(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcocci C, Bartalena L. Role of oxidative stress and selenium in Graves’ hyperthyroidism and orbitopathy. J Endocrinol Invest. 2013;36(10, Suppl):15–20. [PubMed] [Google Scholar]
- 6. Schulz E, Wenzel P, Münzel T, Daiber A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal. 2014;20(2):308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I. Free radical biology of the cardiovascular system. Clin Sci (Lond). 2012;123(2):73–91. [DOI] [PubMed] [Google Scholar]
- 8. Münzel T, Sørensen M, Schmidt F, Schmidt E, Steven S, Kröller-Schön S, Daiber A. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxid Redox Signal. 2018;28(9):873–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. [DOI] [PubMed] [Google Scholar]
- 10. Abalovich M, Llesuy S, Gutierrez S, Repetto M. Peripheral parameters of oxidative stress in Graves’ disease: the effects of methimazole and 131 iodine treatments. Clin Endocrinol (Oxf). 2003;59(3):321–327. [DOI] [PubMed] [Google Scholar]
- 11. Sedeek M, Nasrallah R, Touyz RM, Hébert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol. 2013;24(10):1512–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59(9):1428–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S4–S8. [DOI] [PubMed] [Google Scholar]
- 14. Mikhed Y, Görlach A, Knaus UG, Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 2015;5:275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014; 2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leschik JJ, Diana T, Olivo PD, König J, Krahn U, Li Y, Kanitz M, Kahaly GJ. Analytical performance and clinical utility of a bioassay for thyroid-stimulating immunoglobulins. Am J Clin Pathol. 2013;139(2):192–200. [DOI] [PubMed] [Google Scholar]
- 17. Diana T, Brown RS, Bossowski A, Segni M, Niedziela M, König J, Bossowska A, Ziora K, Hale A, Smith J, Pitz S, Kanitz M, Kahaly GJ. Clinical relevance of thyroid-stimulating autoantibodies in pediatric graves’ disease-a multicenter study. J Clin Endocrinol Metab. 2014;99(5):1648–1655. [DOI] [PubMed] [Google Scholar]
- 18. Diana T, Kanitz M, Lehmann M, Li Y, Olivo PD, Kahaly GJ. Standardization of a bioassay for thyrotropin receptor stimulating autoantibodies. Thyroid. 2015;25(2):169–175. [DOI] [PubMed] [Google Scholar]
- 19. Kahaly GJ, Diana T. TSH receptor antibody functionality and nomenclature. Front Endocrinol (Lausanne). 2017;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y, Kim J, Diana T, Klasen R, Olivo PD, Kahaly GJ. A novel bioassay for anti-thyrotrophin receptor autoantibodies detects both thyroid-blocking and stimulating activity. Clin Exp Immunol. 2013;173(3):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diana T, Li Y, Olivo PD, Lackner KJ, Kim H, Kanitz M, Kahaly GJ. Analytical performance and validation of a bioassay for thyroid-blocking antibodies. Thyroid. 2016;26(5):734–740. [DOI] [PubMed] [Google Scholar]
- 22. Diana T, Wüster C, Kanitz M, Kahaly GJ. Highly variable sensitivity of five binding and two bio-assays for TSH-receptor antibodies. J Endocrinol Invest. 2016;39(10):1159–1165. [DOI] [PubMed] [Google Scholar]
- 23. Diana T, Wüster C, Olivo PD, Unterrainer A, König J, Kanitz M, Bossowski A, Decallonne B, Kahaly GJ. Performance and specificity of 6 immunoassays for TSH receptor antibodies: a multicenter study. Eur Thyroid J. 2017;6(5):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diana T, Krause J, Olivo PD, König J, Kanitz M, Decallonne B, Kahaly GJ. Prevalence and clinical relevance of thyroid stimulating hormone receptor-blocking antibodies in autoimmune thyroid disease. Clin Exp Immunol. 2017;189(3):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease-methodology and clinical applications. Ophthal Plast Reconstr Surg. 2018;34(4SSuppl 1):S13–S19. [DOI] [PubMed] [Google Scholar]
- 26. Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010;162(3):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian L, Wang RE, Fei Y, Chang YH. A homogeneous fluorescent assay for cAMP-phosphodiesterase enzyme activity. J Biomol Screen. 2012;17(3):409–414. [DOI] [PubMed] [Google Scholar]
- 28. Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36(1):101–111. [DOI] [PubMed] [Google Scholar]
- 29. Nazarewicz RR, Bikineyeva A, Dikalov SI. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J Biomol Screen. 2013;18(4):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandes DC, Wosniak J Jr, Pescatore LA, Bertoline MA, Liberman M, Laurindo FR, Santos CX. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2007;292(1):C413–C422. [DOI] [PubMed] [Google Scholar]
- 31. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Müller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Münzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10(8):1435–1447. [DOI] [PubMed] [Google Scholar]
- 32. Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149(12):5945–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu JL, Hu SS, Hou XX, Zhu X, Cao J, Jiang LH, Ge MH. Abnormal expression of DNA double-strand breaks related genes, ATM and GammaH2AX, in thyroid carcinoma. Int J Endocrinol. 2015;2015:136810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther. 2011;10(12):2224–2233. [DOI] [PubMed] [Google Scholar]
- 35. Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12(1):5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vrca VB, Skreb F, Cepelak I, Romic Z, Mayer L. Supplementation with antioxidants in the treatment of Graves’ disease; the effect on glutathione peroxidase activity and concentration of selenium. Clin Chim Acta. 2004;341(1-2):55–63. [DOI] [PubMed] [Google Scholar]
- 37. Venditti P, Balestrieri M, Di Meo S, De Leo T. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J Endocrinol. 1997;155(1):151–157. [DOI] [PubMed] [Google Scholar]