Abstract
During pregnancy, women experience numerous physiological changes but, to date, there is limited published data that characterize accompanying changes in gene expression over pregnancy. This study sought to characterize the complexity of the transcriptome over the course of pregnancy among women with healthy pregnancies. Subjects provided a venous blood sample during early (6–15 weeks) and late (22–33 weeks) pregnancy, which was used to isolate peripheral blood mononuclear cells prior to RNA extraction. Gene expression was examined for 63 women with uncomplicated, term deliveries. We evaluated the association between weeks gestation at sample collection and expression of each transcript. Of the 16,311 transcripts evaluated, 439 changed over pregnancy after a Bonferroni correction to account for multiple comparisons. Genes whose expression increased over pregnancy were associated with oxygen transport, the immune system, and host response to bacteria. Characterization of changes in the transcriptome over the course of healthy term pregnancies may enable the identification of genes whose expression predicts complications or adverse outcomes of pregnancy.
Introduction
Pregnancy is characterized by extensive physiological changes including increased blood volume, elevated levels of estrogen and progesterone, changes in metabolism, and shifts in the maternal immune system in order to accommodate the demands of the growing fetus [1, 2]. Despite these carefully regulated physiological changes that occur throughout the 40 weeks of an average pregnancy, no study has yet described accompanying changes in maternal blood gene expression among healthy women with full term, uncomplicated pregnancies.
To date, studies of gene expression focus on comparisons of pregnant women who are healthy to those with autoimmune disorders. This has been prompted, in part, by the observation that women with some autoimmune disorders report alleviation of their symptoms during pregnancy [3]. For example, a recent study of peripheral blood from 20 women with rheumatoid arthritis and 5 healthy controls identified 4,710 genes that differed in their expression over pregnancy and the postpartum period. The genes identified were enriched for a variety of pathways including immune pathways, signal transduction, and disease-related pathways. Interestingly, several of the genes identified by this study, are members of the alpha defensin family and are involved in immune and defense responses [4]. Another study identified 1,286 transcripts whose expression levels changed over three timepoints in pregnancy and the postpartum period for women with rheumatoid arthritis and controls. These transcripts were enriched for a variety of pathways including hematopoietic cell lineage and toll-like receptor signaling. The authors concluded that the identified pathways may contribute to immunomodulation and thus a reduction in rheumatoid arthritis symptoms during pregnancy with these changes in gene expression being largely reversed postpartum [5]. Gilli and colleagues sampled multiple sclerosis patients and healthy controls before pregnancy, during each trimester, and post-partum to identify 404 transcripts whose expression differed between multiple sclerosis patients and healthy controls. A refined signature of 347 transcripts was then used to evaluate gene expression in patients and controls in the ninth month of pregnancy, at which time the signature could no longer distinguish patients from controls [6]. These studies indicate that gene expression does change over pregnancy, potentially leading to alleviation of some of the symptoms of autoimmune disorders.
Although such studies have been informative for identifying pathways that change in women with autoimmune disorders during pregnancy, if the groups are not sampled at comparable stages of gestation, the findings may be more difficult to interpret or replicate in independent studies. This study sought to characterize gene expression changes over pregnancy in a cohort of healthy women with uncomplicated term deliveries. The pathways identified provide further insight into the connection between gene expression and physiological changes that occur over the course of a healthy, full term pregnancy, and will serve as a resource for ongoing studies of pregnancy complications and adverse pregnancy outcomes.
Results
Study subjects
63 pregnant African American women provided venous blood samples between 6–15 weeks and again between 22–33 weeks gestation, representing the late first/early second trimesters and the late second/early third trimesters. The clinical and demographic features of these women are summarized in Table 1. At the time of enrollment, 7 women (12%) report using tobacco within the last 30 days, and 4 women (7%) report drinking alcohol within the last 30 days. Despite this, no woman delivered a neonate that was small for gestational age or experienced any complications over their pregnancies (see Methods for additional details).
Table 1. Clinical and demographic characteristics of the 63 women included in the study.
| Mean ± SD | |
| Maternal Age (years) | 25.4 ± 4.5 |
| Parity | 1.2 ± 1.1 |
| Gravidity | 2.8 ± 1.6 |
| Length of Gestation (weeks) | 39.4 ± 1.0 |
| Birthweight (grams) | 3318 ± 411.8 |
| N (%) | |
| Delivery Type | |
| Vaginal | 55 (87) |
| C-section | 8 (13) |
| Insurance Type | |
| Medicaid | 46 (73) |
| Private | 17 (37) |
| Education | |
| Some High School | 11 (17) |
| High School Graduate | 17 (27) |
| Some College | 25 (40) |
| College Graduate | 7 (11) |
| Graduate School | 3(5) |
| Mean ± SD | |
| Maternal Age (years) | 25.4 ± 4.5 |
| Parity | 1.2 ± 1.1 |
| Gravidity | 2.8 ± 1.6 |
| Length of Gestation (weeks) | 39.4 ± 1.0 |
| Birthweight (grams) | 3318 ± 411.8 |
| N (%) | |
| Delivery Type | |
| Vaginal | 55 (87) |
| C-section | 8 (13) |
| Insurance Type | |
| Medicaid | 46 (73) |
| Private | 17 (37) |
| Education | |
| Some High School | 11 (17) |
| High School Graduate | 17 (27) |
| Some College | 25 (40) |
| College Graduate | 7 (11) |
| Graduate School | 3(5) |
Changes in cell proportions over pregnancy
Studies have reported changes in a range of cell types and immune activation profiles over pregnancy [7–9]. Consistent with those previous reports, we observed changes in the proportion of cell types in maternal PBMCs over the course of pregnancy. There was a significant increase in the proportion of monocytes (p = .001), along with a significant decrease in the proportion of B cells (p = .03), and natural killer cells (p = .004) based on week of gestation (S1 Fig). Estimates of cell composition used for this study are not comprehensive, and there may be additional changes in cell composition or function that are not currently captured. To account for such changes in cell type and the potential for other known (i.e. age) or unknown confounders, we used surrogate variable analysis (SVA), which identifies and estimates sources of expression heterogeneity to increase power to detect true and replicable associations. Post-hoc evaluation of surrogate variables suggests that they reflect changes in cell composition, age, and control for technical variables such as batch (S2 Fig).
Gene expression changes over pregnancy
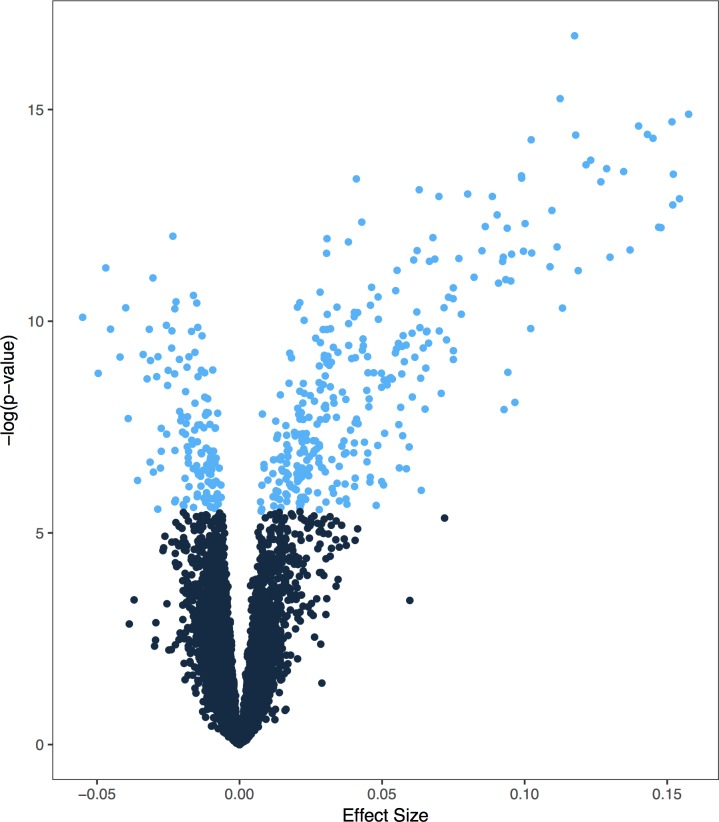
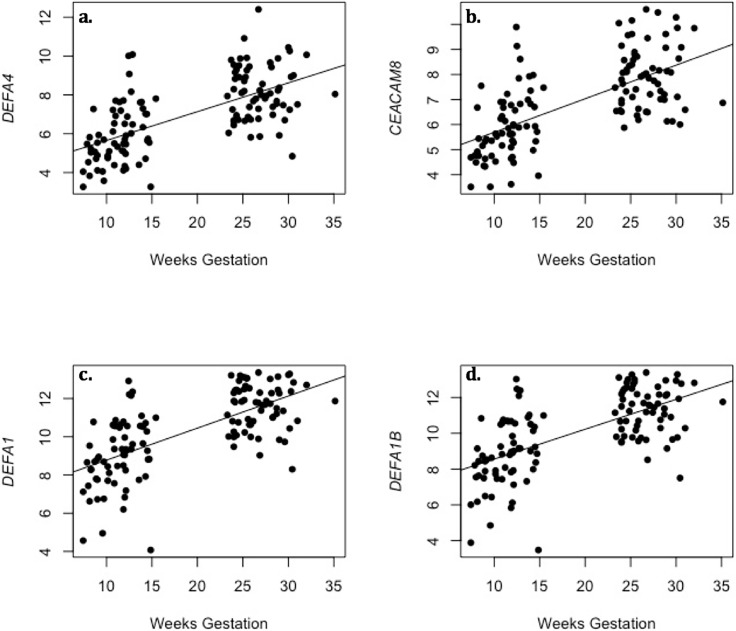
Of the 16,311 transcripts that were evaluated in this transcriptome-wide analysis (Fig 1), 439 associated with weeks of gestation at sample collection, after adjusting for multiple testing (S1 Table). The majority (69.6%) of these genes increased in expression over the course of pregnancy. Genes whose expression changed were enriched for multiple biological processes (Table 2). Of note, many of these genes are in involved in oxygen transport or the hemoglobin complex (e.g. AHSP, HBD, HBM, and HBQ1). In addition to increased expression of genes involved in oxygen transport, other associated biological processes emphasize changes in the immune system known to occur over pregnancy. Several of these immune processes are associated with response to microbes, including the antibacterial humoral response and the innate immune response in mucosa (Table 2, S3 Fig). Key genes in these pathways are members of the alpha defensin family, including DEFA1, DEFA4, and DEFA1B (Fig 2). Similar pathways were identified for genes that increased over pregnancy, though there was no enrichment for biological processes in genes whose expression decreased over pregnancy (S2 Table).
Fig 1. Transcriptome-wide distribution of transcripts that change over pregnancy.
Volcano plot depicting associations with gene transcripts that change over pregnancy. The x-axis represents the effect size. The y-axis represents the significance level for each test. Light blue points represent experiment-wide significance.
Table 2. Biological processes associated with genes whose expression changes over pregnancy.
| Term | GO Identifier | Count | p-value* |
|---|---|---|---|
| oxygen transport | GO:0015671 | 6 | 0.0074 |
| defense response to fungus | GO:0050832 | 7 | 0.0081 |
| antibacterial humoral response | GO:0019731 | 8 | 0.012 |
| leukocyte migration | GO:0050900 | 13 | 0.012 |
| killing of cells of other organism | GO:0031640 | 5 | 0.041 |
| innate immune response in mucosa | GO:0002227 | 6 | 0.044 |
* p-values presented after a Benjamini-Hochberg correction for multiple testing.
Fig 2. Genes in the alpha defensing family that change over pregnancy.
The x-axis represents weeks gestation at sample collection, and the y-axis represents the log2-transfomed expression levels for each transcript. a) DEFA4 (ILMN_1753347; p = 2.45x10-15) b) CEACAM8 (ILMN_1806056; p = 1.57x10-14) c) DEFA1 (ILMN_1679357; p = 3.37x10-14) d) DEFA1B (ILMN_2102721; p = 1.79x10-13).
In contextualizing our study findings with the existing literature, we attempted to compare the genes whose expression levels change in healthy pregnancies to those that have been reported to change in women with autoimmune disorders. In some cases, complete gene lists were not provided [5, 6], limiting our ability to make direct comparisons. For example, Weix and colleagues reported 19 selected candidate genes that differed in pregnancy and differed to a greater degree in pregnant women with rheumatoid arthritis. Of those candidate genes, only 1 (STAT1) differed in our transcriptome-wide analysis (S4 Fig). The study by Mittal and colleagues reported the change in expression of 256 genes over pregnancy in women with rheumatoid arthritis and controls [4], 179 of which were evaluated in our study. Of these, 55.3% change over pregnancy in our cohort of healthy women. This is a substantially higher number than would be expected by chance (99/179; p<2.2x10-16). Of note, both this study and the one conducted by Mittal and colleagues report changes in genes involved in oxygen transport (e.g. AHSP and HBD) and immune response to microbes (CEACAM8 and DEFA4).
Discussion
In this study, we identified 439 transcripts that were associated with weeks gestation at sample collection in uncomplicated term pregnancies. These transcripts were enriched for several key pathways that provide insight into the mechanisms underlying the physiological changes necessary to support a healthy pregnancy.
A widely accepted phenomenon in pregnancy is a change in the maternal immune system. In general, pregnancy is associated with a shift from a pro-inflammatory state in the first trimester to an anti-inflammatory state in the second trimester, with renewed inflammation during the third trimester and at parturition [2]. The pro-inflammatory state in the first trimester is likely a result of implantation and placentation with the anti-inflammatory state of the second trimester being a period of rapid fetal growth and development during which a more symbiotic relationship between mother, fetus, and placenta exists. Finally, in the third trimester, renewed inflammation leads to the processes which can initiate labor and delivery. Consistent with this and other studies, our results suggest that monocytes increase over the course of pregnancy, while B cell and natural killer cell proportions decrease [10, 11]. This shift has been further explored in studies of autoimmune disorders and pregnancy, with several pathways involved being identified that may contribute to reductions, or in other cases, increases in autoimmune symptoms during pregnancy [3–6]. However, such studies often do not control for differences in cellular proportions, increasing the chance that they will identify genes whose expression is not entirely attributable to the disorder of interest or are otherwise difficult to interpret. Thus, it is vital for future studies to estimate and adjust for cellular heterogeneity, as cell type changes over pregnancy.
In this longitudinal analysis of transcriptome-wide changes across gestational week, we identified gene expression changes that supports functional differences in the immune system during pregnancy. Such immune system changes must be carefully regulated so that the mother is protected from bacterial and viral infection without negatively impacting the body’s tolerance of the fetus. However, the immune systems of pregnant women are less able to appropriately respond to several types of bacterial infections and related complications, including Listeria monocytogenes and Neisseria gonorrhoeae [12–14]. Bacterial infections have been associated with preterm birth and spontaneous abortion [15–17]. Evidence of host-microbe interactions in pregnancy is demonstrated in both the gene-level results and through enrichment of genes involved in antibacterial humoral response and innate immunity in the gene ontology analysis. Specific genes related to bacterial response include several alpha defensin genes. These genes encode antimicrobial peptides (AMPs) that have been associated with microbicidal activity and host defense, and are present in the female reproductive tract during pregnancy [18, 19]. Understanding changes in the immune system and host-microbe interactions during pregnancy may provide future insight into how the immune system acts to protect both the mother and the fetus thus allowing us to more effectively treat and prevent potential maternal and fetal effects of systemic infection.
This study also identified genes involved in oxygen transport that change over pregnancy, which support the physiological changes identified in previous studies [20–23]. Some of the most pronounced adaptations in pregnancy are related to increased blood volume, higher rates of erythropoiesis, and increased coagulation. Maternal physiology tends toward a more hypercoagulable state during pregnancy, likely in an effort to limit delivery complications such as post-partum hemorrhage. Previous studies have linked abnormal coagulation, specifically within the placental complex, with preeclampsia, demonstrating the importance of regulation of coagulation for healthy pregnancies [24, 25].
Erythropoiesis increases over the course of pregnancy, potentially to accommodate larger blood volumes and prepare for the acute loss of this volume which occurs at the time of delivery. However, this study evaluated PBMCs, which do not include mature erythrocytes [26]. Still, recent studies have reported the expression of hemoglobin in non-erythroid cells, such as macrophages, epithelial, mesangial, cervical, and endometrial cells, and have also reported functions of hemoglobin other than oxygen transport including antioxidant defense, nitrite reduction, and reactive oxygen species scavenging [27, 28]. We hypothesize that the hemoglobin-associated genes identified in this study reflect non-canonical expression in response to the physiological strain that pregnancy places on the body, potentially resulting in further demands for such alternative functions.
This is the first study to comprehensively characterize transcriptome-wide changes longitudinally over the course of uncomplicated pregnancies. This cohort has been prospectively recruited and characterized, resulting is an invaluable asset for studying pregnancy progression [29]. However, this study does have limitations. Though it is the largest longitudinal study of gene expression changes in pregnancy, it still has a modest sample size. A larger group of subjects is likely to identify more genes that change over the course of pregnancy. Despite this, we were able to identify hundreds of genes whose expression change in PBMCs after accounting for confounding factors and multiple testing. However, we cannot extrapolate these changes to other cell types that may be relevant for pregnancy. This study also uses array-based technology, which does not allow for characterization of novel or alternatively spliced transcripts that may play vary during pregnancy. Finally, we did not have access to samples spanning the entirety of pregnancy, including before 8 weeks and after 33 weeks gestation.
Despite these limitations, we show that gene expression changes significantly over the course of pregnancy, especially in pathways related to oxygen transcript and response to microbes. Our results provide a framework for future studies on changes in gene expression over pregnancy. Knowledge of genes associated with normal changes may potentially allow for identification of abnormal patterns of gene expression associated with pregnancy and delivery complications that could be tested as potential biomarkers. Future studies should examine gene expression over pregnancy in the context of pregnancy complications and other pre-existing conditions, while acknowledging that very subtle changes in the timing of sample collection may confound the results or complicate their interpretation.
Methods
Study subjects
Pregnant African American women were recruited, enrolled, and underwent data collection as part of the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study, as described previously [29]. To summarize, women were recruited from March 2014 through August 2016 at outpatient prenatal care clinics affiliated with two Atlanta metro area hospitals, Emory University Midtown Hospital and Grady Memorial Hospital. Women were eligible for inclusion if they self-identified as African American, were between 18–40 years of age, had a singleton pregnancy, had less than four previous births, and were able to understand written and spoken English. Additional exclusion criteria for this analysis included the following indicators of high-risk pregnancy or pregnancy complications: gestational diabetes, hypertension, intrauterine growth restriction, preterm birth, preeclampsia, eclampsia, hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, hyperemesis gravidarum, oligohydramnios, chorioamnionitis, macrosomia, preterm premature rupture of membranes (pPROM), or fetal intolerance of labor. Fetal death before labor and congenital abnormalities of the fetus were criteria for post-enrollment exclusion. Demographic data were collected through self-report questionnaires. Clinical obstetrical data (including estimated due date, gestational age at delivery, pregnancy complications, labor and delivery course) were ascertained via abstraction of the prenatal and labor and delivery medical chart by a qualified physician. All participants gave written informed consent. This study was approved by the Emory University Institutional Review Board.
Biological sample collection
63 women contributed two samples each over the course of their pregnancy. The first sample was collected at 6–15 weeks, and the second sample was collected at 22–33 weeks. During each prenatal visit, an additional 12 mL of venous blood was drawn using the same needle stick as for the routine blood draws. PBMCs were isolated from whole blood using a Ficoll density gradient and were stored in AllProtect Buffer (Qiagen) at -80°C until a simultaneous DNA and RNA extraction using the AllPrep RNA/DNA Mini Kit (Qiagen) was performed according to manufacturer’s instructions. DNA quantification and quality was assessed using the Quant-it Pico Green kit (Invitrogen). RNA quantification and quality was assessed using the Aligent RNA Nano 6000 Kit and Bioanalyzer 2100.
RNA expression analysis
For each subject, gene expression was assessed for ~47,000 transcripts using the HumanHT-12 v4 BeadChip (Illumina). Briefly, 750 ng of RNA was directly hybridized to the BeadChip according to manufacturer’s instructions. The BeadChips were scanned using the iScan scanner, and the raw data was analyzed using the Expression Module of GenomeStudio Software (Illumina). Two samples with detection p values >.01 for more than 90% of probes were excluded. Probes detected in less than 10% of samples were also excluded; 16,311 probes passed quality control. Data was then quantile-normalized and log2 transformed prior to association testing. RNA expression data can be accessed through NCBI’s Gene Expression Omnibus, GSE107437.
Cell type composition estimation
DNA methylation was interrogated for each subject using either the HumanMethylation450 or MethylationEPIC BeadChip, which measures methylated and unmethylated signal for >450,000 and >850,000 CpG sites across the genome, respectively. Initial data quality control was performed using the R package CpGassoc [30]. Any CpG site with low signal or missing data for greater than 10% of samples was removed, and any sample with missing data for greater than 5% of CpG sites was removed. Cross-reactive probes were removed [31]. Following quality control, 449,094 probes were included in subsequent analyses. Beta values (β) were calculated for each CpG site as the ratio of methylated (M) to methylated and unmethylated (U) signal: β = M/(M+U). Beta-mixture quantile normalization was performed as previously described [32]. Cell type proportions (CD8T+, CD4T+, natural killer, B cell, monocytes, and granulocytes) were estimated as previously described from DNA methylation data [33]. Associations between cell type proportions and gestational age were examined using a linear-mixed effects model with cellular proportions included as fixed effects and a unique identifier for each person included as a random effect. DNA methylation data can be accessed through NCBI’s Gene Expression Omnibus, GSE107459.
Whole transcriptome analysis
We used linear mixed-effects modeling implemented in the R package “nlme” to interrogate associations between gene expression and weeks gestation at sample collection [34].The R package sva was used to estimate surrogate variables to control for potentially confounding factors, including cell type [35]. Surrogate variable analysis was used instead of a covariate adjustment as only a few cellular subtypes are well defined using current methods, and changes in the composition of unmeasured cell types may have a large influence during pregnancy. The 15 significant surrogate variables were included as covariates in the model as fixed effects (Eq 1).
| Eq 1 |
Where SV1-15 represent each included surrogate variable, which model unmeasured factors inferred from the genome-wide methylation signatures (S2 Fig). X represents the independent variable. β1−16 represent fixed effects parameters. u is the individual specific error term, and i represents the unique subject identifier, and j represents the observation number. A random effects term was included in the model to account for repeated sampling of the same person. ε refers to random error. τ2 and σ2 are the variances of the person specific and random error terms, respectively. A Bonferroni correction was applied to account for multiple testing. Pathway analysis was performed using DAVID for both the entire set of associated genes as well as for upregulated and downregulated genes separately [36]. Gene-ontology enrichment p-values presented used a Benjamini-Hochberg correction for multiple testing.
Supporting information
(CSV)
(DOCX)
Monocytes increase over pregnancy, whereas B cells and natural killer cells decrease over pregnancy. Other evaluated cell types do not change. The x-axis represents the weeks of gestation at sample collection and the y-axis represents cell proportions.
(PNG)
The grid consists of the correlation coefficient (r) for each pair on top with the p-value indicating significance of the correlation below. ExpressionRound indicates batch. Comp.1 indicates the first principal component for ancestry. Maternal age was measured in years. Cell composition (NK, CD4+ T cells, monocytes, B cells, granulocytes and CD8+ T cells) were estimated as described in the Methods. The intensity of the shading represents the correlation coefficient, with darker shading being associated with a higher correlation coefficient. Red shading represents a positive correlation coefficient and green shading represents a negative correlation coefficient.
(PNG)
(PNG)
Acknowledgments
We are grateful to the participants enrolled in this cohort who provided samples and medical record data for this study. We are grateful to Misti B. Patel for providing insights into the clinical relevance of this work.
Data Availability
RNA expression data can be accessed through NCBI’s Gene Expression Omnibus, GSE107437. DNA methylation data can be accessed through NCBI’s Gene Expression Omnibus, GSE107459.
Funding Statement
Research reported in this publication was supported in part by the National Institute on Minority Health and Health Disparities (NIMHD, https://nimhd.nih.gov) of the National Institutes of Health, award number R01MD009064 to AKS and ALD, and the National Institute of Nursing Research (NINR, https://www.ninr.nih.gov) of the National Institutes of Health, under award number R01NR014800 to ALD and EJC. The study was also supported by the Office of the Director (grant UG3OD023318 to Anne L Dunlop) and the National Institute of General Medical Sciences (grant T32 GM 8490-24 to Ms. Anna K Knight). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moya J, Phillips L, Sanford J, Wooton M, Gregg A, Schuda L. A review of physiological and behavioral changes during pregnancy and lactation: potential exposure factors and data gaps. J Expo Sci Environ Epidemiol. 2014;24(5):449–58. 10.1038/jes.2013.92 . [DOI] [PubMed] [Google Scholar]
- 2.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. American journal of reproductive immunology. 2010;63(6):425–33. 10.1111/j.1600-0897.2010.00836.x ; PubMed Central PMCID: PMC3025805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccinni MP, Lombardelli L, Logiodice F, Kullolli O, Parronchi P, Romagnani S. How pregnancy can affect autoimmune diseases progression? Clin Mol Allergy. 2016;14:11 10.1186/s12948-016-0048-x ; PubMed Central PMCID: PMC5025626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal A, Pachter L, Nelson JL, Kjaergaard H, Smed MK, Gildengorin VL, et al. Pregnancy-Induced Changes in Systemic Gene Expression among Healthy Women and Women with Rheumatoid Arthritis. PloS one. 2015;10(12):e0145204 10.1371/journal.pone.0145204 ; PubMed Central PMCID: PMC4684291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weix J, Forger F, Haupl T, Surbek D, Ostensen M, Villiger PM. Influence of pregnancy on the adipocytokine and peroxisome proliferator-activated receptor pathways in peripheral blood mononuclear cells from healthy donors and rheumatoid arthritis patients. Arthritis Rheum. 2012;64(7):2095–103. 10.1002/art.34375 . [DOI] [PubMed] [Google Scholar]
- 6.Gilli F, Lindberg RL, Valentino P, Marnetto F, Malucchi S, Sala A, et al. Learning from nature: pregnancy changes the expression of inflammation-related genes in patients with multiple sclerosis. PloS one. 2010;5(1):e8962 10.1371/journal.pone.0008962 ; PubMed Central PMCID: PMC2813302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke SD, Seaward AV, Ramshaw H, Smith GN, Virani S, Croy BA, et al. Homing receptor expression is deviated on CD56+ blood lymphocytes during pregnancy in Type 1 diabetic women. PloS one. 2015;10(3):e0119526 10.1371/journal.pone.0119526 ; PubMed Central PMCID: PMC4368780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo PL, Guo HR. Nucleated red blood cells in maternal blood during pregnancy. Obstetrics and gynecology. 1999;94(3):464–8. . [DOI] [PubMed] [Google Scholar]
- 9.Wegienka G, Havstad S, Bobbitt KR, Woodcroft KJ, Zoratti EM, Ownby DR, et al. Within-woman change in regulatory T cells from pregnancy to the postpartum period. Journal of reproductive immunology. 2011;88(1):58–65. 10.1016/j.jri.2010.06.157 ; PubMed Central PMCID: PMC3008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faas MM, de Vos P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. Journal of reproductive immunology. 2017;119:91–7. 10.1016/j.jri.2016.06.009 . [DOI] [PubMed] [Google Scholar]
- 11.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, et al. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77(5):1032–7. . [DOI] [PubMed] [Google Scholar]
- 12.Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States—1986. Listeriosis Study Group. American journal of epidemiology. 1991;133(4):392–401. . [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease C, Prevention. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morbidity and mortality weekly report. 2013;62(22):448–52. [PMC free article] [PubMed] [Google Scholar]
- 14.Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17(2):201–8. . [DOI] [PubMed] [Google Scholar]
- 15.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. American journal of obstetrics and gynecology. 2003;189(1):139–47. . [DOI] [PubMed] [Google Scholar]
- 16.Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009;116(10):1315–24. 10.1111/j.1471-0528.2009.02237.x . [DOI] [PubMed] [Google Scholar]
- 17.Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21(1):32–40. 10.1177/1933719113488838 ; PubMed Central PMCID: PMC3857766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27(18):1337–47. 10.1007/s10529-005-0936-5 . [DOI] [PubMed] [Google Scholar]
- 19.Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353–77. 10.1093/humupd/dmu065 . [DOI] [PubMed] [Google Scholar]
- 20.Silversides CK, Colman JM. Physiological changes in pregnancy. Heart Disease in Pregnancy, 2nd Edition. 2007:6–17. 10.1002/9780470994955.ch2 PubMed PMID: WOS:000298401200003. [DOI] [Google Scholar]
- 21.Jwa SC, Fujiwara T, Yamanobe Y, Kozuka K, Sago H. Changes in maternal hemoglobin during pregnancy and birth outcomes. BMC pregnancy and childbirth. 2015;15. doi: ARTN 80 10.1186/s12884-015-0516-1 PubMed PMID: WOS:000352346300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heilmann L. Blood rheology and pregnancy. Baillieres Clin Haematol. 1987;1(3):777–99. . [DOI] [PubMed] [Google Scholar]
- 23.Gaillard R, Eilers PH, Yassine S, Hofman A, Steegers EA, Jaddoe VW. Risk factors and consequences of maternal anaemia and elevated haemoglobin levels during pregnancy: a population-based prospective cohort study. Paediatric and perinatal epidemiology. 2014;28(3):213–26. 10.1111/ppe.12112 . [DOI] [PubMed] [Google Scholar]
- 24.Townsley DM. Hematologic complications of pregnancy. Semin Hematol. 2013;50(3):222–31. 10.1053/j.seminhematol.2013.06.004 ; PubMed Central PMCID: PMC3748382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Liu X, Li H, Zou J, Yang Z, Han J, et al. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia. PloS one. 2014;9(12):e114488 10.1371/journal.pone.0114488 ; PubMed Central PMCID: PMC4252147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Bello I, Cilio CM, Wong FS, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163(1):33–49. 10.1111/j.1365-2249.2010.04272.x ; PubMed Central PMCID: PMC3010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha D, Patgaonkar M, Shroff A, Ayyar K, Bashir T, Reddy KV. Hemoglobin expression in nonerythroid cells: novel or ubiquitous? Int J Inflam. 2014;2014:803237 10.1155/2014/803237 ; PubMed Central PMCID: PMC4241286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butcher JT, Johnson T, Beers J, Columbus L, Isakson BE. Hemoglobin alpha in the blood vessel wall. Free Radic Biol Med. 2014;73:136–42. 10.1016/j.freeradbiomed.2014.04.019 ; PubMed Central PMCID: PMC4135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, et al. Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC pregnancy and childbirth. 2017;17(1):161 10.1186/s12884-017-1357-x ; PubMed Central PMCID: PMCPMC5455081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barfield RT, Kilaru V, Smith AK, Conneely KN. CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinformatics. 2012;28(9):1280–1. 10.1093/bioinformatics/bts124 ; PubMed Central PMCID: PMC3577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YA, Lemire M, Choufani S, Butcher DT, Grafodatskaya D, Zanke BW, et al. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics: official journal of the DNA Methylation Society. 2013;8(2):203–9. 10.4161/epi.23470 ; PubMed Central PMCID: PMC3592906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29(2):189–96. 10.1093/bioinformatics/bts680 ; PubMed Central PMCID: PMC3546795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics. 2012;13:86 10.1186/1471-2105-13-86 ; PubMed Central PMCID: PMC3532182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro J BD, DebRoy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models. 2016. [Google Scholar]
- 35.Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS genetics. 2007;3(9):1724–35. 10.1371/journal.pgen.0030161 ; PubMed Central PMCID: PMC1994707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–75. 10.1093/nar/gkm415 ; PubMed Central PMCID: PMC1933169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(DOCX)
Monocytes increase over pregnancy, whereas B cells and natural killer cells decrease over pregnancy. Other evaluated cell types do not change. The x-axis represents the weeks of gestation at sample collection and the y-axis represents cell proportions.
(PNG)
The grid consists of the correlation coefficient (r) for each pair on top with the p-value indicating significance of the correlation below. ExpressionRound indicates batch. Comp.1 indicates the first principal component for ancestry. Maternal age was measured in years. Cell composition (NK, CD4+ T cells, monocytes, B cells, granulocytes and CD8+ T cells) were estimated as described in the Methods. The intensity of the shading represents the correlation coefficient, with darker shading being associated with a higher correlation coefficient. Red shading represents a positive correlation coefficient and green shading represents a negative correlation coefficient.
(PNG)
(PNG)
Data Availability Statement
RNA expression data can be accessed through NCBI’s Gene Expression Omnibus, GSE107437. DNA methylation data can be accessed through NCBI’s Gene Expression Omnibus, GSE107459.