Abstract
Background
Vascular transit time (VTT) is the propagation time of a pulse wave through an artery; it is a measure for arterial stiffness. Because reliable non-invasive VTT measurements are difficult, as an alternative we measure pulse transit time (PTT). PTT is defined as the time between the R-wave on electrocardiogram and arrival of the resulting pulse wave in a distal location measured with photoplethysmography (PPG). The time between electrical activation of the ventricles and the resulting pulse wave after opening of the aortic valve is called the pre-ejection period (PEP), a component of PTT. The aim of this study was to estimate the variability of PEP at rest, to establish how accurate PTT is as approximation of VTT.
Methods
PTT was measured and PEP was assessed with echocardiography (gold standard) in three groups of 20 volunteers: 1) a control group without cardiovascular disease aged <50 years and 2) aged >50 years, and 3) a group with cardiovascular risk factors, defined as arterial hypertension, dyslipidemia, kidney failure and diabetes mellitus.
Results
Per group, the mean PEP was: 1) 58.5 ± 13.0 ms, 2) 52.4 ± 11.9 ms, and 3) 57.6 ± 11.6 ms. However, per individual the standard deviation was much smaller, i.e. 1) 2.0–5.9 ms, 2) 2.8–5.1 ms, and 3) 1.6–12.0 ms, respectively. There was no significant difference in the mean PEP of the 3 groups (p = 0.236).
Conclusion
In conclusion, the intra-individual variability of PEP is small. A change in PTT in a person at rest is most probably the result of a change in VTT rather than of PEP. Thus, PTT at rest is an easy, non-invasive and accurate approximation of VTT for monitoring arterial stiffness.
Introduction
Pulse transit time (PTT) is the sum of the pre-ejection period (PEP) and the vascular transit time (VTT). After opening of the aortic valve, a pulse pressure wave propagates with a certain speed through the blood vessels from the heart to a distal location; this is called the VTT. If an artery is stiff or has a small diameter (or both), the pulse wave will propagate faster and, subsequently, the VTT decreases. Vice versa, if the diameter of the artery increases, the pulse wave propagation is slower and the VTT increases. Therefore, the VTT is a measure for arterial stiffness. Increased stiffness can be either structural (age and atherosclerosis) or functional due to higher sympathetic activity or elevated blood pressure [1–3] It is a promising application for e.g. non-invasive continuous and cuffless blood pressure monitoring, which can also be used in children or in ambulatory setting [4–8].
Because reliable non-invasive VTT measurements are difficult, as an alternative we measure the PTT. PTT can be used to assess a successful loco regional block of an extremity [9, 10], to monitor vasomotor tone, and for assessment of autonomic nervous system response [11]. Another non-invasive measurement to estimate the VTT is the pulse wave velocity (PWV). However, determination of the arterial path length is necessary to calculate the PWV and this may create errors in the measurement [12].
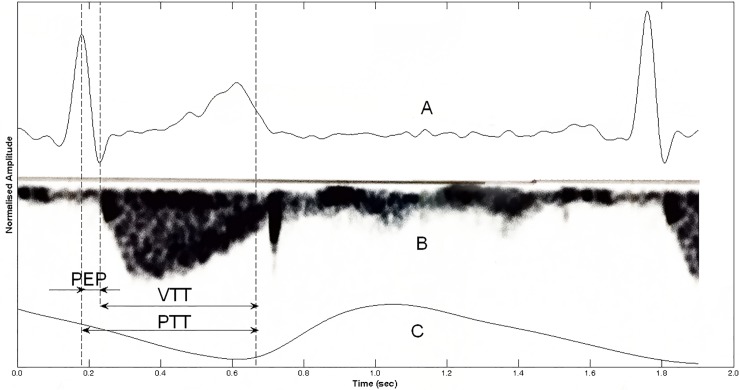
PTT is defined as the time between the R-wave on the electrocardiogram (ECG) and the arrival of the resulting pulse wave measured with photoplethysmography (PPG). The PEP is a component of the PTT measurement (Fig 1).
Fig 1. Pre-ejection period.
A, electrocardiogram; B, Doppler mode echocardiography signal over the aortic valve; C, photoplethysmographic pulse wave. PEP = pre-ejection period, VTT = vascular transit time, PTT = pulse transit time.
The PEP represents the isovolumetric contraction time of the ventricles of the heart. It is the time between electrical activation of the ventricles (Q-wave) and opening of the aortic valve [13]. The R-wave is commonly used instead of the Q-wave [14]. Identification of R-waves is easier and more reliable than Q-waves. Moreover, Seery et al. showed that PEP can be calculated using the R-wave instead of the Q-wave [15]. PEP can be considered as a measure of left ventricular function, as it reflects changes in the contractility of myocardium, left ventricular end-diastolic volume, and aortic diastolic pressure [13, 16–18]. In patients with diabetic chronic kidney disease, fluid overload is a marker for left ventricular systolic dysfunction and is associated with the ratio of brachial PEP and brachial ejection time [19]. Physical activity, as well as respiration or a stress response, can rapidly change the PEP [20, 21]. Therefore, a change in either PEP and/or VTT can result in a change in the PTT value [22].
Cardiothoracic impedance is a measurement that is often used to acquire data for calculation of the PEP and measured PEP values around 75–134 ms [14, 23,24]. However, echocardiography is the gold standard for measuring the PEP [13]. Muehlsteff et al. investigated the role of the PEP on PTT [4]; they measured the PEP in a small group of young healthy volunteers with thorax impedance after exercise and found that PEP dominates the PTT variability after exercise. The use of different measuring tools introduces a discrepancy in literature about the magnitude of PEP and its contribution to the PTT.
The aim of this study was to estimate the variability of PEP at rest, to establish how accurate PTT is as an approximation of VTT. Age and cardiovascular risk factors (arterial hypertension, dyslipidemia, kidney failure, and diabetes mellitus) can increase arterial stiffness and, thereby, decrease VTT and possibly influence the PEP. Therefore, to take these factors into account, the PEP was measured in a control group with no medical history of cardiovascular disease aged ≤50 years and aged ≥50 years, and in a group of persons with at least one cardiovascular risk factor.
Methods
This single-center prospective observational study examined the variability of PEP at rest in 60 volunteers divided into 3 groups (20 per group):
a control group of individuals without a medical history of cardiovascular disease aged under 50 years
a control group aged over 50 years, and
a group of individuals with at least one cardiovascular risk factor, which are defined in the exclusion criteria for the control groups.
The study was approved by the Medical Ethical Committee of the Erasmus University Medical Centre in Rotterdam (MEC-2011-213), and was conducted in accordance with the Declaration of Helsinki. The primary endpoints of the study were PEP, VTT and PTT.
Volunteers were eligible for inclusion when aged ≥18 years and ≤ 75 years. Exclusion criteria were arrhythmia, tremor, muscle or skeletal injuries in upper limb, hematopoietic disease, and incapacitated subjects. Further exclusion criteria for the control group (groups 1 and 2) were history of cardiovascular or peripheral vascular risk factors; these were defined as arterial hypertension, dyslipidemia, kidney failure, and diabetes mellitus. All participants were informed about the aim of the study and gave written informed consent before being enrolled. None of the eligible volunteers refused inclusion in the trial.
Measurement protocol
All measurements were performed under standardized stable conditions in a quiet temperature- controlled room with dimmed light. First, the participant had a short interview to acquire the data such as weight, height and medication and blood pressure was measured. After that the participant was asked to lie down in a left lateral decubitus position and was connected to the measurement equipment (MP100, Biopac Systems, Inc. Goleta, USA). ECG electrodes were placed (ECG100C amplifier, Biopac) to calculate the PTT and for ECG registration during echocardiography. Four PPG sensors (TSD200 and PPG100C amplifier, Biopac) were placed on the index fingers and big toes, for measuring respectively the PTTfinger and PTTtoe. Data were sampled with 2 kHz using the AcqKnowledge 3.7.3 version software (Biopac). Per volunteers, the data acquisition took place in 5 to 10 minutes.
Echocardiography
ECG-gated echocardiography was performed at rest in the left lateral decubitus position by an experienced investigator, using a Vivid-i portable ultrasound system (GE, Solingen, Germany). Measurements were performed during 10 consecutive heartbeats, simultaneous with PTT measurements. A continuous wave Doppler registration through the aortic valve was obtained from a standard apical 5-chamber view. The horizontal sweep was set to 200 mm/s for maximum accuracy. PEP was defined as the time measured from the R-wave of the ECG to the start of the ejection phase. The data were synchronized using the matching R-waves of the simultaneously acquired second lead ECG signals from the echocardiography and PTT measurements.
PTT calculations
Data from the AcqKnowledge software were imported into Matlab R2010a (The MathWorks, Inc., Natick, MA, USA) with the Loadacq syntax for Matlab [25]. In the ECG data, the R-waves were detected using the Rpeakdetect syntax available from the ECGtoolbox [26]. The PPG data were filtered using a fourth-order low-pass Butterworth filter with a cut-off frequency of 9 Hz. For filtering we used a zero-phase digital filtering by processing the input data, PPG, in both the forward and reverse directions. This gives a zero phase delay and is based on a method described by Oppenheim et al. [27].The signals from the PPG were digitally cut between the R-wave and the next R-wave. Thereafter the pulse waves were selected by a ‘7Step PW-filter’ to filter out the pulse waves that strongly deviated in shape for a suitable pulse wave analysis [28]. PTT was determined by calculating the time between the R-wave of the ECG and the foot of the PPG pulse wave. The foot was determined as the maximum value of the second derivative of the pulse wave [29].
Analyses
We calculated the mean PEP of the 10 consecutive heart beats for each participant and of the total group. All data are presented as mean ± standard deviation (SD). Furthermore, to show the magnitude of the contribution of PEP and its variation in the PTT measurements in an individual, the PTTMean, PTTSD, PEPMean and PEPSD were normalized to a percentage of the PTTMean by the following formula (see formula 1):
| (1) |
A similar calculation was used to determine the PEPMean% and PEPSD%. The values of the left and right sensors were compared with the Wilcoxon signed rank test. The difference in PEP and study population characteristics between the 3 groups was tested with ANOVA. To correct for the influence of systolic blood pressure on PTT the ANCOVA was used. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24 (SPSS Inc., Chicago, USA). Figures were made using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA) and Adobe Illustrator (Creative Suits 5 CS5, Adobe Systems Incorporated, San Jose, California, USA).
Results
The present study included 60 volunteers, divided into 3 groups of 20 participants each. As shown in Table 1, the mean age in group 1 is significantly lower than in the other groups. This is mainly caused by the age limit of 50 years in group 1. Also, the BMI was lower in the control group compared to group 2 and 3 and the mean heart rate was higher. The mean systolic blood pressure of group 3 was significantly higher than that of groups1 and 2 (p = 0.048). Of all the participants in group 3, 19 had risk factor arterial hypertension, 13 dyslipidemia, 2 diabetes and 1 kidney failure. The characteristics of the study population are presented in Table 1.
Table 1. Characteristics of the study population.
| Variables | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Age, years | 27 ± 4* | 55 ± 4 | 63 ± 10 |
| Sex (male), no. (%) | 9 (45) | 10 (50) | 10 (50) |
| Weight, kg | 69 ± 12 | 78 ± 12 | 74 ± 12 |
| Height, m | 1.77 ± 0.11 | 1.75 ± 0.09 | 1.71 ± 0.09 |
| Body Mass Index, kg/m2 | 21.8 ± 2.5* | 25.5 ± 3.6 | 25.3 ± 3.2 |
| Blood pressure, mmHg | |||
| Systolic | 131 ± 13 | 131 ± 17 | 141 ± 15* |
| Diastolic | 81 ± 10 | 79 ± 9 | 81 ± 8 |
| MAP, mmHg | 98 10 | 97 ± 10 | 101 ± 9 |
| Heart rate, beats/min | 79 ± 21* | 67 ± 10 | 68 ± 14 |
n = 20 per group. Group 1 = control group <50 years, group 2 = control group >50 years, group 3 = participants with a cardiovascular risk factor. Data are presented as mean ± SD or number (%) of participants. MAP = mean arterial pressure.
* p<0.05
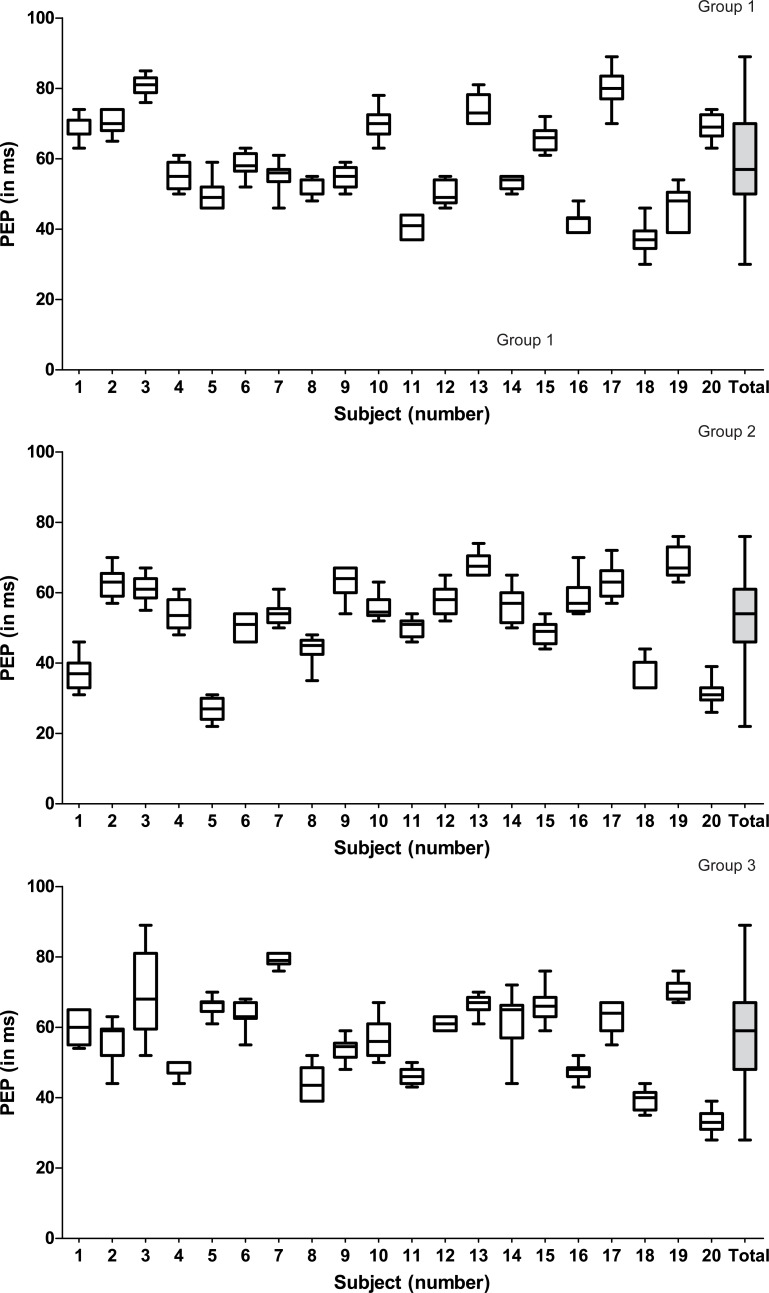
In the total group of 60 participants, the mean PEP was 56.2 ± 12.3 ms. In groups 1, 2 and 3 the mean PEP was 58.5 ± 13.0 ms, 52.4 ± 11.9 ms and 57.6 ± 11.6 ms, respectively. The intra-individual SD of the PEP was much smaller, i.e. 2.0–5.9 ms (group 1), 2.8–5.1 ms (group 2) and 1.6–12.0 ms (group 3) (Fig 2). There were no missing data in the PEP measurement and no significant difference in the mean PEP of the 3 groups (p = 0.235).
Fig 2. Box plots of the pre-ejection period (PEP) of 10 consecutive heartbeats of the 20 participants and of the total group.
Group 1: control group aged <50 years. Group 2: control group aged >50 years. Group 3: participants with a cardiovascular risk factor. The boxes represent the median with interquartile range. The whiskers represent the minimum and maximum value.
For all the PPG measurements of the 3 groups together, 18 sensors showed too many artifacts, modulated waves, for reliable PTT calculation; these measurements were excluded from the analysis, which represents 7.5% of the data (for specification see Figs 3, 4 and 5). Pulse waves were only excluded from analyses when they did not pass the ‘7Step PW-filter’.
Fig 3. Contribution of the PEP (mean and SD) to the PTT for each sensor of group 1 (control group aged <50 years).
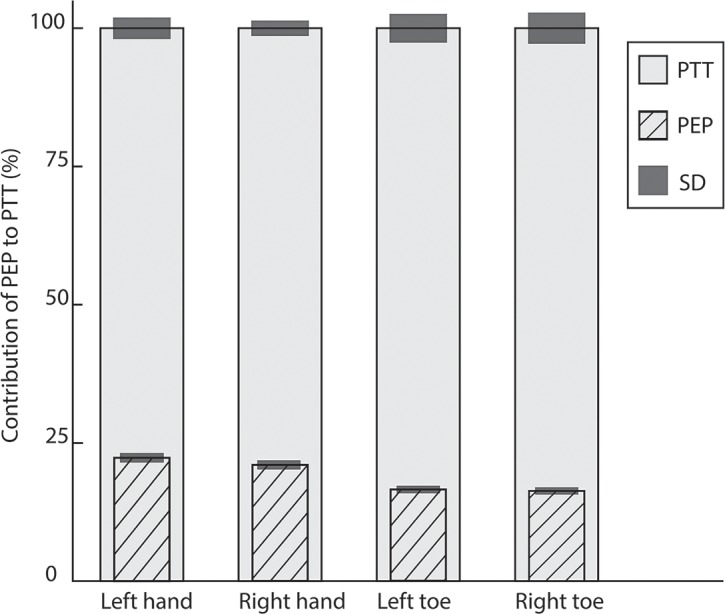
All values are normalized to a percentage (%) of the mean PTT (which is 100%) of the specific sensor. PTTMean% of left hand (n = 19) 100%±3.1%, PTTMean% of right hand (n = 20): 100%±2.4% (p = 0.004). Contribution of PEPMean% for PTT of left versus right hand 22.2%±1.3% versus 21.0%±1.3%. PTTMean% of left big toe (n = 18) 100%±4.7%, PTTMean% right big toe (n = 17) 100%±4.8%. Contribution of PEPMean% for left big toe versus right big toe 16.5%±1.0% versus 16.3%±1.0%.
Fig 4. Contribution of the PEP (mean and SD) to the PTT for each sensor of group 2 (control group aged >50 years).
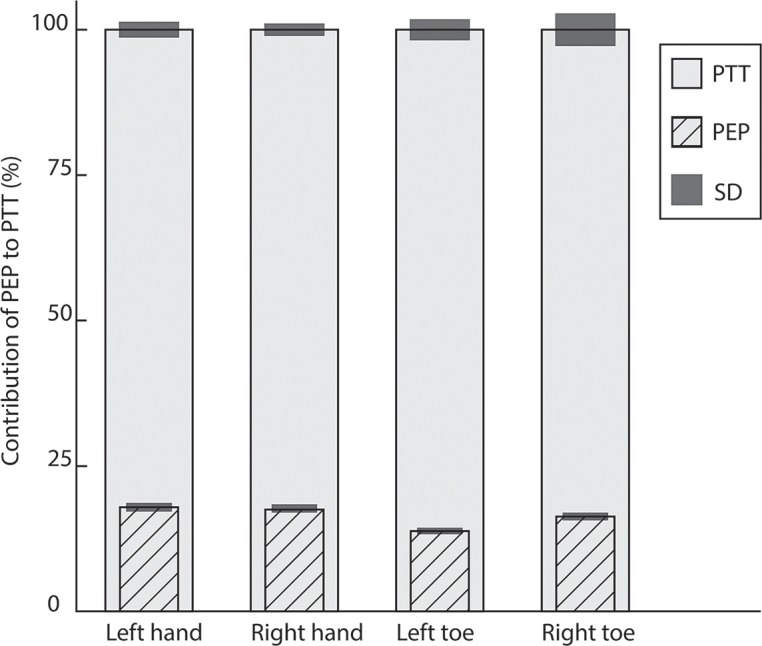
All values are normalized to a percentage (%) of the mean PTT (which is 100%) of the specific sensor. PTTMean% of left hand (n = 19) 100%±3.5%, PTT of right hand (n = 20) 100%±2.6%. Contribution of PEPMean% for PTT of left versus right hand PEP 18.7%±1.4% versus PEP 19.4%±1.4%. PTTMean% of left big toe (n = 18) 100%±5.9%, PTTMean% right big toe (n = 17) PTT 100%±6.3%. Contribution of PEPMean% for left big toe versus right big toe 4.8%±1.1% versus 5.1%±1.1%.
Fig 5. Columns represent the contribution of the PEP (mean and SD) to the PTT for each sensor of group 3 (participants with a cardiovascular risk factor).
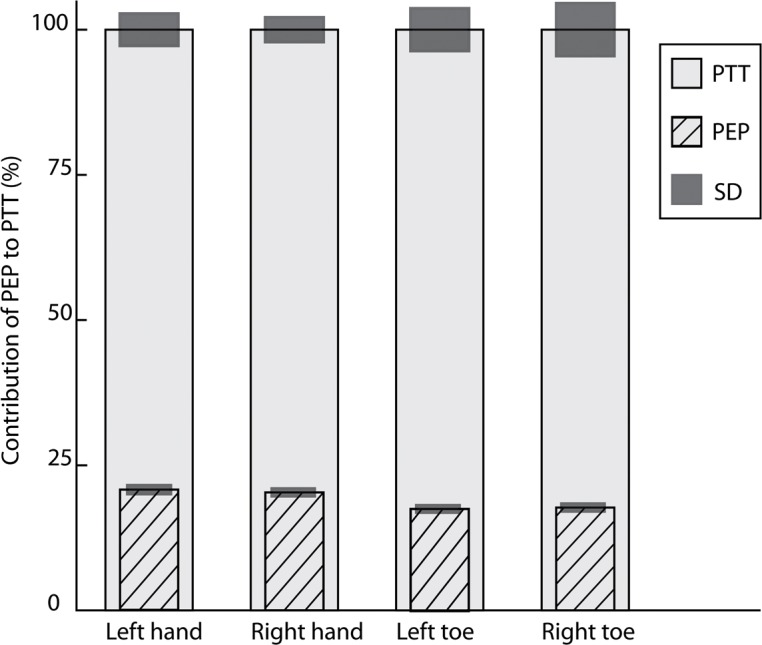
All values are normalized to a percentage (%) of the mean PTT (which is 100%) of the specific sensor. PTTMean% of left hand (n = 19) 100%±5.5%, PTT of right hand (n = 20) 100%±4.2%. Contribution of PEPMean% for PTT of left versus right hand 20.7%±1.5% versus 20.3%±1.4%. PTTMean% of left big toe (n = 15) 100%±7.3%, PTTMean% right big toe (n = 14) 100%±9.1%. Contribution of PEPMean% for left big toe versus right big toe 17.6%±1.3% versus 17.7%±1.4%.
In the normalized data of group 1, the PEPMean% contribution to the PTTMean% of the left hand was significantly different compared to the right hand (p = 0.004) (Tables 2 and 3) (Fig 3). This result is still significant after applying the Bonferroni correction for multiple testing, p<0.008. In groups 2 and 3 there was no significant difference between the left and right measurements (Figs 4 and 5). Between the groups was no significant difference in the contribution of PEPMean% to the PTTMean%, neither after correction for SBP.
Table 2. Data on normalized PTT and PEP variability.
| Normalized PTT in % (PTT mean in ms) |
SD PTT in % of PTT (PTT SD in ms) |
% of PEP with respect to PTT | % of SD PEP with respect to PTT | |
|---|---|---|---|---|
| 1 | 100 (305) | 1.6 (5) | 22.4 | 1.1 |
| 2 | 100 (272) | 2.6 (7) | 25.8 | 1.1 |
| 3 | 100 (285) | 2.5 (7) | 28.3 | 0.9 |
| 4 | 100 (299) | 1.7 (5) | 18.5 | 1.3 |
| 5 | 100 (309) | 1.9 (6) | 16.1 | 1.3 |
| 6 | 100 (268) | 1.1 (3) | 21.8 | 1.3 |
| 7 | 100 (266) | 1.9 (5) | 20.7 | 1.5 |
| 8 | 100 (286) | 1.0 (3) | 17.9 | 0.8 |
| 9 | 100 (230) | 1.7 (4) | 23.8 | 1.3 |
| 10 | 100 (270) | 1.9 (5) | 25.9 | 1.5 |
| 11 | 100 (272) | 1.1 (3) | 14.9 | 1.2 |
| 12 | 100 (274) | 0.7 (2) | 18.3 | 1.2 |
| 13 | 100 (247) | 4.5 (11) | 29.9 | 1.7 |
| 14 | 100 (271) | 3.3 (9) | 19.6 | 0.7 |
| 15 | 100 (292) | 1.0 (3) | 22.5 | 1.2 |
| 16 | 100 (240) | 5.8 (14) | 17.5 | 1.3 |
| 17 | 100 (330) | 2.7 (9) | 24.2 | 1.7 |
| 18 | 100 (294) | 2.0 (6) | 12.7 | 1.5 |
| 19 | 100 (276) | 5.4 (15) | 16.7 | 2.1 |
| 20 | 100 (315) | 3.8 (12) | 22 | 1.2 |
| Mean group | 100 | 2.4 | 21.0 | 1.3 |
Values are normalized as percentage (%) of the PTT sensor of the index finger of the right hand group 1 (control group <50 years). The PTT mean ± SD (in ms) of 10 consecutive heartbeats are given for each person. For calculation of the normalized data see Formula 1. In group 1 the normalized PTT showed a significant difference between the left and right hand, respectively 100%±3.1% versus 100%±2.4% (p = 0.004).
Table 3. Data on normalized PTT and PEP variability per group.
| Normalized PTT in % (PTT mean in ms) |
SD PTT in % of PTT (PTT SD in ms) |
% of PEP with respect to PTT | % of SD PEP with respect to PTT | |
|---|---|---|---|---|
| Group 1 | ||||
| Left hand | 100 (267)* | 3.1 (8) | 22.2 | 1.3 |
| Right hand | 100 (280)* | 2.4 (7) | 21.0 | 1.3 |
| Left foot | 100 (369) | 4.7 (17) | 16.5 | 1.0 |
| Right foot | 100 (375) | 4.8 (18) | 16.3 | 1.0 |
| Group 2 | ||||
| Left hand | 100 (276) | 3.5 (12) | 18.7 | 1.4 |
| Right hand | 100 (275) | 2.6 (7) | 19.4 | 1.4 |
| Left foot | 100 (359) | 5.9 (21) | 14.8 | 1.1 |
| Right foot | 100 (350) | 6.3 (20) | 15.1 | 1.1 |
| Group 3 | ||||
| Left hand | 100 (280) | 5.5 (16) | 20.7 | 1.5 |
| Right hand | 100 (284) | 4.2 (12) | 20.3 | 1.4 |
| Left foot | 100 (339) | 7.3 (22) | 17.6 | 1.3 |
| Right foot | 100 (336) | 9.1 (25) | 17.7 | 1.4 |
Mean values per group, n = 20 per group. Group 1 = control group participants aged <50 years, group 2 = control group participants aged >50 years, group 3 = participants with a cardiovascular risk factor. Values are normalized as percentage (%) of the PTT sensor.
* p<0.05
Discussion
This study aimed to determine the variability of PEP at rest and its contribution to PTT in order to assess the accuracy of measuring VTT with PTT. Since it is difficult to measure PEP with echocardiography during exercise, we measured the PEP at rest only. The absolute value of the variability and SD of the PEP between individuals at rest was large when compared with the variability in one participant. However, in the normalized data, the SD of the PEP is only approximately 1.0–1.5% of the PTT. In an earlier study, we found an increase of the PTT of 17 ms after a successful axillary block from 259 ms to 276 ms [30]. This change is larger than can be expected to be caused by a variation of PEP alone.
This study has some limitations. The participants of this study did not refrain of caffeine, neither for physical activity for 48 hours. This might have an effect on the measurements. Nonetheless, in the study of Kohler et al caffeine had no significant effect on the PEP compared to the baseline measurements [31]. However, after exercise, the hemodynamic stress response is reduced and causes an significant increase in PEP [32]. Moreover, we did not control for the estrogenic phase of the participants. In this study 50% of the participants is female and the female participants in group 2 and 3 are above 50 years old and most likely postmenopausal. Farinatti et al showed in their study that the PEP was similar across age groups in men and women [33].
Furthermore, the PTT was measured bilaterally and the difference between the blocked arm and the contralateral arm was calculated. Other studies have shown that PTT difference is a method to exclude the effect of PEP and can be used to monitor arterial distensibility or pulse wave velocity changes [34]. Therefore, the increase in PTT in the present study is most probably a result of the vascular component of the PTT, the VTT.
Furthermore, in group 1, the contribution of the PEP to the PTTfinger was 22.3% and 21.0% for the left and right hand, respectively; this was a significant difference between the left and right hand. This can be explained by the difference in PTT of the left and right hand. In our earlier study, we found no difference between the PTT of the left and right hand in a person at rest in supine position [30]. For an optimal apical 5-chamber view with echocardiography, a left lateral decubitus position was required with the left hand positioned above their head. PTT is related to the position of the arms [35, 36]. Moreover, this position can introduce a different curvature of the artery to the arm. Therefore, the left lateral decubitus position is a possible explanation for this difference in PTT and, subsequently, the contribution to PTT. However, there was no significant difference between the left and right hand in groups 2 and 3.
The contribution of the PEP to the PTTtoe was smaller compared to the PTTfinger. This difference can be explained by the length of the arterial pathway to the toes and a subsequently longer VTT (and PTT).
In this study, the mean PEP of all 3 groups was 56.2 ± 12.3 ms; this differs from the values found in other studies. The main reason for this is the diverse acquisition techniques used and/or an estimation of PEP made by measuring the left ventricular ejection time [17]. Johansson et al. investigated the use of PTT for respiration rate monitoring; PTT varies with respiration. The authors investigated whether measuring the individual components of the PTT (PEP and VTT) could improve their respiration detection [21]. The PEP was measured with phonocardiography, by using the sound of the closing valves. For technical reasons, they used the first heart sound (S1) which represents the closing of the mitral valve, instead of measuring the opening of the aortic valve. However, after the mitral valve closes (S1) and the isovolumetric contraction starts, the ventricular pressure will rise. When the ventricular pressure is high enough, the aortic valve opens and the ejection of blood to the aorta starts. Therefore, using phonography will introduce an inaccuracy in the measurement. They found a mean PEP at rest of 30.1 ms with a SD of 8.0 ms. Furthermore, the PEP and VTT varied synchronously during respiration; focusing on these components did not improve their respiration detection.
In most of the studies, the PEP was measured with cardiothoracic impedance, which measures the change in blood volume in the thorax, which is primarily caused by the blood flow in the aorta. The advantage of cardiothoracic impedance is that it does not require specially trained personnel and can be measured in every body position and also during movements. It is an indirect measurement and is less accurate than a direct measurement and gold standard echocardiography [37], which was used in the present study. Furthermore, these measurements were performed in three different groups. Age and cardiovascular risk factors such as hypertension and atherosclerosis are important factors for PTT. They influence the stiffness of arteries and can change the contribution of PEP to the PTT. However, our measurements showed no significant difference in the PEP between the groups; moreover, the PEPSD% was very small (between 1.0 and 1.5).
Conclusion
In conclusion, the contribution of PEP to the PTT measured at the finger tips at rest is approximately 20%. Therefore, the VTT is represented by the remaining 80% of the PTT. Since the PEP variability within an individual at rest is small (PEPSD% 1.0–1.5% of the PTT value), its contribution to a change in PTT is also small. Therefore, PTT is an attractive method to non-invasively monitor arterial stiffness at rest. Furthermore, unlike methods such as Doppler ultrasound, it does not need specially trained personnel. ECG and PPG (pulse oximeter) are already available in hospitals and software implementation in the monitors is needed to enable PTT calculation. Therefore, PTT at rest is an easy, non-invasive, low-priced measurement, and accurate approximation of VTT for monitoring arterial stiffness.
Supporting information
(SAV)
Acknowledgments
Special thanks to Mariska Nieuwenhoff and Erik Jan Bakker for their help during the first inclusions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Nitzan M, Khanokh B, Slovik Y. The difference in pulse transit time to the toe and finger measured by photoplethysmography. Physiol Meas. 2002;23(1):85–93. [DOI] [PubMed] [Google Scholar]
- 2.Suurland J, van der Heijden KB, Huijbregts SCJ, van Goozen SHM, Swaab H. Infant Parasympathetic and Sympathetic Activity during Baseline, Stress and Recovery: Interactions with Prenatal Adversity Predict Physical Aggression in Toddlerhood. J Abnorm Child Psychol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang A, Tao J, Guo X, Liu X, Luo Y, Liu X, et al. The product of resting heart rate times blood pressure is associated with high brachial-ankle pulse wave velocity. PLoS One. 2014;9(9):e107852 10.1371/journal.pone.0107852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muehlsteff J, Aubert XL, Schuett M. Cuffless estimation of systolic blood pressure for short effort bicycle tests: the prominent role of the pre-ejection period. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5088–92. 10.1109/IEMBS.2006.260275 [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Gao M, Xu D, Olivier NB, Mukkamala R. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J Appl Physiol. 2011;111(6):1681–6. 10.1152/japplphysiol.00980.2011 [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Shuo C, Tong W, Ting M. Novel blood pressure estimation method using single photoplethysmography feature. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:1712–5. 10.1109/EMBC.2017.8037172 [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim B, Nathan V, Jafari R. Exploration and validation of alternate sensing methods for wearable continuous pulse transit time measurement using optical and bioimpedance modalities. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:2051–5. 10.1109/EMBC.2017.8037256 [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya T, Gupta A, Singh ST, Roy S, Prasad A. Robust motion artefact resistant circuit for calculation of Mean Arterial Pressure from pulse transit time. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:3553–6. 10.1109/EMBC.2017.8037624 [DOI] [PubMed] [Google Scholar]
- 9.Babchenko A, Davidson E, Adler D, Ginosar Y, Kurz V, Nitzan M. Increase in pulse transit time to the foot after epidural anaesthesia treatment. Med Biol Eng Comput. 2000;38(6):674–9. [DOI] [PubMed] [Google Scholar]
- 10.Kortekaas MC, Niehof SP, van Velzen MH, Galvin EM, Huygen FJ, Stolker RJ. Pulse transit time as a quick predictor of a successful axillary brachial plexus block. Acta Anaesthesiol Scand. 2012;56(10):1228–33. 10.1111/j.1399-6576.2012.02746.x [DOI] [PubMed] [Google Scholar]
- 11.Foo JY, Lim CS. Pulse transit time as an indirect marker for variations in cardiovascular related reactivity. Technol Health Care. 2006;14(2):97–108. [PubMed] [Google Scholar]
- 12.Lehmann ED, Hopkins KD, Gosling RG. Comment on: Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension. 1996;27(5):1188–91. [PubMed] [Google Scholar]
- 13.Paiva RP, Carvalho P, Aubert X, Muehlsteff J, Henriques J, Antunes M. Assessing PEP and LVET from heart sounds: algorithms and evaluation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3129–33. 10.1109/IEMBS.2009.5332572 [DOI] [PubMed] [Google Scholar]
- 14.Buxi D, Dugar R, Redoute JM, Yuce MR. Comparison of the impedance cardiogram with continuous wave radar using body-contact antennas. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:693–6. 10.1109/EMBC.2017.8036919 [DOI] [PubMed] [Google Scholar]
- 15.Seery MD, Kondrak CL, Streamer L, Saltsman T, Lamarche VM. Preejection period can be calculated using R peak instead of Q. Psychophysiology. 2016;53(8):1232–40. [DOI] [PubMed] [Google Scholar]
- 16.Etemadi M, Inan OT, Wiard RM, Kovacs GT, Giovangrandi L. Non-invasive assessment of cardiac contractility on a weighing scale. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6773–6. 10.1109/IEMBS.2009.5332508 [DOI] [PubMed] [Google Scholar]
- 17.Paiva RP, Carvalho P, Couceiro R, Henriques J, Antunes M, Quintal I, et al. Beat-to-beat systolic time-interval measurement from heart sounds and ECG. Physiol Meas. 2012;33(2):177–94. 10.1088/0967-3334/33/2/177 [DOI] [PubMed] [Google Scholar]
- 18.Noda K, Endo H, Kadosaka T, Nakata T, Watanabe T, Terui Y, et al. Comparison of the measured pre-ejection periods and left ventricular ejection times between echocardiography and impedance cardiography for optimizing cardiac resynchronization therapy. J Arrhythm. 2017;33(2):130–3. 10.1016/j.joa.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai YC, Chiu YW, Kuo HT, Chen SC, Hwang SJ, Chen TH, et al. Fluid overload, pulse wave velocity, and ratio of brachial pre-ejection period to ejection time in diabetic and non-diabetic chronic kidney disease. PLoS One. 2014;9(11):e111000 10.1371/journal.pone.0111000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong MY, Pickwell-MacPherson E, Zhang YT, Cheng JC. The effects of pre-ejection period on post-exercise systolic blood pressure estimation using the pulse arrival time technique. Eur J Appl Physiol. 2011;111(1):135–44. 10.1007/s00421-010-1626-0 [DOI] [PubMed] [Google Scholar]
- 21.Johansson A, Ahlstrom C, Lanne T, Ask P. Pulse wave transit time for monitoring respiration rate. Med Biol Eng Comput. 2006;44(6):471–8. 10.1007/s11517-006-0064-y [DOI] [PubMed] [Google Scholar]
- 22.Pollak MH, Obrist PA. Aortic-radial pulse transit time and ECG Q-wave to radial pulse wave interval as indices of beat-by-beat blood pressure change. Psychophysiology. 1983;20(1):21–8. [DOI] [PubMed] [Google Scholar]
- 23.Chan GS, Middleton PM, Celler BG, Wang L, Lovell NH. Change in pulse transit time and pre-ejection period during head-up tilt-induced progressive central hypovolaemia. J Clin Monit Comput. 2007;21(5):283–93. 10.1007/s10877-007-9086-8 [DOI] [PubMed] [Google Scholar]
- 24.Fiskum C, Andersen TG, Aslaksen PM, Svendsen B, Flaten MA, Jacobsen K. Cardiac complexity and emotional dysregulation in children. Int J Psychophysiol. 2017;121:38–45. 10.1016/j.ijpsycho.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 25.Moldovan M. Matlab central, file exchange (online) Copenhagen: University of Copenhagen; 2003 [updated 22 October 2008. Available from: http://www.mathworks.com/matlabcentral/fileexchange/4061-loadacq.
- 26.Clifford GD. Rpeakdetect syntax (ECG toolbox) Cambridge (Massachusetts): Harvard- MIT, Division of Health Science & Technology (online); 2008 [Massachusetts Institute of Technology]. Available from: http://www.mit.edu/~gari/CODE/.
- 27.Oppenheim AV, Schafer RW. Discrete-Time Signal Processing: Prentice-Hall; 1989. 284–5 p.
- 28.van Velzen MH, Loeve AJ, Niehof SP, Mik EG. Increasing accuracy of pulse transit time measurements by automated elimination of distorted photoplethysmography waves. Med Biol Eng Comput. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elgendi M, Fletcher RR, Norton I, Brearley M, Abbott D, Lovell NH, et al. Frequency analysis of photoplethysmogram and its derivatives. Comput Methods Programs Biomed. 2015;122(3):503–12. 10.1016/j.cmpb.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 30.Kortekaas MC, Niehof SP, van Velzen MH, Galvin EM, Stolker RJ, Huygen FJ. Comparison of bilateral pulse arrival time before and after induced vasodilation by axillary block. Physiol Meas. 2012;33(12):1993–2002. 10.1088/0967-3334/33/12/1993 [DOI] [PubMed] [Google Scholar]
- 31.Kohler M, Pavy A, van den Heuvel C. The effects of chewing versus caffeine on alertness, cognitive performance and cardiac autonomic activity during sleep deprivation. J Sleep Res. 2006;15(4):358–68. 10.1111/j.1365-2869.2006.00547.x [DOI] [PubMed] [Google Scholar]
- 32.Brownley KA, Hinderliter AL, West SG, Girdler SS, Sherwood A, Light KC. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med Sci Sports Exerc. 2003;35(6):978–86. 10.1249/01.MSS.0000069335.12756.1B [DOI] [PubMed] [Google Scholar]
- 33.Farinatti P, Monteiro W, Oliveira R, Crisafulli A. Cardiorespiratory responses and myocardial function within incremental exercise in healthy unmedicated older vs. young men and women. Aging Clin Exp Res. 2018;30(4):341–9. 10.1007/s40520-017-0776-x [DOI] [PubMed] [Google Scholar]
- 34.Foo JY, Lim CS. Dual-channel photoplethysmography to monitor local changes in vascular stiffness. J Clin Monit Comput. 2006;20(3):221–7. 10.1007/s10877-006-9024-1 [DOI] [PubMed] [Google Scholar]
- 35.Zheng D, Murray A. Non-invasive quantification of peripheral arterial volume distensibility and its non-linear relationship with arterial pressure. J Biomech. 2009;42(8):1032–7. 10.1016/j.jbiomech.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 36.Foo JY, Wilson SJ, Williams GR, Harris MA, Cooper DM. Pulse transit time changes observed with different limb positions. Physiol Meas. 2005;26(6):1093–102. 10.1088/0967-3334/26/6/018 [DOI] [PubMed] [Google Scholar]
- 37.Carvalho P, Paiva RP, Couceiro R, Henriques J, Antunes M, Quintal I, et al. Comparison of systolic time interval measurement modalities for portable devices. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:606–9. 10.1109/IEMBS.2010.5626642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.