Abstract
Helium, a minor component of natural gas and radioactive minerals, is most commonly used as a carrier in gas chromatography-mass spectrometry (GC-MS). Its scarcity leads to limited availability and higher costs. In this experiment, hydrogen from a safe source of a hydrogen generator was tested as a substitutive carrier gas for the detection of adulterant in traditional Chinese medicine (TCM) and food supplements by GC-MS analysis. We found that the limits of detection (LODs) of using hydrogen were from 10 to 1000 μg/g. The levels of LODs tested among 170 drugs remain the same whether hydrogen or helium was used as a carrier gas with the exception of 7 drugs—benzbromarone, estradiol benzoate, bezafibrate, mefenamic acid, oxymetholone, piperidenafil and cetilistat. The real sample analysis results using hydrogen were as satisfactory as those using helium. In addition, the retention time was shortened after the chromatographic performance was optimized. In summary, it is worth considering hydrogen as a carrier gas due to its affordable costs, energy efficiency, carbon reduction and chromatographic advantages to detect adulterated drugs in TCM and dietary supplement using GC-MS.
Introduction
Currently, TCM and dietary supplements are becoming progressively popular in many countries [1]. The worldwide consumption of TCM and dietary supplements has accumulatively increased. Evidence showed that approximately 80% of the world’s population takes herbal medicines. In general, TCM is generally regarded as possessing few side effects, and users are therefore prone to taking it in excess of the recommended dose for an extended period of time. On the other hand, consumers tend to trust natural products, believing that they are safe and free of side effects. This false belief that TCM and food supplements are all natural with no side effects and that they are not harmful to human health has led to the exponential growth of TCM and food supplement remedies in markets worldwide [2–4].
To enhance their efficacy, TCM and food supplements were often illegally adulterated with western medicine. The quantities of adulterants in TCM and food supplements at times even exceeded the normal dosage range, and in many cases, they were not the ones required or responsible for the therapeutic effects advertised on the label [3, 5, 6]. Recently, various scientific and monitoring investigations revealed that undeclared synthetic drugs were found in herbal medicines and dietary supplements. The hidden drugs may cause serious toxic-effects to health. The adulterations of herbal medicine and food supplements are likely to contain indiscernible synthetic medicines, metals, or other toxic substances in high concentrations [7] and has become a problem all over the world. The “Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review, Journal of Internal Medicine” (E. Ernst, 252 (2002) 107–113) showed that 24% of their 2600 Chinese herbal medicines samples contained at least one synthetic medicine [4], and 7% of the 260 Asian patient medicines (one component of TCM) from retail herbal stores in California that were tested were shown to contain an undeclared pharmaceutical [8]. Other than dietary supplements and sexual enhancement remedies, many other traditional herbal products were reported to be adulterated with various types of hidden synthetic chemicals capable of pharmacological activities [2]. These chemicals include the following: steroids (strength enhancers), nonsteroidal anti-inflammatory drugs, PDE-5 inhibitors or their analogues (sexual performance enhancers), antihypertensive agents, sibutramine and its analogues (weight loss products)[9], and many other types of therapeutic synthetic agents [10]. Currently, illegal drug detection in Taiwan is also conducted in accordance with the “Method of Test for Adulterants in Chinese Medicine and Foods,” which is the official inspection protocol by the Taiwan Food and Drug Administration (TFDA) [11]. Therefore, the development of improved analytical methodologies for the detection of adulterants is critically important to protect public health so that the quality of TCM and food supplements can be better insured.
The detection processes of TCM ingredients are complicated and challenging. Several methods are used to determine undeclared adulterants in herbal medicine or food supplements, including the following: LC-PDA [12], GC-MS [13], GC-QQQMS [14], LC-MS [15], LC-MS/MS [12], UPLC-TOF/MS [16], Q-Orbitrap MS [17], NMR [18], X-ray powder diffractometry [19], TLC-image [20], TLC-SERS [21], ATR‑IR [22], and CE-MS [23]. The applicability of GC‑MS is determined by the volatility and thermal stability of analytes. A faster technique for GC‑MS was discovered for the detection of sildenafil, tadalafil, and vardenafil in food and herbal products. A GC‑MS method was also successfully used for the screening of 134 pharmaceuticals in patent medications in China. By searching the NIST Mass Spectral Library and comparing the retention times, one can instantly screen out the ingredients and quantify them at the same time [24].
As soon as an unknown substance is found, GC-MS is performed for further verification. The following three gases are commonly used as carriers in gas chromatography (GC): nitrogen (N2), hydrogen (H2), and helium (He). Most GC studies commonly use He as the carrier gas. Helium on planet earth is generally found in natural gases and radioactive decay and is a relatively rare-5.2 ppm by volume in the atmosphere [25]. In anticipation of a potential helium-shortage crisis in the future, the price of helium is becoming expensive and vagaries in supply are limited. Thus, we describe the use of hydrogen instead of helium as a carrier gas for the analysis of illegal, adulterated drugs in TCM and food supplements using gas chromatography-mass spectrometry with electron ionization. Illicit drugs are investigated with hydrogen and helium to manifest the utility of hydrogen in the detection of the adulterant.
Materials and methods
Samples
Samples were taken from drugstores and herbal medicine stores in China, as well as suppliers and manufacturers by the Health Department of the Tainan County Government in Taiwan, between January 2015 and August 2016. The samples included 83 TCM samples and 40 food supplement samples (Please see S1 Table for sample details including sample name, description of appearance, purchase location and source origin).
Chemicals and solutions
One hundred seventy pharmaceutical standards (purity ≥95%) were purchased from USP, TLC, Sigma-Aldrich, Cerilliant, European Pharmacopoeia, TCI, AK Scientific, AApin and Fluka (Please see S2 Table for standard details including compound name, molecular Weight, purity, brand, lot number, storage and source origin). Individual stock solutions (1,000 mg/L) were prepared by the dissolution of 10 mg of each compound in 10 mL of methanol, which were stored at -18°C. The mixed standard solutions at concentrations of 100 mg/L of each standard were prepared by the additive mixing 1 mL of each stock solution, and diluting it to 10 mL with methanol, respectively. HPLC grade methanol and ethanol were obtained from Merck (Darmstadt, Germany).
Sample preparation
Five grams of sample were dissolved in 15–20 mL of ethanol and homogenized in an ultrasonic shaker for 30 minutes, followed by centrifugation at 3000 g for 5 minutes. The supernatant was filtered through a 0.22-μm PTFE syringe filter prior to being injected into GC-MS.
GC-MS analysis
The analysis was performed on an Agilent 7890B GC system coupled to a 5977A MSD mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) and equipped with a Gerstel Multipurpose sampler (Gerstel, Mülheim an der Ruhr, Germany). For the experiments using helium and hydrogen as a buffer gas, a Peak Precision Hydrogen Trace 500cc generator (Peak Scientific Instruments, Inchinnan, Scotland, UK) was used to generate the hydrogen gas. A silica capillary column, Agilent HP-5MS (30 m x 0.25 mm i.d. 0.25 μm film thickness), was used. The operation conditions were described in Table 1. The compounds’ spectra that were obtained were compared to the spectra of known compounds using the NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library.
Table 1. Gas chromatograph-mass spectrometer settings.
| Parameter | Setting | |
|---|---|---|
| Injector | ||
| Carrier gas | Helium | Hydrogen |
| Flow | 1.4 mL/min | 1.0 mL/min |
| Pressure | 9.4 psi | 1.5 psi |
| Injection mode | Splitless mode | |
| Injection volume | 1 μL | |
| Injector temperature | 250°C | |
| Oven temperature program | ||
| Initial ramp | 80°C at 6°C/min until reaching 120°C | |
| Final ramp | At 8°C/min until reaching 300°C for 29 min. | |
| Mass spectrometer | ||
| Ionization mode | Electron ionization | |
| Acquisition mode | Selected Ion Monitoring (SIM) | |
| Dwell time | 100 ms | |
| Source temperature | 230°C | |
| Quadrupole temperature | 150°C | |
The limit of detection (LOD)
The experiment uses helium as a carrier gas to determine the monitored ion and retention time at 10 μg/mL. If the signal is too low to be detected, we increase the concentration of the drug standard gradually to 100, 500 and 1000 μg/mL until it can be obtained. The same experiment was performed for the comparison using hydrogen as the carrier gas for GC-MS.
Five grams of blank sample powder (Xiao Chai Hu Tong Extract Powder, Sheng Chang, Taipei, Taiwan) was spiked with the abovementioned concentration of drug standard (see 2.5.1) and dissolved in 15–20 mL of ethanol. A sample tube was homogenized in the ultrasonic shaker for 30 minutes and was followed by centrifugation at 3000 g for 5 minutes. The supernatant was filtered through a 0.22-μm PTFE syringe filter prior to being injected into the GC-MS. For the calculation of the method’s LODs, the fortification of three blank samples was performed in a specific concentration of drug standard. The concentration of the standard solution of which the ratio of peak height to noise was over 3 was defined as the LOD.
Proficiency testing TFDA
We have conducted the Proficiency testing using the TFDA standards for the detection of drug-adulterants in traditional Chinese medicine in 2014 and 2015 through the use of hydrogen as a carrier gas by GC–MS. Proficiency testing is another effective tool that can be used to ensure the accuracy and precision of the results when using hydrogen as a carrier gas for the detection of adulterated drugs in Chinese herbal medicine and dietary supplements by GC–MS.
Results
GC-MS analysis
Each drug standard was analyzed with GC using a split-less injection to provide the MS identification of each compound and establish their retention time, LOD and identification ion in both helium and hydrogen as carriers in gas. All the results were obtained using the conditions described in Table 1. According to these results shown in Table 2, the use of hydrogen shortened the analysis time and saved resources. The optimum linear velocity for hydrogen is approximately 40 cm/s, which is about half of that of helium, which leads to a decrease in the analysis time by a factor of four, therefore making it possible to reduce the costs of analysis (less degradation of the capillary columns means a cheaper carrier gas). It is clear that the change from helium to hydrogen as the carrier reduced the run-times in drug analytes. Therefore, according to these results, the use of hydrogen allows for the acceleration of the analysis. The previous study revealed that it should be possible to reduce the length of the capillary column in order to save time and money without a loss of resolution in comparison to a longer column with helium as the carrier [26].
Table 2. Comparison retention time, signal-to-noise ratio(S/N) and monitored ion of drugs when hydrogen or helium is used as the carrier gas.
| Drug | Carrier gas | Retention time (min) |
S/N | Monitored ion (m/z) |
|---|---|---|---|---|
| Acetaminophen | H2 | 16.227 | 562.29 | 151, 109, 80, 83* |
| He | 17.045 | 415.15 | 109, 151, 43*, 80 | |
| Acetildenafil | H2 | 41.707 | 529.77 | 127, 70, 84, 42, 112, 56, 98 |
| He | 44.943 | 159.15 | 127, 70, 84, 42, 112, 56, 98 | |
| Acetohexamide | H2 | 19.500 | 177.45 | 199, 184, 120*, 104, 91, 76, 64*, 51* |
| He | 20.254 | 238.09 | 184, 199, 76, 121*, 43*, 139*, 91, 104 | |
| Allopurinol | H2 | 22.872 | 797.93 | 136, 52, 109, 120, 67 |
| He | 20.159 | 18.05 | 136, 52, 29*, 109, 67,120 | |
| Aminopyrine | H2 | 19.323 | 2492.34 | 231, 97, 77, 56 |
| He | 20.163 | 3188.28 | 231, 56, 97, 77 | |
| Aminotadalafil | H2 | 46.301 | 131.81 | 390, 262, 204, 289, 233, 102, 169, 375, 43*, 405* |
| He | 49.941 | 133.16 | 390, 204, 262, 289, 169, 233, 115*, 169, 102, 375 | |
| Amitripthyline | H2 | 22.689 | 71.28 | 277, 202, 178, 152, 115, 91, 58 |
| He | 23.578 | 144.76 | 277, 58, 202, 178, 115, 91, 152 | |
| Amphetamine | H2 | 6.278 | 84.52 | 44, 91,65,120 |
| He | 6.103 | 575.81 | 44, 91,65,120 | |
| Aspirin | H2 | 8.926 | 138.22 | 120, 92, 152*, 65, 45* |
| He | 9.762 | 80.64 | 92, 120, 138*, 64*, 65 | |
| Atenolol | H2 | 25.113 | 12505.57 | 222, 107, 72 |
| He | 25.693 | 3581.62 | 72, 107, 222 | |
| Atropine | H2 | 22.698 | 1471.07 | 124, 82, 94, 289, 140, 67, 103,42 |
| He | 23.598 | 1486.90 | 124, 82, 94, 289, 140, 67, 42, 103 | |
| Barbital | H2 | 13.361 | 204.84 | 156, 141, 98, 112, 55, 41, 83, 69 |
| He | 14.160 | 776.86 | 156, 141, 98, 55, 112, 41, 83, 69 | |
| Benzbromarone | H2 | 28.290 | 204.84 | 264, 173, 279, 115, 249, 328, 145, 132*, 221 |
| He | 29.169 | 6448.86 | 264, 173, 115, 279, 249, 328, 145, 63*, 221 | |
| Benzocaine | H2 | 14.195 | 302.25 | 165, 120, 92, 65 |
| He | 15.094 | 184.07 | 120, 165, 92, 65 | |
| Betamethasone | H2 | 29.912 | 273.45 | 312, 281*, 207, 160, 122, 91, 55 |
| He | 30.794 | 381.89 | 122, 312, 91, 160, 207, 41, 55, 77* | |
| Bezafibrate | H2 | 26.950 | 6036.33 | 120, 139, 107, 77*, 156 |
| He | 27.690 | 20.97 | 120, 139, 205*, 107, 156 | |
| Bisacodyl | H2 | 29.089 | 23138.37 | 361, 319, 276, 246*, 199, 154 |
| He | 29.996 | 18519.59 | 361, 276, 277*, 199, 319, 43*, 318*, 183*, 278*, 154 | |
| Bromhexine | H2 | 24.672 | 240565.29 | 376, 293, 264, 112, 70, 374* |
| He | 25.592 | 1374605.79 | 376, 293, 264, 305*, 112, 70 | |
| Brompheniramine | H2 | 21.654 | 1070326.01 | 247, 58, 167, 72, 180, 42, 139 |
| He | 22.569 | 572.47 | 247, 58, 167, 72, 180, 42, 139 | |
| Bromvalerylurea | H2 | 12.902 | 18.40 | 137, 44, 100, 55, 83, 69, 120* |
| He | 13.809 | 43670.57 | 137, 44, 83, 180*, 100, 55, 69 | |
| Bucetin | H2 | 20.613 | 1837.10 | 223, 137, 108, 81, 53* |
| He | 21.466 | 1406.13 | 137, 108, 223, 45*, 81 | |
| Caffeine | H2 | 18.055 | 165.90 | 194, 109, 67 |
| He | 18.878 | 283.12 | 194, 109, 67, 55*, 82* | |
| Carbetapentane | H2 | 23.311 | 1995.44 | 86, 144, 115, 100, 58 |
| He | 24.158 | 4056.80 | 86, 144, 115, 100, 58 | |
| Carbimazole | H2 | 15.606 | 417.25 | 186, 114, 72, 81, 42, 56, 127,141 |
| He | 16.519 | 1395.15 | 186, 114, 72, 81, 141, 42, 56, 127 | |
| Carbinoxamine | H2 | 21.362 | 124.32 | 201, 167, 139*, 71 |
| He | 22.248 | 181.70 | 201, 167, 58*, 71 | |
| Carbodenafil | H2 | 43.793 | 249.58 | 84, 56, 70, 381, 452, 339, 311, 42, 113, 136* |
| He | 47.202 | 96.80 | 84, 97*, 56, 70, 381, 452, 339, 311, 42, 113 | |
| Carisoprodol | H2 | 18.770 | 36.02 | 245, 184, 158, 97, 83*, 69*, 55 |
| He | 19.689 | 20.54 | 245, 158, 97, 184, 55, 58*, 43* | |
| Chloramphenicol | H2 | 27.569 | 2199.59 | 207, 172,153, 106, 77 |
| He | 28.303 | 1989.30 | 207, 153, 172, 77, 106 | |
| Chlordiazepoxide | H2 | 29.238 | 170.17 | 282, 247, 220, 190, 165, 124*, 91 |
| He | 30.310 | 246.65 | 282, 241*, 247, 220, 165, 190, 91 | |
| Chlormezanone | H2 | 23.419 | 3689.22 | 208, 174, 152, 125, 98, 69 |
| He | 24.275 | 364.87 | 152, 208, 174, 98, 125, 69 | |
| Chlorpheniramine | H2 | 20.445 | 377.13 | 203, 58, 167, 72, 180, 42 |
| He | 21.356 | 660.96 | 203, 58, 167, 72, 180, 42 | |
| Chlorpromazine | H2 | 25.943 | 393651.65 | 318, 272, 232, 196, 86, 58 |
| He | 26.863 | 1034722.73 | 318, 58, 86, 272, 232, 196 | |
| Chlorpropamide | H2 | 16.426 | 413.58 | 190, 174, 127, 111, 75 |
| He | 17.211 | 334.36 | 190, 111, 174, 127, 75 | |
| Chlorzoxazone | H2 | 16.681 | 1126.98 | 169, 113, 78 |
| He | 17.724 | 428.48 | 169, 113, 78 | |
| Cimetidine | H2 | 5.722 | 101.59 | 45, 116, 55, 70, 60, 74, 42, 88* |
| He | 6.651 | 305.40 | 116, 45, 55, 60, 70, 74, 42, 99* | |
| Cinnarizine | H2 | 31.221 | 640.13 | 201, 117, 167, 251, 152, 91 |
| He | 32.229 | 1610.46 | 201, 117, 167, 251, 152, 91 | |
| Clobenzorex | H2 | 19.892 | 568.16 | 168, 127, 91, 65 |
| He | 20.798 | 829.80 | 168, 127, 91, 65 | |
| Clofibrate | H2 | 13.722 | 657.00 | 242, 169, 128 |
| He | 14.588 | 1238.64 | 128, 242, 169 | |
| Cocaine | H2 | 22.792 | 28.65 | 182, 82, 303, 105, 272, 198, 122, 51 |
| He | 23.671 | 1156.47 | 182, 82, 303, 105, 272, 198, 122, 51 | |
| Colchicine | H2 | 33.875 | 133.19 | 399, 371, 312, 281, 254* |
| He | 35.326 | 126.04 | 312, 399, 371, 297*, 281 | |
| Cortisone | H2 | 27.709 | 131.20 | 122, 300, 91, 256, 105, 77, 147, 161, 55 |
| He | 28.650 | 328.98 | 122, 300, 91, 256, 161, 147, 105, 55, 77 | |
| 7-keto-DHEA | H2 | 28.240 | 616.13 | 302, 161, 91, 79, 105, 134, 187, 55, 41, 205 |
| He | 29.196 | 3160.69 | 302, 161, 91, 79, 105, 134, 187, 55, 41, 205 | |
| N-Desmethylsibutramine | H2 | 18.139 | 8921.42 | 100, 58, 44, 137, 128, 115 |
| He | 18.993 | 57424.38 | 100, 58, 44, 137, 128, 115 | |
| N-Didesmethylsibutramine | H2 | 18.111 | 4950.37 | 137, 115, 86 |
| He | 18.989 | 2657.67 | 86, 137, 115 | |
| Dexamethasone | H2 | 29.940 | 336.90 | 312, 160, 122, 91, 55 |
| He | 30.655 | 478.25 | 122, 312, 160, 91, 55 | |
| Dextromethorphan | H2 | 22.049 | 684403.48 | 271, 150, 214, 59, 171, 203, 128 |
| He | 23.199 | 601.70 | 271, 59, 150, 214, 171, 203, 128 | |
| Diazepam | H2 | 25.356 | 191.73 | 283, 256, 221, 165, 77, 51 |
| He | 26.238 | 790.25 | 283, 256, 221, 165, 77, 51 | |
| Dibucaine | H2 | 27.963 | 325.46 | 116, 86, 58 |
| He | 28.855 | 689.64 | 116, 86, 58 | |
| Diclofenac | H2 | 21.958 | 530.12 | 295, 242, 214, 179, 151 |
| He | 22.816 | 979.97 | 214, 295, 242, 179, 151 | |
| Dicyclomine | H2 | 21.505 | 853.46 | 86, 55 |
| He | 22.375 | 5338.38 | 86, 99*, 55 | |
| Diethylpropion | H2 | 12.933 | 254.89 | 100, 77, 51 |
| He | 13.797 | 995.63 | 100, 77, 51 | |
| Diethylstilbestrol | H2 | 23.917 | 2535.31 | 268, 239, 145, 107 |
| He | 24.749 | 681.06 | 268, 107, 239, 145 | |
| Dimethylsildenafil | H2 | 45.707 | 227.53 | 113, 312, 70, 84, 42, 283, 136 |
| He | 49.312 | 34.59 | 113, 312, 70, 84, 42, 283, 136 | |
| Diphenhydramine | H2 | 18.661 | 728.26 | 165, 58 |
| He | 19.511 | 1728.75 | 165, 58 | |
| Diphenylhydantoin | H2 | 24.361 | 52.21 | 180, 223, 209, 252, 104, 77, 165, 147, 51 |
| He | 25.460 | 47.80 | 180, 104, 223, 209, 252, 77, 165, 51, 147 | |
| Diprophylline | H2 | 24.230 | 165.79 | 254, 223, 180, 137, 109, 81, 54 |
| He | 25.116 | 677.65 | 223, 254, 180, 109, 137, 81, 54 | |
| Econazole | H2 | 29.123 | 312.59 | 299, 207, 125, 81, 54 |
| He | 30.013 | 73.59 | 125, 81, 299, 207, 54 | |
| Estradiol benzoate | H2 | 35.659 | 4236.99 | 376, 105, 77 |
| He | 37.513 | 1810.32 | 376, 105, 77 | |
| Estriol | H2 | 29.054 | 707.09 | 288, 160, 146, 213, 133, 172, 201, 115, 185 |
| He | 30.055 | 124.67 | 288, 160, 146, 213, 133, 172, 201, 115, 185 | |
| Estrone | H2 | 26.785 | 108.53 | 270, 146, 185, 213 |
| He | 27.759 | 247.64 | 270, 146, 185, 213 | |
| Ethinylestradiol | H2 | 27.594 | 109.99 | 213, 296, 160, 133, 145, 228, 172, 185, 115 |
| He | 28.434 | 146.01 | 213, 296, 160, 133, 228, 145, 172, 185, 115 | |
| Ethisterone | H2 | 27.721 | 21.79 | 124, 312, 91, 229, 245, 79, 105, 148, 286, 189, 67 |
| He | 28.612 | 78.02 | 124, 312, 91, 79, 229, 245, 105, 148, 189, 67, 286 | |
| Ethoxybenzamide | H2 | 14.651 | 1578.34 | 165, 150, 120, 92, 65 |
| He | 15.526 | 1168.66 | 120, 92, 150, 165, 65 | |
| Ethylestrenol | H2 | 24.277 | 3402.39 | 216, 241, 201, 288, 91, 270, 79, 121, 147, 105 |
| He | 25.155 | 1896.52 | 216, 201, 241, 91, 79, 288, 270, 121, 147, 105 | |
| Fenfluramine | H2 | 7.506 | 4201.59 | 159, 109, 72, 56* |
| He | 8.267 | 8752.19 | 72, 159, 109, 44* | |
| Finasteride | H2 | 33.484 | 208.98 | 372, 110, 58, 272, 357, 258, 128, 230, 72, 245 |
| He | 34.822 | 168.39 | 372, 58, 110, 272, 357, 258, 128, 230, 72, 245 | |
| Flavoxate | H2 | 32.588 | 218.77 | 263, 234, 147, 98 |
| He | 33.827 | 681.91 | 98, 234, 147, 263 | |
| Fluoxetine | H2 | 18.599 | 4320.42 | 309, 183, 162, 133, 104, 78*, 59 |
| He | 19.489 | 10859.57 | 309, 183, 162, 133, 44*, 104, 59 | |
| Fluoxymesterone | H2 | 29.797 | 83968.78 | 336, 279, 109, 71 |
| He | 30.742 | 145899.76 | 336, 279, 71, 109 | |
| Gemfibrozil | H2 | 19.329 | 1655.20 | 250, 122 |
| He | 20.183 | 1357.76 | 250, 122 | |
| Gendenafil | H2 | 31.414 | 520.43 | 354, 326, 339, 136, 166, 282, 43, 311, 297 |
| He | 31.414 | 514.07 | 354, 326, 339, 136, 166, 282, 43, 311, 297 | |
| Griseofulvin | H2 | 28.131 | 1108.72 | 352, 310, 284, 254, 214, 171, 138, 95, 69 |
| He | 28.949 | 3255.28 | 352, 310, 138, 214, 284, 254, 69, 171, 95 | |
| Guaifenesin | H2 | 15.537 | 1687.05 | 124,109,198,77,95,65,52,167,149 |
| He | 16.375 | 3642.70 | 124, 109, 198, 77, 81, 95, 65, 52, 167 | |
| Homatropine | H2 | 21.349 | 142.55 | 275, 124, 79 |
| He | 22.276 | 514.41 | 124, 275, 79 | |
| Homosildenafil | H2 | 48.785 | 451.37 | 113, 70, 281*, 56, 42, 207, 355, 341, 309, 253 |
| He | 53.164 | 463.73 | 113, 404*, 70, 56, 42, 207, 355, 341, 309, 253 | |
| Hydralazine | H2 | 19.201 | 343.84 | 160, 103, 131, 115, 89, 76, 145, 63, 50 |
| He | 18.044 | 408.02 | 160, 103, 131, 115, 89, 76, 145, 63, 50 | |
| Hydrocortisone | H2 | 29.064 | 187.65 | 305*, 163, 123, 91, 55 |
| He | 29.931 | 458.36 | 285*, 362*, 163, 123, 91, 55 | |
| Ibuprofen | H2 | 15.040 | 1090.68 | 206, 161, 117, 91, 65 |
| He | 15.817 | 674.69 | 161, 206, 117, 91, 65 | |
| Imidazosagatriazinone | H2 | 27.187 | 9066.54 | 312, 284, 136, 240 |
| He | 27.969 | 4361.89 | 312, 284, 136, 240 | |
| Indomethacin | H2 | 30.571 | 1204.53 | 139, 313*, 111, 75 |
| He | 27.765 | 24.57 | 139, 357*, 111, 75 | |
| Ketoprofen | H2 | 22.913 | 54.02 | 105, 177, 209, 77, 254, 45*, 194, 131, 165 |
| He | 23.701 | 158.29 | 105, 77, 177, 209, 254, 51*, 194, 131, 165 | |
| Lidocaine | H2 | 18.667 | 745.66 | 234, 120, 86, 58 |
| He | 19.537 | 2480.92 | 86, 234, 120, 58 | |
| Lorazepam | H2 | 25.073 | 80.39 | 239, 274, 302, 75, 138, 177, 111, 203, 163, 100 |
| He | 25.916 | 448.96 | 239, 274, 302, 75, 138, 177, 111, 203, 163, 100 | |
| Mazindol | H2 | 24.604 | 3833.98 | 266, 231, 204, 176, 128, 102, 75 |
| He | 25.835 | 382.97 | 266, 231, 204, 176, 128, 102, 75 | |
| Mefenamic acid | H2 | 22.179 | 2403.18 | 241, 223, 180, 152, 102* |
| He | 22.732 | 186.25 | 223, 241, 180, 77*, 152 | |
| Melatonin | H2 | 24.765 | 855.84 | 232, 172, 160, 145, 130, 117, 102, 89 |
| He | 25.586 | 834.77 | 160, 172, 232, 145, 117, 130, 102, 89 | |
| Mephenesin | H2 | 14.300 | 44.99 | 182, 108, 91 |
| He | 15.027 | 2776.31 | 108, 182, 91 | |
| Mephentermine | H2 | 9.224 | 3957.33 | 72, 91, 148, 56, 42, 115 |
| He | 8.883 | 1232.24 | 72, 91, 148, 56, 42, 115 | |
| Meprobamate | H2 | 17.741 | 904.61 | 83, 55, 43, 71, 62, 96, 114, 144, 101 |
| He | 18.516 | 2359.60 | 83, 55, 71, 62, 96, 114, 144, 101, 43 | |
| Methamphetamine | H2 | 6.505 | 2216.40 | 58, 91, 65, 134, 42, 115, 119* |
| He | 7.195 | 3184.90 | 58, 91, 65, 56*, 134, 42, 115 | |
| Methandriol | H2 | 26.350 | 74.00 | 253, 213, 271, 304, 105, 145, 286, 228, 119, 159 |
| He | 27.230 | 305.46 | 253, 213, 304, 105, 145, 271, 286, 228, 119, 159 | |
| Methandrostenolone | H2 | 27.749 | 76.18 | 122, 91, 161, 147, 105, 134, 77 |
| He | 28.686 | 360.69 | 122, 91, 161, 147, 105, 134, 77 | |
| Methaqualone | H2 | 22.282 | 1208.48 | 250, 91, 132, 65, 77, 217, 117, 50, 104* |
| He | 23.192 | 3143.27 | 235*, 250, 91, 132, 65, 77, 217, 117, 50 | |
| Metharbital | H2 | 12.212 | 2739.29 | 155, 112, 83, 55 |
| He | 12.953 | 2922.10 | 155, 170*, 112, 83, 55 | |
| Methimazole | H2 | 13.837 | 140.23 | 114, 72, 81, 42, 54, 86, 59 |
| He | 14.903 | 213.63 | 114, 72, 42, 81, 54, 86, 59 | |
| Methylprednisolone | H2 | 29.813 | 457.10 | 136, 91, 55* |
| He | 30.677 | 1796.27 | 136, 91, 121* | |
| Methyltestosterone | H2 | 27.435 | 26731.78 | 302, 229, 202, 161, 124, 91 |
| He | 28.384 | 1780.50 | 302, 124, 91, 229, 202, 161 | |
| Metoclopramide | H2 | 27.217 | 5295.57 | 184, 86, 58 |
| He | 28.034 | 8438.34 | 86, 184, 58 | |
| Metronidazole | H2 | 15.394 | 443.85 | 171, 124, 81, 53 |
| He | 16.174 | 423.83 | 124, 81, 171, 53 | |
| Minoxidil | H2 | 20.855 | 91.48 | 193, 164, 138, 110, 84, 67 |
| He | 21.700 | 1697.28 | 193, 164, 110, 138, 84, 67 | |
| Morphine | H2 | 25.421 | 592.33 | 285, 162, 42, 215, 115, 55, 65, 92, 81 |
| He | 26.311 | 2101.32 | 285, 162, 42, 215, 115, 55, 65, 92, 81 | |
| Nalidixic acid | H2 | 25.020 | 2964.06 | 188, 160, 132, 173, 145, 104, 232, 77 |
| He | 25.602 | 4290.93 | 188, 160, 132, 173, 145, 104, 232, 77 | |
| Nandrolone | H2 | 25.593 | 112.00 | 274, 215, 173*, 147, 119, 91, 67 |
| He | 27.554 | 413.47 | 274, 110*, 91, 67, 119, 215, 147 | |
| Naproxen | H2 | 21.384 | 479.13 | 230, 185, 170, 141, 115 |
| He | 31.254 | 21.76 | 185, 230, 170, 141, 115 | |
| Nifedipine | H2 | 26.652 | 2198.09 | 329, 284, 224, 268, 254, 195, 180 |
| He | 27.496 | 8691.50 | 329, 284, 224, 268, 254, 195, 180 | |
| Noracetildenafil | H2 | 39.756 | 143.98 | 113, 70, 42, 56, 98, 207, 311, 452, 136, 354 |
| He | 42.433 | 144.95 | 113, 70, 42, 56, 98, 207, 452, 311, 136, 354 | |
| Norethisterone | H2 | 27.236 | 237.22 | 298, 283*, 265 |
| He | 28.102 | 855.06 | 298, 231*, 265 | |
| Orphenadrine | H2 | 19.556 | 345.15 | 58, 73, 165, 178, 45 |
| He | 20.419 | 1799.00 | 58, 73, 165, 178, 45 | |
| Oxethazaine | H2 | 26.450 | 198.46 | 114, 86, 213, 56, 133, 72, 304 |
| He | 27.281 | 289.85 | 114, 86, 56, 213, 133, 72, 304 | |
| Oxymetholone | H2 | 28.615 | 8872.16 | 174, 275, 332, 43, 161, 91, 81, 71, 216, 107 |
| He | 29.471 | 16927.40 | 174, 332, 275, 216, 161, 107, 43, 91, 81, 71 | |
| Oxyphenbutazone | H2 | 30.658 | 324.39 | 93, 45*, 55, 69, 161, 193*, 77, 249* |
| He | 31.419 | 312.73 | 93, 199*, 77, 324*, 55, 69, 161 | |
| Pentazocine | H2 | 23.647 | 451.43 | 217, 202, 285, 110, 270, 70, 45, 159, 173 |
| He | 24.505 | 1600.48 | 217, 202, 110, 285, 270, 70, 45, 159, 173 | |
| Phenacetin | H2 | 16.184 | 2534.87 | 179, 137, 108, 80, 65, 53 |
| He | 17.017 | 1248.32 | 108, 179, 137, 80, 65, 53 | |
| Phenazopyridine | H2 | 23.833 | 1046.17 | 213, 108, 81, 54, 136, 97*, 184, 66, 155 |
| He | 24.670 | 660.83 | 213, 108, 81, 136, 54, 184, 66, 51*, 155 | |
| Phenformin | H2 | 14.011 | 274.32 | 146, 104, 91, 77, 65 |
| He | 14.844 | 412.63 | 91, 146, 104, 77, 65 | |
| Phenobarbital | H2 | 20.141 | 3530.71 | 204, 117, 232, 161, 146, 103, 77, 91, 174 |
| He | 20.996 | 2851.29 | 204, 117, 232, 161, 146, 77, 103, 91, 174 | |
| Phenolphthalein | H2 | 31.728 | 255.45 | 318, 274, 225, 181, 152, 104*, 65 |
| He | 32.746 | 765.90 | 274, 318, 225, 181, 152, 121*, 65 | |
| Phentermine | H2 | 6.160 | 2271.97 | 70, 91, 105, 58, 65, 115, 41, 115 |
| He | 6.779 | 3375.10 | 70, 91, 105, 58, 65, 115, 41, 115 | |
| Phentolamine | H2 | 26.987 | 42.57 | 199, 183, 91, 154, 77, 128, 170 |
| He | 27.784 | 117.12 | 199, 183, 91, 154, 77, 128, 170 | |
| Phenylbutazone | H2 | 24.709 | 3051.14 | 308, 252, 183, 152, 105, 77 |
| He | 25.543 | 5464.75 | 183, 308, 252, 77, 152, 105 | |
| Phenylephrine | H2 | 18.847 | 94.46 | 135, 44, 107, 179, 160, 77, 51, 91 |
| He | 16.485 | 224.29 | 135, 44, 107, 179, 160, 77, 51, 91 | |
| Phenylpropanolamine | H2 | 9.731 | 236.24 | 44, 77, 105, 51, 117, 91 |
| He | 10.797 | 1849.14 | 44, 77, 51, 105, 117, 91 | |
| Piperidenafil | H2 | 44.617 | 128.79 | 431, 459, 283, 67, 42, 84, 121, 135, 149, 215 |
| He | 48.328 | 21.36 | 431, 459, 283, 67, 42, 84, 121, 135, 149, 215 | |
| Pirenzepine | H2 | 31.102 | 253.75 | 351, 281, 211, 113, 70 |
| He | 32.009 | 358.14 | 113, 70, 211, 351, 281 | |
| Piroxicam | H2 | 16.877 | 1640.49 | 104, 76, 43, 152, 169, 118, 386*, 211, 91 |
| He | 17.665 | 652.90 | 104, 152, 76, 43, 169, 118, 211, 91 | |
| Prednisolone | H2 | 29.312 | 68.40 | 122, 91, 55 |
| He | 30.225 | 1015.08 | 122, 91, 55 | |
| Prednisone | H2 | 27.889 | 417.77 | 298, 245, 226, 186, 160, 131, 115, 91 |
| He | 28.785 | 534.87 | 298, 160, 91, 245, 226, 186, 131, 115 | |
| Primidone | H2 | 23.472 | 1344.44 | 146, 190, 117, 161, 103, 91, 77, 174 |
| He | 24.067 | 1431.77 | 190, 146, 117, 161, 103, 91, 77, 174 | |
| Probenecid | H2 | 22.941 | 295.85 | 270, 135, 199, 104, 76, 43 |
| He | 23.788 | 142.22 | 270, 135, 199, 104, 76, 43 | |
| Procaine | H2 | 20.737 | 341.47 | 86, 99, 120, 65, 56, 164 |
| He | 21.644 | 523.45 | 86, 99, 120, 164, 65, 56 | |
| Progesterone | H2 | 28.663 | 54.72 | 314, 272, 229, 147, 124, 91, 67 |
| He | 29.599 | 358.43 | 124, 314, 272, 229, 91, 67, 147 | |
| Propantheline | H2 | 24.358 | 735.98 | 86, 181, 310, 99, 152, 44, 58, 325, 127, 71 |
| He | 25.183 | 3169.32 | 86, 181, 44, 99, 310, 152, 58, 325, 127, 71 | |
| Propranolol | H2 | 22.279 | 745.35 | 259, 215, 144, 115, 72 |
| He | 23.188 | 3138.57 | 72, 115, 144, 259, 215 | |
| Quinine | H2 | 28.967 | 2199.60 | 189, 160, 136 |
| He | 29.839 | 4577.02 | 136, 189, 160 | |
| Ranitidine I | H2 | 21.701 | 272.14 | 235, 137, 94, 67 |
| He | 22.514 | 497.94 | 137, 235, 94, 67 | |
| Ranitidine II | H2 | 28.971 | 279.25 | |
| He | 29.960 | 1175.13 | ||
| Rimonabant | H2 | 35.656 | 601.19 | 84, 363, 55, 99, 282, 335, 299, 41, 380, 145, 462* |
| He | 37.392 | 1808.45 | 84, 55, 99, 363, 282, 335, 299, 41, 380, 111*, 145 | |
| Salicylamide | H2 | 12.103 | 1129.40 | 120, 137, 92, 65, 53, 44, 80 |
| He | 12.979 | 457.91 | 120, 137, 92, 65, 53, 44, 80 | |
| Salicylic acid | H2 | 9.100 | 1859.82 | 120, 92, 138, 64, 46 |
| He | 9.655 | 1343.64 | 120, 92, 138, 64, 46 | |
| Scopolamine | H2 | 24.172 | 1502.23 | 94, 138, 108, 154, 303 |
| He | 25.121 | 3631.33 | 94, 138, 108, 154, 303 | |
| Secobarbital | H2 | 17.809 | 2691.55 | 195, 168, 124, 97, 53 |
| He | 18.684 | 1204.76 | 168, 195, 97, 124, 53 | |
| Sibutramine | H2 | 18.276 | 16371.68 | 114, 72, 58, 101, 128, 137 |
| He | 19.109 | 5010.93 | 114, 72, 58, 101, 128, 137 | |
| Sildenafil | H2 | 45.447 | 145.57 | 404, 281*, 207, 99, 56 |
| He | 49.500 | 17.60 | 99, 404, 56, 283*, 207 | |
| Stanozolol | H2 | 31.003 | 163.95 | 96, 328, 257, 270, 133, 119, 175 |
| He | 32.022 | 335.89 | 96, 328, 257, 270, 133, 119, 175 | |
| Strychnine | H2 | 31.979 | 2444.29 | 334, 167, 130, 107, 77, 55 |
| He | 33.167 | 296.67 | 334, 167, 130, 107, 77, 55 | |
| Sulfadiazine | H2 | 26.540 | 512.14 | 185, 92, 65, 108 |
| He | 27.429 | 756.37 | 185, 92, 65, 108 | |
| Sulfadimethoxine I | H2 | 28.890 | 219.77 | 259, 140, 92, 65, 168, 108, 121, 82, 187 |
| He | 29.806 | 639.59 | 259, 140, 92, 65, 168, 108, 121, 82, 187 | |
| Sulfadimethoxine II | H2 | 29.269 | 364.43 | |
| He | 30.114 | 208.79 | ||
| Sulfamerazine | H2 | 27.012 | 801.84 | 199, 92, 65 |
| He | 27.910 | 1340.67 | 200*, 199, 92, 65 | |
| Sulfamethazine | H2 | 27.373 | 2275.83 | 213, 92, 65 |
| He | 28.212 | 1074.36 | 214*, 213, 92, 65 | |
| Sulfamethoxazole | H2 | 25.579 | 1070.19 | 92, 108, 65, 156, 119, 162, 174, 43*, 140* |
| He | 26.156 | 910.57 | 92, 156, 108, 65, 162, 119, 253*, 174, 189* | |
| Sulfamethoxypyridazine | H2 | 28.799 | 157.78 | 215, 92, 108, 65, 53, 80, 280 |
| He | 29.673 | 214.35 | 215, 92, 65, 108, 53, 280, 80 | |
| Sulfanilamide | H2 | 20.112 | 263.20 | 172, 92, 65 |
| He | 20.718 | 202.79 | 172, 92, 65, 108* | |
| Sulfinpyrazone | H2 | 23.429 | 761.20 | 278, 249, 209, 183, 152, 130, 105, 77, 51 |
| He | 24.242 | 1169.11 | 278, 77, 105, 130, 51, 249, 209, 183, 152 | |
| Sulindac | H2 | 29.207 | 198.68 | 233, 297, 312, 248, 67, 123, 47, 133, 220 |
| He | 30.083 | 516.90 | 297, 233, 312, 248,123, 220, 67, 47, 133 | |
| Synephrine | H2 | 15.602 | 43.68 | 135*, 44, 107, 179*, 160*, 77, 51, 91 |
| He | 16.281 | 35.97 | 44, 77, 108*, 107, 65*, 51, 91 | |
| Tadalafil | H2 | 41.729 | 1803.01 | 389, 262, 204, 169 |
| He | 44.673 | 79.24 | 389, 262, 204, 169 | |
| Terbinafine | H2 | 23.292 | 289.12 | 141, 276, 234, 115, 291, 196 |
| He | 24.139 | 1216.53 | 141, 276, 115, 234, 291, 196 | |
| Testosterone | H2 | 27.168 | 65.84 | 288, 246, 203, 147, 124, 91, 55 |
| He | 28.115 | 328.53 | 124, 288, 246, 147, 203, 91, 55 | |
| Tetracaine | H2 | 23.189 | 5752.14 | 58, 71, 176, 150, 105, 193, 92 |
| He | 24.012 | 3991.85 | 58, 71, 176, 150, 105, 193, 92 | |
| Theobromine | H2 | 19.022 | 23.33 | 180, 67, 109, 55, 82, 137, 42, 94 |
| He | 19.157 | 142.02 | 180, 67, 55, 109, 82, 137, 42, 94 | |
| Theophylline | H2 | 20.324 | 33.21 | 180, 95, 68, 53 |
| He | 21.141 | 246.52 | 180, 95, 68, 53 | |
| Thiodimethylsildenafil | H2 | 40.912 | 33.32 | 113, 70, 42*, 84, 328*, 343, 56 |
| He | 43.571 | 48.61 | 113, 340*, 70, 84, 283*, 56, 343 | |
| Thiohomosildenafil | H2 | 50.969 | 202.81 | 113, 70, 56, 475, 98, 42, 327, 341, 269, 84 |
| He | 55.952 | 201.35 | 113, 70, 56, 475, 98, 42, 327, 341, 269, 84 | |
| Thioridazine | H2 | 31.712 | 366.09 | 370, 244, 185, 126, 98, 70 |
| He | 32.798 | 120.18 | 98, 370, 70, 185, 244, 126 | |
| Thiosildenafil | H2 | 47.756 | 296.39 | 99, 448, 56, 489, 425, 70, 207 |
| He | 51.652 | 355.43 | 99, 448, 56, 70, 489, 425, 207 | |
| Tinidazole | H2 | 21.175 | 776.12 | 201, 123, 80, 68, 93, 107, 53, 154, 247 |
| He | 21.921 | 3229.62 | 201, 53, 80, 123, 68, 93, 107, 154, 247 | |
| Tolbutamide | H2 | 15.612 | 426.74 | 91, 171, 155, 65, 107, 77, 197 |
| He | 16.398 | 684.97 | 91, 171, 155, 65, 107, 77, 197 | |
| Vardenafil analogue | H2 | 27.628 | 5472.86 | 284, 312, 256, 67, 297, 120, 269, 93, 135 |
| He | 28.462 | 2912.76 | 284, 312, 256, 297, 67, 120, 269, 93, 135 | |
| Yohimbine | H2 | 32.330 | 1464.36 | 353, 169 |
| He | 33.427 | 1587.82 | 353, 169 | |
| Zolpidem | H2 | 28.784 | 378.38 | 235, 207, 219, 281*, 307, 65, 92, 191 |
| He | 29.717 | 205.59 | 235, 307, 219, 92, 65, 191, 207 | |
| Cetilistat | H2 | 13.943 | 218.91 | 177, 160, 133, 55, 104, 77, 401 |
| He | 14.692 | 234.20 | 177, 160, 133, 104, 55, 77, 401 |
*represent the differences between the H2 and He ions.
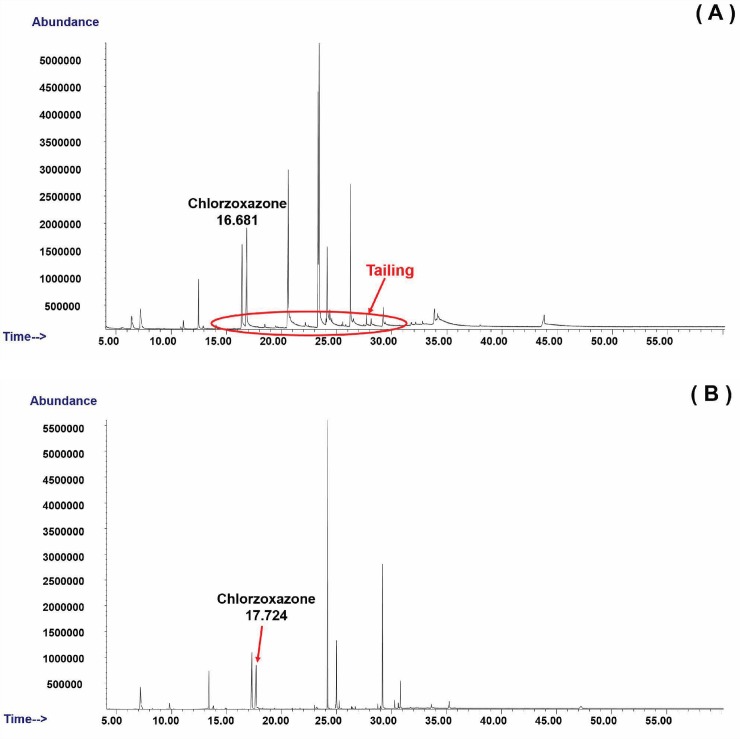
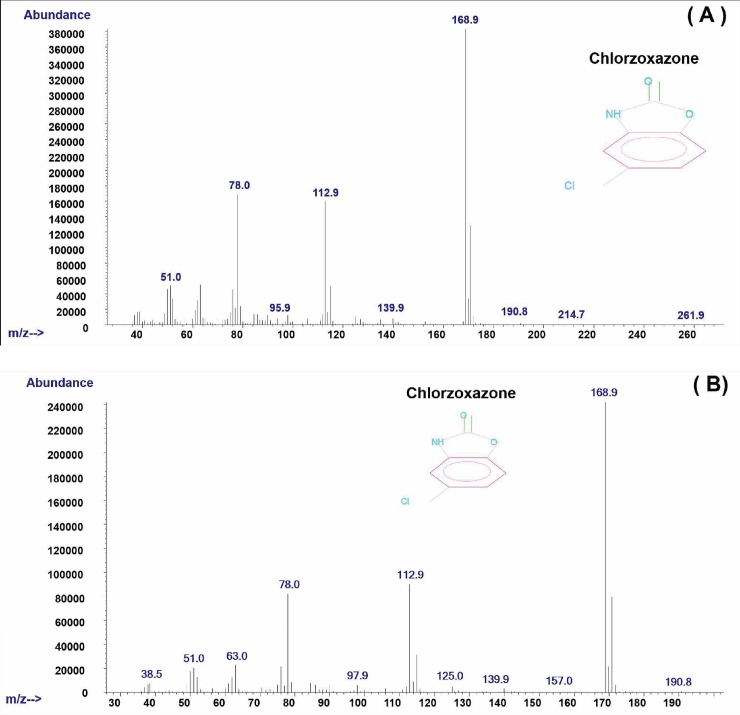
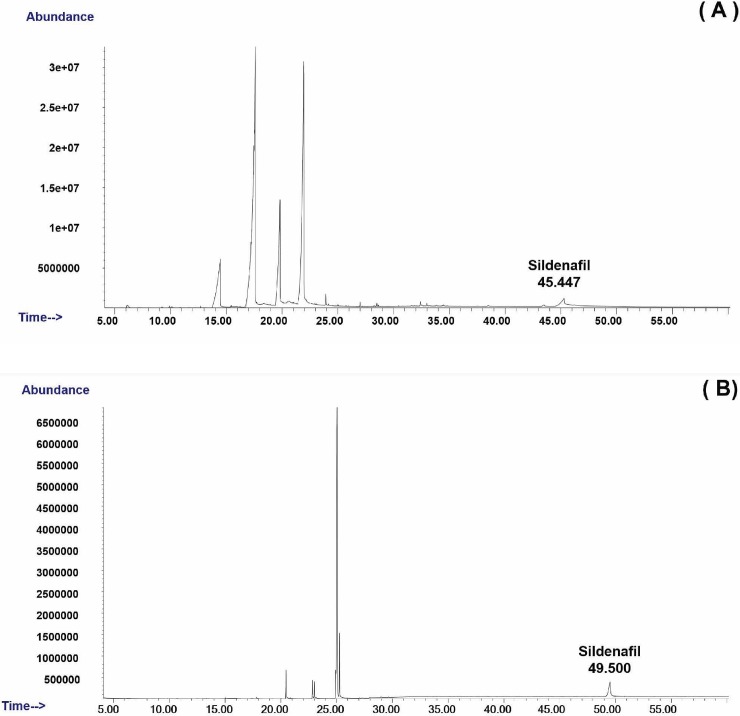
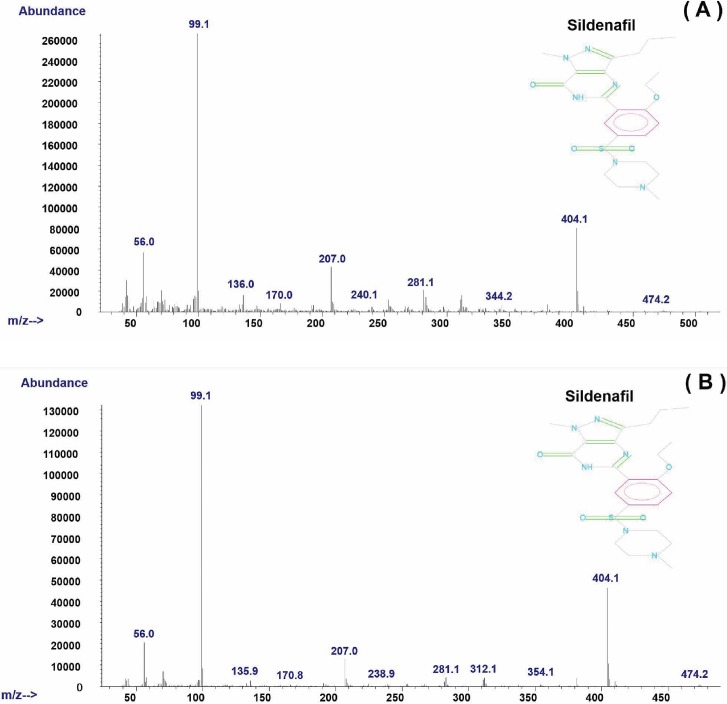
Fig 1 presents the chromatograms of the chlorzoxazone obtained when hydrogen (A) and helium (B) were used as the carrier gas at the retention times of 16.681 and 17.724 min. It can be observed that the use of hydrogen allowed for a reduction in the run-time analysis of 1.043 min and led to a slight peak tailing. However, the same mass spectrum of chlorzoxazone was found by GC-MS when hydrogen (A) and helium (B) were used as the carrier gas in Fig 2. Fig 3 shows a comparison of the chromatograms of sildenafil with hydrogen (A) and helium (B) at the retention times of 45.447 and 49.500 min. An elimination of 4.053 min in the run-time analysis was observed with the use of hydrogen. Similar mass spectra of hydrogen (A) and helium (B) as the carrier in gas are shown in Fig 4.
Fig 1.
Ion chromatogram of chlorzoxazone when (A) hydrogen and (B) helium is used as a carrier gas.
Fig 2.
Mass spectrum of chlorzoxazone when (A) hydrogen and (B) helium is used as a carrier gas.
Fig 3.
Ion chromatogram of sildenafil when (A) hydrogen and (B) helium is used as a carrier gas.
Fig 4.
Mass spectrum of sildenafil when (A) hydrogen and (B) helium is used as a carrier gas.
LOD
In considering the limit of detection (LOD), a signal-to-noise ratio of 3 was defined. The LODs of amitriptyline and 45 other analytes were 10 μg/g, and those of 104 analytes containing acetaminophen were 100 μg/g. The LOD of allopurinol was 500 μg/g, and those of the other 19 drugs—including aminotadalafil—were 1,000 μg/g. Moreover, the monitored ion library of the total analytes was developed for adulterant identification and monitoring. The 170 drugs obtain almost the same level of LODs with the use of hydrogen and helium as a carrier gas as detected by GC–MS for TCM and food supplements. Except for benzbromarone, estradiol benzoate, bezafibrate, mefenamic acid, oxymetholone, piperidenafil and cetilistat, the LODs of those analytes using hydrogen (100, 100, 1000, 1000, 1000, 1000 and 1000 μg/g, respectively) were decoupled as much as those of the previous analytes using helium (10, 10, 100, 100, 100, 100 and 100 μg/g, respectively).
Proficiency testing TFDA
Proficiency testing is another effective tool that can be used to ensure the results using hydrogen as a carrier gas for the detection of adulterated drugs in TCM and dietary supplements by GC–MS. Using the abovementioned detection method performed in testing for Chinese herbal medicine adulteration held by the TFDA revealed satisfactory results in 2015 and 2016. The analytical results were obtained using qualitative analysis. This result also provided the opportunity for the present method to verify the performance in testing for Chinese herbal medicine adulteration.
Analytical results of drugs in TCM and supplement foods on the market
In the present study, forty food supplement samples and eighty-three TCM samples were inspected and detected simultaneously with helium and hydrogen as a carrier gas by GC–MS between 2015 and 2016. Out of all 123 samples, 115 were found to be untainted, and the remaining 8 were TCM samples that were found to contain illegal, adulterated drugs (see in Table 3). The detected drug adulterants of 123 TCM and food supplement samples are shown in Table 4.
Table 3. Analytical results of TCM and food supplement samples.
| Type of sample | TCM | Food supplement | Total |
|---|---|---|---|
| Number of samples | 83 | 40 | 123 |
| Number of samples detected | 8 | 0 | 8 |
| Positive rate (%) | 9.6 | 0 | 6.5 |
Table 4. Detected drug frequency in TCM and food supplement samples.
| Drugs | Detected frequency |
|---|---|
| Acetaminophen | 3 |
| Chlorzoxazone | 3 |
| Ibuprofen | 3 |
| Sulfamethoxazole | 2 |
| Sildenafil | 2 |
| Tadalafil | 1 |
The types, forms and numbers of adulterated drugs in 8 TCM samples were 3 capsules, 3 powders, 1 paste and 1 medicated patch/plaster. The three capsule samples were detected with the kidney-supplement category of sildenafil or tadalafil. The three powder samples were all detected with the antirheumatic-analgesics category of acetaminophen, ibuprofen and chlorzoxazone. One paste and one medicated patch/plaster sample were detected with the antidote category of sulfamethoxazole. The analytical results were consistent with the use of hydrogen and helium as a carrier gas by GC-MS in the current study. In brief, the above evidence demonstrated the availability of the method with the use of hydrogen as a carrier gas for the detection of adulterated drugs in traditional Chinese medicine and dietary supplements using GC-MS for real sample analysis. The previous study also demonstrated the feasibility of using hydrogen as an alternative carrier gas, which has been in use for the routine analysis for government regulations for most estrogens, androgens and gestagens of the Belgium national plan [27].
Discussion
Hydrogen is a highly effective carrier gas because it increases the speed of the analysis and the resolution in GC [28]. Hydrogen offers the chromatographer a number of benefits, including increased speed, lower temperature separations, longer column life, fewer environmental concerns and greater availability [29]. However, some safety concerns are associated with the use of hydrogen cylinders, such as the following: cylinder handling and storage, the flammable nature of hydrogen and the variation in quality of the gas selection of the appropriate gas delivery equipment to ensure the system’s purity. In addition, hydrogen is flammable over a wide concentration range in air from 4% to 74.2% by volume, it has the highest burning velocity of any gas, and it can self-ignite due to very low ignition energy when expanding rapidly from high pressure [27]. As an alternative to cylinders, hydrogen generators provide a continuous source of high purity hydrogen and can eliminate many safety concerns over using hydrogen cylinders. Because hydrogen can be generated on demand, the volume of stored gas in a hydrogen generator is very small. Moreover, it has built-in safety features. In the case of a leak, the flow of hydrogen will be automatically shut down to ensure that it never reaches to the lower explosive limit. In addition to safety concerns, reactions in the ion source, the loss of functionality of the pumping system, and high background noise are also disadvantages of using hydrogen as a carrier gas [30].
Hydrogen is a reactive gas, and it might react with analytes under certain conditions. The major adverse effect of hydrogen is on the GC injector liner activation, which can catalytically degrade samples such as certain simple pesticides [31]. Chromatographers should avoid the use of chlorinated solvents with the hydrogen carrier gas because of the risk of hydrochloric acid (HCl) formation, which can affect the performance of the chromatographic system. All situations should be carefully evaluated when changing to hydrogen as a carrier gas. Furthermore, the ability to switch from He to H2 as a carrier gas will save money and time.
Although hydrogen seems to be an ideal gas for GC/MS and it offers important advantages over helium in terms of efficiency, resolution and the speed of analysis, some disadvantages should be mentioned. The study suggested that hydrogen as a carrier gas had excellent performance that was comparable to using helium for the nonpolar, nonreactive compounds. Thus, the most polar, reactive compounds displayed significantly lower responses with the hydrogen carrier gas. Furthermore, evidence demonstrated that nitrobenzene, which is one of the most reactive compounds, was reduced to aniline when using hydrogen as a carrier [32]. Hydrogen is a reactive compound that might hydrogenate unsaturated and aromatic compounds under certain conditions. Hydrogen reduces metal oxides at the ion source and exposes bare and highly active metal surfaces at the EI (and CI) ion source. Thus, many compounds are degraded at the ion source, lose their molecular ions and are harder to identify by the library [31]. Previous experiments showed that the baseline of the total ion chromatograms is elevated in hydrogen relative to that in helium. The obtained signal-to-noise ratio is poorer with hydrogen compared with helium via both a lower signal and higher noise. The S/N values are approximately 3-5-fold lower when hydrogen is used as a carrier gas compared to the results using helium. Decreased response factors for some analytes may result from chemical interactions with hydrogen in the MS ion source or other causes [32]. The lower signal-to-noise ratio of hydrogen might lead to the lower LOD in a certain compound. Among the 170 analytes studied, 7 drugs using hydrogen as the carrier gas provided an LOD ten times poorer than those using helium as the carrier gas. The same LOD was found for all other 163 drugs. The 7 drugs were benzbromarone, estradiol benzoate, bezafibrate, mefenamic acid, oxymetholone, piperidenafil and cetilistat. [33]. The structure suggested the potential reactivity of the 7 analytes with the hydrogen carrier gas. Therefore, recent work has reported on the unstable signal and reduced accuracies of the pesticides when hydrogen was used as carrier gas, and moreover some compounds were undetectable rather than those when helium was used as a carrier gas [34]. The research indicated that fragmentation patterns are similar whether helium or hydrogen is used as the carrier gas except nitrobenzene. Nitrobenzene can be reduced in the presence of hydrogen, thus resulting in the different fragmentation patterns and peak tailings [35]. Moreover, in general, more abundant fragmentation is observed, and higher relative abundances of the diagnostic ions for the identification of the components using hydrogen as a carrier gas. In the case of Py-GC, using H2 as a carrier gas may cause unwanted protonation or hydrogenation reactions, which may lead to difficulties in the library search when using existing MS libraries [36]. One must carefully consider the chemistry of specific analytes when changing to hydrogen as a carrier gas. The potential reactivity of analytes with the hydrogen carrier gas should be evaluated in the early stages of the method’s development.
In this study, an economical analytical method for the determination of adulterated drugs in traditional Chinese medicine and dietary supplements with GC-MS using hydrogen as a carrier gas was developed. In general, helium is considered to be the most widely used carrier gas for GC–MS analysis. However, the cost of helium is increasingly expensive due to its limited supply. Hydrogen is as an alternative GC-MS carrier gas. The hydrogen generator produces ultrahigh purity hydrogen through the electrolysis of deionized-distill water without cost and usage limits; moreover, there were no safety concerns associated with high pressure cylinders. Hydrogen is not only renewable, abundant and economical, but it also offers important advantages in terms of reduced run-times and performance benefits over helium [27]. The screening of all TCM and dietary supplements proved to be necessary for the detection of pharmaceutical substances to protect consumers from adverse reactions and side effects before the products are made available on the market. The screening of illegal drug adulteration using hydrogen instead of helium as a carrier gas has been in use for routine analysis in our laboratory for 2 years now. Eight products adulterated with acetaminophen, ibuprofen, chlorzoxazone, sulfamethoxazole, tadalafil and sildenafil substances that are prohibited in TCM were detected as the result of 123 TCM and food supplements’ screening. Satisfactory consistency between the hydrogen and helium spectra of illicit drugs in real sample analysis also demonstrates that hydrogen can be used effectively as a buffer gas in GC-MS.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Tainan City Government. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bodeker G, Kronenberg F. A Public Health Agenda for Traditional, Complementary, and Alternative Medicine. American Journal of Public Health. 2002;92(10):1582–91. PMC3221447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khazan M, Hedayati M, Kobarfard F, Askari S, Azizi F. Identification and Determination of Synthetic Pharmaceuticals as Adulterants in Eight Common Herbal Weight Loss Supplements. Iranian Red Crescent Medical Journal. 2014;16(3):e15344 10.5812/ircmj.15344 PMC4005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin M-C, Lin J-H, Wen K-C. Detection and Determination of Phenformin in Chinese Medicinal Capsules by GC-MS and HPLC. Journal of Food and Drug Analysis. 2001;9:139–44. [Google Scholar]
- 4.Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. Journal of Internal Medicine. 2002;252(2):107–13. 10.1046/j.1365-2796.2002.00999.x [DOI] [PubMed] [Google Scholar]
- 5.Ariburnu E, Uludag MF, Yalcinkaya H, Yesilada E. Comparative determination of sibutramine as an adulterant in natural slimming products by HPLC and HPTLC densitometry. Journal of Pharmaceutical and Biomedical Analysis. 2012;64–65:77–81. 10.1016/j.jpba.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.POPESCU AM, RADU GL. Detection of adulterants by FTIR and GC-MS in herbal slimming food supplements. UPB Scientific Bulletin, Series B: Chemistry and Materials Science. 2015;77:221–30. [Google Scholar]
- 7.Marcus DM, Grollman AP. Botanical Medicines—The Need for New Regulations. New England Journal of Medicine. 2002;347(25):2073–6. 10.1056/NEJMsb022858 . [DOI] [PubMed] [Google Scholar]
- 8.Ko RJ. Adulterants in Asian Patent Medicines. New England Journal of Medicine. 1998;339(12):847–. 10.1056/NEJM199809173391214 . [DOI] [PubMed] [Google Scholar]
- 9.Song F, Monroe D, El-Demerdash A, Palmer C. Screening for multiple weight loss and related drugs in dietary supplement materials by flow injection tandem mass spectrometry and their confirmation by liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2014;88:136–43. 10.1016/j.jpba.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Haneef J, Shaharyar M, Husain A, Rashid M, Mishra R, Siddique NA, et al. Analytical methods for the detection of undeclared synthetic drugs in traditional herbal medicines as adulterants. Drug Testing and Analysis. 2013;5(8):607–13. 10.1002/dta.1482 [DOI] [PubMed] [Google Scholar]
- 11.Method of Test for Adulterants in Chinese Medicine and Foods, (2014).
- 12.Kim SH, Lee J, Yoon T, Choi J, Choi D, Kim D, et al. Simultaneous determination of anti-diabetes/anti-obesity drugs by LC/PDA, and targeted analysis of sibutramine analog in dietary supplements by LC/MS/MS. Biomedical Chromatography. 2009;23(12):1259–65. 10.1002/bmc.1248 [DOI] [PubMed] [Google Scholar]
- 13.TSENG M-C, TSAI M-J, LIN J-H, WEN K-C. GC/MS analysis on anorectics adulterated in traditional chinese medicines. Journal of Food and Drug Analysis. 2000;8. [Google Scholar]
- 14.Mokhtar SU, Chin ST, Kee CL, Low MY, Drummer OH, Marriott PJ. Rapid determination of sildenafil and its analogues in dietary supplements using gas chromatography–triple quadrupole mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2016;121:188–96. 10.1016/j.jpba.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Tagami T, Takeda A, Asada A, Aoyama A, Doi T, Kajimura K, et al. Simultaneous Identification of Hydroxythiohomosildenafil, Aminotadalafil, Thiosildenafil, Dimethylsildenafil, and Thiodimethylsildenafil in Dietary Supplements Using High-Performance Liquid Chromatography-Mass Spectrometry. Food Hygiene and Safety Science (Shokuhin Eiseigaku Zasshi). 2013;54(3):232–6. 10.3358/shokueishi.54.232 [DOI] [PubMed] [Google Scholar]
- 16.Damiano F, Silva C, Gregori A, Vacondio F, Mor M, Menozzi M, et al. Analysis of illicit dietary supplements sold in the Italian market: identification of a sildenafil thioderivative as adulterant using UPLC–TOF/MS and GC/MS. Science and Justice. 2014;54(3):228–37. 10.1016/j.scijus.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Shi F, Guo C, Gong L, Li J, Dong P, Zhang J, et al. Application of a high resolution benchtop quadrupole-Orbitrap mass spectrometry for the rapid screening, confirmation and quantification of illegal adulterated phosphodiesterase-5 inhibitors in herbal medicines and dietary supplements. Journal of Chromatography A. 2014;1344:91–8. 10.1016/j.chroma.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Vaysse J, Balayssac S, Gilard V, Desoubdzanne D, Malet-Martino M, Martino R. Analysis of adulterated herbal medicines and dietary supplements marketed for weight loss by DOSY 1H-NMR. Food Additives & Contaminants: Part A. 2010;27(7):903–16. 10.1080/19440041003705821 [DOI] [PubMed] [Google Scholar]
- 19.Stypułkowska K, Błażewicz A, Maurin J, Sarna K, Fijałek Z. X-ray powder diffractometry and liquid chromatography studies of sibutramine and its analogues content in herbal dietary supplements. Journal of Pharmaceutical and Biomedical Analysis. 2011;56(5):969–75. 10.1016/j.jpba.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Phattanawasin P, Sotanaphun U, Sukwattanasinit T, Akkarawaranthorn J, Kitchaiya S. Quantitative determination of sibutramine in adulterated herbal slimming formulations by TLC-image analysis method. Forensic Science International. 2012;219(1):96–100. 10.1016/j.forsciint.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q, Cao Y, Cao Y, Chai Y, Lu F. Rapid on-site TLC–SERS detection of four antidiabetes drugs used as adulterants in botanical dietary supplements. Analytical and Bioanalytical Chemistry. 2014;406(7):1877–84. 10.1007/s00216-013-7605-7 [DOI] [PubMed] [Google Scholar]
- 22.Champagne AB, Emmel KV. Rapid screening test for adulteration in raw materials of dietary supplements. Vibrational Spectroscopy. 2011;55(2):216–23. 10.1016/j.vibspec.2010.11.009. [DOI] [Google Scholar]
- 23.Cheng HL, Tseng M-C, Tsai P-L, Her GR. Analysis of Synthetic chemical drugs in adulterated Chinese medicines by capillary electrophoresis/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2001;15(16):1473–80. 10.1002/rcm.396 [DOI] [PubMed] [Google Scholar]
- 24.Au AM, Ko R, Boo FO, Hsu R, Perez G, Yang Z. Screening Methods for Drugs and Heavy Metals in Chinese Patent Medicines. Bulletin of Environmental Contamination and Toxicology. 2000;65(1):112–9. 10.1007/s0012800102 [DOI] [PubMed] [Google Scholar]
- 25.Oliver BM, Bradley JG, Farrar H. Helium concentration in the Earth's lower atmosphere. Geochimica et Cosmochimica Acta. 1984;48(9):1759–67. 10.1016/0016-7037(84)90030-9. [DOI] [Google Scholar]
- 26.Muñoz-Guerra JA, Prado P, García-Tenorio SV. Use of hydrogen as a carrier gas for the analysis of steroids with anabolic activity by gas chromatography–mass spectrometry. Journal of Chromatography A. 2011;1218(41):7365–70. 10.1016/j.chroma.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Impens S, Wasch KD, Brabander HD. Determination of anabolic steroids with gas chromatography–ion trap mass spectrometry using hydrogen as carrier gas. Rapid Communications in Mass Spectrometry. 2001;15(24):2409–14. 10.1002/rcm.515 [DOI] [PubMed] [Google Scholar]
- 28.Nnaji CN, Williams KC, Bishop JM, Verbeck GF. Hydrogen as a GC/MS carrier and buffer gas for use in forensic laboratories. Science & Justice. 2015;55(3):162–7. 10.1016/j.scijus.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Bartram RJ, Froehlich P. Considerations on Switching from Helium to Hydrogen. LCGC North America. 2010;28(FEV):890–900. [Google Scholar]
- 30.Connor E. Using Hydrogen as a Carrier Gas for GC: Peak Scientific 2015. Available from: http://www.peakscientific.com/articles/using-hydrogen-as-a-carrier-gas-for-gc/. Accessed June 29, 2017. [Google Scholar]
- 31.Avivanalytical. Helium Shortage and Hydrogen as a Carrier Gas for GC-MS 2012. Available from: http://blog.avivanalytical.com/2012/10/helium-shortage-and-hydrogen-as-carrier.html. Accessed June 29, 2017.
- 32.Shimadzu. Evaluation of Hydrogen as a Carrier Gas for Gas Chromatography / Mass Spectrometry 2013. Available from: http://www.ssi.shimadzu.com/products/literature/gcms/GCMS-1303.pdf. Accessed June 29, 2017.
- 33.Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44(D1):D1202–13. Epub 2015/09/25. 10.1093/nar/gkv951 ; PubMed Central PMCID: PMCPMC4702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Zhou S. Feasibility investigation of hydrogen instead of helium as carrier gas in the determination of five organophosphorus pesticides by gas chromatography-mass spectrometry. Chinese journal of chromatography. 2015;33(1):52–7. [In Chinese, English abstract]. Epub 2015/05/12. . [DOI] [PubMed] [Google Scholar]
- 35.Zhao J, Guo Z, Liang X, Zhang X, Kang C. Study on the carrier gas used in GC-MS analysis. Chinese journal of chromatography. 2006;24(6):660 [In Chinese, English abstract]. Epub 2007/02/10. . [PubMed] [Google Scholar]
- 36.Watanabe A, Watanabe C, Freeman RR, Teramae N, Ohtani H. Hydrogenation Reactions during Pyrolysis-Gas Chromatography/Mass Spectrometry Analysis of Polymer Samples Using Hydrogen Carrier Gas. Analytical Chemistry. 2016;88(10):5462–8. 10.1021/acs.analchem.6b00892 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.