Abstract
Placebo analgesia is a robust experimental and clinical phenomenon. While our understanding of the mechanisms of placebo analgesia has developed rapidly, some central questions remain unanswered. Among the important questions is how placebo anal-gesia interacts with active analgesic effects. It is an assumption underlying double-blind randomized placebo-controlled trials (RCTs) that the true effect of a treatment can be determined by examining the effect of the active treatment arm and subtracting the response in the placebo group (“the assumption of additivity”). However, despite the importance of this assumption for the interpretation of RCTs, it has rarely been formally examined. This article reviews the assumption of additivity in placebo analgesia by examining studies employing factorial designs manipulating both the receipt of an active analgesic and instructions about the treatment being delivered. In reviewing the literature, we identified seven studies that allowed a test of additivity. Of these, four found evidence against additivity, while the remaining three studies found results consistent with additivity. While the limited available data are somewhat mixed, the evidence suggests that at least under some conditions the assumption of additivity does not hold in placebo analgesia. The concordance between mechanisms of the active analgesic and placebo analgesia may influence whether additivity occurs or not. However, more research using factorial designs is needed to disentangle the relationship between placebo analgesia and the active effect of analgesic treatments.
1. INTRODUCTION
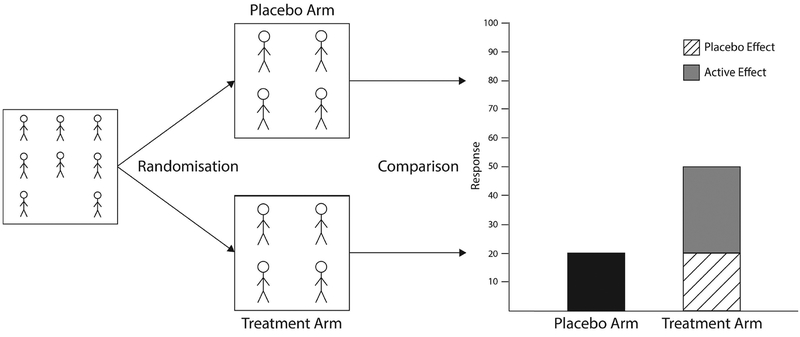
Double-blind randomized placebo-controlled trials (RCTs) remain the gold standard for testing the efficacy and safety of most medical treatments. The primary aim of an RCT is to isolate any improvement that is directly attributable to the specific agent being tested, above and beyond any improvement that may occur simply as a result of the treatment process itself, such as the placebo effect. The placebo effect is the portion of therapeutic improvement not directly attributable to the active effect of a treatment, instead being a product of contextual and psychobiological components following the receipt of a treatment (Benedetti, 2014). As shown in Fig. 1, RCTs aim to isolate treatment efficacy by comparing the active treatment with a matched placebo (placebo control) while keeping participants and researchers unaware of the participants’ treatment allocation (blinding). If the active treatment leads to greater improvement than the placebo, then the active treatment is deemed to be efficacious. If improvement is equivalent in the active treatment and placebo arms, then the active treatment is deemed not be efficacious.
Fig. 1.
A graphical depiction of a placebo-controlled RCT.
The assumption of additivity is central to RCT methodology. It entails that placebo and active treatment effects add onto each quantitatively and without interaction. In essence, this assumption means that a treatment effect and placebo effect should be entirely independent of each other. While the assumption is convenient for the purposes of RCTs, an increasing number of researchers examining the placebo effect have begun to challenge it on both theoretical and empirical grounds (Colagiuri, 2010; Enck, Klosterhalfen, Weimer, Horing, & Zipfel, 2011; Enck, Klosterhalfen, & Zipfel, 2011; Kirsch, 2000; Kube & Rief, 2017). If the assumption of additivity does not hold, then the difference between the active treatment and placebo arm will not represent the true efficacy of the active treatment.
1.1. Subadditivity Versus Superadditivity
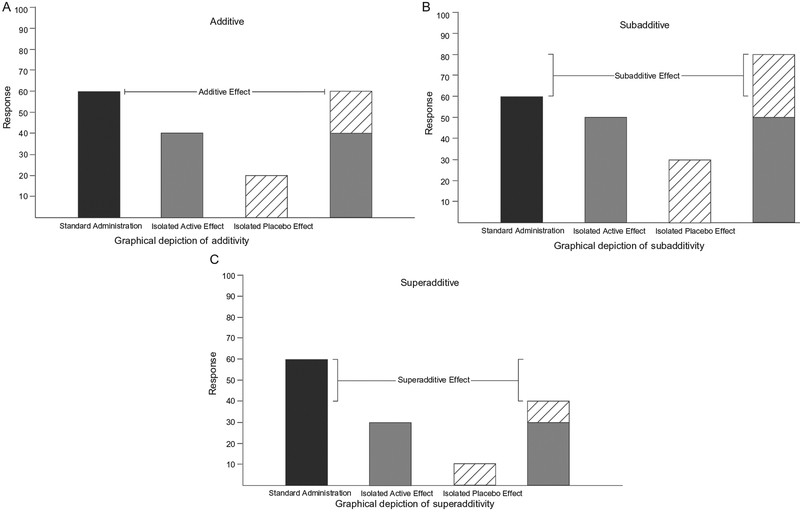
There are two possible nonadditive outcomes in terms of how placebo and active treatment effects may interact. The first is subadditivity (Tallarida, 2001), wherein the combined treatment effect is less than the summed magnitude of the isolated active treatment effect and isolated placebo effect (Fig. 2B). In the case of subadditivity, the relative difference between active treatment and placebo in an RCT may actually underestimate the efficacy of the active treatment (Kirsch, 2000; Kube & Rief, 2017). Hence, subadditivity may result in effective treatments being dismissed as ineffective. The second possibility is superadditivity (Tallarida, 2001), wherein the combined treatment effect is larger than the isolated active treatment effect and isolated placebo effect (Fig. 2C). Superadditivity could suggest a synergistic interaction between the active treatment and the placebo effect and may overestimate the efficacy of the active treatment in an RCT (Kube & Rief, 2017). Hence, superadditivity may result in treatments being adopted because they appear more effective than they actually are.
Fig. 2.
Graphical representations of (A) additive, (B) subadditive, and (C) superadditive outcomes. In these outcomes, the first column indicates treatment efficacy when administered under normal circumstances (i.e., an active treatment is administered with the full awareness of the recipient). In contrast, the following two columns depict the isolated active treatment and placebo effects and the fourth column their summed total.
1.2. Experimental Designs to Examine the Assumption of Additivity
For good ethical and practical reasons, RCTs are generally not equipped to test the assumption of additivity. Instead, additivity must be tested by a factorial design that manipulates both the treatment administered and the instructions delivered along with that treatment. Such factorial designs allow for the separation of both active treatment effects and placebo effects and importantly allow for a test of whether the two interact or not.
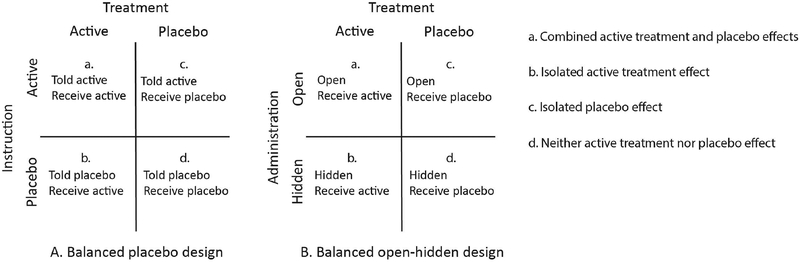
To date, the most common of such methods is the balanced placebo design, a 2 2 factorial design which manipulates the type of treatment (active versus placebo) and instructions (told active versus told placebo) (Enck, Klosterhalfen, & Zipfel, 2011; Fromme & Patel, 2010). As shown in Fig. 3A, this leads to four unique conditions: (a) receive active treatment and told active (combined active treatment and placebo effects), (b) receive active treatment and told placebo (isolated active treatment effect), (c) receive placebo treatment and told active (isolated placebo effect), and (d) receive placebo treatment and told placebo (neither active treatment nor placebo effect).
Fig. 3.
The four cells of the balanced placebo (A) and open-hidden (B) designs.
Another, more recent, design that has been used to examine placebo and drug effects is the open-hidden design, in which a drug is administered either overtly (open) or covertly (hidden, e.g., via automated intravenous infusion) in order to study its effect both with and without expectancy (Benedetti, Maggi, et al., 2003; Benedetti, Pollo, et al., 2003; Colloca, Lopiano, Lanotte, & Benedetti, 2004). A variant of this design, known as the balanced open-hidden design, compares active treatment and placebo treatment under both open and hidden administration (Atlas et al., 2012). Akin to the balanced placebo design and as shown in Fig. 3B, this paradigm has four possible conditions which allow for the interaction between active treatment and placebo effects to be examined: (a) open administration of active treatment (combined active treatment and placebo effect), (b) hidden administration of active treatment (isolated active treatment effect), (c) open administration of placebo (isolated placebo effect), and (d) hidden administration of placebo (neither active treatment nor placebo effect).
In both designs, a lack of interaction between treatment and instruction would indicate support for the assumption of additivity. Conversely, the presence of an interaction would suggest that the magnitude of the active treatment effect differs depending on the instructions accompanying it, and hence that the assumption of additivity is not supported.
1.3. Analgesia as a Model for Understanding Additivity
There are substantial differences between placebo effects in different modalities both in terms of underlying psychological and neurobiological mechanisms (Benedetti, 2008, 2009; Benedetti, Maggi, et al., 2003; Benedetti, Pollo, et al., 2003; Colagiuri, Schenk, Kessler, Dorsey, & Colloca, 2015; Colloca & Benedetti, 2005; Enck, Benedetti, & Schedlowski, 2008), as well as the degree of placebo effect itself (Hróbjartsson & Gøtzsche, 2010). Therefore, it is important to consider the question of additivity in different modalities separately because examining potential additive effects across a range of outcomes may introduce noise and conceal crucial modality-specific effects. Analgesia is a convenient and important condition to investigate additivity, not only because of the relatively well understood mechanisms of both pain and its exogenous modulation by analgesics, but also because placebo analgesia has been the most commonly studied type of placebo effect.
Numerous studies indicate that placebo treatment can ameliorate both experimental (Amanzio, Benedetti, Porro, Palermo, & Cauda, 2013) and clinical pain (Amanzio, Pollo, Maggi, & Benedetti, 2001; Benedetti, Amanzio, & Maggi, 1995; Pollo et al., 2001), with the placebo effect also comprising part of the therapeutic effect of analgesics (Benedetti, Maggi, et al., 2003; Benedetti, Pollo, et al., 2003; Bingel et al., 2011). Recent models propose that placebo analgesia is the result of expectancies induced via the treatment context, such as the verbal, social, and contextual cues surrounding the treatment (Colagiuri et al., 2015; Colloca & Benedetti, 2006, 2009; Stewart-Williams & Podd, 2004). Advances in psychopharmacology and neurobiology indicate that these expectancies trigger changes in the central nervous system that mediate placebo analgesia. Specifically, expectancies are thought to generate the placebo effect via the prefrontal cortices (PFC) (Amanzio et al., 2013; Petrovic et al., 2010). These higher order areas then engage a broad network of regions, notably the descending pain modulatory system which encompasses regions such as the rostral anterior cingulate cortex (ACC), amygdala, hypothalamus, and the periaqueductal grey (Bingel et al., 2011; Eippert et al., 2009; Tracey, 2010). Found in abundance throughout these regions are μ-opioid receptors (Baumgärtner et al., 2006; Wager, Scott, & Zubieta, 2007; Zubieta et al., 2005), which play a crucial role in inhibiting pain perception during placebo analgesia by influencing nociceptive processing at both the subcortical (Wager et al., 2004) and spinal levels in the rostral ventromedial medulla (RVM) (Basbaum & Fields, 1984; Faria, Fredrikson, & Furmark, 2008; Petrovic, Kalso, Petersson, & Ingvar, 2002). The central role of opioid transmission in placebo analgesia is further evidenced by the fact that the competitive opioid antagonist naloxone has been found to attenuate placebo analgesia (Benedetti, 1996; Grevert, Albert, & Goldstein, 1983; Levine, Gordon, & Fields, 1978).
Intriguingly, pharmacological conditioning with the nonsteroidal anti-inflammatory drug (NSAID) ketorolac produces analgesia that is only partially naloxone reversible (Amanzio & Benedetti, 1999). Instead, a placebo effect pre-conditioned in this manner has been found to be attenuated by the CB1 cannabinoid receptor antagonist rimonabant (Benedetti, Amanzio, Rosato, & Blanchard, 2011), indicating the involvement of the endocannabinoid system. This suggests that pharmacological conditioning procedures can alter the recruitment of different pain inhibitory systems during placebo analgesia.
1.4. Mechanisms of Common Analgesics
Pain is a highly heterogeneous condition, and many different types of anal-gesics exist. Summarizing all the various analgesics and their mechanisms of action is beyond the scope of this article; however, we briefly discuss some of the mechanistic differences here in order to highlight that additivity could be influenced by the classification of analgesic being investigated.
In the case of the centralized effect of opioid analgesics, the binding of opioid peptides to μ-opioid receptors throughout pain-processing and modulatory regions (Atlas et al., 2012; Bingel et al., 2011) acts upon pain perception both pre-synaptically and post-synaptically (Koneru, Satyanarayana, & Rizwan, 2009; Ossipov, Dussor, & Porreca, 2010; Snyder & Pasternak, 2003; Vaughan & Christie, 1997). Pre-synaptically this binding decreases the release of excitatory neurotransmitters from nociceptive neurons through hyperpolarization of the cell, leading to a reduction in evoked responses and spontaneous action potential discharge. Post-synaptically this cell hyperpolarization reduces the neurons responsiveness to excitatory input. Furthermore, administration of μ-opioid agonists activates OFF cells in the RVM (Fields, Vanegas, Hentall, & Zorman, 1983; Heinricher, Morgan, Tortorici, & Fields, 1994), while also inhibiting ON cell activity (Heinricher, Morgan, & Fields, 1992) leading to inhibition of ascending nociceptive afferents.
In contrast, other widely used analgesics have entirely distinct mechanisms of action, such as the inhibition of cyclooxygenase enzymes in NSAIDs (Seibert et al., 1994; Vane & Botting, 1996). The inhibition of cyclooxygenase enzymes suppresses prostaglandinformation, with prostaglandins contributing to inflammation through vasodilation (Green, 2001). In addition to this, prostaglandins also have pro-nociceptive effects through the sensitization of peripheral nociceptors, thus reducing the threshold to respond to excitatory input (Ito, Okuda-Ashitaka, & Minami, 2001; Omote et al., 2002). Prostaglandins have also been suggested to have some centralized effect by contributing to central sensitization and the synaptic excitability of spinal nociceptors (Minami et al., 2001; Seybold, 2009; Seybold, Jia, & Abrahams, 2003).
Another example of analgesics are sodium channel blockers such as lido-caine, which have entirely peripheral effects. These drugs modulate the voltage-gated sodium channels on the neuronal membrane, impairing the flow of sodium ions into the neuron during depolarization to a stimulus and preventing conduction or initiation of afferent nociceptive signals (Clare, Tate, Nobbs, & Romanos, 2000; Devor, 2006).
1.5. Potential Moderators of Additivity in Analgesia
The concordance between placebo analgesia and the mechanism of an anal-gesic is a plausible moderator of their additivity. For example, opioid treatments with similar opioid receptor affinities are generally observed to have additive outcomes (Taber, Greenhouse, Rendell, & Irwin, 1969; Tallarida, 2000), although these different opioids may still compete due to differences in receptor binding efficacy (Chen, Smith, Cahill, Cohen, & Fishman, 1993; Trescot, Datta, Lee, & Hansen, 2008). As such, endogenous opioid activity induced by placebo treatment might be either additive or nonadditive depending on the specific opioid receptor affinity of the opioid treatment. In the case of any competition, then one could expect subadditivity as endogenous and exogenous ligands vie to bind with a finite number of receptor sites.
The active effect of analgesics that act on different systems, such as NSAIDs and sodium channel blockers, may not compete with the placebo effect for opioid receptor binding and, as such, may allow for the endogenous and exogenous effects to both maximally contribute to analgesia. While in some cases this may lead to ceiling effects, and, as such, sub-additivity, the use of combination analgesics, wherein a number of analgesics with distinct mechanisms of action are administered together, is common in medicine and is often found to have beneficial effects over the individual treatments (Desmeules, Rollason, Piguet, & Dayer, 2003; Raffa, 2001). Indeed, synergistic effects are frequently observed between different analgesics and may provide some evidence for superadditivity (Christie, Connor, Vaughan, Ingram, & Bagley, 2000; Christie, Vaughan, & Ingram, 1999; Cichewicz, 2004; Miranda, Silva, & Pinardi, 2004; Tallarida, 2001; Vaughan, Ingram, Connor, & Christie, 1997), in which the distinct mechanisms by which the treatments have their effect interact to produce a greater degree of analgesia when administered together than when administered individually. Such evidence adds to the notion that additivity between active and placebo effects may vary based on the congruence or incongruence of their mechanisms.
Beyond the mechanistic similarity between the active treatment and placebo effect in pain, a number of other factors may also influence whether the assumption of additivity holds in particular studies. These may include the specific dosage of the analgesic, with larger dosages increasing the likelihood that ceiling effects may contribute to a nonadditive relationship. Another factor is the time between administration and outcome measurement, as both active treatment and placebo effects contribute to analgesia at different time points (Atlas et al., 2012; Gabka & Price, 1982). Such differences in methods between studies may lead to variability in their findings regarding additivity and should be considered when interpreting the results of a study.
2. CURRENT EVIDENCE
2.1. Available Studies Testing Additivity in Placebo Analgesia
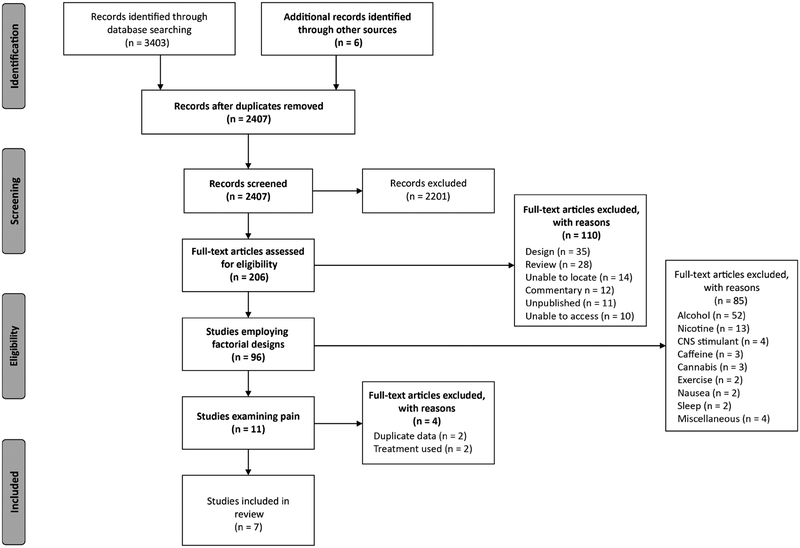
As mentioned above, despite the fact that there have been many studies exploring placebo analgesia, few studies have employed designs that allow for formal testing of additivity. To survey the literature, we conducted a systematic review (see Box 1 for search terms and selection criteria) to identify all balanced placebo designs and all balanced open-hidden designs in placebo analgesia, so that we could review the evidence for additivity. Fig. 4 shows the results of this search, in which only seven studies were found to have used one of these designs in placebo analgesia (Aslaksen, Zwarg, Eilertsen, Gorecka, & Bjørkedal, 2015; Atlas et al., 2012; Berna et al., 2017; Butcher & Carmody, 2012; Kam-Hansen et al., 2014; Lund, Vase, Petersen, Jensen, & Finnerup, 2014; Schenk, Sprenger, Geuter, & Buchel, € 2014).
BOX 1. Systematic Review Methods
Selection criteria
The principle selection criteria were the experimental design employed and the modality being investigated. Studies were required to manipulate receipt of an analgesic treatment, as indicated by the treatment itself and the outcomes, against at least two levels of expectations. These factors could be manipulated both within and between-subjects.
Search strategy
The protocol driven search strategy focused on the electronic search of four online databases, searching each from inception for articles in English:
-
-
EMBASE (1947 to December 2016)
-
-
PsychINFO (1887 to December 2016)
-
-
PUBMED (1966 to December 2016)
-
-
Web of Science (1864 to December 2016)
Registers were searched for “balanced placebo design,” with a specific search strategy of ‘balanced” AND “placebo” AND “design,” “open-hidden design” (“open” AND “hidden” AND “design”), “open hidden administration” (“open” AND “hidden” AND “administration”), “open hidden paradigm” (“open” AND “hidden” AND “paradigm”). The latter three search terms were also performed using hyphenated “open-hidden” variations. Preliminary searches indicated that synonyms and more general criteria for the balanced placebo design failed to contribute any additional studies employing this specific experimental design. Forward and backward search strategies were also employed following the initial screening of studies retrieved by the protocol driven search. Further, this process was also completed with any review papers retrieved by the protocol driven search. Members of the Society for Interdisciplinary Placebo Studies were also contacted requesting citations for studies that met the inclusion criteria.
Fig. 4.
Flow chart depicting the study selection process.
2.1.1. Study Characteristics
As can be seen from the study overview provided in Table 1, the seven identified studies were considerably heterogeneous with respect to the methods employed. Four of the seven studies used the balanced placebo design (Aslaksen et al., 2015; Butcher & Carmody, 2012; Kam-Hansen et al., 2014; Schenk et al., 2014), while two used the balanced open-hidden design (Atlas et al., 2012; Lund et al., 2014). The final study conducted by Berna et al. (2017) used a novel design here described as a “balanced active placebo design.” In this variation of the balanced placebo design, receipt of an active treatment or placebo are factorially crossed with administration of an active placebo or placebo, with the second factor replacing the instructions of treatment allocation typical of the balanced placebo design.
Table 1.
Study Overview
| Study | Sample | Modality | Treatment | Dosage | Administration | Classification | Design | Blinding | Measure | Stimulus |
|---|---|---|---|---|---|---|---|---|---|---|
| Aslaksen et al. (2015) | 93 | Pain | Lidocaine | 3.0 g | Topical | SCBa | BPDb | − | Self-report | Thermode |
| Atlas et al. (2012) | 14 | Pain | Remifentanil | 0.04 μg/kg/min | Intravenous | Opioid | BOHDc | − | Self-report | Thermode |
| Berna et al. (2017) | 100 | Pain | Diclofenac | 100 mg | Oral (pill) | NSAIDd | BAPDe | + | Self-report | Thermode |
| Butcher and Carmody (2012) | 20 | Pain | Ibuprofen | 800 mg | Oral (pill) | NSAID | BPD | + | Multiple outcomes | Electrical |
| Kam-Hansen et al. (2014) | 66 | Migraine | Maxalt | 10 mg | Oral (pill) | Triptan | BPD | NRf | Self-report | Migraine |
| Lund et al. (2014) | 46 | Pain | Lidocaine | 0.1 ml | Intramuscular | SCB | BOHD | + | Self-report | Hypertonic saline |
| Schenk et al. (2014) | 32 | Pain | Lidocaine/prilocaine | NR | Topical | SCB | BPD | + | Self-report | Thermode |
Sodium channel blocker.
Balanced placebo design.
Balanced open-hidden design
Nonsteroidal anti-inflammatory drug.
Balanced active placebo design.
Not reported
The analgesics employed in these studies also varied substantially. Three studies examined lidocaine, a sodium channel blocker (Aslaksen et al., 2015; Lund et al., 2014; Schenk et al., 2014), two examined NSAIDs, namely diclofenac and ibuprofen (Berna et al., 2017; Butcher & Carmody, 2012), one examined maxalt, a triptan used to treat migraines (Kam-Hansen et al., 2014), and one examined the opioid remifentanil (Atlas et al., 2012).
The time course of the treatment relative to outcome assessment was reported in most studies and varied between 13.5 min and 4 h (Aslaksen et al., 2015; Atlas et al., 2012; Berna et al., 2017; Butcher & Carmody, 2012; Kam-Hansen et al., 2014). The two exceptions were Lund et al. (2014) and Schenk et al. (2014), who did not report the time course of treatment administration and outcome assessment.
All but one of the studies examined experimentally-induced pain in healthy volunteers, with the exception being Kam-Hansen et al. (2014) who examined migraine pain in a clinical population. The selected studies also all measured pain intensity/unpleasantness through self-report, with one study also including pain threshold and tolerance (Butcher & Carmody, 2012).
2.1.2. Studies in Experimental Pain With Behavioral Outcomes
Butcher and Carmody (2012) employed a within-subjects balanced placebo design with the NSAID ibuprofen for experimental pain. Participants were either told they were receiving an active analgesic or placebo and then received either 800 mg ibuprofen or placebo orally. The study blinded researchers to both the participants’ treatment allocation and instructions and measured pain using self-report, pain threshold, and pain tolerance across four time points (1, 2, 3, and 4 h post-administration). In the originally reported study, Butcher and Carmody (2012) also analyzed male and female participants separately. Given that there is no a priori reason for performing these analyses separately, only a reanalysis performed on the entire sample is reported here. Despite using sound methods and a design with the potential to examine additivity across the treatment’s time course, this reanalysis failed to observe any main effects of treatment and instruction, or any interaction between the two across all pain outcomes.
Given the absence of an interaction, this study appears to provide evidence for additivity. However, the study also seems to find no effect of either ibuprofen or instructions on pain at all. More specifically, neither the active treatment nor instructions demonstrated any effect whatsoever on pain. Such results may be limited in terms of providing evidence for additivity. This is because the absence of any active treatment or placebo effects across conditions may have meant that the factorial manipulations were ineffective at inducing different levels of NSAID activity or expectancies, leaving no possibility for these different levels to interact. Therefore, the results of this particular study are only weakly in favor of additivity.
Lund et al. (2014) used a within-subjects balanced open-hidden design with intramuscular administration of lidocaine. The nature of the pain model, intramuscular injections of hypertonic saline, allowed for both open and hidden administration of lidocaine alongside the pain stimulus itself. Participants received either 0.1 mL lidocaine or a placebo intramuscularly either with or without information about the treatment being administered. The researcher administering the treatment was blinded to treatment allocation, but not instruction allocation, and self-reported pain was assessed. Further, a subset of the sample underwent pre-conditioning with lidocaine in order to maximize the likely placebo effect, however, no significant effect of pre-conditioning was reported.
The study observed both significant main effects of treatment and instruction, as well as an interaction between the two. In this case, post hoc simple effects analysis suggested that the interaction was indicative of a subadditive outcome. That is, the open administration of lidocaine was associated with less analgesic effect than the sum of the open administration of placebo and the hidden administration of lidocaine. These results, in the context of an RCT, would have underestimated the efficacy of lidocaine. Consistent with the strong analgesic effect of intramuscular lidocaine during both open and hidden administration, it may be that the more efficacious the medication, the more likely that ceiling effects will be observed, with these ceiling effects then contributing to a subadditive outcome.
2.1.3. Studies in Experimental Pain With Both Behavioral and Neuroimaging Outcomes
Only two of the studies found in our systematic search employed neuroim-aging methods, both of which used functional magnetic resonance imaging (fMRI) to examine analgesic effects (Atlas et al., 2012; Schenk et al., 2014).
Atlas et al. (2012) used a within-subjects balanced open-hidden design to examine the opioid agonist remifentanil. Participants received an intravenous infusion of remifentanil or placebo, administered either with or without information that the treatment was being provided. The study used target-controlled infusion to maintain a stable concentration of 0.04 μg/kg/min, with 13.5-min blocks from infusion onset and a 7-min washout period between conditions. The concentration was modeled for each participant to stay below the threshold for sedation to avoid contamination during hidden administration. Researchers were not blinded to treatment allocation, and self-reported pain was assessed across a range of calibrated stimulus intensities.
In terms of the behavioral outcomes, there was a main effect of both treatment and instructions, with pain ratings being lower following remifentanil compared with placebo and instructions that an analgesic was being administered also leading to lower pain ratings. In terms of additivity, the study observed no interaction between treatment and instructions, suggesting evidence in favor of additivity.
The fMRI data were subjected to pharmacokinetic modeling to estimate the relative contribution of active treatment and placebo effects and their potential interaction. As with the behavioral data, the neuroimaging data did not reveal any results indicative of an interaction between the active effect of remifentanil and placebo analgesia, instead finding several potential dissociations in site of action. Specifically, remifentanil produced widespread reductions in activity across pain-processing regions regardless of the presence of instructions. In contrast, knowledge of treatment administration was more closely related to activity in the PFC—one of the regions that is most commonly associated with placebo analgesia (Krummenacher, Candia, Folkers, Schedlowski, & Schönbächler, 2010; Petrovic et al., 2010). Further, the time course of neural activity also varied between the active effect of remifentanil and instruction effects. With the latter being observable from the onset of infusion prior to peak remifentanil concentration. Based on these findings, Atlas et al. (2012) concluded that the active effect of remifentanil and the placebo effect produce additive and dissociable effects in the brain.
Schenk et al. (2014) employed a within-subjects balanced placebo design examining a topical formulation of lidocaine and prilocaine. A clinical model of pain was imitated by pre-treating the treatment sites with capsaicin to cause sensitization to noxious thermal stimuli. Participants received either an unreported dose of a topical lidocaine and prilocaine formulation or placebo to four areas in a double-blind manner. Participants were then instructed that some sites contained active treatment and others placebo by computer-presented instructions within the fMRI scanner before stimulation of each site. Pain outcomes were assessed using self-report. The time course between administration and outcome assessment was not reported. The study found a main effect of treatment, but no statistically significant effect of instruction. However, there was a significant interaction effect between treatment and instruction, indicative of superadditivity. Planned simple effects found that a difference was observed between the administration of the formulation with and without information that it was the active treatment, while no difference was present between the placebo administered with and without information that it was an active treatment. Thus, Schenk et al. observed that the placebo effect only influenced pain outcomes when coupled with the active treatment. As such, this study’s data are inconsistent with the assumption of additivity.
In addition to interactions on the behavioral pain outcomes, Schenk et al.’s (2014) factorial design also revealed a number of patterns in the neuroimaging data. Specifically, greater neural activations were observed in the ACC and ventral striatum during the conditions that isolate the active treatment and placebo effects. Intriguingly, these effects observed in the ACC and ventral striatum appeared to be a product of the incongruence between treatment and expectations of treatment, rather than a synergistic effect. Both of these regions have also been implicated in prediction error processing (Carter et al., 1998; Hare, O’Doherty, Camerer, Schultz, & Rangel, 2008), a mechanism which has been previously suggested to play a role in the instigation of placebo analgesia (Petrovic et al., 2010; Scott et al., 2007). As such, the interactions observed by Schenk et al. (2014) appear to reflect other aspects of the balanced placebo design, that is, the incongruence between the expected and received treatment, rather than a synergistic effect between treatment and instructions on patterns of neural activity.
2.1.4. Studies With Modified Factorial Designs
Aslaksen et al. (2015) used a between-subjects variant of the balanced placebo design in which the instruction that a placebo was to be administered was replaced with information about the treatment having a hyperalgesic effect. In this manner, the study examined the effect of positive and negative instructions on the active effect of lidocaine versus placebo in a factorial design. While this study differs from other studies reviewed, its factorial design still allows the assumption of additivity to be tested. Aslaksen et al. (2015) also included two further conditions, one in which lidocaine was administered alongside no information of its specific medical properties and one where no treatment was provided. The results reported here are a reanalysis of the data including only the factorial conditions manipulating receipt of treatment (lidocaine, placebo) against instructions (analgesia, hyperalgesia).
Participants were given either 3.0 g of lidocaine or placebo topically with instructions of the treatment having either an analgesic or hyperalgesic effect. Self-reported pain outcomes were assessed 18 min after treatment administration, with researchers not being blinded to allocation. The study observed a main effect of instructions, such that those who were told to expect a hyperalgesic effect reported more pain compared to those who were told to expect an analgesic effect. There was no main effect of lidocaine on pain outcomes. However, there was an interaction between treatment and instructions, with inspection of the data suggesting that lidocaine led to reports of more (rather than less) pain in participants who were led to believe it had hyperalgesic properties.
The presence of an interaction effect indicates that the assumption of additivity in this study was not met. However, this study does not reflect a synergistic effect per se, so much as an attenuating influence of the nocebo effect on the active effect of lidocaine. The nocebo effect is the harmful counterpart to the placebo effect, where negative expectations (e.g., increased pain) lead to adverse events or a worsening of symptoms (Colloca & Miller, 2011; Enck et al., 2008; Häuser, Hansen, & Enck, 2012). The study suggests that instructions can significantly diminish the active effects of a medication such to render it ineffective. Such a finding contradicts the implicit assumptions of the additive model that expectations cannot interact with the active effect of a treatment (Kube & Rief, 2017).
The balanced active placebo design employed by Berna et al. (2017) differs substantially from the typical balanced placebo design. Their between-subjects 2 2 factorial design manipulated receipt of analgesic (diclofenac versus placebo) and side effect induction (atropine versus placebo). In this design, the side effects experienced as a result of the atropine were assumed to lead participants to believe that they had received the active analgesic, and as such were argued to be analogous to providing an instruction about treatment allocation in the balanced placebo design. Participants were told they would receive either active treatment or placebo and then received either 100 mg diclofenac or placebo alongside either 1.2 mg atro-pine or placebo in a double-blind manner. Self-reported pain was assessed 1 h after administration.
In this design, additivity may be tested by examining whether the difference in outcomes between the active treatment being administered with and without the active placebo is proportional to the difference between a placebo treatment being administered with and without the active placebo. If no difference is observed, then the increase in the placebo effect brought about by the active placebo combines with the active effect in an additive manner. Conversely, if a difference is observed then there is evidence that the increased placebo effect combines with the active effect in a nonadditive manner. Their results indicated that there were no main effects of analgesic treatment nor were there main effects of the atropine versus no atropine on pain, there was, however, an interaction between the two. By comparing the relative effect sizes of adding atropine either to diclofenac or to placebo, Berna et al. (2017) concluded that diclofenac was made substantially more effective by the addition of atropine, whereas the addition of atropine to the placebo did not increase the placebo effect. As such, this study provides evidence for superadditivity. Further mediation analysis suggested that the analgesic effect of diclofenac was mediated by subjective reports of xerostomia, and the associated beliefs about treatment assignment. That is, diclofenac only had an analgesic effect when participants experienced the side effects associated with atropine and that led them to have an expectation that they had been allocated to the active treatment condition. However, the study also failed to observe any difference in the degree of placebo effect between based on the presence of atropine. Such that, the presence of an analgesic agent was necessary for the side effect induction to induce greater placebo analgesia. As such, their results cast doubt on the assumption of additivity.
2.1.5. Studies With Behavioral Outcomes in Clinical Pain
Kam-Hansen et al. (2014) conducted the only study we could identify in a clinical model of pain. The researchers used the balanced placebo design to examine the effect of maxalt, a serotonin agonist, and the placebo effect on migraine-related pain. Using a within-subjects design, participants were given six labeled treatments which contained either 10 mg of maxalt or placebo to be taken over the course of their next six migraine episodes as a rescue medication. Four of these treatments were labeled either as maxalt or placebo. The remaining two were labeled in a double-blind manner, indicating to participants that it may be either maxalt or placebo, that is, participants were told that the medication could be either active or a placebo (i.e., RCT instructions). The order of these treatments was randomized between participants and blinding was not reported as the participant administered their own treatment and recorded their own outcome. Participants also recorded one untreated episode at the beginning of the study. The primary outcome was change in self-reported pain between 30 min after migraine onset and 2.5 h after onset. The study observed strong evidence for the assumption of additivity, finding main effects of both treatment and instruction, but no interaction between the two factors. The data suggested a robust reduction in migraine pain brought about by both the isolated effect of maxalt and the isolated the placebo effect, which additively combined when maxalt was administered alongside instructions that it was the active treatment.
2.1.6. Summary
Broadly speaking, behavioral findings regarding additivity were mixed (see Table 2 for a summary). Three studies found evidence that confirmed the assumption of additivity between active treatment and placebo effects (Atlas et al., 2012; Butcher & Carmody, 2012; Kam-Hansen et al., 2014), whereas the remaining four studies found evidence against the additivity assumption in the form of statistically significant treatment by expectancy interactions (Aslaksen et al., 2015; Berna et al., 2017; Lund et al., 2014; Schenk et al., 2014). However, the results suggested that where additivity was not supported, the direction of the effect was not consistent. That is, three of the four studies reported superadditive effects (Aslaksen et al., 2015; Berna et al., 2017; Schenk et al., 2014) and the remaining study found evidence of subadditivity (Lund et al., 2014). Similarly, the neuroimaging outcomes regarding additivity were also mixed. Here, one study observed additive patterns of activations between analgesic treatment and placebo effects, consistent with their behavioral findings (Atlas et al., 2012). The second study, in contrast, found nonadditive differences in neural activity between conditions representative of error processing from the incongruence between the expected and received treatment (Schenk et al., 2014). As such, while the neuroimaging outcomes reported by Schenk et al. (2014) do not appear to reflect the synergistic effect observed in their behavioral data, they still suggest that active treatment and placebo effects may nonadditively influence the neural signature of analgesia.
Table 2.
The Main Effects, Interactions, and Additive Outcomes of the Seven Selected Studies
| Study | Treatment | Treatment Effect | Instruction Effect | Interaction | Additivity |
|---|---|---|---|---|---|
| Aslaksen et al. (2015) | Lidocaine | ✖ | ✓ | ✓ | Superadditive |
| Atlas et al. (2012) | Remifentanil | ✓ | ✓ | ✖ | Additive |
| Berna et al. (2017) | Diclofenac | ✖ | ✖ | ✓ | Superadditive |
| Butcher and Carmody (2012) | Ibuprofen | ✖ | ✖ | ✖ | Additive |
| Kam-Hansen et al. (2014) | Maxalt | ✓ | ✓ | ✖ | Additive |
| Lund et al. (2014) | Lidocaine | ✓ | ✓ | ✓ | Subadditive |
| Schenk et al. (2014) | Lidocaine/prilocaine | ✓ | ✖ | ✓ | Superadditive |
A “✓” indicates a statistically significant effect or interaction (P <0.05), while a “✖” indicates an effect or interaction that failed to reach statistical significance (P >0.05).
3. IMPLICATIONS AND LIMITATIONS
Overall, the studies considered in this review suggest that additivity between analgesic and placebo effects does not always hold in pain. To this end, one important consideration is whether additivity between placebo analgesia and an analgesic treatment varies based on the pharmacology of the treatment in question. As previously discussed, research suggests that endogenous opioids are one of the primary systems by which the placebo effect inhibits pain (Levine et al., 1978; Wager et al., 2007; Zubieta et al., 2005), the same system on which opioid analgesics have their exogenous effect. In the studies we reviewed, the one study using exogenous opioids provided strong evidence for additivity with both behavioral and neuroim-aging outcomes (Atlas et al., 2012). This could suggest that exogenous and endogenous opioids may not compete with one another for μ-opioid receptor binding in such a manner as to affect their additive relationship. This finding is consistent with the pharmacology literature, where it is noted that active treatments with similar receptor agonisms tend to have additive outcomes (Taber et al., 1969; Tallarida, 2000). Despite this, given that this was the only study to have examined opioid analgesics, additional research is warranted to confirm that exogenous and endogenous opioids have additive outcomes. Further, as previously discussed, non-opioid mechanisms have been observed for placebo analgesia following conditioning with NSAIDs (Amanzio & Benedetti, 1999; Benedetti et al., 2011). While placebo anal-gesia still appears to be primarily mediated by endogenous opioids in the absence of pharmaceutical conditioning (Amanzio & Benedetti, 1999), the finding that non-opioid mechanisms can be engaged following pharmacological conditioning may have clinical relevance to additivity, where treatment history may influence the recruited mechanism of placebo analgesia.
In contrast, the results from studies using non-opioid analgesics largely failed to support additivity. Of the three studies examining the sodium channel blocker lidocaine, all three reported results that contradicted the assumption of additivity. One study using intravenous administration observed subadditivity (Lund et al., 2014), whereas the remaining two studies applying topical formulations found evidence for superadditivity (Aslaksen et al., 2015; Schenk et al., 2014). It is important to note that the superadditive outcome observed by Aslaksen et al. (2015) did not reflect a synergistic effect of the treatment and placebo effect, so much as an attenuation of lidocaine’s analgesic effect through instructions of hyperalgesia. Regarding the remaining two studies examining lidocaine, Lund et al. (2014) found strong evidence for subadditive outcomes between the active effect of lidocaine and placebo analgesia. Conversely, Schenk et al. (2014) observed super-additive outcomes between the active effect of lidocaine and the placebo effect. One explanation for the differences between these two studies may relate to the different formulations and methods of treatment administration and their relative strength, with Lund et al. (2014) administering lidocaine intravenously and Schenk et al. (2014) a formulation of lidocaine and prilocaine topically. The potent active effect of lidocaine observed across conditions by Lund et al. may have contributed to subadditive outcomes overall. Regardless of the nature of the additive relationship, both of these studies still suggest that the additive model is not supported in the case of lidocaine. Indeed, it is potentially more problematic for the interpretation of RCTs if the assumption of additivity is violated under some conditions, and not others, and in different directions.
Of the two studies that examined NSAIDs, Berna et al. (2017) observed evidence for superadditivity, while Butcher and Carmody (2012) observed some evidence for additivity. Both studies failed to observe any effect of treatment or instruction though, which may be especially problematic when considering the data from Butcher and Carmody (2012) who used a traditional balanced placebo design. The absence of any factorial effects in a traditional balanced placebo design suggests completely ineffective active treatment and instructional manipulations because it means that all outcomes were equivalent to participants being told they were given no treatment and receiving no treatment (cell d in Fig. 3A), which is effectively a natural history group. The absence of any effects above natural history would render a lack of interaction uninformative because there are simply no effects to interact. In contrast, Berna et al.’s (2017) use of a balanced active placebo is distinct because it does not contain a natural history group. The equivalent cell in their design is placebo treatment given under double-blind instructions, which has been shown to induce placebo effects (Colagiuri & Boakes, 2010; Kam-Hansen et al., 2014; Pollo et al., 2001). Their design means that an absence of a main effect of the analgesic does not necessarily mean that the treatment had no “absolute” effect (i.e., relative to natural history). Instead, it only means that the analgesic was not more effective than the placebo effect under double-blind instructions. Similarly, the absence of a main effect of side effect manipulation simply means that the active placebo failed to increase the placebo effect above and beyond benign placebo treatment. As such, both analgesic treatment and side effect/instruction effects could still feasibly present in this design despite the lack of main effects, allowing for their interaction. In light of this, Berna et al.’s (2017) study is likely more informative in terms of evidence for testing NSAIDs’ additivity and would suggest nonadditivity in the form of superadditivity, evidenced by a synergistic effect between the active effect of NSAIDs and placebo analgesia. Further research, however, is required to replicate the findings of Berna et al. (2017).
The one study examining a clinical sample in the present review—Kam-Hansen et al. (2014)—used a triptan in patients suffering from migraines. The study observed an additive effect of this treatment with the placebo effect. In contrast to the previously discussed mechanisms of placebo anal-gesia, less is known about the means by which the placebo effect may relieve the pain associated with migraines. The closest model currently is that of headaches, which appear to employ a similar mechanism to NSAIDs through the suppression of prostaglandins and thus inflammation (Benedetti, Durando, Giudetti, Pampallona, & Vighetti, 2015; Benedetti, Durando, & Vighetti, 2014). It should be noted, however, that these studies found that the placebo effect was either only effective in this manner after pre-conditioning (Benedetti et al., 2015) or in reducing the nocebo-provoked component of headaches (Benedetti et al., 2014), and so may not be a widely recruited mechanism for headaches generally. Despite the above evidence that a lack of correspondence between active and placebo effect mechanisms tends to produce nonadditive outcomes, the model of prostaglandin inhibition proposed here may suggest that different mechanisms produce additive outcomes in migraines. Further research is required to clarify the mechanisms of the placebo effect in migraines to better understand this outcome.
Variables such as treatment dosage and the time course in which outcomes are measured should also be considered for their potential influence on additivity. Treatment dosage, or, for that matter, route of administration, may influence additive outcomes by contributing to ceiling effects through strong isolated active treatment effects. An example of this may have been the subadditive outcome observed by Lund et al. (2014). In contrast, time course may lead studies to observe different additive outcomes depending on when outcomes are measured relative to the peak efficacy of both the active treatment and placebo effects. The small number of studies and the heterogeneity of analgesic medications employed in different studies makes extrapolating from such data difficult. Nonetheless, it remains that such information is important to the interpretation of future research and should be considered when designing, analyzing, and reporting studies.
In addition to observing a number of nonadditive outcomes in traditional factorial designs, Aslaksen et al.’s (2015) comparison of positive versus negative instructions suggests that expectations of increased pain can reduce the active effect of lidocaine. A similar finding has also been reported where the analgesic effect of remifentanil was attenuated by the instruction that no treatment had been administered (Bingel et al., 2011), though without other groups necessary to test additivity. An antithetic relationship has been observed between the opioid and cholecystokinin systems (Noble, Derrien, & Roques, 1993; Noble et al., 1999), the latter being the neuro-biological system underlying nocebo hyperalgesia (Benedetti, Amanzio, Casadio, Oliaro, & Maggi, 1997; Benedetti, Amanzio, Vighetti, & Asteggiano, 2006), which may account for the influence of negative expectations on opioid analgesia. However, this does not explain the attenuation of lidocaine observed by Aslaksen et al. (2015). Such findings may instead suggest that the mechanisms of hyperalgesia may amplify pain to such an extent as to reverse an analgesic’s observable effect. As such, even if further research added evidence to active and placebo analgesic effects being additive when comparing positive instructions to no treatment instructions, models of their relationship must be updated to account for the influence of negative expectations on active effects.
3.1. Limitations of Existing Research
Our review of the available literature also points to some important limitations to existing research. The first and the most obvious is the small number of studies that provide a test of additivity. The balanced placebo design was first described over 50 years ago (Ross, Krugman, Lyerly, & Clyde, 1962), and several reviews have since highlighted the importance of this design to clarify the assumptions underlying RCTs (Enck, Klosterhalfen, Weimer, et al., 2011; Enck, Klosterhalfen, & Zipfel, 2011; Kirsch, 2000; Kleijnen, de Craen, van Everdingen, & Krol, 1994). It is surprising therefore that so few studies have investigated the assumption of additivity in placebo analgesia—an assumption crucial to the interpretation of RCT outcomes. Despite placebo analgesia being the most widely examined and best understood modality of the placebo effect, to the best of our knowledge, only seven studies have been conducted that systematically manipulate both active treatment and instructions as to whether active treatment or placebo has been administered. Thus, there is currently limited data available to assess additivity.
3.1.1. Healthy Versus Clinical Samples and Ethical Considerations
Almost all (six out of seven) of the existing studies were conducted with healthy participants in acute experimental pain settings. This means that the results may not generalize to clinical patient population. Interestingly, the one study that used a clinical sample (Kam-Hansen et al., 2014) found evidence consistent with the additivity assumption. Of course, a single study cannot be taken as definitive evidence in favor of additivity in clinical outcomes. Instead, this further emphasizes the general lack of data to assess additivity. However, the lack of clinical studies assessing additivity may be due to ethical concerns. As noted by a number of reviews (Colagiuri, 2010; Enck, Klosterhalfen, Weimer, et al., 2011; Enck, Klosterhalfen, & Zipfel, 2011; Kube & Rief, 2017; Waring, 2008), the balanced placebo design poses ethical problems due to its reliance on deception. This is because the balanced placebo design entails two conditions in which participants are directly misinformed about the treatment they receive. Firstly, to establish the isolated treatment effect, participants are told that they received placebo despite receiving active treatment. Secondly, to establish the isolated placebo effect, participants who received placebo are told that they received active treatment. While the deception is necessary from a design perspective, it poses concerns, particularly in patients seeking treatment for pain-related problems, where it could be considered unethical to misinform the patients. The same issue also applies to the balanced open-hidden design, where participants are deceived in order to establish the isolated placebo effect.
Some of the ethical limitations of both the balanced placebo and open-hidden designs may be avoided in the recently developed “balanced active placebo design” (Berna et al., 2017). In this design, expectancies are manipulated by administering either an active placebo (with side effects) or a benign placebo. The principle being that the side effects brought about by the active placebo cause participants to believe they have been allocated to receive the active treatment. In this design, then, participants are all accurately and non-deceptively told that they may receive an active treatment or a placebo, which is ethically advantageous in terms of avoiding deception. However, the balanced active placebo design also introduces a new ethical problem, namely intentionally inducing side effects in participants. This might be particularly problematic for those participants who miss out on an active treatment and have side effects induced. Hence, it is questionable as to whether the balanced active placebo design provides a better ethical alternative than the traditional balanced placebo design.
3.1.2. Methodological and Statistical Concerns
Several of the available studies using the balanced placebo or other factorial designs had relatively small sample sizes. This means that these studies may have been statistically underpowered. This could be particularly problematic if the power required to detect a main effect differs from that required to detect an interaction. If so, then some of the studies may have been powered to detect an effect of either the active treatment or instructions, but not an interaction between the two. In addition to this, there are inherent problems with using null hypothesis significance testing (NHST) to test the additivity assumption in general. This is because positive evidence for additivity requires proving the null (i.e., that active treatment and placebo effects do not interact). NHST is limited in this sense because it can only provide evidence for or against an alternative hypothesis, the latter of which cannot be taken as evidence in favor of the null hypothesis (Blackwelder, 1982; Gallistel, 2009; Streiner, 2003). Hence, the reliance on NHST in the studies reviewed means that even the absence of a statistical interaction between active treatment and instruction using NHST cannot be taken as true evidence for additivity. Instead, Bayesian approaches would be needed to compare the evidence for the null versus alternative hypotheses. This is because Bayesian analysis can compare the evidence for and against two or more hypothesis (including a null hypotheses) and provides a numerical estimate of the evidence in favor one of these hypotheses relative to the other.
The balanced placebo and open-hidden designs are also subject to several issues concerning their methodology. First and foremost, these designs rely on an instructional manipulation, and, in the case of placebo analgesia at least, largely use subjective measures of self-report. In this manner, demand characteristics and response bias may contribute to the observed outcomes, such as participants reporting greater pain relief during the open conditions of the balanced open-hidden design as they believe this to be the desired outcome (Colagiuri, 2010; Colagiuri & Lovibond, 2013). Attempting to incorporate objective pain outcomes would obviously overcome this limitation, but pain, of course, is an inherently subjective experience and no good objective measures exist yet.
Necessary variations in the methods of a balanced placebo or open-hidden study based on the analgesic being examined may also introduce confounds. For example, the time course of peak analgesic effect varies between both different analgesics and the placebo effect (Atlas et al., 2012; Gabka & Price, 1982), with a study being designed to assess outcomes in relation to the analgesic being examined. This may lead to variability in additive outcomes, as studies involving different analgesics could assess pain at different time post-administration, and thus the placebo effect may be measured at different time points in relation to its peak efficacy. Ideally, outcomes should be measured across a range of time points to ensure additivity is assessed during both peak analgesic and placebo effects.
Similarly, different analgesics often require different methods of administration. A common notion in the placebo literature is that the means of administering a placebo alters the degree of placebo effect, with methods that a more complex, invasive, or intensive procedure being through to produce a greater response (Kaptchuk, Goldman, Stone, & Stason, 2000). As such, the degree of placebo effect itself may vary between different methods, introducing another source of potential variability. Indeed, the balanced open-hidden design is also often limited by the nature of the treatment administration, which must be feasibly given without the participants’ awareness. The most common method being intravenously through an infusion pump, although other novel methods such as intramuscularly administering the treatment alongside the pain stimulus have been developed (Lund et al., 2014). Not only is this a limitation of the design in that it requires the use of such invasive methods, but such intravenous methods have been shown to produce a stronger placebo effect than pills (de Craen, Tijssen, de Gans, & Kleijnen, 2000). As such, these studies may produce different additive outcomes due to the presence of stronger placebo effects. Such factors should be considered not only when designing a study, but also when interpreting the additive outcomes observed.
4. CONCLUDING REMARKS AND FUTURE DIRECTIONS
The major finding of this article is that the assumption of additivity between analgesics and placebo analgesia cannot be accepted as holding true under all circumstances. There are, however, currently insufficient studies to clearly determine the conditions under which placebo and active treatment effects are additive and those where they are not. Additional studies are required to replicate and extend the current body of evidence examining placebo analgesia using the balanced placebo and other factorial designs. Such research should also be statistically powered to detect an interaction between the two factors, rather than to detect an effect of the active treatment or instruction, and be cognizant of the general problems to do with NHST and providing positive evidence for a null effect. Indeed, the use of Bayesian analyses may prove to be a more reliable method of detecting additive or nonadditive outcomes. With additional studies, meta-analysis may provide further insight into the conditions under which an additive relationship between the active effect of analgesics and placebo analgesia are observed and those when they are not.
Despite the small number of studies, the concordance between mechanisms of the analgesic and placebo analgesia did appear to moderate the additive outcome. Further research is warranted using different classifications of analgesics within the same experimental design in order to provide further evidence for the existence of synergisms between active treatment effects and placebo analgesia, as well as to understand the different instances in which subadditive outcomes may occur. These studies must be carefully designed to take into account the different pharmacodynamics of the anal-gesics and placebo analgesia, ideally using identical methods of administration and a range of time points to assess outcomes.
Not only does this review highlight the surprising paucity of research examining additivity between analgesics and placebo analgesia, but it also indicates that additivity has not been extensively investigated in other health-related outcomes. Indeed, we could identify only 12 studies, which examined heterogeneous samples spanning from the treatment of attention deficit hyperactivity disorder with methylphenidate (Pelham, Hoza, Kipp, Gnagy, & Trane, 1997; Pelham et al., 2002) to the sedative effects of the antihistamine hydroxyzine (Hammami et al., 2016). Given that within a single modality the placebo effect may have different additive relationships with different treatments, the application of findings from one modality to another poses substantial problems. In this manner, studies from other modalities would provide little insight into the nature of additivity between analgesics and placebo analgesia, nor can the findings from this review about pain be readily applied to the relationship between the placebo effect and treatments for other conditions. As such, the current review draws attention not only to the need for further research examining additivity in pain, but also for other health-related outcomes. In particular, examining additivity in outcomes with notably strong placebo effects, such as nausea (Quinn & Colagiuri, 2014) or sleep disturbance (Yeung, Sharpe, Glozier, Hackett, & Colagiuri, 2018) may prove to be particularly consequential for the interpretation of RCTs in these conditions.
The assumption of additivity states that the placebo effect and the active effect of a treatment are quantitatively additive and allows for the active effect to be measured by comparing the active treatment against a placebo control. Together, the studies discussed in this review suggest that there is reasonable evidence that the assumption of additivity does not hold under at least some conditions in pain. The fact that the nature of the nonadditive effect did not appear to be consistent across studies is also potentially more problematic for the interpretation of RCTs. This is because subadditive and superadditive outcomes would lead to very different interpretations of RCT data. This variation may make interpreting RCT data more difficult, as a conservative approach according to one nonadditive outcome cannot necessarily be taken. There was also some evidence that the pharmacology of the analgesic may moderate additivity, however, more rigorous research directly comparing different analgesics using appropriately designed and controlled studies are required. Finally, it was interesting to note that there was only a single study conducted in a clinical population, hence one can question whether these laboratory-based studies would generalize to patient samples. Nonetheless, our review draws attention to the overall dearth of research on pain capable of testing the assumption of additivity. Given the integral nature of this assumption to the interpretation of RCT data, there is an urgent need for more studies to be conducted using either the balanced placebo, active placebo, or open-hidden designs on a wide range of treatments in different conditions.
ACKNOWLEDGMENTS
The authors would like to extend their appreciation to the members of the Society for Interdisciplinary Placebo Studies for suggesting studies for this review to consider, as well as to Dr. Per Aslaksen and Dr. Belinda Butcher for taking the time to reanalyze their data for inclusion in this review.
REFERENCES
- Amanzio M, & Benedetti F (1999). Neuropharmacological dissection of placebo analgesia: Expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of Neuroscience, 19, 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F, Porro CA, Palermo S, & Cauda F (2013). Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Human Brain Mapping, 34(3), 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Pollo A, Maggi G, & Benedetti F (2001). Response variability to analgesics: A role for nonspecific activation of endogenous opioids. Pain, 90, 205–215. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Zwarg ML, Eilertsen HIH, Gorecka MM, & Bjørkedal E (2015). Opposite effects of the same drug: Reversal of topical analgesia by nocebo information. Pain, 156(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, & Wager TD (2012). Dissociable influences of opiates and expectations on pain. Journal of Neuroscience, 32(23), 8053–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, & Fields HL (1984). Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience, 7, 309–338. [DOI] [PubMed] [Google Scholar]
- Baumgärtner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, et al. (2006). High opiate receptor binding potential in the human lateral pain system. NeuroImage, 30(3), 692–699. [DOI] [PubMed] [Google Scholar]
- Benedetti F (1996). The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain, 64(3), 535–543. [DOI] [PubMed] [Google Scholar]
- Benedetti F (2008). Mechanisms of placebo and placebo-related effects across diseases and treatments. Annual Review of Pharmacology and Toxicology, 48, 33–60. [DOI] [PubMed] [Google Scholar]
- Benedetti F (2009). Placebo effects: Understanding the mechanisms in health and disease.New York: Oxford University Press. [Google Scholar]
- Benedetti F (2014). Placebo effects: From the neurobiological paradigm to translational implications. Neuron, 84(3), 623–637. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Casadio C, Oliaro A, & Maggi G (1997). Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain, 71, 135–140. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, & Maggi G (1995). Potentiation of placebo analgesia by proglumide. The Lancet, 346, 1231. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Rosato R, & Blanchard C (2011). Nonopioid placebo anal-gesia is mediated by CB1 cannabinoid receptors. Nature Medicine, 17(10), 1228. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, & Asteggiano G (2006). The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. The Journal of Neuroscience, 26(46), 12014–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Durando J, Giudetti L, Pampallona A, & Vighetti S (2015). High-altitude headache: The effects of real vs sham oxygen administration. Pain, 156(11), 2326–2336. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Durando J, & Vighetti S (2014). Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain, 155(5), 921–928. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Maggi G, Lopiano L, Lanotte M, Rainero I, Vighetti S, et al. (2003). Open versus hidden medical treatments: The patient’s knowledge about a therapy affects the therapy outcome. Prevention & Treatment, 6(1), 1a. [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, & Rainero I (2003). Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. Journal of Neuroscience, 23(10), 4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna C, Kirsch I, Zion SR, Lee YC, Jensen KB, Sadler P, et al. (2017). Side effects can enhance treatment response through expectancy effects: An experimental analgesic randomized controlled trial. Pain, 158(6), 1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh R, Ploner M, & Tracey I (2011). The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Science Translational Medicine, 3(70), 70ra14. [DOI] [PubMed] [Google Scholar]
- Blackwelder WC (1982). “Proving the null hypothesis” in clinical trials. Controlled Clinical Trials, 3(4), 345–353. [DOI] [PubMed] [Google Scholar]
- Butcher BE, & Carmody JJ (2012). Sex differences in analgesic response to ibuprofen are influenced by expectancy: A randomized, crossover, balanced placebo-designed study. European Journal of Pain, 16(7), 1005–1013. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, & Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280(5364), 747–749. [DOI] [PubMed] [Google Scholar]
- Chen JC, Smith ER, Cahill M, Cohen R, & Fishman JB (1993). The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sciences, 52(4), 389–396. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Connor M, Vaughan CW, Ingram SL, & Bagley EE (2000). Cellular actions of opioids and other analgesics: Implications for synergism in pain relief. Clinical and Experimental Pharmacology and Physiology, 27(7), 520–523. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Vaughan CW, & Ingram SL (1999). Opioids, NSAIDs and 5-lipoxygenase inhibitors act synergistically in brain via arachidonic acid metabolism. Inflammation Research, 48(1), 1–4. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL (2004). Synergistic interactions between cannabinoid and opioid analgesics. Life Sciences, 74(11), 1317–1324. [DOI] [PubMed] [Google Scholar]
- Clare JJ, Tate SN, Nobbs M, & Romanos MA (2000). Voltage-gated sodium channels as therapeutic targets. Drug Discovery Today, 5(11), 506–520. [DOI] [PubMed] [Google Scholar]
- Colagiuri B (2010). Participant expectancies in double-blind randomized placebo-controlled trials: Potential limitations to trial validity. Clinical Trials, 7(3), 246–255. [DOI] [PubMed] [Google Scholar]
- Colagiuri B, & Boakes RA (2010). Perceived treatment, feedback, and placebo effects in double-blind RCTs: An experimental analysis. Psychopharmacology, 208(3), 433–441. [DOI] [PubMed] [Google Scholar]
- Colagiuri B, & Lovibond PF (2013). Psychological processes that can bias responses to placebo treatment for pain In Colloca L, Flaten MA, & Meissner K (Eds.), Placebo and pain: From bench to bedside: Elsevier Inc. Chapters. [Google Scholar]
- Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, & Colloca L (2015). The placebo effect: From concepts to genes. Neuroscience, 307, 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, & Benedetti F (2005). Placebos and painkillers: Is mind as real as matter? Nature Reviews. Neuroscience, 6(7), 545. [DOI] [PubMed] [Google Scholar]
- Colloca L, & Benedetti F (2006). How prior experience shapes placebo analgesia. Pain, 124(1), 126–133. [DOI] [PubMed] [Google Scholar]
- Colloca L, & Benedetti F (2009). Placebo analgesia induced by social observational learning. Pain, 144(1–2), 28–34. [DOI] [PubMed] [Google Scholar]
- Colloca L, Lopiano L, Lanotte M, & Benedetti F (2004). Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. The Lancet. Neurology, 3(11), 679–684. [DOI] [PubMed] [Google Scholar]
- Colloca L, & Miller FG (2011). The nocebo effect and its relevance for clinical practice. Psychosomatic Medicine, 73(7), 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Craen AJ, Tijssen JGP, de Gans J, & Kleijnen J (2000). Placebo effect in the acute treatment of migraine: Subcutaneous placebos are better than oral placebos. Journal of Neurology, 247(3), 183–188. [DOI] [PubMed] [Google Scholar]
- Desmeules J, Rollason V, Piguet V, & Dayer P (2003). Clinical pharmacology and rationale of analgesic combinations. European Journal of Anaesthesiology, 20(S28), 7–12. [PubMed] [Google Scholar]
- Devor M (2006). Sodium channels and mechanisms of neuropathic pain. The Journal of Pain, 7(1), S3–S12. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron, 63(4), 533–543. [DOI] [PubMed] [Google Scholar]
- Enck P, Benedetti F, & Schedlowski M (2008). New insights into the placebo and nocebo responses. Neuron, 59(2), 195–206. [DOI] [PubMed] [Google Scholar]
- Enck P, Klosterhalfen S, Weimer K, Horing B, & Zipfel S (2011). The placebo response in clinical trials: More questions than answers. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366(1572), 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enck P, Klosterhalfen S, & Zipfel S (2011). Novel study designs to investigate the placebo response. BMC Medical Research Methodology, 11(1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria V, Fredrikson M, & Furmark T (2008). Imaging the placebo response: A neurofunctional review. European Neuropsychopharmacology, 18(7), 473–485. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, & Zorman G (1983). Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature, 306, 684–686. [DOI] [PubMed] [Google Scholar]
- Fromme K, & Patel AB (2010). Balanced placebo design In Stolerman IP (Ed.), Encyclopedia of psychopharmacology. Berlin, Heidelberg: Springer. [Google Scholar]
- Gabka J, & Price RK (1982). Tooth pulp stimulation: A method of determining the anal-gesic efficacy of meptazinol in man. British Journal of Clinical Pharmacology, 14(1), 104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR (2009). The importance of proving the null. Psychological Review, 116(2), 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green GA (2001). Understanding NSAIDs: From aspirin to COX-2. Clinical Cornerstone, 3(5), 50–59. [DOI] [PubMed] [Google Scholar]
- Grevert P, Albert LH, & Goldstein A (1983). Partial antagonism of placebo analgesia by naloxone. Pain, 16(2), 129–143. [DOI] [PubMed] [Google Scholar]
- Hammami MM, Hammami S, Al-Swayeh R, Al-Gaai E, Farah FA, & De Padua SJ (2016). Drug*placebo interaction effect may bias clinical trials interpretation: Hybrid balanced placebo and randomized placebo-controlled design. BMC Medical Research Methodology, 16(1), 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, & Rangel A (2008). Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience, 28(22), 5623–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser W, Hansen E, & Enck P (2012). Nocebo phenomena in medicine: Their relevance in everyday clinical practice. Deutsches Ärzteblatt International, 109(26), 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, & Fields HL (1992). Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience, 48(3), 533–543. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, & Fields HL (1994). Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventrome-dial medulla. Neuroscience, 63(1), 279–288. [DOI] [PubMed] [Google Scholar]
- Hróbjartsson A, & Gøtzsche PC (2010). Placebo interventions for all clinical conditions. The Cochrane Database of Systematic Reviews, (1), CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Okuda-Ashitaka E, & Minami T (2001). Central and peripheral roles of prostaglandins in pain and their interactions with novel neuropeptides nociceptin and nocistatin. Neuroscience Research, 41(4), 299–332. [DOI] [PubMed] [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, et al. (2014). Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science Translational Medicine, 6(218), 218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Goldman P, Stone DA, & Stason WB (2000). Do medical devices have enhanced placebo effects? Journal of Clinical Epidemiology, 53(8), 786–792. [DOI] [PubMed] [Google Scholar]
- Kirsch I (2000). Are drug and placebo effects in depression additive? Biological Psychiatry, 47(8), 733–735. [DOI] [PubMed] [Google Scholar]
- Kleijnen J, de Craen AJ, van Everdingen J, & Krol L (1994). Placebo effect in double-blind clinical trials: A review of interactions with medications. The Lancet, 344(8933), 1347–1349. [DOI] [PubMed] [Google Scholar]
- Koneru A, Satyanarayana S, & Rizwan S (2009). Endogenous opioids: Their physiological role and receptors. Global Journal of Pharmacology, 3(3), 149–153. [Google Scholar]
- Krummenacher P, Candia V, Folkers G, Schedlowski M, & Schönbächler G (2010). Prefrontal cortex modulates placebo analgesia. Pain, 148(3), 368–374. [DOI] [PubMed] [Google Scholar]
- Kube T, & Rief W (2017). Are placebo and drug-specific effects additive? Questioning basic assumptions of double-blinded randomized clinical trials and presenting novel study designs. Drug Discovery Today, 22(4), 729–735. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, & Fields HL (1978). The mechanisms of placebo analgesia. Lancet, 2, 654–657. [DOI] [PubMed] [Google Scholar]
- Lund K, Vase L, Petersen GL, Jensen TS, & Finnerup NB (2014). Randomised controlled trials may underestimate drug effects: Balanced placebo trial design. PLoS One, 9(1), e84104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Nakano H, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, et al. (2001). Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. British Journal of Pharmacology, 133(3), 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda HF, Silva E, & Pinardi G (2004). Synergy between the antinociceptive effects of morphine and NSAIDs. Canadian Journal of Physiology and Pharmacology, 82(5), 331–338. [DOI] [PubMed] [Google Scholar]
- Noble F, Derrien M, & Roques BP (1993). Modulation of opioid antinociception by CCK at the supraspinal level: Evidence of regulatory mechanisms between CCK and enkephalin systems in the control of pain. British Journal of Pharmacology, 109(4), 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, et al. (1999). International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacological Reviews, 51(4), 745–781. [PubMed] [Google Scholar]
- Omote K, Kawamata T, Nakayama Y, Yamamoto H, Kawamata M, & Namiki A (2002). Effects of a novel selective agonist for prostaglandin receptor subtype EP4 on hyperalgesia and inflammation in monoarthritic model. Anesthesiology: The Journal of the American Society of Anesthesiologists, 97(1), 170–176. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, & Porreca F (2010). Central modulation of pain. The Journal of Clinical Investigation, 120(11), 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Hoza B, Kipp HL, Gnagy EM, & Trane ST (1997). Effects of methylphenidate and expectancy on ADHD children’s performance, self-evaluations, persistence, and attributions on a cognitive task. Experimental and Clinical Psychopharmacology, 5(1), 3. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Hoza B, Pillow DR, Gnagy EM, Kipp HL, Greiner AR, et al. (2002). Effects of methylphenidate and expectancy on children with ADHD: Behavior, academic performance, and attributions in a summer treatment program and regular classroom settings. Journal of Consulting and Clinical Psychology, 70(2), 320. [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, & Ingvar M (2010). A prefrontal non-opioid mechanism in placebo analgesia. Pain, 150(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, & Ingvar M (2002). Placebo and opioid analgesia—Imaging a shared neuronal network. Science, 295(5560), 1737–1740. [DOI] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, & Benedetti F (2001). Response expectancies in placebo analgesia and their clinical relevance. Pain, 93, 77–84. [DOI] [PubMed] [Google Scholar]
- Quinn VF, & Colagiuri B (2014). Placebo interventions for nausea: A systematic review. Annals of Behavioral Medicine, 49(3), 449–462. [DOI] [PubMed] [Google Scholar]
- Raffa RB (2001). Pharmacology of oral combination analgesics: Rational therapy for pain. Journal of Clinical Pharmacy and Therapeutics, 26(4), 257–264. [DOI] [PubMed] [Google Scholar]
- Ross S, Krugman AD, Lyerly SB, & Clyde ADJ (1962). Drugs and placebos: A model design. Psychological Reports, 10(2), 383–392. [Google Scholar]
- Schenk LA, Sprenger C, Geuter S, & Büchel C (2014). Expectation requires treatment to boost pain relief: An fMRI study. Pain, 155(1), 150–157. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, & Zubieta JK (2007). Individual differences in reward responding explain placebo-induced expectations and effects. Neuron, 55(2), 325–336. [DOI] [PubMed] [Google Scholar]
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. (1994). Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proceedings of the National Academy of Sciences of the United States of America, 91(25), 12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS (2009). The role of peptides in central sensitization In Canning B & Spina D (Eds.), Sensory nerves. Handbook of experimental pharmacology. Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Seybold VS, Jia YP, & Abrahams LG (2003). Cyclo-oxygenase-2 contributes to central sensitization in rats with peripheral inflammation. Pain, 105(1–2), 47–55. [DOI] [PubMed] [Google Scholar]
- Snyder SH, & Pasternak GW (2003). Historical review: Opioid receptors. Trends in Pharmacological Sciences, 24(4), 198–205. [DOI] [PubMed] [Google Scholar]
- Stewart-Williams S, & Podd J (2004). The placebo effect: Dissolving the expectancy versus conditioning debate. Psychological Bulletin, 130(2), 324. [DOI] [PubMed] [Google Scholar]
- Streiner DL (2003). Unicorns do exist: A tutorial on “proving” the null hypothesis. The Canadian Journal of Psychiatry, 48(11), 756–761. [DOI] [PubMed] [Google Scholar]
- Taber RI, Greenhouse DD, Rendell JK, & Irwin S (1969). Agonist and antagonist interactions of opioids on acetic acid-induced abdominal stretching in mice. Journal of Pharmacology and Experimental Therapeutics, 169(1), 29–38. [PubMed] [Google Scholar]
- Tallarida RJ (2000). Drug synergism and dose-effect data analysis. CRC Press. [Google Scholar]
- Tallarida RJ (2001). Drug synergism: Its detection and applications. Journal of Pharmacology and Experimental Therapeutics, 298(3), 865–872. [PubMed] [Google Scholar]
- Tracey I (2010). Getting the pain you expect: Mechanisms of placebo, nocebo and reappraisal effects in humans. Nature Medicine, 16, 1277–1283. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, & Hansen H (2008). Opioid pharmacology. Pain Physician, 11, S133–S153. [PubMed] [Google Scholar]
- Vane JR, & Botting RM (1996). Mechanism of action of anti-inflammatory drugs. Scandinavian Journal of Rheumatology, 25, 9–21. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, & Christie MJ (1997). Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. The Journal of Physiology, 498, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, & Christie MJ (1997). How opioids inhibit GABA-mediated neurotransmission. Nature, 390(6660), 611. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. (2004). Placebo-induced changes in FMRI in the anticipation and experience of pain. Science, 303(5661), 1162–1167. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, & Zubieta JK (2007). Placebo effects on human μ-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring DR (2008). The antidepressant debate and the balanced placebo trial design: An ethical analysis. International Journal of Law and Psychiatry, 31(6), 453–462. [DOI] [PubMed] [Google Scholar]
- Yeung V, Sharpe L, Glozier N, Hackett ML, & Colagiuri B (2018). A systematic review and meta-analysis of placebo versus no treatment for insomnia symptoms. Sleep Medicine Reviews, 38, 17–27. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. (2005). Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. The Journal of Neuroscience, 25(34), 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]