Abstract
Introduction:
Tobacco use causes one premature death every six seconds. Current smoking cessation aids include nicotine replacement therapies and bupropion. Although more than 70% of smokers express a desire to quit, fewer than 3% remain abstinent for more than one year, highlighting a critical need for more efficacious smoking cessation treatments.
Areas Covered:
The authors discuss the rationale, preclinical and clinical development of varenicline for smoking cessation. They cover the formulation of varenicline as a partial agonist at α4β2 receptors, the primary neural substrate for nicotine reward. Then, they discuss evidence from preclinical studies indicating varenicline’s efficacy in blocking nicotine reward, followed by clinical trials demonstrating safety and efficacy in sustaining abstinence in smokers. Finally, they cover post-market surveillance, including contraindications in heavy machine operators, putative cardiovascular risk, and the repealed warning for adverse neuropsychiatric events.
Expert opinion:
Varenicline development was based on strong theoretical rationale and preclinical evidence. Clinical studies indicate that varenicline is safe and more effective in sustaining abstinence than placebo, bupropion or nicotine replacement therapies. However, given that continuous abstinence rates across studies remain low (18~30% with varenicline; 4~10% with placebo), novel and more effective medications targeting other nicotinic or glutamate receptors for smoking cessation are required.
Keywords: Acetylcholine receptor, addiction, nicotine, smoking cessation, varenicline
1. Introduction
Tobacco use results in one premature death every six seconds globally 1. Every year 7 million people die worldwide from tobacco-related health problems, including cardiovascular and respiratory disease. Of these, 6 million deaths are estimated to result directly from tobacco use, while the remaining represent non-smokers exposed to second-hand smoke 2. In the United States alone, 16 million individuals are living with a disease caused by smoking 3, and 35.6 million people are current smokers aged 18 or older (15.1% of the U.S. population; 3. With an estimated economic cost of 300 billion U.S. dollars per year, smoking is the second most expensive chronic health condition next to cardiovascular disease in the U.S. 3, 4. Despite the massive individual and public costs of smoking, more than one billion people worldwide continue to use tobacco and nicotine-related products regularly 2.
Tobacco use disorder, as defined by the DSM-V, includes symptoms such as consuming larger quantities of tobacco over time than originally intended, tobacco cravings, unsuccessful attempts to quit or reduce tobacco use, withdrawal symptoms during cessation, and continued tobacco use despite adverse social and health consequences 5. Although up to 70% of smokers express a desire to quit smoking, 80% of those who try to quit return to smoking within the first month, less than 20% quit for six months, and only 3% remain abstinent for more than one year 6, 7. With more than 3,200 youth initiating cigarette use each day 7, more people are likely to become addicted to tobacco every year than manage to quit. Taken together, these observations highlight an urgent need for efficacious treatments of tobacco addiction to reduce the individual and public costs of smoking. In this mini-review, we do not intend to give a comprehensive review for the use of varenicline as a tobacco smoking cessation aid, but rather focus on the rationale for varenicline’s development as a smoking cessation aid and major findings regarding varenicline use in both preclinical and clinical studies, as well as the current challenges and future research directions in medication discovery for treatment of nicotine addiction.
2. Preclinical development of varenicline for smoking cessation
2.1. Neural mechanisms of tobacco addiction
The primary addictive component in tobacco is nicotine. Nicotine acts by binding to nicotinic acetylcholine receptors (nAchRs) located in the central and peripheral nervous systems 8. In the brain, nAchRs are pentameric structures composed of a combination of five different subunits, including nine α-subunits (α2-α210) and three β-subunits (β2- β4), which result in at least 12 unique nAchR subtypes that have been identified thus far 8–10. There are also non-neuronal nicotinic receptor subunits, including α1, β1, γ, δ, and ε, which comprise nAchRs in the peripheral nervous system, most notably at the neuromuscular junction 11. Neuronal nAChRs are typically composed of either two α and three β subunits (Figure 1), or five α7 subunits. The α4β2 and α7 receptor subtypes are the most common in the brain, and are localized both pre- and post-synaptically 8, 9. Activation of nAChRs by nicotine, or the endogenous ligand acetylcholine, increases neuronal excitability and neurotransmitter release via opening of a gated ion channel and subsequent Ca2+ and Na+ influx intracellularly (Figure 1) 10, 12, 13.
Figure 1.
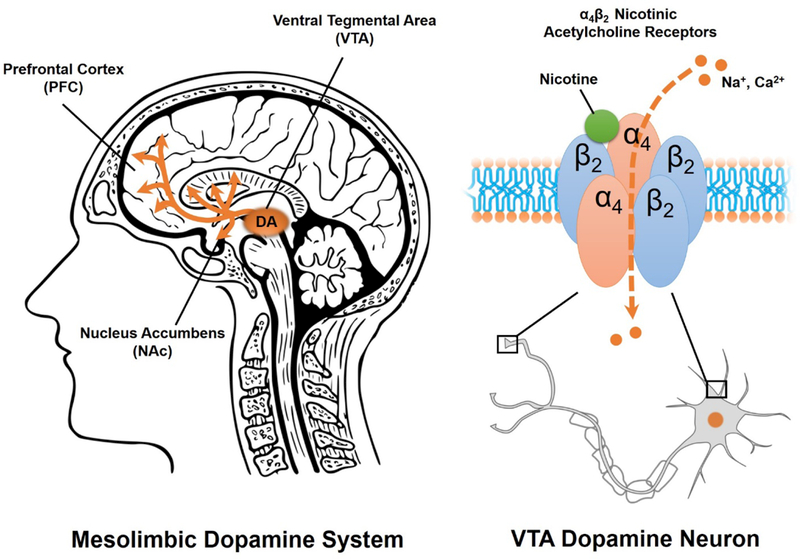
The mesolimbic dopamine (DA) system (left) is comprised of ventral tegmental area (VTA) projections to the nucleus accumbens (NAc) and prefrontal cortex (PFC). Nicotine stimulates DA release in the mesolimbic system by activating pre- and post-synaptic nicotinic acetycholine receptors (nAchRs) in the VTA (right). Activation of pentameric α4β2 nAchRs in the VTA opens an ion pore, allowing cation (Na+ and Ca2+) influx and depolarization of the VTA DA neuron.
About one third of α4β2 nAchRs are located on dopamine (DA) cells of the mesolimbic DA system 10, 14. This mesolimbic DA system is comprised of projections from DA neurons in the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and prefrontal cortex (PFC; Figure 1) 15. Nicotine binding to α4β2 receptors on VTA DA cells stimulates DA release to the NAc 16, 17, an effect which is thought to underlie nicotine’s rewarding and reinforcing effects 16. Evidence for α4β2 receptor involvement in nicotine addiction derives from both human and animal studies. For example, positron emission tomography (PET) studies in humans indicate that smoking a full nicotine cigarette nearly saturates α4β2 receptor occupancy 18. In rodents, systemic blockade of the α4β2 receptor by mecamylamine or dihydro-β-erythroidine (DHβE) suppresses nicotine self-administration 19. Moreover, genetic deletion (knockout) of either the α4 subunit or the β2 subunit, but not the α7 subunit, in mice eliminates intravenous nicotine self-administration 20 as well as nicotine-induced VTA DA neuron firing 21. In contrast, genetic modifications that increase α4 sensitivity to nicotine augment nicotine conditioned place preferences, facilitate tolerance to nicotine’s hypothermic effects, and escalate nicotine-induced locomotor sensitization in mice 8, 22. Finally, selective restoration of either α4 or β2 expression in the VTA, but not the neighboring substantia nigra, using lentiviral vectors also restores nicotine self-administration in knockout mouse models 20. Taken together, these observations strongly implicate VTA α4β2 nAchRs in nicotine addiction, and suggest the α4β2 receptor represents an attractive medicinal target for the treatment of tobacco use disorders.
2.2. Rationale of developing a partial agonist for smoking cessation
Prior to the development of varenicline, available pharmacotherapies for smoking cessation included nicotine replacement therapies (NRTs) such as gum, patches, nasal sprays and inhalers, as well as bupropion, an antidepressant which inhibits DA and norepinephrine reuptake as well as nAchR activity 23. Modulating nAChRs can be accomplished using full agonists, antagonists, or partial agonists. Full agonists like NRTs mimic nicotine’s effects. Since the cessation of nicotine use is the major reason for producing unpleasant withdrawal symptoms, NRTs can help relieve some physical withdrawal by providing supplemental low doses of nicotine, without the other harmful chemicals in tobacco. Antagonists, such as mecamylamine and DHβE, can compete with nicotine to bind to nAChRs and thus block nicotine reward 19. However, nAchR antagonists produce precipitated withdrawal symptoms including irritability, depression, insomnia, fatigue, headaches, constipation, and weight gain 24, 25. In contrast, partial agonists bind to nAchRs but do not elicit the maximum response of a full agonist 26, instead depending upon receptor occupancy by other ligands. For example, in the presence of a full agonist like nicotine a partial nAchR agonist will behave as an antagonist, attenuating nicotine’s effects at the receptor. However, in the absence of nicotine a partial nAchR agonist will behave as an agonist and mitigate withdrawal symptoms. Partial nAchR agonists therefore have potential to block nicotine reward, minimize craving, and prevent withdrawal, suggesting this mechanism may be an ideal medicinal target for nicotine addiction.
2.3. Cytisine: An initial discovery of a partial agonist for smoking cessation
Cytisine is an alkaloid with a molecular structure similar to nicotine (Figure 2) and has high affinity for the α4β2 nAchR subtype 27. Cytisine is derived from the plant Cytisus laburnum, a deciduous shrub that is native to central and southern Europe. Preclinically, cytisine produces locomotor activation and substitutes for nicotine in self-administration studies, suggesting it may have modest abuse potential 28. However, cytisine does not induce reinstatement to nicotine-seeking behaviors following extinction and at higher doses blocks the discriminative effects of nicotine 28, suggesting cytisine is unlikely to induce relapse to nicotine seeking during abstinence.
Figure 2.
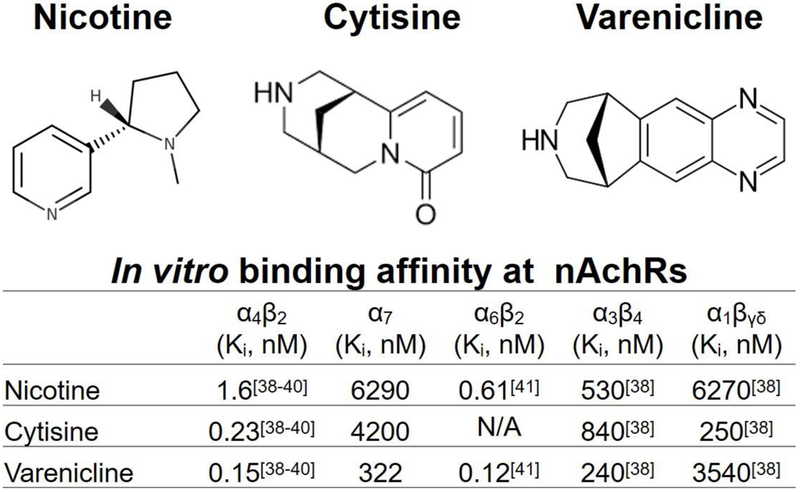
Chemical structures of nicotine, cytisine, and varenicline (top). Comparison of in vitro binding affinities of nicotine, cytisine, and varenicline at nicotinic acetylcholine receptor subtypes (nAchRs; bottom).
Cytisine was first discovered in 1818 and was isolated in 1865 (see timeline in Figure 3) 29. In 1912, cytisine’s biological effects were reported to be nearly identical to those of nicotine, and cytisine was proposed as an inexpensive, readily available replacement for tobacco 30. Consequently, the Cytisus plant was reportedly smoked during World War II by German and Russian soldiers as a tobacco substitute 29. In 1964, a Bulgarian pharmaceutical company named Sopharma marketed cytisine as a smoking cessation aid under the brand name “Tabex” 29. Although clinical trials during the 1960’s and 1970’s reported that cytisine produced quit rates between 21–30% at six-month follow-up, these studies were not conducted systematically. A later placebo-controlled trial published in the New England Journal of Medicine reported more modest effects of cytisine on sustained abstinence at 12-month follow up: 8.4% of subjects remained abstinent on cytisine compared to 2.4% on placebo 31. Similarly, a more recent meta-analysis based on two studies deemed to be of high quality reported a ~three-fold increase (random ratio = 3.29) in the likelihood of maintaining abstinence at six-month follow-up with cytisine use compared to placebo 32. Cytisine’s low efficacy may be due in part to poor absorption and crossing of the blood brain barrier 33. Although cytisine is superior to NRTs in maintaining abstinence from smoking, it is also associated with more adverse events and side effects, including hospitalizations, mild, moderate and severe adverse events (such as symptoms of cold or flu), nausea and vomiting, and sleep disorders 34. Today there remains a “call for action” to provide cytisine as a smoking cessation agent in lower income countries, but cytisine is not approved by the European Union or the United States’ Food and Drug Administration (FDA) for human use 35. Taken together, these observations indicate a need for a more efficacious α4β2 receptor partial agonist for the treatment of nicotine addiction and smoking cessation.
Figure 3.
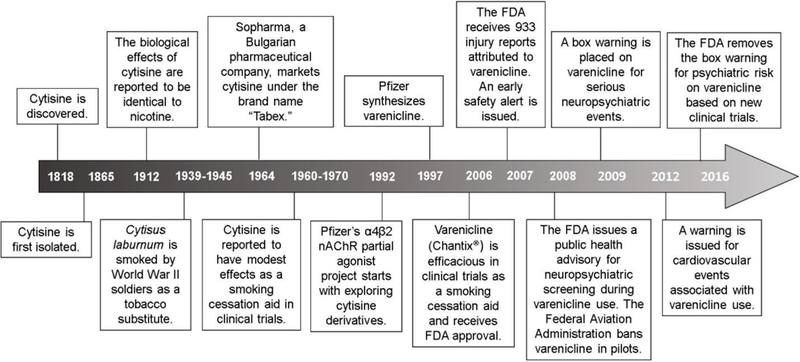
A summarized history of varenicline discovery and development (see text for details).
Note: Timeline is not presented at scale.
2.4. Varenicline: a novel partial agonist for the treatment of nicotine addiction
Varenicline tartrate was originally developed as a smoking cessation agent by Pfizer in 1997 based on the molecular structure of cytisine (Figure 2). There are currently 3 patents protecting varenicline under the brand name Chantix®, which are set to expire in the year 2020 in the U.S. 36. Varenicline is a highly selective and potent partial agonist at the α4β2 nAchR, with 500-fold selectivity for α4β2 over α3β4, 3500-fold selectivity over α7, and 20,000-fold selectivity over α1βγδ 37. In vitro binding assays indicate that varenicline affinity for α4β2 (Ki=0.15 nM) is higher than that of cytisine (Ki=0.23 nM) as well as nicotine (Ki=1.6 nM; see Figure 2) 38–40. Consistent with a partial agonist profile, patch clamp studies using HEK cells expressing human nAchRs show that varenicline exhibits ~45% of nicotine’s maximal efficacy at the α4β2 receptor, with an EC50 of 3.1 μM 39. When co-administered with nicotine, varenicline blocks nicotine-induced DA release in the NAc through activity at the α4β2 and α6β2 receptor subtypes 38, 39, 41.
Preclinical studies indicate that varenicline is superior to cytisine in reducing nicotine addiction-related behaviors (Table 1). In rodents, varenicline attenuates nicotine-induced locomotor sensitization, blocks nicotine conditioned place preferences, reduces nicotine self-administration under fixed- and progressive-ratio schedules, and suppresses nicotine-primed as well as cue-induced reinstatement of nicotine seeking following intraperitoneal, subcutaneous, or oral routes of administration 39, 42–45. Varenicline also selectively blocks nicotine’s effects on brain stimulation reward in intracranial self-stimulation (ICSS) paradigms through activity at the α4β2, but not the α7, nAchR 46. Finally, varenicline attenuates the dysphoria associated with nicotine withdrawal as measured by ICSS thresholds 47. Taken together, these preclinical findings indicate varenicline may not only attenuate nicotine reward and intake, but could reduce the risk of relapse and mitigate withdrawal symptoms in nicotine-dependent subjects.
Table 1.
Preclinical studies of nicotine and varenicline on addiction-related behaviors.
| Assay | Nicotine Alone | Varenicline Alone | Varenicline Effects on Nicotine |
|---|---|---|---|
| Locomotor Sensitization 43, 50 | ↑ | No effect | ↓ |
| Conditioned Place Preferences 42 | ↑ | --- | ↓ |
| Intravenous Self-Administration 81, 102–107 | ↑ | ↑ | ↓ |
| Brain Stimulation Reward 46, 47 | ↑ | ↑ | ↓ |
| Reinstatement (Relapse) 39, 44, 45 | ↑ | No effect | ↓ |
| Marble Burying Test (Anxiety) 51 | ↓ | ↓ | --- |
| Dopamine Release in the Nucleus Accumbens 38, 39, 41 | ↑ | ↑ | ↓ |
| α4β2 nAchR binding 51, 52 | ↑ | ↑ | --- |
Preclinical studies also suggest that varenicline has low abuse liability itself (Table 1). When administered alone, varenicline is 40–60% less efficacious in stimulating DA release in the NAc than nicotine, as shown by in vitro slice preparations as well as in vivo microdialysis 39. Although varenicline partially substitutes for nicotine in drug-discrimination studies 48, it is not readily self-administered by drug-naïve animals without prior training to respond for food reinforcement 49, and does not induce reinstatement of nicotine-seeking behaviors 45. Unlike nicotine, varenicline does not induce locomotor sensitization 43, 50. Chronic varenicline treatment has anxiolytic effects in rodents as measured in the marble-burying test 51. Chronic varenicline treatment also upregulates nAchR binding in the cortex, hippocampus, thalamus and striatum in mice 51, 52. These findings suggest that varenicline has low abuse liability even in the absence of nicotine.
3. Clinical development of varenicline for smoking cessation
3.1. Pharmacokinetics and side effects of varenicline
The strong rationale for targeting the α4β2 nAchR with a partial agonist, coupled with promising findings from preclinical studies described above, lead to the initiation of clinical trials evaluating the safety and efficacy of varenicline in humans. Early studies indicated that varenicline has a longer half-life than cytisine (24 hours vs. 5.8 hours) 53, and a volume of distribution three-fold greater than cytisine 27, 53. Peak plasma concentrations of varenicline occur approximately 3–4 hours after oral administration, and steady-state levels are observed after 4 days of repeated use 37. Protein binding of varenicline is less than 12% and metabolism is less than 10%. Varenicline is eliminated renally via the organic cation transporter OCT-2, and because metabolism is limited over 90% of varenicline is excreted unchanged in the urine. Patients with moderate to severe renal impairment are advised to use caution when taking varenicline, but geriatric (aged 65+ years) and pediatric (aged 12–17 years) efficacy and pharmacokinetics of varenicline do not differ from healthy adult populations. There are no reported drug interactions with varenicline, and it does not interact with cytochrome P450 enzymes in human hepatocytes or human renal transport proteins. Varenicline does not have any carcinogenic, genotoxic, or teratogenic effects, although use during pregnancy is advised only if the potential benefit to the fetus justifies putative risks 37.
Nausea is the most common side effect of varenicline and is largely dose-dependent, occurring in approximately 30% of patients taking 1 mg BID (twice daily) in the first week and 16% of patients taking 0.5 mg BID in the first week 37, 54. Insomnia is the second most-common side effect, reported in 14–37.2% of patients, but is most frequent during the first month of treatment and subsides with extended administration 54. Headache, abnormal dreams, sleep disturbances, dizziness, dry mouth, weight gain, and constipation are less common side effects, and are usually temporary and mild to moderate in severity 10, 55. Varenicline can be used alongside NRTs, but combined therapy does not appear to increase varenicline’s efficacy in smoking cessation 56. In addition, some side effects, such as nausea, headache, vomiting, dizziness, and fatigue are exacerbated with concurrent varenicline and NRT use, and patients may be more likely to discontinue treatment as a result 37.
3.2. Clinical trials evaluating varenicline as a smoking cessation aid
The FDA approved varenicline as a smoking cessation aid on May 11, 2006 (see timeline in Figure 3). By 2008, approximately 3.5 million people in the U.S. and 5 million worldwide were taking varenicline 57. FDA approval was largely based on six randomized clinical trials conducted in 3,659 subjects in the U.S, which are summarized in Table 2 alongside more recent studies 55, 58–60. Sample populations in these trials were composed of approximately equal numbers of men and women, aged 43 years old on average. All subjects were chronic cigarette smokers, reporting smoking 21 cigarettes per day on average for the past 25 years. The primary outcome measure was abstinence from smoking, as assessed by self-report in terms of continuous abstinence rate (CAR) or continuous quit rate (CQR), and verified by exhaled carbon monoxide levels of 10 parts per million or less. Secondary outcomes included the urge to smoke, withdrawal symptoms, and the reinforcing effects of nicotine, as measured by the Brief Questionnaire of Smoking Urges and the Minnesota Nicotine Withdrawal Scale 10, 37. In two seminal 12-week long phase III trials, 0.5 and 1 mg varenicline BID resulted in ~44% abstinence rates from weeks 9–12, a significant improvement over bupropion (~30%) and placebo (~18%) 37, 61. Varenicline-treated subjects were also more likely to remain abstinent at 28- and 40-week follow-ups 37, 61. Secondary outcome measures, including the urge to smoke, were likewise improved in varenicline-treated subjects over placebo 37, 61. Although randomized, double-blind and placebo-controlled, these early trials involved some limitations. For example, the sample population was comprised of healthy volunteers and excluded individuals with medical illnesses or major depression, and may thus not be representative of a typical primary care population 55, 59. Moreover, all participants received individual counseling sessions for 12 weeks, which may not be available in standard health care settings. Finally, follow-up was not completed in 35–43% of participants, although retention rates were comparable or lower in the placebo groups compared to varenicline or bupropion treatments 55, 59.
Table 2.
Clinical Trials Supporting FDA Approval of Varenicline for Smoking Cessation and Post-Approval Trials Conducted on Varenicline Relevant to Post-Market Surveillance Events
| Continuous Abstinence Rate (CAR) |
|||||||
|---|---|---|---|---|---|---|---|
| n | Treatment Duration | Purpose | Treatment Regimen | Weeks 9–12 | Weeks 24+ | ||
| Nides et al. 2006111 | 638 | 6–7 weeks | Evaluation of efficacy, safety and tolerability of 3 varenicline dosages | Varenicline: | 0.3 mg QD 1.0 mg QD 1.0 mg BID |
Not available (#See table note). |
|
| Oncken at al. 200658 | 627 | 12 weeks + 40 week follow-up | Comparison of dosing regimens on tolerability | Varenicline: | 0.5 mg BID with/without 1 week titration | 44% | 18.5% |
| Varenicline: | 1 mg BID with/without 1 week titration | 49.4% | 22.4% | ||||
| Placebo | --- | 11.6% | 3.9% | ||||
| Niaura et al. 200860 | 312 | 12 weeks + 40 week follow-up | Flexible dosing | Varenicline: | 0.5 mg BID, voluntarily adjusted up to 1 mg BID* | 40.1% | 22.3% |
| Placebo | --- | 11.6% | 7.7% | ||||
| Gonzales et al. 200659 | 1022 | 12 weeks + 40 week follow-up | Comparison to bupropion sustained release (SR) and placebo | Varenicline: | 0.5 mg QD for 3 days, 0.5 mg BID for 4 days, to 1 mg BID | 44% | 21.9% |
| Bupropion SR: | 150 mg QD for 3 days, followed by 150 mg BID | 29.5% | 16.1% | ||||
| Placebo | --- | 17.7% | 8.4% | ||||
| Jorenby et al. 200655 | 1023 | 12 weeks + 40 week follow-up | Replication of Gonzales et al.59 | Varenicline: | 0.5 mg QD for 3 days, 0.5 mg BID for 4 days, to 1 mg BID | 43.9% | 23% |
| Bupropion SR: | 150 mg QD for 3 days, followed by 150 mg BID | 29.8% | 14.6% | ||||
| Placebo | --- | 17.6% | 10.5% | ||||
| Cincirpini et al. 201365 | 294 | 12 weeks + 24 week follow-up | Addition of more intense behavioral counseling | Varenicline: | 0.5 mg QD for 3 days, 0.5 mg BID for 4 days, to 1 mg BID | 43% | 23% |
| Bupropion SR: | 150 mg QD for 3 days, followed by 150 mg BID | 37.2% | 27.9% | ||||
| Placebo | --- | 17% | 14.1% | ||||
| Aubin et al.62 Ebbert et al. 201666 |
376 93 |
12 weeks + 40 week follow-up 12 weeks + 12 week follow-up |
Comparison to nicotine replacement therapy (NRT) Evaluation in light smokers (5–10 cig/day) Comparison to nicotine replacement therapy (NRT) Evaluation of combined varenicline + NRT |
Varenicline: | 1 mg BID for 12 weeks | 55.9% | 26.1% |
| Transdermal NRT: | 21 titrated to 12mg/kg/day, 10 weeks | 43.2% | 20.3% | ||||
| Varenicline: | 40% | 31.1% | |||||
| Placebo | 8.3% | 8.3% | |||||
| Aubin et al.62 Ramon et al. 201456 |
376 341 |
12 weeks + 40 week follow-up 12 weeks |
Varenicline/Transdermal NRT | 1 mg BID titrated varenicline + 21 mg daily patch | 39.1% | --- | |
| Varenicline + Placebo | 1 mg BID titrated varenicline + inactive patch | 31.8% | --- | ||||
| Ebbert et al. 201666 Tonstad et al. 2006108 |
93 1927 |
12 weeks + 12 week follow-up 24 weeks + 28 week follow-up |
Evaluation in light smokers (5–10 cig/day) Long-term abstinence |
Open-label varenicline: 1 mg BID for 12 weeks, followed by blind randomization to varenicline (1mg BID) or placebo for 12 weeks^ | ^See table note. | ||
| Rigotti et al. 2014109 | 714 | 12 weeks + 52 week follow-up | Efficacy in cardiovascular disease‡ | Varenicline | 1 mg BID | 47% | 19.2% |
| Placebo | --- | 13.9% | 7.2% | ||||
| Anthenelli et al. 201677 | 8144 | 12 weeks + 12 week follow-up | Evaluation of neuropsychiatric adverse events | Varenicline: | 1 mg BID | 33.5% | 21.8% |
| 22.6% | 16.2% | ||||||
| 23.4% | 15.7% | ||||||
| 12.5% | 9.4% | ||||||
| †See table note. | |||||||
| Rigotti et al. 2014109 | 714 | 12 weeks + 52 week follow-up | Efficacy in cardiovascular disease‡ | Bupropion: | 150 mg BID | 47% | |
| NRT: | 21 mg/day | 13.9% | |||||
| Anthenelli et al. 201677 | 8144 | 12 weeks + 12 week follow-up | Evaluation of neuropsychiatric adverse events | Placebo | --- | 33.5% | 21.8% |
| 22.6% | 16.2% | ||||||
| 23.4% | 15.7% | ||||||
| 12.5% | 9.4% | ||||||
| †See table note. | |||||||
Table Note. BID: Twice daily. QD: Once daily.
Continuous quit rates were higher for 1.0 mg varenlicine QD (37.3%) and 1.0 mg varenicline BID (48%) compared to placebo (17.1%). Overall varenicline was well tolerated.
69% of subjects titrated to maximum dose.
CAR at weeks 13–24 was higher for varenicline-treated subjects (70.5%) than those switching to placebo (49.6%). Varenicline’s efficacy over placebo was maintained at week 52 (43.6% CAR vs. 36.9% CAR, respectively)108.
Varenicline and placebo groups with stable cardiovascular disease showed no significant differences in cardiovascular mortality or adverse cardiovascular events109.
In participants with a history of psychiatric illness 9–12 weeks and 9–24 weeks CARs with varenicline (38%, 25.5%) were higher than that in participants without psychiatric illness (29.2%, 18.3%). Neither varenicline nor bupropion increased moderate-to-severe neuropsychiatric events. At weeks 9–12 follow-up CAR was higher in participants receiving varenicline compared to all other treatment groups (odds ratio vs. placebo: 3.61; vs. NRT 1.68; and vs. bupropion 1.75)77.
Since FDA approval in 2006, a number of reports have continued to demonstrate varenicline’s efficacy for smoking cessation over other available pharmacotherapies. For example, in a randomized phase III trial involving 376 participants followed over 52 weeks, varenicline resulted in significantly higher abstinence rates from smoking (55.9%) compared to a transdermal NRT (43.2%), as well as reduced cravings, attenuated withdrawal, and diminished pleasure from smoking 62. However, it is worth noting that this study involved an open-label design and varenicline treatment was continued 2 weeks longer than NRT, such that some bias may have influenced treatment outcomes and participant drop-out rates after treatment assignment 62. In another study, varenicline was more efficacious as measured by CAR for 9–12 weeks compared to bupropion and placebo 63, and more than doubled the rate of smoking cessation at one year with comparable or fewer side effects and adverse events 64, 65. Varenicline was also favorable to bupropion and placebo in promoting abstinence at six-month follow-up and was associated with fewer depression symptoms and reduced subjective nicotine reward 65. However, the generalizability of this latter study may be limited to due overlap in the population sample with other phase-III trials 65. More recently varenicline’s efficacy has been demonstrated in light smokers (5–10 cigarettes per day), with 12 weeks of treatment producing abstinence rates of 31% compared to 8% following placebo at six-month follow-up 66.
3.3. Pooled and meta-analyses comparing varenicline outcomes across clinical trials
Large-scale analyses comparing outcomes across trials confirm varenicline’s efficacy as a smoking cessation aid 10. For example, pooled analyses of phase III trials 55, 59 show that cravings (urge to smoke) are reduced following varenicline or bupropion treatment compared to placebo 67. Both varenicline and bupropion attenuate nicotine withdrawal symptoms such as depression, anxiety, irritability, insomnia, and attentional deficits 67. Varenicline-treated patients also express reduced smoking satisfaction and pleasurable effects from smoking, as measured by the Modified Cigarette Evaluation Questionnaire 67. One major limitation of this analyses, however, was that the three treatment groups exhibited differences in abstinence rates that could have influenced outcomes 67. Nonetheless, a separate pooled analysis of phase III trials further confirmed that abstinence rates are greater for varenicline-treated patients (44%) compared to bupropion (29.7%) and placebo (17.7%) 63l.
A meta-analysis of nine clinical trials, involving over 7,000 participants, indicated that the odds ratio for abstinence on varenicline vs. placebo was 2.33, varenicline vs. bupropion 1.52, and varenicline vs. NRT 1.31 68. Similarly, a meta-analysis of 101 trials spanning more than 40,000 participants reported that the odds ratio of abstinence at four-weeks post target quit date for varenicline was 3.16, for bupropion 2.25, and for NRT 2.05, with an odds ratio of varenicline vs. bupropion of 1.86 69. A more recent analysis of trials identified in the Cochrane Tobacco Addiction Group, involving a total of ~25,000 participants, reported a pooled risk ratio of 1.39 for varenicline vs. bupropion and 1.25 for varenicline vs. NRT at six-month follow-up 70. In this report, serious adverse events including infection, cancer, and injury, were increased by 25% in varenicline-treated patients, although these were not expected to be a result of the trial itself 70. Further, no significant relationship was found between varenicline treatment and depressed mood, agitation, or suicidal behaviors or ideation 70. Taken together, the results of several clinical trials involving tens of thousands of patients testify to varenicline’s safety and efficacy in aiding smoking cessation and maintaining abstinence rates long-term.
3.4. Cognitive effects of varenicline
On a neural systems level, varenicline’s efficacy as a smoking cessation aid may derive in part from its positive effects on cognition and processing of smoking-related cues, which are important triggers for relapse. For example, in a functional magnetic resonance imaging (fMRI) study using a region of interest (ROI) analysis, varenicline increased the blood oxygen level dependent (BOLD) signal in the anterior cingulate cortex and dorsolateral PFC during a working memory task and improved response time in heavily dependent smokers 71. In smokers who remained abstinent, five weeks of varenicline treatment increased BOLD activation in regions involved in attention, learning and memory, including the insula, putamen, thalamus, and cingulate cortex 72. Impulsivity and mesocorticolimbic dysfunction were also restored following varenicline treatment in abstinent smokers 73.
In the absence of treatment, smoking-related cues (e.g., videos of individuals discussing smoking, images of lighters and cigarettes, etc.) increase smokers’ cravings and BOLD responses in the ventral striatum and orbitofrontal cortex (OFC), two regions in the mesocorticolimbic DA reward pathway 74. However, three weeks of varenicline treatment attenuated ventral striatal and OFC activation by smoking cues and reduced subjective craving 74. An event related potential study found that smokers with greater activation to cigarette-related cues over other pleasant stimuli had a 95–98% greater chance of benefit for varenicline over bupropion, and were more likely to remain abstinent at three-month follow-up 75. Moreover, at six-month follow-up all smokers maintained abstinence on varenicline significantly more than on placebo, an effect driven largely by increased activation to cigarette cues 75. Together, these findings suggest that attenuation of the salience attributed to smoking and cigarette-related cues may critically underlie varenicline’s efficacy as a smoking cessation aid.
4. Post-launch surveillance following varenicline approval
4.1. Neuropsychiatric events and varenicline use
In 2009 the FDA placed a box warning on varenicline for “serious neuropsychiatric events,” including depressed mood, suicidal ideation and attempts, hostility, agitation, psychosis, and severe injuries. The warning was based largely on post-marketing surveillance reports analyzed by the Institute for Safe Medication Practices regarding adverse events between May and December of 2007. During this time, 988 serious injuries reported to the FDA were attributed to varenicline, more than any other drug 57. Of these, 227 were suicidal acts, 397 were psychoses, and 525 were hostility or aggression. Other prominent events associated with varenicline included 173 accidental injuries such as traffic accidents and falls, 148 reports of vision disturbances, 224 heart rhythm disturbances, 86 seizures or abnormal spasms, 338 skin reactions, and 544 cases of diabetes 57. In November 2007, the FDA issued an early alert about an ongoing safety review of varenicline 76. Later, in February 2008, a Public Health Advisory was issued calling for pre-screening and continual monitoring of patients taking varenicline for psychiatric illnesses, and discontinuation of treatment if any changes were observed 10, 76. The box warning was subsequently issued in July 2009. Post-marketing reports described a resolution of symptoms following cessation of varenicline, but in some cases continual monitoring and support were required 37. In 2015, the warning was updated to include more recent cases in the FDA Adverse Event Reporting System regarding patients who experienced reduced alcohol tolerance and increased drunkenness, aggression and memory loss following alcohol consumption while taking varenicline, as well as a potentially increased risk of seizures 76. This information was subsequently included in the Warnings and Precautions section of the drug label as well as in the patient Medication Guide.
In 2016, a joint FDA advisory committee voted to remove the box warning on varenicline based on the results of a large clinical trial conducted by Pfizer 76. This EAGLES trial, which compared varenicline, bupropion, NRT and placebo, included two large cohorts of more than 4000 participants each: one cohort with a psychiatric illness diagnosis (4116 participants), and one without (4028 participants). In patients without a history of psychiatric illness, neither varenicline nor bupropion was associated with a significant increase in moderate-to-severe neuropsychiatric adverse events (the primary outcome measure) compared to placebo (1.5% increase) 77. More specifically, 1.3% of the varenicline-treated group, 2.2% of the bupropion group, 2.5% of the NRT group, and 2.4% of the placebo group reported neuropsychiatric adverse events 77. Similarly, in patients with a history of psychiatric illness, varenicline or bupropion did not increase moderate-to-severe neuropsychiatric events by more than 4% 77. Within the psychiatric cohort, 6.5% of varenicline-treated participants, 6.7% of bupropion-treated participants, 5.2% of NRT-treated participants, and 4.9% of placebo-treated participants reported a moderate-to-severe neuropsychiatric adverse event. Consistent with prior studies, participants receiving varenicline achieved higher abstinence rates compared to all other treatment groups (odds ratio vs. placebo: 3.61; vs. NRT 1.68; and vs. bupropion 1.75). The FDA committee concluded that the neuropsychiatric risks of varenicline are low and may be limited to subjects with a pre-existing illness, such as depression, anxiety, or schizophrenia 76.
While the EAGLES trial demonstrated comparable efficacy of varenicline for smoking cessation regardless of pre-existing psychiatric status, it nonetheless remains possible that individual differences in psychological traits may influence abstinence rates. To address this possibility, a recent study evaluated the influence of trait characteristics including depression, impulsivity, agreeableness, conscientiousness, neuroticism and openness on the efficacy of varenicline for smoking cessation or reduction 78. Participants achieving continuous abstinence with varenicline scored lower on non-planning and motor impulsivity (as measured by the Barrett Impulsivity Scale) at 12 and 36-week follow-up. Modest relationships between personality traits and smoking cessation were also observed, such that participants scoring high on agreeableness and openness were more likely to maintain continuous abstinence and smoke fewer cigarettes at follow-up when treated with varenicline. In addition, those who had not previously experienced a major depressive episode experienced greater benefit (smoked fewer cigarettes) from varenicline compared to placebo. Lower depressive symptoms at treatment onset (as measured by the Beck Depressive Inventory-II) were also associated with higher continuous abstinence rates at 12-week follow-up. However, no relationships between conscientiousness or neuroticism and varenicline efficacy or smoking reduction were observed. Taken together, these findings suggest that the efficacy of varenicline for smoking cessation may vary depending on individual personality and psychological traits and history of depressive episodes 78. In the future, personalized medicine and individualized therapeutic strategies will also inevitably rely on advances in genetics, genomics, and proteomics to maximize smoking cessation rates and treat other neuropsychiatric conditions 79.
4.2. Operation of heavy machinery and varenicline use
In addition to post-marketing reports regarding caution for neuropsychiatric events, varenicline is not recommended for people operating vehicles and heavy machinery. As described above, in 2007 prominent adverse events attributed to varenicline included 173 accidents or falls, as well as vision and heart rhythm disturbances. Based on these reports, the Institute for Safe Medication Practices issued safety concerns regarding the use of varenicline by aircraft, train, bus, and other vehicle operators, as well as individuals working with nuclear power reactors, construction cranes, and life-saving medical devices 57. The Federal Aviation Administration banned varenicline use in pilots in May 2008 80, and an accidental injury warning remains on the package insert today 37.
4.3. Cardiovascular events and varenicline use
In December 2012, the FDA released the results of a large meta-analysis of 15 clinical trials involving more than 7,000 patients taking varenicline. Varenicline use was associated with increased cardiovascular events, including death, nonfatal heart attack, and nonfatal stroke after 30-days of treatment, compared to patients receiving placebo (13% vs. 6%, respectively, hazard ratio=1.95) 76. Although the group difference was not statistically significant, the FDA nonetheless cautioned patients and healthcare professionals to weigh the risks and benefits of varenicline treatment for smoking cessation, and to remain vigilant if new or worsening cardiovascular symptoms develop 76. In rodent models, chronic varenicline exposure caused a reduction in the weight of the heart (0.92 mg following chronic treatment vs. 0.99 mg with acute exposure, p<0.05), impaired oxygen saturation and prolonged QT intervals among other negative cardiovascular side effects 81. However, in a randomized, double-blind placebo-controlled study involving 714 patients with existing cardiovascular disease, no additional adverse cardiovascular events were reported following varenicline treatment compared to placebo, and varenicline was associated with significant improvements in continuous abstinence rates (19.2% of participants on varenicline vs. 7.2% of participants receiving placebo) 109. Moreover, another recent randomized, placebo-controlled trial involving a subset of participants in the EAGLES study (n=4595)77 and reported at the 2017 Society for Research on Nicotine and Tobacco indicated that the incidence of cardiovascular adverse events following varenicline, bupropion, or transdermal nicotine patch (an NRT) did not significantly differ from placebo after 12 weeks of treatment or 1-year follow-up 82. Notably, smoking is associated with cardiovascular disease in itself and cessation can significantly improve long-term outcomes. In randomized trial of 400–700 smokers with cardiovascular disease, varenicline more than doubled abstinence rates and no new safety concerns were noted 76.
5. Summary and Conclusions
Varenicline is the first medication developed to selectively target nicotine activity at the α4β2 nAchR, the primary mechanism of action in nicotine addiction. As a potent partial agonist, varenicline attenuates nicotine-induced DA release and reduces the reinforcing value of nicotine. Because varenicline elicits partial activation of the α4β2 nAchR in the absence of nicotine, it also mitigates withdrawal symptoms during abstinence. In preclinical animal models varenicline blocks nicotine-induced locomotor sensitization, conditioned place preferences, self-administration, and nicotine-primed or cue-induced reinstatement of nicotine seeking. When administered alone, varenicline has modest reinforcing value and is unlikely to have significant abuse liability.
Clinical trials have demonstrated that varenicline is safe and well-tolerated in diverse patient populations. The most common side effect, nausea, occurs in approximately 30% of patients and is often alleviated with continued use and/or dose titration. Varenicline has consistently been found to be more effective than bupropion and NRTs in sustaining abstinence from smoking, both during initial trials as well as in subsequent post-market surveillance and meta-analyses. Although early post-marketing reports identified potentially adverse neuropsychiatric and cardiovascular events associated with varenicline use, subsequent studies have demonstrated that the probability of these events is low in individuals without a pre-existing disorder. Nonetheless, caution is warranted for varenicline use in individuals who routinely operate heavy machinery.
6. Expert opinion
The development of varenicline was successful largely due to the strong theoretical rationale on which its discovery strategy was based. Varenicline was formulated to target the primary mechanism of nicotine addiction, activity at α4β2 nAchRs in the mesolimbic DA system. Varenicline synthesis was based on existing knowledge of cytisine, an alkaloid derived from a deciduous shrub historically used as a tobacco replacement. As a selective and potent partial agonist at the α4β2 nAchR, varenicline conveys significant advantages over full agonists such as NRTs, which do not eliminate nicotine addiction, and antagonists, which precipitate withdrawal symptoms during abstinence. Moreover, converging evidence in preclinical and clinical studies support the utility of varenicline for smoking cessation.
While varenicline is the most efficacious smoking cessation aid currently available, it is worth noting that a majority of clinical trials evaluating varenicline have been sponsored by its manufacturer, Pfizer. Although these trials are randomized and double-blind, additional studies by independent parties are warranted 10. Going forward, it will be critical to evaluate the efficacy of varenicline as a cessation aid for nicotine addiction fueled by e-cigarette use, particularly in adolescent and young adult populations in whom e-cigarette consumption is growing rapidly. From 2011 to 2015 alone, e-cigarette use among high school students increased seven-fold 83. In the 2015 National Youth Tobacco Survey, 27.1% of adolescents in middle school and high school reported using e-cigarettes, representing approximately 7,260,500 teens 83. While the safety of e-cigarettes remains controversial, the American Lung Association has emphasized concern regarding the effects of e-cigarette use on public health, particularly among youth and adolescents, and urge for additional oversight of e-cigarette production by the Food and Drug Administration 84. In addition to delivering nicotine, e-cigarette liquid cartridges contain toxic and carcinogenic ingredients (albeit at lower levels than combustible tobacco and cigarettes), and fruity, sweet flavorings appeal to young people and contribute to adolescent consumption 84, 85. Although large-scale, systematic studies are needed to further determine the long-term health consequences of e-cigarettes, the rise in youth and adolescent use of these nicotine delivery products nonetheless remains a concern. Adolescent drug use more than doubles the likelihood of developing substance use disorder in adulthood 86, 87, suggesting thousands of young people are at elevated risk for nicotine addiction in the future. Evaluation of the efficacy of varenicline and other smoking cessation aids in this vulnerable population will be urgently needed.
An additional concern regarding varenicline is that chronic treatment upregulates α4β2 nAchR binding much like nicotine, suggesting continued varenicline use may prevent α4β2 levels from returning to baseline in heavy smokers 52. Alternative mechanisms for the treatment of nicotine addiction may therefore be desirable. In addition to α4β2 activity, varenicline is a potent partial agonist at the α6β2 nAchR subtype (Ki=0.12 nM) 41, and unlike α4β2, chronic varenicline reduces α6β2 binding levels 52. Accumulating evidence also supports a role for the α6 subunit in nicotine reward. For example, genetic deletion of α6 eliminates nicotine self-administration and suppresses nicotine-induced VTA DA neuron firing, while selective re-expression of α6 in the VTA restores nicotine self-administration 20, 21. These findings suggest α6 may represent a viable target for the development of future smoking cessation aids.
In addition to targeting alternative nicotinic receptor subunits, metabotropic glutamate receptor (mGluR) 2/3 agonists and mGluR5 antagonists have recently shown efficacy in attenuating nicotine self-administration preclinically, and may have promise in human studies for smoking cessation 88. Group 2 mGluRs, including mGluR2 and 3, are located pre-synaptically and on glial cells and are coupled to inhibitory Gi/o-proteins, such that activation of these receptors inhibits adenyl cyclase signaling and can suppress glutamatergic neuron firing. In contrast, group 1 mGluRs, including mGluR1 and 5, are located post-synaptically, are Gq-coupled, and serve to activate phospholipase C 88. Nicotine binding to nAChRs located on pre-synaptic glutamatergic neurons in the VTA increases glutamate excitation of DA cells in this region, contributing to nicotine’s rewarding and reinforcing effects 16, 88, 89. Activation of pre-synaptic mGluR2/3 via a pharmacological agonist, or blockade of post-synaptic mGluR5 with an antagonist, would therefore counteract nicotine’s effects by suppressing glutamate release and binding at VTA DA neurons as well as in the NAc 90. Preclinical studies using LY379268, an mGluR2/3 agonist, and MPEP, an mGluR5 antagonist, support this hypothesis. For example, LY379268 reduced nicotine self-administration and nicotine-primed reinstatement of drug seeking in rats and squirrel monkeys 91, 92, and blocked nicotine-induced DA release in the NAc shell when animals were in the presence of a nicotine-associated context and cues 93. However, LY379268 also increased somatic signs of nicotine withdrawal in rats 92, suggesting this drug may have dysphoric effects that limit its therapeutic efficacy in humans. More recently a specific mGluR2 positive allosteric modulator, AZD8529, has been found to reduce nicotine self-administration and reinstatement in both rats and monkeys 94, 95, although the dysphoric effects of this drug remain to be evaluated. Similar to mGluR2/3 agonists, MPEP (2-methyl-6-(phenylethynyl)-pyridine), a selective mGluR5 antagonist, has been shown to reduce nicotine self-administration, nicotine-primed reinstatement, and nicotine-induced locomotor sensitization in rats and mice 96–99. In addition, knockout mice with genetic deletion of mGluR5 showed attenuated somatic signs of nicotine withdrawal 100, providing further support for mGluRs as new therapeutic targets for nicotine dependence.
In conclusion, the strong theoretical approach used in varenicline’s development can inform new medication discovery strategies for addictions as well as other central nervous system disorders, which have been notoriously difficult to treat 101. On the contrary, the review cites many studies in animals, non-human primates, and humans to indicate that much of the evidence surrounding varenicline’s efficacy as a smoking cessation agent converges across species. Future drug discovery will also inevitably incorporate technological advances in genetics, genomics and proteomics for highly specific, individualized approaches to smoking cessation 79.
Article Highlights:
Smoking is the leading cause of preventable death in the United States. While more than 70% of smokers express a desire to quit, fewer than 3% remain abstinent more than one year. There is a critical need for more efficacious smoking cessation aids.
The primary addictive component in tobacco is nicotine. In the brain, nicotine produces rewarding and psychostimulant effects mainly by binding to α4β2 nicotinic acetylcholine receptors on midbrain dopaminergic neurons, causing an increase in dopamine release in the nucleus accumbens, a key reward processing region.
Varenicline was developed by Pfizer as a selective partial agonist at the α4β2 receptor based on previous reports that cytisine, a plant-derived alkaloid, was used as a tobacco substitute during World War II.
Preclinical studies indicate that varenicline suppresses nicotine-induced dopamine release and attenuates nicotine conditioned place preferences, nicotine self-administration, and nicotine- or cue-induced reinstatement of drug-seeking behaviors.
Clinical studies indicate that varenicline is safe, well-tolerated and more efficacious in sustaining abstinence in smokers compared to placebo as well as to other currently available smoking cessation aids, including bupropion and nicotine replacement therapies.
Although early post-marketing surveillance suggested that potentially adverse neuropsychiatric and cardiovascular events were associated with varenicline use, subsequent studies indicate that the probability of these events is low. Nonetheless, caution is warranted for varenicline use in individuals who routinely operate heavy machinery.
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (Z1A DA000389), National Institutes of Health, USA.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. One referee declares that they have received honoraria for consulting and for lectures for the manufacturer of varenicline (Pfizer) as well as from other manufacturers of drugs for smoking cessation (GlaxoSmithKline and Novartis).
References
- 1.World Health Organization, Gender, women, and the tobacco epidemic. 2010.
- 2.World Health Organization. Tobacco Fact Sheet. 2015 [cited 2017 October 11]; Available from: http://www.who.int/mediacentre/factsheets/fs339/en/.
- 3.Center for Disease Control and Prevention. Tobacco Fact Sheet. 2017 [cited 2017 October 11]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm.
- 4.Center for Disease Control and Prevention. Chronic Disease Overview. 2017 [cited 2017 November 2]; Available from: https://www.cdc.gov/chronicdisease/overview/index.htm.
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed ed. 2013, Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 6.Messer K, Trinidad DR, Al-Delaimy WK, et al. Smoking cessation rates in the United States: a comparison of young adult and older smokers. Am J Public Health 2008. February;98(2):317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services., The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- 8.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2009;49:57–71.*This review summarizes key pharmacology underlying nicotine addiction.
- 9.Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci 1999. December;22(12):555–61. [DOI] [PubMed] [Google Scholar]
- 10.Xi ZX. Preclinical Pharmacology, Efficacy and Safety of Varenicline in Smoking Cessation and Clinical Utility in High Risk Patients. Drug Healthc Patient Saf 2010. April 01;2010(2):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 2002. December;53(4):447–56. [DOI] [PubMed] [Google Scholar]
- 12.Garduno J, Galindo-Charles L, Jimenez-Rodriguez J, et al. Presynaptic alpha4beta2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J Neurosci 2012. October 24;32(43):15148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 2003. June 26;423(6943):949–55. [DOI] [PubMed] [Google Scholar]
- 14.Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res 1985. December 02;348(2):355–8. [DOI] [PubMed] [Google Scholar]
- 15.Ikemoto S Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory . Neurosci Biobehav Rev 2010. November;35(2):129–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontieri FE, Tanda G, Orzi F, et al. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996. July 18;382(6588):255–7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Zhang L, Liang Y, et al. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci 2009. April 01;29(13):4035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brody AL, Mandelkern MA, London ED, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 2006. August;63(8):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins SS, Epping-Jordan MP, Koob GF, et al. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 1999. April;62(4):743–51. [DOI] [PubMed] [Google Scholar]
- 20.Pons S, Fattore L, Cossu G, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 2008. November 19;28(47):12318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Zhao-Shea R, McIntosh JM, et al. Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Mol Pharmacol 2012. April;81(4):541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 2004. November 05;306(5698):1029–32. [DOI] [PubMed] [Google Scholar]
- 23.Carroll FI, Blough BE, Mascarella SW, et al. Bupropion and bupropion analogs as treatments for CNS disorders. Adv Pharmacol 2014;69:177–216. [DOI] [PubMed] [Google Scholar]
- 24.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther 2003. November;307(2):526–34. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction 1994. November;89(11):1461–70. [DOI] [PubMed] [Google Scholar]
- 26.Jackson A Partial Agonist. In: Stolerman IP, ed. Encyclopedia of Psychopharmacology 2010. [Google Scholar]
- 27.Jeong SH, Newcombe D, Sheridan J, et al. Pharmacokinetics of cytisine, an alpha4 beta2 nicotinic receptor partial agonist, in healthy smokers following a single dose. Drug Test Anal 2015. June;7(6):475–82. [DOI] [PubMed] [Google Scholar]
- 28.Radchenko EV, Dravolina OA, Bespalov AY. Agonist and antagonist effects of cytisine in vivo. Neuropharmacology 2015. August;95:206–14. [DOI] [PubMed] [Google Scholar]
- 29.Prochaska JJ, Das S, Benowitz NL. Cytisine, the world’s oldest smoking cessation aid. BMJ 2013. August 23;347:f5198. [DOI] [PubMed] [Google Scholar]
- 30.Dale HH, Laidlaw PP. The physiological action of cytisine, the active alkaloid of laburnum (cytisus-laburnum). Journal of Pharmacology and Experimental Therapeutics 1912;3(3):22. [Google Scholar]
- 31.West R, Zatonski W, Cedzynska M, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med 2011. September 29;365(13):1193–200. [DOI] [PubMed] [Google Scholar]
- 32.Hajek P, McRobbie H, Myers K. Efficacy of cytisine in helping smokers quit: systematic review and meta-analysis. Thorax 2013. November;68(11):1037–42. [DOI] [PubMed] [Google Scholar]
- 33.Reavill C, Walther B, Stolerman IP, et al. Behavioural and pharmacokinetic studies on nicotine, cytisine and lobeline. Neuropharmacology 1990. July;29(7):619–24. [DOI] [PubMed] [Google Scholar]
- 34.Walker N, Howe C, Glover M, et al. Cytisine versus nicotine for smoking cessation. N Engl J Med 2014. December 18;371(25):2353–62. [DOI] [PubMed] [Google Scholar]
- 35.Walker N, Bullen C, Barnes J, et al. Getting cytisine licensed for use world-wide: a call to action. Addiction 2016. November;111(11):1895–98. [DOI] [PubMed] [Google Scholar]
- 36.Drug Patent Watch. Chantix Drug Profile. 2017 [cited 2017 Nov. 22]; Available from: https://www.drugpatentwatch.com/p/tradename/CHANTIX.
- 37.Pfizer Labs, P., Chantix (Varenicline) Tablets Medication Guide. 2009.
- 38.Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005. May 19;48(10):3474–7.** This article describes the synthesis of varenicline as a smoking cessation agent.
- 39.Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 2007. March;52(3):985–94. [DOI] [PubMed] [Google Scholar]
- 40.Rollema H, Coe JW, Chambers LK, et al. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci 2007. July;28(7):316–25. [DOI] [PubMed] [Google Scholar]
- 41.Bordia T, Hrachova M, Chin M, et al. Varenicline is a potent partial agonist at alpha6beta2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther 2012. August;342(2):327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol 2010. April;381(4):361–70. [DOI] [PubMed] [Google Scholar]
- 43.Goutier W, Kloeze MB, McCreary AC. The effect of varenicline on the development and expression of nicotine-induced behavioral sensitization and cross-sensitization in rats. Addict Biol 2015. March;20(2):248–58. [DOI] [PubMed] [Google Scholar]
- 44.Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 2012. October;15(9):1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor EC, Parker D, Rollema H, et al. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2010. February;208(3):365–76. [DOI] [PubMed] [Google Scholar]
- 46.Spiller K, Xi ZX, Li X, et al. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology 2009. July;57(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igari M, Alexander JC, Ji Y, et al. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 2014. January;39(2):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Moura FB, McMahon LR. The contribution of alpha4beta2 and non-alpha4beta2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice. Psychopharmacology (Berl) 2017. March;234(5):781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson NE, Min W, Hackett A, et al. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol Biol Psychiatry 2010. December 01;34(8):1455–64. [DOI] [PubMed] [Google Scholar]
- 50.Biala G, Staniak N. Varenicline and mecamylamine attenuate locomotor sensitization and cross-sensitization induced by nicotine and morphine in mice. Pharmacol Biochem Behav 2010. August;96(2):141–7. [DOI] [PubMed] [Google Scholar]
- 51.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 2011. January;13(1):41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marks MJ, O’Neill HC, Wynalda-Camozzi KM, et al. Chronic treatment with varenicline changes expression of four nAChR binding sites in mice. Neuropharmacology 2015. December;99:142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 2006. January;34(1):121–30. [DOI] [PubMed] [Google Scholar]
- 54.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther 2009. March;31(3):463–91. [DOI] [PubMed] [Google Scholar]
- 55.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006. July 05;296(1):56–63.* This article reports a seminal clinical trial that contributed to approval of varenicline for smoking cessation.
- 56.Ramon JM, Morchon S, Baena A, et al. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med 2014. October 8;12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore TJ. Strong Safety Signal Seen for New Varenicline Risks. 2008. [cited 2017 Nov. 24]; Available from: http://www.ismp.org/docs/vareniclinestudy.asp
- 58.Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006. August 14–28;166(15):1571–7. [DOI] [PubMed] [Google Scholar]
- 59.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006. July 05;296(1):47–55.* This article reports a seminal clinical trial that contributed to approval of varenicline for smoking cessation.
- 60.Niaura R, Hays JT, Jorenby DE, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin 2008. July;24(7):1931–41. [DOI] [PubMed] [Google Scholar]
- 61.Waknine Y FDA Approvals: Chantix. Medscape 2006. [cited 2017 Nov. 24]; Available from: https://www.medscape.org/viewarticle/532569
- 62.Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 2008. August;63(8):717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav 2008. Nov-Dec;32(6):664–75. [DOI] [PubMed] [Google Scholar]
- 64.Benli AR, Erturhan S, Oruc MA, et al. A comparison of the efficacy of varenicline and bupropion and an evaluation of the effect of the medications in the context of the smoking cessation programme. Tob Induc Dis 2017;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cinciripini PM, Robinson JD, Karam-Hage M, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013. May;70(5):522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebbert JO, Croghan IT, Hurt RT, et al. Varenicline for Smoking Cessation in Light Smokers. Nicotine Tob Res 2016. October;18(10):2031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West R, Baker CL, Cappelleri JC, et al. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008. April;197(3):371–7. [DOI] [PubMed] [Google Scholar]
- 68.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2008. July 16(3):CD006103. [DOI] [PubMed] [Google Scholar]
- 69.Mills EJ, Wu P, Spurden D, et al. Efficacy of pharmacotherapies for short-term smoking abstinance: a systematic review and meta-analysis. Harm Reduct J 2009. September 18;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cahill K, Lindson-Hawley N, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016. May 09(5):CD006103.*This large-scale review compares the efficacy of cytisine, varenicline, bupropion, and NRTs in smoking cessation.
- 71.Loughead J, Ray R, Wileyto EP, et al. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry 2010. April 15;67(8):715–21. [DOI] [PubMed] [Google Scholar]
- 72.Hartwell KJ, Lematty T, McRae-Clark AL, et al. Resisting the urge to smoke and craving during a smoking quit attempt on varenicline: results from a pilot fMRI study . Am J Drug Alcohol Abuse 2013. March;39(2):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lesage E, Aronson SE, Sutherland MT, et al. Neural Signatures of Cognitive Flexibility and Reward Sensitivity Following Nicotinic Receptor Stimulation in Dependent Smokers: A Randomized Trial . JAMA Psychiatry 2017. June 01;74(6):632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franklin T, Wang Z, Suh JJ, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 2011. May;68(5):516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cinciripini PM, Green CE, Robinson JD, et al. Benefits of varenicline vs. bupropion for smoking cessation: a Bayesian analysis of the interaction of reward sensitivity and treatment. Psychopharmacology (Berl) 2017. June;234(11):1769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Food and Drug Administration. Varenicline (marketed as Chantix) Information. 2016. [cited 2017 Nov. 24]; Available from:https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106540.htm.
- 77.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016. June 18;387(10037):2507–20.**This comprehensive clinical trial indicates that varenicline does not augment the risk of adverse neuropsychiatric events compared to bupropion, NRT, or placebo.
- 78.Littlewood RA, Claus ED, Wilcox CE, et al. Moderators of smoking cessation outcomes in a randomized-controlled trial of varenicline versus placebo. Psychopharmacology (Berl) 2017. December;234(23–24):3417–29. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Mancilla B, Marrer E, Kehren J, et al. Central nervous system drug development: an integrative biomarker approach toward individualized medicine. NeuroRx 2005. October;2(4):683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Food and Drug Administration. Anti-Smoking Medicine Chantix Banned. 2008. [cited 2017 Nov. 24]; Available from: https://www.faa.gov/news/updates/?newsId=56363.
- 81.Selcuk EB, Sungu M, Parlakpinar H, et al. Evaluation of the cardiovascular effects of varenicline in rats. Drug Des Devel Ther 2015;9:5705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benowitz NL, West R, Pipe A, Hays T, St. Aubin L, McRane T, Anthenelli R PA1–3: Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch: A Double-Blind, Randomized, Placebo Controlled Clinical Trial. Society for Research on Nicotine and Tobacco; 2017; Florence, Italy; 2017.* This conference proceeding suggests that varenicline does not convey additional risk over bupropion or NRT for adverse cardiovascular events.
- 83.U.S. Department of Health and Human Services., E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. 2016. [PubMed]
- 84.American Lung Association. E-cigarettes and Lung Health. 2016. [cited 2018 March 7]; Available from: http://www.lung.org/stop-smoking/smoking-facts/e-cigarettes-and-lung-health.html
- 85.Rehan HS, Maini J, Hungin AP. Vaping versus smoking: A quest for efficacy and safety of E-cigarette. Curr Drug Saf 2018. February 26. [DOI] [PubMed] [Google Scholar]
- 86.Jordan CJ, Andersen SL. Sensitive periods of substance abuse: Early risk for the transition to dependence. Dev Cogn Neurosci 2017. June;25:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Substance Abuse and Mental Health Services Administration, 2014. National Survey onDrug Use and Health: Detailed Tables. 2015, Center for Behavioral Health Statistics and Quality: Rockville, MD. [Google Scholar]
- 88.Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin 2009. June;30(6):723–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998. January 08;391(6663):173–7. [DOI] [PubMed] [Google Scholar]
- 90.Cross AJ, Anthenelli R, Li X. Metabotropic Glutamate Receptors 2 and 3 as Targets for Treating Nicotine Addiction. Biol Psychiatry 2017. November 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Justinova Z, Le Foll B, Redhi GH, et al. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology (Berl) 2016. May;233(10):1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liechti ME, Lhuillier L, Kaupmann K, et al. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 2007. August 22;27(34):9077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D’Souza MS, Liechti ME, Ramirez-Nino AM, et al. The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology 2011. September;36(10):2111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, D’Souza MS, Nino AM, et al. Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats. Psychopharmacology (Berl) 2016. May;233(10):1801–14. [DOI] [PubMed] [Google Scholar]
- 95.Justinova Z, Panlilio LV, Secci ME, et al. The Novel Metabotropic Glutamate Receptor 2 Positive Allosteric Modulator, AZD8529, Decreases Nicotine Self-Administration and Relapse in Squirrel Monkeys. Biol Psychiatry 2015. October 1;78(7):452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kenny PJ, Paterson NE, Boutrel B, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci 2003. November;1003:415–8. [DOI] [PubMed] [Google Scholar]
- 97.Paterson NE, Semenova S, Gasparini F, et al. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003. May;167(3):257–64. [DOI] [PubMed] [Google Scholar]
- 98.Tessari M, Pilla M, Andreoli M, et al. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol 2004. September 19;499(1–2):121–33. [DOI] [PubMed] [Google Scholar]
- 99.Bespalov AY, Dravolina OA, Sukhanov I, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology 2005;49 Suppl 1:167–78. [DOI] [PubMed] [Google Scholar]
- 100.Stoker AK, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 2012. May;221(2):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wegener G, Rujescu D. The current development of CNS drug research. Int J Neuropsychopharmacol 2013. August;16(7):1687–93. [DOI] [PubMed] [Google Scholar]
- 102.Funk D, Lo S, Coen K, et al. Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse . Behav Brain Res 2016. January 1;296:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panlilio LV, Hogarth L, Shoaib M. Concurrent access to nicotine and sucrose in rats. Psychopharmacology (Berl) 2015. April;232(8):1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schassburger RL, Levin ME, Weaver MT, et al. Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology (Berl) 2015. March;232(5):975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costello MR, Reynaga DD, Mojica CY, et al. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology 2014. July;39(8):1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mello NK, Fivel PA, Kohut SJ, et al. Effects of chronic varenicline treatment on nicotine, cocaine, and concurrent nicotine+cocaine self-administration. Neuropsychopharmacology 2014. April;39(5):1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levin ME, Weaver MT, Palmatier MI, et al. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res 2012. March;14(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tonstad S, Tonnesen P, Hajek P, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006. July 5;296(1):64–71. [DOI] [PubMed] [Google Scholar]
- 109.Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010. January 18;121(2)221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]


