Abstract
Barrier surfaces such as the epithelium lining the respiratory and gastrointestinal (GI) tracts, the endothelium comprising the blood-brain barrier (BBB), and placental trophoblasts provide key physical and immunological protection against viruses. These barriers utilize nonredundant mechanisms to suppress viral infections including the production of interferons (IFNs), which induce a strong antiviral state following receptor binding. However, whereas type I IFNs control infection systemically, type III IFNs (IFN-λs) control infection locally at barrier surfaces and are often preferentially induced by these cells. In this review, we focus on the role of IFN-λ at barrier surfaces, focusing on the respiratory and GI tracts, the BBB, and the placenta and how these IFNs act to suppress viral infections.
Keywords: Barrier Surfaces, Placenta, Interferon, Innate Immunity
Barrier Surfaces
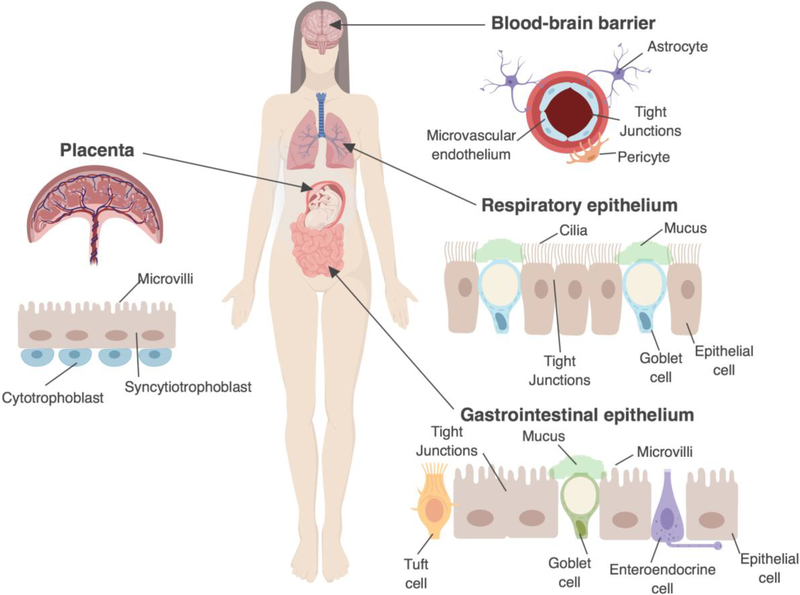
Cellular barriers establish both physical and immunological defenses to prevent viruses from breaching key entry portals into the human body. These barriers can include the epithelium lining the gastrointestinal (GI) and respiratory tracts, the microvasculature that forms the blood brain barrier (BBB), and fetal-derived trophoblasts that constitute the placental barrier during pregnancy (Figure 1). In addition to forming a physical barrier, these cell types sometimes also function as conduits at key cellular interfaces in order to exchange gases, small molecules, and nutrients. Thus, cell types that constitute barrier surfaces have evolved unique mechanisms to defend against viral infections, while retaining their critical role in maintaining cellular homeostasis. Breakdown of these barriers can have far-reaching impacts. For example, disruption of the placental barrier can allow for pathogenic microorganisms to gain access to the fetal compartment, which can induce fetal demise and/or congenital malformations in some cases [1].
Figure 1: Protective cellular barriers of the human body.
The cell composition of the blood-brain barrier, respiratory tract, gastrointestinal tract, and placenta are shown. The blood-brain barrier is made up in part of microvascular endothelial cells which form a physical barrier between the brain and the blood. The respiratory epithelium is composed of epithelial cells and goblet cells, which secrete mucus. The respiratory epithelial cells have cilia which beat in concert to clear mucus. The gastrointestinal tract contains enterocytes which have microvilli and goblet cells which secrete mucus. The human placenta is composed in part by the outermost syncytiotrophoblasts and inner cytotrophoblasts. Syncytiotrophoblasts form a dense brush border, but unlike the respiratory and GI epithelium, does not contain junctional complexes between cells (as the syncytium is a continuous layer).
Each of the barrier cell types mentioned above have evolved unique defense mechanisms to limit access by viruses. In addition, these cell types have, in some cases, also co-evolved by sharing defensive strategies, despite their disparate locations throughout the body. These shared mechanisms include goblet cell-derived mucus secretions in the GI and respiratory tracts, which coat the cell surface with a protective barrier, the formation of junctional complexes limiting paracellular transport, and the formation of complex apical actin networks that limit direct passage of molecules across the cell surface, amongst others [2,3]. Secondary to physical (or natural) protective strategies is the innate immune system. The innate immune system is essential in alerting the body to pathogen infection and is highly evolutionarily conserved. The innate immune system is activated by the recognition of ‘non-self’ from ‘self’ through diverse pattern recognition receptors. The recognition of a foreign substance induces complex signaling pathways that are essential for mounting an immune response to the pathogen and, if necessary, to induce adaptive immunity. Interferons (IFNs) are key cytokines produced during innate immune detection of viral infections. IFNs play a primary role in barrier defenses and are important for barrier function and integrity in the face of viral infections. In this review, we discuss disparate barrier surfaces in the body and how type III IFNs play a critical role in antiviral defenses at these surfaces.
Interferons: discovery, induction, and signaling
IFNs are a diverse family of cytokines with potent antiviral activity against many classes of viruses [4]. IFNs consist of three families: type I, type II, and type III IFNs. In this review, we focus mainly on the antiviral activities of type I and III IFNs given their involvement at the interface of barrier surfaces. Type I IFN was discovered in 1957 by Isaacs and Lindenmann, who named the factor because of its ability to interfere with viral replication [5]. This family of IFNs includes many different subtypes, including 13 IFN-α subtypes and a single IFN-β subtype. Type I IFNs are located on chromosome 9 in humans and on chromosome 4 in mice [6]. In humans, type I IFN is located in an intron-less region of the chromosome where the alpha subtypes are located on the 3’ end, with IFN-β on the 5’ end of the locus [7,8]. Type I IFNs signal through the heterodimeric type I IFN receptor (IFNAR1/2) complex to induce hundreds of antiviral interferon stimulated genes (ISGs). IFNAR is expressed on all nucleated cells, which allows type I IFNs to produce a potent systemic antiviral state.
Type III IFNs are the most recently discovered family of IFNs. This family includes IFN-λ1, IFN-λ2, and IFN-λ3, also known as interleukin IL-29, IL-28A, and IL-28B [9,10]. In 2013, a fourth type III IFN, IFN-λ4, was discovered [11,12]. IFN-λ4 has been shown to induce antiviral activity against hepatitis C virus in cultured Huh7 liver cells [12]. However, it is nonfunctional in a large subset of the world’s human population due to a single nucleotide polymorphism (SNP) that causes a frameshift in the gene [11,12]. Type III interferons are located on chromosome 19 in humans and on chromosome 7 in mice [13]. Unlike the type I IFN locus, the type III genetic cluster consists of introns and exons within each IFN-λ gene [13,14]. Each IFN-λ gene has 5 exons; this characteristic shares homology with the IL-10 cytokine family [15]. Overall, aside from sharing homology with the IL-10 cytokine family, type III IFNs share the IL-10R2 receptor subunit, leading to the speculation that these cytokines might be evolutionarily related [4,16]. The receptor is heterodimeric and includes the other lambda receptor subunit, IFNLR1. However, whereas virtually all cells express the functional type I IFN receptor IFNAR, the expression of the type III receptor IFNLR complex is most commonly restricted to cells at mucosal and other barrier surfaces (e.g. in mice) [17]. Although the full repertoire of immune cells that do or do not respond to type III IFNs has yet to be fully elucidated, it is becoming clear that some immune cell populations may not be responsive to type III IFNs due to their lack of IFNLR expression [18]. Neutrophils are one of the few immune cells that express IFNLR and can respond to IFN-λ, representing an important bridge between innate and adaptive immunity [19,20]. Thus, whereas type I IFNs function in a broad systemic manner, type III IFNs produce a more localized antiviral state, which may be largely restricted to barrier-associated cell types.
Both type I and III IFNs are induced through the recognition of pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs). PRRs, such as Toll-like receptors (TLRs), recognize common features of microorganisms, thus providing them with a strategy to detect diverse and unrelated pathogens [21]. When PRRs recognize a PAMP, an intracellular signaling cascade is induced, thus altering the transcriptional profile of the cell and leading to the upregulation of transcription factors such as Interferon Regulatory Factors (IRFs) and NF-κB, which in turn induce IFNs. The induction of each class of IFNs has been shown to require slightly different proteins to bind to the promoter of a given gene [22]. For example, IFN-β induction requires the binding of NF-κB, AP-1, and phosphorylated IRF3 [23]. On the one hand, IRF7 does not typically bind to the promoter of IFNB in unstimulated cells, largely due to the fact that it is itself an ISG and must be upregulated before it can become fully expressed and activated. If IRF7 is present and phosphorylated, it can then bind to the IFNB promoter in the place of IRF3 [22,23]. On the other hand, a recent study showed that the promoter coding for IFN-λ1 has multiple NF-κB binding sites, suggesting that binding of multiple NF-κB proteins can induce expression. This study concluded that IFN-λ1 could be induced by the binding of multiple NF-κB proteins to the binding sites within the promoter region in human cells in vitro, without the requirement for IRF3 binding or another factor [24]. Once IFNs are expressed, they can then initiate a positive feedback loop acting through both autocrine and paracrine manners [25].
As discussed above, type I IFNs signal through the heterodimeric IFN-α/β receptor (IFNAR1/2) whereas type III IFNs signal through the heterodimeric IFN-λ receptor (IFNLR1/IL10R2) [26]. However, despite their distinct induction differences and usage of receptor complexes, once type I or III IFNs bind to their respective receptors, the downstream signaling process is almost identical and leads to the induction of hundreds of ISGs through the canonical signaling pathway [26,27]. However, the kinetics of ISG induction have been shown to differ in some cell types. For example, human primary hepatocytes express IFNLR and are able to respond to type III IFN; however, type I and III IFNs vary in the magnitude and induction pattern of ISGs induced in these cells [28]. Another group showed that both whereas both type I and III IFNs induce a similar number of ISGs, type III IFN induced a slightly different subset of ISGs in a polarized murine intestinal epithelial cell line [29]. Other studies have indicated that human stem cell-derived enteroids treated with either IFN-β or IFN-λ induce ISGs in a similar manner [30]. These results indicate that ISG induction by type I and III IFN is dependent on many factors, including IFN concentration, cell and tissue type, and time points assessed. Of relevance, the expression of IFNs and ISGs in various cells can trigger a state of antiviral immunity, and here we focus on barrier surfaces.
Antiviral Activity of IFN-λs at epithelial and endothelial barriers
The Respiratory Tract
The respiratory tract is a pseudostratified columnar epithelium composed of ciliated epithelial cells, mucus-secreting goblet cells, and basal cells (Figure 1). The epithelium forms a physical barrier in part due to the presence of tripartite junctional complexes (composed of tight and adherens junctions, and desmosomes) that form between neighboring cells to restrict the free flow of ions and solutes [31]. Two mechanical defense mechanisms in the respiratory tract are the beating of cilia on the apical surface of all ciliated cells and mucus secretion from goblet cells. Cilia beat in a synchronized motion to move mucus out of the respiratory tract in order to clear pathogens [32]. In contrast, junctional complexes form a belt-like structure along the apical-most domains of the paracellular cell surface to restrict viruses (and other pathogens ) from accessing subcellular domains [3]. Collectively, these defensive strategies work in concert to directly clear pathogens (e.g. viruses) from the lungs or to prevent their penetration into the bloodstream should they bypass other physical defenses. However, if these physical barriers are breached or weakened, which can occur in the context of both normal and abnormal physiologic states, IFNs represent a key antiviral defense.
The respiratory epithelium secretes type III IFNs as an antiviral response to viruses that might be damaging to this barrier [33–35]. Studies have shown that primary murine tracheal respiratory epithelial cells and murine lung epithelial cell lines are able to respond to both type I and III IFN [36,37]. Although these cells can respond to both types of IFNs, they preferentially induce type III IFNs in response to influenza A virus (IAV), respiratory syncytial virus (RSV), M. tuberculosis, and other viral infections [38–41]. In primary cultures of human airway epithelium grown at an air liquid interface (ALI), type III IFNs are preferentially secreted into both the apical and basolateral compartments in response to IAV infection [37,39]. Additionally, when ALI epithelial cell cultures were pretreated with recombinant IFN-λ, IAV replication was reduced relative to untreated cells [36]. Although recombinant type I IFN can restrict IAV replication, it is not typically secreted by these cells during a natural infection [36,42]. In mice, type III IFNs are also preferentially induced by IAV infection [33] and mice deficient in IL28RA exhibit higher levels of IAV replication compared to wild type controls [43]. Similar findings have been shown in the context of RSV infection [41]. Collectively, these studies indicate that IFN-λ is an important mediator of antiviral defenses in the respiratory tract.
The Gastrointestinal Tract
The GI tract is a complex surface that acts as a protective and immunological barrier in a diverse microbial environment. The GI epithelium is composed of at least seven distinct cell types, including Paneth cells, goblet cells, enterocytes, and enteroendocrine cells, amongst others (Figure 1). The physical barriers that comprise the respiratory epithelial barrier (described above) are largely shared by the GI epithelium, with the exception of beating cilia. In contrast to beating cilia, the GI epithelium contains a dense brush border at the apical surface of the epithelium, which is supported by a dense cortical actin network that acts to prevent viral access to the cytosol [3].
Historically, the role of IFNs in the GI tract has mostly been studied in the context of cell lines [44]. These cell line-based studies have shown that both type I and III IFNs can be rapidly induced upon the recognition of PAMPs and that these cells are able to mount an antiviral response against enteric viruses [29,45]. Type III IFNs have been shown to induce ISGs in intestinal-derived cell culture models in response to many important enteric viruses, including rotavirus, reovirus, norovirus and enteroviruses, tropic for the GI tract [46–49]. These studies have shown that IFN-λ has an important role at the GI epithelium; however, immortalized cell lines are often derived from malignancies, in which native healthy cell signaling pathways are inherently altered and therefore, these cell lines do not fully recapitulate the diversity of cell types present in the epithelium or their functionality.
Recently, new advances in primary intestinal stem cell-derived in vitro enteroid and organoid models have provided new systems to study enteric virus infections in the setting of a multicellular GI epithelium [reviewed in 50]. Several studies have shown the ability of human intestinal enteroids and organoids to respond to both type I and III IFNs and to induce IFNs and/or ISGs in response to enteric viral infections [47–49,51,52]. However, although human intestinal organoids induce the expression of both type I and III IFNs at the transcript level in response to rotavirus infections, only type III IFNs are secreted from infected cells [53,54], suggesting that the GI epithelial cells are preferentially secreting type III IFNs over type I IFNs. When intestinal enteroids or organoids are pretreated with either type I or III IFN, rotavirus replication is decreased, indicating that type I and III may induce similar antiviral states [46,52,55]. In mice, IFN-λ restricts norovirus and reovirus replication in the intestine [47,48,54]. Indeed, a growing body of work in cell lines -- primary stem cell-derived organoids-- as well as in vivo experiments in mice have demonstrated the prominent role of type III IFNs in restricting enteric virus infections. However, given that the GI epithelium is composed of multiple cell types, it remains unclear whether type I and III IFNs function equivalently in these cell types, or whether cell type specificity governs their functions. Concomitantly, given that enteric viruses exhibit a tropism for the cell types in which they infect the GI epithelium, whether related or unrelated enteric viruses exhibit differential susceptibility to IFN treatment remains unclear.
The Blood Brain Barrier
The BBB is composed of microvascular endothelial cells, pericytes, and astrocytes and is a selective transport membrane that serves as the protective barrier surrounding the brain (Figure 1). The BBB protects the central nervous system (CNS) from a wide variety of toxins and microorganisms in the blood, while allowing for the selective exchange of ions and solutes. Similar to polarized epithelial cells, the microvascular endothelial cells that comprise the BBB are connected by junctional complexes between adjacent cells. In addition to its barrier properties, the BBB microvasculature is important for the exchange of signals between the brain and the circulatory system. Beneath the endothelium is a continuous basement membrane that connects the microvasculature to the pericytes and endfoot astrocytes that further limit permeability of the barrier [56]. Disruption of the BBB is induced upon infection with several neurotropic viruses, such as West Nile Virus (WNV), and damage is caused by both host and viral factors [57,58].
Type III IFNs play important roles in antiviral defenses at the BBB [57,59]. However, the mechanisms by which type III IFNs restrict viral infections at this barrier site may be unique. Unlike the canonical mechanism of IFN-induced antiviral defenses through ISG induction, type III IFNs also function to protect mice from WNV infection through non-ISG-dependent mechanisms. For instance, mice lacking functional type III IFN signaling (Ifnlr1−/−) exhibit increased BBB permeability and higher viral titers after WNV infection relative to wild type controls [57]. However, unlike the canonical pathway of ISG induction, IFN-λ appears to exert its antiviral activity at least in part from a direct increase in endothelial barrier properties. Specifically, treatment of cultured mouse brain microvascular cells with recombinant IFN-λ increased transendothelial resistance values (TEER) in vitro, a measure of the ability of the endothelium to resist ion flow, through a transcription-independent mechanism [57]. This study suggested that these IFNs could elicit antiviral defenses of barrier cell types through ISG-independent mechanisms; however, whether this property is shared amongst other barrier cell types has yet to be determined.
The role of type III IFNs in the human BBB are less clear, owing in part to the difficulties of modeling this complex system ex vivo. Studies utilizing cultured human BBB microvascular endothelial cells suggest that type III IFNs might also play a key role in defense; human BBB endothelial cells could respond to synthetic ligands of viral RNA or to viral infections by potently inducing type III IFNs [60]. When immortalized human BBB microvascular endothelial cells were stimulated with the synthetic vRNA ligand poly(I:C), they responded by secreting high concentrations of IFN-λ compared to mock-treated cells. At low doses of poly(I:C), these cells also secreted higher concentrations of IFN-λ than IFN-β compared to mock-treated cells, suggesting that type III IFNs might be preferentially released in these cells, similar to what was surmised for epithelial-derived cell types [60].
Human and Murine Placental Structures
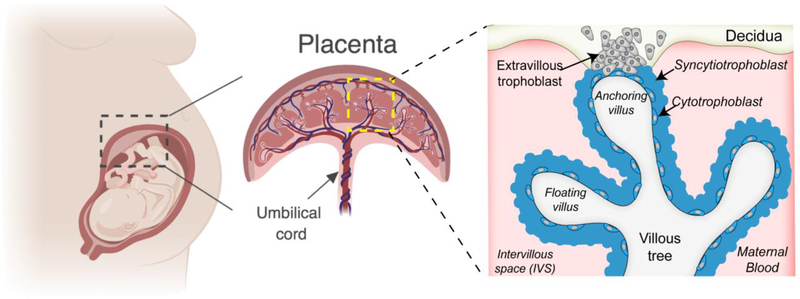
The placenta is a complex cellular barrier that forms the key interface between a mother and fetus during pregnancy (Figure 2). Unlike the cellular barriers described above, which are largely conserved functionally and morphologically between species, the structural complexity of the placenta varies greatly between eutherian organisms [reviewed in 32,33]. However, despite these differences, the placentas of these organisms must still form a protective barrier to prevent any infectious agents present in the maternal circulation from accessing the immunologically underdeveloped fetus. The human placenta is hemochorial, meaning that the fetal-derived chorion is in direct contact with maternal blood. The human placenta is composed of distinct types of fetal-derived trophoblast cells, macrophages (termed Hofbauer cells), endothelial cells, and fibroblasts. The syncytiotrophoblast (SYN) forms the outermost barrier of the human placenta and lines the chorionic villi, which fully develop in the first trimester and become bathed in maternal blood by the end of the first trimester, supplying nutrients to the developing fetus (Figure 2) [1]. The SYN is a highly unique cell type given that it does not contain any cellular junctions and instead exists as a large syncytium of shared cytosolic space and multiple nuclei. Subjacent to the SYN layer are cytotrophoblasts (CYTs), which are mononuclear cells that fuse through the activity of the endogenous retrovirus fusion protein syncytin/HERV-W to form the SYN [63]. Although the CYT layer is largely continuous through the first half of pregnancy, this layer becomes more discontinuous as the placenta becomes larger in later stages of gestation, because CYTs rapidly fuse to replenish the SYN layer to meet the demands of the growing placenta [1]. Extravillous trophoblasts (EVTs) reside at the tips of anchoring villi and imbed directly into the maternal decidua to anchor the fetal placenta to the uterus and are also responsible for remodeling the maternal microvasculature [1]. The mouse placenta is structurally distinct from the human placenta and differs in select cell types and architecture. For example, although the mouse placenta is also hemochorial, it contains spongiotrophoblasts, which are not found in the human placenta [64]. In addition, the mouse placenta contains two SYN layers, which are formed by distinct endogenous retrovirus fusion proteins [65] and in contrast to the human placenta, do not directly contact maternal blood [66]. However, in all cases, the placentas of eutherian organisms form the an interface between maternal and fetal blood and must therefore form a powerful protective barrier to protect the fetus from viral infections.
Figure 2: Human placental Structure.
A zoomed in villous tree shows floating and anchoring villi. The villous trees are lined by syncytiotrophoblasts and an inner layer of cytotrophoblasts (that become more discontinuous throughout pregnancy) that fuse to replenish the outer syncytial layer. Invasive extravillous trophoblasts extend from the villous tree into the maternal decidua and both anchor the placenta to the uterine wall and remodel the maternal microvasculature.
Type III IFNs in placental antiviral defenses
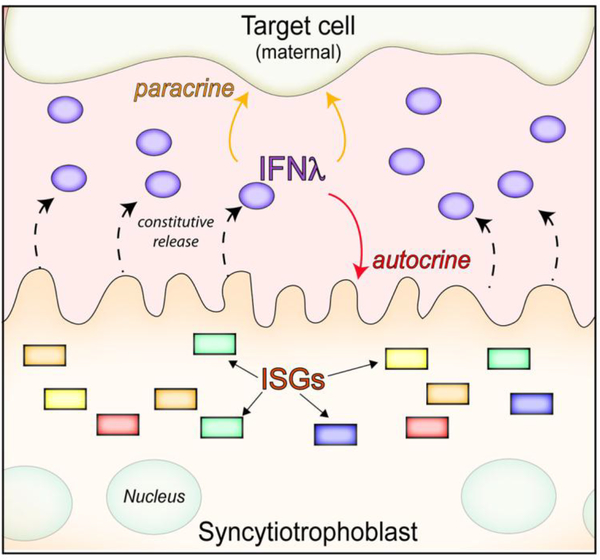
Growing evidence suggests that type III IFNs play essential roles in the protection of the human placenta from viral infections. Of note, unlike the other barrier cell types described above, which require PAMP-mediated IFN-λ induction, type III IFNs are constitutively released from human trophoblasts in the absence of any viral infections [67–69] (Figure 3). Consistent with this, medium isolated from uninfected primary full-term human trophoblast cells or from chorionic villi isolated from human mid-gestation placentas can exert potent antiviral activity against disparate RNA and DNA viruses, including teratogenic viruses such as Zika virus (ZIKV), Rubella virus (RuV), human cytomegalovirus (hCMV), varicella zoster virus (VZV), and herpesviruses (HSV-1) [67–70]. In contrast, this medium does not restrict infection by non-viral pathogens including Toxoplasma gondii and Listeria monocytogenes [69]. Subsequent studies revealed that this medium contained IFN-λs1–3, although other antiviral components such as placental-specific chromosome 19 miRNA cluster (C19MC) miRNAs were also present in the medium [67,68,70]. However, type III IFNs have been reported to play an important role in the paracrine-mediated antiviral effects of trophoblast conditioned medium, because the protective antiviral effects (e.g to ZIKV) are lost in medium-treated cells known to be unresponsive to IFN-λs I (e.g. human placental fibroblasts), or, upon RNAi mediated reduction of IFNLR expression in human brain microvascular endothelial cells [67,68]. The major cell type responsible for IFN-λ release appears to be the SYN layer, as inhibiting fusion of CYTs to SYN by treatment with DMSO reduces ISG induction in cells exposed to placental conditioned medium relative to controls, and reciprocally, enhancing CYT-SYN fusion upon treatment with epidermal growth factor (EGF), triggers ISG induction [67]. Release of IFN-λ from the SYN layer in vivo might provide a mechanism by which the placenta delivers antiviral IFNs directly into maternal blood, given the direct contact of SYN with circulation. Consistent with a possible autocrine feedback of released IFN-λs on the placenta itself, isolated placental trophoblasts from full-term placentas and explants of mid-gestation chorionic villi expressed high levels of ISGs under basal (uninfected) states, suggesting that released IFN-λ might function to protect the placenta from viral infections [67,68]. Accordingly, treatment of isolated mid-gestation chorionic villi with a small molecule inhibitor of JAK1/2 (ruxolitinib), reduced the basal expression levels of ISGs and sensitized the SYN to infection with ZIKV, compared to DMSO-treated controls [68]. It is important to note that the release of type III IFNs from placental trophoblasts is not conserved in human trophoblast cell lines, including BeWo, JEG-3, and JAR cells [67]. However, these cell lines can robustly induce type III IFNs when treated with synthetic ligands of viral RNA or when infected with ZIKV [67]. Although the trophoblast cell lines mentioned above do not constitutively release type III IFNs under standard culture conditions, our group has developed a JEG-3-based three-dimensional (3-D) explant culture model that recapitulates the constitutive release of IFN-λs from primary trophoblasts [68]. Accordingly, JEG-3 cultured in 3-D become resistant to infection by viruses (vesicular stomatitis virus (VSV) and ZIKV) and conditioned medium isolated from these cells has been shown to induce ISGs and to confer protection from ZIKV infection in non-placental cells responding to type III IFNs [68,71]. Collectively, these human-based models point to an important role for type III IFNs in the protection of the human placenta from viral infections throughout gestation.
Figure 3: Syncytiotrophoblasts constitutively release IFN-λs.
Fetal derived syncytiotrophoblasts constitutively release IFN-λs that lead to the upregulation of ISGs in both autocrine (in the syncytium itself) and paracrine (presumably in maternal-derive tissue) manners.
The development of mouse-based models to delineate the role of type III IFNs in murine pregnancy have also provided important insights and support a pan-species role for type III IFNs in placental antiviral defenses. Utilizing a mouse model wherein the fetal-derived placenta lacks functional IFN-λ signaling in the setting of an IFN-λ competent pregnant dam, a recent study investigated whether type III IFNs protected against transplacental ZIKV transmission [72]. Infection of pregnant dams at day E6 of pregnancy (prior to full placentation) resulted in fetal demise in both IFN-λ signaling competent and deficient placentas, suggesting that type III IFNs played little to no role in placental antiviral defenses prior to placentation. In contrast, when pregnant dams were infected later in gestation (following complete placental development at ~E9.5), only placentas lacking functional type III IFN signaling displayed high rates of ZIKV vertical transmission, which correlated with high fetal viral loads, fetal demise, and/or congenital malformations. Analogously, treatment of pregnant dams carrying wild-type fetuses with recombinant IFN-λ2 decreased ZIKV infection by 2,500 fold relative to untreated pregnant dams [72]. This murine study showed that type III IFNs could protect against ZIKV vertical transmission in a gestational age-dependent manner. Another in vivo study also showed that recombinant IFN-λ2 treatment of pregnant dams restricted the vertical transmission of ZIKV [73]. However, it remains unknown whether the mouse placenta constitutively releases type III IFNs in a manner that recapitulates the human placenta, or whether these IFNs are induced systemically or in response to placental infection. Nonetheless, these studies have provided fundamental advances in our understanding of the in vivo role of type III IFNs in placental antiviral defenses and suggest that these IFNs may play functional roles in many eutherian organisms, although this remains to be determined more broadly.
Collectively, human and mouse studies have provided insights into IFN-λ signaling at the maternal-fetal interface and suggest that placental trophoblasts are key cellular components in this process. However, is it less clear what the paracrine-mediated impact of constitutive IFN-λ release could be in the context of pregnancy. It should be noted that human placental chorionic villi are contained within a maternal blood-filled cavity called the intervillous space (IVS). The IVS contains as much as 150mL of maternal blood at the later stages of pregnancy, which is delivered by hundreds of maternal spiral arteries in the uterus [1]. This space is highly contained and given this structure, it is possible that IFN-λ released from the placenta within this space remains localized and acts on key cells located at the maternal-fetal interface, rather than being transported systemically. Consistent with this, maternal-derived decidua tissue and fetal-derived amnion and chorion membranes and isolated epithelial cells are highly responsive to recombinant IFN-λ treatment, which suppresses ZIKV infection [72,73]. Thus, although the precise cellular targets of placental-derived IFN-λ remain unclear, several pieces of evidence indicate that both maternal- and fetal-derived tissues are likely to benefit from its protective effects during pregnancy.
Whereas type III IFNs promote antiviral defenses, which might protect the fetus and placenta from certain viral infections, the activity of type I IFNs appears to exert an opposing effect, with these types of IFNs damaging placental structure and function. Indeed, a recent study showed that mouse placentas derived from mouse fetuses expressing a single copy of IFNAR (Ifnar+/−) were resorbed at much higher rates than littermates homozygously lacking type I IFN signaling (Ifnar−/−) when dams were infected intravaginally with ZIKV [74]. As expected, the levels of ZIKV replication in Ifnar−/− fetuses was much higher than in Ifnar+/− littermates, supporting the hypothesis that this phenotype was not the result of enhanced viral replication in the Ifnar+/− fetus itself. Instead, fetal demise resulted from hypoxia and reabsorption, suggesting adverse impacts on placental function [74]. Consistent with this, human chorionic villi isolated from the second trimester of human pregnancy treated with recombinant type I, but not type III, IFN (IFN-β) displayed high numbers of syncytial knots, which are associated with placental damage and reduced production of essential pregnancy hormones [74]. This study suggests that type I IFNs might damage the placenta whereas type III IFNs might exert protective effects. It is intriguing that pregnant women with interferonopathies (which result from genetic alterations leading to constitutive production of type I IFNs in the absence of infection) [75] exhibit a range of pregnancy complications, including increased rates of preeclampsia, miscarriage, and pre-term birth, and their infants display congenital malformations reminiscent of teratogenic pathogens (such as microcephaly and growth restriction) in the absence of any maternal infections [76,77]. Although further studies are required to fully delineate the roles of type I and III IFNs during pregnancy, it is possible that critical differences may exist in type I versus III IFN signaling at the placental barrier.
Concluding Remarks
In addition to their role as physical barriers, it is becoming increasingly clear that the cell types comprising barriers in the human body are also dynamic and highly reactive chemical barriers that use type III IFNs to protect these sites from viral infections. The role of IFN-λ in the protection of the BBB and the GI and respiratory tracts have clearly established these molecules as essential in antiviral defenses in these critical tissues. The recent identification of type III IFNs as antiviral effectors released from placental trophoblasts also establishes a new framework in our understanding of how the placenta can restrict the vertical transmission of viruses. Furthermore, these findings suggest that defects in IFN-λ production or signaling could have profound impacts on maternal-fetal health and could sensitize the fetus to viral infections. However, many important questions remain unanswered (see Outstanding Questions). For example, future research into the intrinsic and/or extrinsic factors that might weaken placental type III IFN-mediated defenses could provide important insights into the mechanisms by which viruses may be vertically-transmitted to the developing fetus. Additionally, the pathway(s) and molecules that regulate the constitutive expression of IFN-λs from placental trophoblasts remain to be defined, as do the mechanism(s) by which these cells resist the possible cytotoxic effects of some ISGs expressed basally at high concentrations. Despite these outstanding questions, the emerging role of these IFNs at the maternal-fetal interface suggests that these cytokines might play a fundamental role in antiviral fetal defenses during pregnancy.
Highlights.
The epithelium comprising the respiratory and gastrointestinal (GI) tracts induce IFN-λs to enhance antiviral defenses.
IFN-λ signaling enhances junctional barrier function at the blood brain barrier (BBB) to protect against neurotropic viral infections.
Placental trophoblasts constitutively releases type III IFNs as a mechanism to protect the placenta from viral infections.
Recombinant IFN-λ treatment is protective from vertical transmission of Zika virus in vivo.
Outstanding Questions.
Do IFNs control enteric virus infections in a cell-type specific manner?
What is the impact of excessive IFN signaling in barrier cells?
Hows do type III IFNs enhance the barrier function of microvascular endothelial cells?
Do defects in IFN-λ signaling sensitize the fetus to viral infections?
What controls the constitutive release of IFN-λs from placental trophoblasts?
How do placental trophoblasts avoid the cytotoxic impact of high basal expression of ISGs?
Acknowledgements
We apologize to those whose work we did not cover in detail due to space restrictions. Our work on cellular barriers is supported by NIH R01-AI081759, R21-AI139576 , a Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award, and the UPMC Children’s Hospital of Pittsburgh Health System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kay H et al. (2011) The Placenta: From Development to Disease, Wiley-Blackwell. [Google Scholar]
- 2.Cornick S et al. (2015) Roles and regulation of the mucus barrier in the gut. Tissue Barriers 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delorme-Axford E and Coyne CB (2011) The Actin Cytoskeleton as a Barrier to Virus Infection of Polarized Epithelial Cells. Viruses 3, 2462–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider WM et al. (2014) Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol 32, 513–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaacs A and Lindenmann J (1987) Virus interference. I. The interferon. J. Interferon Res 7, 429–438 [DOI] [PubMed] [Google Scholar]
- 6.Hardy MP et al. (2004) Characterization of the type I interferon locus and identification of novel genes. Genomics 84, 331–345 [DOI] [PubMed] [Google Scholar]
- 7.Henco K et al. (1985) Structural relationship of human interferon alpha genes and pseudogenes. J. Mol. Biol 185, 227–260 [DOI] [PubMed] [Google Scholar]
- 8.Díaz MO et al. Structure of the Human Type-I Interferon Gene Cluster Determined from a YAC Clone Contig. , Genomics, 22 (1994) , 540–552 [DOI] [PubMed] [Google Scholar]
- 9.Kotenko SV et al. (2002) IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. DOI: 10.1038/ni875 [DOI] [PubMed]
- 10.Sheppard P et al. (2003) Il-28, IL–29 and their class II cytokine receptor IL-28R. Nat. Immunol 4, [DOI] [PubMed] [Google Scholar]
- 11.Prokunina-Olsson L et al. (2013) A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet 45, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming OJ et al. (2013) Interferon lambda 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J 32, 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wack A et al. Guarding the frontiers: The biology of type III interferons. , Nature Immunology (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotenko SV and Durbin JE (2017) Contribution of type III interferons to antiviral immunity: Location, location, location. J. Biol. Chem 292, 7295–7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabat R (2010) IL-10 family of cytokines. Cytokine Growth Factor Rev 21, 315–324 [DOI] [PubMed] [Google Scholar]
- 16.Walter MR (2014) The Molecular Basis of IL-10 Function: From Receptor Structure to the Onset of Signaling. Curr. Top. Microbiol. Immunol 380, 191–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommereyns C et al. (2008) IFN-Lambda Is Expressed in a Tissue-Dependent Fashion and Primarily Acts on Epithelial Cells In Vivo. PLoS Pathog 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly A et al. (2016) Immune Cell Profiling of IFN-lambda Response Shows pDCs Express Highest Level of IFN-lR1 and Are Directly Responsive via the JAK-STAT Pathway. J. Interf. Cytokine Res 36, 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blazek K et al. (2015) IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J. Exp. Med 212, 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broggi A et al. (2017) IFN-λ suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat. Immunol 18, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uematsu S and Akira S Toll-like receptors and innate immunity DOI: 10.1007/s00109-006-0084-y [DOI] [PubMed]
- 22.Durbin RK et al. (2013) Interferon induction and function at the mucosal surface. Immunol. Rev 255, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T et al. (1998) Structure and function of the interferon-beta enhanceosome. Cold Spring Harb. Symp. Quant. Biol 63, 609–20 [DOI] [PubMed] [Google Scholar]
- 24.Thomson SJP et al. (2009) The role of transposable elements in the regulation of IFN-lambda1 gene expression. Proc. Natl. Acad. Sci. U. S. A. 106, 11564–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seth RB et al. (2006) Antiviral innate immunity pathways. Rev. Cell Res. Cell Res. 16, [DOI] [PubMed] [Google Scholar]
- 26.De Weerd NA and Nguyen T (2012) The interferons and their receptors-distribution and regulation. Immunol. Cell Biol. 90, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingle HI et al. (2018) Distinct Effects of Type I and III Interferons on Enteric Viruses. Viruses 10, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolen CR et al. (2014) Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology 59, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvakumar TA et al. (2017) Identification of a Predominantly Interferon-λ-Induced Transcriptional Profile in Murine Intestinal Epithelial Cells. Front. Immunol 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhushal S et al. (2017) Cell polarization and epigenetic status shape the heterogeneous response to type III interferons in intestinal epithelial cells. Front. Immunol. 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima T et al. (2012) Regulation of tight junctions in upper airway epithelium. Biomed Res. Int. 2013, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghi A and Dolovich M (2016) Airway Epithelial Cell Cilia and Obstructive Lung Disease. Cells 5, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jewell NA et al. (2010) Lambda Interferon Is the Predominant Interferon Induced by Influenza A Virus Infection In Vivo. J. Virol 84, 11515–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mordstein M et al. (2010) Lambda Interferon Renders Epithelial Cells of the Respiratory and Gastrointestinal Tracts Resistant to Viral Infections. J. Virol 84, 5670–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreakos E et al. (2017) Interferon- λs: Front-Line Guardians of immunity and Homeostasis in the Respiratory Tract. Front. Immunol 8, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson S et al. (2016) IFNλ is a potent anti‐influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol. Med 8, 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox JM et al. (2015) Interferon Lambda Upregulates IDO1 Expression in Respiratory Epithelial Cells After Influenza Virus Infection. J. Interf. Cytokine Res 35, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki A and Pillai PS (2014) Innate immunity to influenza virus infection. Nat. Publ. Gr 14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J et al. (2009) Human Alveolar Type II Cells Secrete Antiviral IL-29 (IFN-λ1) in Response to Influenza A Infection. J Immunol 182, 1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travar M et al. (2016) Type I, II, and III Interferons: Regulating Immunity to Mycobacterium tuberculosis Infection. Arch. Immunol. Ther. Exp 64, 19–31 [DOI] [PubMed] [Google Scholar]
- 41.Villenave R et al. (2015) Induction and Antagonism of Antiviral Responses in Respiratory Syncytial Virus-Infected Pediatric Airway Epithelium. J. Virol 89, 12309–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galani IE et al. (2017) Interferon-λ Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity 46, 875–890.e6 [DOI] [PubMed] [Google Scholar]
- 43.Mordstein M et al. (2008) Interferon-λ contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog 4, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambuy Y et al. (2005) The Caco-2 cell line as a model of the intestinal barrier : Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol 21, 1–26 [DOI] [PubMed] [Google Scholar]
- 45.Kotredes KP et al. (2017) The Protective Role of Type I interferons in the Gastrointestinal Tract. Front. Immunol 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pott J et al. (2011) IFN-λ determines the intestinal epithelial antiviral host defense. PNAS 108, 7944–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldridge MT et al. (2017) Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus. J. Virol 91, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nice TJ et al. (2015) Interferon Lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science (80-.). 347, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drummond CG et al. (2017) Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. PNAS 114, 1672–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanik W et al. (2018) Stem Cell-Derived Models of Viral Infections in the Gastrointestinal Tract. Viruses 10, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakim MS et al. (2018) Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids. Sci. Rep 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saxena K et al. (2017) A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. PNAS DOI: 10.1073/pnas.1615422114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pervolaraki K et al. (2017) Type i and Type iii interferons Display Different Dependency on Mitogen- activated Protein Kinases to Mount an antiviral state in the human gut. Front. Immunol 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahlakõiv T et al. (2015) Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections. PLoS Pathog DOI: 10.1371/journal.ppat.1004782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin X et al. (2016) Engineering Stem Cell Organoids. Cell Stem Cell 18, 25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbott NJ et al. (2009) Structure and function of the blood–brain barrier. Neurobiol. Dis 37, 13–25 [DOI] [PubMed] [Google Scholar]
- 57.Lazear HM et al. (2015) Interferon-l restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniels BP and Klein RS (2015) Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System. PLoS Pathog DOI: 10.1371/journal.ppat.1005096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douam F et al. (2017) Type III Interferon-Mediated Signaling Is Critical for Controlling Live Attenuated Yellow Fever Virus Infection In Vivo. MBio 8, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bramley JC et al. (2017) A Three-Dimensional Cell Culture System To Model RNA Virus Infections at the Blood-Brain Barrier. mSphere 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts RM et al. (2016) The evolution of the placenta. Reproduction DOI: 10.1530/REP-16-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maltepe E and Fisher SJ (2015) Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol 31, 523–52 [DOI] [PubMed] [Google Scholar]
- 63.Mi S et al. (2000) Syncytin is captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785–789 [DOI] [PubMed] [Google Scholar]
- 64.Croy B et al. (2014) The Guide to Investigation of Mouse Pregnacy, Academic Press, Elsevier. [Google Scholar]
- 65.Dupressoir A et al. (2005) Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci 102, 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Georgiades P et al. (2002) Comparative Developmental Anatomy of the Murine and Human Definitive Placentae. Placenta 23, 3–19 [DOI] [PubMed] [Google Scholar]
- 67.Bayer A et al. (2016) Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 19, 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corry J et al. (2017) Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal–fetal interface. Proc. Natl. Acad. Sci 114, 9433–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayer A et al. (2015) Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol 212, 71e1–71e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delorme-Axford E et al. (2013) Human placental trophoblasts confer viral resistance to recipient cells. PNAS 110, 12048–12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McConkey CA et al. (2016) A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci. Adv 2, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jagger BW et al. (2017) Gestational Stage and IFN-λ Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe DOI: 10.1016/j.chom.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J et al. (2017) Outcomes of Congenital Zika Disease Depend on Timing of Infection and Maternal-Fetal Interferon Action. Cell Rep 21, 1588–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yockey LJ et al. (2018) Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crow YJ (2011) Type I interferonopathies: A novel set of inborn errors of immunity. Ann. N. Y. Acad. Sci 1238, 91–98 [DOI] [PubMed] [Google Scholar]
- 76.Meuwissen MEC et al. (2016) Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med 213, 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrade D et al. (2015) Interferon-α and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol 67, 977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]