Abstract
Objective:
Paclitaxel and carboplatin (PC) is a standard initial therapy for advanced endometrial cancer. We evaluated the efficacy and tolerability of incorporating three novel agents into initial therapy.
Methods:
In this randomized phase II trial, patients with chemotherapy-naive stage 11I/IVA (with measurable disease) and stage IVB or recurrent (with or without measurable disease) endometrial cancer were randomly assigned to treatment with PC plus bevacizumab (Arm 1), PC plus temsirolimus (Arm 2) or ixabepilone and carboplatin (IC) plus bevacizumab (Arm 3). The primary endpoint was progression-free survival (PFS). Comparable patients on the PC Arm of trial GOG209 were used as historical controls. Secondary endpoints were response rate, overall survival (OS), and safety.
Results:
Overall, 349 patients were randomized. PFS duration was not significantly increased in any experimental arm compared with historical controls (p>0.039). Treatment HRs (92% CI) for Arms 1, 2, and 3 relative to controls were 0.81 (0.63–1.02), 1.22 (0.96–1.55) and 0.87 (0.681.11), respectively. Response rates were similar across arms (60%, 55% and 53%, respectively). Relative to controls, OS duration (with censoring at 36 months), was significantly increased in Arm 1 (p<0.039) but not in Arms 2 and 3; the HRs (92% CIs) were 0.71 (0.550.91), 0.99 (0.78–1.26), and 0.97 (0.77–1.23), respectively. No new safety signals were identified. Common mutations and rates of mismatch repair protein loss are described by histotype. Potential predictive biomarkers for temsirolimus and bevacizumab were identified.
Conclusion:
PFS was not significantly increased in any experimental arm compared to historical controls. NRG Oncology/Gynecologic Oncology Group Study GOG-86P
Keywords: endometrial cancer, paclitaxel, carboplatin, bevacizumab, temsirolimus, ixabepilone
INTRODUCTION
Paclitaxel and carboplatin (PC) is a standard initial therapy for advanced endometrial cancer. A large randomized, noninferiority study (Gynecologic Oncology Group study 209 [GOG 209]) of initial therapy for endometrial cancer showed that PC was not inferior to a three drug combination of paclitaxel, doxorubicin and cisplatin (TAP) in terms of progression free survival (PFS) or overall survival (OS) [1]. Bevacizumab, temsirolimus and ixabepilone have shown single agent activity in recurrent endometrial cancer [2–6]. We evaluated the efficacy and tolerability of incorporating three novel agents into the initial therapy of advanced endometrial cancer. The Cancer Genome Atlas (TCGA) recently reported common somatic mutations and molecular subtypes for endometrioid and serous endometrial carcinomas [7]. Here results of genomic testing of clinical trial samples for common somatic mutations and microsatellite instability were examined for associations with patient outcomes to identify potential predictive biomarkers of response.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients had FIGO stage III or IVA (with measurable disease) or Stage IVB or recurrent (with or without measurable disease) endometrial cancer [8]. Measurable disease was defined by RECIST 1.1 [9]. Histologic confirmation of the original primary tumor was required via central pathology review.
Patients were required to have a GOG performance status of 0, 1, or 2 and to have recovered from effects of recent surgery or radiotherapy. Adequate bone marrow function (platelet count ≥ 100,000/μL; absolute neutrophil count ≥ 1,500/μL), renal function (creatinine ≤ 1.5× institutional upper limit of normal [ULN]), hepatic function (bilirubin ≤1.5× ULN; ALT, AST, alkaline phosphatase ≤2.5× ULN), and neurologic function (neuropathy grade <1) were required. Baseline urine protein:creatinine ratio (UPCR) was required to be less <1. International normalized ratio (INR) and PTT were required to be ≤1.5× ULN (or an in therapeutic range INR, usually between 2 and 3, on therapeutic warfarin). Fasting cholesterol and triglycerides had to be grade 1 or less (<300 mg/dl and ≤ 2.5* ULN, respectively). Patients were excluded if they had active bleeding or pathologic conditions that carried a high risk of bleeding, including tumor that involved major vessels. Patients with known brain metastases or poorly controlled seizures were excluded. Patients must not have had major surgery or significant traumatic injury within 28 days before study entry or a history of abdominal fistula or perforation within the past 3 months. Patients with a current serious nonhealing wound, ulcer, or bone fracture were excluded. Patients with hypertension were permitted on study provided their blood pressure was ≤150/90 mmHg. Use of blood pressure medications to achieve and maintain blood pressure control was permitted. Patients were excluded for a history of myocardial infarction, unstable angina, cerebrovascular accident, transient ischemic attack, or subarachnoid hemorrhage within 6 months of the first date of study treatment; a history of New York Heart
Association grade 2 or worse congestive heart failure; or significant peripheral vascular disease. Patients were also excluded if they had known prior history of interstitial pneumonitis, baseline hypoxemia (grade 2 or greater), baseline dyspnea (grade 2 or greater) or uncontrolled diabetes (baseline HgbA1C > 8).
All patients signed an institutional review board (IRB) approved informed consent and research authorization permitting release of personal health information. The protocol was reviewed and approved annually by IRBs of the participating institutions.
Study Treatment
Patients were to received chemotherapy in combination with bevacizumab (Arms 1 and 3) or temsirolimus (Arm 2) for 6 cycles (one cycle = 3 weeks), followed by maintenance therapy with bevacizumab (Arms 1 and 3) or temsirolimus (Arm 2) until disease progression or adverse events prohibited further therapy. Patients who entered and were treated within <12 weeks of surgery, started bevacizumab or temsirolimus with cycle 2. If bevacizumab or temsirolimus were discontinued, patients continued on trial with PC (Arms 1 and 2) or ixabepilone and carboplatin (IC) (Arm 3). Up to two dose reductions of chemotherapy agents and temsirolimus were permitted for significant protocol defined toxicities. Bevacizumab was held or discontinued for significant protocol defined toxicities.
Arm 1: On day 1 of each cycle, patients received paclitaxel 175 mg/m2 intravenously (IV) over 3 hours, carboplatin area under the curve (AUC) 6 IV over 30 minutes, followed by bevacizumab 15 mg/kg IV. Patients with prior pelvic radiation received paclitaxel at 135 mg/m2 and carboplatin at AUC 5.
Arm 2: On day 1 of each cycle, patients received paclitaxel 175 mg/m2 IV over 3 hours and carboplatin AUC 5 IV over 30 minutes. Temsirolimus was given at 25 mg IV on days 1 and 8 (concurrent with chemotherapy) and days 1, 8 and 15 (during maintenance). Patients with prior pelvic radiation therapy received paclitaxel at 135 mg/m2 and temsirolimus at 20 mg.
Arm 3: On day 1 of each cycle, patients received ixabepilone 30 mg/m2 IV over 1 hour, carboplatin AUC 6 IV over 30 minutes, followed by bevacizumab 15 mg/kg IV. Patients with prior pelvic radiation received ixabepilone at 25 mg/m2 and carboplatin at AUC 5.
Response and Progression Assessment
A computed tomography scan (CT) of chest, abdomen, and pelvis was required within 4 weeks of the start of treatment and was repeated every 9 weeks for 2 years of protocol therapy or followup, then every 3 months; until disease progression. Disease progression and best response to study treatment were determined by RECIST 1.1.
Study Design
The study was designed as three Arm, single stage, historically controlled, randomized phase II study (Supplemental Figure S1). The purpose of this trial was to eliminate insufficiently active regimens that do not warrant further investigation. Patients enrolled to the PC Arm of GOG 209 with similar disease characteristics (patients with recurrent, Stage IV, or measurable Stage III/IVA endometrial carcinoma) were utilized as an historical reference for this trial. The three experimental regimens in this study are compared individually to the historical reference Arm. There was no concurrent enrollment to a reference Arm. A dynamic randomization allocation procedure was used that tends to balance the Arms across strata (1:1:1); with stratification factors defined by presence of measurable disease at study entry, recurrent disease at study entry, and history of pelvic or extended field radiation therapy. All patients were registered centrally at the GOG Statistical and Data Center. The randomized treatment assignment was only revealed following patient registration.
The primary endpoint is PFS. PFS is defined as the time alive, progression free from date of study entry. Patients with a status of alive, progression free are censored at their date of last followup. The date of last tumor assessment was not available electronically in the historical control Arm; therefore the date of last contact was used for all patients. Survival is defined as the duration of time from date of study entry until date of death. Patients with a status of living are censored at the date of last contact.
Secondary endpoints include OS and best confirmed response using RECIST 1.1. The frequency and severity of acute adverse effects was graded according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
A 35% decrease (relative hazard=0.65) in the progression or death rate was considered to be clinically significant. This relative decrease in progression or death rate translates into increasing the 12 month PFS from 39% to 54%. Fifty-eight PFS events in an experimental Arm would provide at least 85% power to detect a hazard ratio (experimental/ historical reference) of 0.65 in a one tail log rank test allowing for 3.9% type I error [10,11] when independence between PFS and treatment is assessed with a log rank test for an intent to treat analysis of all enrolled patients. GOG 209 required tumor assessment for patients with measurable or recurrent disease: within 28 days prior to initiating protocol therapy, prior to cycle 7, every 3 months for 1 year, every 6 months for Year 2, then annually for Years 3–5. GOG 209 required tumor assessment for patients with non-measurable and non-recurrent disease: within 28 days prior to initiating protocol therapy, prior to cycle 7, every 6 months for Years 1 and 2, then annually for Years 3–5. GOG 86P required tumor assessment: within 28 days prior to initiating protocol therapy, every 9 weeks for 2 years, then every 3 months. In both studies, scans were required until documentation of disease progression (or death). To lessen the potential for bias in the progression evaluation times between treatment Arms and historical controls, progression/death times were grouped over six consecutive 18 week time intervals [12]. Progressions were carried forward to the end of the interval. All progressions or deaths occurring after the 6th 18-week interval were censored at 25 months for this analysis.
Simulation suggested statistical power of 86% for a log rank test on grouped data to detect a 35% reduction in the hazard of progression or death when a sample size of 110 patients for each experimental Arm is targeted with a minimum followup of 2 years.
For OS, due to the followup differential between the experimental Arms and the reference Arm, all survival durations that were past 36 months were censored at 36 months when data were compared using statistical hypothesis tests. With this censoring, the minimum number of death events in each of the experimental Arms was at least 58.
Treatment hazard ratio estimates from proportional hazards models are reported for each experimental treatment and each endpoint with their associated confidence intervals (confidence level of 92.2%, 3.9% on each bound).
Translational Research Methods
Biospecimens were collected from patients who consented to participate in the translational research component of the study. Collection of archival formalin fixed paraffin embedded (FFPE) tumor and DNA from whole blood was coordinated by the GOG Tissue Bank. Tumor was macrodissected from FFPE tissues, enriching for regions with at least 60% tumor cell nuclei. Germline and somatic DNA were extracted using standard laboratory protocols.
Somatic mutation detection was performed using a panel of 25 candidate genes including targets in the PI3K/PTEN and RAS pathways, as well as other significantly mutated genes identified by TCGA endometrial cancer study. Paired normal and tumor DNA underwent massively parallel sequencing using a custom Roche Nimblegen SeqCap EZ system to enrich for targeted regions. Alignment was completed using BWA-MEM [13] with duplicate reads marked and removed using Picard tools (broadinstitute.github.io/picard/). Variant calling was completed using VarScan [14] and MuTect [15] on each tumor and normal pair. False positive filtering was performed as described in the VarScan2 paper [14] and implemented for SomaticSniper [16]. The AKT1 hotspot mutation E17K was assessed through a combination of digital droplet PCR and Sequenom based assays in cases with adequate samples.
An immunohistochemical analysis (IHC) using MSH6 and PMS2 was performed on representative sections of tumor for cases having available archival FFPE tumor as previously described [17]. Briefly, primary monoclonal antibodies against MSH6 (clone GRBP.P1/2.D4, diluted 1:200; Serotec Inc, Raleigh, NC) and PMS2 (clone A16–4, diluted 1:200; BD PhArmingen) were applied to 5 um thick FFPE tissue sections. Loss of expression of DNA mismatch repair (MMR) proteins MSH6 and PMS2 was recorded when there was no labeling of tumor cell nuclei and a positive internal control was present. A diagnosis of mismatch repair deficient (dMMR) was inferred from loss of either PMS2 or MSH6.
RESULTS
Accrual of 349 patients from 47 main members and Community Clinical Oncology Program (CCOP) sites was completed in 2.3 years, from 9/14/09 to 1/9/12 (Supplemental Figure S1). There were 11 patients deemed ineligible by central review. Ten patients refused all protocol treatment. Patient and tumor characteristics were well balanced between the Arms, although there was an imbalance of histologic type (Table 1). There were fewer patients with serous histology on Arm I (14% vs. 23–26%) and more with grade 2 endometrioid (31% vs. 2123%).
Table 1:
Patient Characteristics
| ARM 1 PC + Bevacizumab |
ARM 2 PC + Temsirolimus |
ARM 3 IC + Bevacizumab |
Historical Reference from GOG 0209 |
|
|---|---|---|---|---|
| Enrolled (N=349) | 116 | 115 | 118 | 462 |
| Median Age (Range) | 62 (36,87) | 63 (38,82) | 65 (37,89) | 61 (25–83) |
| Performance Status | ||||
| 0–1 | 106 (91%) | 109 (95%) | 113 (96%) | 435 (94%) |
| 2 | 10 (9%) | 6 (5%) | 5 (4%) | 27 (6) |
| FIGO 2009 Stage | ||||
| III | 12 (10%) | 13 (11%) | 10 (9%) | 79 (17%) |
| IVA | 1 (1%) | 0 (0%) | 2 (1%) | 1(1%) |
| IVB | 57 (49%) | 58 (51%) | 60 (51%) | 196 (42%) |
| Recurrent | 46 (40%) | 44 (38%) | 46 (39%) | 186 (40%) |
| Prior Pelvic Radiation Therapy | ||||
| Yes | 17 (15%) | 20 (17%) | 20 (17%) | 122 (26%) |
| No | 99 (85%) | 95 (83%) | 98 (83%) | 340 (74%) |
| Prior Hormonal Therapy | ||||
| Yes | 3 (3%) | 3 (3%) | 7 (6%) | 20 (4%) |
| No | 113 (97%) | 112 (97%) | 111 (94%) | 442 (96%) |
| Measurable Disease | ||||
| Yes | 89 (77%) | 85 (74%) | 85 (72%) | 369 (80%) |
| No | 27 (23%) | 30 (26%) | 33 (28%) | 93 (20%) |
| Histology | ||||
| Endometrioid, Grade 1 | 17 (15%) | 13 (11%) | 15 (13%) | 51 (11%) |
| Endometrioid, Grade 2 | 36 (31%) | 24 (21%) | 27 (23%) | 128 (28%) |
| Endometrioid, Grade 3 | 30 (26%) | 30 (26%) | 22 (19%) | 109 (24%) |
| Serous | 16 (14%) | 26 (23%) | 31 (26%) | 96 (21%) |
| Clear Cell | 6 (5%) | 4 (3%) | 6 (5%) | 13 (3%) |
| Other | 11 (9%) | 18 (16%) | 17 (14%) | 65 (14%) |
At least 70% received 6 cycles of carboplatin (70–83%) or paclitaxel (74–82%); 68% received at least 6 cycles of ixabepilone. Patients on Arm 1 received a median of 12 cycles (range, 0 to 78) of bevacizumab compared with a median of 9 cycles (range, 0 to 53) on Arm 3. A median of 8 cycles (range, 0 to 62) of temsirolimus was given on Arm 2. Disease progression was the most common reason for discontinuing treatment. However, there was a significant amount of discontinuation due to patient refusal, 13%, 15%, and 13%, or toxicity, 23%, 21% and 26%, in Arms 1, 2 and 3, respectively. Ten patients total, 7 on Arm 1, continue on maintenance treatment.
Treatment was to be continued until disease progression was documented. Acute adverse events were tabulated for all patients who initiated treatment. Overall, grade 4 was the maximum grade of acute adverse effects in 65%, 53% and 51% in Arms 1, 2 and 3 respectively. There were 16 deaths reported during the period of active treatment or within 30 days of last study treatment. Among these, 9 deaths were thought to be attributable to study treatment. These deaths resulted from sepsis (n=3); pulmonary embolism (n=1); SVT, febrile neutropenia, nausea and vomiting (n=1); dyspnea with infection (n=1); death not otherwise specified possibly due to sepsis, pneumonia or cardiac collapse (n=1); possibly treatment or other but not otherwise specified (n=1); and intestinal perforation (n=1). Safety summary with pertinent selected adverse events is shown in Table 2.
Table 2:
Safety Summary with Pertinent Selected Adverse Events
| PC + bevacizumab | PC + temsirolimus | IC + bevacizumab | ||||
|---|---|---|---|---|---|---|
| Type of Adverse Event | No. | % | No. | % | No. | % |
| Any Adverse Event, Any Grade | 112 | 100.0 | 113 | 100.0 | 114 | 100.0 |
| Any Adverse Event, Grades ≥3 | 105 | 93.7 | 111 | 98.2 | 109 | 95.6 |
| Any Adverse Event, Grade 5 | 4 | 3.6 | 6 | 5.3 | 6 | 5.3 |
| Adverse Event Leading to Study Drug Cessation | 30 (bev) | 26.8 | 26 (tem) | 23.0 | 28 (bev) 18 (ixa) | 24.6 15.8 |
| Serious Adverse Events, Any Grade | 48 | 42.8 | 57 | 50.4 | 53 | 46.5 |
| Serious Adverse Event, Grades ≥3 | 45 | 40.2 | 50 | 44.2 | 51 | 44.7 |
| Selected Adverse Events | ||||||
| Venous Thromboembolic Event, Grades ≥3 | 9 | 8.0 | 11 | 9.7 | 9 | 7.9 |
| Arterial Thromboembolic Event, Grades ≥3 | 1 | 0.9 | 0 | 0.0 | 1 | 0.9 |
| Bleeding, CNS, Any Grade | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Bleeding, Non-CNS, Grades ≥3 | 3 | 2.7 | 1 | 0.9 | 5 | 4.4 |
| Congestive Heart Failure, Grades ≥3 | 0 | 0.0 | 1 | 0.9 | 2 | 1.7 |
| Hypertension, Grades ≥3 | 18 | 16.1 | 3 | 2.7 | 19 | 16.7 |
| GI Fistula, Leak , Perforation, Any Grade | 3 | 2.7 | 2 | 1.8 | 5 | 4.4 |
| Proteinuria Grades ≥3 | 6 | 5.4 | 0 | 0.0 | 5 | 4.4 |
| Reversible Posterior Leukoencephalopathy Syndrome, Any Grade | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Anemia, Grades ≥3 | 28 | 25.0 | 35 | 31.0 | 40 | 35.1 |
| Thrombocytopenia, Grades ≥3 | 22 | 19.6 | 31 | 27.4 | 31 | 27.2 |
| Neutropenia, Grades ≥3 | 96 | 85.7 | 90 | 79.6 | 89 | 78.1 |
| Febrile neutropenia, Any Grade | 4 | 3.6 | 4 | 3.5 | 6 | 5.3 |
| Mucositis oral, Grade 3 | 0 | 0.0 | 7 | 6.2 | 0 | 0.0 |
| Pneumonitis, Any Grade | 0 | 0.0 | 7 | 6.2 | 1 | 0.9 |
| Hyperglycemia, Grades 1–2 | 34 | 30.3 | 32 | 28.3 | 34 | 29.8 |
| Hyperglycemia, Grades 3–4 | 10 | 8.9 | 16 | 14.1 | 4 | 3.5 |
| Hyperlipidemia (Cholesterol or Triglycerides), Grade 1 | 9 | 8.0 | 25 | 22.1 | 6 | 5.3 |
| Hyperlipidemia (Cholesterol or Triglycerides), Grade 2 | 2 | 1.8 | 10 | 8.8 | 0 | 0.0 |
| Hyperlipidemia (Cholesterol or Triglycerides), Grade 3 | 0 | 0.0 | 5 | 4.4 | 0 | 0.0 |
| Rash, Grades ≥2 | 3 | 2.7 | 19 | 16.8 | 4 | 3.5 |
| Neuropathy, Grades ≥3 | 5 | 4.5 | 4 | 3.5 | 4 | 3.5 |
Adverse events in the Arms with bevacizumab were compared with those in the Arm with temsirolimus. A greater proportion of patients had grade 3 or higher hypertension in the two Arms containing bevacizumab (16.1%, 16.7%) than in the temsirolimus Arm (2.7%) (Fisher’s exact 2 tailed test, p<0.001). Similarly, a greater proportion of patients had grade 3 or higher proteinuria in the two Arms containing bevacizumab (5.4%, 4.4%) than in the temsirolimus Arm (0%). There was more frequent pneumonitis (p=0.002), grade 2 or higher rash (p<0.001), grade 2 or higher oral mucositis (p<0.001) and grade 3 or higher hypertriglyceridemia (p=0.004) in the Arm containing temsirolimus than in the other two Arms. There were no statistically significant differences between the bevacizumab Arms and the temsirolimus Arms with respect to grade 3 or higher arterial thromboembolic events, venous thromboembolic events and non-CNS bleeding or any GI fistula, leak or perforations. All three Arms were compared in a global chi squared test for differences in the proportions of grade 3 or higher neutropenia, thrombocytopenia, and anemia; there were no statistically significant differences.
Response rates among patients with measurable disease did not differ significantly when each Arm was compared to historical controls. The overall response rates were 59%, 55% 53% and 51% in Arms 1, 2 and 3 and the historical reference Arm, respectively.
PFS, compared using a log-rank test on data grouped by time intervals, was not statistically significantly better in any experimental Arm (p>0.039) when each Arm was compared to historical controls (Figure 1). The hazard ratios (92.2% confidence intervals) for Arms 1, 2 and 3 relative to the historical reference Arm are 0.81 (0.63 to 1.02), 1.22 (0.96 to 1.55) and 0.87 (0.68 to 1.11), respectively.
Figure 1:

Progression-Free Survival Kaplan-Meier Plot for all Arms
OS duration with censoring at 36 months was statistically significantly (p<0.039) increased in Arm 1 relative to the historical reference Arm but was not significantly increased in Arms 2 or 3 (Figure 2). The hazard ratios (92.2% confidence intervals) for Arms 1, 2 and 3 relative to the historical reference Arm are 0.71 (0.55 to 0.91), 0.99 (0.78 to 1.26) and 0.97 (0.77 to 1.23), respectively.
Figure 2:

Overall Survival Kaplan-Meier Plot for all Arms
Translational Research Results
Tumor tissue was received from 325 patients, with corresponding matched normal DNA available for 282 patients. After tumor DNA extraction and quality control processing, high quality paired normal and tumor DNA was available for 243 patients and sequenced to a mean depth of at least 600x for each target gene. A minimum sequence depth of 100x was achieved for 96.1% of the targeted regions. The most commonly mutated genes based on histologic subtypes reflected previously published data [7,18] (Table 3). IHC was performed on 305 patient samples with available tissue. Evidence of MMR loss was found in 24% of 240 evaluable cases predominantly restricted to endometrioid cases, as expected. The response rate among the sequenced cases was not different between tumors with and without MMR loss. Using a proportional hazards model, PFS was also similar between patients with and without MMR loss (HR = 1.17, 95% CI: 0.84 – 1.64). These data also confirm the utility of a panel of IHC biomarkers or somatic mutations, including PTEN, ARID1A and DNA MMR, in the differential diagnosis of endometrioid and serous carcinomas [19].
Table 3:
Mutation frequencies and mismatch repair loss for common histologic subtypes
| Endometrioid, G1 (n=37) |
Endometrioid, G2 (n=69) |
Endometrioid, G3 (n=59) |
Serous (n=45) |
|||||
|---|---|---|---|---|---|---|---|---|
| Target | No. | % | No. | % | No. | % | No | % |
| PTEN | 27 | 73.0 | 49 | 71.0 | 34 | 57.6 | 4 | 8.9 |
| TP53 | 5 | 13.5 | 24 | 20.3 | 26 | 44.1 | 39 | 86.7 |
| PIK3CA | 14 | 37.8 | 37 | 53.6 | 31 | 52.5 | 13 | 28.9 |
| ARID1A | 14 | 37.8 | 33 | 47.8 | 23 | 39.0 | 1 | 2.2 |
| CTNNB1 | 21 | 56.8 | 22 | 31.9 | 18 | 30.5 | 1 | 2.2 |
| PIK3R1 | 10 | 27.0 | 18 | 26.1 | 11 | 18.6 | 3 | 6.7 |
| CTCF | 6 | 16.2 | 16 | 23.2 | 17 | 28.8 | 1 | 2.2 |
| KRAS | 7 | 18.9 | 14 | 20.3 | 14 | 23.7 | 1 | 2.2 |
| CHD4 | 10 | 27.0 | 8 | 11.6 | 12 | 20.3 | 5 | 11.1 |
| KMT2B | 3 | 8.1 | 16 | 23.2 | 13 | 22.0 | 1 | 2.2 |
| ARHGAP35 | 1 | 2.7 | 7 | 10.1 | 7 | 11.9 | 8 | 17.8 |
| PP2R1A | 4 | 10.8 | 3 | 4.3 | 3 | 5.1 | 13 | 28.9 |
| FBXW7 | 0 | 0 | 4 | 5.8 | 6 | 10.2 | 7 | 15.6 |
| ATM | 2 | 5.4 | 8 | 11.6 | 7 | 11.9 | 1 | 2.2 |
| MTOR | 2 | 5.4 | 6 | 8.7 | 8 | 13.6 | 2 | 4.4 |
| POLE | 4 | 10.8 | 4 | 5.8 | 5 | 8.5 | 1 | 2.2 |
| SPOP | 1 | 2.7 | 7 | 10.1 | 4 | 6.8 | 2 | 4.4 |
| TSC1 | 3 | 8.1 | 2 | 2.9 | 10 | 16.9 | 0 | 0 |
| FGFR2 | 4 | 10.8 | 5 | 7.2 | 5 | 8.5 | 0 | 0 |
| NF1 | 2 | 5.4 | 6 | 8.7 | 5 | 8.5 | 1 | 2.2 |
| TSC2 | 1 | 2.7 | 7 | 10.1 | 5 | 8.5 | 0 | 0 |
| ATR | 1 | 2.7 | 4 | 5.8 | 3 | 5.1 | 0 | 0 |
| ARID5B | 2 | 5.4 | 4 | 5.8 | 3 | 5.1 | 0 | 0 |
| PIK3R2 | 0 | 0 | 5 | 7.2 | 1 | 1.7 | 0 | 0 |
| RICTOR | 0 | 0 | 2 | 2.9 | 0 | 0 | 0 | 0 |
| PMS2* | 6 | 14.6 | 27 | 34.2 | 28 | 36.8 | 1 | 1.6 |
| MSH6* | 0 | 0 | 4 | 5.0 | 4 | 5.3 | 0 | 0 |
| Any loss* | 6 | 14.6 | 29 | 37.7 | 30 | 39.5 | 1 | 1.6 |
Immunohistochemistry results - number of samples tested are greater than shown in the header, which reflects sequencing data only.
TSC2
Mutations in TSC2, although uncommon, have previously been reported in endometrial cancer as well as other tumor types to be associated with clinical response to mTOR inhibition [20, 21]. TSC2 somatic mutations were identified here in 14 (5.8%) patients. Patients with TSC2 mutated tumors were represented in all treatment Arms: 4 (5.1%) of Arm 1, 4 (5.0%) of Arm 2, and 6 (7.1%) of Arm 3. Most, 13 (93%), patients with TSC2 mutations had endometrioid tumors. There was no difference in PFS based on TSC2 mutations. However, patients with TSC2 mutated tumors appeared to have a better outcome on Arm 2. Among temsirolimus treated patients, TSC2 mutation was predictive of an improved PFS (HR = 0.11, 95% CI: 0.02 – 0.79, Figure 3), but not among patients who did not receive temsirolimus (HR = 1.34, 95%CI: 65 – 2.74).
Figure 3:
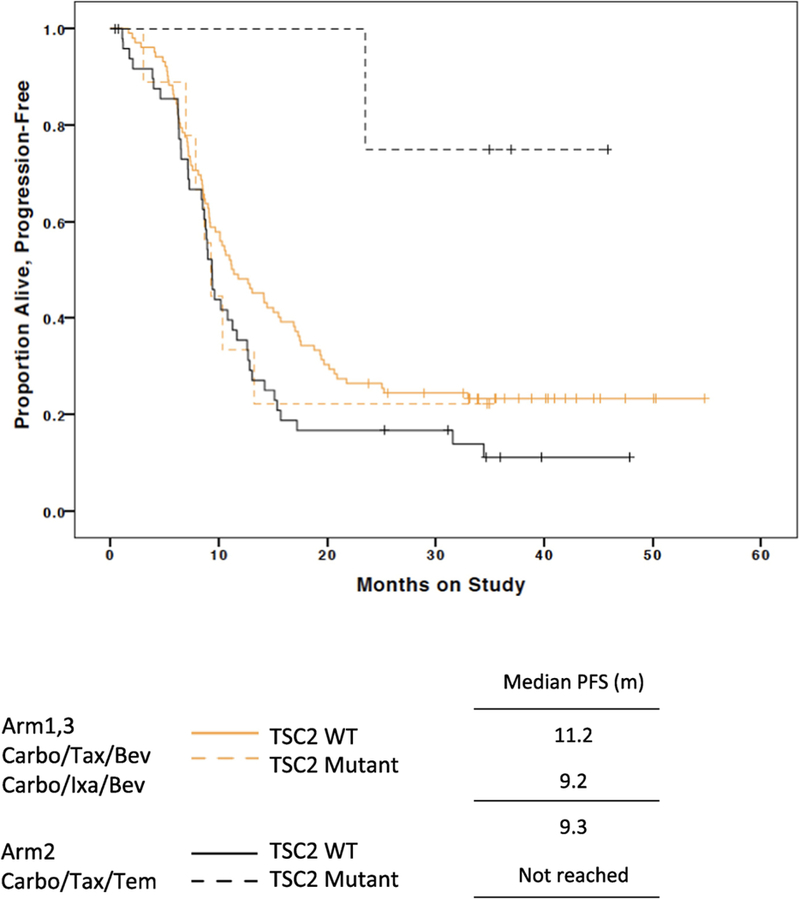
Progression-Free Survival Kaplan-Meier Plot for TSC2 mutations and temsirolimus treatment
CTNNB1
CTNNB1 mutations were identified in 64 (26%) of patients and 94% were in the canonical exon 3 region. Most patients with CTNNB1 mutated tumors, 61 (95%), had endometrioid tumors, including 21 (56.8%), 22 (31.9%) and 18 (30.5%) of grade 1, 2 and 3 endometrioid cases, respectively. CTNNB1 mutation appeared to be associated with longer PFS, similar to previous reports [20]. Patients with CTNNB1 mutated tumors appeared to have the greatest benefit when treated with bevacizumab. Patients with CTNNB1 mutated tumors treated on either of the bevacizumab Arms had longer PFS compared to patients without mutations that received bevacizumab (HR = 0.73, 95%CI: 0.60 – 0.91). However, CTNNB1 mutation was not associated with PFS seen in patients who did not receive bevacizumab ((HR = 1.06, 95%CI: 0.81 – 1.40).
AKT1
Prior work has suggested that AKT1 E17K mutations are associated with response to temsirolimus and longer PFS [20]. AKT1 E17K mutations were identified in this study in 6 (2.8%) of 217 evaluated patients—one was treated on Arm 1, two were treated on Arm 2, and three were treated on Arm 3. Two patients had a clinical response—one on Arm 1 and one on Arm 2. Two patients did not respond, and two were not evaluable for response.
DISCUSSION
Response rate and PFS is not significantly increased in any Arm. OS is significantly increased in the PC plus bevacizumab Arm when compared to historical controls treated with PC. The lack of contemporaneous control, the lack of improvement in response rate and PFS, and the imbalance of histotypes, necessitates interpreting the OS results with caution. A randomized phase II study of PC compared to PC plus bevacizumab was performed by the MITO group (END-2 trial) [22]. In the END-2 trial, all patients had one prior line of platinum based chemotherapy and progressed >6 months after completion of prior platinum. The END-2 trial showed increased response rate with PC plus bevacizumab (54% vs 73%) and improved PFS (8.7 vs 13 months, HR 0.57 [0.34, 0.96], p=0.036). The discordance between the two trials may reflect the different patient population (initial chemotherapy vs second line chemotherapy) and/or statistical fluctuations due to small same sizes in heterogenous patient populations. The ixabepilone results are consistent across studies. In the IXAMPLE2 study, usual care chemotherapy (doxorubicin or paclitaxel) was shown to have favorable OS outcome compared to ixabepilone in patients with 1–2 prior lines of chemotherapy [23]. Overall, toxicity in the current study was high, including 9 treatment related deaths, largely related to sequelae of myelosuppression, leading GOG/NRG Oncology to recommend carboplatin AUC 5 (instead of 6) for future studies in this patient population.
Previous work has demonstrated that patients with TSC2 mutated endometrial tumors have responded to mTOR inhibition in a phase II study without a non mTOR comparator [20]. However, this study demonstrated that the TSC2 associated response was restricted to mTOR treatment and not seen in other clinical trial Arms without mTOR inhibition indicating that TSC2 is a predictive biomarker for endometrial cancer response. CTNNB1 mutations can activate angiogenesis potentially making these tumors more responsive to VEGF inhibition with bevacizumab [24, 25]. The VEGF promoter has many binding sites for CTNNB1 and there is a direct correlation between VEGF expression activation of p-catenin signaling [26]. These findings support the role of CTNNB1 mutations as a potential predictive biomarker for bevacizumab treatment in endometrial cancer.
Pembrolizumab was granted accelerated approval by the US Food and Drug Administration for unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors in 2017. In this study, all patients had advanced or recurrent disease and represent a group likely to be eligible for evaluation for immune therapy based on MSI-H or dMMR. Our results, confirm rates of dMMR in this patient population similar to those reported in studies of primary tumor specimens [7,27]. In the current study, dMMR was noted in 33.5% (65/194) of endometrioid tumors and 1.6% (1/61) of serous tumors tested. Microsatellite instability testing was performed on all TCGA samples using seven repeat loci and found MSI-H in 40% of endometrioid tumors and 2% of serous tumors [7]. In the NRG Oncology/Gynecologic Oncology Group 210 study, 1,024 endometrioid tumors were assessed for MSI, MLH1 methylation and MMR protein expression, resulting in 36% MSI-H; 26% epigenetic MMR deficient (MSI-H with MLH1 methylation) and 10% as probable genetic MMR mutation (MSI-H and/or dMMR with absence of MLH1 methylation) [27].
This study did not identify an improvement in PFS for any of the study arms when compared to historical controls. An unplanned analysis of PFS stratified by stage/disease status did suggest a benefit (projected 2.8-month increase in median PFS of 8.3) for PC plus bevacizumab compared to historical controls (HR = 0.75, 92.2% CI: 0.58 – 0.95). Translational analyses were able to identify predictive biomarkers of reponse due to the randomized nature of this phase II study. Despite some limitations due to sample size, TSC2 mutations appear to be predictive of response to temsirolimus, consistent with known mechanisms of action. CTNNB1 mutations appear to be predictive of response to bevacizumab, possibly due to activation of VEGF; a hypothesis in need of future testing. The study overall supports the concept that even with a lack of difference in clinical outcome between study arms, proper design and conduct of translational research can yield important findings that should help to stratify this patient population for future treatment options.
Supplementary Material
Research Highlights.
Paclitaxel + carboplatin is standard initial therapy for advanced endometrial cancer
We assessed combinations with bevacizumab, temsirolimus or ixabepilone
PFS was not significantly improved compared to historical control
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office and Tissue Bank (U10 CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), the Gynecologic Oncology Group Tissue Bank (U24 CA114793), NRG Oncology (1 U10 CA180822), NRG Operations and Biospecimen Bank (U10 CA180868), the NRG Oncology Biospecimen Bank (U24 CA196067), and American Recovery and Reinvestment Act (ARRA) 3 U10 CA027469–29S1. Drs. Aghajanian, Gao and Soslow are supported in part by the MSK Cancer Center Support Grant P30 CA008748. Translational Research was funded by a Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C- AACR-DT0209).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Washington University School of Medicine, Duke University Medical Center, University of California Irvine Medical Center, University of Iowa Hospitals and Clinics, Women and Infants Hospital, Ohio State University Comprehensive Cancer Center, Rush University Medical Center, Walter Reed National Military Medical Center, University of Cincinnati, Cleveland Clinic Foundation, The Hospital of Central Connecticut, UCSF-Mount Zion, Memorial Sloan Kettering Cancer Center, Mayo Clinic, Cancer Research for the Ozarks NCORP, University of Texas Southwestern Medical Center, Georgia Center for Oncology Research and Education (CORE), Northwestern University, University of North Carolina at Chapel Hill, MD Anderson Cancer Center, University of Wisconsin Hospitals and Clinics, Roswell Park Cancer Institute, University of Colorado Cancer Center - Anschutz Cancer Pavilion, Women’s Cancer Center of Nevada, University of Hawaii, Abington Memorial Hospital, University of Mississippi Medical Center, State University of New York Downstate Medical Center, Cooper Hospital University Medical Center, Carolinas Medical Center/Levine Cancer Institute, William Beaumont Hospital, Abramson Cancer Center of the University of Pennsylvania, University of Chicago, Aurora Women’s Pavilion of Aurora
West Allis Medical Center, Virginia Commonwealth University, Penn State Milton S. Hershey Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Stony Brook University Medical Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, University of Virginia, Case Western Reserve University, Yale University, Yale University, University of Texas - Galveston, Michigan Cancer Research Consortium Community Clinical Oncology Program, Delaware/Christiana Care CCOP, University of Minnesota Medical Center-Fairview, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, University of Kentucky, Moffitt Cancer Center and Research Institute, Saint Joseph’s Hospital and Medical Center, Scott and White Memorial Hospital, Kalamazoo CCOP, Northern Indiana Cancer Research Consortium, and Iowa-Wide Oncology Research Coalition NCORP.
Footnotes
CONFLICT OF INTEREST STATEMENT
Outside the submitted work, C. Aghajanian has participated on the advisory boards of Tesaro, Clovis, Cerulean, Bayer, and VentiRx; she has also participated on the steering committee of Mateon Therapeutics. Outside the submitted work, M. A. Powell has received consultancy fees from Roche/Genentech, AstraZeneca, Clovis Oncology, Tesaro, and Merck; he has also received payments for lectures (e.g., speaker’s bureau) from Roche/Genentech, AstraZeneca, Clovis Oncology, Tesaro, and Merck; he has also received royalties from Up to Date. For the work under consideration, A. Alvarez Secord has received grant funding from NRG Oncology/Legacy GOG; outside the submitted work, she has also received consultancy fees from Alexion, Astex, AstraZeneca, Boerhinger Ingelheim, GlaxoSmithKline, Clovis, Janssen/Johnson & Johnson, Myriad, Roche/Genentech, and Tesaro; her institution has also received grants/pending grants from AbbVie, Amgen, Astellas Pharma Inc., Astex Pharmaceuticals Inc., AstraZeneca, Boerhinger Ingelheim, Bristol Myers Squibb, Eisai, Endocyte, Exelixis, Incyte, Merck, PharmaMar, Prima Biomed, Roche/Genentech, and Tesaro. Outside the submitted work, K. S. Tewari has participated in a speaker’s bureau for Roche. For the work under consideration, D. M. O’Malley’s institution has received grant funding from NRG Oncology/GOG; outside the submitted work, he has received consultancy fees (e.g., advisory board, steering committee) from Clovis, Tesaro, AstraZeneca, Abbvie, Immunogen, GOG, Myriad, and Amgen; outside the submitted work, his institution has received funding for clinical trials from Clovis, AstraZeneca, Serono, Iovance Biotherapeutics, Inc., GOG, Tesaro, Ludwig Institute for Cancer Research Ltd, inVentiv Health Clinical, ImmunoGen, Inc., Stemcentrx, Inc., Agenus, Inc., Bristol-Myers Squibb, Array BioPharma, Inc., TRACON Pharmaceuticals, Janssen Research and Development, LLC, PRA Intl, Ajinomoto Co, Inc., INC Research, Inc., and ERGOMED Clinical Research Ltd. Outside the submitted work, R. A. Soslow has received royalties from Cambridge Press and Springer Publishing. Outside the submitted work, K. Moore has participated on the advisory boards of Clovis, Astra Zeneca, Tesaro, Immunogen, Genentech/Roche, VBL Therapeutics, and Merck.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 125: 771–773, 2012. (Abstract) [Google Scholar]
- 2.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol 29(16): 2259–2265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 129(1):22–27, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Fleming GF, Filiaci VL, Marzullo B, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol 132(3):585–592, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol 29(24):3278–3285, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dizon DS, Blessing JA, McMeekin S, et al. Phase II trial of Ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group trial 129P. J Clin Oncol 27(19): 3104–3108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet 105:103–104, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Emrich LJ. Required duration and power determinations for historically controlled studies of survival times. Stat Med 8(2):153–160, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Dunnett CW. Multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association 50(272):1096–1121, 1955. [Google Scholar]
- 12.Freidlin B, Korn EL, Hunsberger S, et al. Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol 25(15):2122–2126, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5):589–595, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koboldt DC, Zhang Q, Larson DE, et al. VarScan2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22(3):568–576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31(3):213–219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson DE, Harris CC, Chen K, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 28(3):311–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soslow RA, Wethington SL, Cesari M, et al. Clinicopathologic analysis of matched primary and recurrent endometrial carcinoma. Am J Surg Pathol 36(12):1771–1781, 2012. [DOI] [PubMed] [Google Scholar]
- 18.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 228(1):20–30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Husain A, Nelson GS, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol 36(2):128–139, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Myers AP, Filiaci VL, Zhang Y, et al. Tumor mutational analysis of GOG248, a phase II study of temsirolimus or temsirolimus and alternating megestrol acetate and tamoxifen for advanced endometrial cancer (EC): An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 141(1):43–48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perini GF, Campregher PV, Ross JS, et al. Clinical response to everolimus in a patient with Hodgkin’s lymphoma harboring a TSC2 mutation. Blood Cancer J 6(5):e420, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorusso D, Ferrandina G, Colombo N, et al. Ramdozimed phase II trial of carboplatin- paclitaxel (CP) compared to carboplatin-paclitaxel-bevacizumab (CP-B) in advanced (stage III-IV) or recurrent endometrial cancer: The MITO END-2 trial. J Clin Oncol 33, 2015. (suppl; abstr 5502) [Google Scholar]
- 23.McMeekin S, Dizon D, Barter J, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol 138(1):18–23, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Ilyas M Wnt signaling and the mechanistic basis of tumour development. J Pathol 205(2):130–44, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res 61(16):6050–4, 2001. [PubMed] [Google Scholar]
- 26.Easwaran V, Lee SH, Inge L, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res 63(12):3145–53, 2003. [PubMed] [Google Scholar]
- 27.McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 34(25):3062–3068, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


