Abstract
Ultrahigh speed optical coherence tomography (OCT) systems with >100 kHz A-scan rates can generate volumes rapidly with minimal motion artifacts and are well suited for 4D imaging (volumes through time) applications such as intra-operative imaging. In such systems, high OCT data acquisition efficiency (defined as the fraction of usable A-scans generated during the total acquisition time) is desired to maximize the volumetric frame rate and sampling pitch. However, current methods for beam scanning using non-resonant and resonant mirror scanners can result in severe scan distortion and transverse oversampling as well as require acquisition dead times, which limit the acquisition efficiency and performance of ultrahigh speed 4D OCT. We introduce constant linear velocity spiral scanning (CLV-SC) as a novel beam scanning method to maximize the data acquisition efficiency of ultrahigh speed 4D OCT systems. We demonstrate that CLV-SC does not require acquisition dead times and achieves more uniform transverse sampling compared to raster scanning. To assess its clinical utility, we implement CLV-SC with a 400 kHz OCT system and image the anterior eye and retina of healthy adults at up to 10 volumes per second with isotropic transverse sampling, allowing B-scans with equal sampling pitch to be extracted from arbitrary locations within a single volume. The feasibility of CLV-SC for intra-operative imaging is also demonstrated using a 800 kHz OCT system to image simulated retinal surgery at 15 volumes per second with isotropic transverse sampling, resulting in high quality volume renders that enable clear visualization of surgical instruments and manipulation of tissue.
1. Introduction
Optical coherence tomography (OCT) is a non-contact tomographic imaging modality [1] that has achieved widespread adoption in clinical ophthalmology [2]. Recently developed ultrahigh speed OCT systems (>100 kHz A-scan rate) can rapidly acquire volumes with minimal motion artifacts without the need for external tracking technologies [3]. Ultrahigh speed OCT also enables volumetric imaging with isotropic transverse sampling, allowing post-acquisition visualization of high resolution B-scans from arbitrary orientations from a single volume [4,5]. In addition, these systems are well suited for 4D OCT imaging (volumetric imaging through time) applications [6,7], such as intra-surgical guidance [8–11], in which isotropic and high pitch transverse sampling would optimize volume render quality and system efficiency. While systems that are capable of generating A-scans at fast rates are critical for ultrahigh speed OCT imaging, it is also important to consider the fraction of usable A-scans during the image acquisition period. Transverse oversampling, vignetting, and acquisition dead times limit the efficiency of ultrahigh speed OCT (defined here as the fraction of usable (displayable) versus total acquired A-scans) and lead to reduced effective A-scan rates and imaging speed.
The vast majority of ophthalmic OCT systems to date raster scan a single focused spot across the sample surface to generate B-scans and volumes. Raster scanning is typically performed with galvanometer-based scanners (GS) [12] due to their precise positioning, large aperture-scan angle product, and ability to operate over a range of frequencies. Raster scanning optimally translates the beam along the imaging fast axis with constant velocity to generate B-scans with uniformly spaced A-scans (i.e. uniform transverse sampling pitch). However, the high frequency content of drive voltage waveforms often surpasses the bandwidth of the GS, resulting in scan distortion, non-uniform scan velocity and transverse oversampling [13]. Resonant scanners are increasingly used for high speed raster scanning but these are necessarily driven with sinusoidal waveforms that result in continuously varying scan velocity and severe lateral oversampling [6], in addition to restricting the B-scan acquisition rate to the resonant frequency of the scanner. Raster scanning protocols also require acquisition dead times in between B-scans and volumes to allow the scanner time to stop and reverse directions, which may comprise a substantial fraction of the total acquisition time and reduce the data acquisition efficiency (up to 25% for large amplitude and high speed raster scanning [14]).
Continuous two-dimensional (2D) point scanning is an alternative to raster scanning in which the OCT beam is continuously scanned about the 2D transverse imaging plane without acquisition dead times during a volumetric acquisition. Continuous 2D scan patterns have been previously demonstrated for forward-imaging OCT endoscopes, including Lissajous scanning [15] and constant angular velocity spiral scanning [16]. Lissajous scanning was also recently introduced for eye motion correction in retinal OCT [17]. The primary limitation of the aforementioned continuous 2D scan patterns was the inherent non-uniform transverse sampling. In Lissajous scanning, the edges of the transverse FOV are more densely sampled than the middle, while in constant angular velocity spiral scanning the center is more densely sampled than the edges. Consequently, these scan protocols cannot uniformly sample the transverse FOV.
We introduce constant linear velocity spiral scanning (CLV-SC) for ultrahigh speed OCT imaging. CLV-SC is a continuous 2D scan pattern, and we demonstrate that properly configured and sequenced, it requires zero acquisition dead time and achieves more uniform transverse sampling for 4D OCT imaging compared to raster scanning. CLV-SC can be readily applied in most commercial and research-grade OCT scanners and has the potential to improve the efficiency of such systems. We demonstrate the utility of CLV-SC using 400 kHz and 800 kHz swept source OCT systems for near video rate 4D ophthalmic and surgical imaging, respectively.
2. Theory
2.1 Scan parameters for bidirectional raster scanning with isotropic transverse sampling
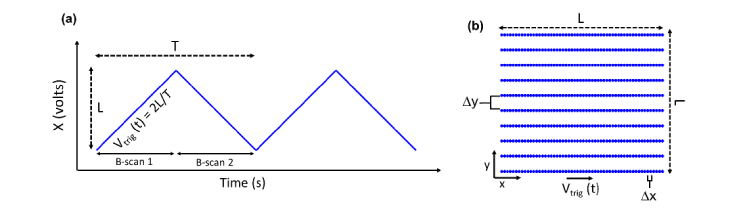
We first provide expressions relating scan rate, velocity, length, and sampling pitch for bidirectional raster scanning (BRS) with isotropic transverse sampling. BRS will serve as a reference when assessing the performance of CLV-SC. A general expression for the B-scan acquisition rate required to generate an OCT volume with equal number of pixels along the slow and fast lateral axes using raster scanning (uniform transverse sampling) is:
| (1) |
where fA-scan is the A-scan rate of the OCT system, and fvolume is the volume acquisition rate. To generate B-scans, one channel of dual-axis GS may be driven using a triangle voltage waveform, as illustrated in Fig. 1. Note that B-scans are acquired during both positive and negative slopes of the triangle waveform for BRS. The peak-to-peak voltage amplitude of the triangle waveform is proportional to scan length L along the B-scan axis and the proportionality constant is determined by the voltage-to-mm calibration of the GS and scan lens system. Assuming the scan length along the slow scan axis to also be L, the transverse scan FOV is L2. An ideal triangle waveform generates a scan with uniform velocity Vtrig during the B-scan acquisition, and Vtrig is related to the fundamental frequency of the triangle wave ftrig as Vtrig = 2Lftrig, as shown in Fig. 1. For BRS 2ftrig = fB-scan since two B-scans are acquired during each period of the triangle waveform. The sampling pitch, or distance between adjacent pixels, ∆x along the B-scan is L/(A-scans/B-scan), which can be expressed as:
| (2) |
∆x is thus proportional to Vtrig and the B-scan sampling uniformity is determined by the scan velocity uniformity, assuming a constant fA-scan. That is, non-uniform Vtrig during the B-scan acquisition time will result in non-uniform ∆x. Moreover, Vtrig is in part determined by ftrig and thus fB-scan calculated using Eq. (1). The sampling pitch along the slow axis ∆y is L/(B-scans/Volume) and is assumed to be equal to ∆x. In raster scanning, ∆y is controlled by the step size of the staircase voltage waveform used to drive the slow axis GS, which will be assumed as constant for this analysis.
Fig. 1.
Bidirectional raster scanning (BRS) with triangle voltage drive waveforms. (a) Representative triangle scanning voltage waveform. (b) Two-dimensional raster scan pattern. L: scan length; T: triangle waveform period; Vtrig: scan velocity; ∆x: sampling pitch along x (fast) scan axis; ∆y: sampling pitch along y (slow) scan axis. The raster scan pattern illustration is undersampled along y for clarity.
2.2 Constant linear velocity spiral scanning
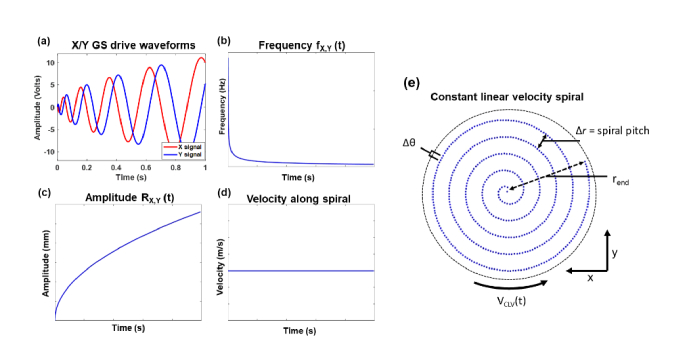
CLV-SC was first introduced for laser disk recording [18], and was recently applied in atomic force microscopy [19] and magnetic resonance imaging [20]. Modified versions of equations first described in these publications are presented here. A CLV-SC pattern can be constructed by driving the x and y channels of dual-axis GS with and [19], where
| (3) |
| (4) |
| (5) |
In Eqs. (3-5), is instantaneous phase, r is instantaneous amplitude, VCLV is the linear velocity along the spiral, t is time, and ∆r is the spiral pitch or sampling pitch along the radial dimension. We use the term linear velocity to differentiate VCLV from angular velocity ω. Equations (3-4) state that instantaneous frequency and instantaneous amplitude of x(t) and y(t) are nonlinear functions of time, as illustrated in Fig. 2. Equation (3) expresses the relationship between VCLV (in units of m/s) and ω (in units of radians/s) of the spiral, and VCLV is constant for ideal CLV-SC. and ∆r are the sampling pitches along the angular (fast) and radial (slow) axes of the spiral scan, respectively, and can be expressed as:
| (6) |
| (7) |
Equation (6) is analogous to sampling pitch along the fast axis of a raster scan (Eq. (2) and Eq. (7) was derived by solving Eq. (4) for ∆r. Setting = ∆r yields isotropic transverse sampling. In a CLV-SC pattern with equivalent 2D transverse sampling as raster scanning, VCLV = Vtrig (or = ∆x), ∆r = ∆y, rend = L/ so that transverse FOVs of the raster scan and CLV-SC are equal to L2 (where rend is the radius of the circular FOV produced by CLV-SC and L is the raster scan length), and the CLV-SC period is 1/fvolume. Note that if VCLV is constant during the CLV-SC scan period, and ∆r would be constant, corresponding to uniform transverse sampling.
Fig. 2.
Constant linear velocity spiral drive waveforms and scan pattern. (a) Galvanometer scanner drive waveforms. (b) Instantaneous frequency and (c) instantaneous amplitude of the drive waveforms as a function of time. (d) Linear velocity along spiral as a function of time. Velocity along spiral is constant resulting in uniform sampling pitch. GS: galvanometer scanners; sampling pitch in angular dimension (along spiral); ∆r: sampling pitch in radial dimension r; rend: radius of CLV spiral scan circular field of view; VCLV: linear scan velocity. Scan pattern is undersampled along r for clarity.
For 4D OCT imaging, CLV-SC can be designed such that the starting location of the scan alternates between the center and edge of the spiral, thus the direction of rotation remains constant between spirals. For example, the second CLV-SC pattern of a two-volume acquisition would start at the edge, where the first CLV-SC pattern ended, and scan towards the center of the FOV. This way, the drive voltage waveforms are smooth during the transition between spirals, ensuring that the GS will be driven continuously and smoothly during 4D OCT imaging to avoid acquisition dead times in between volumes.
3. Methods
3.1 Experimental setup to characterize scan performance
The following experimental setup was used to characterize CLV-SC and compare its performance to BRS. Voltage waveforms were designed in MATLAB (MathWorks; Natick, MA) and relayed to the servo driver of commercially available dual-axis GS (ScannerMax, Pangolin Laser Systems, Inc.; Florida, USA). The voltage waveforms were generated with a DAC (NI-PCIe 6323, National Instruments; Tx, USA) using two analog output channels each with 900 kS/s sampling frequency. The analog position sensors of the x and y axes of the GS generated voltage waveforms with amplitudes proportional to the GS angular position. The voltage waveform outputs of the position sensors in response to drive GS waveforms were digitized using two analog input channels of the same DAC, each with a sampling frequency of 60 kS/s. Using this setup, the GS response to arbitrary drive voltage waveforms could then be analyzed by recording both drive and corresponding response voltage waveforms. The GS were incorporated into a telecentric imaging system comprised of a 100 mm focal length objective for scanning. The GS were placed at the back focal plane of the objective for image-side telecentricity and the voltage-to-mm calibration of the GS was 0.191 volts/mm. This calibration was measured using a 1 Hz sinusoidal drive waveform. Note that this calibration was performed only for a single low frequency scan trajectory. For CLV-SC, the frequency at which the GS were driven varied throughout the scan pattern, with higher frequencies towards the center of the spiral. Therefore, a more accurate voltage-to-mm calibration for CLV-SC could be performed by accounting for the frequency response of the GS over the scan frequency range of interest. The distance between the GS x and y mirrors was 10 mm, and the back focal plane of the objective was placed in between the x and y axial mirror positions. The physical separation of the x and y mirrors did not have a noticeable impact on image-side telecentricity, as determined by imaging a grid target (see Section 3.4), likely due to the low numerical aperture and long depth of focus of the objective. Alternatively, an additional 4f optical relay could be used to separately image the scan pivots of the x and y mirror to the same conjugate plane if high image-side telecentricity is desired.
3.2 Performance comparison of bidirectional raster scanning and constant linear velocity spiral scanning
To establish a performance benchmark for CLV-SC, the GS were first driven with triangular waveforms corresponding to BRS. We were particularly interested in testing scan frequencies and velocities that would generate high speed OCT volume scans with isotropic transverse sampling, as described in Theory, for fvolume 1-10 Hz. The x channel of the GS was driven using triangle waveforms with varying ftrig and a peak-to-peak voltage amplitude corresponding to L = 10 mm, which corresponded to a peak-to-peak voltage amplitude of 1.91 V. The y channel of the GS was driven step-wise with a staircase voltage waveform, with step periods and amplitudes designed such that ∆x = ∆y. The transverse scan FOV corresponded to 100 mm2. For each BRS, 10 x and y GS voltage waveform pairs were tested spanning the frequency and velocity ranges listed in Table 1. Next, 10 CLV-SC waveforms were designed using Eqs. (3-5) to match the transverse sampling parameters of BRS. To match the transverse FOV for CLV-SC to the FOVs of BRS, rend = L. The corresponding parameters are given in Table 1.
Table 1. List of scan parameters used to test and compare the performance of bidirectional raster scanning (BRS) and constant linear velocity spiral scanning (CLV-SC). Parameters corresponding to isotropic transverse sampling for volume rates of 1-10 Hz were tested. The transverse field of view for the three scan patterns was 100 mm2.
| Volume rate fvolume (Hz) | A-scan rate fA-scan (kHz) |
B-scan rate fB-scan (Hz) |
Triangle waveform fundamental frequency ftrig (Hz) |
Ideal scan velocity along fast axis (m/s) Vtrig |
Ideal sampling pitch along fast and slow axes (µm) | |
|---|---|---|---|---|---|---|
| BRS | 1-10 | 400 | 632.5-2000 | 316.3-1000 | 6.4-20 | 15.8-50 |
| CLV-SC | 1-10 | 400 | N/A | N/A | 6.4-20 | 15.8-50 |
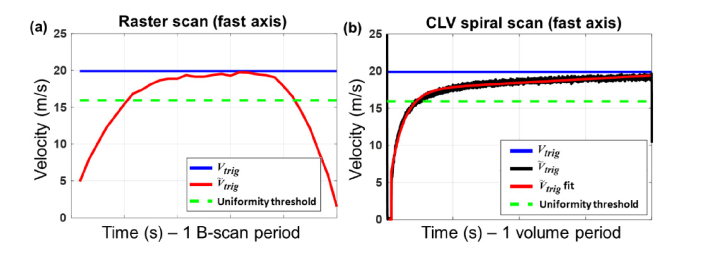
The performance of BRS was assessed by analyzing the GS response to drive waveforms corresponding to the parameters in Table 1, estimating the resulting scan velocity and comparing to the ideal scan velocity Vtrig. could then be used to estimate the sampling pitch achievable by BRS scan patterns generated using the ideal sampling pitch parameters given in Table 1. To estimate for BRS, voltage waveforms generated by the position sensor of the x channel of the GS in response to drive waveforms were recorded, and the recorded waveforms were truncated to correspond to a B-scan acquisition period. The derivative of the waveforms was numerically computed and the result was interpreted as . Similarly, voltage waveforms generated by the position sensors of the x and y channels of the GS in response to a CLV-SC drive waveforms were also recorded. To estimate along the fast axis of CLV-SC, the Hilbert transform of the recorded waveforms was computed and the magnitude and phase of the result was interpreted as the instantaneous amplitude and instantaneous phase of the CLV-SC pattern. was then estimated using Eq. (5). The estimated waveforms were fit to double exponential functions to reduce the impact of high frequency noise amplified during Hilbert transformation. The mean RMSE for the double exponential fits used was 0.179 ± 0.058 mm/s for all estimated . Representative Vtrig and for BRS and VCLV and for CLV-SC are shown in Fig. 3. Note that for BRS, is slowest at the beginning and end of the scan (corresponding to the edges of a B-scan), while for CLV-SC is slowest at the start of the scan (corresponding to the center of the spiral).
Fig. 3.
Representative scan velocity waveforms for bidirectional raster scanning (BRS) (a) and constant linear velocity spiral scanning (CLV-SC) (b). Blue curves are ideal scan velocities Vtrig and VCLV for BRS and CLV-SC, respectively. Constant Vtrig and VCLV correspond to uniform sampling pitch. Red curves show scan velocities or for BRS and CLV-SC, respectively, estimated from the recorded response GS waveforms. The red curve in (b) is the double exponential fit to the data shown in black. Green dotted lines denote threshold over which / Vtrig > 0.8 or / VCLV > 0.8, which was chosen as a metric to characterize the degree of sampling uniformity for each scan pattern.
Using equation Eq. (2), for BRS was used to estimate the sampling pitch along a B-scan assuming an OCT system with fA-scan = 400 kHz. Similarly, was used to estimate the sampling pitch along the fast and slow axes of CLV-SC using Eqs. (6) and 7, respectively. As detailed in Theory, constant scan velocity suggests uniform sampling pitch. However, because the scan velocity may not be constant during the scan period, which would correspond to non-uniform sampling pitch, a metric for sampling pitch uniformity is required that accounts for this non-ideal behavior and allows us to compare sampling pitch uniformity across the two scan types of interest. The degree of sampling pitch uniformity was defined as the fraction of the scan period in which / Vtrig > 0.8 and / VCLV > 0.8, corresponding to the fraction of the scan period in which the estimated sampling pitch was within 20% of the ideal sampling pitch. Conversely, the fraction of the scan period in which / Vtrig < 0.8 and / VCLV < 0.8 would correspond to severe transverse oversampling. The green dotted lines in Fig. 3 show the degree of sampling uniformity threshold overlaid on the representative scan velocity profiles.
3.3 Swept source OCT system at 400 kHz and 800 kHz A-scan rates
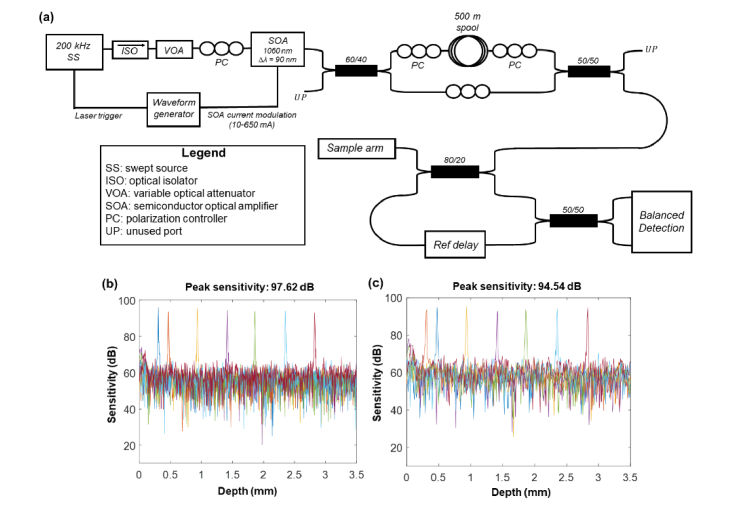
The ultrahigh speed OCT engine utilized for this study employed a frequency-swept laser with a 200 kHz sweep rate and sweeping bandwidth of 90 nm centered at 1034 nm (Axsun Technologies; MA, USA). The output of the laser was optically buffered to double the sweep rate to 400 kHz and the laser output was amplified using a semiconductor optical amplifier (SOA) (Innolume, GmbH; Dortmund, Germany) to compensate for optical power losses in the buffering stage. The un-buffered and buffered laser sweep copies were recombined using a 50/50 fiber splitter after the buffering stage to generate a 400 kHz laser sweep. The duty cycle of the usable portion of the un-buffered laser sweep was 47% while the duty cycle of the entire un-buffered sweep was ~55%. To suppress potential crosstalk between the un-buffered and buffered sweeps from the unusable portions of the laser sweep, the gain of the SOA was modulated to suppress the laser output during the last 51% of the sweep period. The average optical power relayed to the OCT interferometer after the buffering stage was 30 mW. The OCT interferometer used in these studies was previously described in [21]. A schematic of the complete OCT system is provided in Fig. 4. The measured peak sensitivity and axial resolution of the 400 kHz OCT system were 97.62 dB and 8.3 µm, respectively, and the −6dB sensitivity roll-off was 3.9 mm. This OCT system configuration was used to image adult volunteers and assess the clinical utility of CLV-SC.
Fig. 4.
Ultrahigh speed swept source OCT engine schematic and performance. (a) Schematic of optical buffering stage and OCT interferometer. All abbreviations are defined in the table. (b) OCT sensitivity fall-off plot for 400 kHz engine. (c) OCT sensitivity fall-off plot for 800 kHz engine using temporal spectral splitting.
For 4D OCT imaging of simulated surgical maneuvers, the fA-scan of the OCT configuration described above was doubled to 800 kHz using temporal spectral splitting (TSS) [22], a technique that was originally introduced for OCT angiography [23,24]. Conveniently, TSS is a software-based technique that can be applied in post-processing to increase the effective A-scan rate (at the expense of axial resolution) and did not require modification to the hardware of the 400 kHz SS-OCT system. In this configuration, un-buffered and buffered spectra were each windowed into two spectra using Gaussian windows prior to Fourier transformation. This resulted in four spectra with ~100% usable duty cycle and a period of 1.25 µs. The bandwidth of each spectra after TSS was ~45 nm. Therefore, combined with continuous lateral scanning, the four spectra generated four A-scans equidistantly spaced along the scan dimension, after standard OCT processing performed on each spectrum. The measured axial resolution of the 800 kHz A-scans was 17.1 µm, which was ~2x worse compared to the 400 kHz A-scans. The measured peak sensitivity of the 800 kHz system was 94.54 dB.
3.4 OCT data acquisition and processing
The following protocol was implemented to process volumetric OCT data acquired with CLV-SC. Voltage waveforms corresponding to CLV-SC were designed as described in Theory. The voltage waveforms were used to drive the GS and the corresponding response voltage waveforms generated by the position sensors of the GS were digitized and stored (see Methods (Section 3.1) for additional details). Raw interferograms were then processed into A-scans using standard OCT processing. To display conventional B-scans, summed voxel projections (SVPs), and volumes, the recorded response voltage waveforms from the x and y GS channels were used to transform the OCT data (which acquired as a function of and r) to Cartesian coordinates using bilinear interpolation, which was applied a single depth slice at a time. All processing was performed in MATLAB and the computation time was ~3 minutes for a single volume comprised of 400,000 A-scans. The processing was performed on a CPU and the algorithm was not optimized for speed. OCT volumes were rendered using our previously published algorithms [25].
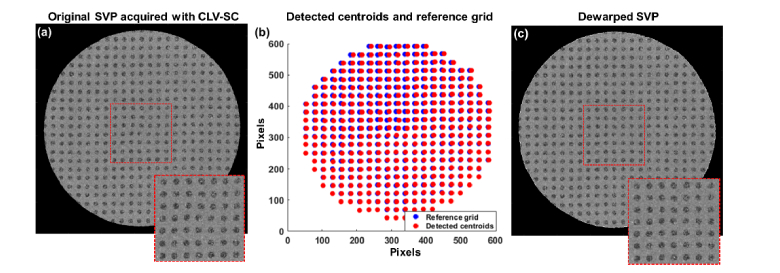
To assess potential image distortion caused by CLV-SC and aberrations in the optical imaging system, a 20 x 20 mm low reflection rectangular grid target (Edmund Optics Inc., Barrington, NJ) was imaged with a telecentric scanner and the 400 kHz OCT system. OCT volumes of the grid target were acquired using CLV-SC and SVPs were rendered from the processed volumetric data sets to generate en face images of the grid target. To estimate residual distortion present in the scans, intensity-based thresholding and blob centroid detection was first used to localize the dot centroids of the grid target, and a reference grid of dots was created using the known spacing of the dots in the grid target. Using the detected dot centroids and the reference dot grid, a piecewise linear transform was created to dewarp SVPs or depth slices of a volume data set [26]. Representative SVPs before and after dewarping acquired with protocol #1 (listed in Table 2) are shown in Fig. 5. Image distortion detected and corrected for using this procedure did not vary significantly among the different imaging protocols. Volumetric images generated with CLV-SC and the 400 kHz OCT system were dewarped using this protocol.
Table 2. Constant linear velocity spiral scanning (CLV-SC) imaging protocols for human and surgical imaging. FOV: field of view; r: radial dimension;: angular dimension. Effective sampling pitch denotes transverse sampling pitch after interpolation in CLV-SC processing corresponding to 80% of ideal sampling pitch to account for nonlinearities described in Results(4.1).
| Scan protocol # | A-scan rate (kHz) |
Volume acquisition time / rate (s / Hz) |
Transverse FOV (mm2) |
Ideal sampling pitch (µm) |
Effective sampling pitch (µm) |
|---|---|---|---|---|---|
| #1 Retinal (Fig. 7) |
400 | 0.2 s | ~22 | 16.6, isotropic |
20.8, isotropic |
| #2 Retinal (Fig. 8) |
400 | 0.4 s | ~85 | 23.0, isotropic |
28.8, isotropic |
| #3 Retinal with 4x avg. (Fig. 9) |
400 | 1.0 s | ~22 |
r: 13.3 : 3.3 |
r: 16.6 : 4.1 |
| #4 Anterior eye 4D (Fig. 10) |
400 | 10 Hz | 225 | 75.0, isotropic |
93.8, isotropic |
| #5 Surgical (Fig. 11) |
800 | 15 Hz | 25 | 21.7, isotropic | 27.1, isotropic |
Fig. 5.
Estimation and correction of residual distortion present in OCT volumes acquired with constant linear velocity spiral scanning (CLV-SC). (a) Summed voxel projection (SVP) generated from an OCT volume of a grid target acquired with CLV-SC. Inset shows distortion present near the center of the scan. (b) Detected centroids (red) corresponding to dot positions in (a) and reference grid (blue) used to generate a transform for image dewarping. (c) SVP of grid target after dewarping. Inset in (c) corresponds to approximately the same location as inset in (a).
3.5 Human and surgical imaging protocols
Retinal and anterior eye OCT imaging was performed in healthy adult volunteers to test the potential clinical utility of CLV-SC. Consent from each volunteer was acquired after explanation of risks associated with the study in accordance with the Declaration of Helsinki. All human imaging was performed under a protocol approved by the Duke Medical Center Institutional Review Board. The optical power incident on the cornea was less than 1.80 mW, which was below the safety limit determined following the ANSI Z136 standard. Retinal imaging was performed with an optical scanner comprised of a 4f telescope to relay the optical scan pivot the subject’s ocular pupil plane. Anterior eye imaging was performed with a telecentric optical scanner comprised of an objective lens with a focal length of 75 mm. At the beginning of each imaging session, the subjects were first aligned using orthogonal B-scans, and volumes with CLV-SC were acquired after alignment and proper fixation was achieved. Human imaging was performed with the 400 kHz OCT engine. To assess the potential utility of CLV-SC for 4D OCT surgical guidance, the 800 kHz OCT engine was used to image simulated surgical maneuvers performed in ex vivo porcine eyes. In this case, retinal imaging was performed using a telecentric scanner with a 100 mm focal length objective lens and a surgical contact lens placed on the porcine cornea. Prior to imaging with CLV-SC, the scans were first aligned to the area of interest using B-scans and SVPs acquired with raster scanning. Imaging protocols parameters are detailed in Table 2.
4. Results
4.1 Scan performance comparison
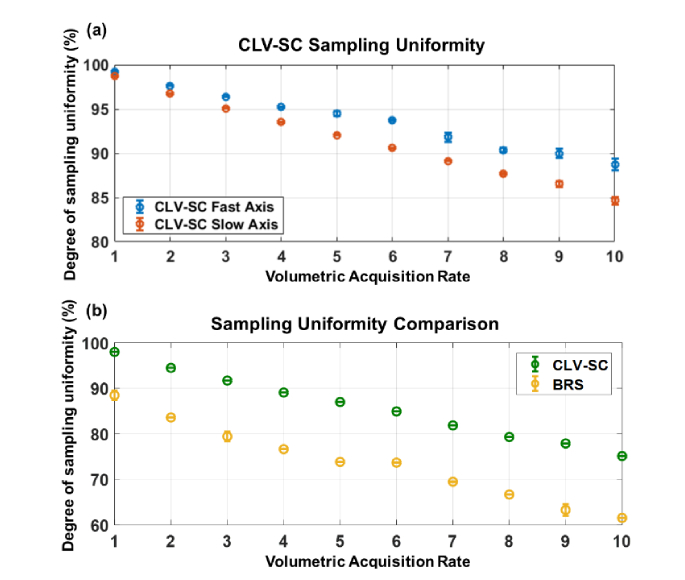
The degree of sampling uniformity along the fast and slow axes of CLV-SC for fvolume = 1-10 Hz are plotted in Fig. 5(a). The scan parameters used to generate the CLV-SC patterns at each fvolume are given in Table 1. The CLV-SC patterns were tested 5 times at each fvolume and the mean degree of sampling uniformity was estimated as described in Methods (Section 3.2). As fvolume increased, the degree of sampling uniformity along each axis decreased monotonically, and the degree of sampling uniformity was greater along the fast axis of the CLV-SC.
The degree of sampling uniformities for BRS and CLV-SC for fvolume = 1-10 Hz are plotted in Fig. 5(b). Each scan type was tested 5 times and the mean and standard deviation of the degree of sampling uniformity was estimated at each fvolume. Note that sampling uniformity along the slow axis of BRS was assumed to be constant and the degree of sampling uniformity was only calculated along the fast axis. In contrast, sampling uniformity varies along the both the fast and slow axes for CLV-SC. The overall degree of sampling uniformity for CLV-SC was estimated for each fvolume by calculating the product of the degree of sampling uniformity distributions along each axes and assuming independent and normal distributions. For all fvolume tested, the degree of sampling uniformity of CLV-SC was at least 10.1% greater than the degree of sampling uniformity for BRS. The superior performance of CLV-SC compared to BRS was more drastic for faster fvolume: At fvolume = 10 Hz, the CLV-SC degree of sampling uniformity was 13.5% greater than the degree of sampling uniformity for BRS.
4.2 OCT Imaging of healthy adult volunteers with constant linear velocity spiral scanning
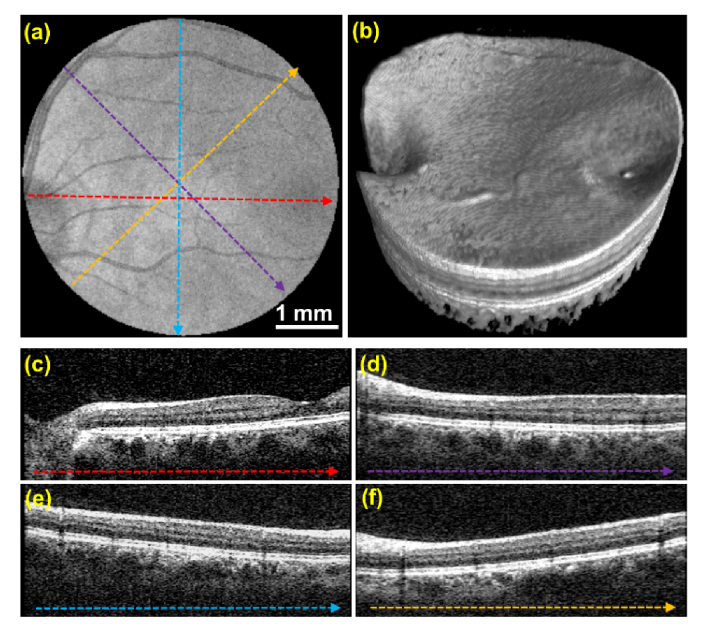
Healthy adult volunteers were imaged with protocols listed in Table 1 to assess the clinical utility of CLV-SC. Images were first acquired using protocol #1. The volume acquisition time was 0.2 s and the CLV-SC pattern was centered in between the optic nerve and fovea. The short volume acquisition time likely reduced the potential for artifacts due to fixational eye motion, and motion artifacts were not apparent in the volume. Isotropic transverse sampling enabled extraction of linear B-scans from arbitrary orientations (Fig. 7). Orthogonal cross-sectional B-scan video fly-throughs are provided in Visualization 1 (4.7MB, mpg) and Visualization 2 (4.6MB, mpg) . A video fly-through of radial B-scans extracted from the volumetric data is provided in Visualization 3 (3.1MB, mpg) .
Fig. 7.
Retinal OCT volume acquired using CLV-SC and isotropic transverse sampling in 0.2 seconds (protocol #1 in Table 1). (a) SVP generated from the OCT volume. Dashed colored lines correspond to locations of the circular and linear B-scans shown in (c-f). (b) Volume render. (c-f) Un-averaged linear B-scans extracted from volume. Orthogonal cross-sectional B-scan fly-throughs are provided in Visualization 1 (4.7MB, mpg) and Visualization 2 (4.6MB, mpg) . A fly-through of radial B-scans extracted from the volumetric data is provided in Visualization 3 (3.1MB, mpg) .
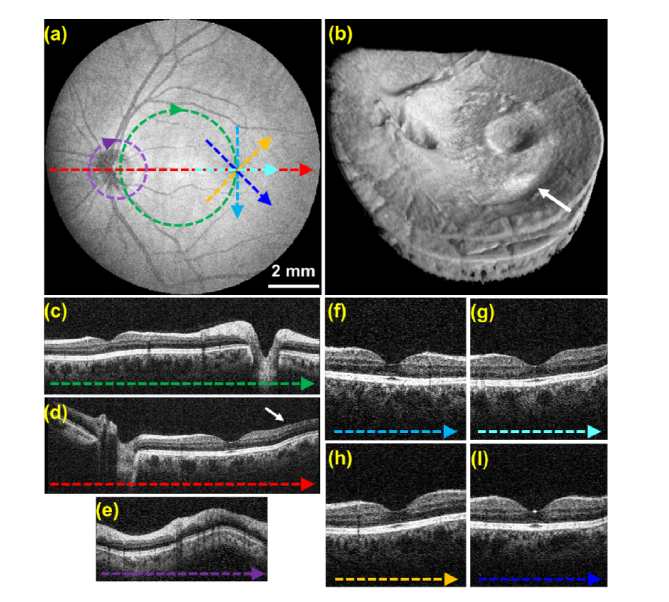
Figure 8 shows an OCT volumetric scan acquired from a healthy volunteer using protocol #2. The scan’s transverse FOV was sufficient to image both the optic nerve and fovea, and the volume acquisition time was 0.4 s. Isotropic transverse sampling of the volume in the transverse plane allowed extraction of radial B-scans centered on the fovea and circumpapillary B-scans. Although the volume acquisition time was only 0.4 s, image artifacts likely due to fixation eye motion were present in the linear B-scans and volume (denoted by white arrows in Fig. 8).
Fig. 8.
Retinal OCT volume acquired using constant linear velocity spiral scanning and isotropic transverse sampling in 0.4 seconds (protocol #2 in Table 1) from a healthy subject. (a) SVP generated from the OCT volume. Dashed colored lines correspond to the locations of the circular and linear B-scans shown in (c-i). (b) OCT volume render. (c-i) Un-averaged linear and circular B-scans extracted from the volumetric data set. White arrows denote artifacts likely due to fixational eye motion.
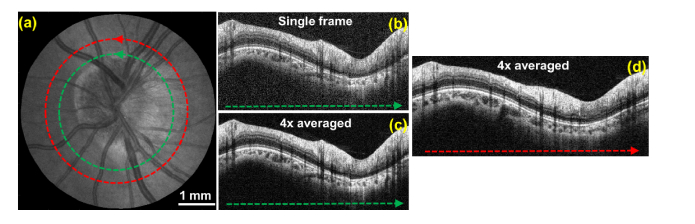
Next, a high resolution OCT volume centered on the optic nerve disk was acquired with CLV-SC and scan protocol #3 (Fig. 9). The volume was oversampled by 4x in the angular dimension and averaged circumpapillary B-scans were generated by averaging 4 adjacent A-scans. Figure 9(b-c) show the same B-scan before averaging (4x oversampled) and after averaging for comparison. The averaged B-scan exhibited increased signal to noise compared to the single frame B-scan but did not substantially reduce speckle noise.
Fig. 9.
Volumetric OCT image centered on the optic nerve of a healthy subject acquired in 1 second using constant linear velocity spiral scanning (protocol #3 in Table 1). The volume was oversampled along the angular dimension by 4x and 4 adjacent A-scans were averaged to generate averaged circular B-scans. (a) SVP generated from the volumetric data set. Dashed colored lines correspond to the locations of the circular B-scans. (b) Un-averaged circular B-scan. (c-d) 4x averaged circular B-scans.
Lastly, 4D OCT imaging of a subject’s pupillary response to light stimulus was performed using CLV-SC and protocol #4. The ability to study intra-ocular dynamics with micrometer scale resolution volumetric images adds a new dimension to OCT imaging (real time volume acquisition) and could help improve understanding of diseases such as glaucoma [27]. To image the pupillary response of a healthy adult volunteer, the room lights were turned off to ensure that the subject was dark adapted and the room lights were turned on that the start of the image acquisition. The 4D OCT video consisted of volumes acquired at fvolume = 10 Hz. The focal plane of the telecentric imaging system was placed near the subject’s iris and the cornea was made translucent using our rendering algorithm improve visualization of the iris. Excerpts from the 4D OCT video are provided in Fig. 10 and illustrate the utility of CLV-SC for imaging three-dimensional ocular dynamics with high temporal resolution.
Fig. 10.
Excerpts from a 4D OCT movie acquired with a volume rate of 10 Hz and isotropic transverse sampling enabled by constant linear velocity spiral scanning (protocol #4 in Table 1). Excerpts show pupil constriction of a healthy subject in response to light stimulus. The focus of the imaging system was near the subject’s iris. Time stamps in seconds are shown for each frame. Corresponding movie is provided in Visualization 4 (822KB, mpg) .
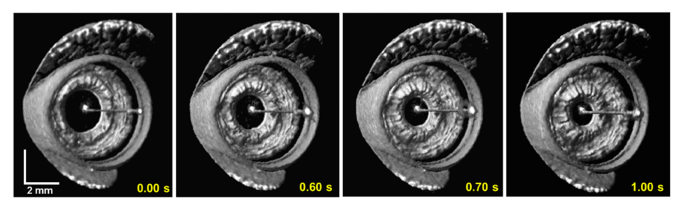
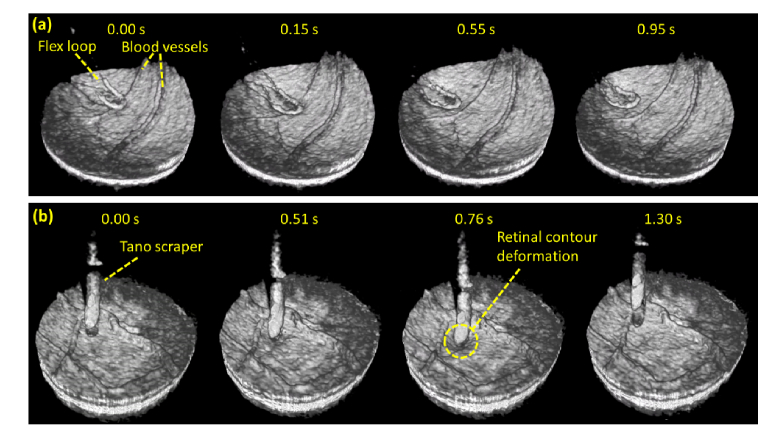
4.3 4D OCT imaging of simulated surgical maneuvers with constant linear velocity spiral scanning at 15 volumes/second
The utility of CLV-SC for near video rate (fvolume = 15 Hz) 4D OCT surgical imaging was tested using the 800 kHz OCT engine and protocol #5. Simulated surgical maneuvers were performed in ex vivo porcine eyes, which are commonly used models for ophthalmic surgical training [28]. Retinal surface brushing was performed using a diamond dusted membrane scraper (Synergetics, Inc.; O’Fallon, MO) and Finesse flex loop (Alcon; Fort Worth, TX), two commercially available and widely used retinal surgical instruments. Isotropic transverse sampling with CVL-SC yielded high quality volumetric renders and fast volume frame rates enabled smooth visualization of the instruments contacting the retinal contour, as shown in Fig. 11.
Fig. 11.
Excerpts from 4D OCT movies of simulated surgical maneuvers that were acquired with a volume rate of 15 Hz and isotropic transverse sampling enabled by CLV-SC (protocol #5 in Table 1). The excerpts show retinal brushing with a surgical flex loop (a) and a diamond dusted tano scraper (b). Near video rate 4D OCT imaging with CLV-SC enabled smooth visualization of the maneuvers. Corresponding movies are provided in Visualization 5 (766KB, mpg) and Visualization 6 (1.4MB, mpg) .
5. Discussion
We have introduced CLV-SC as a novel scanning technique for ultrahigh speed 4D OCT imaging. CLV-SC inherently obviates the need for inter-B-scan and inter-volume acquisition dead times that are required in conventional raster scanning. In addition, we have demonstrated that CLV-SC achieves more uniform transverse sampling compared to BRS for equal transverse sampling parameters and volume rates up to 10 Hz. These characteristics of CLV-SC can improve the data acquisition efficiency of OCT systems that conventionally use raster scanning. The advantages of CLV-SC are even more apparent for high speed scanning, making it especially well-suited for 4D imaging applications. Furthermore, we have demonstrated imaging protocols and visualization methods that leverage isotropic and highly uniform transverse sampling enabled by CLV-SC. Linear and circular B-scans from arbitrary orientations and with equal sampling pitch could be extracted from a single CLV-SC OCT volume. The ability to assess retinal structure using these different visualization modes generated from a single volume could be a powerful tool to reduce patient imaging time in clinical ophthalmology, in which each visualization mode would have likely required separate image acquisitions. Moreover, highly uniform transverse sampling with CLV-SC yielded high quality volumetric renders that enabled clear visualization of ocular dynamics and simulated surgical maneuvers.
The primary goal of this work was to implement, for the first time, CLV-SC for OCT and characterize the achievable performance relative to the theoretical CLV-SC performance and to bidirectional raster scanning. The presented CLV-SC implementation achieved a scanning velocity which was within 20% of linear for at least 75% of the scanning period for volume rates up to 10 Hz. The CLV-SC performance was likely limited by the GS, and future implementations should yield superior results as the performance of commercial GS technology continues to improve. Alternatively, improved performance could likely be obtained using frequency-dependent driving waveforms, in which the drive amplitude and phase are compensated according to the measurements in Fig. 12, such that constant linear velocity scanning could be experimentally achieved over the whole volume with current GS technology. We have also introduced a method for characterizing transverse sampling uniformity of OCT images by estimating the instantaneous scan velocity and calculating the degree of sampling uniformity. In general, non-uniform instantaneous scan velocity, stemming either from distortion of the scan pattern or using sinusoidal drive waveforms, leads to both optical sampling non-uniformity and distortion of en face OCT images. Image distortions may be corrected post-acquisition by imaging a test target with known features, estimating distortion from the resulting image, and using the known features as reference for dewarping as demonstrated here and in other studies. Optical sampling non-uniformity, however, cannot be corrected post-acquisition and it can negatively impact image quality and increase the acquisition time required to critically sample the entire transverse FOV of interest. We have experimentally demonstrated that although CLV-SC exhibited non-uniform instantaneous scan velocity which led to oversampling at the center of the scan, it still achieved greater degree of sampling uniformity compared to BRS given equivalent transverse sampling parameters for both scan types. This is significant because even after image distortion is corrected in post-processing, CLV-SC has the potential to produce higher quality OCT images than BRS due to superior optical sampling uniformity.
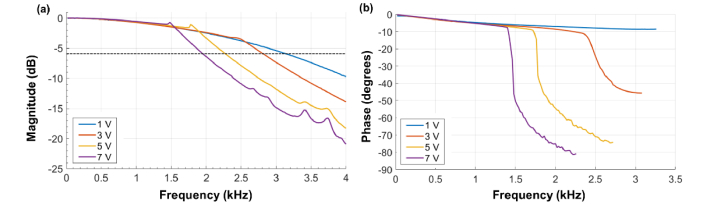
Fig. 12.
Magnitude (a) and phase (b) spectra of galvanometric scanners for 1-7 V peak-to-peak sinusoid amplitudes. The dashed black horizontal line in (a) denotes −6 dB. V: Volts; dB: 20log10(amplitude ratio).
High speed raster scanning is often performed with resonant scanners, which are necessarily driven with sinusoidal voltage waveforms at the resonant frequency of the scanners. To compare the performance of BRS with resonant scanners to CLV-SC, the instantaneous scan velocity for resonant scanners was theoretically approximated by calculating the first derivative of a 1 kHz sinusoidal drive waveform, which resulted in a 1 kHz cosine function. The degree of sampling uniformity of BRS with resonant scanners was then estimated by calculating the fraction of the scan period in which the instantaneous scan velocity was greater than 80% of the peak (or ideal) instantaneous scan velocity. This is the same metric that was used to assess CLV-SC performance in Results 4.1. The theoretical degree of sampling uniformity for 1 kHz resonant scanners was ~40%. To compare the performance of resonant scanners to CLV-SC, note that a 1 kHz resonant scanner would yield a 10 Hz volume rate with the same isotropic transverse sampling as CLV-SC (as listed in Table 1). For a volume rate of 10 Hz, CLV-SC achieved 75% degree of sampling uniformity (Fig. 6(b)). Therefore, this theoretical analysis suggests that CLV-SC should provide superior performance than BRS with resonant scanners for a 10 Hz volume rate with isotropic transverse sampling. However, the degree of sampling uniformity with resonant scanners should remain constant for > 1 kHz and resonant scanners may exhibit superior performance than CLV-SC at higher frequencies.
Fig. 6.
Degree of sampling uniformity achievable with constant linear velocity spiral scanning (CLV-SC) and bidirectional raster scanning (BRS) for volume acquisition rates of 1-10 Hz and isotropic transverse sampling. (a) Degree of sampling uniformity along the fast (angular) and slow (radial) axes of CLV-SC. (b) Sampling uniformity comparison of CLV-SC and BRS.
CLV-SC also offers advantages associated with scanning a circular transverse FOV though optical lenses and other apertures that conventionally exhibit cylindrical symmetry. In applications in which the beam is scanned throughout the entire limiting aperture of the system, such as wide field OCT imaging [29], CLV-SC could reduce vignetting compared to raster scanning methods that produce rectangular FOVs in which the beam is clipped at the corners.
The performance of CLV-SC was assessed by estimating the instantaneous scan velocity during a volumetric scan. As discussed above, the presented implementation of CLV-SC exhibited a discrepancy between the measured instantaneous scan velocity and the desired (ideal) instantaneous scan velocity. This discrepancy was particularly marked at and near the center of the spiral, which corresponded to high frequency and small amplitude GS drive waveforms. To investigate this discrepancy, the magnitude and phase spectra of the GS were measured by driving the GS using sinusoidal voltage waveforms with frequencies ranging from 20 Hz to 4 kHz (with constant amplitude) and recording the response waveforms generated by the position encoders of the GS. The magnitude spectrum was estimated by calculating the ratio of the amplitude of the response waveforms to the amplitude of the drive waveforms. The phase spectrum was estimated as the instantaneous phase difference between response and drive waveforms obtained from their analytic signal representations calculated using the Hilbert transformation, averaged over the measurement time of 0.25 seconds. An additional instrumentation-related fixed time delay between drive and response of 200 µs, measured at the lowest test frequency, was subtracted from all response measurements. The GS magnitude and phase spectra were measured for four peak-to-peak voltage sinusoidal amplitudes ranging from 1 to 7.5 V. The results of these experiments are shown in Fig. 12. The GS magnitude spectra for all amplitudes were similar for frequencies < 1 kHz but markedly different for frequencies > 1 kHz, with faster frequency rolls-offs corresponding to larger amplitudes. The phase response was approximately flat for all amplitudes and for frequencies < 1 kHz but showed clear roll-off for frequencies > 1 kHz. The phase estimates became unstable at frequencies higher than shown in Fig. 12 due to the strong response signal attenuation at those frequencies. We postulate that the reduced performance near the center of CLV-SC pattern was likely a result of high frequency attenuation by the system transfer function of the GS. The phase response of the GS could have also contributed to CLV-SC performance, although further experiments are required to estimate the phase response at frequencies > 1 kHz more accurately. Interestingly, precise measurements of the magnitude and phase spectra of the GS at amplitudes of interest could provide sufficient information for pre-shaping the input signals to yield the desired GS response and improve CLV-SC performance. Similar studies have been performed for BRS using GS [30] and MEMS scanners [31] and therefore it may be a promising strategy for optimizing CLV-SC performance.
There are several other limitations of CLV-SC that could be addressed with further technical development. CLV-SC requires transformation of OCT data from polar to Cartesian coordinates prior to display. In this study, we performed bilinear interpolation during this transformation on a CPU using an algorithm that was not optimized for speed. GPU-based processing and rendering, which is now standard for various applications of OCT imaging, could offer a solution for real time processing of CLV-SC OCT scans. Additionally, pixel reassignment with nearest-neighbor interpolation, instead of bilinear interpolation used in this study, could be a more computationally efficient approach for real time processing. Conventional OCT motion correction algorithms (such as x-fast and y-fast image acquisition and processing [32]) may also not be directly applicable to CLV-SC. However, CLV-SC would still be compatible with real time eye motion correction using external retinal tracking technology. In addition, the performance results for the fast (angular) and slow (radial) axes of CLV-SC were different, as illustrated in Fig. 6(a). This was likely because the characteristics of the GS drive waveforms contributed differently to the sampling pitches along each axis, as shown in Eqs. (6) and (7). Therefore, distortion of the drive waveforms due to the system response of the GS should have different effects on the performance along each CLV-SC axis.
6. Conclusions
CLV-SC is a novel scan pattern for ultrahigh speed OCT. We have experimentally demonstrated that CLV spiral scanning obviates the need for acquisition dead time and exhibits superior sampling uniformity compared to raster scanning using BRS. Ophthalmic and surgical 4D OCT imaging was performed with CLS-SC to demonstrate its potential utility.
Acknowledgments
The authors would like to thank Ruobing Qian for fruitful discussions and Alexandria Drandridge for assistance during simulated surgeries.
Funding
National Institutes of Health/National Eye Institute Biomedical Research Partnership (#R01-EY023039); National Institutes of Health/National Eye Institute (#R01-EY24312); Department of Defense CDMRP (Contract W81XWH-16-1-0498); NSF Graduate Research Fellowship; Ford Foundation Predoctoral Fellowship; NIH T-32 Training Grant.
Disclosures
JAI: Leica Microsystems (P, R).
References
- 1.Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A., Fujimoto J. G., “Optical Coherence Tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuman J. S., Puliafito C. A., Fujimoto J. G., Duker J. S., Optical Coherence Tomography of Ocular Diseases, 3rd ed. (SLACK Incorporated, 2013). [Google Scholar]
- 3.Grulkowski I., Liu J. J., Potsaid B., Jayaraman V., Lu C. D., Jiang J., Cable A. E., Duker J. S., Fujimoto J. G., “Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers,” Biomed. Opt. Express 3(11), 2733–2751 (2012). 10.1364/BOE.3.002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An L., Li P., Shen T. T., Wang R., “High speed spectral domain optical coherence tomography for retinal imaging at 500,000 A‑lines per second,” Biomed. Opt. Express 2(10), 2770–2783 (2011). 10.1364/BOE.2.002770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu C. D., Kraus M. F., Potsaid B., Liu J. J., Choi W., Jayaraman V., Cable A. E., Hornegger J., Duker J. S., Fujimoto J. G., “Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror,” Biomed. Opt. Express 5(1), 293–311 (2014). 10.1364/BOE.5.000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieser W., Draxinger W., Klein T., Karpf S., Pfeiffer T., Huber R., “High definition live 3D-OCT in vivo: design and evaluation of a 4D OCT engine with 1 GVoxel/s,” Biomed. Opt. Express 5(9), 2963–2977 (2014). 10.1364/BOE.5.002963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi D. H., Hiro-Oka H., Shimizu K., Ohbayashi K., “Spectral domain optical coherence tomography of multi-MHz A-scan rates at 1310 nm range and real-time 4D-display up to 41 volumes/second,” Biomed. Opt. Express 3(12), 3067–3086 (2012). 10.1364/BOE.3.003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K., Kang J. U., “Real-time intraoperative 4D full-range FD-OCT based on the dual graphics processing units architecture for microsurgery guidance,” Biomed. Opt. Express 2(4), 764–770 (2011). 10.1364/BOE.2.000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco-Zevallos O. M., Keller B., Viehland C., Shen L., Seider M. I., Izatt J. A., Toth C. A., “Optical Coherence Tomography for Retinal Surgery: Perioperative Analysis to Real-Time Four-Dimensional Image-Guided Surgery,” Invest. Ophthalmol. Vis. Sci. 57(9), OCT37 (2016). 10.1167/iovs.16-19277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco-Zevallos O. M., Keller B., Viehland C., Shen L., Waterman G., Todorich B., Shieh C., Hahn P., Kuo A. N., Toth C. A., Izatt J. A., “Live volumetric (4D) visualization and guidance of in vivo human ophthalmic microsurgery with intra-operative optical coherence tomography,” Sci. Rep. 6, 316891 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrasco-Zevallos O. M., Viehland C., Keller B., Draelos M., Kuo A. N., Toth C. A., Izatt J. A., “Review of intraoperative optical coherence tomography: technology and applications [Invited],” Biomed. Opt. Express 8(3), 1607–1637 (2017). 10.1364/BOE.8.001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson E. A., Izatt J. A., Hee M. R., Huang D., Lin C. P., Schuman J. S., Puliafito C. A., Fujimoto J. G., “In vivo retinal imaging by optical coherence tomography,” Opt. Lett. 18(21), 1864–1866 (1993). 10.1364/OL.18.001864 [DOI] [PubMed] [Google Scholar]
- 13.Duma V. F., Tankam P., Huang J., Won J., Rolland J. P., “Optimization of galvanometer scanning for optical coherence tomography,” Appl. Opt. 54(17), 5495–5507 (2015). 10.1364/AO.54.005495 [DOI] [PubMed] [Google Scholar]
- 14.Duma V. F., Lee K. S., Meemon P., Rolland J. P., “Experimental investigations of the scanning functions of galvanometer-based scanners with applications in OCT,” Appl. Opt. 50(29), 5735–5749 (2011). 10.1364/AO.50.005735 [DOI] [PubMed] [Google Scholar]
- 15.Park H.-C., Seo Y.-H., Jeong K.-H., “Lissajous fiber scanning for forward viewing optical endomicroscopy using asymmetric stiffness modulation,” Opt. Express 22(5), 5818–5825 (2014). 10.1364/OE.22.005818 [DOI] [PubMed] [Google Scholar]
- 16.Huo L., Xi J., Wu Y., Li X., “Forward-viewing resonant fiber-optic scanning endoscope of appropriate scanning speed for 3D OCT imaging,” Opt. Express 18(14), 14375–14384 (2010). 10.1364/OE.18.014375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Hong Y.-J., Makita S., Yasuno Y., “Three-dimensional eye motion correction by Lissajous scan optical coherence tomography,” Biomed. Opt. Express 8(3), 1783–1802 (2017). 10.1364/BOE.8.001783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labinsky A., Reynolds G., Halliday J., “A disk recording system and a method of controlling the rotation of a turntable in such a disk recording system,” U.S. patent PCT/GB1992/002370 (1993).
- 19.Mahmood I. A., Moheimani S. O. R., Bhikkaji B., “A new scanning method for fast atomic force microscopy,” IEEE Trans. NanoTechnol. 10(2), 203–216 (2011). 10.1109/TNANO.2009.2036844 [DOI] [Google Scholar]
- 20.Delattre B. M. A., Heidemann R. M., Crowe L. A., Vallée J. P., Hyacinthe J. N., “Spiral demystified,” Magn. Reson. Imaging 28(6), 862–881 (2010). 10.1016/j.mri.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 21.Carrasco-Zevallos O. M., Nankivil D., Viehland C., Keller B., Izatt J. A., “Pupil tracking for real-time motion corrected anterior segment optical coherence tomography,” PLoS One 11(8), e0162015 (2016). 10.1371/journal.pone.0162015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrasco-Zevallos O. M., Viehland C., Keller B., Kuo A. N., Toth C. A., Izatt J. A., “High-speed 4D intrasurgical OCT at 800 kHz line rate using temporal spectral splitting and spiral scanning (Conference Presentation),” Proc. SPIE 1005, 10053 (2017). [Google Scholar]
- 23.Jia Y., Tan O., Tokayer J., Potsaid B., Wang Y., Liu J. J., Kraus M. F., Subhash H., Fujimoto J. G., Hornegger J., Huang D., “Split-spectrum amplitude-decorrelation angiography with optical coherence tomography,” Opt. Express 20(4), 4710–4725 (2012). 10.1364/OE.20.004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginner L., Blatter C., Fechtig D., Schmoll T., Groschl M., Leitgeb R. A., “Wide-Field OCT Angiography at 400 KHz Utilizing Spectral Splitting,” Photonics 1(4), 369–379 (2014). 10.3390/photonics1040369 [DOI] [Google Scholar]
- 25.Viehland C., Keller B., Carrasco-Zevallos O. M., Nankivil D., Shen L., Mangalesh S., Viet T., Kuo A. N., Toth C. A., Izatt J. A., “Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT,” Biomed. Opt. Express 7(5), 1815–1829 (2016). 10.1364/BOE.7.001815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nankivil D., Waterman G., LaRocca F., Keller B., Kuo A. N., Izatt J. A., “Handheld, rapidly switchable, anterior/posterior segment swept source optical coherence tomography probe,” Biomed. Opt. Express 6(11), 4516–4528 (2015). 10.1364/BOE.6.004516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y., Sung K. R., Na J. H., Sun J. H., “Dynamic changes in anterior segment (AS) parameters in eyes with primary angle closure (PAC) and PAC glaucoma and open-angle eyes assessed using AS optical coherence tomography,” Invest. Ophthalmol. Vis. Sci. 53(2), 693–697 (2012). 10.1167/iovs.11-8389 [DOI] [PubMed] [Google Scholar]
- 28.Todorich B., Shieh C., DeSouza P. J., Carrasco-Zevallos O. M., Cunefare D. L., Stinnett S. S., Izatt J. A., Farsiu S., Mruthyunjaya P., Kuo A. N., Toth C. A., “Impact of microscope integrated OCT on ophthlamology resident performance of anterior segment surgical maneuvers in model eyes,” Invest. Ophthalmol. Vis. Sci. 57(9), OCT146 (2016). 10.1167/iovs.15-18818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polans J., Keller B., Carrasco-Zevallos O. M., LaRocca F., Cole E., Whitson H. E., Lad E. M., Farsiu S., Izatt J. A., “Wide-field retinal optical coherence tomography with wavefront sensorless adaptive optics for enhanced imaging of targeted regions,” Biomed. Opt. Express 8(1), 16–37 (2016. 7. 10.1364/BOE.8.000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo H. W., Ito S., Schitter G., “High speed laser scanning microscopy by iterative learning control of a galvanometer scanner,” Control Eng. Pract. 50, 12–21 (2016). 10.1016/j.conengprac.2016.02.007 [DOI] [Google Scholar]
- 31.Cogliati A., Canavesi C., Hayes A., Tankam P., Duma V. F., Santhanam A., Thompson K. P., Rolland J. P., “MEMS-based handheld scanning probe with pre-shaped input signals for distortion-free images in Gabor-domain optical coherence microscopy,” Opt. Express 24(12), 13365–13374 (2016). 10.1364/OE.24.013365 [DOI] [PubMed] [Google Scholar]
- 32.Kraus M. F., Potsaid B., Mayer M. A., Bock R., Baumann B., Liu J. J., Hornegger J., Fujimoto J. G., “Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns,” Biomed. Opt. Express 3(6), 1182–1199 (2012). 10.1364/BOE.3.001182 [DOI] [PMC free article] [PubMed] [Google Scholar]