Abstract
More than 200 million prescriptions are written annually for opioid analgesics despite limited evidence of their long-term efficacy. These medications currently are prescribed to 10% – 15% of Americans with use of long-acting opioids projected to double in the next three to four years. Despite this widespread use, little is known about the risks of opioids, particularly with chronic use. New data from our research group published in the Journal of General Internal Medicine provides clear evidence that prescription opioid used for non-cancer, non-HIV pain increases significantly the risk of development of major depressive disorder in opioid naïve individuals with no recent history of depression and substance used disorders. The risk of depression increased as the dose and/or the duration of opioid use increased. The purpose of the present paper is to elucidate the details of this study, to examine potential neurobiological mechanisms responsible for the depressogenic effect of opioid analgesics, and to discuss management options that emphasize depression prophylaxis.
“We accept responsibility for those past misstatements and regret they were made.”
-Purdue Pharma Spokesman1
Introduction
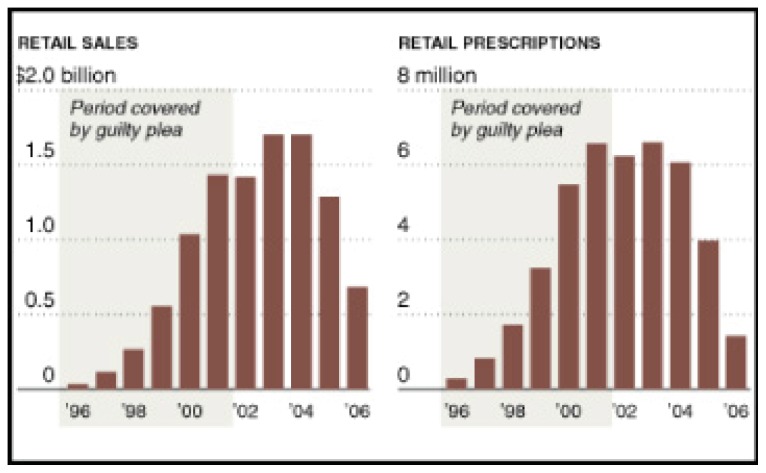
The Opioid ‘Pharmageddon’. The quote above is taken from a statement released by Purdue Pharma, the maker of OxyContin (oxycodone), coincident to its conviction in federal court in 2007 on criminal charges related to the marketing of OxyContin. The company had deliberately misled doctors and patients when it claimed and promoted this opioid analgesic as less likely to be abused than traditional narcotics. The company agreed to pay $600 million in fines to resolve the criminal charges related to “misbranding” of OxyContin. Prosecutors claimed that the fine represented approximately 90% of profits from the drug over the interval during which it was fraudulently promoted (See Figure 1).1
Figure 1.
Oxycontin Retail Sales and Prescriptions
As is evident from the figure, however, the conviction had little acute impact on widespread use of or Purdue Pharma’s profits from prescription OxyContin. This notwithstanding, the lawsuit against OxyContin underscores the need for a fuller understanding of the effects (both adverse and therapeutic) of opioid analgesics. Pharmaceutical companies are not solely responsible for current widespread use of these medications. Medical professionals and patients share part of the responsibility as well. Regardless, there is little doubt that opioid analgesic use (OAU) itself has become a significant public health problem.
Despite their highly addictive potential, opioids have become a popular primary treatment of chronic non-cancer pain, such as back or joint pain. This practice has created a growing public health problem as addiction and misuse of opioids have reached epidemic proportions. Medical use of opioid analgesics has increased by a factor of 10 since the 1990s, and these drugs have become the second leading cause of accidental death in the U.S.2 These increases in OAU occurred while the number of outpatient visits for conditions associated with chronic pain and activity limitations (e.g., arthritis) remained relatively stable.3 According to SAMSHA, there were a total of 1.9 million new illicit users of opioid analgesics in 2012 (about 5,150 new abusers per day). Americans comprise only 4.6% of the world population, but 80% of the global consumption of opioids takes place in the U.S.4 Due to the current high rates of abuse, the FDA is now recommending that supplies of medications containing hydrocodone be restricted to a single 90-day prescription (similar to oxycodone). Coincidentally, our research has found that prescription opioid use for more than 90 days significantly increases risk of Major Depressive Disorder (MDD), as discussed below.
Terminology
Opium is one of the oldest drugs of abuse, largely due to its main natural alkaloid constituents (i.e., natural opiates), such as morphine, codeine, and thebaine.5 Semisynthetic opiates are developed by chemical modification of natural opiates, and include heroin, hydrocodone, oxycodone, among others. The term opiate refers to a diverse group of natural or semisynthetic substances with morphine-like pharmacological activity that bind to mu, kappa, and delta opioid receptors in the body. The umbrella term opioid refers to any natural, semisynthetic or fully synthetic substance that binds to the opioid receptors, irrespective of its structural similarity with naturally occurring molecules and agonistic or antagonistic action (e.g., pentazocine, methadone, naltrexone, or endogenous opioid peptides such as beta-endorphine, among others). Opioids act upon the central and the peripheral nervous system and most have analgesic, mood altering, “sleep inducing” (hence “narcotic”) effects that acting singly or in concert render them as potentially addictive. Opium also contains non-analgesic, non-narcotic, non-addictive alkaloids such as papaverine and noscapine, which act only to relax involuntary (smooth) muscles and are used to treat vasospasm, erectile dysfunction, and for cough suppression, respectively.
Understanding Causal Interactions Among Pain, Opioid Use and Depression
Depression resulting from opioid use would at first appear paradoxical, as opioids are known to have euphoric and analgesic effects. Dysphoria that occurs in <5% of exposed patients is thought to be transient and is not known to result in more serious affective illness. The tri-directional causal interactions among pain, OAU, and depression confounded attempts by researchers to determine whether opioids interposed between pain and depression could independently contribute to the development of new episodes of depression.6 Some studies have found an increased risk of depression only in patients with opioid dependence.7,8 Others have shown an increased risk of opioid misuse in patients with depression.9 Also, chronic pain conditions can cause depression independent of opioid use.10
Advanced methods to control for bias by indication and residual confounding now allow investigators to leverage massive medical record data including prescription records to disentangle pain from the opioid-MDD relationship. Because these data contain thousands of patients, we are able to build regression models to predict the probability of opioid receipt from hundreds of covariates resulting in propensity score. The propensity score, i.e. the probability of getting an opioid for different durations of time, is then used to weight the medical record data to balance pain diagnosis and other factors associated with opioids and depression, across opioid using patients who differ in duration of OAU. 11 Our research group employed a new user retrospective cohort design in combination with propensity score analysis and demonstrated a clear depressogenic impact of OAU, the effect detectable despite the sample (by virtue of being 85% male and 55 years of age on average and free of depression diagnosis for the first two years prior to opioid receipt) being presumably at low risk of MDD. The study is described below in detail.
Prescription Opioid Analgesics Increase the Risk of Depression
We studied the impact of new opioid prescriptions on the subsequent risk of development of depression in a large sample of patients who used the Veterans Health Administration (VA) (N=49,770)12. The study was the first to examine this relationship in subjects who had no recent history of MDD and no OAU (recent history was defined as 24 months prior to the beginning of the study). Propensity scores were used to control for bias by indication, and the data were weighted to balance the distribution of covariates by duration of incident opioid exposure. In other words, propensity score analyses were used to control for potential confounding effects of pain and other factors that affect exposure to opioid analgesics and the occurrence of depression. Cox proportional hazard models with adjustment for painful conditions were used to estimate the association between the duration of prescription opioid use and the subsequent risk of development of MDD. The cohort was evaluated over a seven-year interval from 2001 to 2007. The subjects ranged in age from 18 to 80, although most were middle age (mean age = 54.6 years).
Preliminary analyses indicated that incident MDD was associated with any opioid use as compared to patients who never received opioids. After adjusting for PTSD, substance use disorder, painful conditions, and socio-demographic factors in these preliminary analysis, initiating an opioid remained a significant risk factor for MDD (HR=1.51). The risk of MDD in the subjects was increased with longer duration of treatment and was dose-dependent, i.e., higher doses were associated with higher risk.
To determine if the risk for depression remained after accounting for bias by indication, we used propensity scores and inverse probability of treatment weighting to balance the distribution of covariates in patients who were prescribed opioid analgesics for 1–89, 90–180, and > 180 days, respectively, longer duration of opioid prescription was associated with increased risk of development of depression. Patients using for 90–180 days had a 25% increased risk of depression, and those using them for more than 180 days had a more than a 50% increased risk. The effects remained significant after additional adjustment for chronic pain due to neuropathies, headaches, musculoskeletal diseases, back pain, and arthritis.
We also computed the proportion of incident depression cases by high vs. low opioid dosage stratified on duration of use. Within each duration-of-use-group, patients receiving a high daily dose were at significantly increased risk of depression. However, the proportion of subjects with depression remained similar among low dose patients across duration of use. In contrast, in patients receiving a high dose, the proportion of depressed patients’ increases across duration of use from 9.3% in 1–89 day users, to 13.1% in 90–180 day users to 15.0% in > 180 day users.
Although the patients were free of opioids and MDD during the two-year period before baseline assessment, it is possible that some had a remote lifetime history of MDD or had subclinical symptoms of depression that were made worse by opioid exposure. In these cases, opioid use was associated with a new episode of MDD or an exacerbation of symptoms that become severe enough to result in a diagnosis. In patients who developed MDD without a history or without subclinical symptoms of depression, opioid use was associated with a new onset of MDD. In both instances, the temporal sequence of events (opioids were prescribed first, followed by MDD) supports the hypothesis that prescription opioid analgesics may be a causal contributor to depression.
Neurobiology of Endogenous Opioids and Opioid Receptors
Our data12 indicates that use of opioid analgesics in opioid naïve subjects without depression at the time of opioid initiation may increase the risk of developing MDD. However, the biological mechanisms of this effect in humans have not been systematically studied. Here we review relevant molecular biology of opioids and opioid receptors.
At the molecular level, opioid analgesics act on specific opioid receptors (mu, kappa, nociceptin, or delta) with different physiological roles and functions (See Table 1).
Table 1.
Opioid receptor subtypes, distribution and function.
| Receptor | Subtypes & Symbols* | Endogenous ligands | Distribution | Function |
|---|---|---|---|---|
| delta (δ) | DOR1 DOR2 (δ1, δ2) |
enkephalin | cortex hippocampus amygdala basal ganglia hypothalamus35 nucleus accumbens36 |
analgesia antidepressant effects regulation of anxiety convulsant effects mydriasis |
| kappa (κ) | KOR1a KOR1b KOR2 KOR3 (κ1, κ2, κ3) |
dynorphin | hippocampus hypothalamus thalamus cortex caudate olfactory tubercle37 nucleus accumbens36 |
analgesia anticonvulsant effects dysphoria reward processing response to stress miosis sedation spinal anti-nociception |
| mu (μ) | MOR1 MOR2 MOR3 (μ1, μ2, μ3) |
β-endorphin enkephalins |
thalamus reticular core nuclei periaqueductal gray of the midbrain ventral tegmentum area nucleus accumbens36 dorsal column of the spinal cord gastrointestinal tract |
euphoria reward processing analgesia spinal and supra-spinal antinociception respiratory depression response to stress regulation of the hypothalamic-pituitary-gonadal axis miosis cough suppression reduced GI motility pruritus vasodilation |
| Nociceptin (formerly orphanin) | NOP1 | Nocipeptin / Orphanin FQ (N/OFQ) | cortex striatum thalamus hypothalamus38 spinal cord heart lungs kidneys intestine immune cells37 |
modulation of nociception reward processing appetite regulation learning and memory regulation opioid-induced hyperalgesia |
Former nomenclature is shown in parenthesis.
Opioid receptors belong to the class of G protein coupled receptors, a large family of transmembrane receptors that react to various ligands (e.g. neurotransmitters, hormones, peptides) outside the cell and activate various intracellular signal pathways - ultimately leading to specific cellular responses.13 The ligands that bind to opioid receptors include endogenous opioid peptides such as β-endorphin, enkephalins, endomorphins, nociceptin/ orphanin, and dynorphins.14,15 Opioid receptors and peptides are abundant throughout the brain and less so in the peripheral nervous system16 and gastrointestinal tract. Opioid receptor-rich areas include the limbic system, hypothalamus, pituitary, and brainstem. Limbic areas of the brain are essential in modulation of mood and reward-based learning15 both important in the neuropathology of addiction. Pharmacological agonists or antagonists at different receptors can cause a spectrum of physiological and pathological (undesired) effects,14 both somatic and mental.
Depressogenic Effects of Opioids: Possible and Plausible Mechanisms
The mechanisms by which opioid use contributes to incident depression are not fully understood, but are likely multifactorial. In preliminary post hoc analyses, it appeared that about 25% of the cases of incident MDD were diagnosed during treatment with opioids, but in most cases incident MDD was diagnosed after the treatment ended. Several plausible mechanisms for the depressogenic effects of opioids that might account for both early-onset MDD occurring during opioid use and delayed-onset MDD are described below.
-
Disturbance of the brain reward system and anhedonia in the absence of opioids. The resetting of the brain “reward pathway” to a higher threshold is widely believed to be a common brain response to addictive drugs, including opioids.17–19 The term “reward pathway” refers to a collection of brain structures that regulate behavior via the reinforcing effects of pleasurable stimuli. The neuroanatomical backbone of the reward system consists of dopamine projections from the ventral tegmentum (VTA) to the nucleus accumbens (NAc) (See Image 1) and parts of the prefrontal cortex.17
Opioid receptors are abundantly expressed in the NAc,20 (See Table 1) one of the brain structures of the reward pathway, which is critical in reinforcement learning and reward.21 Every type of reward that has been studied, including the vast majority of addictive drugs, increases the level of dopamine in the reward pathway.18,21 Cessation of drug use frequently leads to dysphoria, anhedonia, and low motivation, all hypothesized to reflect low baseline cortical activity and low baseline striatal dopaminergic activity22 in the absence of drugs. Such neuroplasticity could account for some cases of MDD in our sample that had occurred after opioids were stopped.
Epigenetic misregulation of the endogenous opioid system. Another plausible mechanism to explain our findings is opioid-induced kappa receptor hyperactivity, which has well documented depressogenic effects. Kappa opioid receptors modulate the processing of reward and reinforcement as well as some mood states,14 among other functions (See Table 1). Empirical research in animals23 and humans15 implicates increased kappa receptor activity in mediation of depressed mood, in response to stress and social defeat. Opioid use leads to an early increase in CNS production of dynorphin (an endogenous kappa receptor agonist) and thus to kappa receptor hyperactivity, conceivably explaining early onset MDD in our sample. On the other hand, as drug use continues long term, a different epigenetic mechanism suppresses the production of dynorphin and thus leads to decreased kappa receptor activity.21 Depression, body aches, and anxiety occurring for months and even years after opioid use has been discontinued (“protracted abstinence syndrome”), may be attributed to the rebound kappa receptor hyperactivity and MDD associated with the cessation of opioid treatment.24
Opioid-induced vulnerability to stress. At a system level, opioids modulate a variety of functional brain networks. For example, opioids affect the brain through their effects on the hypothalamic-pituitary-adrenal (HPA) axis and the hippocampus (a brain structure that is associated with learning, memory, and detection of novelty).14 The hippocampus modulates the HPA axis by controlling cortisol production in response to stress. Chronic administration of morphine affects the hippocampus by decreasing the density of dendritic spines.25 This can lead to impaired hippocampal control of the HPA axis and ultimately to depression and anxiety. Similarly, stress associated with MDD can decrease or inhibit the neurogenesis in the hippocampus and can result in atrophy of hippocampal neurons.26 Antidepressants, however, promote neurogenesis in the dentate gyrus region26 suggestive of potential beneficial effects of antidepressants for opioid induced depression.
Opioid-induced somatic correlates of depression. A spectrum of peripheral pathophysiological effects of opioids could underlie their depressogenic properties. For example, endocrine abnormalities are well recognized in chronic opioid users,27 and include, among others, adrenal insufficiency, vitamin D deficiency, and reduced testosterone levels. Many of these abnormalities negatively affect mood14 and clinically present with symptoms that mimic MDD, most prominently with low energy, low motivation, among others. These “physical correlates” of MDD could explain the delayed diagnosis of depression in our study.
Serotonergic deficit associated with opioid use. Opioids modulate several brain neurotransmitter circuits, mainly the serotonin and the dopamine networks14 with major roles in the regulation of mood, appetite, and cognitive functions. Given the well-known role of serotonin in mood and anxiety states, opioid induced disturbances of the serotonin system could conceivably mediate their depressogenic effects. Indeed, morphine injection in rodents releases serotonin in the limbic region, part of the brain that regulates memory and emotion.28 However, frequent morphine injections over an extended period of time decrease serotonin activity28, a well-documented causative factor in depression. Low serotonin activity in the context of long term opioid use may explain the correlation between increased risk of major depression and duration of use.
Psychosocial consequences of chronic opioid use. Although we controlled for opioid abuse and misuse, as well as for other addictions, it is possible that undetected early stages of substance use disorder could have occurred in patients using opioids for a longer period of time at a high dosages. At least some patients could have developed opioid use disorder during the trial with subsequent personal and/ or adverse life events, such as low self-esteem, sense of helplessness, isolation, or family, employment, and relationship dysfunction. These are frequently seen in chronic users and may have resulted in incident diagnosis of MDD. Unfortunately, major life events and daily functioning measures are not available in medical record data and this hypothesis will be explored in planned prospective data collection.
Image 1.
Nucleus Accumbens and Vental Tegmental Area
Finally, as an unlikely possibility, acute opioid withdrawal with mood, anxiety, and physical symptoms, could have contributed to some patients receiving the diagnosis of MDD shortly after opioids were discontinued.
Treatment and Prevention of “Opioid Depression”
The treatment recommendations outlined below are drawn from our clinical experience, and the depressogenic mechanisms described above, and are offered tentatively as they need further empirical testing. Due to the likely multifactorial etiology of MDD in the context of opioid use, treatment of these patients is unlikely to be uniform. Common medical adverse effects associated with opioid use, are first to be investigated and, if identified, treated. In patients who have developed opioid-induced MDD or are considered for chronic treatment with opioid analgesics (i.e., for more than 90 days), antidepressant medications could help to prevent or treat MDD. Chronic opioid use can result in decreased serotonin activity,28 an effect conceivably ameliorated with selective serotonin reuptake inhibitors (SSRI). As SSRIs promote neurogenesis in the hippocampus26 their administration may help re-instate hippocampal control of the HPA vulnerability to stress29 and prevent or treat depression in chronic opioid use. The same may hold for depression occurring after opioid use is stopped. In animal models, depressive symptoms following morphine abstinence are attenuated by fluoxetine.30 Analogous prophylactic approach is employed with interferon therapy for hepatitis C, where antidepressants are co-administered to treat or prevent interferon induced depression with some success.31 Whether co-administration of an antidepressant and opioid analgesics would help to prevent or delay incident depression in humans taking opioids for pain requires further study.
At least some opioid-induced depressive syndromes may have a unique underlying pathophysiology, such as overactive kappa receptors, and thus would require treatments not used in regular MDD syndromes. Buprenorphine, a partial mu receptor agonist and kappa receptor antagonist with powerful analgesic, stimulating, and antidepressant properties has been approved for opioid addiction and may prove useful for opioid induced MDD.32,33 Due to its stimulating effects, buprenorphine may be useful in many cases of “psychomotor” depression. As a potent analgesic and a high affinity partial mu receptor agonist (making it functionally a “blocker” of full opiate agonists), buprenorphine may be an effective alternative to traditional opioids in chronic pain management with reduced risk to further expand the current opioid epidemics.
Discussion
Out of control prescription opioid use is creating a public health problem of epidemic proportions. Our study was the first to demonstrate that OAU taken as prescribed for non-cancer, non HIV pain significantly increases the risk of MDD in individuals free of depression and substance use at the initiation of opioid treatment.12 With this in mind, practical strategies must be developed to deal with iatrogenic depression caused by long-term use of opioids for chronic pain.
The risk of incident depression increases as a function of both opioid dose and duration of treatment over 90 days. The clinical implication is that the duration of opioid analgesic use and the dose should be minimized whenever possible. In many cases, adding adjuvants for pain management makes it easier to reduce the dose and the duration of opioid treatments. Specifically, adjuvant treatment with SSRIs reduces pain severity and pain interference in opioid-dependent patients with depressive symptoms.34 Also, alternative, non-narcotic analgesics for acute pain syndromes may help reduce the frequency and the duration of opioid trials and thus decrease the risk of MDD.
Conclusion
Our data suggest that opioid analgesics should be prescribed for a short duration and at the lowest effective dose, be exclusively reserved for severe pain syndromes, with as much reliance on adjuvant therapy for pain management and non-narcotic analgesics as possible. We also advocate screening for depression and substance use disorder for all patients during opioid trials. Practitioners should consider antidepressant prophylaxis and treatment of MDD in patients entering chronic opioid treatment. More research is needed to determine whether co-administration of anti-depressants and opioids would help to prevent incident depression in humans taking opioid analgesics.
Biography
Katherine Semenkovich, BA, is in the Department of Pediatrics at Washington University School of Medicine. Ravikumar Chockalingam, MD and Kenneth E. Freedland, PhD are in the Department of Psychiatry at Washington University School of Medicine. Jeffrey F. Scherrer, PhD, is in Research Service, John Cochran Hospital, St. Louis VA Medical Center. John M. Ray, MA, is in Mental Health Service, St. Louis VA Medical Center. Vassilis N. Panagopoulos, MD, Dragan M. Svrakic, MD, PhD, (above), Patrick J. Lustman, PhD, and are with the Bell Street Clinic Opioid Treatment Program and at the at the Washington University School of Medicine and the Veterans Administration Medical Center in St. Louis.
Contact: Dragan.Svrakic@va.gov

Footnotes
Disclosure
None reported.
References
- 1.Meier B. Narcotic Maker Guilty of Deceit Over Marketing. The New York Times. May 11, 2007. [Accessed January 5, 2014]. Available at: http://www.nytimes.com/2007/05/11/business/11drug.html?pagewanted=all.
- 2.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 3.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- 5.Reisine T, Pasternak GW. Opioid analgesic and antagonists. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 1996;9:521–556. [Google Scholar]
- 6.Banks S, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychol Bull. 1996:95–110. [Google Scholar]
- 7.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56:793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abus. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 9.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10:304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery PC, Wilson KG, Kowal J. Major depressive disorder and sleep disturbance in patients with chronic pain. [Accessed Dec 23 2013];Pain Res Manag. 2013 doi: 10.1155/2014/480859. pii: 15542 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 12.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, et al. Prescription Opioid Analgesics Increase the Risk of Depression. J Gen Intern Med. 2013 doi: 10.1007/s11606-013-2648-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wettschureck N, Offermanns S. Mammalian G Proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 18.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse. 2001;42:185–192. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- 21.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacol. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 23.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharm Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 24.The Buprenorphine Effect on Depression, National Alliance of Advocates for Buprenorphine Treatment (NAABT) Newsletter 2007
- 25.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 26.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 27.George SM, Murali V, Pullickal R. Review of neuroendocrine correlates of chronic opiate misuse dysfunctions and pathphysiological mechanisms. Addict Disord Their Treat. 2005;4:99–109. [Google Scholar]
- 28.Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharm Exp Ther. 2002;303:704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- 29.Tanti A, Westphal WP, Girault V, Brizard B, Devers S, Leguisquet AM, et al. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus. 2013;23:797–811. doi: 10.1002/hipo.22134. [DOI] [PubMed] [Google Scholar]
- 30.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvao-de Almeida A, Guindalini C, Batista-Neves S, de Oliveira IR, Miranda-Scippa A, Quarantini LC. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32:401–405. doi: 10.1016/j.genhosppsych.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, et al. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J Subst Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 34.Fishbain D. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32:305–316. doi: 10.3109/07853890008995932. [DOI] [PubMed] [Google Scholar]
- 35.Simonin F, Befort K, Gaveriaux-Ruff C, Matthes H, Nappey V, Lannes B, et al. The human delta-opioid receptor: genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol Pharmacol. 1994;46:1015–1021. [PubMed] [Google Scholar]
- 36.Ikeda H, Kamei J, Koshikawa N, Cools AR. Nucleus accumbens and dopamine-mediated turning behavior of the rat: role of accumbal nondopaminergic receptors. J Pharmacol Sci. 2012;120:152–164. doi: 10.1254/jphs.12r02cr. [DOI] [PubMed] [Google Scholar]
- 37.George SR, Zastawny RL, Brionesurbina R, Cheng R, Nguygen T, Heiber M, et al. Distinct distributions of mu, delta, and kappa opioid receptor mRNA in the rat brain. Biochem Biophys Res Commun. 1994;205:1438–1444. doi: 10.1006/bbrc.1994.2826. [DOI] [PubMed] [Google Scholar]
- 38.Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol. 1998;81:184–192. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]