Abstract
Our current environment has led to a vicious cycle of physical inactivity, obesity, and chronic inflammation, creating the “perfect storm” for metabolic diseases. White adipose tissue (WAT) is the major source of obesity/inactivity-related inflammation; in turn, inflammation leads to insulin resistance and metabolic dysfunction. Inactivity, even in the absence of weight gain, disrupts WAT metabolism, while exercise mitigates WAT inflammation. The antiinflammatory mechanism(s) of exercise require additional study. Two current hypotheses include: (1) exercise-mediated antiinflammatory cytokine secretion, and (2) exercise-mediated improvements in adipocyte oxidative capacity.
Adipose Tissue Inflammation Leads to Metabolic Disease
Inflammation is an organism’s multi-level response to protect itself from harmful stimuli and begin the healing process. Although in most cases inflammation is resolved quickly, when elimination of the initial trigger is not accomplished, or when the trigger is ongoing, acute inflammation becomes chronic. Obese individuals typically have a two to threefold increase in plasma concentrations of immune factors such as tumor necrosis factor (TNF)-α, Interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, C-reactive protein (CRP), and others. Such chronic low-grade inflammation is an underlying cause of cardiovascular disease (CVD), Type 2 Diabetes (T2D), some cancers, and many other metabolic diseases associated with obesity, inactivity, and aging.
Research over the past two decades has demonstrated that the major source of inflammation associated with obesity is the WAT. No longer considered an inert storage organ, the WAT is an active endocrine organ producing and secreting a plethora of cytokines, hormones and other factors collectively called “adipokines”. When under metabolic stress, adipocytes produce inflammatory mediators and chemoattractant molecules (e.g., MCP-1) that can both recruit and activate resident and nonresident immune cells. It is these immune cells, namely macrophages (Mφ) and T-lymphocytes (i.e., T-cells), that perpetuate the inflammatory situation in the WAT. WAT Mφ-derived inflammatory molecules such as TNF-α directly disrupt insulin signaling, leading to the development of insulin resistance (IR) and eventually T2D. Cytokine-mediated IR is one known mechanism behind the strong relationship, evidenced by both human and animal studies, between WAT Mφ infiltration and somatic IR.1
An important distinction that has emerged relatively recently is that obesity is associated with “classical” or “M1” Mφ activation in WAT. When Mφ take on an “M1” activation state, they secrete more inflammatory molecules such as TNF-α. It is speculated that these M1 Mφ in turn activate T-cells to also secrete pro-inflammatory signals such as IFN-γ, and that these cytokines lead to Mφ-T-cell cross activation. It is proposed that it is this immune cell/adipocyte cross-activation that leads to tissue specific and eventually systemic IR. Just as M1 Mφ are inflammatory, alternatively activated “M2” Mφ are anti-inflammatory and have been shown to mitigate IR. Similarly, T-cells can take on a more anti-inflammatory/regulatory role under certain environmental conditions. Thus, the metabolic dysfunction associated with WAT inflammation is not merely a consequence of immune cell infiltration per se, but of complex immune cell-adipocyte interactions that take place under specific environmental conditions.
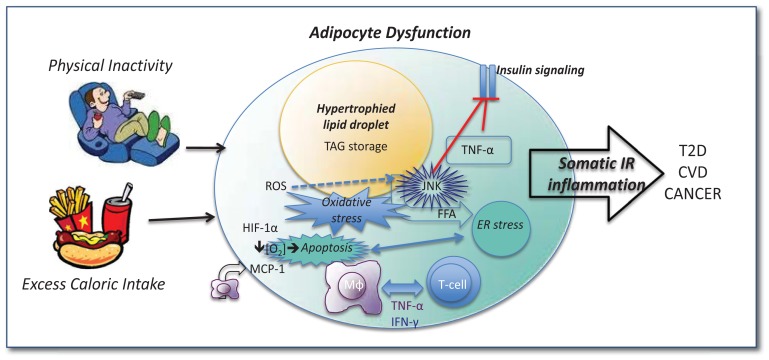
Several mechanisms have been proposed to explain the etiology of WAT inflammation in obesity; however, the brevity of this review precludes a thorough discussion of these. Among the prevailing theories are: (1) increased adipocyte death secondary to hypoxia associated with adipocyte size expansion, (2) adipocyte-immune cell priming (as explained in a very simplified context above) and (3) intra-adipocyte metabolic stress. While the exact etiology of WAT inflammation remains unclear, inflammation arising from the WAT is the “common soil” that unites obesity, physical inactivity and metabolic disease (See Figure 1).
Figure 1.
Physical inactivity and excess caloric intake lead to adipocyte dysfunction. Adipoycte hypertrophy results from excess TAG storage within lipid droplets. Excess FFA leads to adipocyte stress (e.g., ER stress) and the rapid expansion results in hypoxia and oxidative stress via activation of transcription factors such as HIF-1α. The “stressed” adipocyte expresses chemoatt ractant molecules (e.g., MCP-1) which help recruit Mφ and T-cells; these immune cells become “primed” by inflammatory molecules (e.g., TNF-α, IFN-γ) produced both by the adipocyte as well as resident and recruited immune factors, some of which directly inhibit insulin signaling (e.g., TNF-α). Oxidative stress increases ROS which activate inflammatory transcription factors including JNK which directly inhibits insulin signaling. Under such metabolic stress, apoptosis may occur; dead adipocytes in turn recruit inflammatory Mφ which exacerbate the inflammatory situation, leading to continued inflammation/IR both in the adipocyte and systemically. TAG = triacylglycerol; IR = insulin resistance; ROS = reactive oxygen species; HIF-1α = hypoxia inducible factor; MCP = monocyte chemoatt ractant protein; TNF = tumor necrosis factor; IFN = interferon; Mφ = macrophage; FFA = free fatty acids; JNK = c-Jun activated protein kinase; ER = endoplasmic reticulum.
Obesity, Inflammation and Physical Inactivity – A Vicious Cycle
While physical inactivity and obesity tend to coexist, a growing body of evidence supports the concepts that: (1) physical inactivity is a direct cause of the inflammation and metabolic dysfunction associated with obesity; and (2) exercise training/physical activity can mitigate inflammatory and metabolic disturbances even in the absence of weight loss. Support for the first concept is that physical inactivity is associated with inflammation and metabolic dysfunction even in lean individuals. In a study of non-obese patients who exhibited low-grade systemic inflammation evidenced by elevated plasma CRP and MCP-1, and lower insulin sensitivity, a brief period of complete inactivity exacerbated their inflammatory state, as indicated by a reduction in circulating adiponectin (an anti-inflammatory, insulin-sensitizing protein secreted from adipocytes) and increased abdominal WAT inflammation.2 Even the healthy controls in that study exhibited an increase in gene markers of WAT inflammation after the inactive period. On the other hand, for any given body mass index, those who exercise regularly have lower levels of inflammatory markers.3 This may help explain why ~20% of obese individuals are not afflicted with T2D, CVD, or various types of cancer traditionally associated with obesity.4 In some cases, obese individuals who are physically active present a healthier metabolic profile than their inactive, yet leaner, counterparts. This is true across ages.
One study of overweight children showed that, compared to equally obese unfit children, aerobically fit children had lower systemic inflammation.5 Another randomized controlled trial of overweight and obese asthmatics compared the effects of weight loss via diet, exercise, or the combination on asthma-related clinical outcomes.6 While asthma control improved with each intervention, WAT reduction per se (assessed via Dual-energy X-ray Absorptiometry, “DXA”) was associated with reduced airway inflammation. What’s more, exercise per se was associated with a reduction in a specific subtype of bronchial inflammation. Those findings suggest that there are unique benefits of both fat loss and exercise training on inflammatory outcomes. A growing number of animal studies aim to determine the unique mechanisms behind exercise-related improvements in insulin sensitivity, ectopic lipid accumulation, systemic inflammation, and immune cell function. Many have demonstrated that the inflammatory and metabolic improvements occur in the absence of overall weight loss, suggesting that the mechanism goes beyond the obvious obesity-mitigating effect of exercise.7,8 Our hypothesis is that exercise training may improve the overall health of the WAT, in part via anti-inflammatory mechanisms, and that such exercise-related improvements contribute to systemic metabolic improvements associated with exercise.
This is Your Adipocyte on Exercise: An Overview
Before a discussion of how exercise may reduce WAT inflammation, it is essential to provide a brief summary of exercise physiology pertaining to the adipocyte. Adipose tissue is the body’s largest energy reserve, storing upwards of 80,000 kilocalories of potential energy. With positive energy balance, adipocytes enlarge as they fill with lipid in the form of triacylglycerol (TAG); 9 excess energy storage over time leads to obesity and adipocyte dysfunction/inflammation, in part via mechanisms described above and illustrated in Figure 1.10 Given its huge supply of stored energy, it is not surprising that adipose tissue is immensely affected by exercise, especially endurance exercise, which requires much more energy than that stored as glycogen in skeletal muscle and liver.10 With negative energy balance, fatty acid utilization increases to meet energy demands and adipocytes shrink. As blood glucose levels become limited (e.g., during endurance exercise), WAT lipolysis increases as the body relies more on fatty acid oxidation.11 Net whole body fat oxidation is determined by many factors that regulate both WAT lipolysis and skeletal muscle oxidative capacity. WAT lipolysis and skeletal muscle fatty acid oxidation are tightly linked such that WAT lipolysis increases plasma non-esterified fatty acid (NEFA) concentration, and NEFAs are taken up by exercising muscles in a concentration-dependent manner. NEFA oxidation is dependent on both the intensity and the duration of the exercise.12
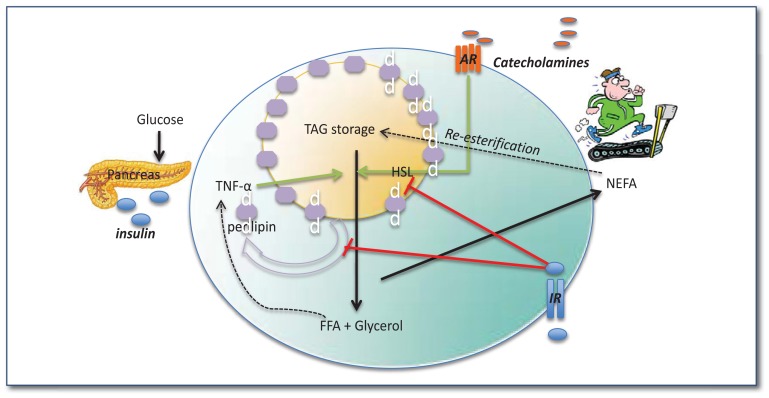
Adipocyte lipolysis is stimulated strongly by catecholamines (e.g., epinephrine/norepinephrine released during exercise) and potently inhibited by insulin, as illustrated in Figure 2.13 Briefly, catecholamines bind to adipocyte cell surface adrenergic receptors, triggering intracellular signaling events culminating in the phosphorylation of hormone sensitive lipase (HSL) by protein kinase A; phosporylated HSL mediates the hydrolysis of TAG resulting in the release of NEFAs and free glycerol from the adipocyte.14,15 Lipid droplet proteins such as perilipin are also important regulators of lipolysis and, when active, allow for the hydrolysis of lipid droplets within adipocytes. Other important players in the stimulation of lipolysis are adipose triglyceride lipase (ATGL) and CG158. ATGL is a TAG hydrolase and the rate-limiting enzyme that promotes the catabolism of fat in both adipose and non-adipose tissues. Efficient ATGL activity requires activation by CG158, and upon stimulation of ATGL, release of fatty acids is increased.16,17 On the other hand, insulin (which decreases during exercise) de-activates both HSL and lipid droplet proteins thereby inhibiting lipolysis.
Figure 2.
Adipocyte lipolysis is stimulated during exercise and inhibited by insulin. Catecholamines released during exercise bind to adrenergic receptors (AR) on the cell surface of adipocytes which ultimately phosphorylate (i.e., activate) lipases such as hormone sensitive lipase (HSL) allowing for TAG breakdown into FFA and glycerol. Lipid droplet proteins including perilipin are phosphorylated allowing them to dissociate from the lipid droplet surface; together these events allow for NEFAs to leave the adipocyte and enter the circulation where they are taken up by exercising skeletal muscle. Insulin inhibits this process while inflammatory cytokines (e.g., TNF-α) activate it. In the presence of high blood glucose, NEFAs are not taken up and therefore get re-esterified and re-enter the storage depot. Under IR conditions, insulin-mediated inhibition of lipolysis is diminished leading to a dyslipidemic blood profile as well as exacerbated inflammation within the adipocyte.
Insulin Resistance (IR), Lipolysis and Exercise
WAT IR is associated with dysregulated lipolysis; meaning, insulin’s ability to suppress lipolysis is reduced. What’s more, inflammatory cytokines produced and secreted by the WAT stimulate lipolysis, thus leading to hyperlipidemia and a vicious cycle of inflammation, IR, and adipocyte dysfunction (See Figure 2). As adipocytes enlarge, they themselves become IR and resilient to further lipid accretion, an insulin-dependent process. This failure to store fatty acids leads to ectopic lipid accumulation in various tissues including skeletal muscle, liver, and pancreatic islets. Moreover, FFAs themselves induce IR via the activation of serine kinases, including c-jun N-terminal kinase (JNK), which disrupt downstream insulin signaling; this is referred to as lipotoxicity.18–21
One of the best-known metabolic effects of regular physical activity is increased whole body insulin sensitivity. It is well appreciated now that insulin-stimulated glucose uptake increases in the immediate post-exercise period; this is true in humans and rodents. The mechanism(s) by which exercise acutely enhances insulin sensitivity are not completely defined, although evidence has demonstrated improvements in skeletal muscle insulin signaling (i.e., enhanced expression of insulin signaling proteins including insulin receptor, insulin receptor substrates (e.g., IRS-1), and Akt phosphorylation) after acute exercise in both humans and rodents.22,23 Such insulin-signaling improvements have been related to a reduction in skeletal muscle JNK activation, suggesting an anti-inflammatory mechanism by which acute exercise improves insulin signaling.22
Another mechanism by which exercise has been associated with improvements in skeletal muscle IR is through amelioration of de novo lipogenesis.24 Obesity and inactivity are associated with ectopic lipid storage in liver and skeletal muscle, which contributes to systemic IR. Exercise training, by increasing both WAT lipolysis and skeletal muscle NEFA oxidation, may reduce the proportion of FFAs towards detrimental pathways, such as ectopic storage of fatty acid metabolites.25 Since intramuscular triglyceride (IMTG) is an important source of fuel for skeletal muscle during high-intensity, exhaustive endurance exercise, training increases IMTG synthesis and storage, especially when NEFA accessibility is high-- this is known as the “athlete’s paradox”.26 This metabolic paradox is likely explained by the fact that it is not the size of the IMTG pool, but rather the balance between fatty-acid availability, uptake, and oxidation, which determines IR. That is, by improving the balance between skeletal muscle FA uptake and oxidation, exercise may prevent the development of skeletal-muscle IR.27
Similar to the effects in skeletal muscle, exercise improves insulin sensitivity in the WAT. While the mechanism(s) behind exercise-mediated improvements in WAT insulin sensitivity are not fully understood, some work has shown improvements in insulin signaling, similar to what happens in skeletal muscle with exercise training. Effects of exercise (i.e., two 3-hour bouts of moderate exercise) on insulin signaling in WAT (i.e., increased insulin-induced tyrosine phosphorylation of the insulin receptor and IRS-1; serine phosphorylation of Akt) have also been documented in rodents.28
Another possibility is that exercise improves IR in WAT by decreasing endoplasmic reticulum (ER) stress (i.e., increased expression of mutant proteins disrupt protein folding in the ER, activating a signaling network called the unfolded protein response which may ultimately trigger cell death). ER stress is triggered by obesity and/or cellular metabolic dysfunction and is associated with inflammation via activation of the JNK signaling pathway.22 In one study, exercise training reduced phosphorylation of eukaryotic translation initiation factor 2-alpha kinase3 (PERK) and eukaryotic initiation factor 2 (eIF2), two markers of ER stress, in WAT and hepatic tissue of rats fed an high fat diet (HFD), which was associated with an improvement in insulin signal transduction and an inhibition of JNK activity.22 Similarly, in another rat study, eight weeks of swim training resulted in JNK suppression and improvements in insulin signal transduction in WAT.22 In summary, it is well understood that adipocyte physiology is intimately affected by exercise and exercise training; the effects of exercise go far beyond the regulation of lipolysis.
WAT as a Target Tissue for the Anti-inflammatory Effects of Exercise
A number of human trials and animal studies have demonstrated an inverse relationship between systemic inflammation (e.g, circulating CRP) and exercise training.29,30 The mechanism driving this anti-inflammatory effect of exercise is not known, but may involve direct anti-inflammatory effects in the WAT. Certainly, by way of reducing total visceral adipose tissue, exercise serves to counter obesity-related WAT inflammation, since the visceral adipose tissue depot is most metabolic and inflammatory.31 However, it appears that the antiinflammatory effects of exercise go beyond simply reducing visceral adiposity.
Weight loss-independent, exercise-mediated reductions in WAT inflammation have been documented in humans32 and rodents.7,33 One study investigated the effects of a 15-week exercise intervention (i.e., 2–3 hours of moderate intensity walking, swimming, or aerobics, five days per week) on weight loss, insulin sensitivity, and inflammation (systemic, as well as gene expression in WAT and skeletal muscle biopsy samples) in severely obese subjects.32 Those subjects experienced improvements in insulin sensitivity (as indicated by HOMA) and reduced systemic inflammation (e.g., decreased CRP, MCP-1; increased adiponectin).32 Importantly, that study also found that WAT inflammation decreased with exercise, an effect that was not found in the skeletal muscle. In a similar study with animals, a reduction in WAT gene expression of CD68 and CD14 (Mφ markers) and TNF-α were observed in morbidly obese rats exposed to acute swimming exercise for 12 weeks.28 Similarly, in obese mice, moderate exercise training (6 or 12 weeks of treadmill running) lowered systemic and as well as WAT inflammation, anti-inflammatory effects that predicted improvements in IR and hepatic steatosis.7 Another group showed that 6 weeks of voluntary wheel running exercise decreased WAT inflammation in obese mice, even in the presence of HFD.33 Likewise, in genetically-obese rats, endurance exercise training in combination with the drug, metformin positively altered WAT secretion and plasma concentrations of leptin and IL-10, shifting the WAT toward an anti-inflammatory phenotype.34 In another study using HFD-fed mice by Kawanishi et al., exercise training resulted in a marked reduction in WAT inflammation despite not weight-reducing effect of exercise.35 Those authors went on to show that exercise was associated, not only with a reduction in total WAT Mφ content, but an M1–M2 Mφ phenotype switch.
Acute exercise has also been reported to have antiinflammatory effects in WAT: a mild-moderate 3-h exercise bout reduced inflammation in the epididymal WAT (a major visceral fat depot) of rats as indicated by decreased mRNA levels of TNF-α, IL-1β, and MCP-1. Similar to the study by Kawanishi et al., there was also a switch in Mφ phenotype from pro-inflammatory “M1” to antiinflammatory “M2” phenotype in that study, suggesting that the mechanism may involve the acute hormonal/physiological/immunological changes associated with exercise.28
Cytokine-Mediated Effect of Exercise: A Possible Anti-inflammatory Mechanism
One hypothesis by which exercise improves WAT inflammation is via the secretion of myokines, which are cytokines released from exercising muscle cells. Similar to adipokines, myokines have been shown to exhibit their effects both locally and peripherally. IL-6 is one such recently identified myokine that has shown potential in possessing a protective metabolic role. IL-6 may exert beneficial metabolic effects via enhancement of lipolysis and mitigation of the inflammatory response in WAT (See Figure 3). Interestingly, IL-6 is commonly secreted by enlarged adipocytes and has been associated with reduced insulin action in WAT and systemically. 36 However, the identification of IL-6 as a myokine has brought a renewed interest in this cytokine.
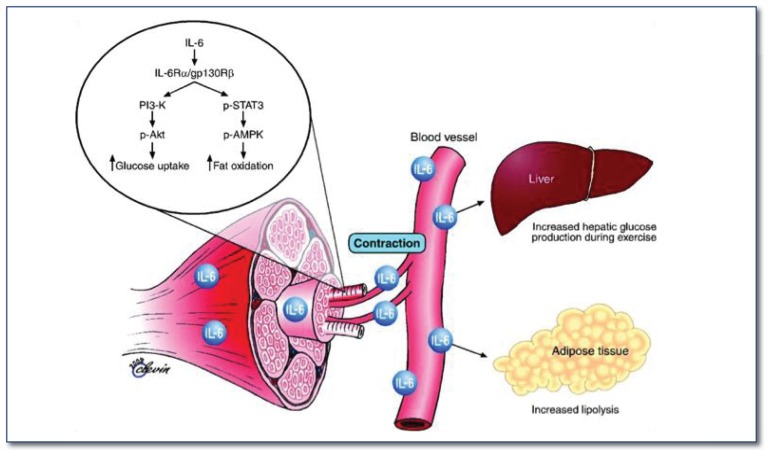
Figure 3.
Biological role of skeletal muscle-contraction induced IL-6. Figure from Pedersen et al. 33 In response to muscle contraction, the myokine IL-6 exerts its effects both locally within the muscle through activation of AMPK, and peripherally in several organs in a hormone-like manner. IL-6 acts in an autocrine or paracrine manner to signal through a gp130Rβ/IL-6Rα homodimer, contributing to activation of AMPK and PI3 kinase to enhance glucose uptake and fat oxidation.
Pedersen et al. 36 were the first to demonstrate that IL-6 is markedly produced by exercising muscle cells and released into the circulation during and after exercise. Plasma IL-6 levels increase exponentially with exercise and is related to exercise intensity, duration, mass muscle recruited, and endurance capacity of an individual. Produced by both type I and II muscle fibers, IL-6 acts to regulate glucose and lipid metabolism. In skeletal muscle, IL-6 activates AMPK and PI3 kinase to enhance glucose uptake and fat oxidation, and also exerts effects in a hormone-like fashion affecting other tissues such as liver (e.g., hepatic glucose production) and WAT (e.g., enhanced lipolysis). Additionally, IL-6 lessens circulating TNF-α levels, implying an anti-inflammatory role. Starkie et al.37 used a model of low-grade inflammation established with a low dose of E.coli endotoxin administered to healthy individuals. The TNF-α response induced by endotoxin was totally blunted by a 3h bout of cycling exercise or an infusion with recombinant human IL-6. Consistent with these multiple beneficial effects, mice with IL-6 deficiency develop obesity and IR, effects that are reversed with IL-6 administration.38 Thus, provocation of an increase in muscle IL-6 secretion may help explain some of the metabolic and anti-inflammatory effects of exercise.
Similar to anti-inflammatory effects of myokine during exercise, adipocyte-released adiponectin levels, which are inversely proportional to abdominal fat mass and IR, significantly increase with both acute and short-term aerobic exercise training.39 Adiponectin inhibits nuclear factor κB (NFκB), an essential transcription factor for the expression of inflammatory and stress related proteins.40 What’s counter-intuitive is that adiponectin has been shown to stimulate rapid TNF-α secretion in human and mouse Mφ. This, in turn, stimulates IL-10, an anti-inflammatory cytokine, thus creating tolerance to further LPS stimulation.41 In summary, regular exercise training may protect against chronic low-grade systemic inflammation as well as adipocyte inflammation via the increased secretion of immuno-modulatory factors such as adiponectin from adipocytes, and IL-6 produced by skeletal muscle cells.
Exercise Makes Fat ‘Fit’: Role of PGC1-α
Transcriptional co-activator PPAR-γ co-activator-1 α (PGC1-α) can mediate many biological programs associated with energy metabolism, and has been shown to modulate oxidative metabolism via mitochondrial biogenesis. PGC-1α is highly expressed in brown fat tissue (BAT) and up-regulated with cold exposure. Unlike WAT, which is used mostly for energy storage, BAT increases energy expenditure via heat generation due to inefficient fatty acid oxidation. This function of BAT is attributed to its greater mitochondrial content combined with its capacity to uncouple respiration and ATP production, via up-regulated uncoupling protein (UCP)-1.42 This thermogenic capacity of BAT allows mammals to live below thermo-neutral conditions without shivering. Moreover, animals with abundant natural or induced BAT are resistant to obesity.43
In one study, mice with up-regulated PGC-1α were not only resistant to obesity and diabetes, but also had a prolonged life-span.44 Human white adipocytes transfected with PGC-1α present increased levels of UCP-1, suggesting PGC-1α can remodel WAT into BAT; this is called “browning” of WAT.45 Exercise up-regulates skeletal muscle PGC1-α, which is linked to mitochondrial biogenesis, angiogenesis, and fiber-type switching. In individuals with T2D, skeletal muscle PGC-1α mRNA levels are reduced 46 whereas the attenuated levels are restored by various types of exercise ranging from acute to chronic endurance.47 Sutherland et al.48 demonstrated that two hours of daily swimming exercise training for a month led to increases in markers of WAT mitochondrial biogenesis such as cytochrome-C oxidase (COXIV) and Core1 expression and citrate synthase activity, which are all driven by increased PGC-1α and mitochondrial transcription factor A (Tfam). Recently, a newly identified “exercise hormone”, irisin, has received much attention due to its potential browning effect on WAT. The Fndc5 gene stimulated by PGC-1α in exercising muscle forms irisin, which is secreted into the circulation, and stimulates a biological program making WAT more like BAT via up-regulated PGC-1α and UCP-1 expression. Spiegelman and Bostrom44 demonstrated that exercise (i.e., voluntary wheel running and swimming) in mice drives the subcutaneous and visceral fat pads into a thermogenic gene program (e.g., enhanced mitochondrial content and increased levels of UCP-1 gene expression) via triggering an increase in circulating irisin.
Exercise-related improvements in WAT mitochondrial function (i.e. enhanced PGC-1α) have been associated with reductions in visceral adiposity through greater lipolysis. What’s more, enhanced WAT browning is associated with reduced inflammation, offering a hypothetical mechanism by which exercise is anti-inflammatory. A possible mechanism between browning and suppressed inflammation may be that PGC-1α directly suppresses reactive oxygen species (ROS) in WAT.49 ROS are often associated with obesity-associated diseases; they increase oxidative stress and trigger inflammatory cytokine production (Figure 1).49 Anti-inflammatory effects of PGC-1α have been demonstrated in vitro using skeletal muscle cell lines. That is, enhanced expression of PGC-1α in C2C12 myotubes suppressed inflammatory cytokine production.50 It is possible that, via up-regulation of PGC-1 in WAT, exercise may reduce WAT inflammation thus constituting another possible anti-inflammatory mechanism.
Summary/Conclusions
Obesity and physical inactivity are associated with chronic low-grade inflammation, which provides the common soil from which a myriad of metabolic diseases develop. The main source of this inflammation is WAT, an active endocrine organ largely responsive to changes in physical activity and metabolism. An emerging body of evidence suggests that physical inactivity may directly and adversely affect the WAT, therefore leading to local and systemic inflammation. Exercise mitigates both systemic and WAT inflammation, an effect likely independent of exercise’s obesity-reducing effects. That is, exercise training may reduce inflammation both by reducing total body fat, and by fat loss independent mechanisms. While the mechanism(s) by which exercise reduces WAT inflammation are not completely defined, there are convincing data to support several specific molecular pathways. Exercise may trigger immune-mediated cross-talk between adipocytes and skeletal muscle cells via exercise-mediated myokine secretion, enhance the metabolic capacity of adipocytes via increasing mitochondrial function/capacity, or directly affect immune cells (e.g., promote M2 Mφ activation, decrease inflammatory cytokine production) within WAT.
Biography
Young-Min Park, Margo Myers, and Victoria J. Vieira-Potter, PhD, are in the Department of Nutrition and Exercise Physiology, College of Human Environmental Sciences, at Missouri University.
Contact: vieirapotterv@missouri.edu

Footnotes
Disclosure
None reported.
References
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Hojbjerre L, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 34:2265–2272. doi: 10.2337/dc11-0631. doi:dc11-0631 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA: the journal of the American Medical Association. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 4.Bergman D. The endocrinology of exercise. Internal and emergency medicine. 2013;8(Suppl 1):17–21. doi: 10.1007/s11739-013-0921-2. [DOI] [PubMed] [Google Scholar]
- 5.Halle M, Korsten-Reck U, Wolfarth B, Berg A. Low-grade systemic inflammation in overweight children: impact of physical fitness. Exercise immunology review. 2004;10:66–74. [PubMed] [Google Scholar]
- 6.Logue J, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54:3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieira VJ, et al. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. American journal of physiology. Endocrinology and metabolism. 2009;296:E1164–1171. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 46:339–345. doi: 10.1016/j.cyto.200903.0062009. doi:S1043-4666(09)00094-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Cinti S. The adipose organ at a glance. Disease models & mechanisms. 2012;5:588–594. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. The American journal of clinical nutrition. 2000;72:558S–563S. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- 12.Stich V, et al. Adipose tissue lipolysis is increased during a repeated bout of aerobic exercise. Journal of applied physiology. 2000;88:1277–1283. doi: 10.1152/jappl.2000.88.4.1277. [DOI] [PubMed] [Google Scholar]
- 13.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiological reviews. 2012;92:157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 14.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–4. doi: 10.1152/ajpgi.00554.2006. doi:00554.2006[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djouder N, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. The EMBO journal. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kratky D, Obrowsky S, Kolb D, Radovic B. Pleiotropic regulation of mitochondrial function by adipose triglyceride lipase-mediated lipolysis. Biochimie. 2013 doi: 10.1016/j.biochi.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lass A, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell metabolism. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascular pharmacology. 2012;57:91–97. doi: 10.1016/j.vph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Dey D, Bhattacharya A, Roy S, Bhattacharya S. Fatty acid represses insulin receptor gene expression by impairing HMGA1 through protein kinase Cepsilon. Biochemical and biophysical research communications. 2007;357:474–479. doi: 10.1016/j.bbrc.2007.03.183. [DOI] [PubMed] [Google Scholar]
- 20.Gruzdeva O, et al. Insulin resistance and inflammation markers in myocardial infarction. Journal of inflammation research. 2013;6:83–90. doi: 10.2147/JIR.S43081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu X, et al. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes. 2013;62:561–571. doi: 10.2337/db12-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Luz G, et al. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. European journal of applied physiology. 2011;111:2015–2023. doi: 10.1007/s00421-010-1802-2. [DOI] [PubMed] [Google Scholar]
- 23.Ropelle ER, et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. The Journal of physiology. 2006;577:997–1007. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavros Y, et al. Changes in Insulin Resistance and HbA1c Are Related to Exercise-Mediated Changes in Body Composition in Older Adults With Type 2 Diabetes: Interim outcomes from the GREAT2DO trial. Diabetes care. 2013;36:2372–2379. doi: 10.2337/dc12-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. The Journal of clinical investigation. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimber NE, Cameron-Smith D, McGee SL, Hargreaves M. Skeletal muscle fat metabolism after exercise in humans: influence of fat availability. Journal of applied physiology. 2013 doi: 10.1152/japplphysiol.00824.2012. [DOI] [PubMed] [Google Scholar]
- 27.Timmermans RJ, Saris WH, van Loon LJ. [Insulin resistance: the role of intramuscular triglyceride and the importance of physical activity]. Nederlands tijdschrift voor geneeskunde. 2006;150:122–127. [PubMed] [Google Scholar]
- 28.Oliveira AG, et al. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity. 2013 doi: 10.1002/oby.20402. [DOI] [PubMed] [Google Scholar]
- 29.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunology and allergy clinics of North America. 2009;29:381–393. doi: 10.1016/j.iac.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 30.You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity : current evidence and potential mechanisms. Sports Med. 2013;43:243–256. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 31.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 32.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. American journal of physiology. Endocrinology and metabolism. 2006;290:E961–967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 33.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. American journal of physiology Endocrinology and metabolism. 2008;295:E586–594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins NT, et al. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. Journal of applied physiology. 2012;113:1873–1883. doi: 10.1152/japplphysiol.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise immunology review. 2010;16:105–118. [PubMed] [Google Scholar]
- 36.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 37.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 38.Wallenius V, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nature medicine. 2002;8 doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 39.Saunders TJ, et al. Acute exercise increases adiponectin levels in abdominally obese men. Journal of nutrition and metabolism. 20122012:148729. doi: 10.1155/2012/148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94:2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Tsatsanis C, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochemical and biophysical research communications. 2005;335:1254–1263. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 42.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 43.Vitali A, et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of lipid research. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bostrom P, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiraby C, et al. Acquirement of brown fat cell features by human white adipocytes. The Journal of biological chemistry. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- 46.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summermatter S, Handschin C. PGC-1alpha and exercise in the control of body weight. Int J Obes (Lond) 2012;36:1428–1435. doi: 10.1038/ijo.2012.12. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. The Journal of physiology. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Handschin C, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. The Journal of clinical investigation. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]