Abstract
Tobacco smoking remains the highest cause of preventable deaths worldwide. Electronic cigarettes have recently become popular as nicotine alternatives. With public use on the rise and recent tobacco industry interest, field experts and regulatory agencies voiced concerns about their safety and unregulated production. Electronic cigarettes are safer than conventional cigarettes and at least as safe as other approved nicotine replacement therapies. Further evidence is needed as their popularity increases amidst controversy over safety and efficacy.
Introduction
The percentage of U.S. adults who smoke cigarettes has been steadily decreasing over the past several decades, down to 18.1% in 2012.1 Globally however, prevalence remains much higher, with 50% of young men and 10% of young women currently smoking cigarettes. There were five million deaths attributed to cigarette smoking in 2010, making it the leading cause of preventable deaths. Based on current use patterns this number will double within the next several decades.2 Approximately 69% of U.S. smokers report a desire to quit and 52% attempt to quit yearly.1 Unfortunately, success rates for different smoking cessation strategies (nicotine replacement therapy or NRT, pharmacologic agents and combinations of these) remain low, with six-month abstinence rates of 19%–36.5%.3 Despite not being approved for this indication, electronic cigarettes (EC) have caused a nationwide debate regarding their potential use for smoking cessation. Studies of EC have failed to show a clear benefit regarding smoking cessation when compared to other accepted strategies. However, they have shown a trend towards decreasing number of cigarettes smoked. In this way ECs present a harm-reduction strategy. Safety, efficacy, behavioral and regulatory concerns have been justifiably raised by field experts and regulatory agencies such as the Food and Drug Administration (FDA). We will review these particular issues, in order to provide guidance for clinicians charged with counseling patients on the use of ECs.
The Electronic Cigarette and Market Trends
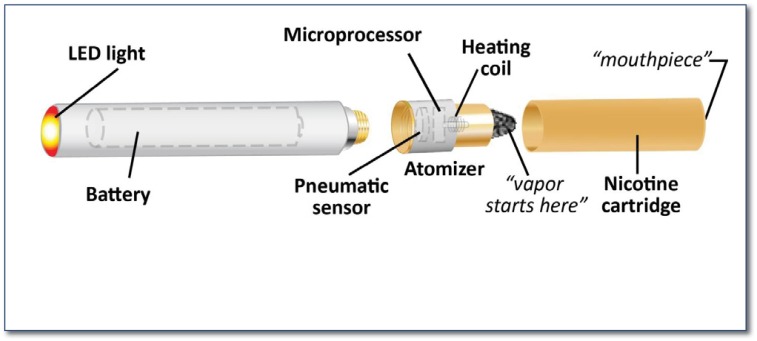
Electronic cigarettes (E-cigarettes; personal vaporizer; electronic nicotine delivery system or ENDS) were first introduced to the public in 2004 by the Chinese company Ruyan (Golden Dragon Holdings). The E-cigarette consists of a rechargeable battery with an optional light emitting diode (LED) simulating a burning cigarette tip. This battery is attached to a vaporizer (or atomizer) composed of a microprocessor, a pneumatic sensor and a heating device; and lastly a replaceable cartridge containing a solvent mixture of water, propylene glycol, glycerol with additive flavoring and nicotine (See Figure 1). When the user inhales, the pneumatic sensor activates the microprocessor, which in turn activates the heater to raise temperature in the chamber, creating an aerosol resembling cigarette smoke. Cartridges can contain varying nicotine concentrations from zero to 19.5mg. One cartridge may provide 300 puffs, an estimated equivalent to one pack of cigarettes.4 Therefore, 15 puffs of EC have been equated to one conventional cigarette. Users can choose to purchase a refill solution bottle, known as “juice.” Colloquially, the use of E-cigarettes (EC) is termed “vaping”; and the smoker of the electronic cigarette is referred to as a “vaper.”
Figure 1.
Anatomy of the electronic cigarette. The vaper (or smoker) inhales at the mouthpiece, triggering the pneumatic sensor to activate the microprocessor, which in turn triggers the heating coil to heat the liquid and release a vapor.
ECs are sold in some mall kiosks, but mainly through internet distributors using online affiliate-based marketing schemes. These consist of commercial networks that run content websites redirecting viewers to vendors. Forensic analysis of this marketing strategy for ECs suggested that affiliate websites, but not linked sellers, made smoking cessation claims.5 This approach allows sellers to distance themselves from unsubstantiated claims, such as smoking cessation efficacy and reduced harm in comparison with conventional cigarettes.
The EC market has grown exponentially, with 2.7% of Americans having tried them at some point. Total sales in 2008 approached $20 million U.S. dollars (USD). Sales soared in 2013 to over $1–2 billion USD.6 Initially handled by small companies, projected profits have attracted large tobacco companies who are now entering the competition. Lorillard (maker of Kent and Newport cigarettes) was the first tobacco company to engage in the EC market by purchasing blueCigs® in April 2012. In 2014, Reynolds (maker of Camel and Pall Mall) and Altria (maker of Marlboro) will each launch their own EC to the market,7 Vuse and MarkTen, respectively. Each EC could cost a minimum of $10 USD for basic supplies, with additional cost for batteries and cartridges.
Public Perceptions and Use
Public interest in smoking cessation methods, particularly EC, has rapidly grown. By 2011 Google searches for EC had increased by 5000% in two years.8 Most common reasons for EC use in adults include: perceived lower toxicity compared with cigarettes (84%), control of tobacco craving (79%), and smoking cessation or relapse avoidance (77%).9
Amongst adolescents and young adults the main source of information on EC is the Internet.10 Concerns of EC as a gateway drug grew, particularly due to recent increase in their availability and use within this age group. A recent survey of ninth through twelfth grade students showed a prevalence of only 0.9% in 2010; however this doubled within a 16-month period.11 By 2009, 4.9% of college students had tried EC at some point. Twelve percent of these students had never smoked conventional cigarettes. This study also suggests that EC use in this population was not for desire to quit smoking cigarettes, but possibly as a novel form of recreational activity.12
Experienced and long-term use of EC has transcended into a cultural phenomenon, including emergence of associations such as the National Vapers Club and American Vapers Club, which hold frequent national meetings. A survey of 104 EC aficionados at one such convention revealed that 73% started using them with the intention to quit smoking and 99% felt that EC helped them succeed.13 Interestingly, most subjects surveyed in this study were not using the most widely known EC brands, instead they were using customized variations intended to allow the atomizer to achieve a higher and more consistent temperature, probably resulting in hotter and more intense vapor.
Public views regarding EC have polarized the conversation. For harm reduction organizations such as the Consumer Advocates for Smoke-Free Alternatives Association (CASAA) and for smokers themselves, ECs present a healthier alternative to tobacco smoke14 and a promising smoking cessation tool. However, much concern has been raised by the FDA regarding the presence of toxic substances, such as tobacco-specific nitrosamines (TSNA) and diethylene glycol (DG).15 There is difficulty in defining EC due to a conceptual uncertainty of this product as either a medicinal nicotine drug-delivery device or a form of smokeless tobacco. The FDA initially tried to obtain regulatory control over them as a drug-delivery device. However, the U.S. Courts of Appeals overturned the decision in 2010.16 On April 24 2014, the FDA proposed a rule to regulate electronic cigarettes (along with cigars, pipe tobacco, nicotine gels and waterpipes) as a form of tobacco product, and impose similar requirements to those applied to conventional cigarettes. These requirements include: a) registration with the FDA and ability to market the product only after their review; b) reporting of ingredients; c) not making claims of reduced health risk until confirmation by the FDA; d) not distributing free samples; e) enforcing a minimum age for purchase; and f) including health warnings.17
In the U.S., the degree of state regulation is variable. As of April 2014, only three states (New Jersey, North Dakota, and Utah) have banned the use of EC in public places that currently ban cigarettes. Large cities such as Chicago, Los Angeles, and New York implemented similar bans. On a more local level, three counties in Missouri (Creve Coeur, Jefferson and Washington) have prohibited their use in public places where conventional cigarettes are currently banned.18
Internationally, the World Health Organization already recognized EC as a form of smokeless tobacco.14 French officials believe that further investigation is needed into long-term implications of EC; thus they banned EC use in public places, and all media advertising to their sale to minors.19 After analysis by the Medicines and Healthcare Products Regulatory Agency in June 2013, EC and other ENDS will be regulated as medications in the UK starting 2016.20 Other countries such as Mexico, Brazil, Uruguay, Singapore, and Turkey have gone further and banned them completely until further information is available.
Safety
Safety concerns have been raised regarding the substances in EC and effects of smoke exposure on health. The temperature required to create a mist varies depending on the type of EC and the carrier substance. Glycerol requires higher temperatures than PG. In some instances temperatures higher than 100 degrees Celsius needed to heat glycerol may lead to production of toxic substances such as acrolein,21 which is a strong irritant of skin, eyes and nasal passages.
Chronic exposure to theatrical fogs (PG or mineral oil) released by EC has been associated with signs of airway obstruction and systemic inflammation. Theatrical fogs may result in small reductions in the lung volumes forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). Increased prevalence of acute and chronic respiratory symptoms, including asthma-like symptoms, work-related wheezing and chest tightness, has also been noted.22 In a recent study, use of EC for five minutes was not associated with changes in spirometry data, but was associated with decreased exhaled nitric oxide (FeNO) and increased pulmonary impedance. These changes may be markers of early obstructive lung disease.23 However, PG is currently used as a carrier for multiple medications (including nebulizers) without known adversities. Therefore the clinical significance of described adverse findings is unclear.
Cigarette smoking is linked to elevations in inflammatory markers and low-grade systemic inflammation;24 a proposed mechanism for atherogenesis. When compared to conventional cigarettes, acute EC exposure (active or passive) was not associated with increases in total white blood cell, lymphocyte or granulocyte counts.25 This suggests lack of correlation between acute EC use and systemic inflammation. The chronic effects however, remain unknown.
Another area of concern is the potential toxicity of EC refill fluid ingestion. According to the U.S. Environmental Protection Agency 2008 report, nicotine doses of 40–60 mg may be lethal to humans.26 Opponents of EC use have touted toxicity based on EC refill bottles containing nicotine in excess of 60 mg. The lethal dose quoted by the EPA has been questioned. A recent report based on post-mortem analysis of fatal cases suggested a much higher lethal dose of 0.5 – 1 g.27 The risk of childhood accidental ingestion is one of the reasons for above discussion which is prompting stricter regulation of EC. But without a clearly defined and reliable lethal dose of nicotine, claims of EC nicotine toxicity may be weak.
The effects of EC use during pregnancy are not known. A recent cytotoxicity analysis showed that embryonic and neonatal stem cells are more sensitive than lung fibroblasts to EC refill fluid. The flavor additives rather than the nicotine, glycerin or PG28 are culprits of stem cell toxicity.
In 2009, the FDA tested and reported toxic substances in e-cigarettes. They reported presence of TSNAs in five of ten EC cartridges tested, and detectable DG in 1 of 18 cartridges tested.15 DG is a potentially lethal substance with gastrointestinal, cardiac and neurologic toxicity. Based on these results the FDA warned consumers about EC use. Further studies showed that vapor generated from EC indeed contains toxic substances, particularly carbonyl compounds (formaldehyde, acetaldehyde, acrolein, 0-methylbenzaldehyde), volatile organic compounds (toluene and m,p-xylene), TSNAs (N′-nitrosonornicotine, nicotine-derived nitrosamino ketone) and heavy metals (cadmium, nickel, lead). However, levels of these compounds in EC were 9–450 times lower than in cigarette smoke (See Table 1) and, in many cases, comparable to a 10 mg nicotine Nicorette inhaler, an already approved form of NRT.29
Table 1.
Comparison of toxin levels between conventional and electronic cigarettes
| Toxins | Conventional cigarette (mcg in smoke) | Electronic cigarette (mcg per 15 puffs) | Average ratio (conventional vs. electronic cigarette) |
|---|---|---|---|
| Formaldehyde | 1.6–52 | 0.20–5.61 | 9 |
| Acetaldehyde | 52–140 | 0.11–1.36 | 450 |
| Acrolein | 2.4–62 | 0.07–4.19 | 15 |
| Toluene | 8.3–70 | 0.02–0.63 | 120 |
| NNN | 0.005–0.19 | 0.00008–0.00043 | 380 |
| NNK | 0.012–0.11 | 0.00011–0.00283 | 40 |
Source: Goniewicz ML, et al. Tobacco Control 2014; 23(2): 133–139.
NNN and NNK are the tobacco-specific nitrosamines, nicotine-derived nitrosamino ketone and N′-nitrosonornicotine, respectively. Comparison of toxin levels between conventional and electronic cigarettes was made using a standardized approach to puff time and puff inhalation.
Subjective undesirable effects of EC use have also been variable between studies. Based on multiple surveys and observational studies to date, the main undesirable effects of EC use are mouth and throat irritation, nausea, vertigo/dizziness, dry cough and headache.30 On one survey, the frequency of these adverse events was comparable to a Nicorette inhaler (See Table 2).31 A 12-month randomized controlled trial (RCT) on smokers not intending to quit showed no significant adverse events in EC users. In fact, the study showed a decrease in frequency of cough, dry mouth, shortness of breath and headache from baseline 32. Overall there may be minor adverse effects related to EC use but these are, at least in short-term analyses, much less significant than those related to cigarette smoke, and need further structured investigations.
Table 2.
Percent of reported side effects after 9 hours of EC vs. Nicorette inhaler use
| EC 16mg | Nicorette inhaler | |
|---|---|---|
| Mouth/throat irritation | 38 | 88 |
| Aching jaws | 8 | 5 |
| Nausea | 29 | 18 |
| GI discomfort | 5 | 23 |
| Vertigo/feeling high | 21 | 18 |
| Headache | 18 | 18 |
| Sweatiness/clammy skin | 4 | 3 |
| Palpitations | 5 | 0 |
Bullen C, et al. Tobacco Control 2010; 19(2): 98–103. Table was altered from original to illustrate differences in side effects between EC and Nicorette inhaler. Data above reflects percent of participants that reported the specific side effect. When comparing electronic cigarette (EC) to Nicorette inhaler, significant difference was seen only for mouth/throat irritation, p<0.001.
Efficacy
Electronic cigarettes have many potential benefits; therefore careful distinction should be made when evaluating their efficacy as a smoking cessation tool, a nicotine delivery device, or a harm-reduction strategy. The latter refers to the development of principles and strategies designed to minimize the adverse consequences of behaviors that may pose serious health risks,33 rather than a “complete abstinence” approach.
The main objective of EC is to simulate use of conventional cigarettes through creation of a mist containing varying concentrations of nicotine. A recent analysis showed variability amongst different brands of EC on total levels of nicotine present in vapor (0.5–15.4 mg) for a single cartridge, most of which was delivered within 150–180 puffs (equivalent to half a pack of cigarettes). When compared to smoking one conventional cigarette, 15 puffs of an EC (the equivalent to one conventional cigarette) deliver considerably less nicotine (0.025–0.77mg vs. 1.54–2.60mg).4
Survey of current EC users found that 63% had recently quit smoking and 95% of these ex-smokers attributed cessation to their use of EC. Most commonly reported beneficial effects of EC use were: improved breathing, decreased cough and sore throats, and help with smoking cessation.34 On a different survey of first-ever EC use amongst subjects with three previous failed smoking cessation attempts, two-thirds had reduced the number of cigarettes smoked, almost half had at least a period of abstinence and almost one-third were not smoking at six months. Abstinence rates were higher with more EC use.35 Amongst more experienced users (more than one year) with multiple prior failed attempts, 99% felt that EC helped them quit cigarette smoking.13 Use of EC has been associated with decreased desire to smoke cigarettes when compared to placebo.31 This effect was also seen despite using EC with no nicotine content suggesting the importance of behavioral cues in cigarette addiction.36
A prospective six-month study in subjects not trying to quit showed that 32.5% of them had a 50% reduction in cigarette smoking by 24 weeks. Sustained abstinence at 24 weeks occurred in 22.5% of subjects.37 More recently, a 12-month RCT of 300 subjects comparing EC with nicotine and placebo showed that 23% of participants had decreased smoking by half at 12 weeks. Only 14.5% of participants maintained this reduction at 52 weeks. Quit rate at 52 weeks was 11%.32 Even though there was no significant difference when compared to placebo, these results are comparable to success rates for some forms of NRTs. A post-hoc analysis of an RCT by Bullen et. al, using a 5% non-inferiority limit for the risk difference, reported that EC were at least as effective as nicotine patches.38
Conclusion
The electronic cigarette market has expanded rapidly and will continue to do so as large tobacco companies enter the competition. The general population will be increasingly exposed to publicity and many will become potential users despite the lack of well-established safety analyses and regulatory measures. Perceptions and impact of EC amongst adolescents differ from that of adults, potentially subjecting them to targeted marketing campaigns.
In light of the high prevalence of EC use, and expected increase in the near future, their safety is clearly the biggest concern. Initial reports about the presence of toxicants in EC cartridges were validated in further studies. But levels of toxins were similar to already approved NRTs and much less than those found in tobacco smoke. As multiple brands enter the market, safety and uniformity of ECs would be difficult to guarantee. However, stringent bans, as implemented in other countries, may not be an optimal alternative as they could negatively impact subjects already deriving benefit from their use.
Currently, ECs appear to be as safe as other forms of NRTs and considerably less toxic than cigarette smoking in the short-term. Their long-term safety, however, remains unknown. Despite safety concerns, they have tangible potential as an alternative to cigarette smoking and perhaps a smoking cessation tool. Current evidence suggests ECs are at least effective in reducing quantity of cigarette consumption, as experienced by many current users. They may also be as effective as nicotine patches for smoking cessation. More studies are urgently needed comparing EC to NRTs and other smoking cessation tools, and determining the long-term effects of EC use. They have not been compared to other forms of smoking-cessation products such as bupropion or varenicline.
E-Cigarettes Not All They’re Puffed Up To Be.
by Jim Blaine, MD
Tobacco Prevention Expert
Chair, MSMA Public Affairs Commission
As a primary care physician, I have ambivalent thoughts about E-Cigarettes. The vendors for E-Cigarettes claim that they are selling a safer alternative to cigarettes, and a valuable ally to help our patients quit smoking. We are told that Electronic Cigarettes emit only harmless water vapor, and are safe to use anywhere without concern for the health of others. They seem, on the surface, to be worthy of consideration.
However; E-cigarettes manufacturers (tobacco companies) have refused to seek FDA approval as a tobacco cessation device. E-cigarettes are clearly being marketed to children with flavors that include Cherry, and Creamy Milk Shake, and, since they contain Nicotine, are potentially as addictive as any other tobacco product.
Additionally, there are no objective studies that prove the safety of either active or passive vapors, and no studies that prove the efficacy of tobacco cessation with E-Cigarettes. In an effort to protect children from becoming addicted to the nicotine found in E-Cigarettes, Senator Jay Wasson introduced SB841 to make it illegal to sell them to children under the age of 18. However, tobacco industry inspired language was added in committee that would prohibit the regulation or taxation of E-Cigarettes as tobacco products. This simple phrase puts E-Cigarettes in a class by itself, and would allow them to be used in any place where smoking is currently prohibited.
The MSMA, the Greene County Medical Society, the Campaign for Tobacco Free Kids, the Missouri Academy of Family Physicians, the American Heart Association, the American Lung Association, the American Cancer Society and the Tobacco Free Missouri Coalition support the veto of SB841. This product cries out for additional study, and not the free reign provided by this bill; please ask your Senators and Representatives to support its veto, and to encourage FDA oversight of E-Cigarettes.
Another “Marlboro Man” Dies From Smoking.
Eric Lawson, 72, died January 10, 2014, in San Luis Obispo, California, of chronic obstructive lung disease. Lawson began smoking at age 14. He is at least the fifth “Marlboro Man” to die prematurely of smoking related health problems. The Marlboro Man advertising campaign lasted for 45 years and officially burned out in 1999.
Photo Source: www.pikabu.ru
Article Source: People.com http://www.people.com/people/article/0,,20780930,00.html
As ECs address not only nicotine delivery, but also the behavioral cues related to this addiction, they should be considered a potential tool in harm-reduction and smoking cessation. As of April 2014, the FDA is attempting to regulate EC similar to conventional cigarettes. This oversight is intended to assure uniform and safe production of EC and to guarantee limited exposure to targeting children and adolescents, while continuing important research on its safety and efficacy as a smoking cessation tool and its impact on public health.
While regulatory entities continue to debate on this topic, physicians will increasingly be confronted with the need to answer questions and counsel patients regarding EC use. Based on current information, the use of EC in patients interested in quitting smoking should probably not be encouraged as a first line aid. However, in patients who have decreased or stopped smoking while using them, it would seem reasonable to continue their use along with other approved measures for short durations. For patients not interested in quitting smoking who inquire about EC, they should be counseled regarding possible lower harmful effects when compared to conventional cigarettes. They should also be reminded that the long-term safety of EC remains unknown. The main focus of interaction with patients who smoke cigarettes should continue to be smoking cessation with already approved strategies. Since we only know that EC is safer than conventional cigarettes, possibly comparable to other NRTs, but more harmful than not smoking at all, health care providers should definitely discourage non-smokers from initiating EC as a new recreational tool. Unfortunately, adolescents and young adults are at greatest risk to fall into this last group.
Biography
A. Rolando Peralta, MD, (left) is a Pulmonary and Critical Care Fellow and Vamsi P. Guntur, MD, MSc, is an Associate Professor of Clinical Medicine, Division of Pulmonary and Critical Care Medicine, School of Medicine, University of Missouri, Columbia, Missouri.
Contact: gunturv@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Smoking and Tobacco Use. 2013. [Accessed 11/23/2013]. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/
- 2.Jha P, Peto R. Global Effects of Smoking, of Quitting, and of Taxing Tobacco. New England Journal of Medicine. 2014;370(1):60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 3.Fiore MC. Treating Tobacco Use and Dependence: 2008 Update. 2008. [Accessed 04/26/2014]. http://www.ncbi.nlm.nih.gov/books/NBK63952/toc/
- 4.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2013 Jan;15(1):158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 5.Cobb NK, Brookover J, Cobb CO. Forensic analysis of online marketing for electronic nicotine delivery systems. Tobacco control. 2013 Sep 13; doi: 10.1136/tobaccocontrol-2013-051185. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.UBS WF. Tobacco Vapor Electronic Cigarette Association: Electronic cigarette statistics. 2013. [Accessed 11/23/13.]. http://www.statisticbrain.com/electronic-cigarette-statistics/
- 7.Esterl M. Altria To Launch MarkTen E-Cigarette Nationally. The Wall Street Journal. 2014. [Accessed 04/20/2014, 2014]. http://online.wsj.com/news/articles/SB10001424052702304914204579393083711733854.
- 8.Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Annals of Internal Medicine. 2010;153(9):607–609. doi: 10.7326/0003-4819-153-9-201011020-00011. [DOI] [PubMed] [Google Scholar]
- 9.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 10.Cho JH, Shin E, Moon SS. Electronic-cigarette smoking experience among adolescents. The Journal of adolescent health. 2011;49(5):542–546. doi: 10.1016/j.jadohealth.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Camenga DR, Delmerico J, Kong G, et al. Trends in use of electronic nicotine delivery systems by adolescents. Addictive Behaviors. 2014;39(1):338–340. doi: 10.1016/j.addbeh.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutfin EL, McCoy TP, Morrell HE, Hoeppner BB, Wolfson M. Electronic cigarette use by college students. Drug and alcohol dependence. 2013;131(3):214–221. doi: 10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/publichealth perspectives. International journal of clinical practice. 2011;65(10):1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell K, Keane H. Nicotine control: E-cigarettes, smoking and addiction. The International journal on drug policy. 2012;23(3):242–247. doi: 10.1016/j.drugpo.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 15.BJW . FDA. U.S. Food and Drug Administration Web site: FDA. 2009. Summary of results: laboratory analysis of electronic cigarettes. [Google Scholar]
- 16.Sottera, Inc vs Food and Drug Administration; USCoAftDoC, editor. Circuit. 2010. [Google Scholar]
- 17.U.S. Food and Drug Administration Website: FDA. Apr 24, 2014. FDA proposes to extend its tobacco authority to additional tobacco products, including e-cigarettes. [press release] 2014. [Google Scholar]
- 18.State US. Local Laws Regulating Use of Electronic Cigarettes. AMERICAN NONSMOKERS’ RIGHTS FOUNDATION; Apr 29, 2014. 2014. [Google Scholar]
- 19.Baker H. Regulation of electronic cigarettes. The Lancet Oncology. 2013;14(8):e301. [Google Scholar]
- 20.Torjesen I. E-cigarettes are to be regulated as medicines from 2016. BMJ. 2013;346:f3859. doi: 10.1136/bmj.f3859. [DOI] [PubMed] [Google Scholar]
- 21.Bertholon JF, Becquemin MH, Annesi-Maesano I, Dautzenberg B. Electronic Cigarettes: A Short Review. Respiration. 2013;86(5):433–438. doi: 10.1159/000353253. [DOI] [PubMed] [Google Scholar]
- 22.Varughese S, Teschke K, Brauer M, Chow Y, van Netten C, Kennedy SM. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. American Journal of Industrial Medicine. 2005;47(5):411–418. doi: 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- 23.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 24.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM. Systemic effects of smoking. CHEST. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 25.Flouris AD, Poulianiti KP, Chorti MS, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food and Chemical Toxicology. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Reregistration Eligibility Decision for Nicotine. United States Environmental Protection Agency. 2008 [Google Scholar]
- 27.Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Archives of Toxicology. 2014;88:5–7. doi: 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology. 2012;34(4):529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odum LE, O’Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? Journal of Pharmacy Practice. 2012;25(6):611–614. doi: 10.1177/0897190012451909. [DOI] [PubMed] [Google Scholar]
- 31.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tobacco Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 32.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marlatt GA. Harm Reduction: Pragmatic Strategies for Managing High-Risk Behaviors. Guilford Press; 2002. [Google Scholar]
- 34.Etter JF. Electronic cigarettes: a survey of us. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. American Journal of Preventive Medicine. 2011;40(4):472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors. 2012;37(8):970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]