Abstract
Purpose
Predictive value and accuracy of the acute pain trajectory were compared with those of pain intensity at 1 day after the surgery for pain prevalence at 6 months after the surgery.
Materials and methods
Female patients scheduled for breast cancer surgery were eligible for this study. Patients were questioned about pain intensity daily during the 7 days after surgery. Presence of pain, its location, and intensity as well as the Japanese version of the quality of the recovery-40 (QOR-40) were determined in an interview prior to and at 6 months after the surgery. Acute pain trajectory was determined by a group-based trajectory modeling analysis that was based on the pain intensity at 1–7 days after surgery. Predictive value of the acute pain trajectory for the presence of pain at 6 months after the surgery was assessed by a logistic regression model. The predictive value was compared with pain intensity at 1 day after the surgery.
Results
A total of 123 participants completed the 6-month follow-up. The three-cluster model (mild, moderate, and severe pain) was considered to be the most statistically appropriate model for the acute pain trajectory. After 6 months, 51.2% and 8.9% of participants reported pain and severe pain, respectively. Presence of pain at 6 months after the surgery was associated with poor recovery. The severe pain cluster was significantly associated with the presence of pain at 6 months after the surgery (adjusted odds ratio, 9.40; P<0.001 vs mild pain cluster).
Conclusion
Classification of patients according to the acute pain trajectory, when compared with the classification according to pain intensity at 1 day after the surgery, made it possible to predict with better precision those patients who will develop persistent postsurgical pain.
Keywords: group-based trajectory analysis, acute postsurgical pain, chronic postsurgical pain, quality of recovery, breast cancer surgery
Introduction
Persistent postsurgical pain frequently occurs and has a serious negative impact on the health of patients undergoing surgery,1 which includes breast cancer surgery patients.2,3 Transition from acute to persistent postsurgical pain, or failure of acute pain resolution, is a consequence of complex pathophysiology.4 Because there are often multiple conditions that lead to pain chronicity after surgery,5 it has been difficult to establish an approach that can prevent this pain.6 Thus, identifying a patient at risk and then implementing adequate treatments early after the surgery can be beneficial in helping to reduce these negative effects.
The resolution of acute postsurgical pain is a dynamic process. It is affected by the degree of tissue damage, the perioperative pharmacological interaction, and the specific characteristics of the patient. Chronological analysis of the trajectory can provide precise information on the intensity and duration of acute postsurgical pain, as opposed to measuring pain intensity only once, eg, pain assessment at 1 day after the surgery.7,8 Evidence has suggested that the acute pain trajectory can be better described when using several different subclass patterns (clusters) rather than following a single typical trajectory.9 Recently, group-based trajectory modeling (GBTM) has been established as a statistical method that can be used to determine the number and characteristics of the trajectory clusters for individuals who will have a similar outcome progression over time.10,11 In the present study, we attempted to use GBTM analysis to establish clusters of trajectories representing the progression of acute pain after breast cancer surgery.
Acute postsurgical pain is one of the major predictive factors for persistent postsurgical pain.2,12–14 The initial pain intensity as well as the rate of resolution from the initial pain independently influenced the prevalence of persistent pain.15 Acute pain trajectory can be retrospectively classified based on the presence of persistent postsurgical pain.16 These lines of evidence led us to hypothesize that GBTM-based clusters of acute pain trajectory can prospectively predict a cluster of patients who are likely to develop persistent postsurgical pain in the future. To test this hypothesis, we investigated the association between the GBTM-based cluster of acute pain trajectory and pain prevalence at 6 months after breast cancer surgery. The predictive value of the pain trajectory was compared with the pain assessment at 1 day after the surgery.
Materials and methods
Participants were collected as part of a research program that was designed to address persistent pain and the quality of recovery after breast cancer surgery. The data collected by this program have not been published elsewhere. The program was designed as a prospective observational investigation of patients who underwent breast cancer surgery at a single university hospital. The protocol for the whole program was approved by the Institutional Ethics Committee of the Kyoto Prefectural University of Medicine (ERB-C-244-2). Informed consent was obtained using a written format and recorded by an electronic or paper document from all the patients. Patients were consecutively recruited from January 1, 2015, to April 31, 2016.
Participants
Inclusion criteria consisted of women aged 18 to 90 years old who were scheduled for breast cancer surgery under general anesthesia. Exclusion criteria included other malignancies, medical history of psychological or neurological disorder, history of breast surgery within 3 months on the same side, bilateral surgery, pregnancy or lactating, uncontrolled diabetes mellitus, severe obesity [body mass index (BMI) >35], cognitive impairment, or being unable to respond to the pain questionnaire. Although participants who underwent reconstructive surgery were enrolled into the overall research project, these patients were excluded from the analysis of the present study because acute pain intensity after breast cancer surgery with reconstruction differs from that seen in breast cancer surgery without reconstruction.17
Surgery, anesthesia, and postsurgical pain management
Each patient underwent either breast-conserving surgery or a mastectomy. Patients with clinically negative nodes underwent sentinel lymph node biopsy (SLNB). If the intra-operative diagnosis of SLNB revealed positive metastasis, axillary lymph node dissection (ALND) was additionally performed. Patients with clinically positive nodes underwent ALND without SLNB.
All patients underwent surgery while under general anesthesia. None of the patients received neuraxial or peripheral nerve blockade. Three surgeons independently performed the surgery during the study period. Two or three oncologists were also involved in the patients’ care. Anesthesia induction was performed using 2–3 mg/kg of propofol, 1–2 µg/kg of fentanyl, and 6–8 mg/kg of rocuronium. Anesthesia was maintained by continuous propofol infusion or sevoflurane inhalation with a continuous infusion of remifentanil or intermittent infusion of fentanyl. At the end of anesthesia, 1–2 µg/kg of fentanyl with or without 1,000 mg of acetaminophen or 50 mg of flurbiprofen was intravenously administered for postanesthesia pain management. Patients were extubated and transferred to the surgical ward for post-surgical care. Standard postsurgical analgesics included oral or intravenous acetaminophen, intravenous flurbiprofen, and oral loxoprofen. All patients treated with breast-conserving surgery underwent whole breast irradiation at a standard dose of 50.0 Gy in 25 fractions. In cases of microscopically incomplete resection (ie, cancer-free margin of the specimen was smaller than 5 mm), a boost dose of 10.0 Gy in five fractions was added on the tumor bed. Postmastectomy radiotherapy (PMRT) was administered to patients who had locally advanced cancer or had more than four positive axillary lymph nodes. PMRT included the chest wall, axillary fossa, and the supraclavicular fossa with a total dose of 50.0 Gy in 25 fractions. If there was a microscopically incomplete margin, even in the mastectomy cases, a 10.0 Gy boost was added on the tumor bed. PMRT was usually started between 1 and 2 months after the surgery.
Patients with a high risk of recurrence were treated with either pre- or postoperative chemotherapy. The regimen was at the discretion of the oncologists in accordance with the recommendation of the National Comprehensive Cancer Network Guidelines and which included an anthracycline and a taxane. Trastuzumab was administered to patients with human epidermal growth factor receptor type 2 positive cancer. Endocrine therapy was administered to patients who were estrogen receptor–positive and/or progesterone receptor–positive. Premenopausal women were treated with tamoxifen, while postmenopausal women were treated with aromatase inhibitors. All participants underwent postsurgical rehabilitation in order to facilitate arm movement by a physical therapist and/or nurse. All participants were encouraged to continue the same exercises after their hospital discharge. Four participants underwent a part of their postsurgical treatments at a regional hospital, with the remainder of the subjects completing the treatments at the university hospital.
Data collection
Baseline data were collected 2–3 weeks before the surgery during the interview that took place at the preoperative anesthesia clinic. Three investigators (MMori, MMatsuda, and FA) were responsible for interviewing the participants. All patients were interviewed for demographic data, presence or absence of any sort of pain, pain intensity measured on a 100 mm visual analog scale (VAS), and the Japanese version of the short-form McGill Pain Questionnaire (SF-MPQ).18 Pain intensity at the site of surgery was assessed every day starting from 1 to 7 days after the surgery. Every measurement was done at the end of day at the surgical ward. Participants were asked to recall their pain intensities on each day during stretches, arm swings, deep breaths, and coughing episodes and then rate them using a numerical rating scale (NRS) to define the movement-evoked pain. Pain conditions at 6 months after the surgery were determined during the postsurgical follow-up clinic that was conducted at our hospital. Presence of pain or severe pain prior to surgery and at 6 months after the surgery was determined based on the question, “Do you have any pain or severe pain at the location associated with your surgery?” The classification of pain into three categories (no, mild, and severe) was adopted from a previous study.19 Participants were assessed for the pain intensity (VAS and SF-MPQ) as well as for the pain location. Quality of recovery was also assessed by the Japanese version of the quality of the recovery-40 (QOR-40).20 QOR-40 is a standard measure of the quality of the recovery after surgery and consists of a 40-item questionnaire that covers the emotional state, physical comfort, psychological support, physical independence, and pain.21 Each question is graded on a five-point Likert scale. QOR-40 scores range from 40 (which indicates poor quality) to 200 (which indicates excellent quality). Postsurgical assessment was performed by one investigator (AO).
Study outcome
The aim of our present study was to compare the predictive accuracy outcomes of the GBTM-based acute pain trajectory and pain intensity at 1 day after the surgery for pain prevalence at 6 months after the surgery. This was determined based on answers to questions regarding whether the participants had any pain (mild or severe) in the area associated with the surgical procedure.
Sample size
The sample size for the entire project was determined in order to detect differences in the QOR-40 score between participants with and without pain at 6 months after breast cancer surgery. We considered a 10-point change to be indicative of a significant difference for the average QOR-40 with regard to the effect of persistent pain. Estimated SD of the QOR-40 was 20. Pain prevalence at 6 months after breast cancer surgery was estimated to be 30%. Power analysis demonstrated that the study required at least 156 participants. Therefore, this study was designed to enroll at least 160 participants into the project.
Acute pain trajectory
Acute pain trajectory, which was based on the longitudinal data of the pain intensity and measured as the NRS starting from 1 to 7 days after the surgery, was constructed using GBTM. We utilized the pain intensity during movement for determining the acute pain trajectory because it has been shown that movement-evoked pain is more closely associated with persistent pain.22,23 We utilized a semiparametric GBTM strategy using the “traj” procedure of the Stata Statistics/Data Analysis software (version 12.1 SE; StataCorp, College Station, TX, USA), which is equivalent of the “proc traj” procedure of the Statistical Analysis System (SAS) software. This procedure implements a longitudinal data analysis methodology for estimating the GBTM. GBTM is a specialized form of the finite mixture modeling that analyzes repeated-measures data using the maximum likelihood to identify distinct clusters of individuals following statistically similar outcome progressions over time. The NRS of postoperative pain (0–10) starting from 1 to 7 days after the surgery was used to describe the postoperative acute pain trajectory. We adopted quadratic function models for each cluster because the mean pain of all patients decreased steeply at first followed by a mild decrease afterward. Bayesian information criteria (BIC), Akaike information criteria (AIC), and log likelihood were calculated for each model. Entropy was calculated using the posterior probability.24 Lower values of BIC and AIC indicate that there is a better statistical model in terms of balance of model fitting and model complexity. BIC and AIC use different penalties for the number of parameters (model complexity). A higher value of entropy indicates a better classification model. We selected the model that best described simple and characteristic trajectories according to these indices, taking into account the number of patients in each group and the trajectory patterns.
Association between acute pain trajectory and persistent pain prevalence
Predictive value of the acute pain trajectory for the presence of pain at 6 months after the surgery was assessed by a logistic regression analysis. Age, presurgical pain, ALND procedure, total mastectomy, chemotherapy, and radiation therapy were used as adjusting factors. These factors were determined prior to the analysis based on the risk factors reported in previous studies.3,12,19,25 Preoperative information including age and presurgical pain were included, as these factors can have an influence on the severity of acute pain26 and on the prevalence of chronic pain after the surgery.27
As a reference, the prediction ability of pain assessed at a point in the acute postsurgical period was determined using the same procedure. Participants were categorized into three groups based on the pain intensity measured at 1 day after the surgery by NRS (0–3, 4–6, and 7–10 for the no/mild, moderate, and severe pain groups, respectively) in order to make a direct comparison with the acute pain trajectory. Cutoff points of 3/4 and 6/7 were determined based on previous studies.14,28–30 The adjusted odds ratio for the presence of pain at 6 months after the surgery was compared between the pain trajectory and the pain at 1 day after the surgery. The predictive accuracy was determined using a receiver operating characteristic (ROC) curve analysis. Area under the curve (AUC) of the ROC curve was calculated in each model.
Statistical analysis
Numerical data were expressed in terms of median and interquartile range (first quartile to third quartile), while the categorical data were expressed with numbers and percentages. GBTM was performed according to the “traj” procedure of the Stata Statistics/Data Analysis software (version 12.1 SE; StataCorp). All analyses other than the GBTM analysis were performed using R (version 3.3.1; The R Foundation for Statistical Computing, Vienna, Austria) and R commander (version 2.3-0). We considered P<0.05 to indicate statistical significance.
Results
Of the 224 patients screened during the whole study registry, there were 175 patients who were considered to be eligible for the present study. After the surgery, 168 patients completed a 6-month follow-up. The 45 participants who received reconstructive surgery were excluded from the present analysis (Figure 1). Table 1 summarizes the demographic data of the participants (n=123). ALND was performed in 24.4% (n=30) of the participants. Chemotherapy, radiation therapy, and endocrine therapy were performed in 43.1% (n=53), 32.5% (n=40), and 79.7% (n=98) of the participants, respectively.
Figure 1.
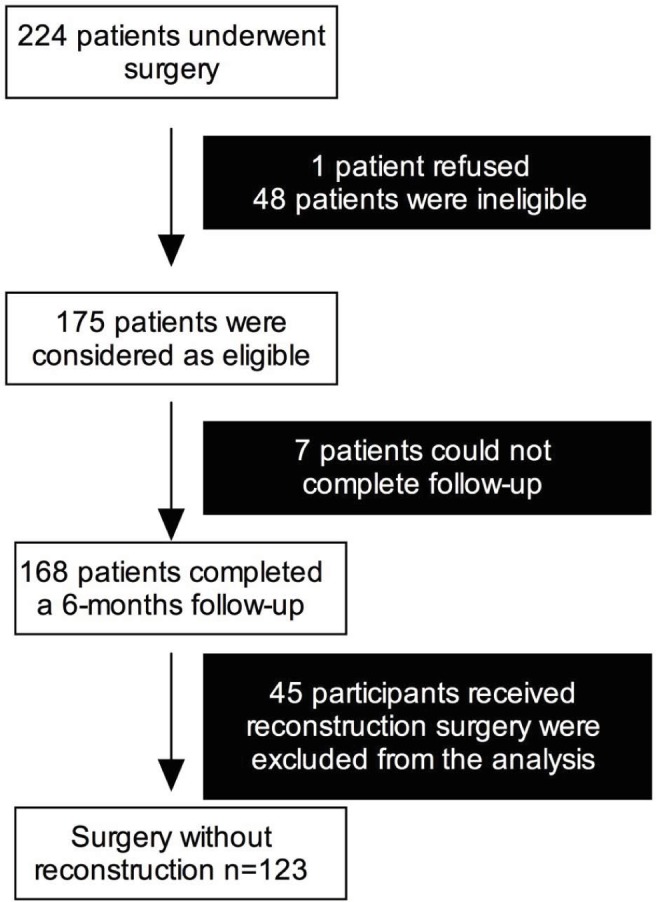
Participants enrolled in the study.
Table 1.
Demographic and patient characteristics
| Overall participants (N=123) | |
|---|---|
| Patient-related data | |
| Age (years) | 59 (48, 68) |
| BMI, median | 22.1 (19.7, 25.1) |
| ASA-PS | |
| 1 | 68 (55.3%) |
| 2 | 55 (44.7%) |
| Preoperative VAS (mm), median | 0 (0, 6.9) |
| Preoperative SF-MPQ, median | 0 (0, 1) |
| Preoperative pain | 44 (35.8%) |
| Treatment-related data, n (%) | |
| Mastectomy | |
| Partial mastectomy | 48 (39%) |
| Total mastectomy | 75 (61%) |
| ALND | 30 (24.4%) |
| SLNB | 103 (83.7%) |
| Operation time (min) | 113 (97, 139.5) |
| Intraoperative blood loss (g) | 52 (20.5, 86) |
| Nonsurgical treatment data | |
| Perioperative chemotherapy | 53 (43.1%) |
| Radiation therapy | 40 (32.5%) |
| Endocrine therapy | 98 (79.7%) |
Abbreviations: ALND, axillary lymph node dissection; ASA-PS, American Society for Anesthesiologist-physical status; BMI, body mass index; SF-MPQ, short-form McGill Pain Questionnaire; SLNB, sentinel lymph node biopsy; VAS, visual analog scale.
Acute pain trajectory modeling
We have developed two- to six-cluster models as candidates for acute pain trajectory. From a statistical perspective, the two- to six-cluster models were tested using goodness of fit analyses (Table 2). Entropy was highest in the three- and four-cluster models. There was a continuous decrease in the BIC and AIC from the two- to six-cluster models. In addition to these statistical results, we considered the three (mild, moderate, and severe pain) cluster model to be the most acceptable model because it is a simple and commonly accepted model for grading pain. Figure 2 presents the three-cluster model for the acute pain trajectory. The severe pain cluster was characterized by a high initial pain with an NRS of more than 6. The initial pain intensity was approximately 4 and 1 for the moderate and mild pain clusters, respectively. At 7 days after the surgery, pain intensity of the severe pain cluster was still high, with an NRS of more than 4. Among the participants, 16.3% (n=20), 53.7% (n=66), and 30.0% (n=37) belonged to the mild, moderate, and severe pain cluster, respectively. Table 3 presents the demographic data of the three clusters. GBTM analysis based on the pain at rest identified a three-cluster model that was similar to movement-evoked pain (data not shown). Categorization according to the pain at 1 day after the surgery resulted in 46.3% (n=57) of the participants being allocated to the no/mild pain group, while 33.3% (n=41) of the participants were allocated to the moderate pain group and 20.3% (n=25) were allocated to the severe pain group.
Table 2.
Indices of group-based trajectory modeling
| Number of clusters | BIC (n=861) |
BIC (n=123) |
AIC | LL | Entropy |
|---|---|---|---|---|---|
| 2 | 3,275.88 | 3,260.30 | −1,618.90 | −1,610.90 | 0.93 |
| 3 | 3,089.12 | 3,065.76 | −1,516.01 | −1,504.01 | 0.96 |
| 4 | 2,958.36 | 2,927.22 | −1,441.12 | −1,425.12 | 0.96 |
| 5 | 2,900.46 | 2,861.56 | −1,402.65 | −1,382.65 | 0.92 |
| 6 | 2,895.20 | 2,848.50 | −1,390.51 | −1,366.51 | 0.93 |
Notes: Likelihood AIC=−2×log-likelihood+2×(the number of parameters); BIC=−2×log-likelihood+(the number of parameters)×log(sample size). BIC (n=123) represents as sample size-adjusted BIC.
Abbreviations: AIC, Akaike information criteria; BIC, Bayesian information criteria; LL, Log likelihood.
Figure 2.
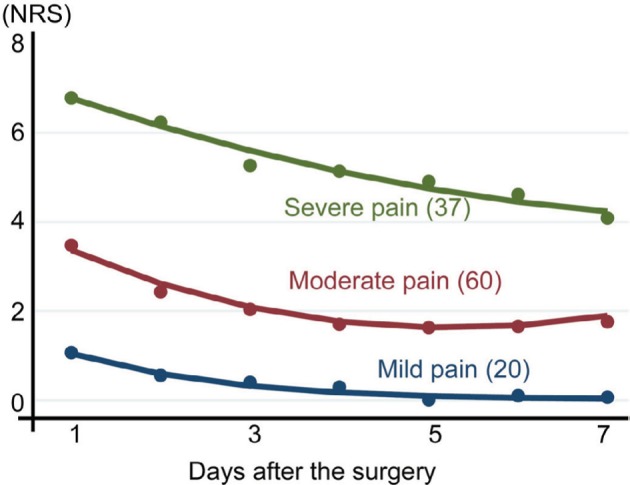
Acute pain trajectory three-cluster model.
Notes: Severe, moderate, and mild pain clusters demonstrated different intensities and durations of acute pain after breast cancer surgery without reconstruction. The numbers indicates the number of participants belonging to each cluster.
Abbreviation: NRS, numerical rating scale.
Table 3.
Patient characteristics of three trajectory clusters
| Acute pain trajectory clusters | Mild pain (N=20) | Moderate pain (N=66) | Severe pain (N=37) |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 67.5 (53.8, 72.3) | 58 (48.3, 67) | 56 (46, 65) |
| BMI, median | 23 (21.2, 25.3) | 21.3 (19.2, 24.5) | 21.9 (20.2, 26.2) |
| ASA-PS | |||
| 1 | 10 (14.7%) | 36 (52.9%) | 22 (32.4%) |
| 2 | 10 (18.2%) | 30 (54.5%) | 15 (27.3%) |
| Preoperative VAS (mm), median | 0 (0, 0) | 0 (0, 1) | 1 (0, 1) |
| Preoperative SF-MPQ, median | 0 (0, 0.6) | 0 (0, 0.8) | 3.8 (0, 16.2) |
| Preoperative pain | 4 (9.1%) | 19 (43.2%) | 21 (47.7%) |
| Surgical treatment data | |||
| Mastectomy | |||
| Partial mastectomy | 8 (16.7%) | 29 (60.4%) | 11 (22.9%) |
| Total mastectomy | 12 (16%) | 37 (49.3%) | 26 (34.7%) |
| ALND | 3 (10%) | 14 (46.7%) | 13 (43.3%) |
| SLNB | 18 (17.5%) | 56 (54.4%) | 29 (28.2%) |
| Operation time (min) | 104 (80.5, 119.8) | 111.5 (99, 132.8) | 123 (103, 158) |
| Intraoperative blood loss (g) | 26.5 (19, 76.3) | 42 (20, 70.8) | 72 (41, 127) |
| Nonsurgical treatment data | |||
| Perioperative chemotherapy | 5 (9.4%) | 29 (54.7%) | 19 (35.8%) |
| Radiation therapy | 5 (12.5%) | 27 (67.5%) | 8 (20%) |
| Endocrine therapy | 16 (16.3%) | 54 (55.1%) | 28 (28.6%) |
Abbreviations: ALND, axillary lymph node dissection; ASA-PS, American Society for Anesthesiologist-physical status; BMI, body mass index; SF-MPQ, short-form McGill Pain Questionnaire; SLNB, sentinel lymph node biopsy VAS, visual analog scale.
Prevalence, severity, and location of persistent pain
Table 4 lists the prevalence of pain, VAS, SF-MPQ, and QOR-40 score at 6 months after the surgery. Overall pain prevalence at 6 months after surgery was 51.2% (n=63). Severe pain was observed in 8.9% (n=11) of the participants. VAS value, total SF-MPQ score, and sensory pain subscale, but not affective pain subscale, were higher in participants with pain compared with the no-pain participants. QOR-40 score was significantly lower in participants with pain compared with the no-pain participants. Table 5 lists the location of pain in the participants at 6 months after the surgery. Common pain locations were chest (39.8%, n=49), axilla (27.6%, n=34), and arm (13.8%, n=17).
Table 4.
Pain prevalence and severity at 6 months after surgery
| No pain | Pain
|
|||
|---|---|---|---|---|
| Mild | Severe | Overall | ||
| Pain prevalence, n (%) | 60 (48.8%) | 52 (42.3%) | 11 (8.9%) | 63 (51.2%) |
| SF-MPQ total | 0 (0, 1) | 2 (1, 4) | 18 (7, 24) | 2 (1, 7) |
| SF-MPQ APS | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 2 (1, 6) |
| SF-MPQ SPS | 0 (0, 1) | 2 (1, 5) | 2 (1, 4) | 11 (5, 16) |
| VAS | 0 (0, 2) | 12 (4, 23) | 59 (36, 68) | 16 (4, 35) |
| QOR-40 | 198 (189, 200) | 183 (173, 195) | 153 (140, 164) | 177 (166, 193) |
Abbreviations: APS, affective pain subscale; QOR-40, quality of recovery-40; SF-MPQ, short-form McGill Pain Questionnaire; SPS, sensory pain subscale; VAS, visual analog scale.
Table 5.
Location of pain at 6 months after surgery
| Location | N (%) |
|---|---|
| Arm | 17 (13.8%) |
| Axilla | 34 (27.6%) |
| Chest | 49 (39.8%) |
| Back | 9 (7.3%) |
| Other | 12 (9.8%) |
Relationship between acute pain trajectory and persistent pain after the surgery
Table 6 presents the results of the univariate analysis of the perioperative factors for the pain at 6 months after the surgery. Presurgical pain, ALND procedure, a severe pain cluster in the acute pain trajectory, and severe pain group in acute pain at 1 day after the surgery were significantly associated with pain at 6 months after the surgery.
Table 6.
Unadjusted association between acute pain and pain at 6 months after the surgery
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Acute pain trajectory | ||
| Mild pain cluster | 1 | |
| Moderate pain cluster | 3.33 (1.09, 12.6) | 0.049* |
| Severe pain cluster | 14.5 (4.09, 63.01) | <0.001* |
| Acute pain 1 day after the surgery | ||
| No/mild pain group | 1 | |
| Moderate pain group | 1.71 (0.76, 3.85) | 0.194 |
| Severe pain group | 3.80 (1.37, 10.55) | 0.010* |
| Age (stratified by 10 years old) | 1.07 (0.80, 1.44) | 0.659 |
| Presurgical pain | 4.13 (1.89, 9.49) | <0.001* |
| Mastectomy | ||
| Partial mastectomy | 1 | |
| Total mastectomy | 1.24 (0.60, 2.58) | 0.558 |
| ALND | 2.83 (1.20, 7.13) | 0.021* |
| Chemotherapy | 1.67 (0.82, 3.47) | 0.162 |
| Radiation therapy | 1.25 (0.59, 2.69) | 0.519 |
Notes:
Indicates statistical significance.
Abbreviation: ALND, Axillary lymph node dissection.
Table 7 presents the results of the logistic regression analysis of the acute pain trajectory or pain at 1 day after the surgery for the prediction of pain at 6 months after the surgery. The severe pain cluster in the acute pain trajectory was associated with the presence of pain at 6 months after the surgery (P<0.001). The odds ratio of the severe pain cluster was larger than that of the moderate pain cluster (9.40 and 2.31 for the severe pain cluster and moderate pain cluster; P<0.001 and P=0.092 for the severe pain cluster and moderate pain cluster, respectively). The severe pain group at 1 day after the surgery was not associated with the presence of pain at 6 months after the surgery in the multivariate analysis (P=0.063). In the acute pain at 1 day after the surgery model, the adjusted odds ratios for the severe and moderate pain groups were similar (3.26 and 2.40 for the severe and moderate pain groups, respectively; P=0.063 and P=0.062 for the severe and moderate pain groups, respectively) and did not reach statistical significance compared with the no/mild pain group. The finding that the ROC-AUC value of acute pain trajectory was larger than the pain at 1 day after the surgery means that the acute pain trajectory model predicts persistent pain more accurately than pain at 1 day after the surgery.
Table 7.
Adjusted association between acute pain and pain at 6 months after the surgery
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Acute pain trajectory | ||
| Mild pain cluster | 1 | |
| Moderate pain cluster | 2.31 (0.89, 6.31) | 0.092 |
| Severe pain cluster | 9.40 (2.73, 37.07) | <0.001* |
| Age (by 10 years) | 1.28 (0.89, 1.16) | 0.190 |
| Presurgical pain | 3.73 (1.43, 10.36) | <0.009* |
| Mastectomy | ||
| Partial mastectomy | 1 | |
| Total mastectomy | 1.36 (0.44, 4.46) | 0.597 |
| ALND | 2.17 (0.71, 7.03) | 0.182 |
| Chemotherapy | 1.80 (0.66, 5.07) | 0.260 |
| Radiation therapy | 4.53 (1.29, 18.59) | 0.025* |
| ROC-AUC | 0.81 (0.73, 0.89) | |
| Acute pain 1 day after the surgery | ||
| No/mild pain group | 1 | |
| Moderate pain group | 2.40 (0.97, 6.13) | 0.062 |
| Severe pain group | 3.26 (0.96, 11.86) | 0.063 |
| Age (stratified by 10 years old) | 1.18 (0.84, 1.68) | 0.330 |
| Presurgical pain | 4.50 (1.74, 12.56) | 0.002* |
| Mastectomy | ||
| Partial mastectomy | 1 | |
| Total mastectomy | 1.51 (0.51, 4.62) | 0.460 |
| ALND | 2.92 (1.01, 9.10) | 0.054 |
| Chemotherapy | 1.80 (0.66, 5.07) | 0.260 |
| Radiation therapy | 3.84 (1.16, 14.58) | 0.036* |
| ROC-AUC | 0.78 (0.69–0.86) |
Notes:
Indicates statistical significance.
Abbreviations: ALND, axillary lymph node dissection; ROC-AUC, receiver operating curve-area under the curve.
Discussion
In the present study, we developed a three-cluster trajectory model based on the GBTM analysis that proved to be an adequate model for the progression of acute pain after breast cancer surgery. We observed a high prevalence of persistent postsurgical pain in the participants who were categorized as the severe pain cluster. These results show that classifying patients according to the acute pain trajectory makes it possible to determine a more accurate prediction of which patients will develop persistent postsurgical pain compared with classifying patients according to pain intensity at 1 day after the surgery.
Characteristics of acute pain trajectory
GBTM analysis enabled us to model several subclasses of patients who showed similar progress of their longitudinal data.31 In the present analysis, we identified three distinctive pain trajectories (mild, moderate, and severe pain clusters) that best represented a subclass of patients who had a similar intensity, duration, and resolution of acute pain after breast cancer surgery. To the best of our knowledge, this is the first study to demonstrate clusters of individuals who exhibited similar pain progression after breast cancer surgery. The severe pain cluster demonstrated poor pain resolution, with the pain intensity at 7 days after surgery found to be even higher than the initial pain intensity seen for the mild or moderate pain clusters. Although it is theoretically possible for no-pain clusters to exist, we did not identify any of these clusters in the present analysis. Acute pain trajectory was developed as a way of better understanding day-by-day pain progression after the initial surgery. It is our belief that participants are able to very precisely figure out their daily pain experience at the end of day.
Pain prevalence and severity after breast cancer surgery
ICD-11 defined persistent postsurgical pain as a pain that persists for at least 3 months after surgery.32 In breast cancer treatments, postsurgical radiation therapy is usually not completed until 4–5 months after breast cancer surgery. Based on the definition and the clinical schedule of the invasive procedure, we considered surveillance at 6 months after the surgery to be adequate for obtaining generalized information about persistent postsurgical pain. According to previous studies performed in European countries, 44%–65% of the patients reported pain while 13%–16% reported moderate to severe pain at 6–12 months after the breast cancer surgery.14,19,33 In the present study, we observed that 51% and 9% of the participants complained of pain and severe pain, respectively, which demonstrated a similar prevalence of persistent postsurgical pain across countries,34 even in countries with different ethnicities and cultures.
Persistent pain is believed to decrease a patients’ quality of life. In our study, presence of pain at 6 months after surgery was associated with a poor quality of recovery after the surgery. Differences in the QOR-40 score between participants with and without pain exceeded the differences in the QOR-40 between patients with or without comorbidity.21 It is also remarkable that the lower QOR-40 was sustained for at least 6 months after surgery in participants with pain, as it has been previously shown that the decline of the QOR-40 is generally transient and indicates immediate recovery after breast cancer surgery.35
Acute pain trajectory and prevalence of persistent pain
Initial intensity1,36 and rate of resolution15 of acute postsurgical pain have been reported to be independently associated with persistent postsurgical pain. Our present study investigated the predictive value of the acute pain trajectory for persistent pain after surgery. After adjusting for the perioperative factors, the severe pain cluster showed a strong association with persistent pain. The odds ratio of the severe pain cluster was strikingly high compared with that seen for the severe pain group in the pain at 1 day after the surgery model. These results suggest that the GBTM analysis of acute pain was able to identify the subgroup of patients with a high risk for persistent pain after breast cancer surgery. This result seems to be reasonable, as the acute pain trajectory is characterized by both the initial pain intensity and the rate of resolution. Furthermore, the ROC-AUC analysis confirmed that there was a better precision for persistent pain by the acute pain cluster.
Our trajectory included daily pain intensity for 7 days after the surgery. A previous investigation demonstrated the importance of the pain intensity at 7 days after the surgery with regard to the prediction of persistent pain.27 Another analysis that examined daily pain measurements for up to 60 days after the surgery suggested that the change of pain progression occurred during the 10–20 days after the surgery.37 Taken together with the present observations, these data highlight the important role of pain progression up to the subacute phase for the prediction of persistent pain after surgery. It is worth noting, however, that while the longer observational period gave us a better chance to determine the prediction of persistent pain with a better precision, it also increased the risk of missing the chance to start an adequate treatment in the patient. Thus, we consider that 7 days for the trajectory was an adequate and feasible tool for identifying patients at risk for persistent postsurgical pain.
GBTM-based trajectory analysis has been used to explain the heterogeneity of pain progression10 and its association with a lower functional recovery.38,39 The present study provides scientific evidence on the usefulness of GBTM-based trajectory analysis of acute pain for the prediction of persistent postsurgical pain with better precision. The strong association found between the severe pain cluster and the presence of persistent pain indicates the biological significance of the transition from acute to chronic pain in the pathogenesis of persistent postsurgical pain.
Radiation therapy and chemotherapy have been recognized as candidate risk factors for persistent pain after breast cancer surgery.14,19,25 In our study, radiation therapy but not chemotherapy was identified as an independent factor that was associated with pain at 6 months after surgery.14 Therefore, we consider that radiation therapy increased the risk of chronic pain after the surgery in our study population. Our results are in accordance with the previous study that showed radiotherapy but not chemotherapy that was associated with chronic pain after the surgery. However, we cannot exclude the possibility of chemotherapy as a risk factor, as the aim of our present study was not designed to focus on this issue.
Strengths and limitations of this study
This study was performed in a prospective manner in order to avoid any data collection bias. Pain prevalence was measured at 6 months after the surgery, which fulfilled the definition of persistent pain as defined by the ICD-11. This is the first study to investigate pain prevalence for postmastectomy patients with Asian ethnicity. Our data clearly demonstrated that pain prevalence and risk factors were similar between Asian and European ethnicities.
However, the present study had several limitations. The sample size of this study was relatively small. While we found a significant association between the acute pain trajectory and persistent pain after the surgery, we did not investigate any causality relationship. In addition, as this investigation was performed at a single university hospital, some of our results will require further validation in order for generalization of the present results. Another study limitation is that in order to follow the recommendations of the Japanese Breast Cancer Society clinical practice guidelines, we did not perform identification of the intercostobrachial nerve during the lymph node dissection.40 Consequently, it was not possible to distinguish the ALND participants who underwent sectioning or preserving of the intercostobrachial nerve. Moreover, a previous study has also shown that persistent postsurgical pain was associated with preoperative psychosocial conditions.12,33 However, our present study did not investigate the presence of these conditions. VAS was used to measure pain for 7 days after the surgery, whereas NRS was used to analyze pain prior to and at 6 months after surgery. Generally, the VAS value correlates well with NRS score. Even so, the use of a different analytical tool might have had an influence on the results of this study. While the multivariable regression analysis performed in this study had sufficient statistical power, we cannot exclude the possibility that determination of our sample size based on information other than that for our primary aim could have influenced the study outcome. We did not evaluate the contribution of the neuropathic component in our participants. Postsurgical pain with a neuropathic component is known to be associated with severe postsurgical pain.
Conclusion
In conclusion, we prospectively characterized the presence of pain after breast cancer surgery. Half of the patients complained about the presence of pain that was associated with a lower QOR-40. GBTM analysis of the pain trajectory demonstrated that participants with poor pain resolution from acute pain have an increased risk of pain at 6 months after surgery.
Acknowledgments
The authors would like to thank Professor Henrik Kehlet, Department of Clinical Medicine, Copenhagen University, for his valuable suggestions and comments. FA was supported by a Grant-in-Aid, Japan Society for the Promotion of Science (Tokyo, Japan).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 2.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 4.Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017;18359(4):359.e1e338–359. doi: 10.1016/j.jpain.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J. 2014;90226(1062):222–227. doi: 10.1136/postgradmedj-2013-132215. [DOI] [PubMed] [Google Scholar]
- 6.Kalso E. IV. Persistent post-surgery pain: research agenda for mechanisms, prevention, and treatment. Br J Anaesth. 2013;111(1):9–12. doi: 10.1093/bja/aet211. [DOI] [PubMed] [Google Scholar]
- 7.Chapman CR, Zaslansky R, Donaldson GW, Shinfeld A. Postoperative pain trajectories in cardiac surgery patients. Pain Res Treat. 2012;2012:608359–608368. doi: 10.1155/2012/608359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: the pain trajectory. J Pain. 2011;12(2):257–262. doi: 10.1016/j.jpain.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tighe PJ, Le-Wendling LT, Patel A, Zou B, Fillingim RB. Clinically derived early postoperative pain trajectories differ by age, sex, and type of surgery. Pain. 2015;156(4):609–617. doi: 10.1097/01.j.pain.0000460352.07836.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downie AS, Hancock MJ, Rzewuska M, Williams CM, Lin CW, Maher CG. Trajectories of acute low back pain: a latent class growth analysis. Pain. 2016;157(1):225–234. doi: 10.1097/j.pain.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 11.Kannampallil T, Galanter WL, Falck S, et al. Characterizing the pain score trajectories of hospitalized adult medical and surgical patients: a retrospective cohort study. Pain. 2016;157(12):2739–2746. doi: 10.1097/j.pain.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce J, Thornton AJ, Scott NW, et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer. 2012;107(6):937–946. doi: 10.1038/bjc.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13(12):1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meretoja TJ, Leidenius MHK, Tasmuth T, Sipilä R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311(1):90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 15.Althaus A, Arránz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J Pain. 2014;18(4):513–521. doi: 10.1002/j.1532-2149.2013.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavand’homme PM, Grosu I, France MN, Thienpont E. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: an observational study. Clin Orthop Relat Res. 2014;472(5):1409–1415. doi: 10.1007/s11999-013-3389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni AR, Pusic AL, Hamill JB, et al. Factors associated with acute postoperative pain following breast reconstruction. JPRAS Open. 2017;11:1–13. doi: 10.1016/j.jpra.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arimura T, Hosoi M, Tsukiyama Y, et al. Pain questionnaire development focusing on cross-cultural equivalence to the original questionnaire: the Japanese version of the Short-Form McGill Pain Questionnaire. Pain Med. 2012;13(4):541–551. doi: 10.1111/j.1526-4637.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 19.Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156(12):2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y, Wakita T, Fukuhara S, et al. Validation of the Japanese version of the quality of recovery score QoR-40. J Anesth. 2011;25(4):509–515. doi: 10.1007/s00540-011-1151-2. [DOI] [PubMed] [Google Scholar]
- 21.Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi: 10.1093/bja/aet014. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12(1):50–55. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Tasmuth T, Kataja M, Blomqvist C, von Smitten K, Kalso E. Treatment-related factors predisposing to chronic pain in patients with breast cancer–a multivariate approach. Acta Oncol. 1997;36(6):625–630. doi: 10.3109/02841869709001326. [DOI] [PubMed] [Google Scholar]
- 24.Ramaswamy V, Desarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science. 1993;12(1):103–124. [Google Scholar]
- 25.Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. 2013;14(10):1185–1195. doi: 10.1016/j.jpain.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111(3):657–677. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 27.Meretoja TJ, Andersen KG, Bruce J, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35(15):1660–1667. doi: 10.1200/JCO.2016.70.3413. [DOI] [PubMed] [Google Scholar]
- 28.Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120(5):1237–1245. doi: 10.1097/ALN.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain. 2010;149(2):194–201. doi: 10.1016/j.pain.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MP, Smith DG, Ehde DM, Robinsin LR. Pain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe pain. Pain. 2001;91(3):317–322. doi: 10.1016/S0304-3959(00)00459-0. [DOI] [PubMed] [Google Scholar]
- 31.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 32.Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232–243. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama Y, Iida H, Amaya F, et al. Prevalence of chronic postsurgical pain after thoracotomy and total knee arthroplasty: a retrospective multi-center study in Japan (Japanese Study Group of Subacute Postoperative Pain. J Anesth. 2018;32(3):434–438. doi: 10.1007/s00540-018-2481-0. [DOI] [PubMed] [Google Scholar]
- 35.Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: A randomised controlled trial. Eur J Anaesthesiol. 2018;35(3):215–223. doi: 10.1097/EJA.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 36.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 37.Houle TT, Miller S, Lang JE, et al. Day-to-day experience in resolution of pain after surgery. Pain. 2017;158(11):2147–2154. doi: 10.1097/j.pain.0000000000001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015;156(11):2383–2389. doi: 10.1097/j.pain.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagé MG, Katz J, Romero Escobar EM, et al. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain. 2015;156(3):460–468. doi: 10.1097/01.j.pain.0000460327.10515.2d. [DOI] [PubMed] [Google Scholar]
- 40.Jinno H, Inokuchi M, Ito T, et al. The Japanese Breast Cancer Society clinical practice guideline for surgical treatment of breast cancer, 2015 edition. Breast Cancer. 2016;23(3):367–377. doi: 10.1007/s12282-016-0671-x. [DOI] [PubMed] [Google Scholar]


